Abstract
Dependency of taste buds and taste papillae on innervation has been debated for a long time. Previous research showed neurotrophins, Brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3), play an important role for the establishment of the lingual gustatory and somatosensory innervation. BDNF null mutant mice showed severe deficits in gustatory innervation and loss of taste buds while NT-3 null mutation reduced lingual somatosensory innervation to tongue papillae. These results proved BDNF or NT-3 null mutations affected different sensory modalities (i.e. gustatory and somatosensory, respectively). In this study, we analyzed taste bud development in BDNFxNT-3 double knockout mice to examine the relationship between taste bud development and gustatory/somatosentory innervation. Our results demonstrate that, at the initial stage, before nerve fibers reached the appropriate areas in the papilla, taste bud formation did not require innervation. However, at the synaptogenic stage, after nerve fibers ramified into the apical epithelium, innervation was required and played an essential role in the development of taste buds/papillae.
Keywords: gustation, neurotrophin, development, taste buds, BDNF, NT-3
INTRODUCTION
Taste buds are chemosensory organs that transduce chemical stimulation to neuronal signal. The connections between taste buds and their innervating afferents are essential for the function. Their relationship has been debated for a long time [1, 13, 17]. Due to lack of appropriate experimental models, functional and morphological relationships between taste bud development and gustatory/somatosensory innervation at embryonic stages have not been evaluated.
Appropriate expression of neurotrophins BDNF and NT-3 are required for proper development of taste buds and proper innervation [11, 20]. BDNF null-mutated mice have severe gustatory deficits while NT-3 knockout mice exhibit loss of lingual somatosensory innervation [11, 19]. Therefore, BDNFxNT-3 double knockout (DK) mice should be an ideal animal model to examine the relationship between developing taste buds and innervation. We showed that BDNFxNT-3 DK mice exhibited severe deficits in their gustatory system at birth compared to BDNF single knockout mice [12]. Here, we extended our previous studies and investigated the relationship between the development of taste buds and gustatory/somatosensory innervation at multiple embryonic and neonatal stages.
MATERIALS AND METHODS
BDNF−/−xNT-3−/− mice were generated by crossbreeding BDNF+/−xNT-3+/− mice. NT-3 mice were kindly provided by Dr. Kevin Jones (University of Colorado). Polymerase chain reaction (PCR) was used for genotyping [2, 5]. Midday on the day of an observed sperm plug was noted as embryonic day (E) 0.5 and the birth day as postnatal day (P) 0. Mice were collected on E13.5, E15.5, E17.5, and P0. Procedures were approved by the Institutional Animal Care and Use Committee at the University of Tennessee Health Science Center. Tissue preparation, procedures for immunohistochemistry, tongue papillae size and number measurements have been previously described in detail [6]. At least three wild-type and three transgenic mice were used for each stage/comparison and to perform statistical analysis. Statistical analysis used one-way ANOVA and Bonferroni post-hoc test utilizing GraphPad Instat software (GraphPad Software, Inc., San Diego, CA). For scanning electron microscopy (SEM), E16.5 tongues were rinsed in PBS and dehydrated, sputter-coated and examined with a scanning electron microscope.
RESULTS
Tongue surface morphology
To examine whether neurotrophin manipulation would lead to morphological changes, we performed scanning electron microscopy on E16.5 tongues of wild-type and neurotrophin double knockout mice. By E16.5 in wild-type mice, afferent nerve fibers have clearly penetrated and have established ramifications in the lingual epithelium. While a large number of developing fungiform papillae covered the entire portion of the anterior tongue in wild-type mice (Figure 1 A), BDNF−/−xNT-3−/− mice had only a few (Figure 1 B). Fungiform papillae in wild-type were clearly elevated above the surrounding lingual epithelium and appeared well-developed for this stage (Figure 1 A1). In DK mice, papillae were smaller and misshaped compared to those in the wild-type mice (Figure 1 B2). The circumvallate papillae in wild-type mice were large and well-developed (Figure 1 C) but were smaller and showed a distorted morphology in BDNF−/−xNT-3−/− mice (Figure 1 D).
Figure 1.
