Abstract
Purkinje cell protein 2 (PCP2), a member of the family of guanine dissociation inhibitors and a strong interactor with the G-protein subunit Gαo, localizes to retinal ON bipolar cells. The retina-specific splice variant of PCP2, Ret-PCP2, accelerates the light response of rod bipolar cells by modulating the mGluR6 transduction cascade. All ON cone bipolar cells express mGluR6 and Gα o, but only a subset expresses Ret-PCP2. Here we test the hypothesis that Ret-PCP2 contributes to shaping the various temporal bandwidths of ON cone bipolar cells in monkey retina. We found that the retinal splice variants in monkey and mouse are similar and longer than the cerebellar variants. Ret-PCP2 is strongly expressed by diffuse cone bipolar type 4 cells (DB4; marked with anti-PKCα), and weakly expressed by midget bipolar dendrites (labeled by antibodies against Gα o, Gγ13, or mGluR6). Ret-PCP2 is absent from diffuse cone bipolar type 6 (DB6; marked with anti-CD15) and blue cone bipolar cells (marked with anti-CCK precursor). Thus, cone bipolar cells that terminate in stratum 3 of the inner plexiform layer (DB4) express more Ret-PCP2 than those that terminate in stratum 3+4 (midget bipolar cells), and these in turn express more than those that terminate in stratum 5 (DB6 and blue cone bipolar cells). This expression pattern approximates the arborization of ganglion cells (GC) with different temporal band-widths: parasol GCs stratifying near stratum 3 are faster than midget GCs stratifying in strata 3+4, and these are probably faster than the sluggish GCs that arborize in stratum 5.
Keywords: PCP2, retina, ON bipolar, PKC, temporal bandwidth
Purkinje cell protein-2 (PCP2, also called L7 or GPSM4) is a member of the G-protein modulator family known as guanine nucleotide dissociation inhibitors (GDI). The brain expresses two splice variants of PCP2, and these localize only to cerebellar Purkinje cells (Nordquist et al., 1988; Berrebi et al., 1991; Vandaele et al., 1991; Berrebi and Mugnaini, 1992; Zhang et al., 2002). The retina expresses a third splice variant (Ret-PCP2) whose N-terminus has 16 more amino acids than the cerebellar long form. In the mouse retina, Ret-PCP2 localizes to rod bipolar cells and to a subset of ON cone bipolar cells (Xu et al., 2008; Kim et al., 2008).
PCP2 is known to interact with Gα of the Gi/o family (Luo and Denker, 1999), and has been shown by biochemical assay to behave as a GDI (Natochin et al., 2001; Natochin et al., 2002; but see Luo and Denker, 1999). This function can be supported by expression in heterologous systems (Kinoshita-Kawada et al., 2004) and in cultured mammalian cells (PC12) (Willard et al., 2004; Guan et al., 2005). Examining PCP2-null mice by relatively crude criteria showed them to lack a discernable phenotype (Mohn et al., 1997; Vassileva et al., 1997). However, examining light responses in their rod bipolar cells revealed that these cells display a more depolarized dark resting potential and a slower light response (Xu et al., 2008). These effects are most likely due to Ret-PCP2's modulation of Gαo1, a G protein that couples mGluR6 to its transduction cascade (Nawy, 1999; Dhingra et al., 2000; Dhingra et al., 2002).
The discovery that Ret-PCP2 localizes to a subset of mouse ON cone bipolar cells prompted us to suggest that Ret-PCP2 contributes to shaping the temporal bandwidth of the different cone bipolar cell types. As an example, mouse type 7 cone bipolar cells exhibit a relatively slow light response and express a low level of Ret-PCP2. To further explore the function of Ret-PCP2, our study examines the expression and localization of Ret-PCP2 in monkey retina. Monkey retina possesses six types of ON bipolar cells: rod bipolar cell, invaginating midget cone bipolar cell, diffuse cone bipolar type 4 cell (DB4) that stratifies in sublamina 3, diffuse cone bipolar type 5 cell (DB5) that stratifies in sublamina 4, diffuse cone bipolar type 6 cell (DB6) that stratifies narrowly in sublamina 5, and the blue cone bipolar cell that also stratifies in sublamina 5, but has larger axon terminals. About half of these types can be identified by cell markers. Here we sequenced monkey Ret-PCP2, and found it to be similar to that of mouse. This protein colocalized with PKCα throughout rod bipolar cells and DB4s, and in the dendrites of midget bipolar cells. Ret-PCP2 was absent from DB6s and blue cone bipolar cells. Thus, we suggest that the differential expression of Ret-PCP2 contributes to the differential temporal responses of these cells.
Materials and Methods
Monkey (Macaca mulatta and facicularis) eyes were obtained from Covance Research Products Inc. (Alice, TX) following unrelated experiments. For RT-PCR and Western blotting, a total of 3 retinas were detached quickly and frozen in liquid nitrogen; for immunocytochemistry, a total of 6 were fixed by immersion in buffered paraformaldehyde at room temperature. Fixed retinas were cryoprotected with 30% sucrose, and small pieces (about 5×5 mm) were cut. Images that are labeled “central” came from the retinal piece that included the fovea at its center; neighboring pieces were considered either “mid periphery” or “periphery”. Tissue was embedded in a mixture of Tissue Freezing Medium (Electron Microscopy Sciences, Ft. Washington, OA) and 20% sucrose (1:2), and was cryosectioned at 10-15 μm thickness. For most experiments, sections were taken randomly either from the peripheral or the central region of the retina; thus the precise eccentricity is not known. To observe changes of staining pattern across different eccentricities, a long piece (5×12 mm extending from 1.5 mm nasal to fovea to far periphery) was embedded in agar, placed vertically in Tissue Freezing Medium on a microtome metal chuck, and frozen with liquid nitrogen.
RT-PCR
PCP2 was amplified by RT-PCR from whole retinal RNA prepared using a Nucleospin RNA II kit (Clontech, Mountain View, CA). Reverse transcription was performed on 1μg of total RNA with oligo dT primers using Moloney murine leukemia virus reverse transcriptase (BD Biosciences, San Jose, CA). For the PCR reaction, the forward primer was: 5’ – ggg aca tga tgg atc agg ag– 3’and the reverse primer was: 5’ – cga gta agg ccc agg atg –3’. The PCR reaction used thirty-five cycles (94°C for 1 min, 58°C for 30 seconds, and 72°C for 1 min), and was performed on a programmable thermocycler (PerkinElmer LifeSciences, Boston, MA).
Western Blotting
Frozen monkey retinas were homogenized in 0.2-1 ml ice cold homogenization buffer (5 ml per g wet weight) (in mM: 320 sucrose, 5 Tris-HCl, and 2 EDTA, 2.5 β-mercaptoethanol, pH adjusted to 7.4 at room temperature) containing protease inhibitor cocktails (P8340; Sigma Aldrich, St. Louis, MO). They were centrifuged at 6,000 × g for 10 min and the supernatant was collected. The protein assay was carried out using a BCA protein reagent (Biorad). Twenty μg of protein was mixed with NuPAGE LDS sample buffer (Invitrogen, Carlsbad, CA), was incubated for 10 min at 95°C, and was then loaded onto a 10% Tris-glycine gel (Bio-Rad, Hercules, CA). Electrophoresis was performed with a mini-protean II electrophoresis cell (Bio-Rad) under reduced denaturing conditions. The resolved proteins were transferred to a nitrocellulose membrane (Bio-Rad) in transfer buffer (192 mM glycine, 25 mM Tris-Cl, pH 8.3, and 20% methanol) for 40-50 min at 80V using a Bio-Rad Trans-Blot semi-dry transfer cell. The membrane was blocked with 7% skim milk in TBST (10 mM Tris-HCl, 150 mM NaCl, 0.1% Tween 20, PH 7.4) for 1 hr, and was incubated with the anti-PCP2 antibody (1:10000) overnight at 4°C in TBST-milk. After extensive washes in TBST, the membrane was incubated with the secondary antibody conjugated to peroxidase (Protos immunoresearch, San Francisco, CA, 1:3000) in TBST-milk for 2 hr at room temperature. After extensive washes in TBST, bound antibody was detected using SuperSignal West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL).
