Summary
Extensive efforts have been made to determine the status on neural progenitor cell proliferation in specific pathological conditions and to evaluate the therapeutic efficacy of drugs for preventing neurogenic deficits in neurodegenerative diseases. However, the most commonly used stereological analysis using 5-bromo-2′-deoxyuridine (BrdU) immuno-positive sections is a time consuming and labor intensive process and is often a bottle neck in neurogenic drug development, particularly when large sample sizes are needed. In addition, BrdU is toxic to new born neurons and also labels DNA damage in old cells. In this study, we established a method that quantitatively measures the number of Ki-67, an endogenous cell proliferation marker, positive cells by flow cytometry which analyzes extracted cell nuclei from rodent hippocampi in suspension. Our results demonstrate that this approach can be applied to a large number of rodent samples, can be accomplished in a short period of time (1-3 days), and can be completed in a more accurately objective manner than by using 3-D cell counting with immunohistochemically processed sections.
Keywords: Neurogenesis, high throughput screen, hippocampus, flow cytometry, Ki-67
1. Introduction
The adult brain has two stable regions of mitotic activity, the subventricular zone (SVZ) of the lateral ventricle in the frontal cortex and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus (1,2). Active neurogenesis in hippocampi lead to the incorporation of thousands of new granule cells into the dentate gyrus every day (3). While the regenerative potential of the mammalian brain is sustained throughout the life span, the magnitude of the proliferative efficacy of neural progenitors declines with age and diseases, such as Alzheimer's disease (AD) (4-7). Therefore, to reverse and/or to prevent from neurogenic deficits becomes a potential therapeutic strategy for anti-neurodegenerative diseases, including AD. For example, extensive efforts have been made to experimentally evaluate the efficiency of potential neurogenic enhancers using transgenic mouse AD models (8-11).
The most common method for in vivo analysis of neurogenesis is the unbiased stereology of 5-bromo-2′-deoxyuridine (BrdU) immunohistochemical labeled serial brain sections under microscopy, which are both labor and time extensive. In addition, stereological analysis uses the optical fractionator (12), a combination of optical dissector with statistically optimized spatial sampling protocols, where the estimates are obtained from cell densities, must be restricted to well defined structures of isotropic architecture and measurable volume (12). Moreover, the actual positive cell numbers are achieved by multiplying cell density by volume, which is determined by precisely drawing the structural boundary and accurately estimating the tissue volume change during section preparation. The numbers obtained are not independent variables and therefore are limited statistically to compare against volume (13). Thus, extreme importance is placed on establishing a high throughput evaluation for in vivo neurogenic efficiency screening that can be completed in a short time and used for a large sample size. Of more objective importance in particular, is the development of potential neurogenic drugs.
The thymidine analog, BrdU, is a commonly used molecule to measure cell proliferation in different tissues, including the CNS, based on the stable incorporation occurring in S-phase of the cell cycle (3). However, BrdU is toxic to newborn neurons and triggers cell death by altering DNA stability and lengthening the cell cycle. Additionally BrdU has various mitogenic, transcriptional, and translational effects on cells that incorporate the nucleoside (14). Therefore, difficulty is found in giving a clear interpretation of the 5 times less amount of BrdU positive cells in mice 21 days after BrdU injection than that detected 24 hours after BrdU injection (11,15). In determining whether the apoptosis of the newly formed cells is a natural phenomenon or is triggered by the BrdU incorporation, a recent in vitro study, in which human neural progenitor cells were used, reported that BrdU doses in the concentration range that is recommended for cell proliferation studies (1-10 μM) interfered with the survival of newborn neurons (BrdU/TuJ1+ cells), and high doses of BrdU activated the classical apoptosis pathways in newly formed neurons (16). When administered to pregnant mice and rats, BrdU interfered with embryonic brain development, caused bodily defects in embryos, and caused postnatal behavioral abnormalities (17). In addition, BrdU is not only a marker of the S-phase of the cell cycle but is also a marker of DNA synthesis, including DNA repair, and that, on the other hand, may induce a false positive. Therefore, importance is placed on using a less toxic and efficient molecule, ideally an endogenous marker, to probe neurogenesis in establishing a high throughput screen.