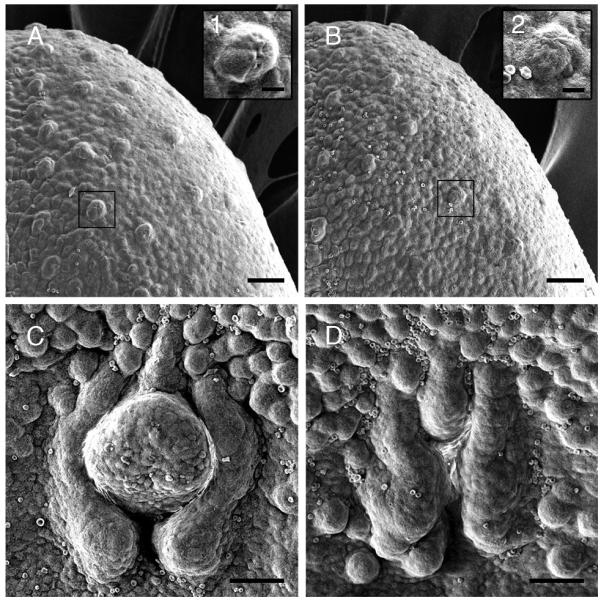
Scanning electron microscopy (SEM) photomicrographs of wild-type and BDNF−/−xNT-3−/− mouse tongue at E16.5. Scale bars represent 50 μm in (A-D) and 10 μm in insets. Insets are higher magnification of boxed areas in A and B. (A) In wild-type mice, many well-developed fungiform papillae were observed on the anterior part of the tongue. (B) BDNF−/−xNT-3−/− mice exhibited fewer and less developed fungiform papillae that in wild-type mice. (C) Circumvallate papilla in wild-type mice at E16.5. The trench system was clearly visible and the centerpiece of the papilla had a dome-shape. (D) The circumvallate papilla in BDNF−/−xNT-3−/− mice at E16.5 was smaller and significantly underdeveloped compared to that in wild-type mice. The midportion of the papilla was malformed and the trench system had an unclear boundary.
Fungiform papillae
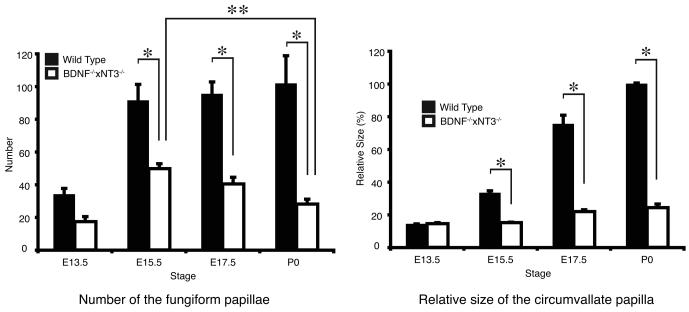
At E13.5, clusters of Troma-1 positive cells formed distinct thickenings in the tongue epithelium. The central portion of these epithelial thickenings had more intensely labeled cells that might represent taste cell progenitors. Size and morphology of the presumptive Troma-1 positive taste bud progenitors in both mice were similar at this stage (Figure 2 A1, B1). Growth Associated Protein 43 (GAP43) positive fibers were located in the subepithelial nerve plexus subjacent to most Troma-1 positive clusters in the wild-type mice, but very few nerve fibers were observed in BDNF−/−xNT-3−/− mice (Figure 2 A2-A3, B2-B3). To examine the identity of the Troma-1 positive epithelial thickenings, we used antibodies to Sonic Hedgehog (Shh) and SRY-box containing gene 2 (Sox2) as markers for taste cell progenitors. Shh and Sox2 labeling were observed in the central portion of the epithelial thickenings in both wild-type and BDNF−/−xNT-3−/− mice (Figure 3 A-F). The number of developing papillae in wild-type mice (34.0 ±3.6) did not show significant difference to BDNF−/−xNT-3−/− mice (17.3 ±3.1) (P>0.05) (Figure 4 A). At E15.5, many GAP43-positive nerve fibers were observed in the intragemmal areas of the developing fungiform papillae in wild-type mice (Figure 2 C2-C3), but not in BDNF−/−xNT-3−/− mice (Figure 2 D2-D3). Troma-1 labeling appeared similar in both mice, and was confined to the central portions of the developing fungiform papillae (Figure 2, C1, D1). The number of fungiform papillae was larger in wild-type mice (91.3±9.9) than in BDNF−/−xNT-3−/− mice (49.7±3.1). However, the number increased from E13.5 to E15.5 in both wild-type (P<0.001) and BDNF−/−xNT-3−/− mice (P<0.01) (Figure 4 A). At E17.5, there were 95.3±7.4 fungiform papillae in wild-type and 40.3±4.0 in DK mice (P<0.001) (Figure 4 A). At P0 in wild-type mice, the papillae were heavily innervated (Figure 2 E2-E3). Troma-1 positive taste buds were well-developed