Abstract
OBJECTIVES
We sought to identify clinical and/or plaque characteristics that affect atherosclerotic disease progression and arterial remodeling in the carotid artery with subclinical stenosis.
BACKGROUND
Increasing severity of stenosis has been associated with a higher risk of stroke. Factors that drive subclinical lesions to become stenotic plaques remain ambiguous. Carotid magnetic resonance imaging (MRI) has been validated with histology to accurately quantify in vivo arterial morphology and plaque composition.
METHODS
A total of 67 asymptomatic participants with 16% to 49% carotid stenosis as demonstrated by duplex ultrasonography were imaged at 1.5-T with a carotid MRI protocol at baseline and at 18-month follow-up. Clinical and/or intra-arterial metrics with a significant association with change in plaque burden during multivariate analysis were evaluated for effects on lumen, wall, and total vessel volume.
RESULTS
From multiple regression analysis, intraplaque hemorrhage (IPH) (p < 0.001) and statin therapy (p = 0.015) were identified as key determinants of change in plaque burden. The group with IPH compared with the group without IPH demonstrated luminal narrowing, with a mean ± SD decrease in lumen volume (−24.9 ± 21.1 mm3/year vs. −0.5 ± 26.9 mm3/year; p = 0.005), a larger increase in wall volume (44.1 ± 36.1 mm3/year vs. 0.8 ± 34.5 mm3/year; p < 0.001), and no difference in total vessel volume (19.3 ± 27.4 mm3/year vs. 0.4 ± 42.4 mm3/year; p = 0.15). The nonstatin group compared with the statin group demonstrated outward remodeling, with an increase in wall volume (22.4 ± 35.6 mm3/year3/year vs. 0.9 ± 38.0 mm3/year; p = 0.026) and total vessel volume (19.2 ± 36.9 mm3/year vs. −4.9 ± 40.4 mm3/year; p = 0.019) and no difference in lumen volume (−5.8 ± 26.6 mm3/year vs. −3.2 ± 29.5 mm3/year; p = 0.72).
CONCLUSIONS
IPH may represent an indication of accelerated plaque growth and impending luminal compromise in the subclinical carotid artery. Statin therapy may stabilize lesions by slowing or halting lesion progression. This phase of plaque stenosis (16% to 49%) may be a critical stage for intrinsic and extrinsic factors to affect the atherosclerotic disease process.
Keywords: atherosclerosis, carotid arteries, magnetic resonance imaging, remodeling
From natural history studies and multiple large clinical trials, increasing severity of stenosis has been associated with a higher risk of stroke. In 1987, Glagov et al. (1) proposed that luminal narrowing occurred after plaque burden exceeded the ability of the artery to outwardly remodel. From observations of postmortem coronary arteries, they identified luminal narrowing to occur after the atherosclerotic lesion occupied greater than 40% of the internal elastic lamina area (1).
Support for the hypothesis of Glagov et al. (1) has been reported in studies of the in vivo carotid arteries. In a prospective magnetic resonance imaging (MRI) study, Saam et al. (2) found an association between plaque burden and remodeling patterns in individuals with advanced carotid disease (50% to 79% stenosis). Evidence from histological studies (3), however, suggests that factors beyond lesion size (e.g., plaque composition) may have an integral role in progression and remodeling. In a case-control study of individuals with 50% to 79% carotid stenosis, Takaya et al. (4) found that lesions with intraplaque hemorrhage (IPH) at baseline had a greater increase in wall volume and reduction in lumen volume compared with arteries without IPH. Although both studies (2,4) provide insight into plaque features associated with patterns of remodeling in more advanced carotid atherosclerosis, neither considers arterial changes that occur in carotid arteries at a subclinical stage of atherosclerosis development (<50% stenosis). In addition, the study by Saam et al. (2) does not investigate the role of plaque composition, whereas the study by Takaya et al. (4) does not evaluate the impact of clinical risk factors on progression. As such, factors that drive earlier stage lesions to become stenotic plaques remain ambiguous.
In this study, we sought to identify the natural history of subclinical carotid atherosclerotic disease and to identify both clinical and intraplaque factors that contribute to disease progression and arterial remodeling. In accord, we designed a prospective, observational study to serially evaluate in vivo carotid atherosclerotic disease in individuals with <50% stenosis.
