Abstract
CD4+ and CD8+ T cell functions are rapidly aborted during chronic infection, preventing viral clearance. CD4+ T cell help is required throughout chronic infection so as to sustain CD8+ T cell responses; however, the necessary factor(s) provided by CD4+ T cells are currently unknown. Using a mouse model of chronic viral infection, we demonstrated that interleukin-21 (IL-21) is an essential component of CD4+ T cell help. In the absence of IL-21 signaling, despite elevated CD4+ T cell responses, CD8+ T cell responses are severely impaired. CD8+ T cells directly require IL-21 to avoid deletion, maintain immunity, and resolve persistent infection. Thus, IL-21 specifically sustains CD8+ T cell effector activity and provides a mechanism of CD4+ T cell help during chronic viral infection.
During chronic viral infections, antiviral CD4+ and CD8+ T cells become non-responsive to viral antigens and are either physically deleted or persist in a nonfunctional “exhausted” state, unable to produce important antiviral and immunostimulatory cytokines such as interleukin-2 (IL-2), tumor necrosis factor–α (TNF-α), and interferon-γ (IFN-γ); lyse virally infected cells; or proliferate (1–4). To date, the majority of work analyzing T cell exhaustion during chronic viral infection has focused on CD8+ T cells; however, CD4+ T cells also exhibit reduced function, thus further exacerbating viral persistence. CD4+ T cells are required throughout chronic viral infection in order to sustain CD8+ T cell function and control infection, prevent deletion of high-affinity antiviral CD8+ T cells, and eventually resolve the infection (5–7). The loss of CD4+ and CD8+ T cell function is associated with viruses that establish chronic viral infections in their hosts: HIV and hepatitis B and hepatitis C virus (HCV) in humans and lymphocytic chorio-meningitis virus (LCMV) in rodents (8–12). Chronic LCMV infection is eventually controlled in the periphery 60 to 80 days after infection in a CD4+ T cell–dependent manner, suggesting that exhausted CD4+ T cells retain helper activity. Expression of the key CD4+ T cell helper cytokine IL-2, however, is rapidly extinguished during viral persistence (4, 13), indicating that an alternative helper mechanism is being implemented.
To determine how CD4+ T cells help CD8+ T cells to clear a viral infection, we infected mice with the Armstrong strain of LCMV (Arm) that induces a robust T cell response, resulting in viral clearance within 8 to 10 days or with the Clone 13 strain of LCMV (Cl 13) that generates a chronic infection due to the up-regulation of immunosuppressive factors that induce a dramatic depletion and inactivation of virus-specific T cells (14–18). LCMV-Arm and –Cl 13 share identical CD4+ and CD8+ T cell epitopes, enabling direct comparison of virus-specific T cell responses during acute and persistent infection (15, 17). In initial experiments, we observed high amounts of IL-21 mRNA expression in the spleen during LCMV–Cl 13 infection (Fig. 1A). On the basis of the known immunomodulatory effects of IL-21 on T cells (19), we further investigated the function of IL-21 during viral persistence.
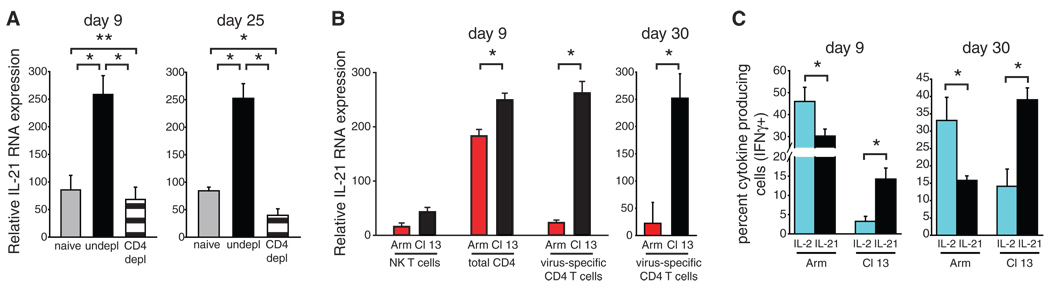
Fig. 1.