Immunocytochemistry
Sections were soaked in diluent containing 10% normal goat serum, 5% sucrose, and 0.5% Triton X-100 in 0.1M phosphate buffer pH 7.4. They were then incubated in primary antibodies (Table 1) at 4°C overnight, washed, incubated in secondary antibodies conjugated to a fluorescent marker (3 hr), rinsed, and mounted in Vectashield (Vector Laboratories, Burlingame, CA). For double staining with two primary antibodies raised in different species, incubations followed the protocol for single labeling, but both primary antibodies were applied together and so were both secondary antibodies. For double staining with two primary antibodies raised in rabbit (Ret-PCP2- and CCK or Gγ13), the sections were incubated sequentially with first anti-Ret-PCP2, then anti-rabbit Fab fragment conjugated to FITC, then anti-CCK (or anti-Gγ13), and finally whole anti-rabbit IgG conjugated to rhodamine. Control experiments omitted the second primary antibody (i.e., anti-CCK). Retinas were photographed with an Olympus confocal microscope (FV-1000) under 40x (NA 1.3) and 60x (NA 1.42) with a zoom of 2 or 3. Most experiments were performed on at least 2 different retinas; the double labeling for Ret-PCP2 and mGluR6 or blue opsin was done on 1 retina. Two fixation protocols were used for cryosectioned retinas: 4% paraformaldehyde for 30 min or 4% paraformaldehyde and 0.01% glutaraldehyde for 1 hr. The antibodies against PCP2, Gαo, Gγ13, and CD15 worked well with either fixative; the antibodies against PKCα, CCK precursor, and mGluR6 gave better results with less fixation. Retinal sections were imaged with an Olympus confocal microscope. All images were taken with the default pinhole (of one airy disk) calculated by the confocal microscopes. These give a 500-600 nm approximate z-resolutions for the 60x NA1.42 objectives and 650-750 nm for the 40x NA 1.3 objective. For publication, images were contrast adjusted with Adobe Photoshop; no other manipulations were performed. Figures were prepared with Adobe Illustrator. To examine localization at the electron microscope level, fixed tissue (4% paraformaldehyde for 1 hour) was frozen and thawed 3 times prior to Vibratome sectioning (100 μm thick). Sections were incubated in diluent and primary antibody as above (with 0 - 0.3% Triton-X 100), then in secondary antibody conjugated to HRP. They were then rinsed, developed in 0.05% 3, 3’-diaminobenzidine (DAB) + 0.01% hydrogen peroxide in phosphate buffer for 15 minutes, washed, and postfixed. DAB reaction product was intensified with gold-substituted silver and sections were osmicated, dehydrated, and mounted in Epon 812. Ultrathin sections were poststained with uranyl acetate and lead citrate and photographed with an electron microscope.
Table 1.
| Antigen | Immunogen | Manufacturer (catalog no.), Host species | Dilution |
|---|---|---|---|
| PCP2 | NT peptide of mouse PCP2: magspdqegffnllthvqgdr | Prepared by Rod Feddersen, Rabbit polyclonal | 1:1000 |
| Gαo | Purified bovine protein | Millipore (MAB3073; clone 2A), Mouse monoclonal | 1:300 |
| Gγ13 | aa 47–59 of mouse Gγ13: cflnpdlmknnpwv | Gift of Robert Margolskee, Mount Sinai School of Medicine, Rabbit polyclonal | 1:500 |
| PKCα | Purified bovine PKCα | Sigma-Aldrich (P 5704; Clone MC5), Mouse monoclonal | 1:500 |
| CCK precursor | G6-gly peptide: ygwmdfg | Gift of John DelValle, University of Michigan, Rabbit polyclonal | 1:1000 |
| CD15 | U-937 histiocytic cell line (human) | ATCC, Manassas, VA (HB-78-MMA)*, Mouse monoclonal | 1:1-2 |
| mGluR6 | CT of human protein: c-katstvaappkgedaeahk | Prepared by our lab, Rabbit polyclonal | 1:100 |
| Blue opsin | A peptide within the NT of human blue-opsin: efylfknissvgpwdgpqyh | Santa Cruz Biotechnical Inc. Europe (OPN1SW (N-20):sc-14363), Goat polyclonal | 1:50 |
| ChAT | Human placental enzyme | Millipore/Chemicon (AB144P-200ul) Goat Polyclonal | 1:200 |
Catalog number is for the hybridoma cell line; antibody was collected from the medium according to manufacturer's protocol.
CT, C-terminus; NT. N-terminus.
Antibody Characterization
The list of antibodies used in this study, their immunogens, and their sources are provided in Table 1. Here we provide evidence for their specificity. The antibody against PCP2, when used for Western blotting of monkey retina, gave a single prominent band with the same mobility as PCP2 of mouse retina (depending on gel concentration these ran between 17-30 kDa; see Results section). In addition, the band and staining pattern were eliminated in PCP2 knockout mice (Xu et al., 2008). The antibody against Gαo was used to label the somas and dendrites of all ON bipolar cells; in the IPL it also stained amacrine cell processes. This antibody gave staining that was identical to the rabbit anti-Gαo staining and this staining was absent in the Gαo knockout mouse (Li et al., 1995; Dhingra et al., 2000; Dhingra et al., 2002). The antibody against Gγ13 was used to mark all ON bipolar cells; in mouse, its immunostaining could be correlated with the expressed transcript (Huang et al., 1999; Huang et al., 2003). The antibody against protein kinase C alpha subunit (PKCα) is routinely used to identify rod bipolar cells; it specifically recognized a 79 kDa protein and its staining could be blocked with peptide 296-317. The antibody against cholecystokinin (CCK) precursor was used to mark blue cone bipolar cells (Kouyama and Marshak, 1992; Kouyama and Marshak, 1997) and it may also stain midget cone bipolar cell dendrites (Wässle et al 1994). It recognized the glycine extended form of CCK; its labeling was blocked with the immunogenic peptide G6-gly (Marshak et al., 1990). Anti-CD15 (Forutan et al., 2001) labels the diffuse cone bipolar type 6 cell (Wässle et al., 1994; Chan et al., 2001; Jusuf et al., 2004). The antibody against human mGluR6 recognizes this protein in transfected HEK cells; in Western blots of monkey retina, it gave a monomer and a dimer at about 95 and 190 kDa. Both the immunostaining of fixed tissue and the bands were blocked by application of immunogenic peptide (Vardi et al., 2000). Anti-blue-opsin strongly stains the blue-sensitive cones (recognizable by their shape and density). Anti-choline acetyltransferase (ChAT) is specific since immunoblotting gave a single 68 kDa protein (manufacturer's technical information) and since it stains only the known cholinergic amacrine cells in retinas from a variety of species including monkey (Yamada et al. 2003).
Counting
To estimate the percent colocalization of two stains at the dendritic tips, we first looked at the marker stain (with the aid of Fluoview 1000) and identified the pedicles by the characteristic position of the clustered cone bipolar dendritic tips that contact them. We counted all the twigs stained with marker within each pedicle, then looked at the Ret-PCP2 stain and counted the twigs that showed this stain, and then checked if there were any “new” twigs that stained for Ret-PCP2 but not for the marker. For each region of the retina that was photographed, a stack of images was taken through the z-axis, and counting was performed only on the brightest confocal plane within a stack. We restricted our analysis to a single plane because resolution in the z-axis is inferior to that in the x-y axes, and we needed to avoid a duplicate count of the same twig. Due to scarcity, twigs near the blue cone pedicle were counted from the full stack.
RESULTS
Retina expresses a new splice variant of PCP2
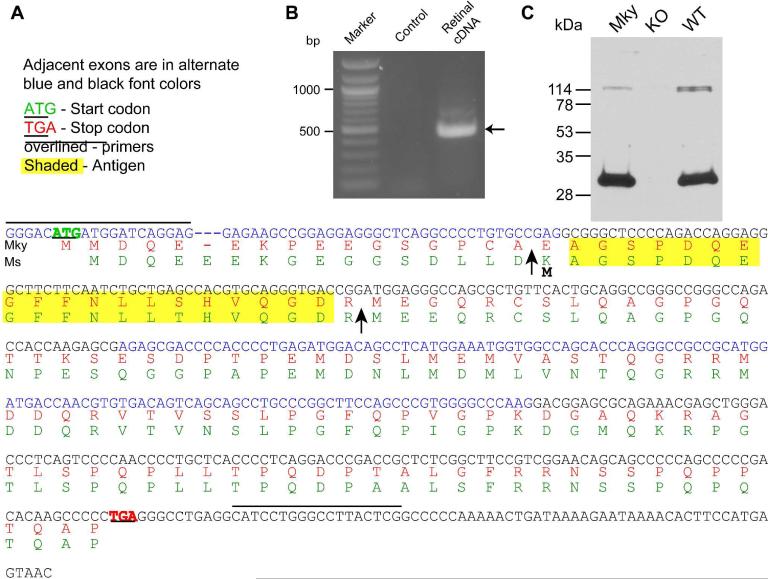
We previously found that the mouse PCP2 expressed in retina (Ret-PCP2) is longer than the cerebellar forms. In human cerebellum, two splice variants are expressed: a shorter form-A and a longer form-B. There is no information regarding the PCP2 splice variants in monkey. Consequently, we PCR amplified monkey retinal cDNA using the predicted ENSEMBL sequence ENSMMUT00000038822 as a reference. PCR amplification of retinal cDNA followed by sequencing revealed the monkey retinal PCP2 sequence to be similar to mouse Ret-PCP2 (Xu et al., 2008) (Fig. 1). The primers shown in Figure 1A yielded a reaction product of ~450 bp (Fig. 1B) whose sequencing revealed a putative exon not present in the known human cerebellar splice variants (Fig. 1A). This sequence appears to represent a new exon (henceforth referred to as exon 1ret) because it has a splice donor site (GU) following it and an initiation codon with Kozak consensus to facilitate translation. Exon 1ret followed by exons 2-4 yields a protein variant Ret-PCP2 (Genbank accession number EU676240). As in mouse, Ret-PCP2 is predicted to encode a 15 kDa protein that is longer than the cerebellar long form by 16 amino acids at the N-terminus. Western blots of homogenates of monkey retina probed with an antibody raised against the N-terminus of form-B PCP2 confirmed the expression of Ret-PCP2 since it revealed a protein band with mobility similar to that of mouse Ret-PCP2 (Fig. 1C).
