Abstract
Pyruvate dehydrogenase from pig heart exists in active and inactive forms. Interconversion from the active (dephospho) form into the inactive (phospho) form is catalyzed by an ATP-dependent kinase. Conversely the enzyme is reactivated by a phosphatase which removes the phosphate group from the protein. By gradient centrifugation pyruvate dehydrogenase was prepared free of phosphatase but still containing the kinase. Reactivation of pyruvate dehydrogenase is stimulated by adenosine 3′,5′-cyclic phosphate. There is incorporation of 32P from γ-32P-ATP into the protein fraction containing the phosphatase and this phosphorylation reaction is also stimulated by adenosine 3′,5′-cyclic phosphate. The participation of this phosphate in the pyruvate dehydrogenase interconversion system suggests that, in heart muscle, pyruvate oxidation may be under hormonal control by a mechanism similar to that involved in the regulation of glycogen synthesis and breakdown.
Full text
PDF

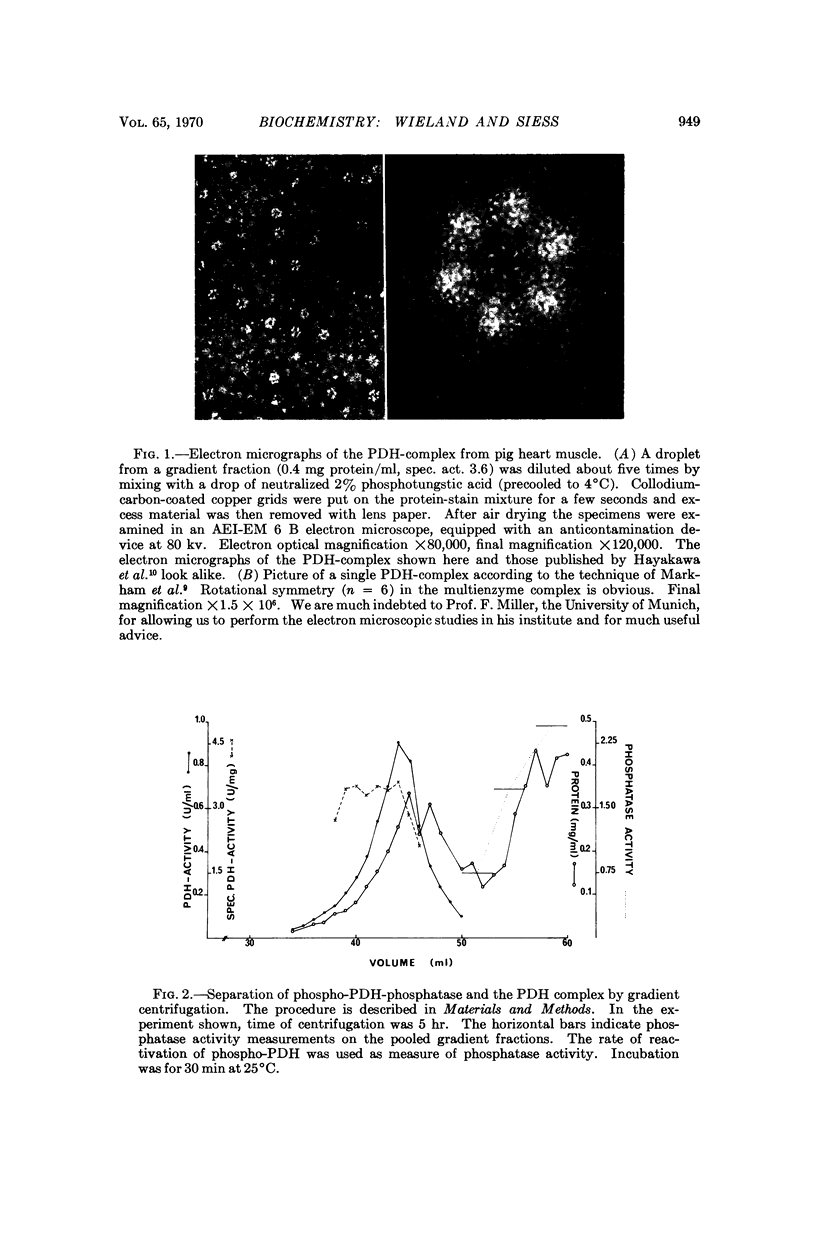
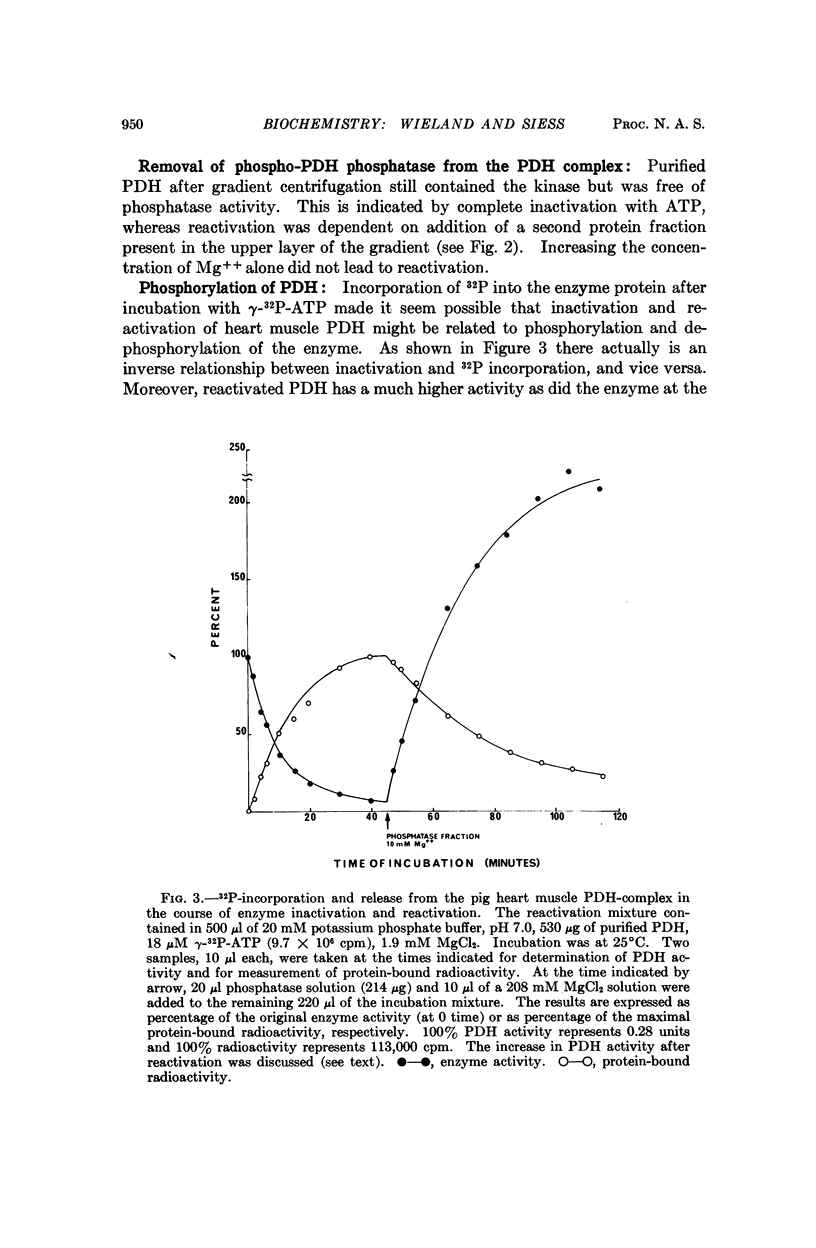
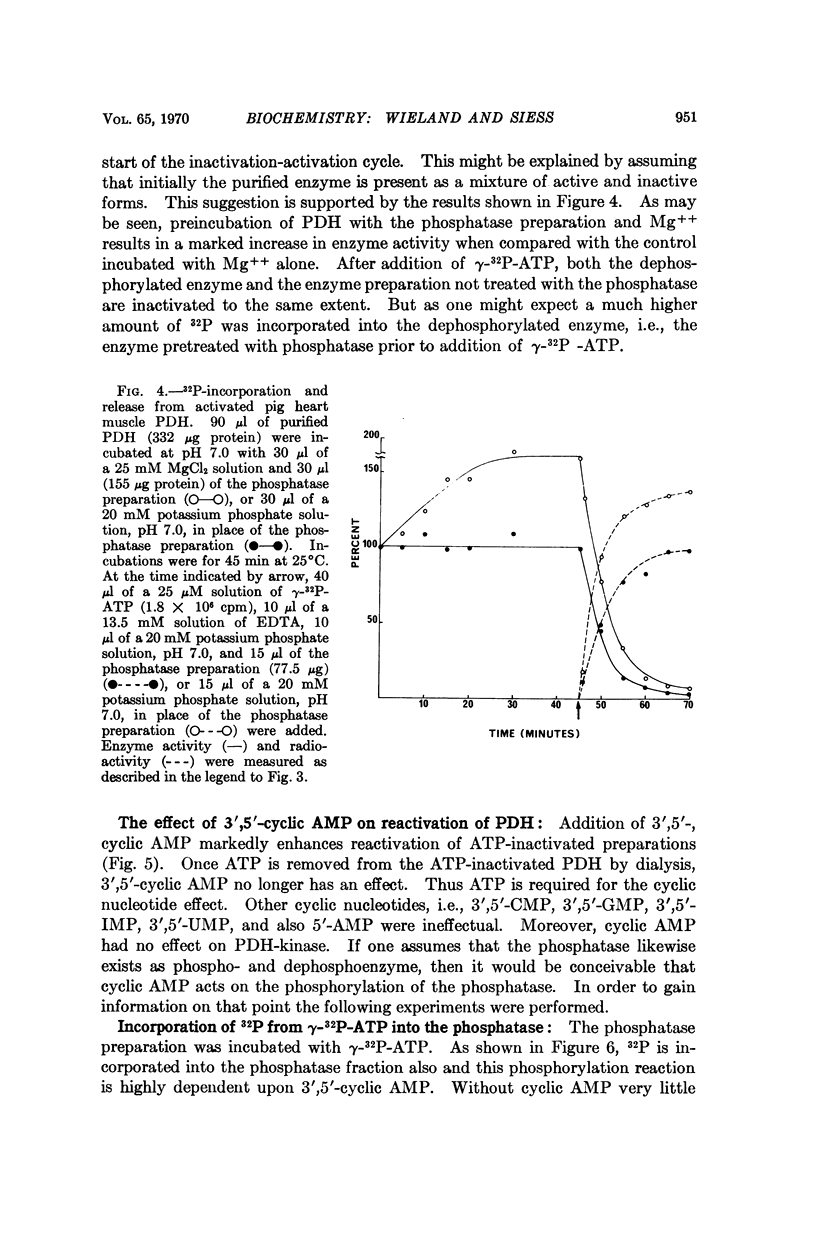
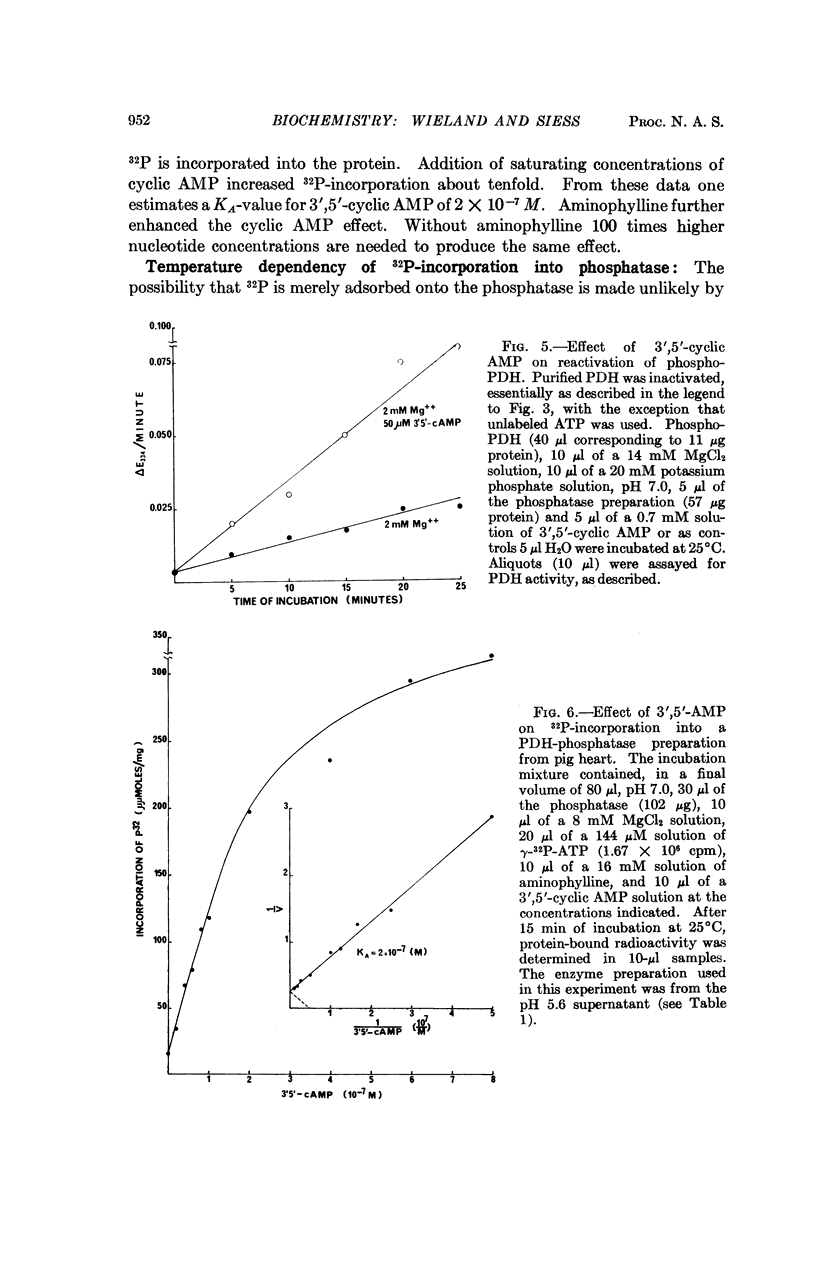


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. S., Larner J. Presence in liver of a 3':5'-cyclic AMP stimulated kinase for the I form of UDPG-glycogen glucosyltransferase. Biochim Biophys Acta. 1969 Feb 11;171(2):374–377. doi: 10.1016/0005-2744(69)90176-4. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Krebs E. G. A cyclic AMP--stimulated protein kinase in adipose tissue. Biochem Biophys Res Commun. 1969 Jul 23;36(2):328–336. doi: 10.1016/0006-291x(69)90334-9. [DOI] [PubMed] [Google Scholar]
