Abstract
This experiment monitored eyelid responses bilaterally during delay eyeblink conditioning in rats. Rats were given paired or unpaired training with a tone or light conditioned stimulus (CS) and a unilateral periorbital shock unconditioned stimulus (US). Rats given paired training acquired high levels of conditioned responses (CRs), which occurred in both eyelids. However, acquisition was faster, and the overall percentage of CRs was greater in the eyelid that was ipsilateral to the US. CRs in the eyelid ipsilateral to the US also had shorter onset latencies and larger amplitudes than CRs in the contralateral eyelid. Both eyelids consistently showed high percentages of unconditioned responses (UR) to the US, and the UR amplitude decreased across training sessions in the paired group. The present study demonstrated that CRs occur robustly in both eyelids of rats given eyeblink conditioning, which is similar to previous findings in humans and monkeys. The results also showed that conditioning occurs more prominently in the eyelid that is ipsilateral to the US, which is similar to previous findings in humans, monkeys, dogs, and rabbits.
Keywords: Learning, Pavlovian Conditioning, Cerebellum, Timing
Pavlovian eyeblink conditioning procedures have been used extensively to investigate the behavioral and neural mechanisms of associative learning (e.g., Christian & Thompson, 2003; Gormezano, Kehoe, & Marshall, 1983; Gormezano, Schneiderman, Deaux, & Fuentes, 1962; Schneiderman, Fuentes, & Gormezano, 1962; Spence, 1953). Eyeblink conditioning is established using presentations of a conditioned stimulus (CS), such as a tone, that is paired with an unconditioned stimulus (US), such as a periorbital shock or air-puff directed at one eye. Before training only the US elicits the blink reflex, but after sufficient CS-US pairings conditioned blink responses (CRs) are elicited by the CS, which precede the onset of the US.
Eyelid and/or nictitating membrane (NM) response conditioning has been established in various mammalian species including rabbits (e.g., Gormezano et al., 1962; Gormezano et al., 1983; Schneiderman et al., 1962; Thomas & Wagner, 1964), rats (e.g., Hughes & Schlosberg, 1938; Skelton, 1988; Stanton, Freeman, & Skelton 1992), humans (e.g., Hilgard & Campbell, 1936; Hilgard & Marquis, 1936; Spence & Trapold, 1961; Woodruff-Pak & Steinmetz, 2000), monkeys (e.g., Clark & Zola, 1998; Hilgard & Campbell, 1936; Marquis & Hilgard, 1937), ferrets (e.g., Ivarsson & Hesslow, 1993; Ivarsson, Svensson, & Hesslow, 1997; Jirenhaed, Bengtsson, & Hesslow, 2007), cats (e.g., Gruart, Blázquez, & Delgado-García, 1995; Harrison & Buchwald, 1983; Jiménez-Díaz, Navarro-López, Gruart, & Delgado-García, 2004), dogs (e.g., Hilgard & Marquis, 1935; 1936; Vardaris & Fitzgerald, 1969), and mice (e.g., Bao, Chen, & Thompson, 1998; Kishimoto, Suzuki, Kawahara, & Kirino, 2001; Park, Onodera, Nishimura, Thompson, & Itohara, 2006). Acquisition and expression of the eyeblink CR is similar across species. For example, CR percentage and amplitude both increase as a function of training, and in well-trained subjects eyelid and/or nictitating membrane closure reaches maximal amplitude immediately preceding the onset of the US.
A difference in eyeblink conditioning that has been found among species involves the expression of the CR in the reinforced and nonreinforced eyes. Eyeblink conditioning is typically established with a US that is directed at one eye, but unilateral US presentations produce URs in both eyelids (Hilgard & Campbell, 1936; Hilgard & Marquis, 1936; McCormick, Lavond, & Thompson, 1982; Ivarsson, & Hesslow, 1993; Brandon, Betts, & Wagner, 1994). However, the percentage and amplitude of bilateral CRs that develops during training differs across species. For example, early studies of eyeblink conditioning found that humans and monkeys express CRs in both eyes throughout training, whereas dogs exhibit relatively few CRs in the nonreinforced eye (Hilgard & Campbell, 1936; Hilgard & Marquis, 1936). Subsequent research has shown that rabbits also express significantly fewer CRs in the nonreinforced eye relative to the reinforced eye (Disterhoft, Kwan, & Lo, 1977; Lee, Kim, & Wagner, 2008; McCormick, et al., 1982; Brandon et al., 1994). A consistent finding in most species is that CRs in the nonreinforced eye have smaller amplitudes and longer onset latencies than CRs in the reinforced eye (Hilgard & Marquis, 1936; Disterhoft et al, 1977; McCormick et al. 1982). These findings demonstrate bilateral CR expression varies across species, but that conditioning is consistently stronger in the reinforced eye.
