Abstract
Novel dendritic trityl radicals (DTR1 and DTR2) with a TAM radical core, PAMAM branching and carboxylate exterior surface exhibit high stability towards oxidoreductants as evidenced by their electrochemical and EPR properties, offering potential application as dual oxygen and pH probe.
There has been great interest in the development of tetrathia-triarylmethyl (TAM) radicals, a class of trityl radicals, since these compounds have found wide application in electron paramagnetic resonance (EPR) spectroscopy and imaging.1 Due to the stability as well as the narrow EPR line width of TAM radicals under physiological conditions, they provide high sensitivity and resolution for the measurement of oxygen concentrations, redox status and reactive oxygen species (ROS) in biological systems.2 However, their stability has been problematic when exposed to biological oxidoreductants,3 whose generation is often due to changes in cellular oxygen level,4 thus limiting their application in the investigation of ROS-mediated diseases.5 Although TAM radicals have been proposed for pH measurement,6 these radicals are prone to self-aggregation at acidic pH resulting in broad and weak EPR signals thereby decreasing their EPR sensitivity and limiting their potential for biomedical imaging applications.2b Therefore, there is a pressing need to develop new trityl radicals with improved properties in aqueous solution.
Dendrimers with highly symmetric and branched architecture are especially useful for covalent encapsulation of active core moieties.7 Dendritic encapsulation changes the thermodynamic properties of these cores and builds kinetic barriers, resulting in their stabilization.8 Moreover, encapsulation has been shown to prevent self-aggregation of dye molecules,9 or led to the isolation of reactive functional molecules from biomolecules in in vivo systems, thereby reducing their toxicity.10
Herein, we report the first example of dendritic trityl radicals (Chart 1), in which the TAM radical CT-03 is used as a core for the synthesis of first (DTR1) and second (DTR2) generation PAMAM dendrimer11 with carboxylate groups on the exterior surface. Electrochemical properties, stability towards biological oxidoreductants, and non-aggregation under acidic conditions were investigated. Moreover, the potential use of a DTR2-Cu2+ complex as pH probe was also explored.
Chart 1.
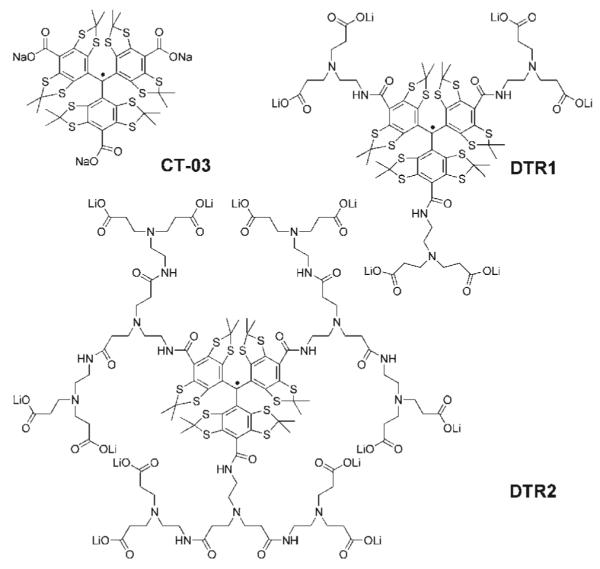
Molecular structure of dendritic trityl radicals.
The synthesis of DTR1 and DTR2 is described in the ESI.† As expected, each EPR spectrum of DTR1 and DTR2 exhibits a singlet signal (Fig. 1). Almost identical linewidths were observed for DTR1 (826 mG) and DTR2 (812 mG) suggesting that increasing dendrimer generation has only a minor effect on their linewidths and spin relaxation. These broader linewidths for DTR1 and DTR2 relative to CT-03 (190 mG) could be partly due to the presence of resolved or unresolved hyperfine splittings from the paramagnetic nitrogen and hydrogen nuclei of the three amide groups that are directly linked to the aromatic rings.
Fig. 1.