and included many spindle-shaped Troma-1 positive cells (Figure 2, E1). In BDNF−/−xNT-3−/− fungiform papillae, almost no GAP43 positive fibers were observed (Figure 2, F2-F3). Troma-1 positive taste buds and papillae proper were smaller in DK mice compared to those in wild-type mice (Figure 2 F1). The number of fungiform papillae in wild-type mice at P0 was 101.7±17.0, similar to the numbers at previous stages. The number in DK mice at P0 (28.0±3.1) was slightly reduced compared to E17.5 (P>0.05), and significantly reduced compared to E15.5 (P<0.01) (Figure 4 A).
Figure 2.
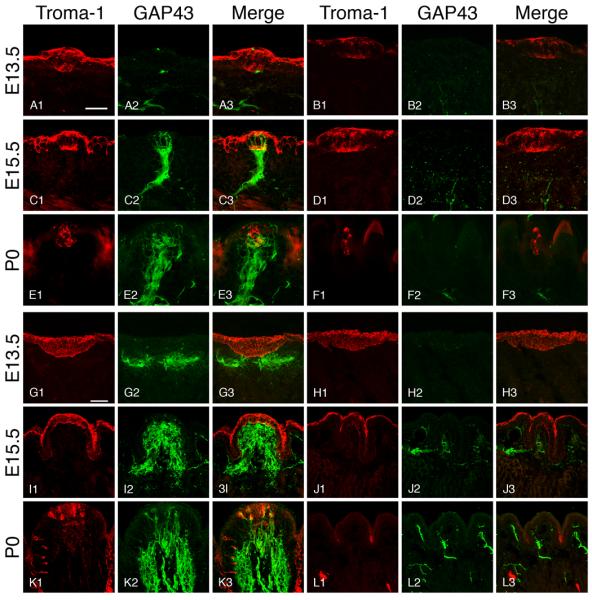
Photomicrographs of fungiform (A-F) and circumvallate papillae (G-L) in wild-type mice (A, C, E, G, I, K) and BDNF−/−xNT-3−/− mice (B, D, F, H, J, L). Scale bar (A, G) is 40 μm. Scale bar in (A) applies to pictures (A-F) and in (G) to (G-L). Troma-1 positive cells formed clusters in the lingual epithelium (presumably taste bud progenitors) at E13.5 (A1, B1). While nerve fibers were observed in close proximity to taste bud progenitors in wild-type mice (A2), only a few were seen in double knockout mice (B2). Nerve fibers did not penetrate the epithelium (A3, B3). At E15.5, stronger Troma-1 labeling was observed (C1, D1). Many GAP43 positive nerve fibers entered the developing taste buds in wild-type mice (C2, C3), but not in double knockout mice (D2, D3). At P0, well-developed taste buds were observed in wild-type mice. Some taste buds contained spindle-shaped cells that were Troma-1 positive (E1). The gustatory and somatosensory nerve fibers had clearly ramified into the intra- and perigemmal components (E2, E3). In BDNF-/-xNT-3 mice, taste buds/papillae atrophied (F1). No nerve fibers were innervating fungiform taste buds/papillae (F2, F3). At E13.5, the developing vallate papilla consisted of the placodal lingual epithelial thickness that appeared to be diffusely Troma-1 positive in both wild-type and BDNF−/−xNT-3−/− mice (G1, H1). There was a subepithelial GAP43 positive nerve plexus in wild-type papillae (G2, G3) but not in BDNF−/−xNT-3−/− papillae (H2, H3). At E15.5, circumvallate trench system was clearly visible in wild-type mice (I1). The core part of the papilla contained a GAP43 positive nerve plexuses (I2, I3). The circumvallate papillae had a distorted appearance in BDNF−/−xNT-3−/− mice (J1). Only a few subepithelial nerve fibers were observed (J2, J3). At P0, the circumvallate papilla of wild-type mice had a well-developed shape and contained a larger number of Troma-1-positive taste buds (K1). A large number of nerve fibers extended into lingual epithelium from the subepithelial nerve plexus (K2, K3). Circumvallate papilla in BDNF−/−xNT-3−/− mice had a distorted morphology. There were no Troma-1 positive taste buds (L1). Innervation was clearly reduced compared to wild-type mice (L2, L3).