Fig. 1. Retina expresses a new splice variant of PCP2 transcript, Ret-PCP2.
A. The sequence of the PCR reaction product (in blue, now called Ret-PCP2) and its predicted translation in monkey (Mky, red). Mouse (Ms) Ret-PCP2 (green) is shown for reference. The sequences are similar. The arrows mark the translation start point of human cerebellar form-B (first arrow) and form-A (second arrow). Lines above the sequence mark the primers and yellow highlights mark the amino acid sequence used to generate the antibody.
B. A prominent PCR reaction product of ~450 bp (arrow) results from PCR amplification of retinal cDNA. Control includes water instead of cDNA sample.
C. Western blots of monkey (Mky) and wild type mouse (WT) retinal proteins show a single prominent band that migrates with similar mobility (~29 kDa). This band is missing in the PCP2-knockout mouse (KO).
Ret–PCP2 is expressed throughout diffuse bipolar type 4 cells
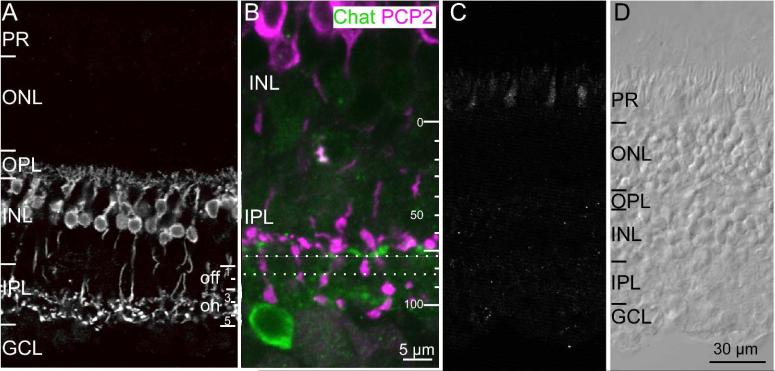
In mouse, Ret-PCP2 is not restricted to rod bipolar cells, but is also expressed in a subset of cone bipolar cells (Xu et al., 2008). This is also the case in monkey. Immunostaining for Ret-PCP2 (using either fixative; see Methods) labeled somas of bipolar cells in the inner nuclear layer (Fig. 2A). These cells sent axons that crossed sublamina a of the inner plexiform layer, and terminated in sublamina b. The stained axon terminals arborized both in stratum 5, where rod bipolar axons arborize, and in the lower portion of stratum 3 where certain ON cone bipolar cells arborize. The arborizations of the ON cone bipolar cells reside just above the substratum where processes of the ON starburst amacrine cells stratify (the OFF starburst amacrine cells are scarce and do not contribute to an observable band, consistent with Yamada et al, 2003) (Fig. 2B). Taking the stratification of the starburst cells as 70% and the interface between the IPL and the ganglion cell layer at 100% (Yamada et al, 2003), the ON cone bipolar terminate at 55-70%. Staining at the depth of ~70-80% (corresponding to stratum 4) of the inner plexiform layer appears to result solely from descending rod bipolar axons without evidence of contributions from arborizing cone bipolar cells. Preincubating the primary antibody with 10x excess of the immunogenic peptide completely abolished staining (Fig. 2C, D).
Fig. 2. Ret-PCP2 is expressed by ON cone bipolar cells.
A. Immunostaining for Ret-PCP2 (radial view). Staining is restricted to bipolar cells whose somas are located high in the INL and whose axons terminate in the ON sublamina of the IPL. Note that the arborizations in the IPL appear in two wavy laminas in stratum 3 and 5. For this and all figures: PR, photoreceptors; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
B. Immunostaining for Ret-PCP (magenta) and choline acetyl transferase (ChAT;green) shows that the level of cone bipolar arborization lies just above the stratification of the starburst amacrine cells at around 65% depth. PCP2-stained bipolar terminals hardly arborize between the dotted lines, but axons of rod bipolar cells do cross through this sublamina to arborize in sublamina 5.
C. Preabsorption control. All staining was eliminated in a retinal section that was incubated in antibody that was pre-absorbed with the antigenic peptide (excess of x10). To show some structure, contrast was enhanced relative to the image in B.
D. Image of the same section as in C taken under differential interference contrast.
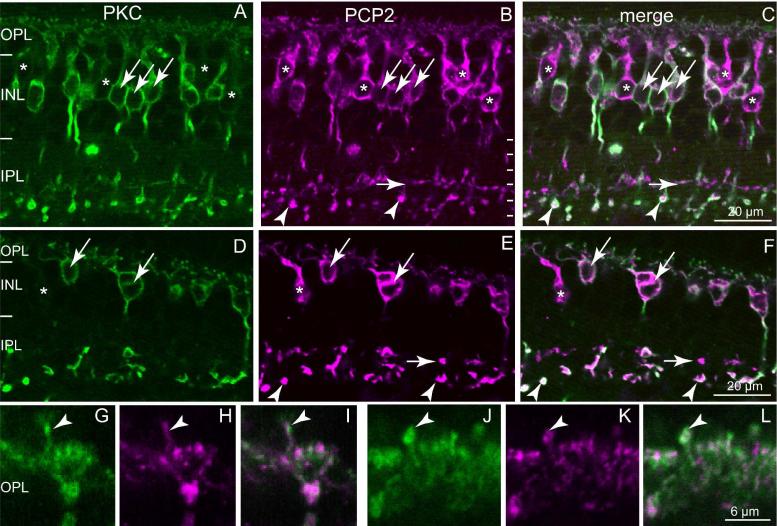
To determine which type of ON cone bipolar cell terminates in stratum 3 and expresses Ret-PCP2, we double labeled the retina for Ret-PCP2 and protein kinase C alpha subunit (PKCα), which in monkey labels both rod bipolar cells and DB4 (Grünert et al., 1994). Ret-PCP2 staining was found in all PKC-positive cells (Fig. 3). However, about 15% of the Ret-PCP2-positive somas were faintly stained or unstained for PKC (40/246). This may result from Ret-PCP2 being expressed in bipolar cell types other than the rod bipolar cell and DB4 cells, or from PKC failing to label all the DB4 cells. In the outer plexiform layer, staining for RET-PCP2 and PKC colocalized one hundred percent (Fig. 3G-L) suggesting that, in some cells, PKC is expressed only in their dendrites and not in their somas.
Fig. 3. Ret-PCP2 is expressed throughout diffuse bipolar type 4 (DB4) cells.
Double-labeling for Ret-PCP2 (magenta) and PKC (green).
A-C. Foveal sections.
D-F. Peripheral sections. All PKC-labeled somas are Ret-PCP2-positive (arrows); certain somas are Ret-PCP2-positive but PKC-negative (*). Rod bipolar terminals in sublamina 5 are strongly stained by both antibodies (arrowheads) whereas DB4 terminals are strongly stained for PCP2 but faintly stained for PKC (horizontal short arrows).
G-L. Two examples at higher magnification of dendrites approaching the cones in the OPL: there is a high degree of correlation between the two stains. Arrowheads point to dendrites of rod bipolar cells recognized by their position above the cone bipolar clusters.