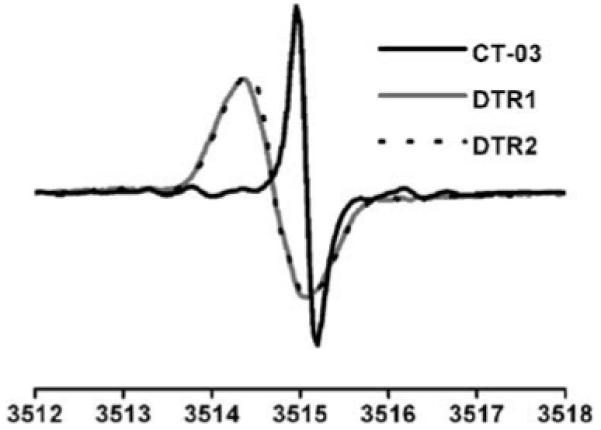
X-Band EPR spectra of aerobic aqueous solutions of DTR1, DTR2 and CT-03. The linewidths are 826, 812 and 190 mG for DTR1, DTR2 and CT-03, respectively.
Fig. 2 shows the cyclic voltammograms of the TAM radicals. CT-03 underwent one-electron quasi-reversible reduction at -0.63 V vs. Ag/AgCl to the corresponding trityl anion and one-electron reversible oxidation at 0.45 V vs. Ag/AgCl to the cation form. However, the voltammetric waves observed for CT-03 disappeared in both DTR1 and DTR2. These changes in electrochemical properties indicate a decrease in the electron-transfer rates between the buried TAM radical and the electrode surface due to dendritic shielding of the radical moiety. This protection is unusual since in most cases12 the disappearance of the voltammetric waves were observed from second or higher generation dendrimers. The highly negatively charged surface and hydrophobic interior could have a significant effect on the electron transfer rate from the electrode to the radical core.
Fig. 2.
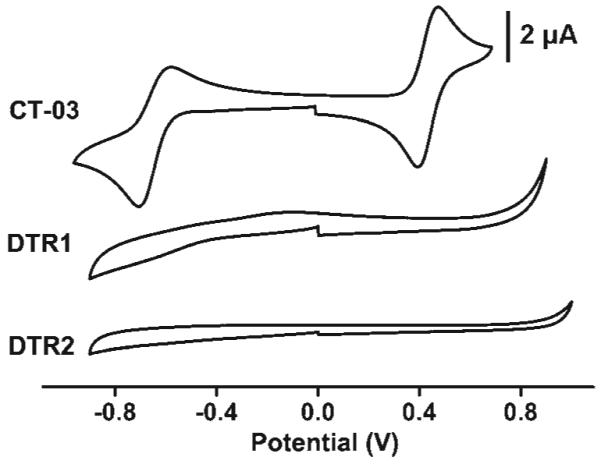
Cyclic voltammograms of CT-03, DTR1 and DTR2 in phosphate buffer solution (PBS) (0.1 M, pH 7.4) containing 0.1 M NaCl; scan rate, 50 mV s-1.
In order to further assess the effects of dendritic shielding on the TAM radicals, their stability towards various biological oxidoreductants was investigated (Table 1). DTR1 and DTR2 have similar stability to CT-03 towards reducing agents such as glutathione (GSH) and ascorbate (Asc), but also exhibit a much higher stability towards several oxidants. For example, while the paramagnetism of CT-03 was quenched to different degrees by ROO·, O2·-, ·CH3 and HO·, DTR1 and DTR2 showed high stability in the presence of the same amount of these radical sources with almost no quenching. Dendritic encapsulation, therefore, provides marked protection of the TAM radicals.
Table 1.
Percentage of CT-03, DTR1 and DTR2 remaining after exposure to various reactive species in PBSa
| Trityl | Asc | GSH | ROO· | O2·- | ·CH3 | HO· | H2O2 |
|---|---|---|---|---|---|---|---|
| CT-03 | 96.7 | 94.6 | 59.7 | 55.2 | 86.5 | 90.5 | 97.1 |
| DTR1 | 99.2 | 100 | 99.4 | 97.8 | 96.6 | 99.7 | 98.8 |
| DTR2 | 99.3 | 99.9 | 99.7 | 99.5 | 99.7 | 100 | 100 |
EPR spectra were recorded 30 min after the radical production was initiated in the presence of trityl (10 μM) in PBS. The percentage was obtained by dividing spectral intensity of each sample by that of the control consisting of the trityl alone. Each value was the average of triplicate measurements. See more details in the ESI.†
Acidic titration reveals that the EPR spectral linewidth of DTR1 and DTR2 remained constant over the pH range of 2.0-7.8 (see ESI†), but for CT-03, the linewidth broadening was observed as the pH was decreased, due to self-aggregation.2b Thus, it can be inferred that the dendritic branches could increase the solubility of DTR1 and DTR2 in acidic conditions, thereby preventing self-aggregation. The non-aggregating property of the dendritic trityls was further confirmed by the UV-Vis spectroscopy (see ESI†).