IL-21 mRNA expression during viral infection. IL-21 mRNA expression was quantified by real-time reverse transcription polymerase chain reaction after LCMV-Arm or LCMV–Cl 13 infection in (A) uninfected (gray bar), LCMV–Cl 13 infected (black bar), or LCMV–Cl 13 infected and CD4+ T cell–depleted (striped bar) splenocytes or in (B) the indicated sorted cell population. Red Bars indicate cells sorted from LCMV-Arm–infected animals; black bars indicate cells sorted from LCMV–Cl 13–infected animals. (C) Frequency of IFN-γ+ SMARTA T cells that simultaneously produced IL-2 or IL-21 on day 9 and day 30 after infection. Error bars represent the average ± SD of 3 to 5 mice per group and 2 to 4 independent experiments. *P < 0.05, **P = 0.4.
IL-21 is primarily produced by activated CD4+ T cells (20–22); however, the cells that produce IL-21 during chronic viral infection are unknown. Natural killer T cells, B cells, macrophages, and dendritic cells expressed low amounts of IL-21 mRNA during both acute and chronic LCMV infection (Fig. 1B and fig. S1). In contrast, IL-21 was highly expressed by total CD4+ T cells after either acute or chronic LCMV infection (Fig. 1B). To determine whether IL-21 production by virus-specific CD4+ T cells was elevated during chronic infection, we transferred LCMV-specific CD4+ T cell receptor (TCR) transgenic T cells (SMARTA), which recognize LCMV-derived peptide GP61–80 in the context of I-Ab, into C57BL/6 mice and then infected the mice with LCMV-Arm or LCMV–Cl 13. Virus-specific CD4+ T cells from LCMV–Cl 13–infected mice exhibited a greater than 11-fold increase in IL-21 mRNA expression as compared with that in CD4+ T cells from LCMV-Arm–infected mice, and the increase was sustained through chronic infection (Fig. 1B). Depletion of CD4+ T cells reduced IL-21 mRNA expression to that observed in uninfected mice (Fig. 1A), indicating that CD4+ T cells are probably the main source of IL-21 during chronic LCMV infection. The modest increase in IL-21 mRNA by total CD4+ T cells during chronic infection as compared with the substantial increase in IL-21 by virus-specific CD4+ T cells is probably due to the decreased frequency of virus-specific CD4+ T cells during chronic infection (4). Whereas the ability of virus-specific CD4+ T cells to produce IL-2 was rapidly extinguished after LCMV–Cl 13 infection, many virus-specific CD4+ T cells produced IL-21 throughout persistent infection, resulting in an inversion from a predominantly IL-2–producing to an IL-21–producing cell population (Fig. 1C and fig. S2). Thus, although the expression of many cytokines by virus-specific CD4+ T cells is decreased during chronic infection, IL-21 is maintained.
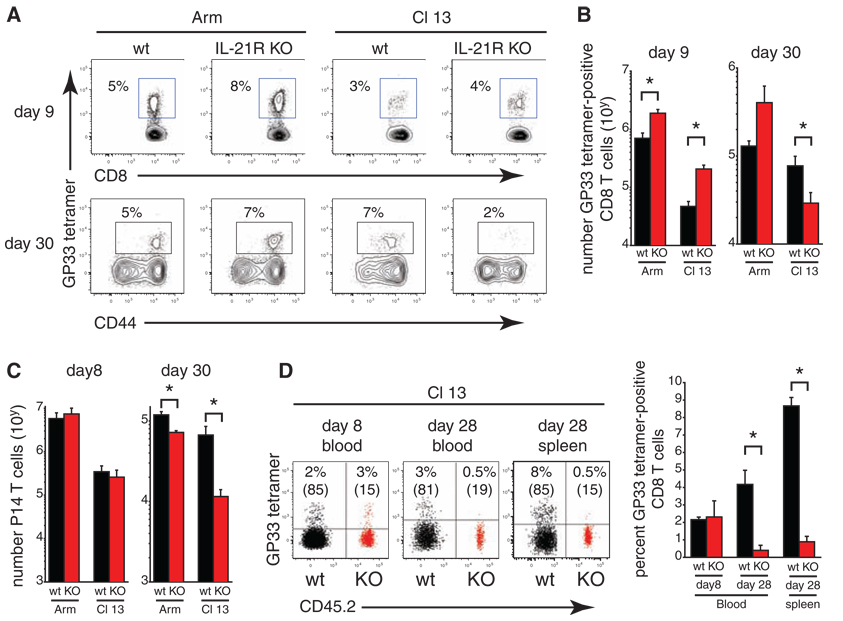
To determine the effect of IL-21 on antiviral CD8+ T cell immunity, we infected wild-type (WT) C57BL/6 mice and IL-21 receptor knock-out (Il21r−/−) mice (23) with LCMV-Armor –Cl 13. In both WT and Il21r−/− mice, LCMV-Arm infection stimulated robust virus-specific CD8+ T cell expansion and production of IFN-γ and TNF-α (fig. S3). Early after LCMV–Cl 13 infection (day 9), the extent of CD8+ T cell exhaustion was similar in WT and Il21r−/− mice (fig. S3). The lack of IL-21 signaling during the chronic phase of Cl 13 infection (day 30), however, resulted in a dramatic decrease of IFN-γ and IFN-γ/TNF-α dual-producing CD8 T cells (fig. S3). To determine whether the reduction in cytokine-producing CD8+ T cells during chronic infection was due to enhanced functional exhaustion or physical deletion, we used LCMV-GP33–41 major histocompatability complex (MHC) class I tetramers to directly enumerate virus-specific T cells. Both the frequency and number of Il21r−/− tetramer+ CD8+ T cells were increased relative to WT mice early (day 9) after LCMV–Cl 13 infection; however, they were significantly diminished in Il21r−/− mice as compared with WT mice as chronic infection progressed (day 30) (Fig. 2, A and B). These results suggested that IL-21 is required to sustain virus-specific CD8+ T cells as chronic infection progressed.
