Abstract
Evidence is presented for two natural transcription initiators positioned internally in the histidine operon of Salmonella typhimurium. They were detected using highly polar S. typhimurium his mutants as recipients for F′ Escherichia coli his episomes. These intergeneric complementation tests provide a sensitive method for the detection of other initiators and of terminators.
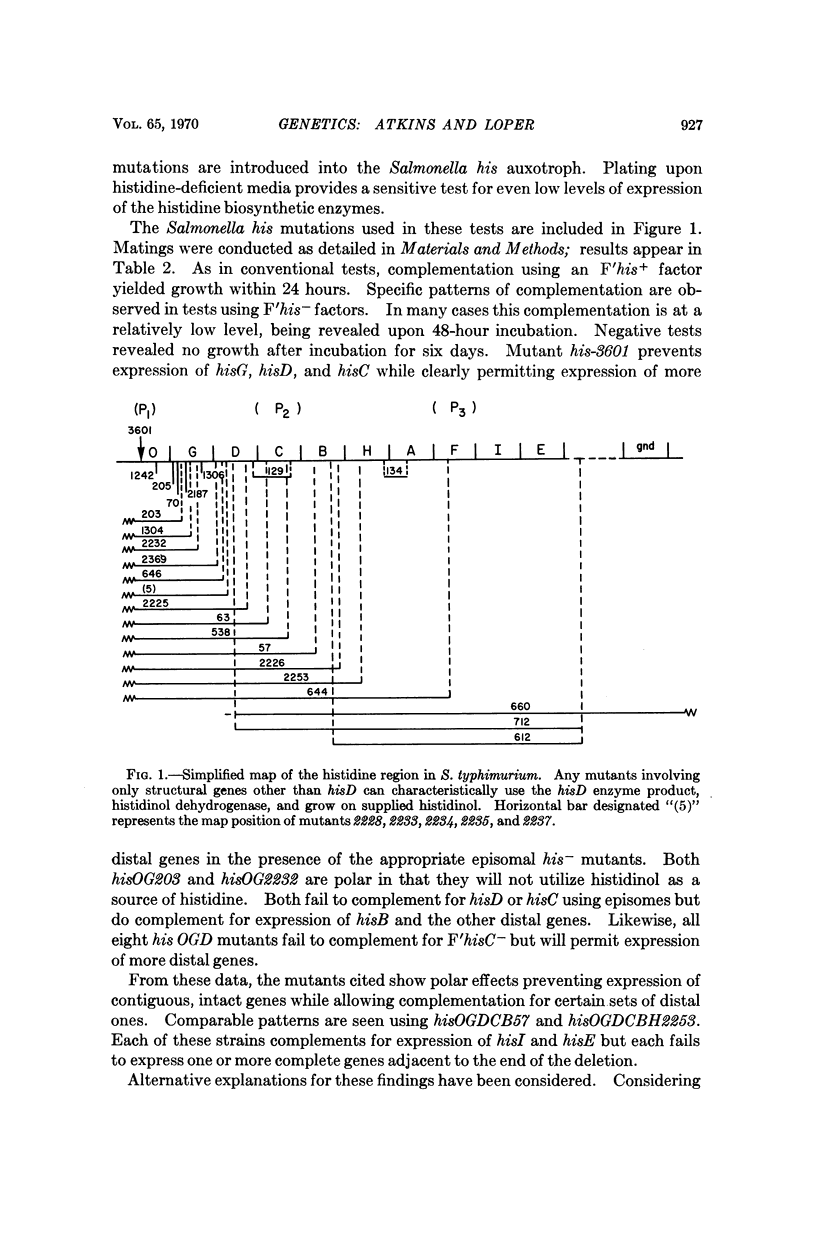
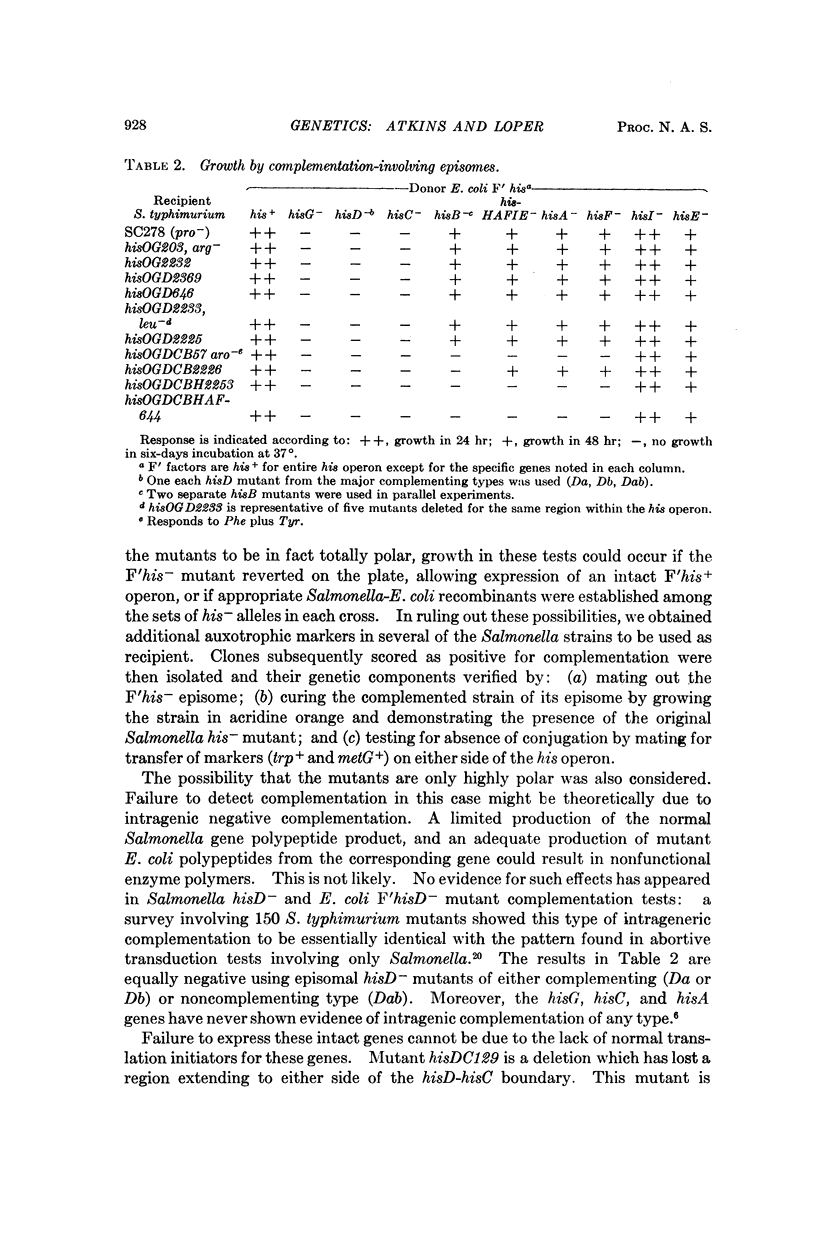
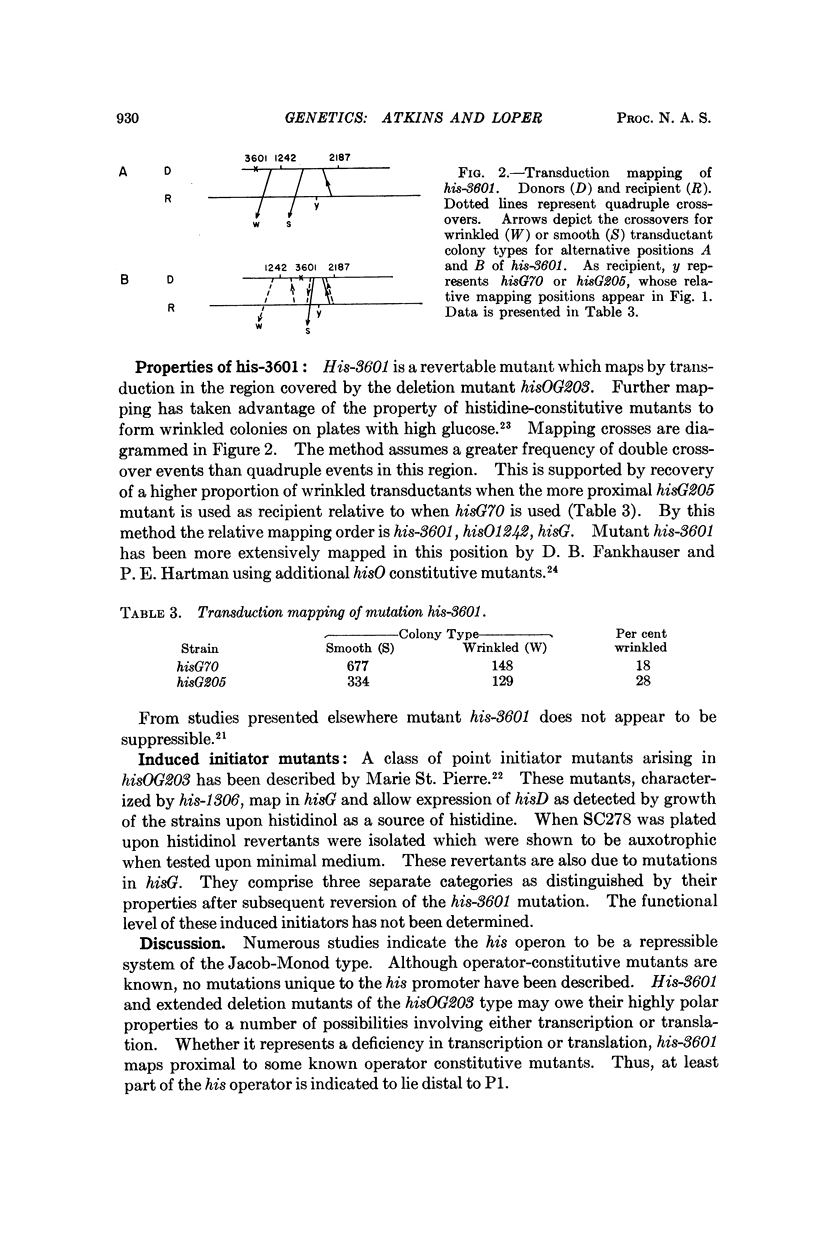
That at least part of the operator for the his operon lies distal to the transcription initiator is suggested by transduction analysis of a new highly polar mutant described in this paper.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., GARRY B., HERZENBERG L. A. The genetic control of the enzymes of histidine biosynthesis in Salmonella typhimurium. J Gen Microbiol. 1960 Apr;22:369–378. doi: 10.1099/00221287-22-2-369. [DOI] [PubMed] [Google Scholar]
- Berberich M. A., Kovach J. S., Goldberger R. F. Chain initiation in a polycistronic message: sequential versus simultaneous derepression of the enzymes for histidine biosynthesis in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1857–1864. doi: 10.1073/pnas.57.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz D., Hushon J. M., Whitfield H. J., Jr, Roth J., Ames B. N. Procedure for identifying nonsense mutations. J Bacteriol. 