Figure 3.
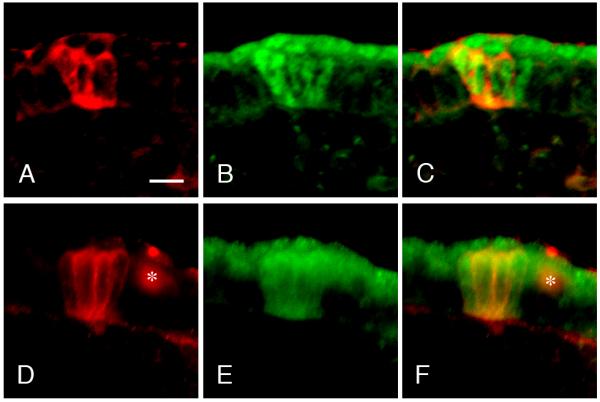
Photomicrographs represent developing fungiform papillae at E13.5 (A-F) in wild-type (A-C) and BDNF−/−xNT-3−/− mice (D-F). The papillae were stained with antibodies to Shh (red) (A, D) and Sox2 (green) (B, E). C and F are merged images. Asterisks in D and F indicate a nonspecific artifact. Scale bar reoresebts 10 μm. Fungiform taste bud progenitors were positive to both Shh and Sox2 antibody in wild- type and BDNF−/−xNT-3−/− mice at E13.5. Merged images showed that the positive cells corresponded well to each other in both mice. Taste bud progenitors appeared to have similar morphology in both wild-type and knockout mice at E13.5.
Figure 4.
(A) In wild-type mice, the number of fungiform papillae increased from E13.5 to E15.5. The number was almost equal from E15.5 to P0. In BDNF−/−xNT-3−/− mice, the number increased from E13.5 to E15.5 even though that number was always less than in wild-type mice. However, the number decreased from E15.5 to E17.5, and the reduction was significant between E15.5 and P0. (B) Relative size of the circumvallate papilla in wild-type and BDNF−/−xNT-4−/− mice from E13.5 to P0. At E13.5, there is no difference. But from E15.5 to P0, the size in wild-type mice was significantly larger than in BDNF−/−xNT-3−/− mice. (A, B) Error bars represent standard deviation. Asterisks (*, **) represent significance levels (P<0.01).
Circumvallate papillae
At E13.5, a Troma-1 positive epithelial thickening was observed in the posterior portion of the tongue in both wild-type and BDNF−/−xNT-3−/− mice (Figure 2 G1, H1). The subepithelial GAP43 positive nerve plexus was subjacent to the developing vallate papilla in wild-type but not in BDNF−/−xNT-3−/− mice (Figure 2 G2-G3, H2-H3). The size of the vallate papilla in BDNF−/−xNT-3−/− mice (14.5%±0.6%) was similar to that in wild-type mice (14.3%±0.9%) (Figure 4 B). At E15.5, developing wild-type circumvallate papillae were well-innervated (Figure 2 I2-I3). However, in BDNF−/−xNT-3−/− mice, the papillae appeared distorted, and the trenches were misshaped (Figure 2 J1) and only a few subepithelial nerve fibers were found in the core part of the papillae (Figure 2 J2-J3). A non-specific Troma-1 positive layer was found on the surface of lingual epithelium in both mice (Figure 2 I1, J1). The size of developing papillae was 33.3%±1.3% in wild-type mice and 15.1%±0.3% in BDNF−/−xNT-3−/− mice (P<0.001) (Figure 4 B). The core size of the developing papilla at E17.5 in knockout mice (21.9%±1.2%) was significantly smaller than that in wild-type mice (75.4%±5.4%) (P<0.001) (Figure 4 B). At P0, many Troma-1 positive taste buds were observed in wild-type mice (Figure 2 K1). Nonspecific Troma-1 positive layer had fully disappeared at this stage in both animals (Figure 2 K1, L1). There was a large subepithelial nerve plexus in the core part of the papillae and a large number of intraepithelial nerve fibers (Figure 2 K2-K3). BDNF−/−xNT-3−/− mice exhibited distorted morphology, there were no Troma-1 positive taste buds, and innervation deficits were significant (Figure 2 L1-L3). The size of the papillae in BDNF−/−xNT-3−/− mice (24.3%±2.2%) was markedly smaller than that in wild-type mice (100.0%± 0.5%) (Figure 4 B).
