Abstract
The past few years of research on leptin have provided important information on the link between metabolism and immune homeostasis. Adipocytes influence not only the endocrine system but also the immune response through several cytokine-like mediators known as adipokines, which include leptin. It is widely accepted that leptin can directly link nutritional status and pro-inflammatory T helper 1 immune responses, and that a decrease of leptin plasma concentration during food deprivation can lead to an impaired immune function. Additionally, several studies have implicated leptin in the pathogenesis of chronic inflammation, and the elevated circulating leptin levels in obesity appear to contribute to the low-grade inflammatory background which makes obese individuals more susceptible to increased risk of developing cardiovascular diseases, type II diabetes, or degenerative disease including autoimmunity and cancer. Conversely, reduced levels of leptin such as those found in malnourished individuals have been linked to increased risk of infection and reduced cell-mediated immune responses. We discuss here the functional influences of leptin in the physiopathology of inflammation, and the effects of leptin in the modulation of such responses.
Keywords: leptin, inflammation, autoimmunity, infection
Introduction
Immune cells can secrete cytokines that affect the activity of adipocytes. Conversely, adipocytes can produce soluble mediators called adipocytokines (or adipokines) that not only influence energy homeostasis but also immune responses.
Leptin is an adipokine that was originally identified as a key molecule in the regulation of food intake and body weight [1]. Circulating leptin is found both as a biologically active free form and presumably as an inactive form that is bound to plasma proteins and/or with to the soluble leptin receptor (Ob-R) isoform Ob-Re [2] (see below). The levels of circulating leptin levels directly reflect the amount of energy stored in the adipose tissue, and are proportional to the body adipose mass, both in mice and in humans. It derives that obese individuals typically produce higher levels of leptin than leaner individuals [3–5], yet obese subjects are resistant to the inhibitory activity that this molecule has on food intake and on the control of satiety.
Notably, an exquisite gender bias typically characterizes the expression of leptin, considering that this hormone is found in females at much higher concentrations than in males matched for body mass, and leptin production is inhibited by testosterone and possibly promoted by ovarian sex steroids [6].
Structurally, leptin is a 16 kDa non-glycosylated polypeptide found primarily in adipocytes, although it can be also detected (at lower levels) in the hypothalamus, pituitary [7], stomach and intestine [8], skeletal muscle [9], mammary epithelium [10], chondrocytes [11], placenta [12], cartilage and bone cells [13], and immune cells [14–15].
The most evident function of leptin is the control of appetite, and this is classically demonstrated in phenotypically obese mice which carry a mutation of the gene that encodes leptin (ob/ob mice), or in mice that are deficient in the gene that encodes the leptin receptor (db/db mice). Both ob/ob and db/db mice have an obese phenotype secondary to the lack of the perception of satiety, together with hyperglycemia and insulin-resistance [1]. interestingly, the hyperglycemic, hyperinsulinemic and obese phenotype of ob/ob mice are all reversed to a normal range following the administration of leptin (because these mice respond to exogenous leptin), but that does not occur in db/db mice (which have a mutated receptor and are thus unresponsive to leptin) [16–19].
The functions of leptin do not stop to the control of food intake. Leptin is as a key regulator not only of the endocrine system and of the hypothalamic-pituitary-adrenal axis, but also of energy homeostasis, insulin secretion, angiogenesis, bone formation and reproduction [20–24]. Importantly, leptin also has a critical role in the modulation of innate and adaptive immune responses. This is likely because of the structural similarities of leptin with the cytokines of the long-chain helical family that include interleukin (IL)-6, IL-11, IL-12, and oncostatin M [25]. Because of its immune activities, leptin can link the nutritional status with T helper (Th)1 immune responses, and a decrease of plasma leptin levels typically leads to an impaired immune function [26]. For example, during starvation and malnutrition, the levels of leptin are low, and those conditions associate with thymic atrophy and impaired immune responses [27].
Genetics of leptin
The obese gene is located on human chromosome 7 (ob) and on mouse chromosome 6 (OB) [28].
In the white adipose tissue, leptin gene expression is regulated at the transcriptional level depending on the adipocyte size, which in turn reflects the energy stores [29–30].
Mice with mutation in the ob gene have early onset obesity due to hyperphagia and subsequent hyperinsulinemia, hyperglycemia and diabetes, along with low body temperature, hypercortisonemia, hypothalamic hypogonadism, and reduced immune function [1, 17–18, 28].
Mutations in the human OB gene are rather rare and phenotypically similar to those found in the mouse (i.e. they associate with morbid obesity, hyperinsulinemia, increased adrenocorticotrophic hormone and cortisol levels, hypothalamic hypogonadism, mild hypothyroidism and alterations in growth hormone and parathyroid hormone functions, in addition to an impaired immune function) [31–33].
Immunologically, leptin can manifest its biological actions on the immune subpopulations that express the leptin receptor (Ob-R), a molecule encoded by the diabetes (db) gene that is homologousto the gp-130 signal-transducing subunit of the IL-6-type cytokinereceptors of the class-I cytokine receptor family [34].
Mutations in the db gene results in morbid obesity and other abnormalities similar to those observed in ob/ob mice, and db/db mice have a mutation in their leptin receptor leading to a premature stop codon that impairs leptin-mediated intracellular signaling [35].
Human leptin receptor deficiency is similar to the murine leptin-deficient phenotype [36] and is more frequent that the OB/OB mutation, as indicated by a recent study showing that the prevalence of pathogenic congenital leptin-receptor deficiency mutations in a cohort of subjects with severe, early-onset obesity was about 3% [37]. Incidentally, in those individuals the serum levels of leptin were not particularly elevated, indicating that serum leptin alone may not be used as a marker for leptin-receptor deficiency [37].
Leptin signaling
The reciprocal influence between the immune system and the nutritional status has recently been investigated to understand how the energy status can affect and is affected by immune responses, and the Ob-R signaling pathways have come under scrutiny.
Six splice variants of leptin receptors have been identified in the mouse. They are designated Ob-Ra through Ob-Rf. [38]. In humans, only OB-Ra, OB-Rb, and OB-Rc have been reported [39]. Of those isoforms, only OB-Rb is responsible for the anorexigenic effects of leptin, and is abundant in the hypothalamic centers regulating food intake and body weight [15]. OB-Rb can also be found on immune cells including subpopulations of T cells, B cells, dendritic cells (DC), monocytes, neutrophils, macrophages, and natural killer (NK) cells [40–44].
