Abstract
The face inversion effect, or impaired recognition of upside down compared to upright faces, is used as a marker for the configural processing of faces in primates. The inversion effect in humans and chimpanzees is strongest for categories of stimuli for which subjects have considerable expertise, primarily conspecifics' faces. Moreover, discrimination performance decreases linearly as faces are incrementally rotated from upright to inverted. This suggests that rotated faces must be transformed, or normalized back into their most typical viewpoint before configural processing can ensue, and the greater the required normalization, the greater the likelihood of errors resulting. Previous studies in our lab have demonstrated a general face inversion effect in rhesus monkeys that was not influenced by expertise. Therefore, the present study examined the influence of rotation angle on the visual perception of face and nonface stimuli that varied in their level of expertise to further delineate the processes underlying the inversion effect in rhesus monkeys. Five subjects discriminated images in five orientation angles. Results showed significant linear impairments for all stimulus categories, including houses. However, compared to the upright images, only rhesus faces resulted in worse performance at rotation angles greater than 45°, suggesting stronger configural processing for stimuli for which subjects had the greatest expertise.
Keywords: Face processing, Expertise, Configural cues, Rotation
Introduction
One of the most replicable aspects of face processing in humans is the general impairment that occurs when faces are inverted 180°, typically referred to as the face inversion effect (Yin 1969). The inversion effect has been widely studied in humans and suggests that faces are processed using configural cues, or the spatial arrangement of features within the face, as opposed to specific facial features (Valentine 1988). When faces are inverted, the configural arrangement of facial features is disrupted and recognition accuracy and memory for faces declines, while information pertaining to individual features is preserved (Farah et al. 1995). Moreover, the inversion effect is stronger for categories of faces for which subjects have expertise, such as same-race faces, and even other stimulus categories for which subjects have considerable years of experience, such as dog show judges when viewing their breed of expertise (Diamond and Carey 1986). Thus, there appears to be a strong and stable relationship between expertise and configural processes in humans.
Several studies in humans have examined whether processing inverted faces involves the same perceptual mechanisms as upright face processing. The two-component mode, for example, suggests upright faces are processed using configural cues, i.e., the spatial arrangement of features within the face, whereas inverted faces are processed using a component strategy, i.e., extracting information pertaining to specific individual features (Carey and Diamond 1977; Diamond and Carey 1986). However, people's expertise with faces, that which depends on the use of configural cues, is limited to their perceptual experience. Faces are normally seen in an upright orientation, with only a small range of deviation in real-life encounters, so any other orientation would be atypical and would not be expected to recruit configural processing. People, for example, have as little experience viewing faces at right angles as they do inverted, but some experience exists for viewing minimal degrees of rotation, such as that comparable to a head-tilt. The prediction that results from the two-component/expertise hypothesis is a nonlinear relationship between discrimination performance and stimulus orientation. As a stimulus is rotated from upright to inverted, there should be a shift in performance at a specific angle of orientation that represents the switch between an upright/configural strategy to an inverted/feature-based strategy, and after which expertise with faces is minimal.
The alternative hypothesis is that because faces are seen most often in one orientation (upright), there is only a single processing strategy, that will be called the unified configural strategy, that is maximized to process faces in the orientation in which they are most often encountered. While this is similar to the assumptions based on expertise, described above, the mechanisms are different. If a face is encountered in an unusual orientation, the unified configural strategy predicts that it must first be normalized into a more typical viewpoint before recognition processes can ensue. For faces, this can be achieved using visual imagery, where the image is mentally rotated back to its upright orientation. However, the process of mental rotation is expected to be more difficult as a greater degree of transformation is required, leading to more difficult recognition where the main dependent measure is traditionally increased reaction time (Rock 1973). However, with greater transformation also comes the likelihood that discrimination errors may occur. Thus, the more the face is oriented away from its typical upright orientation, the greater the mental transformation, and the more difficult the recognition process (Shepard and Metzler 1971). This is expected to lead to a significant linear relationship between angle of orientation and mental transformation effort such that performance would decrease as faces are rotated away from their veridical orientation.