Fig. 2.
IL-21 sustains virus-specific CD8+ T cells during viral persistence. (A) LCMV-GP33–41 MHC class I tetramer staining on days 9 or 30 after LCMV-Arm or LCMV–Cl 13 infection. Numbers indicate the frequency of tetramer+ CD8+ T cells in WT and Il21r−/− (KO) mice. (B) Number of tetramer+ CD8+ T cells on days 9 and 30 after LCMV-Arm or LCMV–Cl 13 infection of WT and Il21r−/− mice. (C) Number of WT or Il21r−/− P14 T cells after infection of Il21r+/+ mice. (D) Frequency of WT and Il21r−/− LCMV-GP33–41 tetramer+ CD8+ T cells in bone-marrow chimeric mice on days 8 and 28 in the blood and on day 28 in the spleen after LCMV–Cl 13 infection. Numbers in parentheses indicate the total proportion of WT and Il21r−/− CD8+ T cells. Bar graphs represent the average ± SD of 3 to 5 mice per group and represent 2 to 4 independent experiments. *P<0.05.
Using two approaches, we next assessed whether IL-21–mediated help was directly acting on virus-specific CD8+ T cells. We performed the first approach by transferring WT or Il21r−/− LCMV-specific CD8+ TCR transgenic P14 cells, which recognize LCMV-peptide GP33–41 in the context of H-2Kb, into separate Il21r+/+ mice. We observed comparable functional and numerical responses after LCMV-Arm infection and in the early phase (day 8) of LCMV–Cl 13 infection (Fig. 2C and fig. S4) similar to that in Il21r−/− mice. In contrast, Il21r−/− P14 T cells exhibited enhanced functional exhaustion and deletion as compared with WT P14 T cells as chronic infection progressed (day 30) (Fig. 2C and fig. S4). In the second approach, irradiated WT mice were reconstituted with a mixture of WT (Ly5.1+) and Il21r−/− (Ly5.2+) bone marrow, allowing for both WT and Il21r−/− virus-specific CD8+ T cells to be exposed to identical inflammatory signals and cell interactions. We observed a similar frequency of LCMV-GP33–41–specific tetramer+ CD8+ T cells on day 8 after LCMV–Cl 13 infection; however, although the frequency of WT bone marrow–derived tetramer+ cells was maintained as the infection progressed, Il21r−/− bone marrow–derived virus-specific CD8+ T cells were deleted (Fig. 2D).We did not observe deletion of Il21r−/− bone marrow–derived tetramer+ CD8+ T cells after LCMV-Arm infection (fig. S5). After LCMV-Arm or LCMV–Cl 13 infection of the bone-marrow chimeric mice, the ratio of WT to Il21r−/− CD8+ T cells rapidly decreased from 60:40 before infection to 80:20 by day 8 after infection that was then stably maintained (fig. S5), suggesting a growth advantage for IL-21R–expressing total CD8+ T cells during viral infection. Any growth advantage of total IL-21R–expressing CD8+ T cells did not affect the frequency of tetramer+ CD8+ T cells in response to LCMV-Arm infection (fig. S5) and occurred before the loss of Il21r−/− bone marrow–derived tetramer+ cells during LCMV–Cl 13 infection (Fig. 2D and fig. S4). Thus, IL-21 directly sustains virus-specific CD8+ T cells during chronic infection.