DB6 and blue cone bipolar cells do not express Ret-PCP2
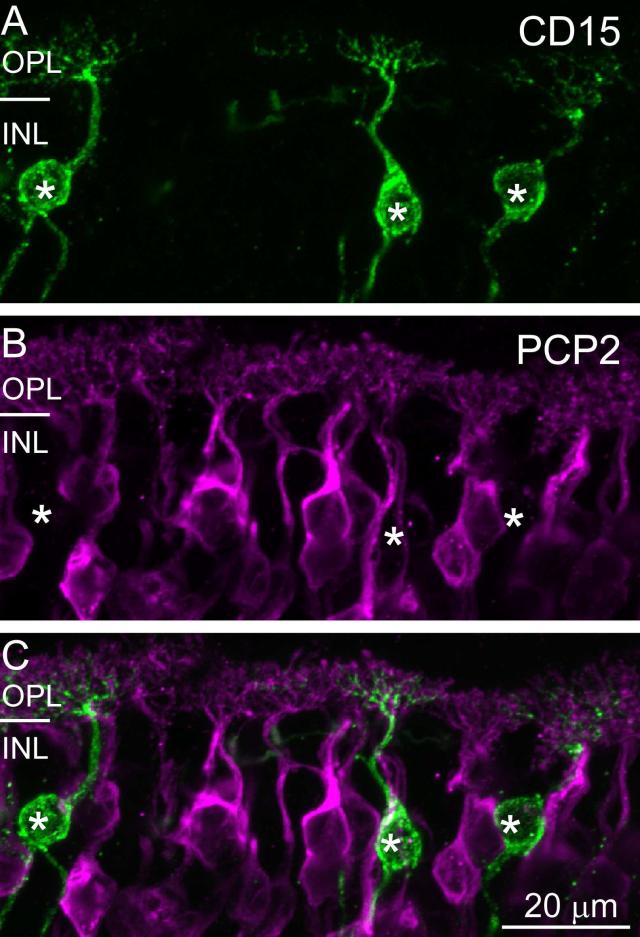
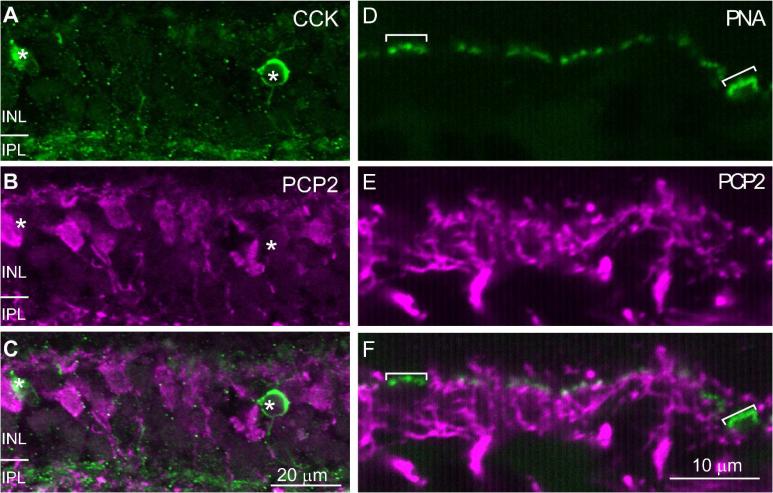
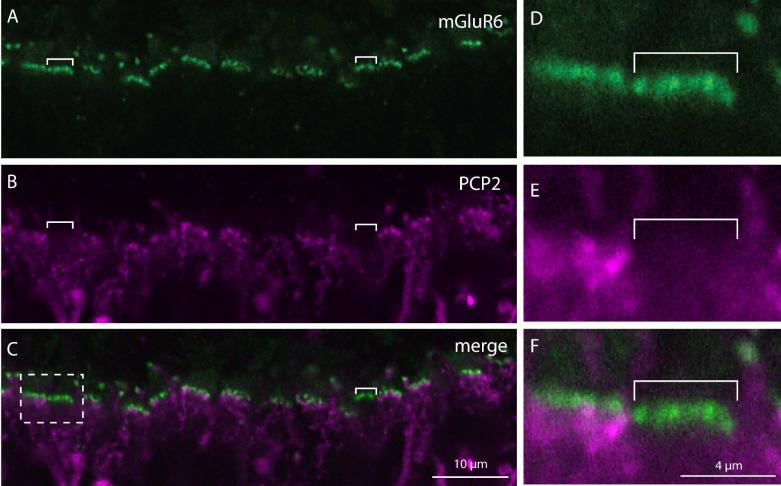
To further test which cell types express Ret-PCP2, we costained the retina with anti-CD15, a marker for DB6 cells, and with anti-CCK precursor, a marker for the blue cone bipolar cell. Ret-PCP2 staining did not colocalize with CD15 either in the soma or in the dendrites, indicating that DB6 cells do not express Ret-PCP2 (Fig. 4A-C). It also did not colocalize with CCK precursor-positive somas (Fig. 5A-C). However, since the staining for CCK precursor in the outer plexiform layer did not reveal the bipolar dendrites, we used a different method to assess whether blue bipolar dendrites stain for Ret-PCP2. We identified the blue-sensitive cone terminal either by staining with an antibody against the blue opsin which gave us low signal to noise ratio, or by staining with peanut agglutinin which stains blue cone terminals more strongly than red/green terminals (Lee et al., 2004; Lee et al., 2005). We found that the blue cone terminal received very few Ret-PCP2-stained dendrites (1.5; 9 full or partial pedicles were analyzed) (Fig. 5D-F). Consistent with this observation, costaining for mGluR6 and Ret-PCP2 showed few clusters that stained for mGluR6 but were negative for Ret-PCP2 (Fig. 6). This suggests that the blue bipolar cell dendrites are unstained for Ret-PCP2, and that the few Ret-PCP2 stained contacts that the blue cone pedicles do receive are from DB4 dendrites that contact this pedicle sparsely (Lee and Grünert, 2007).
Fig. 4. Ret-PCP2 is not expressed in DB6 cells.
Double staining for Ret-PCP2 (magenta) and CD15 (green). The images represent a projection of 2-3 confocal images. The two stains did not colocalize in either the somas (*) or the dendrites.
Fig. 5. Ret-PCP2 is not expressed in blue cone bipolar cells.
A-C. Double staining for Ret-PCP2 (magenta) and CCK (green). The images represent a projection of 2-3 confocal images. The two stains did not colocalize in the somas (*). D-F. Double staining using peanut agglutinin (PNA; green) and anti-Ret-PCP2 (magenta). Peanut agglutinin highlights the blue cone pedicles more brightly (brackets) than the other pedicles. These pedicles did not receive Ret-PCP2-stained dendrites.
Fig. 6. A subset of cones (probably blue-sensitive) does not receive Ret-PCP2-expressing dendrites.
Double staining for Ret-PCP2 (magenta) and mGluR6 (green).
A-C. An example of a section where two clusters of mGluR6 puncta are not approached by Ret-PCP2 stained dendrites (brackets).
D-F. Higher magnification of the dashed square in C. The pedicle on the left received multiple Ret-PCP2-expressing cone bipolar dendrites while that on the right received none.
Midget ON cone bipolar cells and possibly DB5 cells express Ret-PCP2 only in their dendritic tips
So far, the only cone bipolar cell that expresses Ret-PCP2 in its soma is DB4. If this is indeed the only cone bipolar that expresses this protein, we would expect a relatively low density of stained dendrites under the cone pedicle. However, the density of Ret-PCP2-stained dendrites appears high. We therefore considered the possibility that certain bipolar cell types express Ret-PCP2 only in their dendrites. Lacking a marker for the remaining cone bipolar cell types (DB5 or midget ON bipolar cells), we could not address this question directly. Instead, we assessed this possibility by co-staining with markers that stain all ON bipolar dendrites.
