Abstract
The role of sensory feedback in the early ontogeny of motor coordination remains a topic of speculation and debate. On E20 of gestation (the 20th day after conception, 2 days before birth), rat fetuses can alter interlimb coordination after a period of training with an interlimb yoke, which constrains limb movement and promotes synchronized, conjugate movement of the yoked limbs. The aim of this study was to determine how the ability to express this form of motor learning may change during prenatal development. Fetal rats were prepared for in vivo study at 4 ages (E18–21) and tested in a 65-min training-and-testing session examining hind limb motor learning. A significant increase in conjugate hind limb activity was expressed by E19, but not E18 fetuses, with further increases in conjugate hind limb activity on E20 and E21. These findings suggest substantial development of the ability of fetal rats to modify patterns of interlimb coordination in response to kinesthetic feedback during motor training before birth.
Historically, the study of motor development has been dominated by efforts to chart predictable stages and the orderly emergence of motor skills during the first years after birth (Adolph & Berger, 2006; Gesell, 1946). Comparatively little work has been devoted to examining how experience may contribute to the development of motor behavior, particularly at very early ages. However, Esther Thelen has exhorted researchers to reevaluate the study of child motor development with a new focus on the process and mechanisms of developmental change (Thelen, 1995; Thelen & Smith, 1994). The research of Thelen and her students and colleagues on infant kicking, walking, reaching, and other basic motor skills has revealed the complex, subtle, and dynamic interplay among multiple determinants of motor behavior in human infants. From this perspective, motor development emerges from the expression of motor behavior in real time. At any point in time, organized movement is codetermined by neural resources available for motor control, biomechanical constraints on movement, active exploration and selection of motor patterns during spontaneous activity, context at the time of motor performance, changes in task demands, and experience with past motor performance.
The research perspective exemplified by Thelen in the domain of child development offers a rich set of conceptual and experimental tools that provide new insights into long-standing questions in developmental and behavioral neuroscience. Application of this research approach to nonhuman animals, such as the rat, presents many advantages to researchers interested in fundamental problems in motor development, most notably the ability to use experimental methods and technologies that are not feasible with human infants and children. This advantage is particularly salient in efforts to trace the developmental origins of behavior. For example, the newborn rat does not display adultlike motor skills, but does show organized motor behavior that is essential for its survival and subsequent development. The expression of organized motor behavior within minutes or hours of birth implies a developmental history antecedent to birth. Study of fetal behavior in the rodent model thus provides a unique window into the ontogenetic origins of motor behavior and the codeterminants of developmental change in the motor system (Alberts & Ronca, 1993; Smotherman & Robinson, 1996).
Norway rats (Rattus norvegicus), like most rodents in the family Muridae, are a prototypical example of an altricial mammal. Rats are born after a relatively brief gestation (22 days) in a very immature condition—what Aristotle referred to as being born “imperfect”—and are completely dependent on caregivers for their survival. Blind and deaf for the first 2 weeks after birth, infant rats rely on tactile, thermal, and olfactory cues to interact with their mother, siblings in the nest, and other features of their environment (Alberts & Cramer, 1988). Similar to newborn humans, rat pups also show limited motor skills after birth: They are unable to support the body off the ground and show poor crawling abilities (Altman & Sudarshan, 1975), and orient within the nest environment with simple motoric responses such as contact righting (Eilam & Smotherman, 1998; Pellis, Pellis & Teitelbaum, 1991) and punting, an unusual form of locomotion in which the head and forelimbs participate in a tripod gait (Altman & Sudarshan, 1975; Smotherman & Robinson, 1990).
Given these limited motor abilities, newborn rats were long thought to have comparable limitations in learning abilities. However, since the late 1970s, psychobiologists have developed a diverse assortment of age-appropriate tasks to apply different learning paradigms to progressively younger animals. This work has confirmed that newborn rats can express both classical (e.g., Bachevalier & Blozovski, 1980; Bordner & Spear, 2006; Spear & Rudy, 1991) and instrumental conditioning (Johanson & Hall, 1979) as early as 1 day after birth. Moreover, nonassociative and associative learning also has been demonstrated in the rat fetus, in the forms of habituation (Smotherman & Robinson, 1992), sensory exposure learning (Chotro & Arias, 2003; Hepper, 1988; Molina, Chotro, & Dominguez, 1995; Smotherman, 1982), taste or odor aversion learning (Mickley, Remmers-Roeber, Crouse, Walker, & Dengler, 2000; Smotherman & Robinson, 1985; Stickrod, Kimble, & Smotherman, 1982), and classical conditioning of motor activity (Smotherman & Robinson, 1991b) and physiological responses (Robinson, Arnold, Spear, & Smotherman, 1993). Much of the past work on perinatal learning has focused on questions of sensory function, discrimination, preference, and aversion, and their neural substrates during early development (Wilson & Sullivan, 1994). This research has confirmed that basic forms of learning can be expressed by the fetus and newborn despite the general immaturity of the perinatal rat’s sensory and motor systems.
Growth of the fetus and changes in the intrauterine environment present substantial challenges to the fetal motor system as well as opportunities for motor experience (Robinson & Kleven, 2005; Ronca, Lamkin, & Alberts, 1993). The period of late gestation from postconception day 18 (E18) to E21 is a time of rapid growth and development in the rat. Physically, the rat fetus grows at a nearly exponential rate over this span, with crown–rump length increasing 182% (20.5 mm to 37.4 mm) and body mass 344% (1.6 g to 5.5 g; Robinson, 1989; Robinson & Smotherman, 1992a). Although the earliest movements are expressed only 2 days earlier, on E16, general motor activity rises to a peak by E18 (Narayanan, Fox, & Hamburger, 1971; Robinson, 1989; Smotherman & Robinson, 1986). Over the next 3 days (E18–21), fetal movements are transformed quantitatively and qualitatively from a seemingly random assortment of jerks and twitches to a repertoire of coordinated action that foreshadows functional behavior of the neonate and adult (Robinson & Brumley, 2005; Robinson & Smotherman, 1992b). Synchronous movement of the limbs—perhaps the earliest form of motor coordination—rises to a peak of expression on E19 in the forelimbs and E20 in the hind limbs (Kleven, Lane, & Robinson, 2004), and elements of suckling (Robinson et al., 1992; Robinson & Smotherman, 1992c), grooming (Robinson & Smotherman, 1991), head orientation (Robinson et al., 1992), postural control (Ronca & Alberts, 1994), and locomotion (Bekoff & Lau, 1980; Brumley & Robinson, 2005) can be expressed for the first time. In terms of motor development, the short span from E18 to E21 represents a crucial period in which the foundations of motor control and coordination are being established.
For experience to contribute directly to prenatal motor development, fetuses must possess a functional kinesthetic sense, which is essential for motor control and sensorimotor integration. Most research on prenatal sensory function has been concerned with exteroceptive senses, such as tactile and chemical senses in the rat and sheep fetus (Ronca & Alberts, 1994; Schaal, 2005), and audition in avian embryos (Lickliter, 2005) and the human fetus (Fifer & Moon, 1995). Much less is known about the prenatal development of functional proprioception. Anatomical evidence suggests that muscle spindles in major hind limb muscles of the rat begin to receive primary afferent innervation on E18, secondary afferents on E19, and innervation of motor neurons on E20 (Kucera, Walro, & Reichler, 1988, 1989; Milburn, 1973). Differentiation of spindles from primary myotubes is induced by afferent innervation, suggesting that functional afferent feedback from spindles may not be possible earlier than E19. Moreover, the timing of spindle development is shifted in different hind limb muscles, occurring earlier in tibialis anterior than soleus or medial gastrocnemius (Kucera & Walro, 1994). Neural recording from primary afferents in the anesthetized rat fetus suggests that spindles in major limb muscles respond to muscle stretch as early as E17 (Fitzgerald, 1987), prior to complete differentiation of sensory end organs and long before spindles are innervated by gamma motor neurons. However, the responsiveness of these sensory neurons to changes in limb position may be confounded by high levels of spontaneous neural activity before E19. Thus, anatomical and physiological evidence suggests that the rudiments of a kinesthetic sense are functional by E18 to E20 of gestation.
In avian embryos, efforts to chart the development of functional proprioception have led to mixed findings. Early studies suggested that proprioceptive feedback exerts little influence on the spontaneous activity of chick embryos (Hamburger, Wenger, & Oppenheim, 1966; Oppenheim, 1972). However, Bekoff and Sabichi (1987) elegantly demonstrated the necessary role of proprioceptors in the neck of the chick embryo in triggering the onset of hatching behavior. More recent work by Bradley has further reported that chick embryos are responsive to changes in the biomechanical context of movement, such as reduced buoyancy created by reducing the level of amniotic fluid around the E9 embryo (Bradley, 1997) or immobilization of the ankle joint of E12 embryos with an external brace (Bradley & Sebelski, 2000). Because spindle organs in the chick are poorly developed earlier than E13 (Maier, 1993), some of these proprioceptive responses may be mediated by cutaneous and not muscle afferents. However, these findings are sufficient to imply that functional proprioception emerges sometime between E9 and E13 in the chick embryo.