The expression of bilateral CRs and URs during eyeblink conditioning has not been investigated in rats, although evidence suggests that the cerebellar processing important for eyeblink conditioning in rats is lateralized. For example, unilateral lesions and reversible inactivation in the ipsilateral cerebellar hemisphere to the trained eye have been shown to prevent acquisition and expression of delay eyeblink conditioning in rats (Campolattaro & Freeman, 2008, 2009; Freeman, Carter, & Stanton, 1995; Freeman, Halverson, & Poremba, 2005). A recent metabolic mapping investigation of eyeblink conditioning in rats has also shown that increased glucose uptake is greater in the cerebellar cortical hemisphere ipsilateral to the trained eye relative to contralateral eye (Plakke, Freeman, & Poremba, 2007). It is, therefore, possible that expression of eyeblink CRs is strongly lateralized to the reinforced eye in rats, as previously observed with rabbits and dogs (Brandon et al., 1994; Disterhoft et al., 1977; Hilgard & Campbell, 1936; Hilgard & Marquis, 1936; McCormick et al., 1982). Alternatively, CR expression may occur robustly in both eyelids, which would be more consistent with the findings in humans and monkeys (Hilgard & Campbell, 1936; Hilgard & Marquis, 1936). The present experiment was designed to examine the extent of bilateral UR and CR expression during eyeblink conditioning with rats. Here, activity from both eyelids of rats was monitored simultaneously during ten sessions of paired or unpaired training with a CS (tone or light) and a unilateral US.
Method
Subjects
Subjects were 24 male Long Evans rats (200–250g), approximately 150 days old at the beginning of the experiment. The rats were housed in Spence Laboratories of Psychology at the University of Iowa with a 12-hr light-dark cycle, with light onset at 07:00am.
Surgery
One week prior to training, rats were removed from their home cage and anesthetized by isoflurane. The rats were fitted with differential electromyograph (EMG) electrodes that were implanted in the upper left and right eyelid muscles (orbicularis oculi) and ground electrodes were attached to a stainless steel skull screw. The EMG electrode leads terminated in gold pins held in a plastic connector, which were secured to the skull with bone cement. Bipolar stimulating electrodes (for delivering the shock US) were implanted subdermally, immediately caudal to each eye. The bipolar electrodes terminated in a plastic connector and were secured to the skull with bone cement.
Conditioning Apparatus
The conditioning apparatus and eyelid EMG recording procedures used in the present study have been previously described in other reports (e.g., Campolattaro, Schnitker, & Freeman, 2008; Freeman et al., 2005; Nicholson & Freeman, 2002; Plakke et al., 2007).
Conditioning Procedure
All rats in this experiment received ten sessions of training. On each day of training they were given a 100-trail session of either paired (n = 16) or unpaired (n = 8) presentations of a 400-msec CS (2 kHz, 85d tone or 6W light; counterbalanced) and a 25-msec periorbital unconditioned shock stimulus (US; 1–2 mA, DC constant current). Every 10th trial in the paired training was a CS-alone probe trial (10 probe trails per 100-trial session). These trials were necessary to obtain behavioral eyeblink responses without contamination by the presence of the US. Half of the rats in each group were given US presentations to the left periorbital region. The other half of rats in each group were given US presentations to the right periorbital region. For rats given paired training, the offset of the CS coincided with the onset of the US yielding a non-overlapping 400 msec interstimulus interval. For rats given paired training, trials were separated by a variable intertrial interval (ITI) that averaged 30 sec. For rats given unpaired training, CS and US presentations were separated by a variable ITI that averaged 15 sec.
Responses
CRs were defined as electromyography (EMG) activity that exceeded a threshold of 0.4 units (amplified and integrated units in volts) above the baseline mean during the CS period after 80 ms. CRs during CS-US trials were defined as responses obtained after the baseline period, but before the onset of the US. Measurements of CR amplitude, onset latency and peak latency were taken from CS-alone probe trials. Measurements of UR amplitude and peak latency were taken from trials when a US occurred. Onset latency of a CR was defined as the initial EMG activity that exceeded threshold after the baseline period. Peak latency of a CR and UR were defined as the moment when the response reached maximum amplitude.
Results
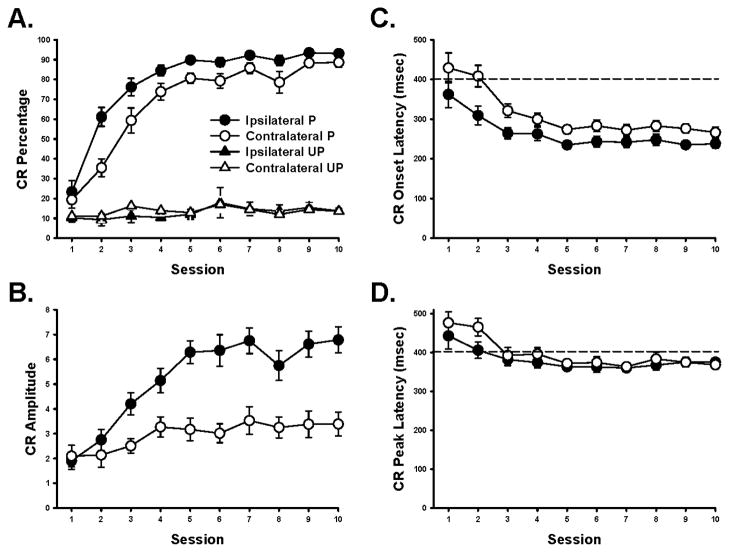
Rats that received paired CS-US training acquired high CR percentages in both eyelids relative to the rats given unpaired training (Figure 1A). Paired training resulted in a high percentage of CRs (~85%) by the tenth session of training in both eyelids, whereas unpaired training only produced baseline responding (~10%). This difference was confirmed with an ANOVA that showed a significant interaction between the group, session and eye (ipsilateral vs contralateral) variables, F(9, 198) = 3.69, p < 0.01. Follow-up tests (Tukey’s honestly significant difference, HSD) showed that the rats in the paired group had significantly more CRs on sessions 2–10 than unpaired control rats (all comparisons, p < 0.05).