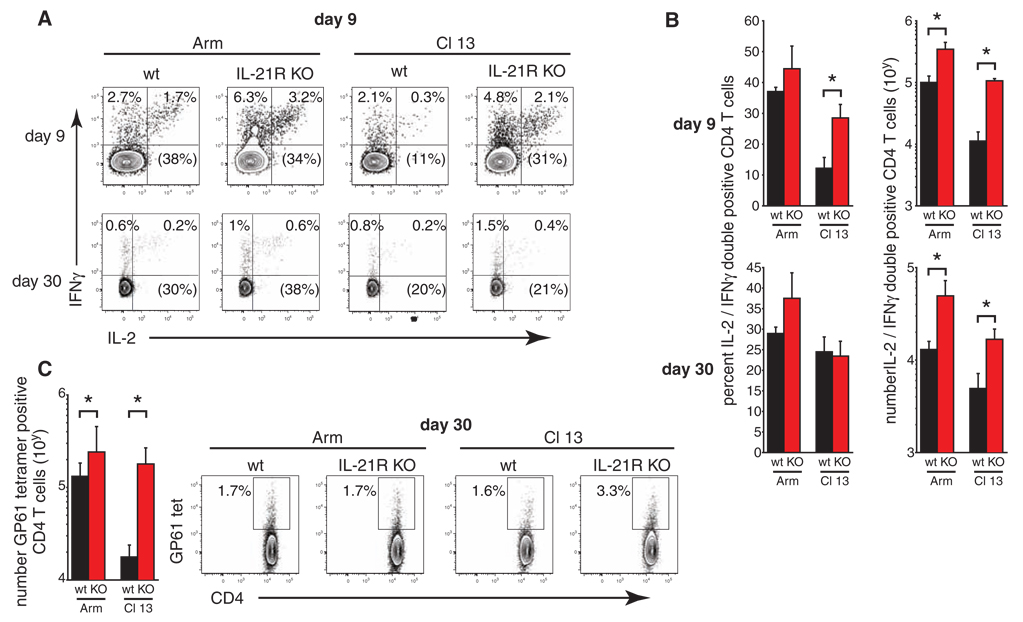
Although IL-21 is produced by CD4+ T cells, how it regulates CD4+ T cell function in the context of an in vivo viral infection is unclear. We observed increased amounts of IFN-γ–producing and LCMV-GP61–80–specific MHC class II tetramer+ CD4+ T cells in Il21r−/− as compared with WT mice at days 9 and 30 after LCMV-Arm or –Cl 13 infection (Fig. 3, A to C). Furthermore, we observed rapid suppression of IL-2 and TNF-α after LCMV–Cl 13 infection in WT mice (Fig. 3, A and B, and fig. S5). TNF-α expression was also rapidly diminished in Il21r−/− mice after LCMV–Cl 13 infection (fig. S6); however, unlike WT mice, virus-specific CD4+ T cells continued to produce IL-2 in LCMV–Cl 13–infected Il21r−/− mice in quantities similar to those observed at day 9 after LCMV-Arm infection (Fig. 3, A and B). These findings indicate that IL-21 signaling results in decreased IL-2 production by virus-specific CD4+ T cells. The elevated frequencies of virus-specific CD4+ T cells producing IL-2 observed early during LCMV–Cl 13 infection in Il21r−/− mice declined as the infection progressed and by 30 days after infection was similar to frequencies observed in WT mice (Fig. 3, A and B). The total number of IL-2–expressing virus-specific CD4+ T cells, however, remained significantly increased in Il21r−/− mice after LCMV-Arm and particularly LCMV–Cl 13 infection, as compared with that in WT mice (Fig. 3B). Thus, IL-21 suppresses distinct facets of CD4+ T cell function during viral persistence, such as early IL-2 expression, whereas other facets, such as TNF-α expression, are not affected.
Fig. 3.
IL-21 suppresses CD4+ T cell function. (A) LCMV-GP61–80–specific CD4+ T cell responses on days 9 and 30 after LCMV-Arm or LCMV–Cl 13 infection of WT or Il21r−/− mice. Numbers in each quadrant indicate the frequency of IFN-γ+ and IL-2+ CD4+ T cells and the numbers in parentheses indicate the frequency of IFN-γ and IL-2 double-positive cells. (B) The frequency and number of CD4+ T cells that simultaneously produce IL-2 and IFN-γ on days 9 or 30 after LCMV-Arm or LCMV–Cl 13 infection. (C) Frequency of LCMV-GP61–80 tetramer+ CD4+ T on day 30 after LCMV-Arm or LCMV–Cl 13 infection. The bar graph represents the number of tetramer+ CD4+ T cells. Bar graphs summarize the average ± SD of 3 to 4 mice per group and 3 to 4 independent experiments. *P < 0.05.
