Abstract
Translational control through the mammalian target of rapamycin (mTOR) is critical for synaptic plasticity, cell growth, and axon guidance. Recently, it was also shown that mTOR signaling was essential for the maintenance of the sensitivity of subsets of adult sensory neurons. Here, we show that persistent pain states, but not acute pain behavior, are substantially alleviated by centrally administered rapamycin, an inhibitor of the mTOR pathway. We demonstrate that rapamycin modulates nociception by acting on subsets of primary afferents and superficial dorsal horn neurons to reduce both primary afferent sensitivity and central plasticity. We found that the active form of mTOR is present in a subpopulation of myelinated dorsal root axons, but rarely in unmyelinated C-fibers, and heavily expressed in the dorsal horn by lamina I/III projection neurons that are known to mediate the induction and maintenance of pain states. Intrathecal injections of rapamycin inhibited the activation of downstream targets of mTOR in dorsal horn and dorsal roots and reduced the thermal sensitivity of A-fibers. Moreover, in vitro studies showed that rapamycin increased the electrical activation threshold of Aδ-fibers in dorsal roots. Together, our results imply that central rapamycin reduces neuropathic pain by acting both on an mTOR-positive subset of A-nociceptors and lamina I projection neurons and suggest a new pharmacological route for therapeutic intervention in persistent pain states.
Introduction
Local activity-dependent protein translation within dendrites, regulated by the kinase mTOR, the mammalian target of rapamycin, plays a critical role in the modulation of long term plasticity and memory processes (Zheng et al., 2001; Hanz et al., 2003; Klann and Dever, 2004; Willis et al., 2005; Murashov et al., 2007; Costa-Mattioli et al., 2009). Activation of mTOR complexed with the protein raptor (mTORC1) promotes the phosphorylation of mTOR downstream targets, 4E-BP1/2 and S6K. mTORC1 regulates cap-dependent translation of some growth related mRNAs (Gingras et al., 1999) via 4E-BP1/2 phosphorylation and the translation of a subset of mRNAs that contain an oligopyrimidine tract in their 5′ end (TOP mRNAs) via S6K phosphorylation (Ruvinsky and Meyuhas, 2006; Costa-Mattioli et al., 2009). TOP mRNAs largely encode components of the translational machinery, including ribosomal proteins and elongation factors. Deletion of either 4E-BP1/2 and S6K gene in mice results in deficits in synaptic plasticity and long-term memory (Banko et al., 2005; Antion et al., 2008; Costa-Mattioli et al., 2009).
Recently, the contribution of mTOR to axonal regeneration and growth has been recognized and ribosomes (Koenig and Giuditta, 1999; Alvarez, 2001; Martin, 2004; Koenig, 2009), mRNAs (Zheng et al., 2001; Willis et al., 2005), and the enzymatic machinery involved in the regulation of translation (Hengst et al., 2006; Murashov et al., 2007) have been localized to the axonal compartment (Price and Géranton, 2009). Most previous research has concentrated on the role of local translation in damaged or developing axons. For example, peripheral nerve injury was shown to induce the axonal transport of mRNAs into damaged fibers to promote regeneration (Verma et al., 2005; Willis et al., 2005; Toth et al., 2009) as well as the local synthesis of NaV1.8 sodium channel that may be responsible for the increased sensitivity of injured nerve fibers (Thakor et al., 2009).
However, we have recently shown that the sensitivity of some primary afferents can be regulated locally through mTORC1 signaling (Jiménez-Díaz et al., 2008). Injury is followed by the spread of sensitivity into undamaged areas around the site of injury (secondary hyperalgesia). This is generated by changes in the superficial dorsal horn that lead to the amplification of the response of a specific subset of capsaicin-insensitive primary afferent A-fibers (Magerl et al., 2001). It is the sensitivity of this population of sensory fibers that is maintained peripherally by the tonically active mTORC1 signaling pathway (Jiménez-Díaz et al., 2008). Moreover, in this study, rapamycin, which specifically inhibits mTORC1 signaling, was shown to reduce the increased mechanical sensitivity seen in a neuropathic pain model when injected in the hindpaw.