Figure 1.
Mean (± SEM) conditioned response (CR) percentage (A), amplitude (B), onset latency (C) and peak latency (D) recorded in the eyelids that were ipsilateral (black plots) and contralateral (white plots) to the location of the unconditioned stimulus (US). Rats were given either paired (P; circles) or unpaired (UP; triangles) training with a tone or light CS and the US. The timescale for the CR onset and peak latency data is relative to the onset of the conditioned stimulus. The horizontal dashed line shows the onset of the US.
Differences in percentage and topography of eyeblink CRs were found between the eyelids that were ipsilateral and contralateral to the US for rats in the paired group. Measurements of CR amplitude, onset latency and peak latency were obtained from CS-alone probe trials when a CR was produced. Topographical measurements of CRs in the unpaired group were not analyzed because these rats produced very few responses (~10%). For rats in the paired group, acquisition was faster (Figure 1A), response amplitudes were greater (Figure 1B) and onset latencies were shorter (Figure 1C) in the eyelid that was ipsilateral to the US. These findings were confirmed with separate ANOVAs that revealed significant interactions between the eye and session factors for CR percentage F(9, 135) = 6.68, p < 0.01, CR amplitude, F(9, 81) = 2.31, p < 0.03, and CR onset latency, F(9, 81) = 2.24, p < 0.03. However, CR peak latencies did not differ significantly indicating that terminal closure of the eyelids was highly synchronized (Figure 1D). Follow-up tests (Tukey’s HSD) showed that CR percentage in the ipsilateral eyelid was greater than the contralateral eyelid on sessions 2–3, p < 0.05. CR amplitudes were also significantly greater in the ipsilateral eyelid than the contralateral eyelid during sessions 3–10, and CR onset latencies were shorter in the ipsilateral eyelid relative to the contralateral eyelid for all ten training sessions (all comparisons, p < 0.05).
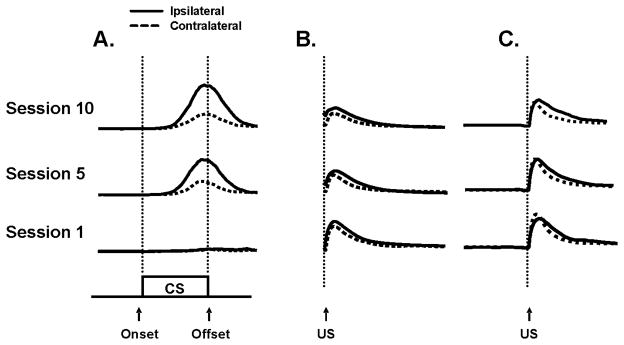
Composite eyelid traces recorded from the ipsilateral and contralateral eyelids from CS-alone probe trials during sessions 1, 5 and 10 are shown in Figure 2A. The eyelid traces in Figure 2A were derived from the averaged eyelid activity recorded during the CS-alone probe trials for all rats that received paired training. Comparison between the eyelid traces revealed an increase in CR amplitude across training sessions in both eyelids, but eyeblink CR amplitudes observed in the ipsilateral eyelid were much larger, and response latencies were shorter, than the responses observed in the contralateral eyelid.
Figure 2.
Composite EMG activity for the eyelids that were ipsilateral (solid line) and contralateral (dashed line) to the location of the unconditioned stimulus (US) during sessions 1, 5 and 10. Eyelid responses were elicited by the conditioned stimulus (CS) on CS-alone probe trials (A) and to the US on paired trials (B) for rats given paired training. Eyelid responses were elicited to US-alone presentations (C) for rats given unpaired training.
The US was effective for eliciting the UR in both eyelids. EMG amplification was gated during the US to avoid the electrical artifact, which resulted in a gap of 25 msec with no response data. It is therefore likely that the UR was predominately the R2 component of the EMG response to the US (Pellegrini, Horn, & Evinger, 1995). Overall the percentage of URs was greater in the eyelid ipsilateral to the US relative to the contralateral eyelid. An ANOVA using the group, session and eyelid factors confirmed this observation, F(1,22) = 15.6, p < 0.01. For rats given paired training the percentage of URs in the eyelid ipsilateral to the US was 99.2 and the percentage of URs in the contralateral eyelid was 92.5. Similar UR percentages were observed between the ipsilateral and contralateral eyelids for the rats given unpaired training (99.2 and 93.4, respectively). UR peak latencies for both eyelids were the same in both groups, again demonstrating that terminal closure of the eyelids was highly synchronized. The UR amplitudes in the ipsilateral eyelid to the US were slightly larger than those observed in the contralateral eyelid for rats given paired training. However, the difference was only marginally significant, F(1, 15) = 4.2, p = 0.059. There was no significant difference in UR amplitude between the eyelids during unpaired during. There was no main effect for the eye factor for UR peak latencies. It was not possible to analyze UR onset data precisely because EMG activity was not recorded during the US. Figure 2 shows composite eyelid traces to the US presentation for the ipsilateral and contralateral eyelids during paired (B) and unpaired (C) training during sessions 1, 5 and 10. An ANOVA with the eyelid and session factors revealed that a decrease in UR amplitude occurred during paired training, F(9, 135) = 3.874, p < 0.01. Follow-up tests (Tukey’s HSD) showed that UR amplitudes were significantly greater on session 1 than sessions 5 and 10 (p < 0.05). No significant changes in UR amplitude were observed throughout unpaired training.