The majority of studies in humans have supported this latter view, reporting a significant linear relationship between discrimination performance and angle of rotation, regardless of task specifics. Valentine and Bruce (1988), for example, found a significant linear relationship between reaction time performance and rotation angle (0°, 45°, 90°, 135°, 180°) for tasks that required subjects to discriminate between photographs of unfamiliar individuals and famous faces, but not objects. Bruyer et al. (1993) presented subjects with unfamiliar and famous faces in ten orientations, ranging from 0° to 180° in 20° increments, rotated in both a clockwise and counterclockwise direction. Subjects were required to discriminate the faces based on gender, familiarity, or semantic judgments. Results showed a linear increase in reaction times for those decisions as faces were rotated away from upright, supporting the prediction that mental rotation takes time and processing effort (Shepard and Metzler 1971). In a more recent study, Collishaw and Hole (2002) attempted to disambiguate whether inverted faces were configurally processed by presenting subjects with blurred faces of famous and unfamiliar individuals in nine rotation angles. The blurring functioned to minimize a feature-based processing strategy, thus highlighting the relationship between configural processing and face orientation. As faces were rotated away from upright, performance accuracy declined in a linear fashion, regardless of stimulus familiarity (Collishaw and Hole 2002). Thus, the majority of the data strongly suggests that faces are processed using a single perceptual strategy dependent on the use of configural cues in a typical orientation.
Evidence for the face inversion effect and its causes in other primate species is less clear. Some studies have reported evidence of the face inversion effect in monkeys and apes (Neiworth et al. 2007, cotton-top tamarins; Overman and Doty 1982, pigtail macaques; Parr et al. 1998; Parr and Heintz 2006; Tomonaga 1999, 2007, chimpanzees; Tomonaga 1994, Japanese macaques; Vermeire and Hamilton 1998, rhesus monkeys), while others have failed to find evidence of orientation-specific processing exclusive for faces (Bruce 1982; Dittrich 1990, longtail macaques; Gothard et al. 2004; Parr et al. 1999; Rosenfeld and van Hoesen 1979, rhesus monkeys; Weiss et al. 2001, cotton-top tamarins). Numerous studies in our lab have supported the relationship between stimulus expertise and configural processing in chimpanzees. Chimpanzees showed the inversion effect only for stimulus categories for which they had considerable expertise, such as human and chimpanzee faces, but not unfamiliar capuchin monkeys or automobiles (Parr et al. 1998). Rhesus monkeys tested using the same stimuli showed no effect of face-specificity or expertise, a significant inversion effect was found for rhesus faces, capuchin monkey faces and automobiles, but not human faces, despite the monkey subjects being raised in captivity by humans (Parr et al. 1999). More recent comparative studies in our lab provide further evidence of prominent species differences in face processing by rhesus monkeys and chimpanzees. In this new group of rhesus monkeys, a general face inversion effect was found for discriminations of all face categories (conspecifics' faces, chimpanzee faces and human faces), regardless of their familiarity, but no performance differences were found for discriminations of upright versus inverted clip art images or houses (Parr, unpublished data).
In an effort to understand whether the inversion effect in chimpanzees involved the same processing strategies as reported for humans, Parr and Heintz (2006) investigated the impact of rotation angle on the discrimination of conspecifics' faces and houses by chimpanzees. This study showed a significant linear decrease in performance as conspecifics' faces were rotated from upright to inverted in increments of 45°, but no significant impairments were found for discriminations of houses (Parr and Heintz 2006). Thus, data from chimapnzees supports the unified configural strategy, similar to that reported in humans. The present study utilizes the same experimental procedures to examine the influence of orientation angle on face discrimination in this more recent group of rhesus monkeys, where an inversion effect was demonstrated for all faces categories (L. A. Parr, unpublished data). The monkeys were presented with three categories of stimuli, unfamiliar conspecifics' faces (expert category), unfamiliar chimpanzee faces (nonexpert category) and houses (nonexpert category) in five orientation angles, 0°, 45°, 90°, 135° and 180°. We hypothesize that because rhesus monkeys have the most experience with their own species' faces, that this stimulus category will reveal the greatest decrements in performance with increasing rotation angle. However, because no significant inversion effect was found previously for houses, and because this is a nonexpert/nonface stimulus category, we do not expect to find a relationship between performance and rotation angle for houses. Finally, because chimpanzee faces are a nonexpert category for the rhesus monkeys, the inversion effect found in previous experiments may have been the results of a general configural strategy for faces when upright, conforming to first-order configural properties found in all faces. However, this experiment will yield information about whether the inverted faces were processed using a different strategy or whether configural processing might extend to all primate faces, regardless of orientation.
Methods
Subjects
Data were collected from five rhesus monkeys (Macaca mulatta, two males) that were born in large social groups at the Yerkes Primate Center field station, Lawrenceville, GA. Two females were 5 years old and the remaining subjects were 6 years old at the time of testing. In the fall 2004, these experimentally naïve monkeys were brought to the main Yerkes' campus in Atlanta, GA where they were pair-housed and trained for participation in computerized experiments on social cognition. Training on matching-to-sample tasks began with discriminations of clip art objects. Once subjects were proficient in this, performing above 85% with novel images, they were moved on to discriminations involving conspecifics' faces and the other categories in these studies. Prior to this study, subjects had exposure to tasks requiring them to match upright and inverted conspecifics' faces, chimpanzee faces, human faces, houses, and clip art (L. A. Parr, unpublished data). However, these studies were limited to only a few exemplars (maximum of 20 faces/images), which would not constitute the exposure or the range of exemplars necessary for expertise.