DISCUSSION
Almost four decades ago and using an in vitro approach, Farbman showed that tongue papillae formation is nerve independent but maintenance requires innervation [3]. However, due to lack of appropriate in vivo experimental models, embryonic taste bud formation and development in the absence of innervation have not been previously studied. To examine nerve dependency of taste bud development in mammals, we utilized BDNFxNT-3 DK mice in the present study. We showed that despite severe loss of both guatatory and somatosensory innervations in BDNFxNT-3 DK mice, early taste bud progenitor markers (Shh, Sox2, and Troma-1) were expressed in the right areas during the appropriate timing for taste bud formation in both wild-type and transgenic mice. However, these taste cell marker positive structures failed to develop and to maintain a mature wild-type taste bud appearance in BDNF−/−xNT-3−/− mice. This suggests a two-stage formation process; an initial nerve independent followed by a nerve dependent stage. It is important to emphasize that despite distinct losses in single neurotrophin knockout mice, due to close proximity and functional interactions between gustatory and somatosensory nerves, both components must be severely affected to justify a transgenic mouse line to function as a model for embryonic taste bud development in the absence of innervation. We propose that this is only achieved in BDNFxNT-3 DK mice. Most importantly, the innervational loss in BDNF−/−xNT-3−/− mice was not absolute. There were still remaining neurons (app. 1/4th to 1/3rd of the total neurons) in the relevant cranial ganglia [9, 11, 12], and few nerve fibers were still found in some instances in the areas of the developing gustatory papillae. However, the neuronal and innervational losses were so severe that it supported the use of BDNF−/−xNT-3−/− mice as a model for the early loss of gustatory and somatosensory innervation. Despite only moderate deficits in single BDNF knockout mice, these mice were promoted to function as a taste bud development model in the absence of innervation by other investigators [16].
BDNF−/−xNT-3−/− mice had the greatest loss of fungiform papillae at P0. Interestingly, fungiform papillae number was statistically similar in both mice at E13.5. Since the papillae and taste buds did not become innervated in BDNF−/−xNT-3−/− mice, the already formed papillae atrophied. It is possible that papillae maintenance role of innervation, as shown in in vitro studies, is based on preserving functional taste buds; i.e. lack of appropriate innervation leads to loss of functional taste buds that in turn fail to preserve normal papillae morphology. We previously showed that taste buds/papillae impairments in BDNFxNeurotrophin-4 (NT-4) DK mice were more severe than in single BDNF mutant mice [6]. It is notable that BDNF−/−xNT-3−/− mice had their highest number of fungiform papillae between E15.5 and E17.5 (Figure 4 A) and BDNF−/−xNT-4−/− at E17.5 [6]. Detailed analysis of possible additional differences in our DK models will shed more light on functional specificity and overlap among neurotrophins.