Discussion
The present study demonstrated that eyeblink conditioning in rats with a unilateral US produces bilateral CRs and URs. The rate of acquisition was faster and overall percentage of CRs was greater in the eyelid that was ipsilateral to the US. These findings show that expression of bilateral eyeblink CRs in rats is more similar to humans and monkeys, than rabbits and dogs. CRs in the eyelid ipsilateral to the US had shorter onset latencies and larger amplitudes than CRs in the contralateral eyelid. These results are consistent with previous findings with humans, monkeys, and rabbits (Hilgard & Marquis, 1936; Disterhoft et al, 1977; McCormick et al. 1982). Both eyelids also consistently showed high percentages of URs to the US, and the UR amplitude decreased across training sessions. The reduction in UR amplitude observed in the current experiment, also referred to as conditioned diminution of the UR, is consistent with findings previously reported in rabbits (Donegan, 1981; Kimble & Ost, 1961; Canli, Detmer, Donegan, 1992). Specifically, increased levels of associative responding to the CS have been shown to produce a reduction in the amplitude of the eyeblink UR.
Rats acquired high percentages of CRs in both eyes during eyeblink conditioning with a unilateral US, which has been found in both humans and monkeys. Rabbits and dogs, however, express substantially fewer CRs in the nonreinforced eye compared to the reinforced eye. Numerous studies with rabbits have shown that the percentage of eyeblink responses in the contralateral eyelid rarely exceed frequencies much greater than spontaneous blinking (Brandon et al., 1994; Disterhoft et al., 1977; Kettlewell, O’Connell, & Berger, 1974; Pearce, Montgomery, & Dickinson, 1981). However, a recent study showed that rabbits can acquire higher percentages of contralateral eyeblink CRs above spontaneous blinking rates, although they still occurred much less frequently than in the ipsilateral eye (Lee et al., 2008). Nevertheless, a consistent finding in most species is that CR amplitudes are smaller and onset latencies are longer in the contralateral eye.
It has been previously shown that elicitation of the reflexive blink response in the contralateral eyelid requires approximately three times the stimulation threshold that is sufficient to elicit a UR in the ipsilateral eyelid (Pellegrini et al., 1995). Although not tested in the present study, it is possible that a lower intensity USs would produce weaker conditioning in the contralateral eyelid. A previous study showed that conditioning of the ipsilateral eye with a weak US produces significant savings in the contralateral eye when the US (of a greater intensity) is subsequently used on that side (Kettlewell et al., 1974). This result implies that a memory of the eyeblink CR may be formed in the contralateral cerebellum even when training is given with a relatively weak US on the opposite side. As previously suggested by Lee et al (2008), additional studies will be necessary to determine the precise neural mechanisms for the lateralization of eyeblink conditioning.
The brain areas necessary for acquiring delay eyeblink conditioning are located within the cerebellum and its interconnected brainstem nuclei (Christian & Thompson, 2003; Hesslow, Svensson, & Ivarsson, 1999; Steinmetz, Lavond, & Thompson, 1989; Steinmetz, Logan, Rosen, Thompson, Lavond, & Thompson, 1987; Mauk, Steinmetz, & Thompson, 1986; Steinmetz, Rosen, Chapman, Lavond, & Thompson, 1986). Functional lateralization has been observed within the cerebellar circuitry that is necessary for eyeblink conditioning in many species. For example, permanent or reversible lesions in one cerebellar hemisphere (i.e., interpositus nucleus) have been shown to prevent eyeblink conditioning of the ipsilateral eye in rabbits (Krupa & Thompson, 1997; Krupa, Thompson, & Thompson, 1993; McCormick, Clark, Lavond, & Thompson, 1982) and also in rats (Campolattaro & Freeman, 2008, 2009; Freeman et al., 2005). Studies with rabbits have also shown that unilateral lesions to the interpositus nucleus have no effect on conditioning of the contralateral eye (Clark, McCormick, Lavond, & Thompson, 1984; Lincoln, McCormick, & Thompson, 1982; Polenchar, Patterson, Lavond, & Thompson, 1985; Steinmetz, Logue, & Steinmetz, 1992).