In this study, we reported a high throughput flow cytometric assay to evaluate the newly formed cells in rodent hippocampi within different conditions. The assay analyzed immunolabeled fluorescent Ki-67, an endogenous protein only expressed in active cell cycles (18-21), positive cells in homogeneous, isotropic suspensions. Hippocampi were first dissected from fixed brain hemispheres. The isotropic nuclear suspensions were then extracted, and immuno-labeling of the newly formed cells by cell proliferation marker Ki-67 was completed. Finally the positive fluorescent cells were analyzed by flow cytometry.
2. Materials and Methods
2.1. Animal
Ovariectomy reduction of hippocampal neurogenesis was demonstrated, and estradiol reversed this decrease (22,23). Therefore, we used female ovariectomized (OVX) mice to evaluate our methods. Female C57/B6 mice were purchased from Harlan Laboratories, Indianapolis, IN). Animals were ovariectomized and the estradiol injection was initiated 5 days after OVX in a dose of 30 μg/kg body weight once/day for 5 days. All experiments were conformed to the Animal Welfare Act, Guide to Use and Care of Laboratory Animals, and the U.S. Government Principles of the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training guidelines on the ethical use of animals. In addition, the minimal number of required animals was used for these experiments, and pain was minimized.
2.2. Tissue dissection
Mice (6/group) were anesthetized with 100mg/kg ketamine and 10 mg/kg xylazine. Cardiac perfusion was performed with saline. This flash perfusion removed the blood cells in the brain and eliminated the contribution of the dividing white blood cells. The decapitation and brain dissection were followed by an immersion fixation with 4% buffered paraformaldehyade in PBS for 16 h. After post fixation, the hippocampus was extracted using consistent anatomical landmarks as criteria for dissection as described by Bilsland and colleagues (24). The rostral 1/3 of the hippocampal lobe was removed to avoid the contribution from subventricular zone and rostral migratory stream proliferative pools.
2.3. Nuclei extraction and immunolabeling
Samples were homogenized using next advance 24 sample homogenizer. Hippocampi were minced into a 1.5 mL heavy duty microcentrifuge tube in phosphate buffered saline (PBS) that was 5 times the hippocampi weight and a bead (ZrSiO) amount that was 1 times the hippocampi weight. The samples were then homogenized for 3 min on speed 7. When performed on fixed tissue, this procedure lyses the plasmalemma but preserves the nuclear envelope intact. The cell sample was collected into a regular 1.5 mL microcentrifuge tube by washing the beads and tube 4 times using 200 μL of PBS. The cells were then centrifuged for 10 min at 10,000 rpm. Once all of the nuclei were collected in a pellet, the supernatant was discarded. The pellet was then re-suspended in 600 μL of PBS plus 0.5% Triton X-100. The number of nuclear density was estimated by counting the propidium iodide (PI), a fluorescent molecule that stoichiometrically binds to DNA by intercalating between the bases with no sequence preference, positive particles. Aliquots of 50 μL are used for immunolabeling with Ki-67, a proliferating marker. Nuclei in the aliquot are collected by centrifugation, resuspended in 200 μL of a 0.2 M solution of boric acid, pH 9.0, and heated for 1 h at 75°C for epitope retrieval. Subsequently, nuclei are again collected by centrifugation, washed in PBS, and incubated for 24 h at 4°C with primary antibodies (1:500 for polyclonal anti-Ki-67, abcam, ab15580). After being washed in PBS (2 times for 5 min at 5,000 rpm), nuclei are incubated in CY2-conjugated goat anti-rabbit IgG secondary antibody (1:100 in PBS; Jackson Immuno Reasearch Labs, Inc.) for 2 h, collected by centrifugation, washed in PBS 2 times, and then suspended in a small volume of PBS. Each of 2.5 μL cell suspension stained with PI or without PI was checked under fluorescent microscope to verify the immunolabeling quality. The remainder of cell suspension is diluted to 500 μL and sent for flow cytometry assay using Beckman FC 500 System with CXP Software. To avoid counting bias, we register the presence or absence of Ki-67 immunoreactivity for all of the PI-stained nuclei samples until 10,000 PI-stained nuclei have been examined.