Subjects were tested either in a dedicated testing room across the hall from their colony room, or directly in their home cage. The testing room was equipped with two custom-made, sound-attenuated touchscreen chambers, 85 × 90 × 125 cm, each equipped with a 17″ flat panel touchscreen (Elo secure touch, surface wave monitors, http://www.elotouch.com), ventilation fan, speakers, and black and white infrared camera. The home cage testing system consisted of a 15″ touchscreen built into a metal frame that attached inside of the door opening of the home cages.
Stimuli
Stimuli consisted of digital photographs of unfamiliar rhesus monkey faces, chimpanzee faces and houses. The photographs of rhesus monkeys and chimpanzees were taken using a hand-held, digital camera (Canon, Digital Rebel, http://www.canon.com) from colonies of individuals living at the Yerkes Primate Center field station, none of which were familiar to the subjects of this study. Moreover, the category of chimpanzee faces was also unfamiliar as the subjects had never seen a live chimpanzee. These images were cropped to 300 pixels height using Adobe Photoshop 6.0 to reveal only the face of each individual. Photographs of 20 different individuals were used in these experiments.
Photographs of houses were downloaded from the internet, converted into 256 greyscale images, saved as 300 dpi, and cropped to a 300 pixel height so as not to show any distinguishing features other than the structure itself. Many of these images were the same as those used in the previous rotation study in chimpanzees (Parr and Heintz 2006). Photographs of 20 different houses were used in these experiments. In order to ensure that subjects would not attempt to match the photographs using the overall shapes of the images, each photograph was cropped individually by hand using Photoshop 7.0 and placed on a black background. This produced stimuli that all differed slightly in their overall shape, even in the case of the matching pair which showed the same images but were cropped individually.
Procedure
Subjects were tested using a matching-to-sample (MTS) procedure. They had already learned the principles of the task and had performed several studies discriminating a range of stimuli from conspecifics' faces to clip art (L. A. Parr, unpublished data). The general format requires subjects to first make an orienting response to a single sample stimulus presented on the monitor on a black background. The orienting response required three touches of this image in rapid succession. The location of the sample was centered randomly against one of the four walls of the monitor. After touching the sample, two comparison stimuli appeared together with the sample on the computer monitor. This is referred to as a simultaneous matching procedure and does not implicate any memory functions. The comparison images were located equidistant from the sample on the opposite wall of the monitor, forming a triangular configuration. To make a correct selection, the subject must touch the comparison image that looks most like the sample. All images in a trial were from the same stimulus category. These responses were food rewarded and followed by an inter-trial interval (ITI) of 2 s, while incorrect responses were not food rewarded and followed by an 8 s ITI.
Subjects were tested using the same stimulus order, rhesus monkey faces, house, and chimpanzee faces, completing each category before moving on to the next. Subjects first discriminated ten trials in their upright orientation, where the correct pair of images was identical photographs. This required photographs of 20 different individuals, 10 for use as the correct pair and 10 for the nonmatching comparisons, consisting of images from the same stimulus category. The order of each unique training trial was pseudo random so that each trial was seen once before any individual trial was repeated. Two testing session were given per day, each consisting of a block of 40 trials. This was repeated until the performance exceeded 80% correct, after which the comparison images in these same ten trials were rotated in one of the four orientations (45°, 90°, 135° or 180°). The samples remained in their upright orientation. Figure 1 shows an example of each stimulus category in the five orientations. These 10 rotated trials were added to the 10 upright training trials and subjects were given a 60 trial test session (3 repetitions of the 10 upright and 10 rotated trials). Each rotation angle was presented separately for the fixed number of trials and all subjects received the same order after reaching criterion on the upright (0°) trials: 135°, 90°, 180°, and finally 45°. Performance was then compared on the upright versus rotated trials for each angle. Testing was voluntary and initiated by the subject. Sessions lasted approximately 15 min.
Fig. 1.