- Hayakawa T., Kanzaki T., Kitamura T., Fukuyoshi Y., Sakurai Y., Koike K., Suematsu T., Koike M. Mammalian alpha-keto acid dehydrogenase complexes. V. Resolution and reconstitution studies of the pig heart pyruvate dehydrogenase complex. J Biol Chem. 1969 Jul 10;244(13):3660–3670. [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. An adenosine 3',5'-monophosphate-dependent protein kinase from Escherichia coli. J Biol Chem. 1969 Jun 25;244(12):3417–3419. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langan T. A. Histone phosphorylation: stimulation by adenosine 3',5'-monophosphate. Science. 1968 Nov 1;162(3853):579–580. doi: 10.1126/science.162.3853.579. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto E., Kuo J. F., Greengard P. Adenosine 3',5'-monophosphate-dependent protein kinase from brain. Science. 1968 Jul 4;165(3888):63–65. [PubMed] [Google Scholar]
- SCRIBA P., HOLZER H. [Production of alpha-hydroxyethyl-2-thiamine pyrophosphate with pyruvate oxidase from pig heart muscle]. Biochem Z. 1961;334:473–486. [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]
- Wieland O., Jagow-Westermann B. v. ATP-dependent inactivation of heart muscle pyruvate dehydrogenase and reactivation by Mg(++). FEBS Lett. 1969 Jun;3(4):271–274. doi: 10.1016/0014-5793(69)80156-0. [DOI] [PubMed] [Google Scholar]
- Wieland O., Siess E. 3',5'-cyclo-AMP als Effektor im Interconvertierungssystem der Herzmuskel-Pyruvat-Dehydrogenase. Hoppe Seylers Z Physiol Chem. 1969 Oct;350(10):1160–1161. [PubMed] [Google Scholar]
- Wieland O., Von Jagow-Westermann B., Stukowski B. Kinetic and regulatory properties of heart muscle pyruvate dehydrogenase. Hoppe Seylers Z Physiol Chem. 1969 Mar;350(3):329–334. doi: 10.1515/bchm2.1969.350.1.329. [DOI] [PubMed] [Google Scholar]