Direct evidence has been lacking for the development of functional pro-prioception in mammalian fetuses, or its possible role in motor development during the prenatal period. However, previous studies have reported that human infants (Thelen, 1994), newborn rabbits (Viala, Viala, & Fayein, 1986), and fetal rats (Robinson & Smotherman, 1994) alter the patterning of leg activity after a period of training in which independent leg movements are biomechanically constrained by a physical linkage or yoke connecting the two legs. Recently, our laboratory has adapted this experimental paradigm for studying motor learning in the rat fetus, which allows inferences about functional proprioception (Robinson, 2005).
The interlimb yoke training paradigm involves attachment of a length of thread to the two hind limbs of the fetus, attached just above the ankle. During a 30-min period of exposure to the interlimb yoke, hind limb movements are constrained, which results in changes in interlimb coordination. In particular, yoke training promotes conjugate movement of the hind limbs, in which both limbs initiate movement at the same time and follow parallel trajectories. Frame-by-frame analysis of video recordings to reconstruct 3D limb trajectories has confirmed this spatial and temporal coordination of the hind limbs during conjugate movements (Robinson & Kleven, 2005). Conjugate hind limb activity increases in occurrence during the period of yoke training, and persists above levels expressed by unyoked control subjects for 20 to 30 min after the interlimb yoke is removed. Changes in interlimb coordination in response to yoke training imply that the fetus can (a) detect the presence of a biomechanical constraint of limb movement, (b) alter the coordination of limbs to adjust to the presence of limb constraint, and (c) maintain altered patterns of coordination in the absence of the physical yoke. This form of motor learning therefore reveals that the rat fetus possesses a functional kinesthetic sense that is capable of mediating changes in motor control at least as early as E20.
In this study, we sought to determine how the capacity to modify limb coordination in this simple form of motor learning may change during prenatal development. Fetal rats were prepared for in vivo behavioral observation and interlimb yoke training at one of four prenatal ages (E18, E19, E20, or E21) to chart developmental changes in fetal responsiveness to yoke training. Preliminary findings from this study have been reported previously in abstract form (Bailey, Kleven, Brumley, & Robinson, 2000).
GENERAL METHODS
Subjects
Sprague-Dawley rats (Harlan) were time-mated in the animal care facilities at the University of Iowa to provide fetal subjects for behavioral study. Owing to the length of experimental sessions, only a single fetal subject was used from each pregnancy. A total of 48 fetuses served as subjects in the four experiments reported here (n = 6 fetuses per experimental condition). Female rats were housed in groups of three with a single male during a 4-day breeding period. Vaginal smears were collected daily and examined for the presence of sperm; the date of conception (E0) was defined as the first day in which sperm were detected. During housing, breeding, and gestation, rats were exposed to a 12-hr light, 12-hr dark photoperiod and provided with food and water ad libitum. All animals were treated in accordance with guidelines established by the National Institutes of Health (NIH, 1986); experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Iowa.
Prenatal Preparation
Pregnant females were prepared for fetal testing on one of 4 days of gestation: E18, E19, E20, or E21. Pregnant rats were anesthetized by placement in an animal jar suffused with ethyl ether. During general anesthesia, the spinal cord was prepared by chemomyelotomy, in which 100 μl of 100% ethanol was injected into the spinal cord between vertebrae L1 and L2. This procedure results in irreversible chemical blockade of transmission within the spinal cord at a low thoracic level. The prepared rat then was placed in a holding device that elevated her body at a 45° angle and the uterus was exteriorized through a midline laparotomy into a warm (37.5° C) bath containing buffered physiological saline (Locke’s solution). The rat and constituent fetuses were permitted to recover from ether anesthesia and acclimate to the bath environment for at least 20 min before fetal testing.
A single fetus from one uterine horn was identified as the test subject and was externalized through a small incision in the uterus into the saline bath. Typically, the subject was selected from the second or third position from the ovarian end of the uterine horn; fetuses in these uterine positions seldom exhibit growth retardation (which sometimes occurs at the ovarian or cervical extremes) and allow good visual access for behavioral study. The embryonic membranes (chorion and amnion) were gently removed to provide experimental access to the subject, taking care to preserve the integrity of the umbilical cord and placental connection to the uterus. The condition of subject fetuses was continuously monitored during the experimental session by noting general coloration of the fetus and umbilical cord (pink indicating good oxygenation), the presence of motor activity, and the absence of stereotypic movements indicative of hypoxia. All fetuses reported in these experiments remained in good physiological condition throughout the experimental session. These methods for preparing fetal subjects for behavioral study, which have become standardized over the past two decades, permit direct observation and experimental manipulation of fetal subjects and creation of high-quality video recordings for subsequent analysis of fetal motor behavior (Smotherman, Richards, & Robinson, 1984; Smotherman & Robinson, 1991a).
Procedures for Yoke Training
The motor learning paradigm in this study involved attachment of an interlimb yoke to both hind limbs to constrain limb movement during a training period. The interlimb yoke consisted of PE-50 polyethylene tubing cut to a specific length to accommodate subjects of different age; the length of the tubing segment was adjusted to approximate the mean distance between the ankles at rest for each age (E18: 3 mm; E19: 5 mm; E20: 8 mm; E21: 10 mm). Silk suture (4–0) then was threaded through the tubing, creating two loops at either end of the tubing. The yoke was fitted to the subject by slipping each loop over a hind foot and tightening the loops to fit snugly at the ankle joint. Interlimb yokes of this design resisted forces of tension, compression, and torsion. Figure 1 graphically depicts an E20 fetus, viewed from a ventral perspective, with an interlimb yoke attached to both hind limbs.
FIGURE 1.
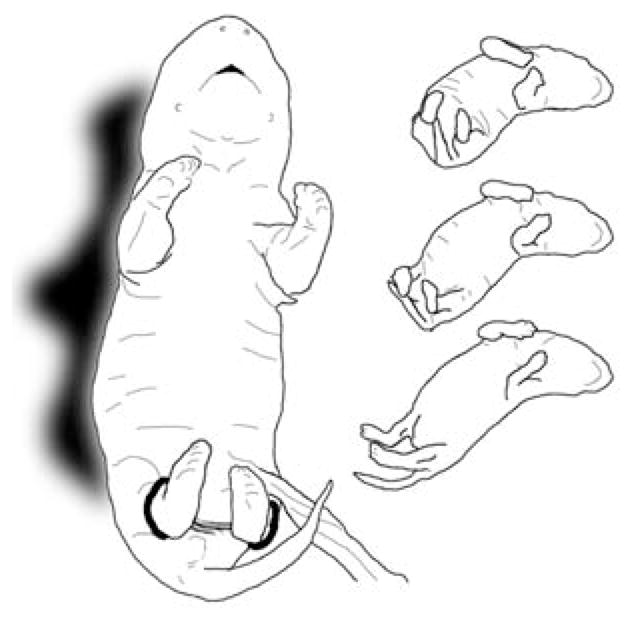
Drawings depicting E20 rat fetuses exposed to interlimb yoke training. The larger drawing on the left, traced from a still photograph of an E20 fetus in a resting posture viewed from a ventral perspective, shows the interlimb yoke attached to both hind limbs at the ankles. The smaller figures on the right present a sequence (top to bottom) of a conjugate limb movement (CLM), in which the two hind legs are extended simultaneously in a caudal direction. These three drawings were traced from individual video frames of an E20 rat fetus recorded after yoke training and removal of the yoke; each image in the sequence is separated from the next by five video frames (0.17 sec).
The purpose of the interlimb yoke was to constrain spontaneous movements of the yoked hind limbs. With the yoke in place, an independent movement by one limb would be hindered by its connection to the other limb. Conversely, the inactive limb could be moved passively by movement of the active limb. Fetuses were assigned to one of two conditions (yoked or unyoked, n = 6 per group) and tested in a 65-min experimental session. Fetuses in both conditions were observed during an initial 5-min baseline, during which they were not explicitly manipulated. After the baseline period, the interlimb yoke was attached to the hindlimbs. In the yoked condition, the yoke remained attached to both hind limbs for 30 min (designated as the training period). At the end of training, the yoke was bisected and fetuses were observed for the remaining 30 min (designated as the testing period). In the unyoked condition, the yoke was immediately bisected with scissors at the beginning of the training period and fetuses were observed without further manipulation for 60 min.
Analysis of Behavioral Data
Fetuses were maintained in a supine posture throughout the experimental session to facilitate clear observation of all four limbs. Fetal behavior was quantified by scoring the movement of each limb, treating each movement as a point event entered into a computer using real-time event recording software. This data acquisition system preserved information about the limb involved in movement (left vs. right, fore vs. hind), the time the movement occurred (± 0.1 sec), and whether the limb movement was independent or conjugate (Robinson, 2005). Conjugate limb movements (CLM) occurred when the two limbs appeared to move as one, with both limbs initiating movement at the same time and following parallel trajectories with similar velocity. An example of CLM by an E20 fetus is depicted in Figure 1 (right), which shows drawings from three individual video frames separated by intervals of five frames (0.17 sec). CLM were distinctive during baseline and testing periods in yoked subjects, and were readily distinguished from passive dragging of one limb during yoke training, because the passive limb appeared to trail the active limb through a movement trajectory. Further, CLM involved movement of both limbs at an angle orthogonal to the line of the interlimb yoke, whereas passive movement involved one limb moving in the same direction as the line of the yoke. In such cases, the active movement of the leading limb was scored as an individual limb movement, but the passive movement of the trailing limb was not. This method of quantifying fetal movement has been used extensively in previous studies of fetal motor behavior and responsiveness to sensory stimuli (Robinson & Brumley, 2005; Smotherman & Robinson, 1991a). Estimates of reliability have been assessed from repeatedly scoring the same session recorded on video; real-time scoring of basic categories of limb movement typically results in intra-rater reliability coefficients exceeding .95 and interrater reliabilities of at least .90.