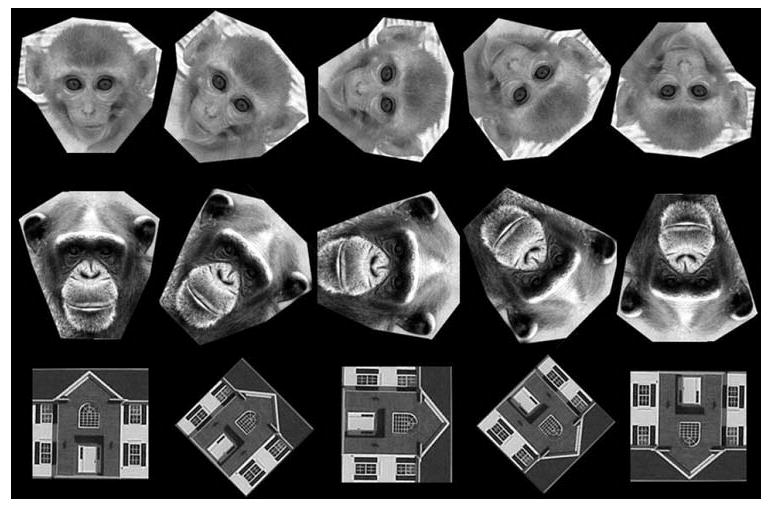
An example of the rhesus faces, chimpanzee faces and house stimuli in each of five orientations
Data analysis
Analyses first compared the number of trials required to reach significance on the upright training trials for all stimulus categories. Mean performance on each of the stimulus categories, rhesus faces, houses and chimpanzee faces, was then analyzed using repeated measures ANOVAs where rotation angle (0°, 45°, 90°, 135° and 180°) was the within-subjects factor. In this analysis, the upright performance (0°) represented the mean for the upright trials presented for each stimulus category. Performance was also evaluated at each angle of rotation compared to upright control trials during that session using paired t-tests. Statistical significance was set at alpha < 0.05.
Results
Training performance on the upright trials revealed no significant difference across stimulus categories, F2,8 = 0.27, P = 0.77. This was assessed as the number of trials, provided in blocks of 40 trials, that was required for each subject to perform above 80% correct. Table 1 lists the mean performance by subjects discriminating rhesus faces, chimpanzee faces and houses in each rotation angle, and their mean performance on the upright training trials for each stimulus category. The means listed for 0° represent the average performance on the control trials during each of the other rotation angles. Repeated measures ANOVAs were performed on these control trials alone to determine whether the addition of rotated comparison images affected overall performance on the upright trials. This analysis revealed no significant effects for control performance over any rotation angle, F3,12 = 1.53, P = 0.26, stimulus category (rhesus faces, chimpanzee faces or houses), F2,8 = 0.16, P = 0.85, nor any interactions between stimulus type or the rotation angles, F6,24 = 0.62, P = 0.72. Thus, performance on upright control trials maintained consistency through the task.
Table 1.
Mean percentage of correct responses (+sem) for discriminations of rhesus faces, chimpanzee faces and houses in five orientation angles
| Stimulus type | Training trials | Angle of rotation (°) | ||||
|---|---|---|---|---|---|---|
| 0° | 45° | 90° | 135° | 180° | ||
| Rhesus faces | 136 | 83.6 | 83.4 | 73.3 | 68.0 | 72.6 |
| Sem | 29.93 | 2.8 | 2.8 | 3.5 | 3.7 | 4.6 |
| Houses | 184 | 83.68 | 84.68 | 74.00 | 68.66 | 72.00 |
| Sem | 82.56 | 2.36 | 3.42 | 5.31 | 3.10 | 3.43 |
| Chimpanzee faces | 150 | 83.68 | 79.33 | 65.33 | 56.67 | 70.53 |
| Sem | 48.00 | 0.98 | 3.23 | 4.16 | 2.11 | 4.86 |
The performance listed under 0° is the overall mean for control trials of that stimulus type. The number of trials required (in 40 trial blocks) for subjects to reach above 80% correct during the upright training is also given for each stimulus category
Rhesus monkey faces
A repeated measures ANOVA revealed a significant main effect of rotation angle for rhesus faces, F4,16 = 9.55, P < 0.001. Polynomial contrasts showed that this effect was significantly linear, F1,4 = 34.73, P < 0.004, with mean performance decreasing as rotation angles increased. Results of the paired t test comparisons between mean performance discriminating rhesus faces at each rotation angle compared to the control trials for that session (see Fig. 2) revealed significant differences for all orientations except 45°, t4 = 0.26, P = 0.81; 90°, t4 = 3.16, P < 0.04; 135°, t4 = 3.39, P < 0.03; 180°, t4 = 7.61, P < 0.002.
Fig. 2.