The memory trace of the eyeblink CR may form in both cerebellar hemispheres during training, although it tends to be dominant on the side ipsilateral to the US. For example, learning-related neuronal activity occurs within both cerebellar hemispheres during eyeblink conditioning with rabbits, but inactivation of the interpositus nucleus ipsilateral to the US abolishes learning-related activity in the contralateral cerebellar hemisphere (Clark, Zhang, & Lavond, 1997). Similarly, functional imaging studies have shown that metabolic changes occur within both cerebellar cortical hemispheres during eyeblink conditioning in rabbits (Miller, Chen, Li et al., 2003) and also humans (Molchan, Sunderland, McIntosh, Herscovitch, & Schreurs, 1994; Schreurs, McIntosh, Bahro, Herscovitch, Sunderland, & Molchan, 1997), but that the changes are larger in the hemisphere that is ipsilateral to the US. The Miller et al., (2003) study specifically showed that the bilateral metabolic changes in deep cerebellar nuclei (i.e., interpositus nuclei) occurred early during training, but became more lateralized to the side that was ipsilateral to the US later in training (Miller et al., 2003). Together, these results support the hypothesis that processing of eyeblink conditioning is lateralized to the cerebellar hemisphere that is ipsilateral to the US, but that learning related changes can occur in both hemispheres as a result of unilateral training.
Learning in the contralateral cerebellar hemisphere may play an important role in establishing savings in the contralateral eye (Clark et al., 1997; Gruart & Yeo, 1995; Lee et al., 2008; Ivarsson et al., 1997; Pearce et al., 1981). That is, relatively few training trials are needed to acquire conditioning when training is subsequently switched to the contralateral eye because some plasticity had already occurred in the contralateral cerebellum during initial training. Consistent with this hypothesis is the finding that post-training lesions of the ipsilateral cerebellar interpositus nucleus do not affect savings of eyeblink conditioning when training is switched to the contralateral eye (McCormick, Lavond, Clark, Kettner, Rising, & Thompson, 1981; Lavond, Kanzawa, Ivkovich, & Clark, 1994). However, savings in the contralateral eye does not occur if lesions are made in the ipsilateral cerebellar hemisphere to the US before initial training, which suggests that plasticity must be established in the ipsilateral hemisphere before it is induced within the contralateral hemisphere (Lincoln et al., 1982).
The typical eyeblink conditioning experiment is conducted with a US directed at one eye and a CS that is presented in the ambient environment. CS information is relayed to the cerebellum as mossy fiber projections from the pontine nuclei (Steinmetz et al., 1986; Steinmetz et al., 1987; Steinmetz et al. 1989; Hesslow et al., 1999), and US information reaches the cerebellum via climbing fibers projections from the inferior olive (IO) (Mauk et al., 1986). Converging activation of the ipsilateral mossy fiber and climbing fiber pathways in the cerebellum induces the plasticity for acquiring and storing the eyeblink CR memory for the trained eye (Gould, Sears, & Steinmetz; 1993; Mauk & Donegan, 1997; Steinmetz et al., 1989). Recently, a similar mechanism has been proposed to account for formation of the eyeblink CR memory in the contralateral eyelid (Lee et al., 2008). Specifically, the Lee et al. (2008) study proposed that CS and US inputs also converge in the contralateral cerebellar hemisphere to induce a memory of the eyeblink CR.
Neuroanatomical studies have shown that the climbing fiber projections from the IO to the contralateral deep cerebellar nuclei (ipsilateral to the US location) are more prominent than projections to the ipsilateral deep cerebellar nuclei (contralateral to the US location) in both rats (Ruigrok & Voogd, 1990; Sugihara, Wu, & Shinoda, 1999) and rabbits (Tracy, Thompson, Krupa, & Thompson, 1998). A sparse projection from the IO to the ipsilateral cerebellar cortex (lobule HVI) has been found in the rat (Sugihara et al., 1998), but not in the rabbit (Yeo, Hardiman, & Glickstein, 1985; Rosenfield & Moore, 1995). A possible mechanism underlying the difference in the magnitude of learning in the contralateral eyelid found between rats and rabbits is the different distribution of climbing fiber projections to the ipsilateral cerebellum in these species.
In conclusion, the present experiment demonstrated that CRs and URs are expressed in both eyelids during unilateral eyeblink conditioning in rats, but conditioning is more prominent in the eyelid that is ipsilateral to the US. The overall lateralization of eyeblink conditioning observed in rats is consistent with previous findings with rabbits, humans, monkeys and dogs. Additional research is needed to investigate the neural mechanisms responsible for the lateralization of eyeblink conditioning, which may provide insights about the mechanisms that underlie the facilitated learning that occurs during transfer of training from one eyelid to the other.