1968 Jul;96(1):215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G. R., Martin R. G. Translation and polarity in the histidine operon. II. Polarity in the histidine operon. J Mol Biol. 1967 Nov 28;30(1):97–107. doi: 10.1016/0022-2836(67)90246-x. [DOI] [PubMed] [Google Scholar]
- HARTMAN P. E., HARTMAN Z., SERMAN D. Complementation mapping by abortive transduction of histidine requiring Salmonella mutants. J Gen Microbiol. 1960 Apr;22:354–368. doi: 10.1099/00221287-22-2-354. [DOI] [PubMed] [Google Scholar]
- HARTMAN P. E., LOPER J. C., SERMAN D. Fine structure mapping by complete transduction between histidine-requiring Salmonella mutants. J Gen Microbiol. 1960 Apr;22:323–353. doi: 10.1099/00221287-22-2-323. [DOI] [PubMed] [Google Scholar]
- Kovach J. S., Berberich M. A., Venetianer P., Goldberger R. F. Repression of the histidine operon: effect of the first enzyme on the kinetics of repression. J Bacteriol. 1969 Mar;97(3):1283–1290. doi: 10.1128/jb.97.3.1283-1290.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolies M. N., Goldberger R. F. Isolation of the fourth (isomerase) of histidine biosynthesis from Salmonella typhimurium. J Biol Chem. 1966 Jul 25;241(14):3262–3269. [PubMed] [Google Scholar]