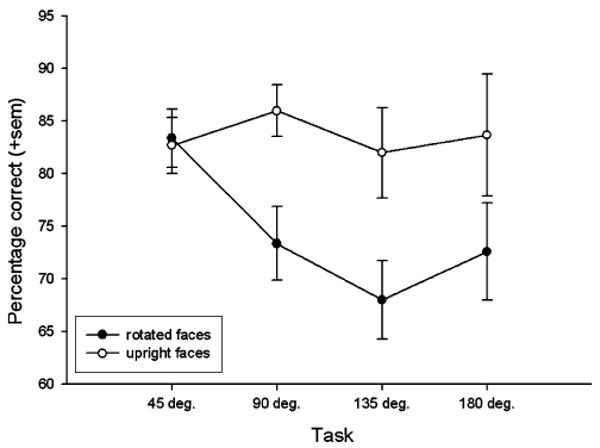
The mean percentage of correct responses (+sem) by subjects for discriminations of unfamiliar rhesus faces in the four orientation angles compared to upright control trials. The data for each angle of orientation represents a single 60 trial session, 30 rotated trials and the 30 upright control trials
Houses
A repeated measures ANOVA revealed a significant main effect of rotation angle for houses, F4,16 = 4.09, P < 0.02. Polynomial contrasts showed that this effect was significantly linear, F1,4 = 11.95, P < 0.03, with mean performance decreasing as rotation angle increased. Results of the paired t test comparisons between mean performance discriminating houses at each rotation angle compared to the control trials for that session (see Fig. 3) revealed no significant differences for any of the orientations, all P's >0.05. Looking at Fig. 3, there is the suggestion that performance on the upright control images also decreased during the rotation sessions, but this effect was not significance.
Fig. 3.
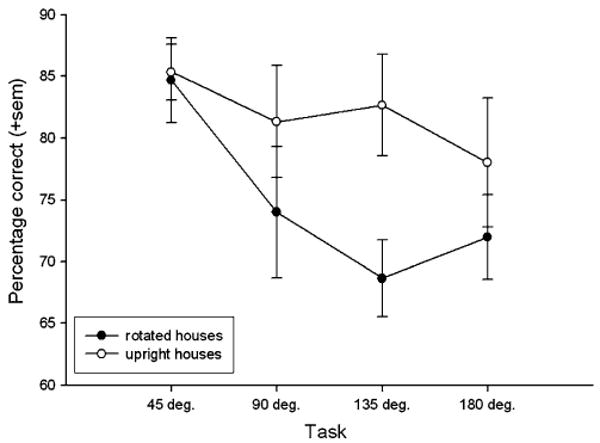
The mean percentage of correct responses (+sem) by subjects for discriminations of houses in the four orientation angles compared to upright control trials during that task session. The data for each angle of orientation represents a single 60 trial session, 30 rotated trials and the 30 upright control trials
Chimpanzee faces
A repeated measures ANOVA revealed a significant main effect of rotation angle for chimpanzee faces, F4,16 = 12.05, P < 0.001. Polynomial contrasts showed a significant linear effect, F1,4 = 13.25, P < 0.03, but also a significant quadratic effect, F1,4 = 159.70, P < 0.001. Paired t-tests revealed significant differences between the rotated images and control trials at 90°, t4 = 3.94, P < 0.02, and 135°, t4 = 6.95, P < 0.002, but no differences at 45° or 180° (see Fig. 4).
Fig. 4.
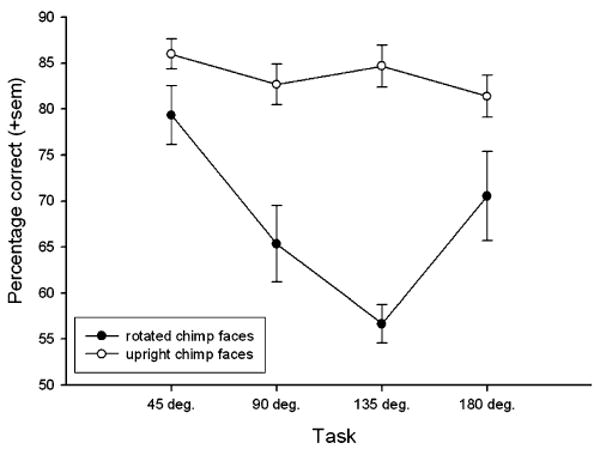
The mean percentage of correct responses (+sem) by subjects for discriminations of chimpanzee faces in the four orientation angles compared to upright control trials during that task session. The data for each angle of orientation represents a single 60 trial session, 30 rotated trials and the 30 upright control trials
Discussion
Previous studies with this group of rhesus monkeys revealed significant inversion effects for all of the primate faces that were presented (conspecific, human, and chimpanzee), but not for the nonface stimulus categories (houses and clip art) (Parr, unpublished data). This suggests that rhesus monkeys have a general configural bias for face processing, but that this is not specific to expert face categories, like conspecifics' faces. However, the inversion effect alone does not allow for an assessment of whether similar perceptual strategies are used for processing upright compared to inverted or otherwise rotated images. One hypothesis proposes that upright and inverted faces are processed using a similar perceptual strategy that first requires the inverted image to be normalized, or mentally rotated back into its typical orientation before recognition can ensue. As faces are subject to greater angles of rotation, more normalization would be required leading to slower response times and linear decreases in recognition accuracy. For face processing, this is presumed to involve configural cues and the previous studies with rhesus monkeys would suggest that this general configural strategy would apply to all faces, not exclusively conspecifics' faces (Parr, unpublished data). Conversely, two distinct processes might be involved, one that is engaged when discriminating upright images, i.e. configural processing, and another for processing inverted, or atypical, images, that may involve the detection of specific features.