The frequencies of individual limb movements and CLM were summarized in 5-min intervals across the baseline, training, and testing periods. Forelimb activity was computed as the sum of both left and right forelimb movements. Hind limb activity similarly was computed as the sum of both left and right hind limb movements. In addition to absolute movement counts, the relative frequency of CLM was calculated as twice the number of conjugate movement events (because two limbs are involved in each event), divided by the total number of all hind limb movements. Changes in limb activity were assessed by mixed model analyses of variance (ANOVAs), with the time factor (5-min intervals) treated as a repeated measure. Following significant interaction effects, one-factor ANOVAs were performed to test for simple main effects, and post-hoc comparisons of means were conducted by the method of Fisher’s protected least significant difference (PLSD). The alpha level was set at p <.05 for all tests of statistical significance.
RESULTS
A preliminary analysis of CLM was conducted to confirm age-related changes in the response of fetal rats to yoke training. The three-factor mixed model ANOVA (4 ages × 2 conditions × 13 5-min intervals) indicated significant main and interaction effects for all combinations of the three factors, including the three-way interaction, F(36, 480) = 5.6, p <.001, ηp2 = 0.297 (partial eta-squared). Significant three-way interactions also were found for overall forelimb and hind limb activity. To interpret these interactions, separate two-factor ANOVAs were conducted to assess fetal responses to yoke training at each gestational age. In the sections that follow, these results are presented beginning with the oldest age (E21), when the effects of yoke training were most pronounced, and progressing to successively younger ages.
E21 Fetuses
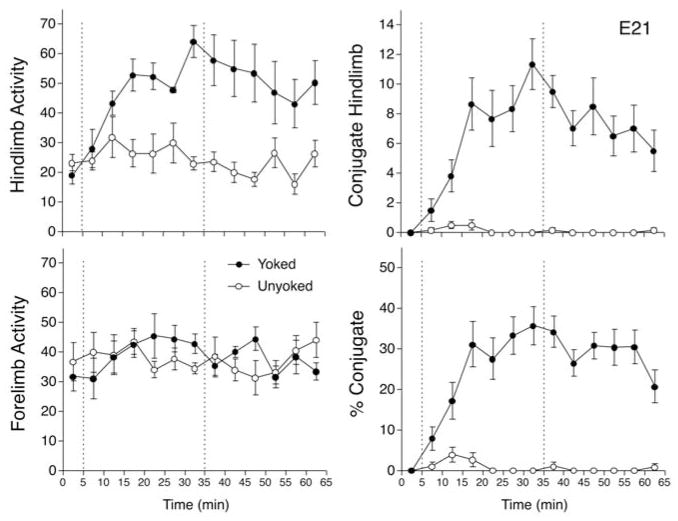
The overall frequency of hind limb activity was compared in a two-factor ANOVA (Conditions × 5-Min Intervals), with the intervals factor treated as a repeated measure. This analysis indicated the significant main effects of condition, F(1,10) = 19.1, p <.005, ηp2 = 0.657, and intervals, F(12, 120) = 3.5, p <.001, ηp2 = 0.257, and the significant interaction between these two factors, F(12, 120) = 3.7, p <.001, ηp2 = 0.270 (Figure 2). To examine the nature of this interaction, a series of unpaired t tests was conducted to assess the simple main effect of condition at each 5-min Interval. These tests revealed that yoked subjects showed significantly more hindlimb movements than unyoked subjects by the third interval of training (min 15) and in all subsequent intervals except min 50 (ps <.05, Cohen d = 1.31–4.03). Forelimb activity also was compared in a two-factor repeated measures ANOVA, which indicated no significant main effects, but did show a significant Condition × Intervals interaction, F(12, 120) = 2.0, p <.05, ηp2 = 0.166 (Figure 2). Post-hoc t tests indicated that yoked subjects showed more forelimb movements than unyoked subjects during the last interval of training (p <.05, Cohen d = 1.27). These findings suggest that a 30-min exposure to an interlimb yoke between the hind limbs affected general activity of both forelimbs and hind limbs, although the effect was more pronounced and more prolonged in hind limbs. Overall, hind limb activity of yoked subjects increased threefold during training, compared to an increase of about 50% in forelimb activity.
FIGURE 2.
Responses of rat fetuses to interlimb yoke training on E21 of gestation. In this and subsequent graphs, the vertical dotted lines divide the experimental session into an initial 5-min baseline period before attachment of the yoke, a 30-min period of yoke training, and a subsequent 30-min test period after removal of the yoke. Two experimental conditions involved fetuses that were exposed to the interlimb yoke attached to both hind limbs during training (yoked), and fetuses exposed to a control procedure that did not constrain interlimb movements (unyoked). Left panels show mean number of limb movements of hind limbs (top left) or forelimbs (bottom left) in successive 5-min blocks during the baseline, training, and test periods. Right panels depict the incidence of conjugate hind limb movements (CLM). The graph at top right shows the mean number of CLM during the session; the graph at bottom right shows CLM expressed as a percentage of overall hind limb activity. In all graphs, error bars depict SEM.
The two-factor ANOVA examining hind limb CLM also indicated the significant main effect of condition, F(1, 10) = 42.6, p <.001, ηp2 = 0.810, and intervals, F(12, 120) = 8.6, p <.001, ηp2 = 0.463, and a significant interaction, F(12, 120) = 8.9, p <.001, ηp2 = 0.470. Unpaired t tests revealed that CLM increased in yoked subjects relative to unyoked by the second interval of training (min 10), and remained elevated through the remainder of the experimental session (ps <.01, Cohen d = 1.75–5.73). As shown in Figure 2, CLM increased gradually to a peak at the end of training, then decreased by about half after the yoke was removed. Although the absolute rate of hind limb CLM was modest—about two movements per minute at the end of training—CLM did not occur at all during the baseline period. The magnitude of the effect of yoke training is apparent in Figure 2: The change in CLM from the first to the last 5-min interval represented a nearly eightfold increase during training, and nearly a hundredfold increase relative to unyoked controls.
To determine whether the apparent increase in CLM could be accounted for by the general increase in hind limb activity during and after training, CLM was expressed as a percentage of hind limb activity and compared in a separate two-factor ANOVA. As before, this analysis indicated the significant main effects of condition, F(1, 10) = 125.1, p <.001, ηp2 = 0.926, and intervals, F(12, 120) = 8.3, p <.001, ηp2 = 0.454, and a significant interaction, F(12, 120) = 8.9, p <.001, ηp2 = 0.472 (Figure 2). No CLM were observed in either condition during baseline, but percentage CLM increased gradually in yoked subjects relative to unyoked in the first two intervals of training (p <.05, Cohen d = 1.31–1.66), and remained elevated for all subsequent intervals (ps <.001, Cohen d = 2.83–10.74). At the peak of expression during training, CLM constituted about 36% of all hind limb activity among yoked subjects.
E20 Fetuses
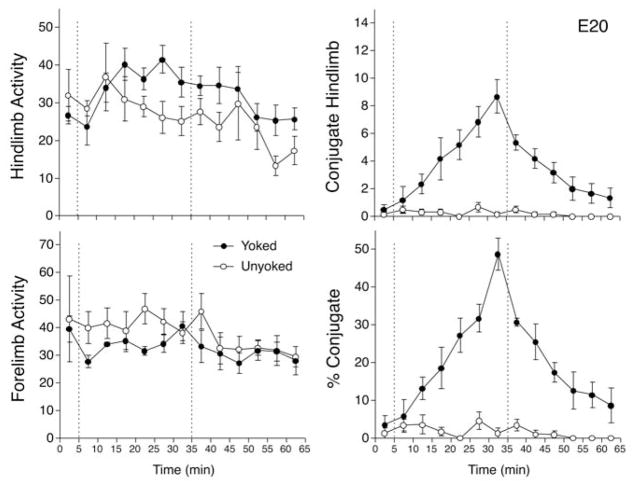
The same plan of analysis reported already for E21 fetuses was used to examine the effect of yoke training on E20. The two-factor (Conditions × Intervals) ANOVA comparing hind limb activity indicated the significant main effect of intervals, F(12, 120) = 3.1, p <.001, ηp2 = 0.239, but neither the main effect of condition nor the interaction were significant (ps >.15, ηp2 = 0.86–0.189; Figure 3). Post-hoc comparisons (Fisher PLSD) indicated that hind limb activity increased modestly during min 10 and 15 of the training period and decreased in min 55 of testing (ps <.05). Overall, hind limb activity increased about 50% during the training period. The ANOVA examining forelimb activity revealed nearly the same pattern of effects, with a significant main effect of intervals, F(12, 120) = 2.9, p <.005, ηp2 = 0.226, but no significant effect of condition nor an interaction (ps >.15, ηp2 = 0.122–0.168). In contrast to the increase in hind limb movements, post-hoc comparisons confirmed that forelimb activity did not change during training and was modestly reduced during the last 25 min of the testing period.