- Margolin P., Bauerle R. H. Determinants for regulation and initiation of expression of tryptophan genes. Cold Spring Harb Symp Quant Biol. 1966;31:311–320. doi: 10.1101/sqb.1966.031.01.041. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. A transcription-initiating mutation within a structural gene of the tryptophan operon. J Mol Biol. 1969 May 14;41(3):317–328. doi: 10.1016/0022-2836(69)90278-2. [DOI] [PubMed] [Google Scholar]
- Riyasaty S., Atkins J. F. External suppression of a frameshift mutant in salmonella. J Mol Biol. 1968 Jun 28;34(3):541–557. doi: 10.1016/0022-2836(68)90179-4. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Antón D. N., Hartman P. E. Histidine regulatory mutants in Salmonella typhimurium. I. Isolation and general properties. J Mol Biol. 1966 Dec 28;22(2):305–323. doi: 10.1016/0022-2836(66)90134-3. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Levine M. A phage P22 gene controlling integration of prophage. Virology. 1967 Feb;31(2):207–216. doi: 10.1016/0042-6822(67)90164-x. [DOI] [PubMed] [Google Scholar]
- Venetianer P., Berberich M. A., Goldberger R. F. Studies on the size of the messenger-RNA transcribed from the histidine operon during simultaneous and sequential depression. Biochim Biophys Acta. 1968 Aug 23;166(1):124–133. doi: 10.1016/0005-2787(68)90496-6. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D. Hybrids of Escherichia and Salmonella. Science. 1960 Mar 18;131(3403):813–815. doi: 10.1126/science.131.3403.813. [DOI] [PubMed] [Google Scholar]