The central application of rapamycin intrathecally over the spinal cord has received some attention and both rapamycin and anisomycin (Kim et al., 1998; Price et al., 2007; Asante et al., 2009) have been shown to reduce formalin-induced pain-related behavior. This was thought to reflect the loss of synaptic plasticity that underlies central sensitization of dorsal horn neurons and accompanies injury and that has been mainly attributed to the inhibition of protein synthesis in spinal neurons. However, it seems likely that reduced mTORC1 activity in the central processes of sensory neurons could also contribute to the attenuation of pain behavior. Here, we examine the subcellular distribution and function of activated mTORC1 in the dorsal horn and dorsal roots and conclude that intrathecal rapamycin has effects at both sites, resulting in a profound reduction in neuropathic pain sensitivity.
Materials and Methods
Subjects
All procedures complied with the UK Animals (Scientific Procedures) Act 1986. Male Sprague Dawley rats [170–200 g; Central Biological Services, University College London, London, UK; postnatal day 18–21 (P18–P21), University of Edinburgh Biological Services, Edinburgh, UK], group housed 5 per cage, were used for all experiments except for electromyographic (EMG) studies when male Wistar rats (280–310 g; University of Bristol, Bristol, UK) were used. Animals were kept in their home cages at 21°C and 55% relative humidity with a light–dark cycle of 12 h (lights on at 08:00 A.M.). Food and water were provided ad libitum. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Behavioral experiments
In all experiments, the observer was blinded to the intrathecal (vehicle vs rapamycin) treatment. Each animal was assigned to one behavioral test only; i.e., was never tested in more than one modality, apart from the spared nerve injury (SNI) groups where Von Frey testing was followed by pinprick.
Mechanical stimulation
Von Frey test.
A series of calibrated Von Frey hairs were applied to the plantar surface of the paw, in ascending order. The threshold was taken as the lowest force required to elicit a response to one of five repetitive stimuli.
Pinprick test.
The point of a safety pin was applied to the plantar surface of the paw at an intensity sufficient to indent but not penetrate the skin. The duration of paw withdrawal was recorded with a minimum arbitrary value of 0.5 s for a brief normal response and a maximum cutoff of 10 s.
Thermal stimulation
Thermal withdrawal latencies were determined as described previously (Hargreaves et al., 1988); four readings were collected from each paw. Withdrawal latencies were defined as the mean of the four readings.
Antibodies and drugs
Anti-phospho-mTOR [P-mTOR, Ser2448; used at a concentration of 1:1000 for immunohistochemistry; catalog number (Cat. No.): 2971], anti-mTOR (1:1000; Cat. No.: 2972), anti-phospho-4E-BP1/2 (P-4E-BP1/2, Thr37/46; 1:1000; Cat. No.: 9459), anti-4E-BP1/2 (1:1000; Cat. No.: 9452), anti-phospho-S6 (P-S6, Ser235/236; 1:1000; Cat. No.: 2211), and anti-S6 (1:1000; Cat. No.: 2217) antibodies were obtained from Cell Signaling Technology. The anti-protein gene product 9.5 antibody (PGP; 1:500; Cat. No.: RA95101 was obtained from Ultraclone. The antibody to mouse anti-neurofilament 200 kDa clone N52 (N52; 1:2000; Cat. No.: N0142) was obtained from Sigma. Anti c-Fos antibody (1:60,000) was obtained from Calbiochem and the anti-peripherin (1:250, Cat. No.: 1530) and anti-Gapdh antibodies from Millipore Bioscience Research Reagents. The antibody against neurofascin (Sherman et al., 2005) (Nfc2) was a gift from Peter Brophy (University of Edinburgh, Edinburgh, UK). Both Fluorogold and the antibody against Fluorogold (1:25,000) were purchased from Fluorochrome. Finally, N-vanillylnonanamide (synthetic capsaicin) was purchased from Sigma and rapamycin from LC Laboratories.