Initial development of taste bud progenitors
Troma-1, Shh, and Sox2 positive fungiform taste buds/papillae did not show significant morphological differences between wild-type and BDNF−/−xNT-3−/− mice at E13.5. The number of fungiform papillae in BDNF−/−xNT-3−/− mice increased from E13.5 to E15.5, but was fewer than in wild-type mice. Despite the lack of vallate nerve plexus in the knockout mice, the morphology and the size of the vallate papilla showed no difference between BDNF−/−xNT-3−/− and wild-type mice at E13.5. These data indicate that the development of taste bud progenitors is independent of gustatory/somatosensory innervation. We did notice a reduction in the number of taste bud-bearing fungiform papillae in E13.5 BDNFxNT-3 compared to wild-type mice. This difference was not statistically significant. It has been reported that there was a similar number of fungiform papillae in BDNF null and wild-type mice at E14.5 [16]. Our current results also indicated that circumvallate papillae size was also similar in both DK and wild-type mice. We previously proposed an auocrine/paracrine role for neurotrophins in the tongue [6] and suggested that neural crest migration deficits might account for some of the deficits in neurotrophin knockout mice. However, further studies are required to elucidate if any of the above-mentioned mechanisms might lead to a developmental deficit in fungiform papillae.
Taste bud development at synaptogenesis
Fungiform taste bud progenitors in BDNF−/−xNT-3−/− mice showed atrophy at E17.5 and this appeared to continue to P0. While GAP43 positive nerves clearly ramified into the intra- and perigemmal areas of the developing fungiform papillae in the wild-type mice at E17.5 and P0, there were almost no gustatory/somatosensory nerve fibers in BDNF−/−xNT-3−/− mice at all. As may be seen in figure 4, the histograms representing the number of the fungiform papillae had a peak at E15.5 and then decreased from E15.5 to P0 in BDNF−/−xNT-3−/− mice. Interestingly, this coincides with the severe loss of innervation that was observed from E15.5 onwards. It has been clearly shown that papillae maintenance requires innervation [4]. It is also conceivable that the loss of taste bud progenitors could be the consequence of the loss of the trophic influence of the nerves on maintaining the gustatory papillae, autocrine/paracrine function of the neurotrophins or defects in neural crest migration. The circumvallate papillae size difference between wild-type and BDNF−/−xNT-3−/− mice became more apparent after E15.5 and only a malformed trench system was observed in the circumvallate papillae of the BDNF−/−xNT-3−/− mice. The severe loss of GAP43 positive nerve fibers reflects a significant loss of both gustatory and somatosensory nerve fibers. Moreover, no Troma-1 positive taste buds were seen in the circumvallate papilla of the DK mice, even at P0. These results suggest that developing vallate taste buds are more sensitive to neurotrophin manipulation than the developing fungiform taste buds. At E15.5 and onwards, some taste cell progenitors start forming synapses. It has been shown that the majority of BDNF expressing cells express the synaptic marker SNAP-25 [18]. This suggests BDNF might be a synaptogenic/target invasion factor for taste cells and gustatory nerves in addition to promoting axonal growth [15] and being a chemoattractant [8]. This was functionally verified when BDNF was expressed by lingual muscle fibers [10, 14] and induced synapse-like structures between gustatory nerves and tongue muscle fibers. We propose that loss of neurotrophins leads to disruption of the innervation due to failure to form synapses between the nerves and their normal targets. In turn, loss of innervation leads to the atrophy of the targets.
It has been shown that almost all epithelial cells irrespective of ecto-, meso- or endodermal origins express Cytokeratin 8 (Troma-1) in 12-15 day-old human embryos [7]. The broad early expression in primitive oral epithelium followed by a restricted presence in specialized cells in only taste buds and glandular epithelium is an interesting phenomenon but requires further studies.
CONCLUSION
At E13.5, taste cell progenitors expressed taste cell markers Troma-1, Shh, and Sox2, indicating that taste cell progenitor differentiation is nerve independent at the initial development stages. From E15.5 to E17.5/P0, we observed morphological disruption of fungiform and circumvallate taste buds/papillae and deficits in gustatory/somatosensory innervation with decreased number of fungiform papillae and smaller size of the vallate papillae in BDNF−/−xNT-3−/− mice. These results indicate a two-stage development of taste buds, initial formation that is nerve independent, and a synaptogenesis stage that requires functional connections between taste cells and nerves.