METHODS
Study sample
Neurologically asymptomatic individuals with at least 1 carotid artery with 16% to 49% stenosis by duplex ultrasonography using Strandness criteria (5) were serially recruited from the diagnostic vascular ultrasound laboratory at the University of Washington Medical Center and the Veterans Affairs Puget Sound Health Care System. Reasons for referral to the ultrasound laboratory of asymptomatic patients included the presence of a cervical bruit and patients scheduled for coronary artery bypass graft or lower extremity bypass. The artery with 16% to 49% stenosis was designated as the index artery and was selected for serial imaging by carotid MRI. If both the right and left carotid arteries had 16% to 49% stenosis, the index artery was randomly assigned. All participants provided answers to a standardized health questionnaire at both imaging sessions. At the baseline scan, all participants had their height, weight, and mean systolic blood pressure (SBP) in both arms recorded. The study procedures and consent forms were reviewed and approved by the institutional review board before study initiation. Subjects were asymptomatic with respect to their carotid disease before enrollment. Exclusion criteria were previous carotid endarterectomy on the index carotid, previous radiation therapy to the neck, age older than 80 years, renal insufficiency, and contraindication for MRI. Medications were not criteria for exclusion, and all patients were managed medically by their primary care providers for the duration of the observation period. During the period of observation, study participants were not enrolled in any additional imaging studies and data from these participants were not previously reported.
MRI protocol
From November 7, 2003, to November 13, 2006, 80 participants were enrolled and underwent carotid MRI at baseline and at 18-month follow-up on a 1.5-T scanner (Signa Horizon EchoSpeed, General Electric Healthcare, Milwaukee, Wisconsin) using bilateral, 4-element, phased-array surface coils (Pathway MRI, Seattle, Washington). A standardized multicontrast protocol (6) for carotid MRI was used to obtain 2-dimensional T1-, proton density-, and T2-weighted black-blood images and 3-dimensional time-of-flight bright-blood angiography. In addition, a contrast-enhanced, black-blood T1-weighted sequence (7) was acquired 5 minutes after intravenous infusion of 0.1 mmol/kg gadolinium–DTPA-BMA (Omniscan, GE Healthcare) at a rate of 2 ml/s. The 12 axial images with an in-plane resolution of 0.62 × 0.62 mm2 were acquired with a 2-mm slice thickness, for a total longitudinal coverage of 24 mm, centered at the bifurcation of the index artery.
Image review
Two reviewers, blinded to time point and clinical information, matched the multicontrast axial images of the index artery between time points based on distance from the bifurcation of the carotid artery. All matched axial locations for the index artery were evaluated for image quality (4-point scale: 1 = poor, 4 = excellent) and interpreted by reaching a consensus. In instances of disagreement, a third blinded reviewer mediated a decision. For images with image quality ≥2 at both time points, image analysis software (CASCADE, Seattle, Washington) was used to draw the lumen and outer wall boundaries (8). Lumen volume, wall volume, total vessel volume (lumen volume + wall volume), and percentage of wall volume (100% × wall volume/total vessel volume) were recorded (the percentage of wall volume is a measure of plaque burden similar to that proposed by Nissen et al. [9] during imaging investigations of the coronary arteries). The presence or absence of calcification, a lipid-rich necrotic core (LRNC), and IPH were determined using multicontrast imaging criteria previously validated with histology (10,11). Volume measurements of the LRNC and calcification, when present, were also collected. IPH was treated as a dichotomous variable (present vs. absent) due to decreased reproducibility of volume measurements compared with the other metrics (10,12).
Data analysis
Summary statistics for each metric are presented as mean ± SD annualized rates for all arteries included in each analysis. Change in the percentage of wall volume was compared with zero using the 1-sample t test. Univariate least-squares regression analysis using each of the clinical and baseline arterial variables identified in Table 1 as an independent variable in separate analyses was conducted to identify statistically significant predictors of the percentage of wall volume change. In the demographics and regression analysis, the presence of statin therapy or diabetes mellitus indicates presence during the observation period. All participants on statin therapy were taking statins for a minimum of 3 months before the baseline scan. No subject had statin therapy initiated or discontinued during the period of observation. Statin therapy and diabetes mellitus were entered as dichotomous variables, and the baseline type and dose were not used. A multivariate regression model was created for the annual change in the percentage of wall volume by using predictors identified during univariate analysis with a statistical association having p ≤ 0.10. This inclusive regression model offers a conservative estimate of the association of each of the variables with the outcome. The regression coefficients are reported as unstandardized B ± SE and standardized coefficient beta for univariate and multivariate models.