From a biochemical standpoint, the receptor of leptin does not have an intrinsic tyrosine kinase domain but its proline-rich box 1 motif binds to Janus kinase (JAK)2. In particular, the binding of leptin to its receptor activates JAK2 which becomes auto-phosphorylated and in turn phosphorylates tyrosine residues on the intracellular domain of the receptor for the binding and activation of signal transducers and activators of transcription (STATS) including STAT1, STAT3, STAT5 and STAT6 (in a variety of cell types). STAT3 activation regulates the expression of different genes that mediate the effects of leptin on cell growth and function as well as on the activation of peripheral mononuclear cells including lymphocytes and macrophages [45–47]. In addition, STAT3 regulates the activation of suppressors of cytokine signaling 3 (SOCS3), a member of a family of anti-inflammatory cytokines that suppress cellular responses to inflammatory cytokines which participate in an inhibitory feedback loop of leptin signaling [48]. In this context, the increased expression of SOCS3 due to high levels of leptin may be a partial explanation of why obese subjects have diminished responsiveness to leptin [49]. In addition to SOCS3, protein tyrosine phosphatase 1B (PTP1B) also participates in the inhibitory regulation of leptin-mediated signaling and acts by dephosphorylating JAK2 [50–51].
Phosphorylated JAK2 also activates other pathways including mitogen-activated-protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase (PI3K)/Akt [52]. The PI3/Akt pathway represents a key signaling cascade that mediates effects of various pro-inflammatory cytokines and bacterial and viral stimuli in a variety of immune cells. Leptin activates PI3K/Akt pathway via phosphorylation of the insulin receptor substrate (IRS)1/2, which binds directly to the regulatory unit p85 of PI3K for the subsequent activation of the catalytic domain [53]. Alternatively, leptin can activate PI3K via phosphorylation of src associated in mitosis protein (Sam)68 in peripheral blood mononuclear cells [54]. By stimulating the uptake of glucose through ERK1/2 and PI3K-dependent pathways, leptin might help to restore the impaired T-cell function that is present during starvation [25].
In another pathway, phosphorylated residues of tyrosine recruit src homology 2 domain-containing phosphatase 2 (Shp2), which activates MAPK/ERK pathways through interaction with adaptor protein growth factor receptor-bound protein (GRB)2 [55–57]. This pathway may be linked to Ras activation, a critical step in cell proliferation and/or differentiation [58].
Leptin, the adipose tissue and the immune system
The anatomical proximity between the adipose tissue and primary lymphoid organs (thymus) and secondary lymphoid organs (lymph nodes, spleen) has classically been explained in terms of support and protection, yet it also allows an active cross-talk between adipocytes and innate and adaptive immune cells at lymphoid sites and, more in general, systemically (through the activity of several soluble mediators) [59]. Typically, the communication between the adipose tissue and the immune system could also be meant to provide a link for a rapid, optimal adjustment of the magnitude and extent of ongoing immune responses in relation to availability of nutrients in the environment.
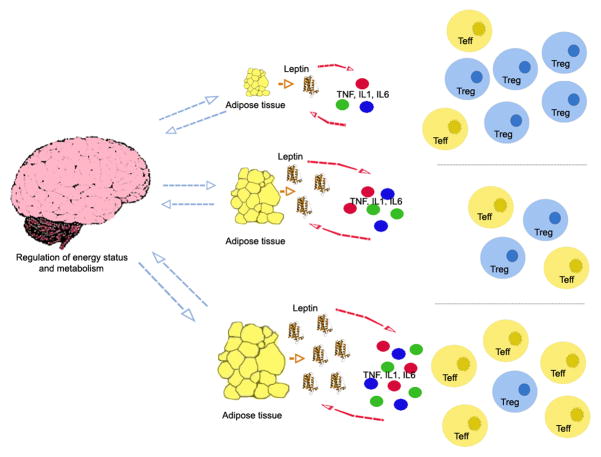
The amount of body fat stored by adipocytes mirrors the bioavailability of energy. Adipocytes inform the body through the brain on whether there is sufficient amount of energy to mount certain responses or rather whether limited availability of energy should constrain certain (i.e. redundant) immune responses (Figure 1). Clearly, immune cells cannot operate properly under energetic restrictions, and the lack of nutrients that leads to energy conservation will associate with a reduced or dysregulated immune response. Conversely, immune cells would typically signal to adipocytes whether to catabolize (and at what extent) the stored fat, according to the cell needs.
Figure 1.
Leptin is at the interface between metabolism and inflammatory responses. Leptin production by the adipose tissue facilitates the secretion of proinflammatory cytokines such as TNF, IL-1 and IL-6, which in turn promote the release of leptin from adipocytes. More leptin is available when the body fat mass is large, such as in obesity. Excess of leptin promotes inflammation and and the expansion of effector T cells (Teff) but it constrains the proliferation of regulatory T cells (Tregs), whereas reduced leptin levels facilitate the activity of the Tregs but associate with reduced number of Teff.
Since leptin serum concentration fluctuates in dependance on the nutritional status, this molecule has a dual role in both controlling metabolism and in modulating immune responses.
The immunology of leptin
Leptin is one of the most abundant adipocytokines produced by adipocytes, together with cytokines such as tumor necrosis factor (TNF)-α, IL-6, IL-1, the CC-chemokine ligand 2 (CCL2) and other mediators [60].
Leptin has pro-inflammatory properties and several actions similar to those of the acute phase reactants, and upregulates the secretion of inflammatory cytokines like TNF-α, IL-6, and IL-12 [61–62]. Conversely, TNF-α and IL-1β increase the expression of leptin mRNA in the adipose tissue, creating a loop whose components influence each other in promoting inflammation [63] (Figure 1).
The levels of expression of leptin in serum and adipose tissue typically increase after the administration of inflammatory stimuli such as lipopolysaccharide (LPS), or in experimental acute inflammation [64–65].