The results reported here provide no support for the role of two distinct processes for discriminating upright images and images that have been rotated. Rather, for all stimulus categories presented, the monkey's performance was significantly and linearly impaired as rotation angle increased. However, there were significant differences for performance within each stimulus category and these were not face-specific. The performance on conspecific faces, for example, differed significantly from the upright control trials at all orientation angles except 45°. Therefore, in this example, the impairments began at an angle greater than 45°. This supports a unified configural processing strategy at orientation angles that deviate from what is typical, as subjects were likely to have ample experiencing viewing conspecifics with some minimal degree of head tilt. Performance on the chimpanzee faces was the most erratic. Performance on rotated chimpanzee face trials differed significantly from the upright trials at 90° and 135° but not at 180°, thus failing to replicate the general inversion effect reported earlier with these same subjects (L. A. Parr, unpublished data). As with the conspecific faces, linear impairments occurred after 45° but then improved for inverted chimpanzee faces, perhaps reflecting a point at which the processing strategy may have shifted, although it is unclear what additional strategy, if any, might have been employed. Finally, performance deficits discriminating rotated houses were also significantly linear, but unlike conspecific face discriminations, no significant differences were found between performance on the upright house control trials and any of the rotated house trials. Therefore, similar to the previous study, the monkeys showed no inversion effect for houses in this task. A visual inspection of the data reveals that the monkeys' performance on the upright control houses decreased slightly across the different orientations tested, but this decrease was not significant. Moreover, these results were not due to overall differences in the monkeys' experimental history with the different stimulus categories, as they were all familiar having been used during previous experiments on the inversion effect (L. A. Parr, unpublished data). Nor is it likely that these results were due to differences in the monkeys overall ability to discriminate these images, as there were no significant differences in the number of trials required to learn each category in their upright orientation.
While the data support a unified strategy for discriminating upright and inverted images, this strategy does not appear to be influenced globally by stimulus expertise, as has been proposed for face processing by chimpanzees and humans, as significant linear deficits were found as all image categories were rotated from upright (Maurer et al. 2002; Parr et al. 1998; Parr and Heintz 2006). The expertise hypothesis suggests that as stimuli become more familiar, subjects are able to utilize second-order relational features, which enable discriminations at the individual, or subordinate-level (Diamond and Carey 1986). This makes conspecifics' faces special, as this is a natural category for which subjects have acquired expertise, although expertise may be acquired with other stimulus categories (Gauthier et al. 2000; Diamond and Carey 1986). However, it is not known how these second-order features are extracted and processed, or whether this would be affected uniquely by rotation in a way that would differ from any other perceptual strategy. A recent study examined discrimination accuracy for whole faces or facial features presented upright, rotated 90°, or inverted. These authors found an advantage for discriminating whole faces when upright compared to isolated features, suggesting that upright faces are processed best using the entire facial configuration (Lewis and Glenister 2003). Moreover, while no differences were found for discriminations of whole faces compared to isolated features when rotated upright to 90°, the inversion effect was strongest for whole faces. The recognition of isolated features was also affected by inversion, but the strongest deficit for parts recognition was when faces were rotated 90°, and thus feature-based processing did not reveal a linear deficit with angular disparity. Moreover, only faces were used in this study, so no inferences can be made as to the processing strategies for others stimulus categories. The data here suggest that monkeys processed all stimuli in a similar way, regardless of whether they were faces or houses, or differences in their experience with the stimuli, being familiar with conspecifics' faces, but never having seen chimpanzees and having no familiarity with houses. However, the results of follow-up comparisons temper this overall conclusion, as only performance discriminating rhesus faces differed significantly when upright compared to angles of rotation greater than 45°. Within the framework of the hypotheses presented, this suggests that rhesus faces may elicit a stronger configural processing strategy than chimpanzee faces or houses.