Table 1.
Baseline Clinical and Arterial Data (n = 67)
| Mean ± SD or % | Range or Dose (Median) | |
|---|---|---|
| Age, yrs | 69.8 ± 9.2 | 48–89 |
| Male sex, % | 76.1 | |
| Body mass index, kg/m2 | 27.6 ± 4.0 | 19.5–37.7 |
| SBP, mm Hg* | 139.7 ± 17.3 | 100.5–191.5 |
| Smoking, % | ||
| Never smoked | 29.9 | |
| Quit | 49.3 | |
| Active | 20.9 | |
| History of coronary artery disease, % | 34.3 | |
| History of claudication, % | 17.9 | |
| Diabetes mellitus, % | 17.9 | |
| Insulin† | 66.7 | |
| Metformin† | 41.7 | |
| Glyburide† | 8.3 | |
| Glipizide† | 8.3 | |
| Statin therapy, mg, % | 64.2 | |
| Simvastatin† | 41.9 | 5–80 (20) |
| Atorvastatin† | 34.9 | 10–80 (20) |
| Lovastatin† | 18.6 | 10–40 (20) |
| Pravastatin† | 4.7 | 10–40 |
| Statin therapy duration, months | ||
| <3 | 0 | |
| 3–12 | 32.6 | |
| >12 | 67.4 | |
| Lumen volume, mm3 | 704.3 ± 226.5 | 236.7–1,299.5 |
| Wall volume, mm3 | 699.3 ± 217.5 | 358.7–1,453.6 |
| Total vessel volume, mm3 | 1,403.7 ± 374.9 | 831.5–2,483.4 |
| Percentage of wall volume | 49.9 ± 8.4 | 33.8–76.7 |
| Presence of LRNC, % | 58.2 | |
| Presence of calcification, % | 68.7 | |
| Presence of IPH, % | 17.9 | |
| LRNC volume (n = 39), mm3 | 80.0 ± 92.3 | 1.9–407.7 |
| Calcification volume (n = 46), mm3 | 35.9 ± 39.0 | 1.2–162.5 |
Missing data for 1 subject.
Percentage of diabetes mellitus or statin users.
IPH = intraplaque hemorrhage; LRNC = lipid-rich necrotic core; SBP = systolic blood pressure.
Each metric with a significant association with the change in the percentage of wall volume during multivariate analysis was evaluated for effects on arterial remodeling. Outward remodeling was defined as an increase in wall volume and total vessel volume without a change in lumen volume. Luminal narrowing was defined as an increase in wall volume, a decrease in lumen volume, and no change in total vessel volume. For dichotomous variables, change in lumen volume, wall volume, and total vessel volume were compared with zero using the 1-sample t test for each subgroup. The independent t test was used to compare changes between subgroups within each metric. For continuous variables, data were partitioned into tertiles and an identical analysis was performed using the first and last tertiles as subgroups. Differences between subgroups of each metric were also evaluated using a multivariate model that controlled for each of the other metrics.
Changes in LRNC volume and calcification volume were compared with 0 using the 1-sample t test. The Pearson correlation coefficient r was used to identify associations between the change in the percentage of wall volume and the change in plaque composition. All statistical calculations were made using SPSS version 12.0 for Windows (SPSS Inc., Chicago, Illinois). Statistical significance, based on 2-sided tests, was defined as a value of p < 0.05.
RESULTS
Of the 80 participants enrolled, 67 (84%) had matched baseline and 18-month carotid MRI scans of sufficient image quality for identification and quantification of the vessel boundaries and plaque composition. All subjects remained asymptomatic during the period of observation. The mean interval between scans was 17.8 ± 0.1 months and the mean coverage was 19.8 ± 0.3 mm. The demographics and baseline arterial characteristics for the assessable study sample are presented in Table 1.