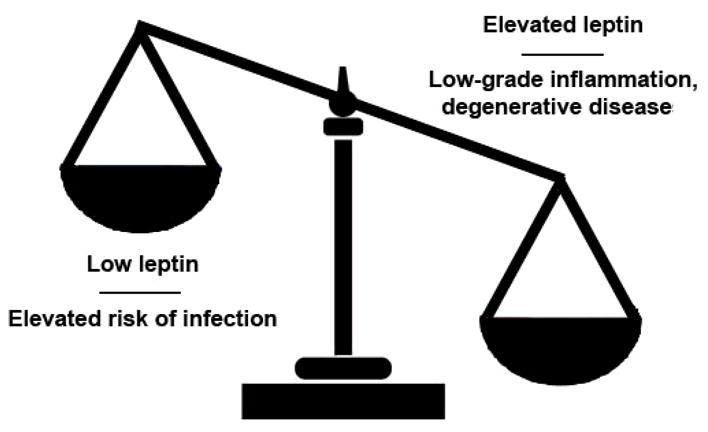
The activity of leptin and its close link with several pro-inflammatory mediators underscores the capacity of this molecule to promote or sustain low-grade inflammation that could ultimately favor the development of chronic disease. This could clearly happen more frequently in the case of obesity, where leptin levels are typically elevated (Figure 2).
Figure 2.
Schematic representation of the dichotomous association between leptin levels and pathological conditions.
Leptin and innate immunity
Congenital leptin-deficient patients have dysfunctional immune responses leading to elevated incidence of childhood infection-related deaths. The therapy with leptin reverses these immunological abnormalities [33, 66].
In innate immunity, leptin can stimulate DC, monocytes, macrophages, neutrophils and NK cells. Leptin is involved in DC maturation and survival, and treatment of DC with exogenous leptin can activate Akt pathway for the induction of CD40 expression [67]. On the other hand, and in the absence of leptin signaling, DC display a Th2-biased cytokine profile while exogenous leptin stimulation skews the cytokine balance towards a Th1 profile [40–41]. Also, DC from db/db mouse bone marrow have increased apoptosis and defective Akt and nuclear factor (NF)κB signaling pathways [41].
In monocytes, leptin activates proliferation of human monocytes in vitro, in which it increases the expression of CD39, CD69, CD25 (IL-2Rα), CD71 (transferrin receptor), and IL-1Rα [68–69]. On monocytes leptin also upregulates the expression of human leukocyte antigen (HLA)-DR [68] and stimulates the production of of IL-6 and TNF-α [68]. Leptin activates macrophages, enhances their phagocytic activity through phospholipase activation [69] and induces them to produce eicosanoids, nitric oxide, leukotriene B4, cholesterol acyl-transferases-1, and cyclooxygenase 2, and other pro-inflammatory cytokines [68, 70–71].
In microglia, leptin induces IL-6 production via IRS-1, PI3K/Akt and the NF-κB pathways, and IL-1β production by activation of STAT-3 [72–73].
In neutrophils, leptin stimulates chemotaxis and the release of reactive oxygen species such as hydrogen peroxide [74]. In addition, leptin protects neutrophils from apoptosis involving PI3K- and MAPK-dependent pathways and a delayed cleavage of Bid and Bax, the mitochondrial release of cytochrome c and the activation of caspases [75].
Finally, leptin promotes NK cell proliferation, differentiation, activation, and cytotoxicity [42, 76] - the last function via an upregulation of IL-2 and perforin expressions through the activation of STAT3 [76].
Leptin and adaptive immunity
Leptin is important for thymic homeostasis and for thymic maturation. Leptin-deficient mice have a high thymocyte apoptosis and low thymic cellularity, which are conditions that can be reversed by leptin administration [27]. The immunosuppressive effects of acute starvation and lymphoid atrophy in mice are also reversed by leptin [27].
Leptin promotes T cell activation and shifts the T-cell cytokine production towards a Th1 response, increasing the production of interferon (IFN)-γ and IL-2, and suppressing the production of the Th2 cytokine IL-4 [26, 33]. In Th1 cells, it increases TNF-α and IFN-γ production and promotes IgG2a switching in B cells, while it exerts inhibitory effects on Th2 cells and IgG1 switching [26].
Leptin administration can lead to a significant increase of both CD4+ and CD8+ T cells as well as NKT cells, as well as cytokine responsiveness [77].
In humans with leptin-deficiency, circulating CD4+ T cells appear decreased in number and impaired in cell proliferation and cytokine release – which are again phenomena that are reversed by recombinant leptin administration [33]. However, in patients with lipodystrophy and low leptin levels, no changes in T lymphocyte counts are observed.
In addition to the finding that leptin can promote survival of both T and B lymphocytes by suppressing Fas-mediated apoptosis [78], recent reports have indicated an ability of leptin to negatively influence the proliferation of naturally occurring human CD4+CD25+FoxP3+ regulatory T cells [15] (Figure 1). Regulatory T cells (Tregs) are critical mediators of peripheral immune tolerance through mechanisms of suppression of the proliferation and secretion of inflammatory cytokines in target cells. Although anergic (hyporesponsive to antigenic stimulation) in vitro, Tregs are capable of self-renewal and proliferation in vivo in conditions of lymphopenia. It has recently been shown that freshly isolated Tregs can produce leptin and express significant levels of OB-R on the cell surface [15]. In vitro neutralization of leptin with anti-leptin antibodies of anti-CD3/28-stimulated Tregs results in the IL-2-dependent proliferation of the Tregs [15]. The expanded Tregs are functional and secondary to the reversal of their anergic state, as suggested by the finding of a downmodulation of the cycline-dependent kinase inhibitor p27 (p27kip1) and the phosphorylation of ERK1/2 [15].
Leptin, nutrition and disease
Nutrition can affect immune responses directly or through the activity of soluble mediators that have effects on both metabolism and immunity. As such, leptin can at the same time regulate energy balance and modulate inflammation.
Individuals with a normal body mass index are typically normoleptinaemic, whereas dysregulated levels of serum leptin are usually found in those individuals whose body fat mass is not within a normal range. By definition, obesity is characterized by an excessive mass of adipose tissue, whereas the limited fat stores that associate with malnutrition may represent the other side of the spectrum.
Obesity is a key contributor to the metabolic syndrome, in addition to being associated with increased risk of cardiovascular disease-related morbidity and mortality. In substance, obesity is a chronic, low-grade inflammatory disease, and obese individuals have elevated serum levels of C-reactive protein (CRP). Obesity represents a risk factor for atherosclerosis via the promotion of other risk factors including dyslipidemia, hypertension and hyperglycemia [79]. Obesity predisposes to an increased risk of developing several diseases including atherosclerosis, diabetes, non-alcoholic fatty liver disease, cancer, and asthma [80–81]. It may be speculated that a common mechanism of immune dysregulation in these subjects may constitute a basis for susceptibility to a variety of inflammatory conditions.