2.4. Flow cytometry protocol
Propidium iodide (PI) cells were first gated on a histogram; the positive cells were visualized on a forward/side scatter plot. PI cells were ‘back-gated’ on the forward/side scatter plot to eliminate debris prior to analysis; this also eliminated auto-fluorescence of the sample. An analysis plot was generated with CY2 fluorescence on the Y-axis and PI fluorescence on the X-axis. Gates were always set using dissociates with cell aliquots lacking the first antibody but having been incubated with second antibody and then processed alongside the experimental procedure. Ten thousand PI expressing cells were analyzed for Ki-67 expressing cells. Data were expressed as total positive cells per hippocampus.
2.5. Statistical assay
The statistically significant differences were determined by a one-way ANOVA followed by a post-hoc t-test (two sample assuming equal variance).
3. Results and Discussion
The expression of the human Ki-67 protein is strictly associated with cell proliferation (20,21). During interphase, the Ki-67 antigen can be exclusively detected within the cell nucleus whereas in mitosis most of the protein is relocated to the surface of the chromosomes. Therefore, Ki-67 protein can be immunohistochemically detected during all active phases of the cell cycle (G1, S, G2, and mitosis), which excludes the resting phase (G0). Ki-67 is now accepted as a cellular marker for proliferation (20,21) and neurogenesis (19,25).
In this study, nuclei extracted from mouse hippocampi were immuno-labeled by antibodies specific for Ki-67 and visualized with secondary antibodies conjugated with CY2 as required by the primary antibody. The results demonstrated an exclusive localization of Ki-67 in the nuclei of mouse hippocampal proliferating cells (Figure 1).
Figure 1. Immuno-labeling.
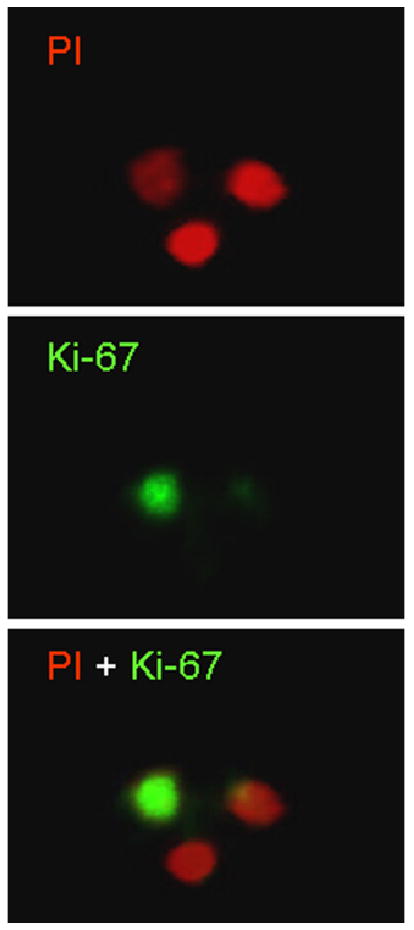
Nuclei extracted from mouse hippocampi were immuno-labeled by antibodies specific for Ki-67 and visualized with secondary antibodies conjugated with CY2 (green, middle panel). The nuclei were counterstained with Propidium Iodide (PI, red, up panel). The merged image showed in the low panel.
When the immunolabeled cells are subjected to flow cytometry, the CY2 positive Ki-67 cells can be gated and counted. Examples of flow cytometry profiles are presented in Figure 2. The x-axis represents the intensity of Propidium Iodide (PI), and the y-axis represents the intensity of CY2-Ki-67. Therefore, area K2 represents the Ki-67 and PI double positive nuclei of newly formed cells. The K4 area represents PI only positive cells which are not newly formed cells. The K3 area contains the cell debris that are double CY2 and PI negative (Figure 2).