Change in plaque burden (percentage of wall volume)
For the study sample as a whole, there was a trend toward an overall increase in the percentage of wall volume (0.4 ± 1.7% per year; p = 0.070). Clinical and arterial metrics associated with annual change in the percentage of wall volume determined from univariate regression analysis are detailed in Table 2. Notably, the baseline lumen volume (B = 0.02 ± 0.05 per 50-mm3 increase; p = 0.66) and the baseline percentage of wall volume (B = 0.11 ± 0.25 per 10% increase; p = 0.65) were not listed in Table 2 due to a statistically non-significant association with change in the percentage of wall volume. Among the metrics identified during univariate analysis, multivariate analysis identified IPH (B = 2.24 ± 0.62% per year with IPH present; p = 0.001), statin therapy (B = −0.99 ± 0.36% per year with statin therapy present; p = 0.008), and SBP (B = 0.22 ± 0.10% per year per 10-mm Hg increase; p = 0.031) as predictors of change in the percentage of wall volume (Table 2).
Table 2.
Predictors of Annual Change in Percentage of Wall Volume (%/year)
| Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|
| B ± SE | Beta | p Value | B ± SE | Beta | p Value | |
| Smoking status (per categorical increase from nonsmoking) | 0.62 ± 0.28 | 0.26 | 0.030 | 0.23 ± 0.20 | 0.12 | 0.25 |
| Presence of statin therapy | −0.69 ± 0.42 | −0.20 | 0.10 | −0.99 ± 0.36 | −0.29 | 0.008 |
| SBP (per 10-mm increase in Hg) | 0.24 ± 0.12 | 0.24 | 0.051 | 0.22 ± 0.10 | 0.22 | 0.031 |
| Presence of IPH | 2.16 ± 0.47 | 0.50 | <0.001 | 2.24 ± 0.62 | 0.52 | 0.001 |
| LRNC volume (per 10-mm3 increase) | 0.08 ± 0.02 | 0.37 | 0.002 | −0.00 ± 0.03 | −0.01 | 0.92 |
Abbreviations as in Table 1.
IPH
There were 12 (17.9%) participants with IPH at baseline. No subject developed IPH during the period of observation. Lesions with IPH present compared with lesions without IPH demonstrated a significant reduction in lumen volume (−24.9 ± 21.1 mm3/year vs. −0.5 ± 26.9 mm3/year, respectively; p = 0.005) (Fig. 1) and an increase in wall volume (44.1 ± 36.1 mm3/year vs. 0.8 ± 34.5 mm3/year, respectively; p < 0.001) without a significant difference in the total vessel volume (19.3 ± 27.4 mm3/year vs. 0.4 ± 42.4 mm3/year, respectively; p = 0.015). The magnitude and statistical significance of these differences (or similarities) were comparable to the results from the multiple regression analysis after controlling for statin therapy and SBP (Table 3).
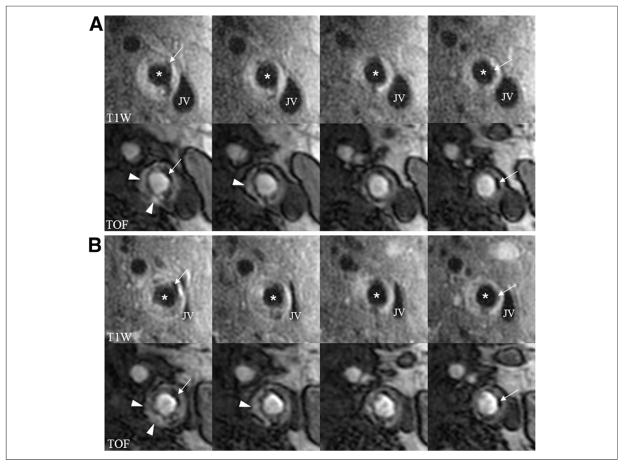
Figure 1. In Vivo Evidence of the Effects of IPH.
Images are serial axial sections through a lesion in the left internal carotid artery from T1-weighted (T1W) and time-of-flight (TOF) sequences. (A) Baseline scan; (B) corresponding matched axial images at 18-month follow-up. The lesion contains IPH (arrowheads) at both time points. Notice the reduction in lumen area most pronounced in the first 2 columns associated with an increase in the wall thickness indicative of luminal narrowing. Also present is calcification (arrows) that can be used to confirm registration between time points. *Lumen of the internal carotid. IPH = intraplaque hemorrhage; JV = jugular vein.