FIGURE 3.
Responses of rat fetuses to interlimb yoke training on E20. The four graphs depict mean number of hind limb movements (top left), mean forelimb movements (bottom left), mean hind limb conjugate limb movements (CLM; top right), and mean CLM expressed as a percentage of overall hind limb activity (bottom right). Conventions follow Figure 1.
The analysis of hind limb CLM in E20 fetuses indicated the significant main effect of condition, F(1, 10) = 26.6, p <.001, ηp2 = 0.727, and intervals, F(12, 120) = 12.9, p <.001, ηp2 = 0.564, and a significant interaction, F(12, 120) = 11.6, p <.001, ηp2 = 0.536 (Figure 3). A series of t tests conducted at each interval indicated that CLM was significantly elevated in yoked subjects during min 10 and 15 (ps <.05, Cohen d = 1.43–1.55), and increased more dramatically during the last 15 min of training (ps <.001, Cohen d = 2.77–4.04). CLM began to decline in frequency after the yoke was removed, but remained elevated in yoked subjects during min 35 to 45 of testing (ps <.001, Cohen d = 2.26–4.64), and were marginally greater than unyoked subjects by min 55 (p <.05, Cohen d = 1.35). CLM did not differ between yoked and unyoked subjects in the last interval of testing. As on E21, the absolute rate of hind limb CLM was modest (somewhat less than two per minute by the end of training). However, the striking pattern evident in Figure 3 is that this increase in CLM was pronounced. The change in CLM represented a sevenfold increase compared to the first 5-min interval of training, and a nearly 50-fold increase relative to baseline or unyoked levels. A second noteworthy pattern among E20 fetuses was the symmetry of response during and after yoke training: CLM increased at a steady rate during training and decreased at a very similar rate to near-baseline levels after the yoke was removed.
A final ANOVA compared CLM expressed as a percentage of hind limb activity. This analysis confirmed the significant main effects of condition, F(1, 10) = 49.0, p <.001, ηp2 = 0.831, and intervals, F(12, 120) = 14.8, p <.001, ηp2 = 0.596, and a significant interaction, F(12, 120) = 13.6, p <.001, ηp2 = 0.576. Percentage CLM increased gradually in yoked subjects relative to unyoked during min 10 to 15 of training (ps <.05, Cohen d = 1.42–1.74), increased more sharply during min 20 to 30 (ps <.001, Cohen d = 3.47–6.18), were well elevated relative to unyoked subjects during min 35 to 45 of testing (ps <.001, Cohen d = 2.93–8.54), and remained significantly greater than unyoked in min 50 to 55(ps <.05, Cohen d = 1.46–2.02; see Figure 3). As in the analysis of the frequency of CLM, percentage CLM did not differ between yoked and unyoked subjects in the last interval of testing (p >.05, Cohen d = 1.08). At the peak of expression, CLM amounted to about 49% of all hind limb movements.
E19 Fetuses
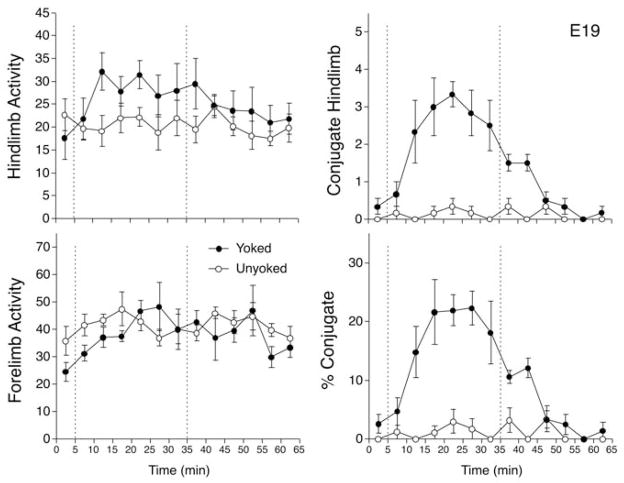
The two-way ANOVA comparing hind limb activity in E19 fetuses indicated no significant changes between conditions or across intervals, with no significant interaction between these factors (ps >.05, ηp2 = 0.045–0.279). However, the analysis of forelimb activity revealed a significant main effect of intervals, F(12, 120) = 2.5, p <.01, ηp2 = 0.202, but no significant effect of condition or interaction (ps >.05, ηp2 = 0.052–0.141). Post-hoc comparisons indicated that forelimb activity increased after the baseline period, but remained stable thereafter (Figure 4). The increase in forelimb activity during training amounted to levels about 30% higher than baseline.
FIGURE 4.
Responses of rat fetuses to interlimb yoke training on E19. The four graphs depict mean number of hind limb movements (top left), mean forelimb movements (bottom left), mean hind limb conjugate limb movements (CLM; top right), and mean CLM expressed as a percentage of overall hind limb activity (bottom right). Conventions follow Figure 1.
The analysis of hind limb CLM in E19 fetuses indicated the significant main effect of condition, F(1, 10) = 334.1, p <.001, ηp2 = 0.971, and intervals, F(12, 120) = 6.9, p <.001, ηp2 = 0.409, and a significant interaction, F(12, 120) = 6.0, p <.001, ηp2 = 0.373 (Figure 4). Post-hoc t tests indicated a significant increase in CLM in yoked subjects during min 10 to 30 (ps <.05, Cohen d = 1.60–4.39). CLM remained modestly elevated in yoked subjects during min 35 to 40 of the testing period (ps <.01, Cohen d = 2.19–3.87), but did not differ from unyoked fetuses during min 45 to 60 (ps >.10, Cohen d = 0.31–0.91). In contrast to older fetuses, peak levels of CLM were achieved in min 20 of training, and amounted to a fivefold increase relative to the first 5-min interval of training, and tenfold compared to baseline. The analysis of CLM expressed as a percentage of hind limb activity revealed the same pattern of effects, with a significant main effect of condition, F(1, 10) = 43.4, p <.001, ηp2 = 0.812, a main effect of intervals, F(12, 120) = 9.5, p <.001, ηp2 = 0.487, and a significant interaction, F(12, 120) = 8.1, p <.001, ηp2 = 0.447 (Figure 4). At peak during training, CLM constituted 22% of all hind limb movements. CLM returned to baseline and unyoked levels by the third interval after removal of the yoke.
E18 Fetuses
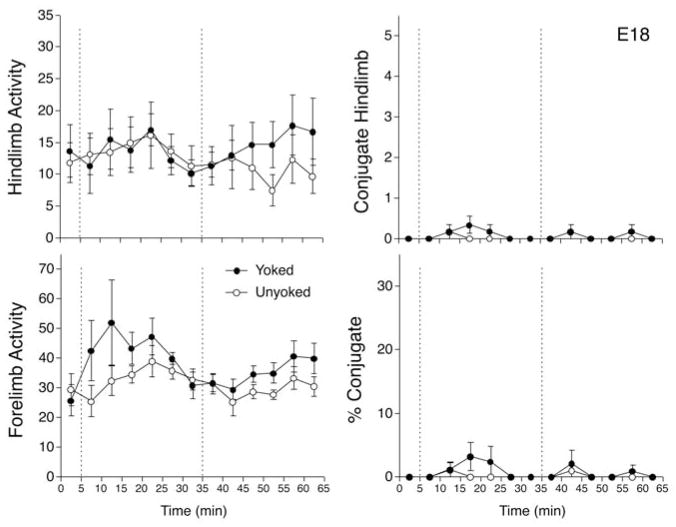
As with other age groups, a series of two-factor ANOVAs were conducted to assess the effects of yoke training on hind limb activity, forelimb activity, hind limb CLM, and CLM expressed as a percentage of hind limb activity in E18 fetuses. There were no indications of significant main or interaction effects for hind limb activity, CLM, or percentage CLM (Figure 5). For forelimbs, there was a significant main effect of intervals, F(12, 120) = 2.4, p <.01, ηp2 = 0.195, but no significant effect of condition nor the interaction (ps >.05, ηp2 = 0.095–0.220). Overall, forelimb activity was higher than baseline during min 10 to 25 of the training period, then returned to baseline levels in subsequent intervals. Because there was no evidence of differential behavioral effects in the yoked and unyoked conditions for any of the four response measures, E18 fetuses appeared to be unresponsive to the constraint of limb movement induced by yoke training.
FIGURE 5.
Responses of rat fetuses to interlimb yoke training on E18. The four graphs depict mean number of hind limb movements (top left), mean forelimb movements (bottom left), mean hind limb conjugate limb movements (CLM; top right), and mean CLM expressed as a percentage of overall hind limb activity (bottom right). Conventions follow Figure 1.