Figure 2. Examples of flow cytometry profiles.
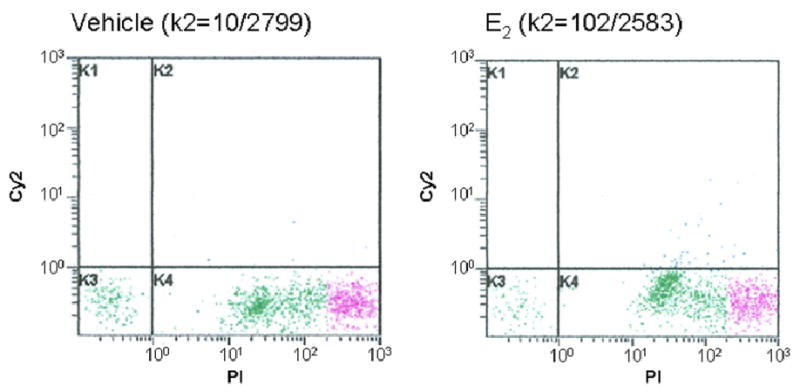
The X axis represents the intensity of Propidium Iodide (PI), and the Y axis represents the intensity of CY2-Ki-67. Area K2 represents the Ki-67 positive and PI positive nuclei of newly formed cells. The K4 area represents only PI positive cells which are not newly formed cells. The K3 area contains the cell debris which is double CY2 and PI negative. The different color in K4 presents different gate areas.
The results of the flow cytometry counting demonstrate a significant decrease of Ki-67 positive cells in OVX mice hippocampi, from 17,350 ± 4,968 to 3,238 ± 1,628, (Figure 3, n = 6 per group, p < 0.001). The positive Ki-67 number in sham OVX mice hippocampi is comparable with the previous reports that there are about 9,000 BrdU positive cells in rodent hippocampi per day (3,26,27), considering that BrdU only labels the s-phase, while Ki-67 is expressed in all the active cycle (G1, S, G2, and M phase). In the E2 treated OVX mice, the positive Ki-67 cells were 26,129 ± 9,683. These results demonstrate that E2 reverses cell proliferation deficits in OVX mice to a level that is compatible to that of the Sham-OVX mice (p = 0.136, n = 6) which is also supported by previous studies (22,23). Our results are also highly comparable with the results reported from other group by immunohistochemical analysis of BrdU positive cells in hippocampus of mice which were injected BrdU once a day for 4 days, which showing ∼5,000 positive BrdU cells per hippocampus in mice which were given saline and ∼17,000 BrdU positive cells per hippocampus in fluoxetine, an anti-depressant of the selective serotonin reuptake inhibitor which showed neurogenic effect, treatment mice (28).
Figure 3. Estradiol-17β (E2) reverses OVX-induced deficits of hippocampal neuroprogenitor cell proliferation.
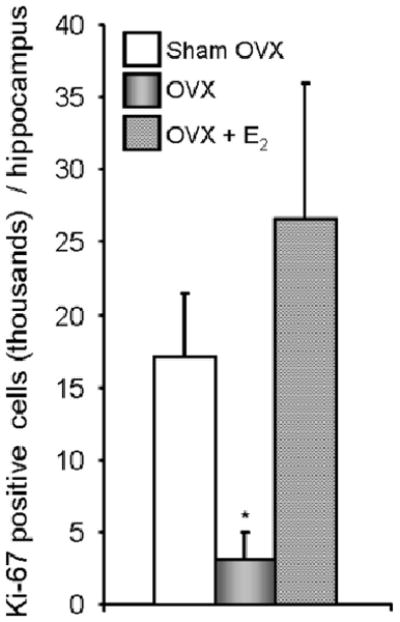
Ki-67 positive cells in mice hippocampi were sorted by flow cytometry. Data were presented as mean ± STD. * p < 0.01 of OVX vs. sham OVX and OVX + E2.