Table 3.
Absolute Change (mm3/year) in Arterial Morphology
| Regression Coefficients |
||||||
|---|---|---|---|---|---|---|
| Present* (Mean ± SD) | Absent* (Mean ± SD) | p Value† | B ± SE | Beta | p Value‡ | |
| IPH | ||||||
| No. of subjects | 12 | 55 | ||||
| Lumen volume | −24.9 ± 21.1 (p = 0.002) | −0.5 ± 26.9 (p = 0.90) | 0.005 | −24.4 ± 8.6 | −0.34 | 0.006§ |
| Wall volume | 44.1 ± 36.1 (p = 0.001) | 0.8 ± 34.5 (p = 0.86) | <0.001 | 48.8 ± 10.1 | 0.49 | <0.001§ |
| Total vessel volume | 19.3 ± 27.4 (p = 0.033) | 0.4 ± 42.4 (p = 0.95) | 0.15 | 24.4 ± 12.5 | 0.23 | 0.055§ |
| Statin therapy | ||||||
| No. of subjects | 43 | 24 | ||||
| Lumen volume | −5.8 ± 26.6 (p = 0.16) | −3.2 ± 29.5 (p = 0.60) | 0.72 | 1.2 ± 6.9 | 0.02 | 0.86|| |
| Wall volume | 0.9 ± 38.0 (p = 0.88) | 22.4 ± 35.6 (p = 0.005) | 0.026 | −28.6 ± 8.1 | −0.36 | 0.001|| |
| Total vessel volume | −4.9 ± 40.4 (p = 0.43) | 19.2 ± 36.9 (p = 0.018) | 0.019 | −27.4 ± 10.0 | −0.33 | 0.008|| |
| SBP | ||||||
| No. of subjects | 23 | 22 | ||||
| Lumen volume | −8.5 ± 36.1 (p = 0.27) | 1.4 ± 21.1 (p = 0.75) | 0.28 | −1.9 ± 1.9 | −0.12 | 0.31¶ |
| Wall volume | 18.4 ± 31.1 (p = 0.009) | −1.0 ± 45.8 (p = 0.92) | 0.10 | 5.9 ± 2.2 | 0.27 | 0.010¶ |
| Total vessel volume | −9.9 ± 40.8 (p = 0.25) | 0.4 ± 45.3 (p = 0.97) | 0.46 | 4.0 ± 2.7 | 0.17 | 0.16¶ |
One-sample t test of change (present or absent; for SBP, present = third tertile [>147 mm Hg] and absent = first tertile [<131 mm Hg]).
Independent t test between groups with feature present or absent.
p value from multiple regression analysis
controlling for statin therapy and SBP,
IPH and SBP, or
IPH and statin therapy.
Abbreviations as in Table 1.
Statin therapy
Subjects not on statin therapy compared with those on statin therapy had a statistically significant progression of wall volume (22.4 ± 35.6 mm3/year vs. 0.9 ± 38.0 mm3 mm3/year, respectively; p = 0.026) and total vessel volume (19.2 ± 36.9 mm3/year vs. −4.9 ± 40.4 mm3/year, respectively; p = 0.019) without a significant difference in change in lumen volume (−3.2 ± 29.5 mm3/year vs. −5.8 ± 26.6 mm3/year, respectively; p = 0.72). The magnitude and statistical significance of the differences increased after controlling for IPH and SBP (Table 3). The similarity in lumen volume change between these 2 subgroups was strengthened during multivariate analysis (Table 3). Neither nonstatin nor statin users demonstrated a significant change in lumen volume compared with baseline (Table 3). Of note, there were no significant differences between the group on statin therapy for >12 months before enrollment (n = 29) compared with the group on statin therapy for 3 to 12 months before enrollment (n = 14) for change in lumen volume (−4.8 ± 25.3 mm3/year vs. −7.7 ± 30.0 mm3/year, respectively; p = 0.74), wall volume (2.7 ± 32.5 mm3/year vs. −3.0 ± 48.7 mm3/year, respectively; p = 0.65), and total vessel volume (−2.1 ± 29.3 mm3/year vs. −10.7 ± 58.2 mm3/year, respectively; p = 0.61).