Age Comparisons
Across the four age groups, comparisons of fetal behavior in yoked and unyoked conditions suggested that yoke training resulted in both immediate and persistent changes in fetal motor behavior on E19, E20, and E21. To assess developmental changes in fetal responses to the interlimb yoke, percentage CLM by subjects in the yoked condition was explicitly compared across ages in a two-factor (4 ages × 13 5-min intervals) ANOVA, with the intervals factor treated as a repeated measure. This analysis indicated the significant main effect of age, F(3, 20) = 34.0, p <.001, ηp2 = 0.836, the main effect of intervals, F(12, 240) = 25.3, p <.001, ηp2 = 0.559, and the Age × Intervals interaction, F(36, 240) = 6.7, p <.001, ηp2 = 0.502 (see Figures 2–5). One-way ANOVAs examining the effect of age at each interval indicated significant differences in percentage CLM among age groups at min 10 (p <.05, ηp2 = 0.746), min 15 (p <.01, ηp2 = 0.886), and all subsequent intervals (ps <.001, ηp2 = 0.839–0.963). Post-hoc comparisons of means suggested that percentage CLM was elevated in E19, E20, and E21 fetuses relative to E18 at all intervals after the first interval of training. Furthermore, percentage CLM was higher on E21 than E19 at min 25 to 40, higher on E20 than any other age at min 30, higher on E20 than E19 at min 35 to 45, and higher on E21 than any other age at min 45 to 60. As already reported, these differences were due to increases in CLM during yoke training on E19 to E21; the interlimb yoke did not result in decreased hind limb activity on E18 relative to unyoked controls. Moreover, pronounced increases in percentage CLM were evident despite general increases in hind limb activity on E20 and E21. This pattern of age-related differences suggested that (a) E18 fetuses failed to respond to yoke training, (b) fetuses from E19 to E21 showed similar rates of acquisition of CLM during the first 20 min of training, (c) fetuses on E20 and E21 achieved a higher peak of CLM performance during training than on E19, and (d) fetuses showed progressively longer periods of retention of CLM after removal of the yoke at older ages (E19–E21).
To further examine age-related differences in CLM performance, the total number of CLM within the 30-min training and testing periods were compared in a two-way ANOVA (4 ages × 2 periods). This analysis confirmed the general pattern of changes in percentage CLM and revealed systematic improvements in CLM performance during training and testing (Figure 6). The ANOVA indicated a significant main effect of ages, F(3, 20) = 23.3, p <.001, ηp2 = 0.836, a main effect of periods, F(1, 20) = 16.6, p <.001, ηp2 = 0.559, and the Ages × Periods interaction, F(3, 20) = 8.8, p <.001, ηp2 = 0.502. Within the training period, the total number of CLM increased progressively from E18 through E21, with all ages differing from all others (ps <.05). The same pattern of progressive increase in total CLM was evident within the testing period across ages, except that the frequency of CLM during testing did not differ between E19 and E18 fetuses. Paired t tests conducted at each age revealed differences in total CLM expressed during training and testing on E19 (p <.001, Cohen d = 6.45) and E20 (p <.01, Cohen d = 1.03), but no significant difference on E21 (p >.50, Cohen d = 0.16). On E19, the number of CLM expressed during testing was about 28% of CLM during training. On E20, CLM during testing persisted at higher levels than on E19, amounting to about 62% of CLM during training. On E21, CLM was maintained at high levels throughout the testing period, and constituted 108% of CLM during training (Figure 6).
FIGURE 6.
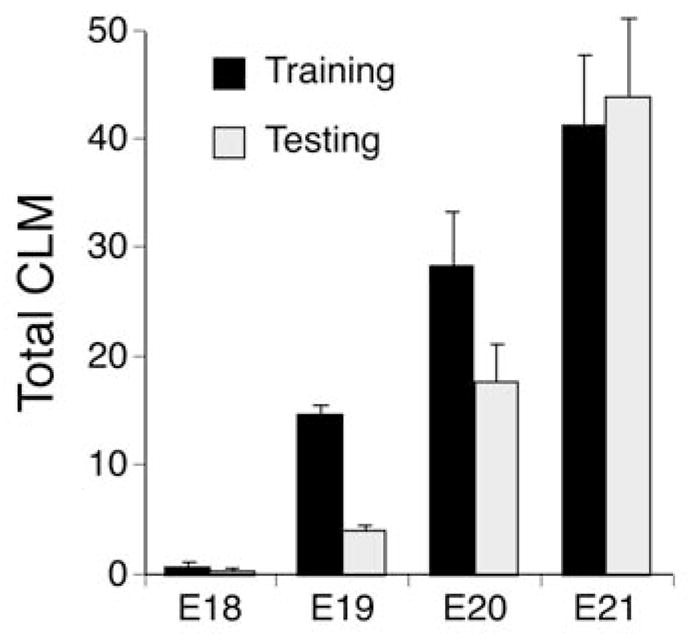
Summary of changes in fetal responses to interlimb yoke training from E18 to E21. The mean number of hindlimb conjugate limb movements (CLM) expressed during the training and testing periods of each session are shown. Vertical lines show SEM. Note that there is steady improvement in both the number of CLM during yoke training and their persistence after the yoke is removed from E19 to E21
DISCUSSION
In the original report of motor learning in the rat fetus, evidence was presented that by E20 of gestation—2 days before birth—the fetus can detect a biomechanical constraint of limb movement, alter the patterning of hind limb activity during exposure to the interlimb yoke, and continue to express changes in interlimb coordination after limb restraint is removed (Robinson, 2005). This study extends these findings by documenting the prenatal emergence of the capacity to adaptively modify limb coordination in response to yoke training. Fetuses on E18 showed no evidence of responsiveness to the interlimb yoke. However, at all subsequent pre-natal ages (E19–E21), fetuses exhibited a significant increase in CLM during yoke training, which persisted above levels shown by unyoked controls for at least 10 min after the yoke was removed. Yoke training also resulted in an increase in overall hind limb activity. However, at all three ages where training effects were evident, the increase in hind limb activity was small relative to the increase in CLM, and CLM still showed a significant increase when expressed as a percentage of hind limb activity. We conclude from these results that motor learning of coordinated hind limb behavior, in response to interlimb yoke training, can be expressed as early as E19 of gestation in the rat.
Although motor learning was evident from E19 to E21, differences were apparent in the responses of fetuses to interlimb yoke training at each age. Responsiveness to yoke training was most pronounced on E20 and E21, when the highest rates of CLM were expressed, both in absolute terms and as a percentage of overall hind limb activity. Fetuses at these two ages showed nearly the same rates of increase in CLM during training, but CLM continued to be expressed at high levels much longer after removal of the yoke on E21. Conversely, E19 fetuses showed diminished responses to yoke training, during both training and test periods, compared to older subjects. E19 subjects achieved lower peak levels of CLM during training, expressed fewer CLM in total, and performed CLM after removal of the yoke only at very low rates. Closer examination of each of the six fetuses in the yoked group on E19 revealed that these lower rates were consistent across all subjects, and did not reflect responses to training by only a subset of subjects at this age. We may infer from the comparatively weak training effect on E19 that the underlying neural mechanisms necessary for this form of motor learning are incompletely developed or exhibit only partial function at this age.
Presumably, the ability of the fetus to control the trajectory of each hind limb must be dependent on afferent information that derives from muscle spindles, tendon organs, joint angle sensors, or cutaneous receptors that provide kinesthetic feedback about motor performance during yoke training. Anatomical studies and neurophysiological recordings suggest that muscle spindles, at least, begin to produce functional afferent signals by E19 (Fitzgerald, 1987; Kucera et al., 1989). Responsiveness to cutaneous stimuli also has been reported in the rat fetus during the last days of gestation, including generalized increases in motor activity to nonspecific stroking (Narayanan et al., 1971; Smotherman & Robinson, 1988), reactions to changes in activity of adjacent siblings in utero (Brumley & Robinson, 2002), and species-typical responses to specific tactile stimuli that mimic conditions encountered during labor (Ronca & Alberts, 1994; Ronca et al., 1993) or after birth (Robinson et al., 1992; Robinson & Smotherman, 1991; Smotherman & Robinson, 1996). Feedback from cutaneous afferents has been implicated in mediating biomechanical effects on motor activity in the chick embryo (Bradley, 2001) and modulating the expression of organized motor patterns in the rat fetus (Robinson & Smotherman, 1994; Smotherman & Robinson, 1989). Changes in cutaneous or proprioceptive feedback also may be responsible for changes in the frequency, duration, and patterning of spontaneous fetal movement in different contexts, such as rat fetuses that remain enveloped by the amniotic sac versus fetuses externalized from the amnion into an unconstrained bath environment (Ronca, Kamm, Thelen, & Alberts, 1994; Robinson & Smotherman, 1988). So it is unclear whether sense organs in muscles, skin, or other elements of the musculoskeletal system are responsible for mediating the effects of yoke training in this study. Regardless of the mediating sense organ(s) in promoting CLM during limb yoking, rats appear to have a functional kinesthetic sense by E19.