The decrease of proliferating cells in OVX mice hippocampi vs. that in sham OVX mice along with the increase of Ki-67 positive cells (Figure 3) in Estrogen treated OVX mice are supported by the previous demonstration that the lack of ovarian hormones reduces hippocampal neural progenitor cell proliferation. The demonstration also supported neural progenitor cell proliferation being enhanced with estradiol replacement (22,23,29).
Hippocampal neurogenesis has now been used as an important indicator for drug development in Alzheimer's disease (10,30,31). In addition, accumulated data demonstrated that neurogenic deficits in the hippocampal dentate gyrus is the neural basis for a number of mental disorders, including depression, schizophrenia, epilepsy, and diabetes (32,33). The time consuming and labor intensiveness of conventional methods of BrdU quantification are limiting factors for progress in drug development. The recently developed flow cytometry counting technique of immunocytochemically labeled BrdU nuclei in homogeneous suspensions make the BrdU positive cells counting more efficient and more objective (28). In parallel, we established that the method of flow cytometrically counting the Ki-67 positive cells will not only increase the efficacy and the objectivity but will also limit the side effect concerns of BrdU, a toxic and mutagenic substance which changes DNA stability and lengthens the cell cycle. In addition, BrdU is not only a marker in the S-phase of the cell cycle but is also a marker of DNA synthesis. Therefore, BrdU may also induce false positives in some disease conditions by showing active DNA repair activity (14).
Because Ki-67 is a protein expressed exclusively in the active cell cycle and in the nucleus (34). The co-localization of Ki-67 with nuclear neuronal markers (NeuN, calbindin) is impossible (35), because they are expressed at different period of the cell cycle. The known, so-called early neuronal markers, such as doublecordtin, Tuj1, the polysialylated form of the neural cell adhesion molecule, are all post-mitotic proteins and located in the cytoplasm of immature neurons (35). In parallel to these early neuronal markers, the known glial cell markers, including glial fibrillary acidic protein, are also cytoplasm proteins. So far, it is difficulty to perform a co-localization for ki-67 with a phenotype marker to identify the phenotype of Ki-67 positive cells using nuclei extracted from fixed brain tissue by flow cytometry analysis. Although this method has limitation for using ki-67 to trace the survival of the newly formed cells, this method generates results that reflect the real proliferation status of cells in hippocampus (6).
In conclusion, we have established a high throughput analytical method to evaluate the proliferation of neuroprogenitor cells within the rodent hippocampus by flow cytometry assay of Ki-67 positive cells. This method provides more accurate, more sensitive results which are closer to endogenous proliferation status than the traditional stereological method using BrdU as a probe, and is far less time-consuming neurogenic agent development.
Acknowledgments
This study was supported by a grant from Alzheimer's Association, a small grant from Public Health Service Grants P20 RR17701, an IRSP grant from UMMC.
References
- 1.Gage FH. Brain, repair yourself. Sci Am. 2003;289:46–53. doi: 10.1038/scientificamerican0903-46. [DOI] [PubMed] [Google Scholar]
- 2.Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 4.Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29:129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hattiangady B, Shuai B, Cai J, Coksaygan T, Rao MS, Shetty AK. Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging hippocampus. Stem Cells. 2007;25:2104–2117. doi: 10.1634/stemcells.2006-0726. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- 8.Brinton RD, Wang JM. Preclinical analyses of the therapeutic potential of allopregnanolone to promote neurogenesis in vitro and in vivo in transgenic mouse model of Alzheimer's disease. Curr Alzheimer Res. 2006;3:11–17. doi: 10.2174/156720506775697160. [DOI] [PubMed] [Google Scholar]