The elevated levels of leptin in obese individuals could help to predispose to the low-grade inflammation, on which the development of chronic conditions and degenerative diseases are promoted (Figure 2). In this sense, obesity has long been known as a major risk factor for atherosclerosis and cardiovascular dysfunction. Leptin pro-atherogenic effects could include the induced expression of CRP in the vascular endothelium [82], as suggested by the finding that defective leptin signaling enhances the regulatory immune response associated with amelioration of atherosclerosis in mice [83]. In addition, obese individuals are more prone than lean individuals to the morbidity and mortality associated with surgical interventions, suggesting again that obesity may be linked to or could favor an impaired immune function.
On the contrary, malnutrition associates with low leptin levels but also with reduced inflammatory and increased risk of infection (Figure 2). For example, during acute starvation, leptin drops and the risk of morbidity from infection increases. Treatment with leptin corrects the host defense and protects from infection, as shown in the case of murine pneumococcal pneumonia [84]. A protective role of leptin in the protection from infection could derive from the fact that leptin promotes the expansion and activity of effector T cells while it reduces the activity of regulatory T cells [15]. With low levels of leptin, an insufficient effector activity of the T cells could not allow a proper control of the pathogens, and a concomitant enhanced Treg function could be detrimental under those conditions.
Leptin and inflammation
Leptin expression is not only regulated by the intake of food, but also by various hormones, as well as by several inflammatory mediators [85–86]. In general, serum leptin levels directly correlate with insulin levels and inversely correlate with glucocorticoid levels, and increase in the course of acute infection and sepsis [85, 87–88].
Additionally, some studies suggest an association of leptin levels and inflammatory markers such as soluble TNF receptors or CRP [89–92], although other studies do not confirm those associations [93]. Recent work has shown that serum leptin levels can correlate with the level of inflammation. The concentration of leptin is elevated during active disease in rheumatoid arthritis patients, and it decreases when the disease is controlled [94–95].
Overall, the pro-inflammatory properties of leptin are similar to those of other acute phase reactants. In this sense, leptin levels are typically elevated during infection and inflammation [25]. Moreover, after exposure to inflammatory stimuli such as LPS, TNF-α, and IL-1, the levels of circulating leptin and leptin expression in the adipose tissue increase [63–64]. Thus, it appears that pro-inflammatory mediators such as TNF-α and IL-1, which upregulate leptin expression, contribute in turn to the creation of a loop of acute phase reactants that influence each other in promoting the development of chronic inflammation (Figure 1).
Another consideration relates to the fact that, as mentioned before, leptin is a sexually dimorphic hormone whose serum levels are higher in females than in males, in physiologic conditions [96]. As such, leptin-induced pro-inflammatory responses can be favored in females. In this regard, it is interesting to note that several epidemiological studies suggest higher prevalence of several autoimmune diseases in females.
Leptin and acute inflammation
In acute inflammation, acute infection and sepsis, the levels of leptin rapidly increase, favored particularly by LPS and cytokines such as TNF-α, IL-6, and IL-1β [85]. However, in some acute inflammatory conditions such as acute experimental endotoxemia and newborn sepsis, there is no increase of serum leptin [97–98].
Of note, infection associates with reduced leptinemia in tuberculosis and in pediatric patients with HIV [99–100]. After highly active anti-retroviral therapy (HAART), the amelioration of the clinical picture and the improvement of the CD4+ T cell count in HIV patients associate with increased levels of serum leptin [101].
A protective role of leptin in the clearance from pathogens is observed in leptin-deficient ob/ob mice, which develop severe disease and die of infection with Klebsiella more rapidly than wild-type mice [102–103]. The ob/ob mice are also highly susceptible to LPS-induced lethality, which can be reversed by the administration of leptin [104–105]. The protective effects of leptin in those cases seem to occur through a modulation of TNF-α and IL-6 responses after the endotoxin priming [106].
In humans, plasma leptin is elevated in septic patients, and a positive correlation between circulating leptin levels and survival after sepsis has been observed [107–108].
Leptin and chronic inflammation
Together with the established function of leptin as a pro-inflammatory cytokine that helps the host against infection, there is at the meantime increasing evidence of an association between leptin and increased risk of chronic inflammatory disease.
Serum leptin is elevated in many chronic inflammatory conditions including inflammatory bowel disease, inflammatory nephritis, pelvic endometriosis, nonalcoholic hepatitis, chronic pulmonary inflammation, Behcet’s disease, Graves’ disease [32–43]. A number of studies have implicated a role of leptin in the pathogenesis of several autoimmune diseases – which are classically typified by chronic inflammation - including type 1 diabetes, inflammatory bowel disease, and possibly rheumatoid arthritis [32–43].
In experimental animal models, the investigation of the effects of leptin on the susceptibility to autoimmunity has clearly indicated that leptin can promote autoreactivity. For example, leptin deficient ob/ob mice and leptin receptor-deficient db/db mice are resistant to the development of several experimentally-induced autoimmune diseases [25].
Following immunization with methylated bovine serum albumin into knee joints, ob/ob and db/db mice develop a milder form of antigen-induced arthritis than wild-type controls, and antigen-specific autoreactive T cell proliferative responses appear markedly decreased in the ob/ob mice concomitantly with a reduced production of pro-inflammatory cytokines [109]. In concanavalin A-induced hepatitis, leptin-deficient ob/ob mice also appear protected from hepatitis secondarily to a decreased production of pro-inflammatory Th1 cytokines and a shifting towards a Th2-type immune response [110]. In models of intestinal inflammation, CD4+CD45RBhi T cells from db/db mice show a reduced capacity to cause colitis than wild-type mice when transferred into severe combined immunodeficient (SCID) mice [111]. Ob/ob mice are also protected from immune-mediated renal disease and accelerated nephrotoxic nephritis [112] and are spontaneously resistant to the development of experimental autoimmune encephalomyelitis, but become susceptible to levels comparable with those of wild-type mice after treatment with recombinant leptin [113]. Finally, in another animal model of spontaneous autoimmunity, the non-obese diabetic female mouse (which develops type-1 diabetes), increased serum leptin levels precede the onset of diabetes, and leptin administration anticipates the development of the disease [114].
A confirmatory role of leptin in autoimmunity is shown by experiments in which blockade of leptin with anti-leptin antibodies or soluble receptor sort beneficial effects on the chronic inflammatory condition, i.e. on the progression of experimental allergic encephalomyelitis [115].