SBP
The tertile with the highest SBP (>147 mm Hg) compared with the tertile with the lowest SBP (<131 mm Hg) demonstrated a trend toward an increase in wall volume (18.4 ± 31.1 mm3/year vs. −1.0 ± 45.8 mm3/year, respectively; p = 0.010). There was not a significant difference between the 2 subgroups for change in lumen and total vessel volume (Table 3). After controlling for IPH and statin therapy, the difference for change in wall volume lessened, but was statistically significant (Table 3). Difference for change in lumen volume and total vessel volume remained statistically non-significant (Table 3).
Change in plaque composition
There were 39 (58.2%) and 46 (68.7%) participants with an LRNC and calcification present at baseline, respectively. No participant without an LRNC (calcification) at baseline developed an LRNC (calcification) during the observation period. There was a significant increase in LRNC volume (8.0 ± 20.1 mm3/year; p = 0.018). There was a strong association between change in the percentage of wall volume and change in LRNC volume (r = 0.75, p < 0.001) (Figs. 2 and 3). There was not a statistically significant change from baseline (1.8 ± 7.2 mm3/year; p = 0.097) in calcification. There was a slight association between change in the percentage of wall volume and change in the volume of calcification (r = 0.36, p = 0.013) (Fig. 2).
Figure 2. Association of Change in the Percentage of Wall Volume With Plaque Composition.
Scatterplots of change in the percentage of wall volume versus change in lipid-rich necrotic core (LRNC) volume (A, B, C) (r = 0.75, p < 0.001) and calcification volume (D, E, F) (r = 0.36, p = 0.013). The solid line represents the best fit through the data. For each row, the plots are identical with data points relabeled according to the presence/absence of intraplaque hemorrhage (IPH) (A, D) and statin therapy (B, E) and tertiles of systolic blood pressure (C, F). Of note, plots in the upper panel indicate that effects of statin therapy are diminished by IPH and may be enhanced in subjects in the lowest tertile of systolic blood pressure.
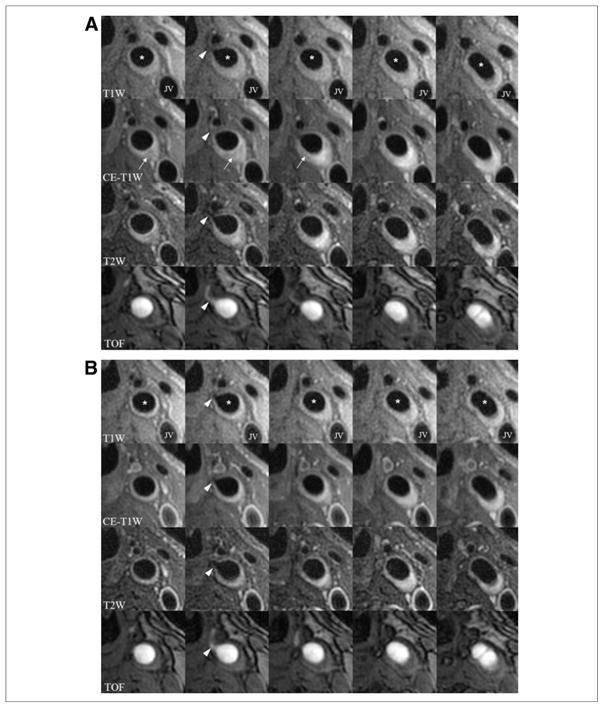
Figure 3. In Vivo Evidence of Plaque Regression in a Statin User.
(A) Baseline scan; (B) 18-month follow-up scan. Multicontrast images (T1-weighted [T1W], contrast-enhanced [CE]-T1W, T2-weighted [T2W], and time-of-flight [TOF]) from serial axial sections through the left common carotid artery of a statin user. A branching vessel (arrowhead, second column) and the tip of the flow divider on TOF (last column) confirm registration. At baseline, there is a lipid-rich necrotic core present (arrows), which has essentially resolved at follow-up along with a reduction in plaque burden. *Lumen of the common carotid. JV = jugular vein.