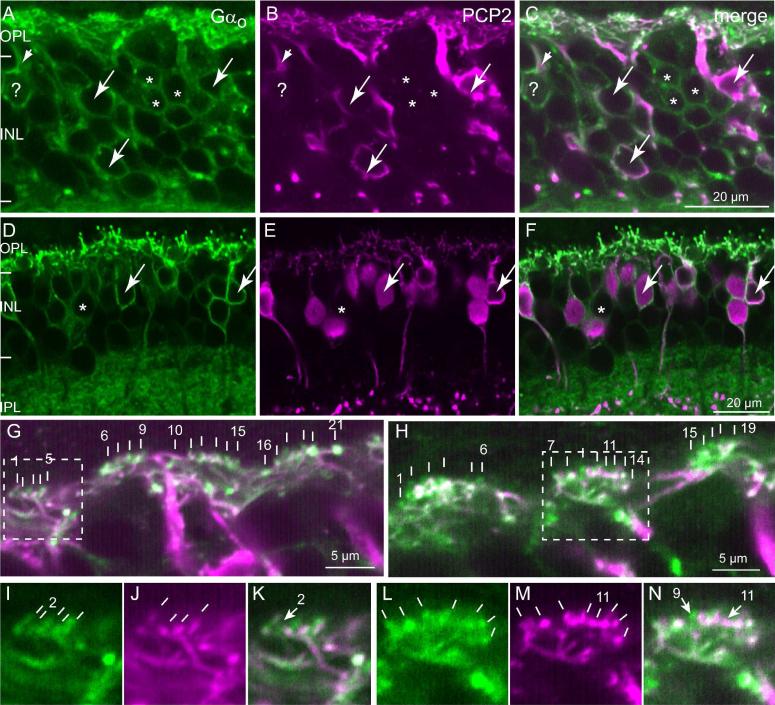
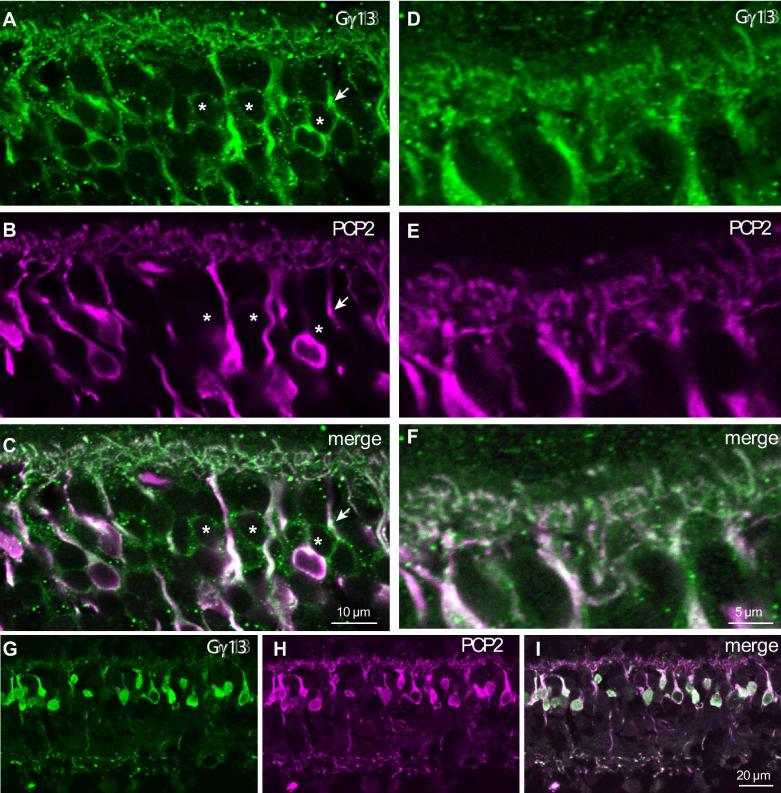
Staining the retina with anti-Gαo (Vardi, 1998; Dhingra et al., 2000) and examining the inner nuclear layer of a foveal region, we found many Gαo-positive somas that were not stained for Ret-PCP2 (Fig. 7A-C). In the periphery, there were fewer such Gαo-positive Ret-PCP2-negative somas (Fig. 7A-F). Since midget bipolar cells’ density quickly declines from fovea to periphery, this finding suggests that these unstained somas belong to the midget ON bipolar cells. Similar results were obtained for the bipolar somas when we double stained for Ret-PCP2 and Gγ13, another ON bipolar cell marker (Fig. 8). However, when we assessed colocalization of Ret-PCP2 with Gαo, Gγ13, or mGluR6 in the outer plexiform layer where the bipolar cell dendrites lie, both in the fovea and the periphery, the correspondence between the two stains was very high (Fig. 7G-N). About 92% (240/262) of Gαo-stained “twigs” co-expressed Ret-PCP2 while 94% (240/256) of Ret-PCP2-stained twigs co-expressed Gαo (43 pedicle profiles were analyzed). Two reasons can explain why not all Ret-PCP2-stained twigs show Gαo staining. First, immunohistochemical staining may be incomplete or weak; and second, if the two proteins concentrate in slightly different regions within a dendrite, the small section of dendrite captured by a confocal plane may have only one of the proteins. Indeed Gαo often appeared to extend further into the dendritic tips than Ret-PCP2 (Fig. 7G-N). Similar results were obtained by double staining for Gγ13 and Ret-PCP2 (Fig. 8) and by double staining for mGluR6 and Ret-PCP2 (Fig. 9). For the latter, concentrated stains were separated since mGluR6 was restricted to the dendritic tips and Ret-PCP2 was strongest slightly below the tip. Again, 92% (133/145) of mGluR6-stained twigs coexpressed Ret-PCP2 and 94% (133/141) of Ret-PCP2-stained twigs coexpressed mGluR6 (29 pedicles).
Fig. 7. Ret-PCP2 is localized to the dendritic tips of midget ON bipolar cells.
Double labeling for Ret-PCP2 (magenta) and Gαo (green).
A-F. All Ret-PCP2-positive somas are positive for Gαo (arrows), but certain Gαo-positive somas are devoid of Ret-PCP2 staining (*); Gαo-positive PCP2-negative somas in the fovea (A-C) is much greater than that in the periphery (D-F). Note the Gαo-labeled soma that is marked by ?; it is stained for Ret-PCP2 in its primary dendrite (very short arrow) but not in its soma. Note that the staining for Gαo is restricted to the soma's outline (membrane associated) while that for Ret-PCP2 is in the cytosol.
G–N. High magnification of the outer plexiform layer. G, H are merged images. Each vertical white bar “points” to an ON bipolar invaginating dendrite. Each cluster of dendrites probably resides under a cone pedicle. The first and last dendrites within a cluster are numbered. Dashed squares denote the areas of higher magnification shown in I-N for each stain separately. In I (Gαo), there are 5 resolvable dendrites; only four can be seen in J (Ret-PCP2) (no. 2 is lacking PCP2; arrow in K). In L (Gαo), 7 dendrites are resolvable, while in M (Ret-PCP2) 8 are resolvable. Dendrite no. 11 is strongly stained for Ret-PCP2, but either unstained or weakly stained for Gαo (arrow in N). The slight white color of twig 11 in the merged image (shown by arrow) is due to diffused green background at this spot; the green appearance of twig 9 results from Gαo in this dendrite that extends beyond Ret-PCP2. The orientation of the lines in I-N intends to show the orientation of the dendrites.
Fig. 8. Ret-PCP2 is localized to a subset of ON bipolar cell somas, but to most bipolar cell dendrites.
Double staining for Ret-PCP2 (magenta) and Gγ13 (green).
A-C. Central retina, certain somas that stain for Gγ13 are not stained (or are very weakly stained) for Ret-PCP2 (*). The arrow points to a primary dendrite that is stained for both Gγ13 and Ret-PCP2, but its soma is stained only for Gγ13.
D-F. Central section shows the OPL at higher magnification. All dendrites appear stained for both proteins.
G-I. In a different region where soma density is lower (mid periphery), all somas stain for both proteins.
Fig. 9. Ret-PCP2 is expressed in most ON bipolar cell dendrites.
Double staining for Ret-PCP2 (magenta) and mGluR6 (green).
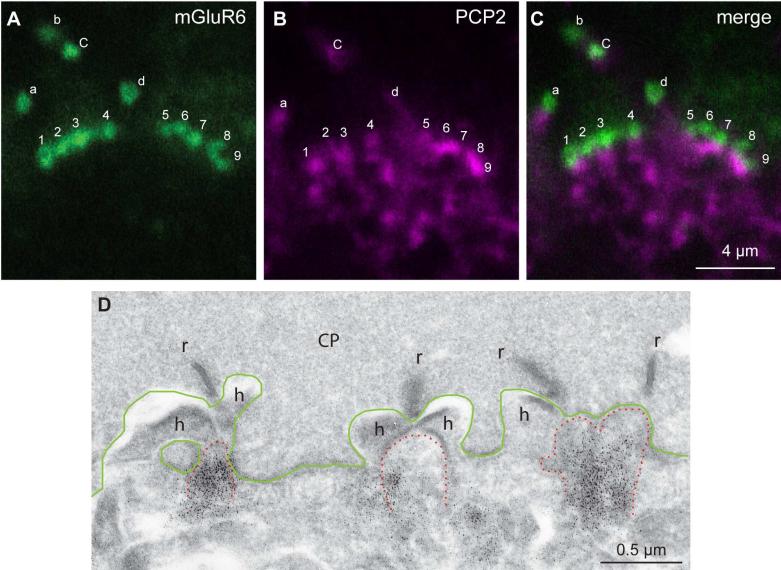
A-C. Two clusters of mGluR6-stained puncta are shown; the lettered puncta are rod bipolar dendritic tips (identified as such because these are large puncta located above the level of the cone pedicles) and the numbered puncta are cone bipolar dendritic tips. mGluR6 concentrates slightly above Ret-PCP2. Rod bipolar magenta punctum b is not visible in this focal plane and d is weak. All cone bipolar puncta can be correlated with Ret-PCP2 staining just below mGluR6.