Although we have yet to specify neural mechanisms involved in the yoke training effect, it is possible to identify general functional relationships that are likely necessary for this form of motor learning. The interlimb yoke constrains limb motion, but does so in a contingent manner. As one limb begins to move, it will encounter resistance from its physical connection to the yoked limb, resulting in increased dynamic load. The magnitude of this load is contingent on the speed and direction of relative movement of both limbs: Movement of the more active limb will be retarded, resulting in underperformance, and induced movement of the inactive limb will generate proprioceptive signals indicating a change in position in the absence of neural commands to move. Thus, the interlimb yoke is likely to create a mismatch between motor commands and motor performance in both yoked limbs, which is communicated to the central nervous system as error signals via afferent feedback. Production of CLM represents one kind of solution to the problem created by the interlimb yoke: CLM would obviate error signals by generating motor commands to both limbs that result in parallel movement trajectories, preventing underperformance or overperformance by either limb.
If this interpretation of yoke motor learning is accurate, then fetuses must monitor ongoing motor performance and adjust motor commands as error signals are generated. But how is this accomplished by an organism in which brain regions conventionally associated with motor learning—including motor cortex, basal ganglia, and cerebellum—are very immature and probably nonfunctional? Perhaps, as suggested by Sporns and Edelman (1993), adaptive changes in fetal motor coordination occur as a result of a “dumb” mechanism, such as overproduction and selective elimination of movement variants. It may be noteworthy in this regard that fetuses expressed a pronounced increase in hind limb activity during yoke training, which may have resulted in enhanced variability of interlimb coordination that would facilitate motor learning by a selectionist mechanism. Enhanced variability of movement in response to perturbation also is consistent with a dynamic systems perspective on early motor development, as exemplified by human infants learning to reach (Thelen & Spencer, 1998). Selectionist or dynamic systems accounts of motor learning do not imply conscious intent or motor planning on the part of the fetus, but do suggest that feedback about motor performance, and not merely activity, is more important than heretofore recognized during prenatal motor development (cf. DiBiasi & Einspieler, 2004; Hamburger, 1973; Haver-kamp & Oppenheim, 1986).
Of course, the ability to modify limb movement to adapt to altered task demands would scarcely be surprising during postnatal development. Learning is presumed to play a crucial role in the development of motor control after birth, and recent research on reaching, crawling, kicking, walking, and other action systems in human infants has confirmed the importance of experience in refining skilled movement (Adolph, 1998; Adolph & Berger, 2006; Galloway & Thelen, 2004; Goldfield, Kay, & Warren, 1993; Thelen, 1984; Thelen, Corbetta, & Spencer, 1996). Indeed, the yoke training paradigm was originally applied in a postnatal context in humans, in which 3-month-old infants were challenged to kick their legs, which were either yoked or unyoked, to move an overhead mobile (Thelen, 1994). Moving the mobile is reinforcing to young infants (Kraebel, Fable, & Gerhardstein, 2004; Rovee & Rovee, 1969; Rovee-Collier, Morrongiello, Aron, & Kupersmidt, 1978), and because the interlimb yoke reduced the amplitude of individual leg kicks, infants learned to kick their legs in a conjugate pattern to more effectively shake the mobile. Variations of this mobile reinforcement paradigm have since been used to train specific patterns of CLM in infants (Angulo-Kinzler, 2001; Angulo-Kinzler & Horn, 2001; Angulo-Kinzler, Ulrich, & Thelen, 2002; Chen, Fetters, Holt, & Saltzman, 2002). In the mobile paradigm, the task is defined by reinforcement controlled by the experimenter—movement of the overhead mobile—and the interlimb yoke constrains kicking strategies that can successfully complete the task.
In contrast to yoke training in human infants, the fetal rats in this study altered their interlimb coordination without explicit reinforcement. The experimenter did not define a particular fetal movement as a goal to be reached, nor were there any experimenter-controlled rewards or punishments associated with different movement patterns. Rather, any contingencies associated with different coordinative patterns must be intrinsic to the production of spontaneous movement in the context of the biomechanical constraint created by the interlimb yoke. For example, the interlimb yoke may result in discomfort in the area of yoke attachment when leg movements are not conjugate, or the fetus may experience greater expenditure of energy than would occur in the absence of yoke constraint. If intrinsic sources of reinforcement such as these are important for yoke training, then fetal motor learning may represent a basic form of instrumental conditioning similar to that reported for human infants. However, until we obtain better information about the consequences of yoke training, it will not be possible to determine whether yoke motor learning as reported in fetal rats and human infants is governed by similar learning mechanisms.
In many respects it is more appropriate to compare 3-month-old infants with rat pups during their second week after birth or later. Conversely, E19 rat fetuses exhibit gross patterns of brain development that are most similar to human fetuses around Week 11 of gestation (Clancy, Darlington, & Finlay, 2001), and anatomical evidence suggests spindle organs may be functional at this age (Cuajunco, 1940). Given the differences in age and state of neural development between studies of yoke motor learning in human infants and rat fetuses, it is interesting to speculate that similarities in their response to the interlimb yoke may be supported by very different neural resources. Because infant learning is dependent on visual feedback and reinforcement, it is likely dependent on limbic and cortical control of leg movement. In contrast, the rat fetus expresses many forms of motor organization after spinal transection, including spontaneous activity, interlimb synchrony, cyclicity, and coordinated stepping, suggesting that motor learning in the fetus may be supported by spinal circuitry alone (Brumley & Robinson, 2005; Robertson & Smotherman, 1990; Robinson, Blumberg, Lane, & Kreber, 2000).
On the other hand, there is growing evidence that the spinal cord exhibits considerable plasticity in adulthood as well as early in development (Edgerton, Tillakaratne, Bigbee, de Leon, & Roy,, 2004; Patterson & Grau, 2001). Animal research has experimentally demonstrated that spinal responses to pain and other afferent signals can be enhanced or diminished by sensitization or habituation, and motor reflexes such as limb withdrawal can be modified through classical or operant conditioning procedures (Grau, 2002; Wolpaw, 2007a). Indeed, spinal plasticity is now recognized as an important mechanism in the acquisition and improvement of motor skills in animals and humans (Wolpaw, 2007b). Therefore, it is possible that apparent differences in yoke training may mask deeper continuities in the mechanisms of motor learning between fetal rats and human infants. It remains for further research to determine whether interlimb yoke training in the rat fetus exhibits developmental continuity with more conventional forms of motor learning later in life.
In conclusion, this report provides the first systematic investigation of the pre-natal development of a simple motor learning task that presumably requires a functional proprioceptive sense. Because motor learning was not evident earlier than E19, we conclude that the ability of the fetal rat to make use of kinesthetic feedback from spontaneous limb movement to modify subsequent motor behavior emerges at this age. Experience that accrues from ongoing motor activity before birth therefore may play a significant role in motor development during the last few days of gestation in the rat fetus, and by inference, during the last two trimesters of gestation in the human fetus. Thus, it is possible that natural features of the intrauterine environment—such as changes in activity or posture of the mother, movement of siblings in utero, dynamic interactions with the elastic membranes that surround the fetus, or the occurrence of uterine contractions—have the potential to modify motor behavior and thereby shape the course of motor development of the mammalian fetus and neonate.
Acknowledgments
Portions of this research were presented at the 2000 meeting of the International Society for Developmental Psychobiology. We thank O. Bailey for her assistance in conducting these fetal experiments, and A. J. Marcano-Reik and S. A. Woller for their comments during preparation of this article. This research was supported by NIH grant HD 33862 to Scott R. Robinson.