DISCUSSION
During this 18-month observational, prospective investigation of 16% to 49% carotid stenosis, both clinical and intra-arterial factors were associated with dynamic carotid atherosclerotic disease. IPH, statin therapy, and SBP were identified as the strongest predictors of change in plaque burden. Although increased SBP was associated with a general increase in plaque burden, IPH and statin therapy had more definite effects. IPH was associated with accelerated plaque growth and luminal narrowing. In contrast, statin therapy slowed plaque progression. Moreover, in the absence of statin therapy, expansion of plaque was associated with outward arterial remodeling. Our findings from this natural history study indicate that specific clinical risk factors and IPH may drive plaque evolution and arterial remodeling in subclinical carotid atherosclerotic disease.
Arterial remodeling was originally described in the context of plaque burden. Glagov et al. (1) described outward remodeling, an increase in the total vessel area, as an adaptive phenomenon that preserves blood flow in response to atherosclerotic disease. Luminal narrowing did not occur until the atherosclerotic lesion occupied >40% of the internal elastic lamina area. In lesions with 50% to 79% stenosis, Saam et al. (2) found changes (i.e., luminal narrowing) in the carotid artery consistent with the hypothesis of Glagov et al. (1). In the absence of statin therapy, we observed outward arterial remodeling in lesions in this study of patients with 16% to 49% stenosis, which further supports the hypothesis of Glagov et al. (1). However, we also observed luminal narrowing in our cohort. Our findings indicate that the accelerated expansion of plaque burden associated with the presence of IPH may induce luminal narrowing. The alterations to luminal morphology may have occurred because the local environment evolved too rapidly to allow compensatory outward remodeling. Alternatively, the hemorrhagic event may signify a fundamental change in the biology of the lesion. For example, a change in the composition of a lesion might produce a change in vascular remodeling, as was described previously in balloon-injured rabbits (13).
IPH was previously associated with lesion instability in the carotid artery. In individuals with a recent history of cerebral ischemia, Murphy et al. (14) found IPH present in 67% of lesions with <70% stenosis. Altaf et al. (15) reported that the presence of IPH in symptomatic patients with 30% to 69% carotid stenosis was associated with a hazard ratio of 9.8 for the development of recurrent neurological symptoms. In asymptomatic subjects with >50% stenosis, Takaya et al. (16) reported that IPH was associated with a hazard ratio of 5.2 for a future ipsilateral ischemic event. IPH has also been reported to accelerate plaque burden progression and increase the size of the LRNC in asymptomatic subjects with 50% to 79% stenosis (4). Our findings expand the integral role of IPH in the natural history of carotid atherosclerosis to lesions with 16% to 49% stenosis. Previous studies combined with the data presented here strongly suggest that IPH may act to destabilize lesion integrity at any stage of plaque burden and stenotic severity.
Multiple long-term, randomized studies have demonstrated that statin therapy decreases the occurrence of clinical events. We found evidence that statin therapy alters the natural history of in vivo carotid atherosclerotic disease. Not all participants, however, responded similarly to statin therapy; there were clear instances of regression (Figs. 2 and 3) and progression (Fig. 2). Interestingly, individuals on statin therapy and in the lowest tertile of SBP (<131 mm Hg) demonstrated regression in both LRNC volume and plaque burden. Furthermore, the effects of statin therapy seemed to be offset by IPH and/or elevated SBP, an established risk factor for cardiovascular disease. Although there were insufficient data to statistically evaluate the full extent of these relationships, these findings form the basis for large, long-term prospective studies that evaluate these hypotheses.
Study limitations
There are 2 limitations related to our analysis of statin therapy that warrant discussion. First, statin therapy and dose were not randomized and/or uniform across the study sample. However, our findings are consistent with those of previous work that did not find a significant difference between high- and low-dose rosuvastatin in carotid disease over a similar period of observation (17). Second, cholesterol levels (cross-sectional or serial) were not available. We did not observe a significant difference between duration of statin therapy before study enrollment, which suggests that cholesterol levels may have been at a steady state during the observation period. In addition, a previous double-blind, randomized, prospective study did not find an association between change in cholesterol levels and change in plaque composition (17). Nevertheless, monitoring cholesterol levels during future investigations may provide insight into the relationships between statin therapy, serological response to statin therapy, and alterations in plaque morphology/composition.