Intraplantar injections of capsaicin
N-Vanillylnonanamide (synthetic capsaicin) solution was made up at a concentration of 10 mm in a vehicle of saline containing 10% ethanol and 10% Tween 80. All injections were given in a volume of 10 μl. To prepare for the injection, rats were gently wrapped in a cotton towel with the left hindpaw exposed. During the injection the needle penetrated the skin just distal to the targeted area which was the center of the plantar surface of the left hindpaw. Care was taken to deliver each injection superficially into the skin. Injection of capsaicin, but not of the vehicle, produced immediate “nocifensive” behavior (lifting, licking, and/or shaking of the paw) that lasted 1–3 min.
Spared nerve injury surgery
The SNI was performed as described previously (Decosterd and Woolf, 2000). The common peroneal and tibial nerves were tightly ligated with 5.0 silk and sectioned distal to the ligation. Care was taken to avoid touching or stretching the spared sural nerve. For sham surgery, the sciatic nerve was exposed but no contact was made with the nerve. Behavioral testing began 48 h after surgery and continued for 6 d postsurgery.
Intrathecal injection of rapamycin (for behavioral studies and immunochemistry)
Dose and pretreatment times were optimized in a previous study (Jiménez-Díaz et al., 2008). Under isoflurane anesthesia [1.5–2% isoflurane combined with 100% O2 (1l/min)], rats were placed in a stereotaxic frame and a small incision was made in the atlanto-occipital membrane. A cannula was inserted into the subarachnoid space, terminating in L4–5 region. Animals received either 10 μl of rapamycin (250 μm, 2.3 μg) or vehicle (saline containing 20% ethanol). The cannula was then withdrawn and the wound closed with suture clips.
Electrophysiology
Electromyographic dissociation of A- and C-fiber responses.
A-fiber (myelinated, capsaicin-insensitive) heat nociceptors or C-fiber (unmyelinated, capsaicin-sensitive) heat nociceptors on the dorsal surface of the hindpaw were preferentially stimulated via fast (7.5 ± 1°C s−1) or slow (2.5 ± 1°C s−1) rates of heating, respectively, using a constant bulb voltage as described previously (McMullan et al., 2004; Jiménez-Díaz et al., 2008). The cutoff temperature of the heat lamp was controlled via a Spike2 script to prevent tissue damage. Alternating fast and slow heat ramps were performed at 8 min intervals and threshold temperature at which the withdrawal reflex occurred recorded. Intrathecal injection of 2.3 μg of rapamycin or the vehicle was made once a steady baseline of paw withdrawal thresholds had been achieved, without disconnecting the animal from the set up. Fast and slow heat ramps were resumed and paw withdrawal thresholds measured: a “pair” of fast and slow heat ramps was performed every 30 min, with an 8 min interstimulus interval, and continued for 6 h postinjection.
Extracellular compound action potential recording from dorsal roots.
Unsexed rats (P18–P21) were anesthetized with isoflurane, decapitated, and lumbar (L4/L5) dorsal roots, without dorsal root ganglion, were removed and placed in ice-cold dissection solution. Isolated roots were preincubated in 200 nm rapamycin (Tang et al., 2002; Ehninger et al., 2008) or vehicle (0.1% DMSO in oxygenated recovery solution) for at least 2.5 h before recording (initially at 36−37°C for 1 h then at room temperature). Roots were transferred to a recording chamber and continuously perfused with 200 nm rapamycin or vehicle (0.1% DMSO in oxygenated Krebs) at a flow rate of 1–2 ml/min and recordings were made at room temperature. Saturated Krebs solution in 95% O2/5% CO2 had the following composition (in mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 25 glucose, 1 MgCl2, 2 CaCl2, pH 7.4. Recovery solution was the same as Krebs but with 1.5 CaCl2 plus 6 MgCl2. Dissection solution was the same as recovery but with 1 mm kynurenic acid.