Contributor Information
Scott R. Robinson, Department of Psychology, University of Iowa
Gale A. Kleven, Department of Obstetrics and Gynecology, Wake Forest University School of Medicine
Michele R. Brumley, Department of Neurological Surgery, University of Miami School of Medicine
References
- Adolph KE. Learning to crawl. Child Development. 1998;69:1299–1312. [PubMed] [Google Scholar]
- Adolph KE, Berger SA. Motor development. In: Damon W, Lerner R, Kuhn D, Siegler RS, editors. Handbook of child psychology: Vol. 2. Cognition, perception, and language. Vol. 6. New York: Wiley; 2006. pp. 161–213. [Google Scholar]
- Alberts JR, Cramer CP. Ecology and experience: Sources of means and meaning of developmental change. In: Blass EM, editor. Handbook of behavioral neurobiology: Vol. 9. Developmental psychobiology and behavioral ecology. New York: Plenum; 1988. pp. 1–39. [Google Scholar]
- Alberts JR, Ronca AE. Fetal experience revealed by rats: Psychobiological insights. Early Human Development. 1993;35:153–166. doi: 10.1016/0378-3782(93)90102-z. [DOI] [PubMed] [Google Scholar]
- Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Animal Behaviour. 1975;23:896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Angulo-Kinzler RM. Exploration and selection of intralimb coordination patterns in 3-month-old infants. Journal of Motor Behavior. 2001;33:363–376. doi: 10.1080/00222890109601920. [DOI] [PubMed] [Google Scholar]
- Angulo-Kinzler RM, Horn CL. Selection and memory of a lower limb motor-perceptual task in 3-month-old infants. Infant Behavior & Development. 2001;24:239–257. [Google Scholar]
- Angulo-Kinzler RM, Ulrich B, Thelen E. Three-month-old infants can select specific leg motor solutions. Motor Control. 2002;6:52–68. doi: 10.1123/mcj.6.1.52. [DOI] [PubMed] [Google Scholar]
- Bachevalier JC, Blozovski D. Acquisition and retention of classical conditioning in the newborn rat. Developmental Psychobiology. 1980;13:519–526. doi: 10.1002/dev.420130511. [DOI] [PubMed] [Google Scholar]
- Bailey OE, Kleven GA, Brumley MR, Robinson SR. Perinatal development of interlimb motor learning in the rat fetus and neonate [Abstract] Developmental Psychobiology. 2000;36:235. [Google Scholar]
- Bekoff A, Lau B. Interlimb coordination in 20-day-old rat fetuses. Journal of Experimental Zoology. 1980;214:173–175. doi: 10.1002/jez.1402140207. [DOI] [PubMed] [Google Scholar]
- Bekoff A, Sabichi AL. Sensory control of the initiation of hatching in chicks: Effects of a local anesthetic injected into the neck. Developmental Psychobiology. 1987;20:489–495. doi: 10.1002/dev.420200503. [DOI] [PubMed] [Google Scholar]
- Bordner KA, Spear NE. Trace conditioning in 1-day-old rats. Developmental Psychobiology. 2006;48:58–70. doi: 10.1002/dev.20108. [DOI] [PubMed] [Google Scholar]
- Bradley NS. Reduction in buoyancy alters parameters of motility in E9 chick embryos. Physiology & Behavior. 1997;62:591–595. doi: 10.1016/s0031-9384(97)00168-6. [DOI] [PubMed] [Google Scholar]
- Bradley NS. Age-related changes and condition-dependent modifications in distribution of limb movements during embryonic motility. Journal of Neurophysiology. 2001;86:1511–1522. doi: 10.1152/jn.2001.86.4.1511. [DOI] [PubMed] [Google Scholar]
- Bradley NS, Sebelski C. Ankle restraint modifies motility at E12 in chick embryos. Journal of Neurophysiology. 2000;83:431–440. doi: 10.1152/jn.2000.83.1.431. [DOI] [PubMed] [Google Scholar]
- Brumley MR, Robinson SR. Responsiveness of rat fetuses to sibling motor activity: Communication in utero? [Abstract] Developmental Psychobiology. 2002;41:73. doi: 10.1002/dev.21615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumley MR, Robinson SR. The serotonergic agonists quipazine, CGS-12066A and α-methylserotonin alter motor activity and induce hindlimb stepping in the intact and spinal rat fetus. Behavioral Neuroscience. 2005;119:821–833. doi: 10.1037/0735-7044.119.3.821. [DOI] [PubMed] [Google Scholar]
- Chen YP, Fetters L, Holt KG, Saltzman E. Making the mobile move: Constraining task and environment. Infant Behavior and Development. 2002;25:195–220. [Google Scholar]
- Chotro MG, Arias C. Prenatal exposure to ethanol increases ethanol consumption: A conditioned response? Alcohol. 2003;30:19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Cuajunco F. Development of the neuromuscular spindle in human fetuses. Contributions to Embryology. 1940;28:97–128. [Google Scholar]
- DiBiasi J, Einspieler C. Load perturbation does not influence spontaneous movements in 3-month-old infants. Early Human Development. 2004;77:37–46. doi: 10.1016/j.earlhumdev.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJK, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annual Review of Neuroscience. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Eilam D, Smotherman WP. How the neonatal rat gets to the nipple: Common motor modules and their involvement in the expression of early motor behavior. Developmental Psychobiology. 1998;32:57–66. doi: 10.1002/(sici)1098-2302(199801)32:1<57::aid-dev7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Fifer WP, Moon CM. The effects of fetal experience with sound. In: Lecanuet J-P, Fifer WP, Krasnegor NA, Smotherman WP, editors. Fetal development: A psychobiological perspective. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1995. pp. 351–366. [Google Scholar]
- Fitzgerald M. Spontaneous and evoked activity of primary afferents in vivo. Nature. 1987;326:603–605. doi: 10.1038/326603a0. [DOI] [PubMed] [Google Scholar]
- Galloway JC, Thelen E. Feet first: Object exploration in young infants. Infant Behavior and Development. 2004;27:107–112. [Google Scholar]
- Gesell A. The ontogenesis of infant behavior. In: Carmichael L, editor. Manual of child psychology. New York: Wiley; 1946. pp. 295–331. [Google Scholar]
- Goldfield EC, Kay BA, Warren WH., Jr Infant bouncing: The assembly and tuning of action systems. Child Development. 1993;64:1128–1142. [PubMed] [Google Scholar]
- Grau JW. Learning and memory without a brain. In: Bekoff M, Allen C, Burghardt GM, editors. The cognitive animal: Empirical and theoretical perspectives on animal cognition. Cambridge, MA: MIT Press; 2002. pp. 77–87. [Google Scholar]
- Hamburger V. Anatomical and physiological basis of embryonic motility in birds and mammals. In: Gottlieb G, editor. Behavioral embryology. Vol. 1. New York: Academic; 1973. pp. 51–76. [Google Scholar]
- Hamburger V, Wenger E, Oppenheim RW. Motility in the chick embryo in the absence of sensory input. Journal of Experimental Zoology. 1966;162:133–160. [Google Scholar]
- Haverkamp LJ, Oppenheim RW. Behavioral development in the absence of neural activity: Effects of chronic immobilization on amphibian embryos. Journal of Neuroscience. 1986;6:1332–1337. doi: 10.1523/JNEUROSCI.06-05-01332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepper PG. Adaptive fetal learning: Prenatal exposure to garlic affects postnatal preferences. Animal Behaviour. 1988;36:935–936. [Google Scholar]
- Johanson IB, Hall WG. Appetitive learning in 1-day-old rat pups. Science. 1979;205:419–421. doi: 10.1126/science.451612. [DOI] [PubMed] [Google Scholar]
- Kleven GA, Lane MS, Robinson SR. Development of interlimb movement synchrony in the rat fetus. Behavioral Neuroscience. 2004;118:835–844. doi: 10.1037/0735-7044.118.4.835. [DOI] [PubMed] [Google Scholar]
- Kraebel KS, Fable J, Gerhardstein P. New methodology in infant operant kicking procedures: Computerized stimulus control and computerized measurement of kicking. Infant Behavior and Development. 2004;27:1–18. [Google Scholar]
- Kucera J, Walro JM. Sequences of intrafusal fiber formation are muscle-dependent in rat hindlimbs. Anatomy and Embryology. 1994;190:273–286. doi: 10.1007/BF00234305. [DOI] [PubMed] [Google Scholar]
- Kucera J, Walro JM, Reichler J. Innervation of developing intrafusal muscle fibers in the rat. American Journal of Anatomy. 1988;183:344–358. doi: 10.1002/aja.1001830408. [DOI] [PubMed] [Google Scholar]
- Kucera J, Walro JM, Reichler J. Role of nerve and muscle factors in the development of rat muscle spindles. American Journal of Anatomy. 1989;186:144–160. doi: 10.1002/aja.1001860205. [DOI] [PubMed] [Google Scholar]
- Lickliter R. Prenatal sensory ecology and experience: Implications for perceptual and behavioral development in precocial birds. Advances in the Study of Behavior. 2005;35:235–274. [Google Scholar]
- Maier A. Development of chicken intrafusal muscle fibers. Cell Tissue Research. 1993;274:383–391. doi: 10.1007/BF00318757. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Remmers-Roeber DR, Crouse C, Walker C, Dengler C. Detection of novelty by perinatal rats. Physiology & Behavior. 2000;70:217–225. doi: 10.1016/s0031-9384(00)00229-8. [DOI] [PubMed] [Google Scholar]
- Milburn A. Early development of muscle spindles in the rat. Journal of Cell Science. 1973;12:175–195. doi: 10.1242/jcs.12.1.175. [DOI] [PubMed] [Google Scholar]
- Molina JC, Chotro MG, Dominguez HD. Fetal alcohol learning resulting from alcohol contamination of the prenatal environment. In: Lecanuet JP, Fifer WP, Krasnegor N, Smotherman WP, editors. Fetal development: A psychobiological perspective. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1995. pp. 419–438. [Google Scholar]
- Narayanan CH, Fox MW, Hamburger V. Prenatal development of spontaneous and evoked activity in the rat (Rattus norvegicus) Behaviour. 1971;40:100–134. doi: 10.1163/156853971x00357. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals (DHEW Publication No. 86–23) Washington, DC: U.S. Government Printing Office; 1986. [Google Scholar]