Acknowledgments
This research was supported by National Institute for Mental Health grant 080005 to JHF.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Bao S, Chen L, Thompson RF. Classical eyeblink conditioning in two strains of mice: Conditioned responses, sensitization, and spontaneous eyeblinks. Behavioral Neuroscience. 1998;112:714–718. doi: 10.1037//0735-7044.112.3.714. [DOI] [PubMed] [Google Scholar]
- Brandon SE, Betts SL, Wagner AR. Discriminated lateralized eyeblink conditioning in the rabbit: An experimental context for separating specific and general associative influences. Journal of Experimental Psychology Animal Behavior Processes. 1994;20:292–307. doi: 10.1037//0097-7403.20.3.292. [DOI] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH. Eyeblink conditioning in 12-day-old rats using pontine stimulation as the conditioned stimulus. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8120–8123. doi: 10.1073/pnas.0712006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH. Cerebellar inactivation impairs cross modal savings of eyeblink conditioning. Behavioral Neuroscience. 2009;123:292–302. doi: 10.1037/a0014483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Schnitker KM, Freeman JH. Changes in inhibition during differential eyeblink conditioning with increased training. Learning & Behavior. 2008;36:159–165. doi: 10.3758/lb.36.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Detmer WM, Donegan NH. Potentiation or diminution of discrete motor unconditioned responses (rabbit eyeblink) to an aversive pavlovian unconditioned stimulus by two associative processes: Conditioned fear and a conditioned diminution of unconditioned stimulus processing. Behavioral Neuroscience. 1992;106:498–508. doi: 10.1037//0735-7044.106.3.498. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: Acquisition and retention. Learning & Memory. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Clark GA, McCormick DA, Lavond DG, Thompson RF. Effects of lesions of cerebellar nuclei on conditioned behavioral and hippocampal neuronal responses. Brain Research. 1984;291:125–136. doi: 10.1016/0006-8993(84)90658-9. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zhang AA, Lavond DG. The importance of cerebellar cortex and facial nucleus in acquisition and retention of eyeblink/NM conditioning: Evidence for critical unilateral regulation of the conditioned response. Neurobiology of Learning and Memory. 1997;67:96–111. doi: 10.1006/nlme.1996.3740. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola S. Trace eyeblink classical conditioning in the monkey: A nonsurgical method and behavioral analysis. Behavioral Neuroscience. 1998;112:1062–1068. doi: 10.1037//0735-7044.112.5.1062. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Kwan HH, Lo WD. Nictitating membrane conditioning to tone in the immobilized albino rabbit. Brain Research. 1977;137:127–143. doi: 10.1016/0006-8993(77)91016-2. [DOI] [PubMed] [Google Scholar]
- Donegan NH. Priming-produced facilitation or diminution of responding to a pavlovian unconditioned stimulus. Journal of Experimental Psychology Animal Behavior Processes. 1981;7:295–312. [PubMed] [Google Scholar]
- Freeman JHJ, Carter CS, Stanton ME. Early cerebellar lesions impair eyeblink conditioning in developing rats: Differential effects of unilateral lesions on postnatal day 10 or 20. Behavioral Neuroscience. 1995;109:893–902. doi: 10.1037//0735-7044.109.5.893. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr, Halverson HE, Poremba A. Differential effects of cerebellar inactivation on eyeblink conditioned excitation and inhibition. The Journal of Neuroscience. 2005;25:889–895. doi: 10.1523/JNEUROSCI.4534-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormezano I, Kehoe EJ, Marshall BS. 20 years of classical-conditioning research with the rabbit. Progress in Psychobiology and Physiological Psychology. 1983;10:197–275. [Google Scholar]
- Gormezano I, Schneiderman N, Deaux E, Fuentes I. Nictitating membrane: Classical conditioning and extinction in the albino rabbit. Science. 1962;138:33–34. doi: 10.1126/science.138.3536.33. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Sears LL, Steinmetz JE. Possible CS and US pathways for rabbit classical eyelid conditioning: Electrophysiological evidence for projections from the pontine nuclei and inferior olive to cerebellar cortex and nuclei. Behavioral and Neural Biology. 1993;60:172–185. doi: 10.1016/0163-1047(93)90285-p. [DOI] [PubMed] [Google Scholar]
- Gruart A, Blázquez P, Delgado-García JM. Kinematics of spontaneous, reflex, and conditioned eyelid movements in the alert cat. Journal of Neurophysiology. 1995;74:226–248. doi: 10.1152/jn.1995.74.1.226. [DOI] [PubMed] [Google Scholar]
- Gruart A, Yeo CH. Cerebellar cortex and eyeblink conditioning: Bilateral regulation of conditioned responses. Experimental Brain Research. 1995;104:431–448. doi: 10.1007/BF00231978. [DOI] [PubMed] [Google Scholar]
- Harrison J, Buchwald J. Eyeblink conditioning deficits in the old cat. Neurobiology of Aging. 1983;4:45–51. doi: 10.1016/0197-4580(83)90053-2. [DOI] [PubMed] [Google Scholar]
- Hesslow G, Svensson P, Ivarsson M. Learned movements elicited by direct stimulation of cerebellar mossy fiber afferents. Neuron. 1999;24:179–185. doi: 10.1016/s0896-6273(00)80831-4. [DOI] [PubMed] [Google Scholar]