In addition to face expertise effects, these results may also be interpreted within the framework of mental rotation studies. Previous studies on mental rotation in animals using object stimuli have reported mixed results. Pigeons, for example, show rotational invariance, or the absence of any mental rotation deficits due to angular disparity (Hollard and Delius 1982). This has been interpreted according to the ecological constraints of bird vision. Because birds use a horizontal frame of reference during flight, i.e., the ground, it is highly advantageous for them to be able to recognize objects quickly in any orientation. The mental rotation deficits seen in humans may, therefore, have evolved as a result of a terrestrial habitat and vertical frame of reference, where images are seen most often in a single orientation. To test these ideas, researchers have compared mostly arboreal with more terrestrial species of nonhuman primates with the hypothesis that ecological adaptations to a vertical plane of reference among terrestrial living primates would result in a different pattern of visual processing than the horizontal plane of reference found among avian and arboreal species, including some marine mammals (Mauch and Dehnhardt 1997). Burmann et al. (2005) report no reaction time deficits with angular disparity in the arboreal lion-tailed macaque. However, the same group of researchers later found an effect of increased reaction time for the discrimination of rotated images in one of three rhesus monkeys (Kohler et al. 2005). Moreover, two of these monkeys also showed significantly linear performance deficits with increasing angular disparity, supporting also the results of the present study using houses as stimuli. Therefore, support was found for the ecological hypothesis and suggests that rotation deficits might increase with angular disparity in terrestrial species.
In conclusion, the present data, along with previous studies of mental rotation in rhesus macaques, suggest that the monkeys use a general visual processing strategy for all image categories tested thus far, where there were no differences found for discriminations of conspecific's faces compared to other species' faces, or faces compared with nonfaces. This general visual processing strategy appears to rely on images first being normalized to match the sample image, resulting in performance deficits that are linearly related to the angular disparity of the images. However, a more detailed examination of the data suggests that this strategy is much stronger and more consistent for rhesus faces than the other nonexpert categories, supporting the role of configural cues when processing images from expert categories in rhesus monkeys.
Acknowledgments
This investigation was supported by RR-00165 from the NIH/NCRR to the Yerkes National Primate Research Center, and R01-MH068791 to L. A. Parr. Thanks to Gauri Pradhan and Daniel Brubaker for assistance with animal testing, the animal care staff at Yerkes National Primate Research Center, and three anonymous reviewers for helpful comments. The Yerkes Primate Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Contributor Information
Lisa A. Parr, Email: parr@rmy.emory.edu, Yerkes National Primate Research Center, 954 Gatewood Rd, Atlanta, GA 30329, USA; Department of Psychiatry and Behavioral Sciences, Emory University, Atlanta, GA 30322, USA.
Matthew Heintz, Yerkes National Primate Research Center, 954 Gatewood Rd, Atlanta, GA 30329, USA.
References
- Bruce C. Face recognition by monkeys: absence of an inversion effect. Neuropsychol. 1982;20:515–521. doi: 10.1016/0028-3932(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Bruyer R, Galvez C, Prairial C. Effect of disorientation on visual analysis, familiarity decision and semantic decision on faces. Br J Psychol. 1993;84:433–441. doi: 10.1111/j.2044-8295.1993.tb02494.x. [DOI] [PubMed] [Google Scholar]
- Burmann B, Dehnhardt G, Mauck B. Visual information processing in the lion-tailed macaque (Macaca silenus): mental rotation or rotational invariance? Brain Behav Evol. 2005;65:168–176. doi: 10.1159/000083626. [DOI] [PubMed] [Google Scholar]
- Carey S, Diamond R. From piecemeal to configurational representation of faces. Science. 1977;195:312–314. doi: 10.1126/science.831281. [DOI] [PubMed] [Google Scholar]
- Collishaw SM, Hole GJ. Is there a linear or a nonlinear relationship between rotation and configural processing of faces? Perception. 2002;31:287–296. doi: 10.1068/p3195. [DOI] [PubMed] [Google Scholar]