D. Electron micrograph of monkey retina stained for Ret-PCP2 shows a region of a cone pedicle (CP, its base is outlined in green) with 4 synaptic ribbons (r) and 3 postsynaptic triads. The triads consists of stained (black dots) central elements (ON bipolar dendrites; tip is outlined in dotted red lines) and two lateral elements (horizontal cell process, h).
The results described above suggest that Ret-PCP2 is expressed in almost all ON bipolar cell dendrites. Since the dendrites of midget ON bipolar cells contribute about 30% of the invaginating contacts, these cells probably express Ret-PCP2, but only in their dendrites. This interpretation is supported by electron microscopy. Although compromised structure makes it hard to quantify, most central elements (and thus ON bipolar cell dendrites) were stained for Ret-PCP2 (Fig. 9D). Further supporting the idea that Ret-PCP2 in some cells is restricted to their dendrites, figures 7A-C and 8 show that some somas of ON bipolar cells are stained for Gαo (or Gγ13) and unstained for Ret-PCP2 while their primary dendrites are stained for both. With this interpretation, the 6% of dendrites not expressing Ret-PCP2 may belong to DB6 and possibly to DB5. Since DB5 dendrites are not as numerous as midget bipolar cell dendrites, it is not clear whether they are stained or not.
The antibody to PKCα labels cell types other than rod bipolar and DB4 cells
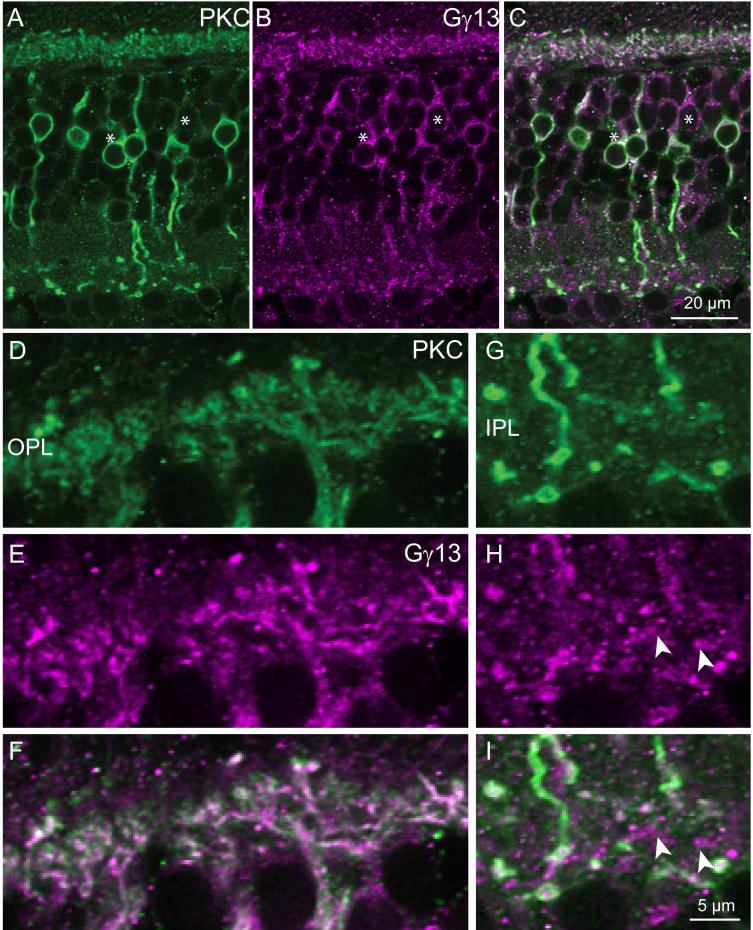
If PKCα and Ret-PCP2 staining colocalizes completely in the outer plexiform layer, and if Ret-PCP2 is not restricted to rod bipolar and DB4 cells, then PKCα must not be restricted to these cell types either. To test this directly, we double stained for Gγ13 and PKCα. In the fovea, many somas that stained for Gγ13 were unstained for PKC, while in the periphery, all cells that stained for one protein also stained for the other. In both fovea and periphery, the stains in the outer plexiform layer were well colocalized (Fig. 10). Thus, staining for PKC is not restricted to rod bipolar and DB4 cells as previously thought, but it also localizes to dendrites of midget bipolar cells.
Fig. 10. PKC colocalizes with Gγ13 in the ON bipolar cell dendrites.
Double staining for PKC (green) and Gγ13 (magenta).
A-C. Low magnification shows that some somas are stained only for Gγ13 (*).
D-F. High magnification of OPL shows close to 100% colocalization.
G-I. High magnification of IPL shows that certain bipolar terminals (arrowheads) are stained only for Gγ13. Scale bar in I applies to D-I
DISCUSSION
In this study, we present evidence that, like the mouse retina, monkey retina expresses a retina-specific slice variant of PCP2 that we name Ret-PCP2. This protein is expressed not only by rod bipolar cells, but also by DB4 cells and the dendrites of midget cone bipolar cells. It is not expressed by DB6 or blue cone bipolar cells.
Compartmentalization of Ret-PCP2
Our high resolution analysis of the localization of Ret-PCP2 reveals that, unlike Gαo or PKCα, Ret-PCP2 is not associated with the plasma membrane but rather is found throughout the cytosol. While in DB4 and rod bipolar cells, Ret-PCP2 is expressed throughout the cell and appears with similar intensities in dendrites and axon terminals, in midget bipolar cells, its staining is restricted to the dendrites. Ret-PCP2 is thought to modulate the mGluR6 cascade, thus its localization in the ON bipolar dendrites is expected, but in axon terminals is not. Nonetheless, Ret-PCP2's presence in locations other than the dendrites follows the distribution of Gαo which concentrates in the dendrites but also localizes to the soma (Vardi, 1998), and of Gγ13 which localizes throughout the ON bipolar cells (Huang et al., 2003).
The localization to the axon terminal may suggest that, in DB4 and rod bipolar cells, Ret-PCP2 also functions to modulate additional G-protein-coupled receptors located at the axon terminal. Currently, we do not have evidence to support or eliminate this possibility. Alternatively, Ret-PCP2 may float in the cytosol in excess and its function may depend on its level relative to that of other cascade elements. Thus, in cells that express a large amount of Ret-PCP2, Ret-PCP2 may simply diffuse to the axon terminals. Such analysis suggests that DB4 cone bipolar cells express more Ret-PCP2 than midget bipolar cells. It is further possible that Ret-PCP2 distributes throughout the bipolar cell to titer its expression at the active site according to environmental conditions, as occurs for several phototransduction elements. For example, in rod photoreceptors, transducin is highly localized to the outer segment of the dark-adapted rod, but diffuses to the inner segment in the light, thus contributing to adaptation by reducing the signal in the light (Philp et al., 1987; Whelan and McGinnis, 1988; Strissel et al., 2005; Calvert et al., 2006). Conversely, arrestin is highly localized to the outer segment in the light-adapted rod and can thus assist in terminating the light response, but it diffuses to the inner segment in the dark to increase integration time and sensitivity.