- Hilgard ER, Campbell AA. The course of acquisition and retention of conditioned eyelid responses in man. Journal of Experimental Psychology. 1936;19:227–247. [Google Scholar]
- Hilgard ER, Marquis DG. Acquisition, extinction, and retention of conditioned lid responses to light in dogs. Journal of Comparative Psychology. 1935;19:29–58. [Google Scholar]
- Hilgard ER, Marquis DG. Conditioned eyelid responses in monkeys, with a comparison of dog, monkey, and man. Psychological Monographs. 1936;47:186–198. [Google Scholar]
- Hughes B, Schlosberg H. Conditioning in the white rat. IV. the conditioned lid reflex. Journal of Experimental Psychology. 1938;23:641–650. [Google Scholar]
- Ivarsson M, Hesslow G. Bilateral control of the orbicularis oculi muscle by one cerebellar hemisphere in the ferret. Neuroreport. 1993;4:1127–30. [PubMed] [Google Scholar]
- Ivarsson M, Svensson P, Hesslow G. Bilateral disruption of conditioned responses after unilateral blockade of cerebellar output in the decerebrate ferret. Journnal of Physiology. 1997;502:189–201. doi: 10.1111/j.1469-7793.1997.189bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Díaz L, Navarro-López Jde D, Gruart A, Delgado-García JM. Role of cerebellar interpositus nucleus in the genesis and control of reflex and conditioned eyelid responses. The Journal of Neuroscience. 2004;24:9138–9145. doi: 10.1523/JNEUROSCI.2025-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirenhed DA, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. The Journal of Neuroscience. 2007;27:2493–2502. doi: 10.1523/JNEUROSCI.4202-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettlewell NM, O’Connell MF, Berger LH. Bilateral nictitating membrane conditioning in rabbits under asymmetrical levels of cutaneous afferent activity. Physiology & Behavior. 1974;13:27–33. doi: 10.1016/0031-9384(74)90302-3. [DOI] [PubMed] [Google Scholar]
- Kimble GA, Ost JW. A conditioned inhibitory process in eyelid conditioning. Journal of Experimental Psychology. 1961;61:150–156. doi: 10.1037/h0044932. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Suzuki M, Kawahara S, Kirino Y. Age-dependent impairment of delay and trace eyeblink conditioning in mice. Neuroreport. 2001;12:3349–3352. doi: 10.1097/00001756-200110290-00040. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Thompson JK, Thompson RF. Localization of a memory trace in the mammalian brain. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Thompson RF. Reversible inactivation of the cerebellar interpositus nucleus completely prevents acquisition of the classically conditioned eye-blink response. Learning & Memory. 1997;3:545–556. doi: 10.1101/lm.3.6.545. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Kanzawa SA, Ivkovich D, Clark RE. Transfer of learning but not memory after unilateral cerebellar lesion in rabbits. Behavioral Neuroscience. 1994;108:284–293. doi: 10.1037//0735-7044.108.2.284. [DOI] [PubMed] [Google Scholar]
- Lee T, Kim JJ, Wagner AR. Bilateral nature of the conditioned eyeblink response in the rabbit: Behavioral characteristics and potential mechanisms. Behavioral Neuroscience. 2008;122:1306–1317. doi: 10.1037/a0013591. [DOI] [PubMed] [Google Scholar]
- Lincoln JS, McCormick DA, Thompson RF. Ipsilateral cerebellar lesions prevent learning of the classically conditioned nictitating membrane/eyelid response. Brain Research. 1982;242:190–193. doi: 10.1016/0006-8993(82)90510-8. [DOI] [PubMed] [Google Scholar]
- Marquis DG, Hilgard ER. Conditioned responses to light in monkeys after removal of the occipital lobes. Brain: A Journal of Neurology. 1937;60:1–12. [Google Scholar]
- Mauk MD, Donegan NH. A model of pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learning & Memory. 1997;4:130–158. doi: 10.1101/lm.4.1.130. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Steinmetz JE, Thompson RF. Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:5349–5353. doi: 10.1073/pnas.83.14.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Lavond DG, Clark GA, Kettner RE, Rising CE, Thompson RF. The engram found? role of the cerebellum in classical conditioning of nictitating membrane and eyelid responses. Bulletin of the Psychonomic Society. 1981;18:103–105. [Google Scholar]
- McCormick DA, Lavond DG, Thompson RF. Concomitant classical conditioning of the rabbit nictitating membrane and eyelid responses: Correlations and implications. Physiology & Behavior. 1982;28:769–775. doi: 10.1016/0031-9384(82)90192-5. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Chen NK, Li L, Tom B, Weiss C, Disterhoft JF, et al. fMRI of the conscious rabbit during unilateral classical eyeblink conditioning reveals bilateral cerebellar activation. The Journal of Neuroscience. 2003;23:11753–11758. doi: 10.1523/JNEUROSCI.23-37-11753.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molchan SE, Sunderland T, McIntosh AR, Herscovitch P, Schreurs BG. A functional anatomical study of associative learning in humans. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:8122–8126. doi: 10.1073/pnas.91.17.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Neuronal correlates of conditioned inhibition of the eyeblink response in the anterior interpositus nucleus. Behavioral Neuroscience. 2002;116:22–36. [PubMed] [Google Scholar]