- Diamond R, Carey S. Why faces are and are not special: an effect of expertise. J Exp Psychol. 1986;115:107–117. doi: 10.1037//0096-3445.115.2.107. [DOI] [PubMed] [Google Scholar]
- Dittrich W. Representation of faces in longtailed macaques (Macaca fascicularis) Ethol. 1990;85:265–278. [Google Scholar]
- Farah MJ, Tanaka JW, Drain HM. What causes the face inversion effect? J Exp Psychol. 1995;21:628–634. doi: 10.1037//0096-1523.21.3.628. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nat Neurosci. 2000;3:191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- Gothard KM, Erickson CA, Amaral DG. How do rhesus monkeys (Macaca mulatta) scan faces in a visual paired comparison task? Anim Cogn. 2004;7:25–36. doi: 10.1007/s10071-003-0179-6. [DOI] [PubMed] [Google Scholar]
- Hollard VD, Delius JD. Rotational invariance in visual pattern recognition by pigeons and humans. Science. 1982;218:804–806. doi: 10.1126/science.7134976. [DOI] [PubMed] [Google Scholar]
- Kohler C, Hoffmann KP, Dehnhardt G, Mauck B. Mental rotation and rotational invariance in the Rhesus monkey (Macaca mulatta) Brain Behav Evol. 2005;66:158–166. doi: 10.1159/000087156. [DOI] [PubMed] [Google Scholar]
- Lewis MB, Glenister TE. A sideways look at configural encoding: two different effects of face rotation. Perception. 2003;32:7–14. doi: 10.1068/p3404. [DOI] [PubMed] [Google Scholar]
- Mauch B, Dehnhardt G. Mental rotation in a California sea lion (Zalophus californianus) J Exp Biol. 1997;2000:1309–1316. doi: 10.1242/jeb.200.9.1309. [DOI] [PubMed] [Google Scholar]
- Maurer D, Le Grand R, Mondloch CJ. The many faces of configural processing. TICS. 2002;6:255–260. doi: 10.1016/s1364-6613(02)01903-4. [DOI] [PubMed] [Google Scholar]
- Neiworth JJ, Hassett JM, Sylvester CJ. Face processing in humans and new world monkeys: the influence of experiential and ecological factors. Anim Cogn. 2007;10:125–134. doi: 10.1007/s10071-006-0045-4. [DOI] [PubMed] [Google Scholar]
- Overman WH, Doty RW. Hemispheric specialization displayed by man but not macaques for analysis of faces. Neuropsychol. 1982;20:113–128. doi: 10.1016/0028-3932(82)90002-1. [DOI] [PubMed] [Google Scholar]
- Parr LA, Dove T, Hopkins WD. Why faces may be special: Evidence for the inversion effect in chimpanzees (Pan troglodytes) J Cog Neurosci. 1998;10:615–622. doi: 10.1162/089892998563013. [DOI] [PubMed] [Google Scholar]
- Parr LA, Heintz M. The perception of unfamiliar faces and houses by chimpanzees: influence of rotational angle. Perception. 2006;35:1473–1483. doi: 10.1068/p5455. [DOI] [PubMed] [Google Scholar]
- Parr LA, Winslow JT, Hopkins WD. Is the inversion effect in rhesus monkeys face specific? Anim Cogn. 1999;2:123–129. [Google Scholar]
- Rock I. Orientation and form. Academic Press; New York: 1973. [Google Scholar]
- Rosenfeld SA, Van Hoesen GW. Face recognition in the rhesus monkey. Neuropsychologia. 1979;17:503–509. doi: 10.1016/0028-3932(79)90057-5. [DOI] [PubMed] [Google Scholar]
- Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science. 1971;171:701–703. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- Tomonaga M. Inversion effect in perception of human faces in a chimpanzee (Pan troglodytges) Primates. 1999;40:417–438. [Google Scholar]
- Tomonaga M. Visual search for orientation of faces by a chimpanzee (Pan troglodytes): face-specific upright superiority and the role of configural properties of faces. Primates. 2007;48:1–12. doi: 10.1007/s10329-006-0011-4. [DOI] [PubMed] [Google Scholar]
- Tomonaga M. How laboratory-raised Japanese monkeys (Macaca fuscata) perceive rotated photographs of monkeys: evidence for an inversion effect in face perception. Primates. 1994;35:155–165. [Google Scholar]
- Valentine T. Upside-down faces: a review of the effects of inversion upon face recognition. Br J Psychol. 1988;79:471–491. doi: 10.1111/j.2044-8295.1988.tb02747.x. [DOI] [PubMed] [Google Scholar]
- Valentine T, Bruce V. Mental rotation of faces. Mem Cogn. 1988;16:556–566. doi: 10.3758/bf03197057. [DOI] [PubMed] [Google Scholar]
- Vermeire BA, Hamilton CR. Inversion effect for faces in split-brain monkeys. Neuropsychologia. 1998;36:1003–1014. doi: 10.1016/s0028-3932(98)00054-2. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Kralik JD, Hauser MD. Face processing in cotton-top tamarins (Saguinus oedipus) Anim Cogn. 2001;4:191–205. [Google Scholar]
- Yin RK. Looking at upside-down faces. J Exp Psychol. 1969;81:141–145. [Google Scholar]