CONCLUSIONS
We conclude that lesions with 16% to 49% stenosis represent a dynamic period of disease evolution. In this demographic group, IPH may be an indication of accelerated plaque growth and impending luminal compromise in the carotid artery. Statin therapy may stabilize carotid lesions by slowing lesion progression, particularly in individuals with well-controlled blood pressure. Both IPH and statin therapy substantially alter the natural history and arterial remodeling of the carotid artery. The subclinical phase of plaque stenosis may be a critical stage for both intrinsic and extrinsic factors to affect the atherosclerotic disease process.
Acknowledgments
This work was supported by a grant from the NIH (P01-HL072262).
ABBREVIATIONS AND ACRONYMS
- IPH
intraplaque hemorrhage
- LRNC
lipid-rich necrotic core
- MRI
magnetic resonance imaging
- SBP
systolic blood pressure
References
- 1.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–5. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 2.Saam T, Yuan C, Chu B, et al. Predictors of carotid atherosclerotic plaque progression as measured by noninvasive magnetic resonance imaging. Atherosclerosis. 2007;194:e34–42. doi: 10.1016/j.atherosclerosis.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–25. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 4.Takaya N, Yuan C, Chu B, et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation. 2005;111:2768–75. doi: 10.1161/CIRCULATIONAHA.104.504167. [DOI] [PubMed] [Google Scholar]
- 5.Roederer GO, Langlois YE, Yager KA, Primozich JF, Phillips DJ, Strandness DE. A simple spectral parameter for accurate classification of severe carotid disease. Bruit. 1984;8:174–8. [Google Scholar]
- 6.Yuan C, Kerwin WS, Yarnykh VL, et al. MRI of atherosclerosis in clinical trials. NMR Biomed. 2006;19:636–54. doi: 10.1002/nbm.1065. [DOI] [PubMed] [Google Scholar]
- 7.Yarnykh VL, Yuan C. T1-insensitive flow suppression using quadruple inversion-recovery. Magn Reson Med. 2002;48:899–905. doi: 10.1002/mrm.10292. [DOI] [PubMed] [Google Scholar]
- 8.Kerwin W, Xu D, Liu F, et al. Magnetic resonance imaging of carotid atherosclerosis: plaque analysis. Top Magn Reson Imaging. 2007;18:371–78. doi: 10.1097/rmr.0b013e3181598d9d. [DOI] [PubMed] [Google Scholar]
- 9.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–80. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 10.Saam T, Ferguson MS, Yarnykh VL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005;25:234–9. doi: 10.1161/01.ATV.0000149867.61851.31. [DOI] [PubMed] [Google Scholar]
- 11.Cai J, Hatsukami TS, Ferguson MS, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–44. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 12.Chu B, Kampschulte A, Ferguson MS, et al. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke. 2004;35:1079–84. doi: 10.1161/01.STR.0000125856.25309.86. [DOI] [PubMed] [Google Scholar]
- 13.Courtman DW, Schwartz SM, Hart CE. Sequential injury of the rabbit abdominal aorta induces intramural coagulation and luminal narrowing independent of intimal mass: extrinsic pathway inhibition eliminates luminal narrowing. Circ Res. 1998;82:996–1006. doi: 10.1161/01.res.82.9.996. [DOI] [PubMed] [Google Scholar]
- 14.Murphy RE, Moody AR, Morgan PS, et al. Prevalence of complicated carotid atheroma as detected by magnetic resonance direct thrombus imaging in patients with suspected carotid artery stenosis and previous acute cerebral ischemia. Circulation. 2003;107:3053–8. doi: 10.1161/01.CIR.0000074204.92443.37. [DOI] [PubMed] [Google Scholar]
- 15.Altaf N, Daniels L, Morgan PS, et al. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg. 2008;47:337–42. doi: 10.1016/j.jvs.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 16.Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI—initial results. Stroke. 2006;37:818–23. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 17.Underhill HR, Yuan C, Zhao XQ, et al. Effect of rosuvastatin therapy on carotid plaque morphology and composition in moderately hypercholesterolemic patients: a high-resolution magnetic resonance imaging trial. Am Heart J. 2008;155:584.e1–8. doi: 10.1016/j.ahj.2007.11.018. [DOI] [PubMed] [Google Scholar]