The potential application of the dendritic radicals as oxygen sensors was explored. DTR2 and DTR1 showed nearly identical oxygen-dependent EPR line broadening response but relatively lower than that of CT-03 (see ESI†). This lower sensitivity is possibly due to the presence of broader linewidths in anaerobic solution but also partly due to the reduced accessibility of the radical center to the oxygen molecule.2c However, the non-aggregation makes the dendritic trityls well-suited for oxygen measurement at acidic pH and would be very useful for the imaging of ischemic tissues and the stomach.
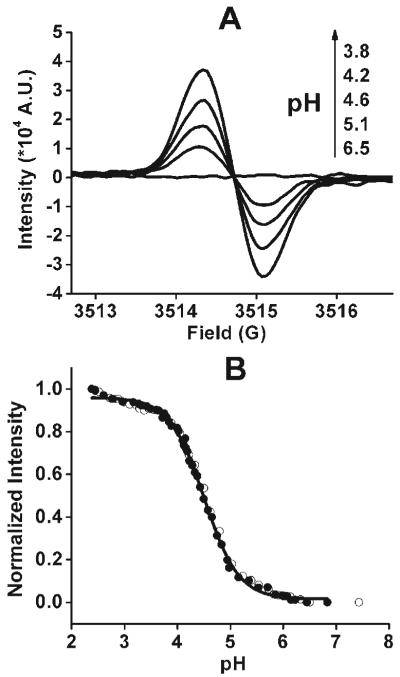
The acid-induced release of Cu2+ from the DTR2-Cu2+ complex was exploited as a means to measure pH by EPR. Similar studies on other dendrimers have been previously reported13 but used spectrophotometric techniques. The EPR signal of DTR2 (10 μM) showed a sharp decrease upon addition of Cu2+ and completely disappeared at the [Cu2+]/[DTR2] ratio of 2 (see ESI†) due to strong spin-spin interaction between the radical and the bound Cu2+. Interestingly, the EPR signal can be regenerated from the above conditions after addition of HCl with an increase in signal intensity as the pH decreases (Fig. 3A). The intensity as a function of pH is shown in Fig. 3B. At neutral pH, Cu2+ binds to one or more tertiary amines of the dendritic branch.14 The protonation of the tertiary amines occurs from 6.5 to 5.0, and leads to the binding of Cu2+ exclusively with carboxylate-O.14 Since Cu2+ is farther away from the central radical at this point, a slight increase in the signal is observed. Further acidification of the solution from pH ∼5.0 to ∼3.8 leads to the release of Cu2+ bound by the carboxylate groups as shown by the dramatic increase of the EPR signal. With decrease of pH below 3.8, only a slight enhancement of the EPR signal was observed due mostly to the release of Cu2+ from its non-selective absorption. Therefore, the pH-dependent EPR signal enhancement from the DTR2-Cu2+ complex can be exploited to measure pH. It provides by far the most sensitive and stable EPR probe known for measurement of pH and is suitable for biomedical spectroscopy and imaging.
Fig. 3.
pH dependence of the EPR spectra (A) and signal intensity (B) of the complex of DTR2 (10 μM) with Cu2+ (25 μM). In Fig. B, titration was carried out from alkaline to acidic pH (●) and acidic to alkaline pH (○), respectively.
Of note, while this manuscript was being processed, novel amino derivatives of trityl radicals were also reported to function as dual pH and oxygen probes.15 These radicals have EPR spectra with complex hyperfine structure which is directly altered by pH. However, their complex spectra may limit sensitivity and ease of use in EPR imaging applications.
In summary, the first example of dendritic TAM radicals was synthesized and characterized. Covalent encapsulation by the PAMAM dendrimer is highly effective in enhancing their stability and improving their solubility over a wide pH range. Importantly, the DTR2-Cu2+ complex is shown to be a highly effective pH probe. These new molecules and their potential for derivatization offer the possibility of new biomedical applications as probes for molecular imaging and site specific oximetry, and pH measurements under both normal and extreme conditions such as highly acidic and reducing/oxidizing environments.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants EB0890, EB4900 (J. L. Z.) and HL81248 (F. A. V.).