Differential expression of Ret-PCP2 in different cone bipolar types
In mouse rod bipolar cells, Ret-PCP2 was shown to accelerate the light response. Since different types of cone bipolar cells serve to transmit different temporal bandwidths (Freed, 2000; Euler and Masland, 2000; Awatramani and Slaughter, 2000), it is possible that level of Ret-PCP2 expression correlates with faster transmission. Indeed, in mouse we find that type 7 cone bipolar cells, that are slower than certain other types, express little or no Ret-PCP2 (Euler and Masland, 2000; Xu et al., 2008). A similar correlation holds for monkey retina. There, DB4, the cone bipolar cell type that expresses the most Ret-PCP2, stratifies just above the starburst amacrine cells, ON parasol ganglion cells, and monostratified Y-like cells that stratify around 65% (Jacoby et al., 1996). These ganglion cells are transient and fast (Croner and Kaplan, 1995; 1996; reviewed by Kaplan and Benardete, 2001; Petrusca et al., 2007; Crook et al., 2008) and may receive input from DB4 cells. Similarly, motion-sensitive starburst amacrine cells are transient and may also receive input from DB4, a hypothesis to be tested in the future. Midget bipolar cells express less Ret-PCP2 than DB4 (inferred from the fact that expression is limited to their dendrites) and provide output to midget ganglion cells that are slower than parasol cells. DB6 cells, a bipolar cell type that does not express Ret-PCP2, may provide output to ganglion cells stratifying in stratum 5, possibly the large, giant, and /or melanopsin-expressing ganglion cells (Dacey et al., 2003; Dacey et al., 2005). Ganglion cells stratifying in this stratum are likely to be slow, as is the case in cat (Cleland and Levick, 1974a; Cleland and Levick, 1974b) and rabbit (Roska and Werblin, 2001). Similarly, the blue cone bipolar cell does not express Ret-PCP2; it stratifies in stratum 5, and contacts the bistratified blue ganglion cell. This ganglion cell shows a sustained response (Field et al., 2007; Crook et al., 2009) and may be slower than midget cells as the blue system is known to be more sluggish than the L/M system (Liu and Wandell, 2005).
Colocalization of Ret-PCP2 with PKCα
For a long time the anti-PKCα antibody was thought to be expressed solely by rod bipolar cells (e.g., Grünert and Martin, 1991; Martin and Grünert, 1992). It was later shown that in monkey, this antibody also labels, albeit more faintly, the DB4 cone bipolar cells (Grünert et al., 1994). Here we show that PKC staining perfectly colocalizes with Ret-PCP2 staining suggesting that the antibody also labels the dendrites of midget cone bipolar cells. Because this PKCα antibody also recognizes PKCß (to a lesser extent), it is currently not clear which of these PKC isoforms is expressed by ON cone bipolar cells. Regardless of the isoform though, the colocalization of PKC and Ret-PCP2 raises the possibility that PKC is involved in modulating the mGluR6 cascade in conjunction with Ret-PCP2. It is premature to speculate on the contribution of PKC to ON bipolar cell light responses. However, it is worth noting that synthesis, transport, and activation of PKC in rat rod bipolar cells is modulated by the light/dark cycle and the adaptation level (Gabriel et al., 2001). Moreover, since PKC activation requires calcium, it may be modulated by calcium entering the cell via the ON bipolar transduction channel.
Acknowledgments
We thank Sharron Fina for assisting in quantifying the immunostains, Dr. Jian Li for assistance with electron microscopy, Dr. Rukmini Rao-Mirotznik for reading and editing the paper, Dr. Robert Margolskee for providing the antibody against Gγ13 and Dr. John DelValle for providing the antibody against the gly-extended CCK precursor. Supported by NIH EY11105 (to NV), NIH R29 NS32320 (to RMF), and NIH NEI P30 EY01583 (Vision Research Core of the University of Pennsylvania).
Reference List
- Awatramani GB, Slaughter MM. Origin of transient and sustained responses in ganglion cells of the retina. J Neurosci. 2000;20:7087–7095. doi: 10.1523/JNEUROSCI.20-18-07087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrebi AS, Mugnaini E. Characteristics of labeling of the cerebellar Purkinje neuron by L7 antiserum. J Chem Neuroanat. 1992;5:235–243. doi: 10.1016/0891-0618(92)90048-u. [DOI] [PubMed] [Google Scholar]
- Berrebi AS, Oberdick J, Sangameswaran L, Christakos S, Morgan JI, Mugnaini E. Cerebellar Purkinje cell markers are expressed in retinal bipolar neurons. J Comp Neurol. 1991;308:630–649. doi: 10.1002/cne.903080409. [DOI] [PubMed] [Google Scholar]
- Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr., Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16:560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Chan TL, Martin PR, Grünert U. Immunocytochemical identification and analysis of the diffuse bipolar cell type DB6 in macaque monkey retina. Eur J Neurosci. 2001;13:829–832. doi: 10.1046/j.0953-816x.2000.01449.x. [DOI] [PubMed] [Google Scholar]
- Cleland BG, Levick WR. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol (Lond ) 1974a;240:421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland BG, Levick WR. Properties of rarely encountered types of ganglion cells in the cat's retina and an overall classification. J Physiol. 1974b;240:457–492. doi: 10.1113/jphysiol.1974.sp010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croner LJ, Kaplan E. Receptive fields of P and M ganglion cells across the primate retina. Vision Res. 1995;35:7–24. doi: 10.1016/0042-6989(94)e0066-t. [DOI] [PubMed] [Google Scholar]
- Crook JD, Davenport CM, Peterson BB, Packer OS, Detwiler PB, Dacey DM. Parallel ON and OFF cone bipolar inputs establish spatially coextensive receptive field structure of blue-yellow ganglion cells in primate retina. J Neurosci. 2009;29:8372–8387. doi: 10.1523/JNEUROSCI.1218-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JD, Peterson BB, Packer OS, Robinson FR, Gamlin PD, Troy JB, Dacey DM. The smooth monostratified ganglion cell: evidence for spatial diversity in the Y-cell pathway to the lateral geniculate nucleus and superior colliculus in the macaque monkey. J Neurosci. 2008;28:12654–12671. doi: 10.1523/JNEUROSCI.2986-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Peterson BB, Robinson FR, Gamlin PD. Fireworks in the primate retina: in vitro photodynamics reveal diverse LGN-projecting ganglion cell types. Neuron. 2003;37:15–27. doi: 10.1016/s0896-6273(02)01143-1. [DOI] [PubMed] [Google Scholar]
- Dhingra A, Jiang M, Wang T-L, Lyubarsky A, Savchenko A, Bar-Yehuda T, Sterling P, Birnbaumer L, Vardi N. Light response of retinal ON bipolar cells requires a specific splice variant of Gαo. J Neurosci. 2002;22:4878–4884. doi: 10.1523/JNEUROSCI.22-12-04878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Lyubarsky A, Jiang M, Pugh EN, Jr., Birnbaumer L, Sterling P, Vardi N. The light response of ON bipolar neurons requires Gαo. J Neurosci. 2000;20:9053–9058. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Masland RH. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol. 2000;83:1817–1829. doi: 10.1152/jn.2000.83.4.1817. [DOI] [PubMed] [Google Scholar]
- Field GD, Sher A, Gauthier JL, Greschner M, Shlens J, Litke AM, Chichilnisky EJ. Spatial properties and functional organization of small bistratified ganglion cells in primate retina. J Neurosci. 2007;27:13261–13272. doi: 10.1523/JNEUROSCI.3437-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forutan F, Mai JK, Ashwell KW, Lensing-Hohn S, Nohr D, Voss T, Bohl J, Andressen C. Organisation and maturation of the human thalamus as revealed by CD15. J Comp Neurol. 2001;437:476–495. doi: 10.1002/cne.1296. [DOI] [PubMed] [Google Scholar]
- Freed MA. Parallel cone bipolar pathways to ganglion cell use different rates and amplitudes of quantal excitation. J Neurosci. 2000;20:3956–3963. doi: 10.1523/JNEUROSCI.20-11-03956.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel R, Lesauter J, Silver R, Garcia-Espana A, Witkovsky P. Diurnal and circadian variation of protein kinase C immunoreactivity in the rat retina. J Comp Neurol. 2001;439:140–150. doi: 10.1002/cne.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünert U, Martin PR. Rod bipolar cells in the Macaque monkey retina: Immunoreactivity and connectivity. J Neurosci. 1991;11:2742–2758. doi: 10.1523/JNEUROSCI.11-09-02742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünert U, Martin PR, Wässle H. Immunocytochemical analysis of bipolar cells in the macaque monkey retina. J Comp Neurol. 1994;348:607–627. doi: 10.1002/cne.903480410. [DOI] [PubMed] [Google Scholar]