Finally, increased serum leptin levels associate with the development of chronic graft-versus-host disease in patients who receive hematopoietic stem cell transplantation [116].
Future perspectives
Leptin has recently emerged as a key link between metabolic responses and inflammation. It is thought that the elevated levels of leptin in obese individuals can contribute to the low-grade chronic inflammation, on which degenerative diseases and autoimmune reactivity could possibly develop. Conversely, starvation or malnutrition would reduce inflammatory response and thus associate with increased risk of infection. Because of this dual role in immune responses, leptin could represent a new immunotherapeutic target. A better understanding of the mechanisms that lead from obesity to inflammation could have significant implications for the design of new modalities of intervention to reduce the morbidity that associates with obesity. For example, consideration could be given to the possibility that leptin antagonists might negatively influence the development and progression of chronic inflammatory diseases including cardiovascular and metabolic diseases. In infection, when low leptin negatively affects the effector immune response that would lead to the clearance of pathogens, the use of recombinant leptin or leptin agonists could possibly favor more effective immune responsiveness.
Thus, at least theoretically, in inflammation, obesity and autoimmunity, the blockade of leptin with antagonists or antibodies or soluble receptor could inhibit the activity of bioavailable leptin and/or reduce its pro-inflammatory effects [117–118]. However, as the majority of the studies using leptin as a target in the treatment of diseases were based on animal models with experimental conditions, and leptin administration did not improve immune function in the normal or obese individuals but only in individuals with congenital leptin deficiency and lipodystrophy, further studies will be required to confirm the effectiveness of managing leptin levels in human autoimmune disease [119].
Alternatively, other targets could possibly be found in the signaling pathways of leptin. SOCS3 is an important factor of leptin resistance and negative feedback, and increasing interests have focused on elucidating the mechanisms of how SOCS3 could contributes to the development of obesity and diabetes. PTP1B dephosphorylates JAK2 on leptin receptor and is an effective target for the treatment of both type 2 diabetes and obesity [120]. Shp2 downregulates the ObRb-STAT3 pathway while promoting ERK activation, and has a critical role in leptin signaling [121]. Other molecules to possibly consider as leptin-related targets could be the ones with antagonizing effects on this molecule, for example ghrelin and NPY [59].
Conclusion
Molecules at the interface between metabolism and immunity such as leptin can either promote an optimal immune response in a balanced metabolic state or, on the other hand, reflect the inappropriate intake of nutrients and associate with abnormal immune responses (Figure 3). Until recently, anti-leptin therapy was targeted towards weight regulation. After the discovery of the immune effects of leptin - particularly in inflammation - the development of new therapeutic approaches has been initiated. Many aspects of the biology of leptin remain unclear, and several mechanistic explanations are needed. Yet, the influence of leptin on immunity in relation to the metabolic status can lead to the development of novel approaches that may specifically focus on nutrition for the modulation of the immune response.
Figure 3.
A healthy immune status associates with balanced serum leptin levels.
Acknowledgments
A. L. C. is supported by the National Institute of Health grants AR53239 and AI63515. G. M. is supported by the Juvenile Diabetes Research Foundation-Telethon Italy, and by the Fondazione Italiana Sclerosi Multipla.
Footnotes
Disclosure
The authors declare no conflict of interests.
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Sinha MK, Opentanova I, Ohannesian JP, et al. Evidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short-term fasting. J Clin Invest. 1996;98:1277–82. doi: 10.1172/JCI118913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 4.Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–4. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 5.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 6.Blum WF, Englaro P, Hanitsch S, et al. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J Clin Endocrinol Metab. 1997;82:2904–10. doi: 10.1210/jcem.82.9.4251. [DOI] [PubMed] [Google Scholar]
- 7.Jin L, Burguera BG, Couce ME, et al. Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence of a regulatory role for leptin on pituitary cell proliferation. J Clin Endocrinol Metab. 1999;84:2903–11. doi: 10.1210/jcem.84.8.5908. [DOI] [PubMed] [Google Scholar]
- 8.Bado A, Levasseur S, Attoub S, et al. The stomach is a source of leptin. Nature. 1998;394:790–3. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393:684–8. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet M, Delavaud C, Laud K, et al. Mammary leptin synthesis, milk leptin and their putative physiological roles. Reprod Nutr Dev. 2002;42:399–413. doi: 10.1051/rnd:2002034. [DOI] [PubMed] [Google Scholar]
- 11.Dumond H, Presle N, Terlain B, et al. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003;48:3118–29. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- 12.Senaris R, Garcia-Caballero T, Casabiell X, et al. Synthesis of leptin in human placenta. Endocrinology. 1997;138:4501–4. doi: 10.1210/endo.138.10.5573. [DOI] [PubMed] [Google Scholar]
- 13.Morroni M, De Matteis R, Palumbo C, et al. In vivo leptin expression in cartilage and bone cells of growing rats and adult humans. J Anat. 2004;205:291–6. doi: 10.1111/j.0021-8782.2004.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanna V, Di Giacomo A, La Cava A, et al. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest. 2003;111:241–50. doi: 10.1172/JCI16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Rosa V, Procaccini C, Calì G, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–55. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–9. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 17.Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–6. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 18.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 19.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–7. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 20.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 21.Emilsson V, Liu YL, Cawthorne MA, Morton NM, Davenport M. Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes. 1997;46:313–6. doi: 10.2337/diab.46.2.313. [DOI] [PubMed] [Google Scholar]