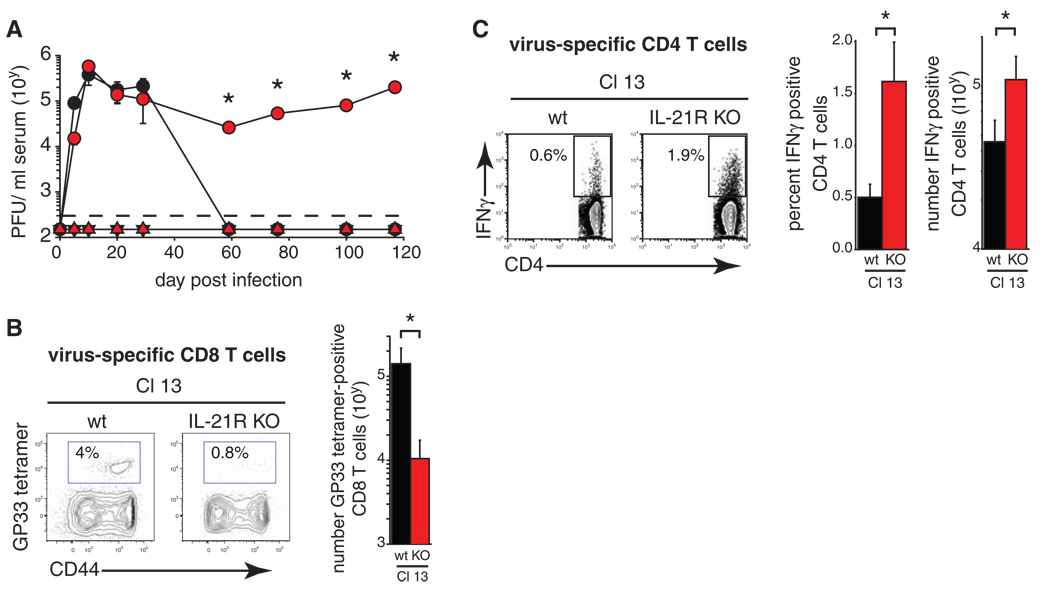
CD8 T+ cells are required to resolve LCMV–Cl 13 infection (5), thus the reduced numbers of virus-specific CD8+ T cells in Il21r−/− mice suggested that they may be insufficient to resolve the infection. The initial generation of productive T cell responses led to acute clearance of LCMV-Arm infection in WT and Il21r−/− mice (Fig. 4A). In contrast, the rapid T cell exhaustion after LCMV–Cl 13 infection led to chronic infection in WT and Il21r−/− mice (Fig. 4A). Consistent with the similar generation of virus-specific CD8+ T cell responses after infection, LCMV–Cl 13 titers were initially equal in WT and Il21r−/− mice; however, whereas infection in WT mice resolved after two months, Il21r−/− mice did not resolve the infection (Fig. 4A). In the presence of chronic infection, virus-specific Il21r−/− CD8+ T cell responses continued to decline as compared with that in WT mice, whereas no further decrease was observed in WT mice over this same time period (Fig. 2D and Fig. 4B). Conversely, the number of IFN-γ+ (virus-specific) CD4+ T cells remained elevated 100 days after LCMV-Arm (fig. S7) and –Cl 13 infection in Il21r−/− mice (Fig. 4C). Thus, despite the increased presence of virus-specific CD4+ T cells in Il21r−/− mice, CD8+ T cell responses are not sustained and the infection is not resolved.
Fig. 4.
IL-21 is required to purge chronic viral infection. (A) Serum viral titers after LCMV-Arm (triangles) or LCMV–Cl 13 (circles) infection of WT (black) or Il21r−/− (red) mice. Data are expressed as plaque-forming units (PFU) per milliliter of serum. The dashed line indicates the lower limit of detection (200 PFU/ml). *P < 0.05 for LCMV–Cl 13 infection of WT versus Il21r−/− mice. (B and C) The frequency and number of (B) LCMV-GP33–41 tetramer+ CD8+ T cells and (C) IFN-γ+ CD4+ T cells on day 100 after LCMV–Cl 13 infection of WT and Il21r−/− mice. Time points and bar graphs represent the average ± SD of 3 to 4 mice per group and 3 independent experiments. *P < 0.05.