- Park JS, Onodera T, Nishimura S, Thompson RF, Itohara S. Molecular evidence for two-stage learning and partial laterality in eyeblink conditioning of mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5549–5554. doi: 10.1073/pnas.0601150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JM, Montgomery A, Dickinson A. Contralateral transfer of inhibitory and excitatory eyelid conditioning in the rabbit. Quarterly Journal of Experimental Psychology Section B-Comparative and Physiological Psychology. 1981;33:45–61. [Google Scholar]
- Pellegrinin JJ, Horn AKE, Evinger C. The trigeminally evoked blink reflex I. Neural circuits. Experimental Brain Research. 1995;107:166–180. doi: 10.1007/BF00230039. [DOI] [PubMed] [Google Scholar]
- Plakke B, Freeman JH, Poremba A. Metabolic mapping of the rat cerebellum during delay and trace eyeblink conditioning. Neurobiology of Learning and Memory. 2007;88:11–18. doi: 10.1016/j.nlm.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polenchar BE, Patterson MM, Lavond DG, Thompson RF. Cerebellar lesions abolish an avoidance response in rabbit. Behavioral and Neural Biology. 1985;44:221–227. doi: 10.1016/s0163-1047(85)90226-2. [DOI] [PubMed] [Google Scholar]
- Rosenfield ME, Moore JW. Connections to cerebellar cortex (Larsell’s HVI) in the rabbit: A WGA-HRP study with implications for classical eyeblink conditioning. Behavioral Neuroscience. 1995;109:1106–1118. doi: 10.1037//0735-7044.109.6.1106. [DOI] [PubMed] [Google Scholar]
- Ruigork TJ, Voogd J. Cerebellar nucleo-olivary projections in the rat: An anterograde tracing study with Phaseolus vulgaris-leucoagglutinin (PHA-L) Journal of Comparative Neurology. 1990;298:315–333. [Google Scholar]
- Schneiderman N, Fuentes I, Gormezano I. Acquisition and extinction of the classically conditioned eyelid response in the albino rabbit. Science. 1962;136:650–652. doi: 10.1126/science.136.3516.650. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, McIntosh AR, Bahro M, Herscovitch P, Sunderland T, Molchan SE. Lateralization and behavioral correlation of changes in regional cerebral blood flow with classical conditioning of the human eyeblink response. Journal of Neurophysiology. 1997;77:2153–2163. doi: 10.1152/jn.1997.77.4.2153. [DOI] [PubMed] [Google Scholar]
- Skelton RW. Bilateral cerebellar lesions disrupt conditioned eyelid responses in unrestrained rats. Behavioral Neuroscience. 1988;102:586–590. doi: 10.1037//0735-7044.102.4.586. [DOI] [PubMed] [Google Scholar]
- Spence KW. Learning and performance in eyelid conditioning as a function of intensity of the UCS. Journal of Experimental Psychology. 1953;45:57–63. doi: 10.1037/h0058815. [DOI] [PubMed] [Google Scholar]
- Spence KW, Trapold MA. Performance in eyelid conditioning as a function of reinforcement schedules and changes in them. Proceedings of the National Academy of Sciences of the United States of America. 1961;47:1860–1868. doi: 10.1073/pnas.47.11.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME, Freeman JHJ, Skelton RW. Eyeblink conditioning in the developing rat. Behavioral Neuroscience. 1992;106:657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Lavond DG, Thompson RF. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3:225–233. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz JE, Logue SF, Steinmetz SS. Rabbit classically conditioned eyelid responses do not reappear after interpositus nucleus lesion and extensive post-lesion training. Behavioural Brain Research. 1992;51:103–114. doi: 10.1016/s0166-4328(05)80317-1. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Rosen DJ, Chapman PF, Lavond DG, Thompson RF. Classical conditioning of the rabbit eyelid response with a mossy-fiber stimulation CS: I. pontine nuclei and middle cerebellar peduncle stimulation. Behavioral Neuroscience. 1986;100:878–887. doi: 10.1037//0735-7044.100.6.878. [DOI] [PubMed] [Google Scholar]
- Sugihara I, Wu HS, Shinoda Y. Morphology of single olivocerebellar axons labeled with biotinylated dextran amine in the rat. Journal of Comparative Neurology. 1999;414:131–148. [PubMed] [Google Scholar]
- Thomas E, Wagner AR. Partial reinforcement of the classically conditioned eyelid response in the rabbit. Journal of Physiological Psychology. 1964;58:157–158. doi: 10.1037/h0048811. [DOI] [PubMed] [Google Scholar]
- Tracy JA, Thompson JK, Krupa DJ, Thompson RF. Evidence of plasticity in the pontocerebellar conditioned stimulus pathway during classical conditioning of the eyeblink response in the rabbit. Behavioral Neuroscience. 1998;112:267–285. doi: 10.1037//0735-7044.112.2.267. [DOI] [PubMed] [Google Scholar]
- Vardaris RM, Fitzgerald RD. Effects of partial reinforcement on a classically conditioned eyeblink response in dogs. Journal of Comparative and Physiological Psychology. 1969;67:531–534. doi: 10.1037/h0027314. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Steinmetz JE. Past, present, and future of human eyeblink classical conditioning. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink classical conditioning: Volume I, applications in humans. Boston: Kluwer; 2000. pp. 1–17. [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit III. Connections of the cerebellar lobule HVI. Experimental Brain Research. 1985;60:114–126. doi: 10.1007/BF00237024. [DOI] [PubMed] [Google Scholar]