- 22.Sierra-Honigmann MR, Nath AK, Murakami C, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–6. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 23.Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 24.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–20. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 25.La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–9. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 26.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 27.Howard JK, Lord GM, Matarese G, et al. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–9. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 29.MacDougald OA, Hwang CS, Fan H, Lane MD. Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 1995;92:9034–7. doi: 10.1073/pnas.92.20.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saladin R, De Vos P, Guerre-Millo M, et al. Transient increase in obese gene expression after food intake or insulin administration. Nature. 1995;377:527–9. doi: 10.1038/377527a0. [DOI] [PubMed] [Google Scholar]
- 31.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 32.Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18:213–5. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- 33.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumann H, Morella KK, White DW, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA. 1996;93:8374–8. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chua SC, Jr, White DW, Wu-Peng XS, et al. Phenotype of fatty due to Gln269Pro mutation in the leptin receptor (Lepr) Diabetes. 1996;45:1141–3. doi: 10.2337/diab.45.8.1141. [DOI] [PubMed] [Google Scholar]
- 36.Clement K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 37.Farooqi IS, Wangensteen T, Collins S, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356:237–47. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fei H, Okano HJ, Li C, et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA. 1997;94:7001–5. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chua SC, Jr, Koutras IK, Han L, et al. Fine structure of the murine leptin receptor gene: splice site suppression is required to form two alternatively spliced transcripts. Genomics. 1997;45:264–70. doi: 10.1006/geno.1997.4962. [DOI] [PubMed] [Google Scholar]
- 40.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–8. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- 41.Lam QL, Liu S, Cao X, Lu L. Involvement of leptin signaling in the survival and maturation of bone marrow-derived dendritic cells. Eur J Immunol. 2006;36:3118–30. doi: 10.1002/eji.200636602. [DOI] [PubMed] [Google Scholar]
- 42.Tian Z, Sun R, Wei H, Gao B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun. 2002;298:297–302. doi: 10.1016/s0006-291x(02)02462-2. [DOI] [PubMed] [Google Scholar]
- 43.Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745–52. doi: 10.4049/jimmunol.176.12.7745. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez-Margalet V, Martin-Romero C, Gonzalez-Yanes C, Goberna R, Rodriguez-Bano J, Muniain MA. Leptin receptor (Ob-R) expression is induced in peripheral blood mononuclear cells by in vitro activation and in vivo in HIV-infected patients. Clin Exp Immunol. 2002;129:119–24. doi: 10.1046/j.1365-2249.2002.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui H, Cai F, Belsham DD. Leptin signaling in neurotensin neurons involves STAT, MAP kinases ERK1/2, and p38 through c-Fos and ATF1. FASEB J. 2006;20:2654–6. doi: 10.1096/fj.06-5989fje. [DOI] [PubMed] [Google Scholar]
- 46.Hübschle T, Thom E, Watson A, Roth J, Klaus S, Meyerhof W. Leptin-induced nuclear translocation of STAT3 immunoreactivity in hypothalamic nuclei involved in body weight regulation. J Neurosci. 2001;21:2413–24. doi: 10.1523/JNEUROSCI.21-07-02413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mansour E, Pereira FG, Araújo EP, et al. Leptin inhibits apoptosis in thymus through a janus kinase-2-independent, insulin receptor substrate-1/phosphatidylinositol-3 kinase-dependent pathway. Endocrinology. 2006;147:5470–9. doi: 10.1210/en.2006-0223. [DOI] [PubMed] [Google Scholar]
- 48.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–25. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 49.Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–65. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 50.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, et al. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2:385–7. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 51.Kaszubska W, Falls HD, Schaefer VG, et al. Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol Cell Endocrinol. 2002;195:109–18. doi: 10.1016/s0303-7207(02)00178-8. [DOI] [PubMed] [Google Scholar]
- 52.Martin-Romero C, Sanchez-Margalet V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: possible role of Sam68. Cell Immunol. 2001;212:83–91. doi: 10.1006/cimm.2001.1851. [DOI] [PubMed] [Google Scholar]
- 53.Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 54.Sanchez-Margalet V, Martin-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clin Exp Immunol. 2003;133:11–9. doi: 10.1046/j.1365-2249.2003.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 56.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–72. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 57.Bjørbaek C, Buchholz RM, Davis SM, et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–55. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 58.Gualillo O, Eiras S, White DW, Diéguez C, Casanueva FF. Leptin promotes the tyrosine phosphorylation of SHC proteins and SHC association with GRB2. Mol Cell Endocrinol. 2002;190:83–9. doi: 10.1016/s0303-7207(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 59.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 60.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen J, Sakaida I, Uchida K, Terai S, Okita K. Leptin enhances TNF-α production via p38 and JNK MAPK in LPS-stimulated Kupffer cells. Life Sci. 2005;77:1502–15. doi: 10.1016/j.lfs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Faggioni R, Jones-Carson J, Reed DA, et al. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor α and IL-18. Proc Natl Acad Sci USA. 2000;97:2367–72. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Landman RE, Puder JJ, Xiao E, Freda PU, Ferin M, Warldaw SL. Endotoxin stimulates leptin in the human and nonhuman primate. J Clin Endocrinol Metab. 2003;88:1285–91. doi: 10.1210/jc.2002-021393. [DOI] [PubMed] [Google Scholar]