Footnotes
Electronic supplementary information (ESI) available: Details of synthesis and spectroscopic characterization.
Notes and references
- 1.(a) Krishna MC, English S, Yamada K, Yoo J, Murugesan R, Devasahayam N, Cook JA, Golman K, Ardenkjaer-Larsen JH, Subramanian S, Mitchell JB. Proc. Natl. Acad. Sci. U. S. A. 2002;99:2216. doi: 10.1073/pnas.042671399. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Deng YM, Petryakov S, He GL, Kesselring E, Kuppusamy P, Zweier JL. J. Magn. Reson. 2007;185:283. doi: 10.1016/j.jmr.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Reddy TJ, Iwama T, Halpern HJ, Rawal VH. J. Org. Chem. 2002;67:4635. doi: 10.1021/jo011068f. [DOI] [PubMed] [Google Scholar]; (b) Dhimitruka I, Velayutham M, Bobko AA, Khramtsov VV, Villamena FA, Hadad CM, Zweier JL. Bioorg. Med. Chem. Lett. 2007;17:6801. doi: 10.1016/j.bmcl.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liu Y, Villamena FA, Sun J, Xu Y, Dhimitruka I, Zweier JL. J. Org. Chem. 2008;73:1490. doi: 10.1021/jo7022747. [DOI] [PubMed] [Google Scholar]
- 3.Rizzi C, Samouilov A, Kutala VK, Parinandi NL, Zweier JL, Kuppusamy P. Free Radical Biol. Med. 2003;35:1608. doi: 10.1016/j.freeradbiomed.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 4.(a) Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. J. Biol. Chem. 2003;278:36027. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]; (b) Zweier JL, Flaherty JT, Weisfeldt ML. Proc. Natl. Acad. Sci. U. S. A. 1987;84:1404. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Kulkarni AC, Kuppusamy P, Parinandi N. Antioxid. Redox Signaling. 2007;9:1717. doi: 10.1089/ars.2007.1724. [DOI] [PubMed] [Google Scholar]; (b) Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Int. J. Biochem. Cell Biol. 2007;39:44. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Bobko AA, Dhimitruka I, Zweier JL, Khramtsov VV. J. Am. Chem. Soc. 2007;129:7240. doi: 10.1021/ja071515u. [DOI] [PubMed] [Google Scholar]
- 7.Lee CC, MacKay JA, Fréchet JMJ, Szoka FC. Nat. Biotechnol. 2005;23:1517. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 8.(a) Hecht S, Fréchet JMJ. Angew. Chem., Int. Ed. 2001;40:74. doi: 10.1002/1521-3773(20010105)40:1<74::aid-anie74>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]; (b) Gorman CB, Smith JC. Acc. Chem. Res. 2001;34:60. doi: 10.1021/ar000044c. [DOI] [PubMed] [Google Scholar]
- 9.(a) Ortiz A, Flora WH, D’Ambruoso GD, Armstrong NR, McGrath DV. Chem. Commun. 2005:444. doi: 10.1039/b413684e. [DOI] [PubMed] [Google Scholar]; (b) Brinas RP, Troxler T, Hochstrasser RM, Vinogradov SA. J. Am. Chem. Soc. 2005;127:11851. doi: 10.1021/ja052947c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, Meijer EW, Paulus W, Duncan R. J. Controlled Release. 2000;65:133. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 11.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. Polym. J. (Tokyo) 1985;17:117. [Google Scholar]
- 12.(a) Hong YR, Gorman CB. Langmuir. 2006;22:10506. doi: 10.1021/la060867w. [DOI] [PubMed] [Google Scholar]; (b) Appoh FE, Thomas DS, Kraatz HB. Macromolecules. 2006;39:5629. [Google Scholar]; (c) Pollak KW, Leon JW, Fréchet JMJ, Maskus M, Abruna HD. Chem. Mater. 1998;10:30. [Google Scholar]
- 13.Zhao M, Sun L, Crooks RM. J. Am. Chem. Soc. 1998;120:4877. [Google Scholar]
- 14.Ottaviani MF, Bossmann S, Turro NJ, Tomalia DA. J. Am. Chem. Soc. 1994;116:661. [Google Scholar]
- 15.Dhimitruka I, Bobko AA, Hadad CM, Zweier JL, Khramtsov VV. J. Am. Chem. Soc. 2008;130:10780. doi: 10.1021/ja803083z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.