- 9.Wang JM, Irwin RW, Liu L, Chen S, Brinton RD. Regeneration in a degenerating brain: potential of allopregnanolone as a neuroregenerative agent. Curr Alzheimer Res. 2007;4:510–517. doi: 10.2174/156720507783018262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang JM, Liu L, Irwin RW, Chen S, Brinton RD. Regenerative potential of allopregnanolone. Brain Res Rev. 2008;57:398–409. doi: 10.1016/j.brainresrev.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Wang JM, Singh C, Liu L, Irwin WR, Chen S, Chung E, Thompson RF, Brinton RD. Allopregnanolone Reverses Neurogenic and Cognitive Deficits in Mouse Model of Alzheimer's Disease. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.1001422107. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 13.Harrison KH, Hof PR, Wang SS. Scaling laws in the mammalian neocortex: does form provide clues to function? J Neurocytol. 2002;31:289–298. doi: 10.1023/a:1024178127195. [DOI] [PubMed] [Google Scholar]
- 14.Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 16.Caldwell MA, He X, Svendsen CN. 5-Bromo-2′-deoxyuridine is selectively toxic to neuronal precursors in vitro. Eur J Neurosci. 2005;22:2965–2970. doi: 10.1111/j.1460-9568.2005.04504.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuwagata M, Ogawa T, Nagata T, Shioda S. The evaluation of early embryonic neurogenesis after exposure to the genotoxic agent 5-bromo-2′-deoxyuridine in mice. Neurotoxicology. 2007;28:780–789. doi: 10.1016/j.neuro.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Bullwinkel J, Baron-Luhr B, Ludemann A, Wohlenberg C, Gerdes J, Scholzen T. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol. 2006;206:624–635. doi: 10.1002/jcp.20494. [DOI] [PubMed] [Google Scholar]
- 19.Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 20.Scholzen T, Endl E, Wohlenberg C, van der Sar S, Cowell IG, Gerdes J, Singh PB. The Ki-67 protein interacts with members of the heterochromatin protein 1 (HP1) family: a potential role in the regulation of higher-order chromatin structure. J Pathol. 2002;196:135–144. doi: 10.1002/path.1016. [DOI] [PubMed] [Google Scholar]
- 21.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481:252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- 23.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bilsland JG, Haldon C, Goddard J, Oliver K, Murray F, Wheeldon A, Cumberbatch J, McAllister G, Munoz-Sanjuan I. A rapid method for the quantification of mouse hippocampal neurogenesis in vivo by flow cytometry. Validation with conventional and enhanced immunohistochemical methods. J Neurosci Methods. 2006;157:54–63. doi: 10.1016/j.jneumeth.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Wojtowicz JM, Kee N. BrdU assay for neurogenesis in rodents. Nat Protoc. 2006;1:1399–1405. doi: 10.1038/nprot.2006.224. [DOI] [PubMed] [Google Scholar]
- 26.Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev Neurosci. 2007;18:93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- 27.Jessberger S, Gage FH. Fate plasticity of adult hippocampal progenitors: biological relevance and therapeutic use. Trends Pharmacol Sci. 2009;30:61–65. doi: 10.1016/j.tips.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balu DT, Hodes GE, Hill TE, Ho N, Rahman Z, Bender CN, Ring RH, Dwyer JM, Rosenzweig-Lipson S, Hughes ZA, Schechter LE, Lucki I. Flow cytometric analysis of BrdU incorporation as a high-throughput method for measuring adult neurogenesis in the mouse. J Pharmacol Toxicol Methods. 2009;59:100–107. doi: 10.1016/j.vascn.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang JM, Liu L, Brinton RD. Estradiol-17beta-induced human neural progenitor cell proliferation is mediated by an estrogen receptor beta-phosphorylated extracellularly regulated kinase pathway. Endocrinology. 2008;149:208–218. doi: 10.1210/en.2007-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: Form and function in brain. Front Neuroendocrinol. 2008 doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 32.Balu DT, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33:232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newton SS, Duman RS. Regulation of neurogenesis and angiogenesis in depression. Curr Neurovasc Res. 2004;1:261–267. doi: 10.2174/1567202043362388. [DOI] [PubMed] [Google Scholar]
- 34.Weissman T, Noctor SC, Clinton BK, Honig LS, Kriegstein AR. Neurogenic radial glial cells in reptile, rodent and human: from mitosis to migration. Cereb Cortex. 2003;13:550–559. doi: 10.1093/cercor/13.6.550. [DOI] [PubMed] [Google Scholar]
- 35.Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