- 64.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1 beta mediates leptin induction during inflammation. Am J Physiol. 1998;274:R204–8. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- 65.Otero M, Lago R, Lago F, et al. Leptin, from fat to inflammation: old questions and new insights. FEBS Lett. 2005;579:295–301. doi: 10.1016/j.febslet.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 67.Lam QL, Zheng BJ, Jin DY, Cao X, Lu L. Leptin induces CD40 expression via the activation of Akt in murine dendritic cells. J Biol Chem. 2007;282:27587–97. doi: 10.1074/jbc.M704579200. [DOI] [PubMed] [Google Scholar]
- 68.Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 69.Gabay C, Dreyer M, Pellegrinelli N, Chicheportiche R, Meier CA. Leptin directly induces the secretion of interleukin 1 receptor antagonist in human monocytes. J Clin Endocrinol Metab. 2001;86:783–91. doi: 10.1210/jcem.86.2.7245. [DOI] [PubMed] [Google Scholar]
- 69.Shen J, Sakaida I, Uchida K, Terai S, Okita K. Leptin enhances TNF-α production via p38 and JNK MAPK in LPS-stimuated Kupffer cells. Life Sci. 2005;77:1502–15. doi: 10.1016/j.lfs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 70.Zarkesh-Esfahani H, Pockley G, Metcalfe RA, et al. High-dose leptin activates human leukocytes via receptor expression on monocytes. J Immunol. 2001;167:4593–9. doi: 10.4049/jimmunol.167.8.4593. [DOI] [PubMed] [Google Scholar]
- 71.Mancuso P, Canetti C, Gottschalk A, Tithof PK, Peters-Golden M. Leptin augments alveolar macrophage leukotriene synthesis by increasing phospholipase activity and enhancing group IVC iPLA2 (cPLA2γ) protein expression. Am J Physiol Lung Cell Mol Physiol. 2004;287:L497–502. doi: 10.1152/ajplung.00010.2004. [DOI] [PubMed] [Google Scholar]
- 72.Tang CH, Lu DY, Yang RS, et al. Leptin-induced IL-6 production is mediated by leptin receptor, insulin receptor substrate-1, phosphatidylinositol 3-kinase, Akt, NF-κB, and p300 pathway in microglia. J Immunol. 2007;179:1292–302. doi: 10.4049/jimmunol.179.2.1292. [DOI] [PubMed] [Google Scholar]
- 73.Pinteaux E, Inoue W, Schmidt L, Molina-Holgado F, Rothwell NJ, Luheshi GN. Leptin induces interleukin-1β release from rat microglial cells through a caspase 1 independent mechanism. J Neurochem. 2007;102:826–33. doi: 10.1111/j.1471-4159.2007.04559.x. [DOI] [PubMed] [Google Scholar]
- 74.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–9. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 75.Bruno A, Conus S, Schmid I, Simon HU. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J Immunol. 2005;174:8090–6. doi: 10.4049/jimmunol.174.12.8090. [DOI] [PubMed] [Google Scholar]
- 76.Zhao Y, Sun R, You L, Gao C, Tian Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem Biophys Res Commun. 2003;300:247–52. doi: 10.1016/s0006-291x(02)02838-3. [DOI] [PubMed] [Google Scholar]
- 77.Oral EA, Javor ED, Ding L, et al. Leptin replacement therapy modulates circulating lymphocyte subsets and cytokine responsiveness in severe lipodystrophy. J Clin Endocrinol Metab. 2006;91:621–8. doi: 10.1210/jc.2005-1220. [DOI] [PubMed] [Google Scholar]
- 78.Fujita Y, Murakami M, Ogawa Y, et al. Leptin inhibits stress-induced apoptosis of T lymphocytes. Clin Exp Immunol. 2002;128:21–6. doi: 10.1046/j.1365-2249.2002.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oda E. Metabolic syndrome and CRP. Circ J. 2007;71:620–1. doi: 10.1253/circj.71.620. [DOI] [PubMed] [Google Scholar]
- 80.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 81.Mannino DM, Mott J, Ferdinands JM, Camargo CA, Friedman M. Boys with high body masses have an increased risk of developing asthma: findings from the National Longitudinal Survey of Youth (NLSY) Int J Obes. 2006;3:6–13. doi: 10.1038/sj.ijo.0803145. [DOI] [PubMed] [Google Scholar]
- 82.Singh P, Hoffmann M, Wolk R, Shamsuzzaman AS, Somers VK. Leptin induces C-reactive protein expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27(9):e302–7. doi: 10.1161/ATVBAHA.107.148353. [DOI] [PubMed] [Google Scholar]
- 83.Taleb S, Herbin O, Ait-Oufella H, et al. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2691–8. doi: 10.1161/ATVBAHA.107.149567. [DOI] [PubMed] [Google Scholar]
- 84.Mancuso P, Huffnagle GB, Olszewski MA, Phipps J, Peters-Golden M. Leptin corrects host defense defects after acute starvation in murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2006;173:212–8. doi: 10.1164/rccm.200506-909OC. [DOI] [PubMed] [Google Scholar]
- 85.Sarraf P, Frederich RC, Turner EM, et al. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–5. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gualillo O, Eiras S, Lago F, Dieguez C, Casanueva FF. Elevated serum leptin concentrations induced by experimental acute inflammation. Life Sci. 2000;67:2433–41. doi: 10.1016/s0024-3205(00)00827-4. [DOI] [PubMed] [Google Scholar]
- 87.Boden G, Chen X, Kolaczynski JW, Polansky M. Effects of prolonged hyperinsulinemia on serum leptin in normal human subjects. J Clin Invest. 1997;100:1107–13. doi: 10.1172/JCI119621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zakrzewska KE, Cusin I, Sainsbury A, Rohner-Jeanrenaud F, Jeanrenaud B. Glucocorticoids as counterregulatory hormones of leptin: toward an understanding of leptin resistance. Diabetes. 1997;46:717–9. doi: 10.2337/diab.46.4.717. [DOI] [PubMed] [Google Scholar]
- 89.Chan JL, Bullen J, Stoyneva V, Depaoli AM, Addy C, Mantzoros CS. Recombinant methionyl human leptin administration to achieve high physiologic or pharmacologic leptin levels does not alter circulating inflammatory marker levels in humans with leptin sufficiency or excess. J Clin Endocrinol Metab. 2005;90:1618–24. doi: 10.1210/jc.2004-1921. [DOI] [PubMed] [Google Scholar]
- 90.Shamsuzzaman AS, Winnicki M, Wolk R, et al. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation. 2004;109:2181–5. doi: 10.1161/01.CIR.0000127960.28627.75. [DOI] [PubMed] [Google Scholar]
- 91.van Dielen FM, van’t Veer C, Schols AM, Soeters PB, Buurman WA, Greve JW. Increased leptin concentrations correlate with increased concentrations of inflammatory markers in morbidly obese individuals. Int J Obes Relat Metab Disord. 2001;25:1759–66. doi: 10.1038/sj.ijo.0801825. [DOI] [PubMed] [Google Scholar]
- 92.Chen K, Li F, Li J, et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med. 2006;12:425–32. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- 93.Gomez-Ambrosi J, Salvador J, Silva C, et al. Leptin therapy does not affect inflammatory markers. J Clin Endocrinol Metab. 2005;90:3803. doi: 10.1210/jc.2005-0558. [DOI] [PubMed] [Google Scholar]