- Oppenheim RW. An experimental investigation of the possible role of tactile and pro-prioceptive stimulation in certain aspects of embryonic behavior in the chick. Developmental Psychobiology. 1972;5:71–91. doi: 10.1002/dev.420050109. [DOI] [PubMed] [Google Scholar]
- Patterson MM, Grau JW. Spinal cord plasticity: Alterations in reflex function. Norwell, MA: Kluwer Academic; 2001. [Google Scholar]
- Pellis VC, Pellis SM, Teitelbaum P. A descriptive analysis of the postnatal development of contact-righting in rats (Rattus norvegicus) Developmental Psychobiology. 1991;24:237–263. [Google Scholar]
- Robertson SS, Smotherman WP. The neural control of cyclic motor activity in the fetal rat (Rattus norvegicus) Physiology & Behavior. 1990;47:121–126. doi: 10.1016/0031-9384(90)90049-a. [DOI] [PubMed] [Google Scholar]
- Robinson SR. Unpublished doctoral dissertation. Oregon State University; Corvallis, OR: 1989. A comparative study of prenatal behavioral ontogeny in altricial and precocial murid rodents. [Google Scholar]
- Robinson SR. Conjugate limb coordination after experience with an interlimb yoke: Evidence for motor learning in the rat fetus. Developmental Psychobiology. 2005;47:328–344. doi: 10.1002/dev.20103. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Arnold HM, Spear NE, Smotherman WP. Experience with milk and an artificial nipple promotes conditioned opioid activity in the rat fetus. Developmental Psychobiology. 1993;26:375–387. doi: 10.1002/dev.420260702. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Blumberg MS, Lane MS, Kreber LA. Spontaneous motor activity in fetal and infant rats is organized into discrete multilimb bouts. Behavioral Neuroscience. 2000;114:328–336. doi: 10.1037//0735-7044.114.2.328. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Brumley MR. Prenatal behavior. In: Whishaw IQ, Kolb B, editors. The behaviour of the laboratory rat: A handbook with tests. New York: Oxford University Press; 2005. pp. 257–265. [Google Scholar]
- Robinson SR, Hoeltzel TCM, Cooke KM, Umphress SM, Murrish DE, Smotherman WP. Oral capture and grasping of an artificial nipple by rat fetuses. Developmental Psychobiology. 1992;25:543–555. doi: 10.1002/dev.420250802. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Kleven GA. Learning to move before birth. In: Hopkins B, Johnson SP, editors. Prenatal development of postnatal functions. Westport, CT: Praeger; 2005. pp. 131–175. [Google Scholar]
- Robinson SR, Smotherman WP. Chance and chunks in the ontogeny of fetal behavior. In: Smotherman WP, Robinson SR, editors. Behavior of the fetus. Caldwell, NJ: Tel-ford Press; 1988. pp. 95–115. [Google Scholar]
- Robinson SR, Smotherman WP. The amniotic sac as scaffolding: Prenatal ontogeny of an action pattern. Developmental Psychobiology. 1991;24:463–485. doi: 10.1002/dev.420240703. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Behavioral response of altricial and precocial rodent fetuses to acute umbilical cord compression. Behavioral and Neural Biology. 1992a;57:93–102. doi: 10.1016/0163-1047(92)90581-n. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Fundamental motor patterns of the mammalian fetus. Journal of Neurobiology. 1992b;23:1574–1600. doi: 10.1002/neu.480231013. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Organization of the stretch response to milk in the rat fetus. Developmental Psychobiology. 1992c;25:33–49. doi: 10.1002/dev.420250104. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Behavioral effects of milk in the rat fetus. Behavioral Neuroscience. 1994;108:1139–1149. [PubMed] [Google Scholar]
- Ronca AE, Alberts JR. Sensory stimuli associated with gestation and parturition evoke cardiac and behavioral responses in fetal rats. Psychobiology. 1994;22:270–282. [Google Scholar]
- Ronca AE, Kamm K, Thelen E, Alberts JR. Proximal control of fetal rat behavior. Developmental Psychobiology. 1994;27:23–38. doi: 10.1002/dev.420270104. [DOI] [PubMed] [Google Scholar]
- Ronca AE, Lamkin CA, Alberts JR. Maternal contributions to sensory experience in the fetal and newborn rat (Rattus norvegicus) Journal of Comparative Psychology. 1993;107:61–74. doi: 10.1037/0735-7036.107.1.61. [DOI] [PubMed] [Google Scholar]
- Rovee CK, Rovee DT. Conjugate reinforcement of infant exploratory behavior. Journal of Experimental Child Psychology. 1969;8:33–39. doi: 10.1016/0022-0965(69)90025-3. [DOI] [PubMed] [Google Scholar]
- Rovee-Collier CK, Morrongiello BA, Aron M, Kupersmidt J. Topographical response differentiation and reversal in 3-month-old infants. Infant Behavior and Devleopment. 1978;1:323–333. [Google Scholar]
- Schaal B. From amnion to colostrum to milk: Odor bridging in early developmental transitions. In: Hopkins B, Johnson SP, editors. Prenatal development of postnatal functions. Westport, CT: Praeger; 2005. pp. 51–102. [Google Scholar]
- Smotherman WP. In utero chemosensory experience alters taste preferences and corti-costerone responsiveness. Behavioral and Neural Biology. 1982;36:61–68. doi: 10.1016/s0163-1047(82)90245-x. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Richards LS, Robinson SR. Techniques for observing fetal behavior in utero: A comparison of chemomyelotomy and spinal transection. Developmental Psychobiology. 1984;17:661–674. doi: 10.1002/dev.420170608. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. The rat fetus in its environment: Behavioral adjustments to novel, familiar, aversive and conditioned stimuli presented in utero. Behavioral Neuroscience. 1985;99:521–530. doi: 10.1037//0735-7044.99.3.521. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Environmental determinants of behaviour in the rat fetus. Animal Behaviour. 1986;34:1859–1873. [Google Scholar]
- Smotherman WP, Robinson SR. Behavior of rat fetuses following chemical or tactile stimulation. Behavioral Neuroscience. 1988;102:24–34. doi: 10.1037//0735-7044.102.1.24. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Cryptopsychobiology: The appearance, disappearance and reappearance of a species-typical action pattern during early development. Behavioral Neuroscience. 1989;103:246–253. doi: 10.1037//0735-7044.103.2.246. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. The prenatal origins of behavioral organization. Psychological Science. 1990;1:97–106. [Google Scholar]
- Smotherman WP, Robinson SR. Conditioned activation of fetal behavior. Physiology & Behavior. 1991b;50:73–77. doi: 10.1016/0031-9384(91)90500-n. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Accessibility of the rat fetus for psychobiological investigation. In: Shair H, Barr GA, Hofer MA, editors. Developmental psychobiology: New methods and changing concepts. New York: Oxford University Press; 1991a. pp. 148–166. [Google Scholar]
- Smotherman WP, Robinson SR. Habituation in the rat fetus. Quarterly Journal of Experimental Psychology. 1992;44B:215–230. doi: 10.1080/02724999208250613. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. The development of behavior before birth. Developmental Psychology. 1996;32:425–434. [Google Scholar]
- Spear NE, Rudy JW. Tests of the ontogeny of learning and memory: Issues, methods and results. In: Shair HN, Hofer MA, Barr G, editors. Developmental psychobiology: Current methodology and conceptual issues. New York: Oxford University Press; 1991. pp. 84–113. [Google Scholar]
- Sporns O, Edelman GM. Solving Bernstein’s problem: A proposal for the development of coordinated movement by selection. Child Development. 1993;64:960–981. [PubMed] [Google Scholar]
- Stickrod G, Kimble DP, Smotherman WP. In utero taste/odor aversion conditioning in the rat. Physiology & Behavior. 1982;28:5–7. doi: 10.1016/0031-9384(82)90093-2. [DOI] [PubMed] [Google Scholar]
- Thelen E. Learning to walk: Ecological demands and phylogenetic constraints. Advances in Infancy Research. 1984;3:213–260. [Google Scholar]
- Thelen E. Three-month-old infants can learn task-specific patterns of interlimb coordination. Psychological Science. 1994;5:280–285. [Google Scholar]
- Thelen E. Motor development: A new synthesis. American Psychologist. 1995;50:79–95. doi: 10.1037//0003-066x.50.2.79. [DOI] [PubMed] [Google Scholar]
- Thelen E, Corbetta D, Spencer JP. Development of reaching during the first year: Role of movement speed. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:1059–1076. doi: 10.1037//0096-1523.22.5.1059. [DOI] [PubMed] [Google Scholar]
- Thelen E, Smith LB. A dynamic systems approach to the development of cognition and action. Cambridge, MA: MIT Press; 1994. [Google Scholar]
- Thelen E, Spencer JP. Postural control during reaching in young infants: A dynamic systems approach. Neuroscience and Biobehavioral Reviews. 1998;22:507–514. doi: 10.1016/s0149-7634(97)00037-7. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Neurobiology of associative learning in the neonate: Early olfactory learning. Behavioral and Neural Biology. 1994;61:1–18. doi: 10.1016/s0163-1047(05)80039-1. [DOI] [PubMed] [Google Scholar]
- Wolpaw JW. The education and re-education of the spinal cord. Progress in Brain Research. 2007a;157:261–281. doi: 10.1016/s0079-6123(06)57017-7. [DOI] [PubMed] [Google Scholar]
- Wolpaw JW. Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiologica. 2007b;189:155–169. doi: 10.1111/j.1748-1716.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- Viala D, Viala G, Fayein N. Plasticity of locomotor organization in infant rabbits spinalized shortly after birth. In: Goldberger ME, Gorio A, Murray A, editors. Development and plasticity of the mammalian spinal cord. New York: Springer-Verlag; 1986. pp. 301–310. [Google Scholar]