- Guan J, Luo Y, Denker BM. Purkinje cell protein-2 (Pcp2) stimulates differentiation in PC12 cells by Gbetagamma-mediated activation of Ras and p38 MAPK. Biochem J. 2005;392:389–397. doi: 10.1042/BJ20042102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Max M, Margolskee RF, Su H, Masland RH, Euler T. G protein subunit Ggamma13 is coexpressed with Galphao, Gbeta3 and Gbeta4 in retinal ON bipolar cells. J Comp Neurol. 2003;455:1–10. doi: 10.1002/cne.10396. [DOI] [PubMed] [Google Scholar]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- Jacoby R, Stafford D, Kouyama N, Marshak D. Synaptic inputs to ON parasol ganglion cells in the primate retina. J Neurosci. 1996;16:8041–8056. doi: 10.1523/JNEUROSCI.16-24-08041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusuf PR, Lee SCS, Grünert U. Synaptic connectivity of the diffuse bipolar cell type DB6 in the inner plexiform layer of primate retina. J Comp Neurol. 2004;469:494–506. doi: 10.1002/cne.11027. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Benardete E. The dynamics of primate retinal ganglion cells. Prog Brain Res. 2001;134:17–34. doi: 10.1016/s0079-6123(01)34003-7. 17-34. [DOI] [PubMed] [Google Scholar]
- Kim DS, Ross SE, Trimarchi JM, Aach J, Greenberg ME, Cepko CL. Identification of molecular markers of bipolar cells in the murine retina. J Comp Neurol. 2008;507:1795–1810. doi: 10.1002/cne.21639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita-Kawada M, Oberdick J, Xi ZM. A Purkinje cell specific GoLoco domain protein, L7/Pcp-2, modulates receptor-mediated inhibition of Cav2.1 Ca2+ channels in a dose-dependent manner. Brain Res Mol Brain Res. 2004;132:73–86. doi: 10.1016/j.molbrainres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Kouyama N, Marshak DW. Bipolar cells specific for blue cones in the Macaque retina. J Neurosci. 1992;12:1233–1252. doi: 10.1523/JNEUROSCI.12-04-01233.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouyama N, Marshak DW. The topographical relationship between two neuronal mosaics in the short wavelength-sensitive system of the primate retina. Vis Neurosci. 1997;14:159–167. doi: 10.1017/s0952523800008841. [DOI] [PubMed] [Google Scholar]
- Lee SC, Grünert U. Connections of diffuse bipolar cells in primate retina are biased against S-cones. J Comp Neurol. 2007;502:126–140. doi: 10.1002/cne.21284. [DOI] [PubMed] [Google Scholar]
- Lee SC, Jusuf PR, Grünert U. S-cone connections of the diffuse bipolar cell type DB6 in macaque monkey retina. J Comp Neurol. 2004;474:353–363. doi: 10.1002/cne.20139. [DOI] [PubMed] [Google Scholar]
- Lee SC, Telkes I, Grünert U. S-cones do not contribute to the OFF-midget pathway in the retina of the marmoset, Callithrix jacchus. Eur J Neurosci. 2005;22:437–447. doi: 10.1111/j.1460-9568.2005.04231.x. [DOI] [PubMed] [Google Scholar]
- Li X, Mumby S, Greenwood A, Jope R. Pertussis toxin-sensitive G-protein □-subunits: production of monoclonal antibodies and detection of differential increases upon differentiation of PC12 and LA-N-5 cells. J Neurochem. 1995;64:1107. doi: 10.1046/j.1471-4159.1995.64031107.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Wandell BA. Specializations for chromatic and temporal signals in human visual cortex. J Neurosci. 2005;25:3459–3468. doi: 10.1523/JNEUROSCI.4206-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Denker BM. Interaction of heterotrimeric G protein Galphao with Purkinje cell protein-2. Evidence for a novel nucleotide exchange factor. J Biol Chem. 1999;274:10685–10688. doi: 10.1074/jbc.274.16.10685. [DOI] [PubMed] [Google Scholar]
- Marshak DW, Aldrich LB, Del Valle J, Yamada T. Localization of immunoreactive cholecystokinin precursor to amacrine cells and bipolar cells of the macaque monkey retina. J Neurosci. 1990;10:3045–3055. doi: 10.1523/JNEUROSCI.10-09-03045.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR, Grünert U. Spatial density and immunoreactivity of bipolar cells in the Macaque monkey retina. J Comp Neurol. 1992;323:269–287. doi: 10.1002/cne.903230210. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Feddersen RM, Nguyen MS, Koller BH. Phenotypic analysis of mice lacking the highly abundant Purkinje cell- and bipolar neuron-specific PCP2 protein. Mol Cell Neurosci. 1997;9:63–76. doi: 10.1006/mcne.1997.0606. [DOI] [PubMed] [Google Scholar]
- Natochin M, Gasimov KG, Artemyev NO. Inhibition of GDP/GTP exchange on G alpha subunits by proteins containing G-protein regulatory motifs. Biochem. 2001;40:5322–5328. doi: 10.1021/bi015505w. [DOI] [PubMed] [Google Scholar]
- Natochin M, Gasimov KG, Artemyev NO. A GPR-protein interaction surface of Gi(alpha): implications for the mechanism of GDP-release inhibition. Biochem. 2002;41:258–265. doi: 10.1021/bi015708k. [DOI] [PubMed] [Google Scholar]
- Nawy S. The metabotropic receptor mGluR6 may signal through Go, but not phosphodiesterase, in retinal bipolar cells. J Neurosci. 1999;19:2938–2944. doi: 10.1523/JNEUROSCI.19-08-02938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordquist DT, Kozak CA, Orr HT. cDNA cloning and characterization of three genes uniquely expressed in cerebellum by Purkinje neurons. J Neurosci. 1988;12:4780–4789. doi: 10.1523/JNEUROSCI.08-12-04780.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrusca D, Grivich MI, Sher A, Field GD, Gauthier JL, Greschner M, Shlens J, Chichilnisky EJ, Litke AM. Identification and characterization of a Y-like primate retinal ganglion cell type. J Neurosci. 2007;27:11019–11027. doi: 10.1523/JNEUROSCI.2836-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp NJ, Chang W, Long K. Light-stimulated protein movement in rod photoreceptor cells of the rat retina. FEBS Lett. 1987;225:127–132. doi: 10.1016/0014-5793(87)81144-4. [DOI] [PubMed] [Google Scholar]
- Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature. 2001;410:583–587. doi: 10.1038/35069068. [DOI] [PubMed] [Google Scholar]
- Strissel KJ, Lishko PV, Trieu LH, Kennedy MJ, Hurley JB, Arshavsky VY. Recoverin undergoes light-dependent intracellular translocation in rod photoreceptors. J Biol Chem. 2005;280:29250–29255. doi: 10.1074/jbc.M501789200. [DOI] [PubMed] [Google Scholar]
- Vandaele S, Nordquist DT, Feddersen RM, Tretjakoff I, Peterson AC, Orr HT. Purkinje cell protein-2 regulatory regions and transgene expression in cerebellar compartments. Genes Dev. 1991;5:1136–1148. doi: 10.1101/gad.5.7.1136. [DOI] [PubMed] [Google Scholar]
- Vardi N. Alpha subunit of Go localizes in the dendritic tips of ON bipolar cells. J Comp Neurol. 1998;395:43–52. [PubMed] [Google Scholar]
- Vardi N, Duvoisin RM, Wu G, Sterling P. Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J Comp Neurol. 2000;423:402–412. doi: 10.1002/1096-9861(20000731)423:3<402::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Vassileva G, Smeyne RJ, Morgan JI. Absence of neuroanatomical and behavioral deficits in L7/pcp-2-null mice. Brain Res Mol Brain Res. 1997;46:333–337. doi: 10.1016/s0169-328x(97)00081-8. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Martin PR, Boycott BB. Immunocytochemical characterization and spatial distribution of midget bipolar cells in the macaque monkey retina. Vision Res. 1994;34:561–579. doi: 10.1016/0042-6989(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Whelan JP, McGinnis JF. Light-dependent subcellular movement of photoreceptor proteins. J Neurosci Res. 1988;20:263–270. doi: 10.1002/jnr.490200216. [DOI] [PubMed] [Google Scholar]
- Willard FS, Kimple RJ, Siderovski DP. Return of the GDI: the GoLoco motif in cell division. Annu Rev Biochem. 2004;73:925–51. doi: 10.1146/annurev.biochem.73.011303.073756. 925-951. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sulaiman P, Feddersen RM, Liu J, Smith RG, Vardi N. Retinal ON bipolar cells express a new PCP2 splice variant that accelerates the light response. J Neurosci. 2008;28:8873–8884. doi: 10.1523/JNEUROSCI.0812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada ES, Dmitrieva N, Keyser KT, Lindstrom JM, Hersh LB, Marshak DW. Synaptic connections of starburst amacrine cells and localization of acetylcholine receptors in primate retinas. J Comp Neurol. 2003;461:76–90. doi: 10.1002/cne.10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang H, Oberdick J. Conservation of the developmentally regulated dendritic localization of a Purkinje cell-specific mRNA that encodes a G-protein modulator: comparison of rodent and human Pcp2(L7) gene structure and expression. Brain Res Mol Brain Res. 2002;105:1–10. doi: 10.1016/s0169-328x(02)00379-0. [DOI] [PubMed] [Google Scholar]