IL-21 signaling also affects antibody production by B cells and antigen-presenting cell responses (19), which in addition to CD8+ T cell deletion could contribute to the failure to resolve infection. Compared with that in WT mice, antigen-presenting cell maturation was not differentially affected in Il21r−/− mice after LCMV-Arm or –Cl 13 infection and by some measurements was increased in the absence of IL-21 signaling (fig. S8). Furthermore, the total number of B cells was similar in WT and Il21r−/− mice during chronic infection (fig. S9).We observed a 1.9-fold decrease in LCMV-specific immunoglobulin G antibody titers in Il21r−/− mice, although substantial antibody titers were observed in both WT and Il21r−/− mice (fig. S9). LCMV-neutralizing antibody titers were undetectable in both WT and Il21r−/− mice at day 30 after LCMV-Cl 13 infection. Together, these data suggest that antigen-presenting cells impairment or impaired humoral immunity are unlikely to underlie the reduced control of chronic infection in Il21r−/− mice.
The requirement for CD4+ T cell help to control chronic viral infection has long been established (5, 6); however, because CD4+ T cells rapidly lose the ability to produce traditional helper cytokines such as IL-2, the specific mechanisms and factors that comprise CD4+ T cell help have remained elusive. Our results suggest that CD4+ T cells do not necessarily “exhaust” or “lose” function during chronic viral infection. Instead, we propose that CD4+ T cell function is diverted toward the production of factors such as IL-21 that sustain effector activity to control infection. Multiple effector mechanisms probably contribute to the long-term development of CD8+ T cell responses. In combination with (or in the absence of) other yet unidentified helper factors, IL-21 maintains the CD8+ T cell effector activity required to control infection and thus provides a mechanism for CD4+ T cell help in response to chronic viral infection. Failure of CD4+ T cell help is associated with the inability to acutely clear HCV infection and with the progression to AIDS after HIV infection (8–12). Thus, loss of IL-21 as CD4+ T cell responses decline may hinder control of these human chronic viral infections. Further identification of helper factors and how they regulate precise immune outcomes will provide valuable insight into the generation and maintenance of antiviral immunity to prevent and treat chronic viral infections.
Supplementary Material
References and Notes
- 1.Zajac AJ, et al. J. Exp. Med. 1998;188:2205. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallimore A, et al. J. Exp. Med. 1998;187:1383. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. J. Virol. 2003;77:4911. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks DG, Teyton L, Oldstone MB, McGavern DB. J. Virol. 2005;79:10514. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battegay M, et al. J. Virol. 1994;68:4700. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matloubian M, Concepcion RJ, Ahmed R. J. Virol. 1994;68:8056. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ou R, Zhou S, Huang L, Moskophidis D. J. Virol. 2001;75:8407. doi: 10.1128/JVI.75.18.8407-8423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerlach JT, et al. Gastroenterology. 1999;117:933. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 9.Thimme R, et al. J. Exp. Med. 2001;194:1395. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grakoui A, et al. Science. 2003;302:659. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 11.Smyk-Pearson S, et al. J. Virol. 2008;82:1827. doi: 10.1128/JVI.01581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klenerman P, Hill A. Nat. Immunol. 2005;6:873. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 13.Fuller MJ, Zajac AJ. J. Immunol. 2003;170:477. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 14.Materials and methods are available as supporting material on Science Online
- 15.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. J. Exp. Med. 1984;160:521. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber DL, et al. Nature. 2006;439:682. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 17.Brooks DG, et al. Nat. Med. 2006;12:1301. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ejrnaes M, et al. J. Exp. Med. 2006;203:2461. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spolski R, Leonard WJ. Annu. Rev. Immunol. 2008;26:57. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 20.Parrish-Novak J, et al. Nature. 2000;408:57. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 21.Holm C, Nyvold CG, Paludan SR, Thomsen AR, Hokland M. Cytokine. 2006;33:41. doi: 10.1016/j.cyto.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Coquet JM, et al. J. Immunol. 2007;178:2827. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 23.Ozaki K, et al. Science. 2002;298:1630. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 24.We thank M. Oldstone for his generous support (AI09484). Our work was supported by the UCLA Center for AIDS Research, the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA, and NIH grants AI077012, AI082975 (to D.G.B.), and AI070845 (to K.S.).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.