- 94.Lee SW, Park MC, Park YB, Lee SK. Measurement of the serum leptin level could assist disease activity monitoring in rheumatoid arthritis. Rheumatol Int. 2007;27:537–40. doi: 10.1007/s00296-006-0253-x. [DOI] [PubMed] [Google Scholar]
- 95.Targonska-Stepniak B, Majdan M, Dryglewska M. Leptin serum levels in rheumatoid arthritis patients: relation to disease duration and activity. Rheumatol Int. 2007 doi: 10.1007/s00296-007-0480-9. In press. [DOI] [PubMed] [Google Scholar]
- 96.Chow VT, Phoon MC. Measurement of serum leptin concentrations in university undergraduates by competitive ELISA reveals correlations with body mass index and sex. Adv Physiol Educ. 2003;27:70–7. doi: 10.1152/advan.00001.2003. [DOI] [PubMed] [Google Scholar]
- 97.Bornstein SR, Preas HL, Chrousos GP, Suffredini AF. Circulating leptin levels during acute experimental endotoxemia and antiinflammatory therapy in humans. J Infect Dis. 1998;178:887–90. doi: 10.1086/515349. [DOI] [PubMed] [Google Scholar]
- 98.Koç E, Ustündağ G, Aliefendioğlu D, Ergenekon E, Bideci A, Atalay Y. Serum leptin levels and their relationship to tumor necrosis factor-alpha and interleukin-6 in neonatal sepsis. J Pediatr Endocrinol Metab. 2003;16:1283–7. doi: 10.1515/jpem.2003.16.9.1283. [DOI] [PubMed] [Google Scholar]
- 99.Yarasheski KE, Zachwieja JJ, Horgan MM, Powderly WG, Santiago JV, Landt M. Serum leptin concentrations in human immunodeficiency virus-infected men with low adiposity. Metabolism. 1997;46:303–5. doi: 10.1016/s0026-0495(97)90258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Crevel R, Karyadi E, Netea MG, et al. Decreased plasma leptin concentrations in tuberculosis patients are associated with wasting and inflammation. J Clin Endocrinol Metab. 2002;87:758–63. doi: 10.1210/jcem.87.2.8228. [DOI] [PubMed] [Google Scholar]
- 101.Pinzone Fox ML, Sastry MK, Parenti DM, Simon GL. Plasma leptin concentration increases early during highly active antiretroviral therapy for acquired immunodeficiency syndrome, independent of body weight. J Endocrinol Invest. 2005;28:RC1–3. doi: 10.1007/BF03345372. [DOI] [PubMed] [Google Scholar]
- 102.Hsu A, Aronoff DM, Phipps J, Goel D, Mancuso P. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin Exp Immunol. 2007 doi: 10.1111/j.1365-2249.2007.03491.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wieland CW, Stegenga ME, Florquin S, Fantuzzi G, van der Poll T. Leptin and host defense against Gram-positive and Gram-negative pneumonia in mice. Shock. 2006;25:414–9. doi: 10.1097/01.shk.0000209524.12873.da. [DOI] [PubMed] [Google Scholar]
- 104.Faggioni R, Fantuzzi G, Gabay C, et al. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol. 1999;276:R136–42. doi: 10.1152/ajpregu.1999.276.1.R136. [DOI] [PubMed] [Google Scholar]
- 105.Faggioni R, Moser A, Feingold KR, Grunfeld C. Reduced leptin levels in starvation increase susceptibility to endotoxic shock. Am J Pathol. 2000;156:1781–7. doi: 10.1016/S0002-9440(10)65049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiao E, Xia-Zhang L, Vulliemoz NR, Ferin M, Wardlaw SL. Leptin modulates inflammatory cytokine and neuroendocrine responses to endotoxin in the primate. Endocrinology. 2003;144:4350–3. doi: 10.1210/en.2003-0532. [DOI] [PubMed] [Google Scholar]
- 107.Arnalich F, Lopez J, Codoceo R, Jimenez M, Madero R, Montiel C. Relationship of plasma leptin to plasma cytokines and human survival in sepsis and septic shock. J Infect Dis. 1999;180:908–11. doi: 10.1086/314963. [DOI] [PubMed] [Google Scholar]
- 108.Bornstein SR, Licinio J, Tauchnitz R, et al. Plasma leptin levels are increased in survivors of acute sepsis: associated loss of diurnal rhythm, in cortisol and leptin secretion. J Clin Endocrinol Metab. 1998;83:280–3. doi: 10.1210/jcem.83.1.4610. [DOI] [PubMed] [Google Scholar]
- 109.Busso N, So A, Chobaz-Peclat V, et al. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol. 2002;168:875–82. doi: 10.4049/jimmunol.168.2.875. [DOI] [PubMed] [Google Scholar]
- 110.Siegmund B, Lear-Kaul KC, Faggioni R, Fantuzzi G. Leptin deficiency, not obesity, protects mice from Con A-induced hepatitis. Eur J Immunol. 2002;32:552–60. doi: 10.1002/1521-4141(200202)32:2<552::AID-IMMU552>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 111.Siegmund B, Sennello JA, Jones-Carson J, Gamboni-Roberson F. Leptin receptor expression on T lymphocytes modulates chronic intestinal inflammation in mice. Gut. 2004;53:921–2. doi: 10.1136/gut.2003.027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tarzi RM, Cook HT, Jackson I, Pusey CD, Lord GM. Leptin-deficient mice are protected from accelerated nephrotoxic nephritis. Am J Pathol. 2004;164:385–90. doi: 10.1016/S0002-9440(10)63128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Matarese G, Di Giacomo A, Sanna V, et al. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol. 2001;166:5909–16. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
- 114.Matarese G, Sanna V, Lechler RI, et al. Leptin accelerates autoimmune diabetes in female NOD mice. Diabetes. 2002;36:1356–61. doi: 10.2337/diabetes.51.5.1356. [DOI] [PubMed] [Google Scholar]
- 115.De Rosa V, Procaccini C, La Cava A, et al. Leptin neutralization interferes with pathogenic T cell autoreactivity in autoimmune encephalomyelitis. J Clin Invest. 2006;116:447–55. doi: 10.1172/JCI26523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tauchmanovà L, Matarese G, Carella C, et al. High serum leptin in patients with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Transplantation. 2004;78:1376–83. doi: 10.1097/01.tp.0000140485.20848.b7. [DOI] [PubMed] [Google Scholar]
- 117.Otero M, Lago R, Gomez R, et al. Towards a pro-inflammatory and immunomodulatory emerging role of leptin. Rheumatology (Oxford) 2006;45:944–50. doi: 10.1093/rheumatology/kel157. [DOI] [PubMed] [Google Scholar]
- 118.Peelman F, Van Beneden K, Zabeau L, et al. Mapping of the leptin binding sites and design of a leptin antagonist. J Biol Chem. 2004;279:41038–46. doi: 10.1074/jbc.M404962200. [DOI] [PubMed] [Google Scholar]
- 119.Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol. 2007;4:1–13. [PubMed] [Google Scholar]
- 120.Zhang S, Zhang ZY. PTP1B as a drug target: recent developments in PTP1B inhibitor discovery. Drug Discov Today. 2007;12:373–81. doi: 10.1016/j.drudis.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 121.Feng GS. Shp2 as a therapeutic target for leptin resistance and obesity. Expert Opin Ther Targets. 2006;10:135–42. doi: 10.1517/14728222.10.1.135. [DOI] [PubMed] [Google Scholar]