Acknowledgments
We thank Waldemar De Reijk and Nancy Pecora for scanning electron microscopy, Michael Whitt for use of the confocal microscope, Michelle Sims for technical assistance, and Thomas Finger for obtaining NT-3 mice. This project was supported by Grant Number R01-RDC007628 from NIH-NIDCD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barlow LA, Northcutt RG. Embryonic origin of amphibian taste buds. Dev. Biol. 1995;169:273–285. doi: 10.1006/dbio.1995.1143. [DOI] [PubMed] [Google Scholar]
- 2.Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 3.Farbman AI. Differentiation of taste buds in organ culture. J. Cell Biol. 1972;52:489–493. doi: 10.1083/jcb.52.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farbman AI, Mbiene JP. Early development and innervation of taste bud-bearing papillae on the rat tongue. J. Comp. Neurol. 1991;304:172–186. doi: 10.1002/cne.903040203. [DOI] [PubMed] [Google Scholar]
- 5.Farinas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- 6.Ito A, Nosrat CA. Gustatory papillae and taste bud development and maintenance in the absence of TrkB ligands BDNF and NT-4. Cell Tissue Res. 2009 doi: 10.1007/s00441-009-0833-7. [DOI] [PubMed] [Google Scholar]
- 7.Kemler R, Brulet P, Schnebelen MT, Gaillard J, Jacob F. Reactivity of monoclonal antibodies against intermediate filament proteins during embryonic development. J Embryol Exp Morphol. 1981;64:45–60. [PubMed] [Google Scholar]
- 8.Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, Yuan XB. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- 9.Liebl DJ, Tessarollo L, Palko ME, Parada LF. Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice. J Neurosci. 1997;17:9113–9121. doi: 10.1523/JNEUROSCI.17-23-09113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L, Lopez GF, Krimm RF. Epithelial-derived brain-derived neurotrophic factor is required for gustatory neuron targeting during a critical developmental period. J Neurosci. 2009;29:3354–3364. doi: 10.1523/JNEUROSCI.3970-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nosrat CA, Blomlöf J, Elshamy WM, Ernfors P, Olson L. Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbances, respectively. Development. 1997;124:1333–1342. doi: 10.1242/dev.124.7.1333. [DOI] [PubMed] [Google Scholar]
- 12.Nosrat IV, Agerman K, Marinescu A, Ernfors P, Nosrat CA. Lingual deficits in neurotrophin double knockout mice. J Neurocytol. 2004;33:607–615. doi: 10.1007/s11068-005-3330-2. [DOI] [PubMed] [Google Scholar]
- 13.Oakley B, Witt M. Building sensory receptors on the tongue. J Neurocytol. 2004;33:631–646. doi: 10.1007/s11068-005-3332-0. [DOI] [PubMed] [Google Scholar]
- 14.Ringstedt T, Ibáñez CF, Nosrat CA. Role of BDNF in target invasion in the gustatory system. J. Neurosci. 1999;19:3507–3518. doi: 10.1523/JNEUROSCI.19-09-03507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rochlin MW, O'Connor R, Giger RJ, Verhaagen J, Farbman AI. Comparison of neurotrophin and repellent sensitivities of early embryonic geniculate and trigeminal axons. J Comp Neurol. 2000;422:579–593. [PubMed] [Google Scholar]
- 16.Thirumangalathu S, Harlow DE, Driskell AL, Krimm RF, Barlow LA. Fate mapping of mammalian embryonic taste bud progenitors. Development. 2009;136:1519–1528. doi: 10.1242/dev.029090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vintschgau MV, Hönigschmied J. Nervus Glossopharyngeus und Schmeckbecher. Arch. ges. Physiol. 1877;14:443–448. [Google Scholar]
- 18.Yee CL, Jones KR, Finger TE. Brain-derived neurotrophic factor is present in adult mouse taste cells with synapses. J Comp Neurol. 2003;459:15–24. doi: 10.1002/cne.10589. [DOI] [PubMed] [Google Scholar]
- 19.Zhang CX, Brandemihl A, Lau D, Lawton A, Oakley B. BDNF is required for the normal development of taste neurons in vivo. Neuroreport. 1997;8:1013–1017. doi: 10.1097/00001756-199703030-00039. [DOI] [PubMed] [Google Scholar]