Two suction electrodes were used: one for electrical stimulation of the root and the other for recording compound action potentials. Electrical stimulation (×10) was performed at 0.2 Hz for 0.1 ms using an ISO-Flex Stimulus Isolator (A.M.P.I., Intracel). The stimulation intensities used were (in μA) 1, 2, 3, 4, 5, 7.5, 10, 15, 20, 25 for Aβ-, 30–100 (in increments of 10) for Aδ- and 150–500 (in increments of 50) for C-fiber input (Nakatsuka et al., 2000). Data were recorded and acquired using a Cygnus ER-1 differential amplifier (Cygnus Technology) and pClamp 10 software (Molecular Devices). Data were filtered at 10 kHz and sampled at 50 kHz.
Three main components of the compound action potentials could be distinguished; fast (Aα/β-), medium (Aδ-) and slow (C-) conducting components, each having a triphasic (positive-negative-positive) profile. Sometimes small intermediate components were present among these three groups but these were not analyzed. The threshold of each component was defined as the lowest stimulation intensity at which the negative component was clearly discernible. The amplitude of each component was calculated by measuring the distance between the negative and positive peaks and the conduction velocity was calculated based on the latency to the negative peak at 25, 100, and 500 μA for Aα/β-, Aδ-, and C-components, respectively.
Retrograde labeling of dorsal horn projection neurons
To label projection neurons in the dorsal horn, a retrograde marker, Fluorogold (FG, 4%, 300 nl) was injected into the parabrachial area (Géranton et al., 2007) of adult rats. Rats were placed in a Kopf stereotaxic frame under isoflurane anesthesia and a small incision was made in the scalp to expose the skull and reveal bregma. Following craniotomy, animals received an injection of Fluorogold into the parabrachial area (mm: AP −9.2, ML ±1.7, DV −6.4, both sides) (Paxinos et al., 1985) delivered by a Hamilton syringe of a total volume of 2.5 μl. Rats were allowed to recover from anesthesia in an incubation chamber and then transferred back to their home cage for 3 d. On the third day after surgery, rats were perfused, as described below.
Immunohistochemistry
For immunohistochemistry, rats were deeply anesthetized with pentobarbital and perfused transcardially. The spinal cord, and dorsal roots were dissected out, postfixed in the same PFA solution for 2 h and transferred into a 30% sucrose solution in PB containing 0.01% azide, until further processing and for a minimum of 24 h. Tissue was cut on a freezing microtome at 40 μm. A tyramide signal amplification based protocol was followed for all primary antibodies apart from c-Fos (Jiménez-Díaz et al., 2008). For double labeling with PGP, N52, Nfc2, peripherin and Fluorogold antibody, P-mTOR (as well as P-S6 and P-4EBP1/2) labeled sections were left overnight in a solution of PGP (1:500), N52 (1:2000), Nfc2 (1:3000), peripherin (1:250) or Fluorogold antibody (1:25,000) and processed as before (Géranton et al., 2007; Jiménez-Díaz et al., 2008).
For c-Fos immunohistochemistry, sections were incubated for 1 h in 3% normal goat serum, 0.3% Triton X-100 and 0.6% of hydrogen peroxide in 0.1 m PB. Then, sections were incubated overnight in a rabbit polyclonal antibody anti-c-Fos (1:60,000; Ab-5; Calbiochem). Sections were washed in 0.1 m PB and incubated for 1 h in a goat anti-rabbit biotinylated antibody (Vector Laboratories; 1:500). Sections were washed again and incubated for 2 h in avidin-biotin peroxidase complex (ABC Elite; Vectastain, Vector Laboratories). Following washes, sections were incubated for 5 min in a solution containing 0.05% of 3,3′ diaminobenzidine (DAB) and 0.2% ammonium nickel sulfate in H2O. The reaction was stopped by washes in H2O and then PB and sections were mounted on gelatin-coated slides and coverslipped.
Controls included omission of the first or second primary antibodies or addition of blocking peptides when available (P-mTOR and P-S6). We also confirmed antibody specificity by Western blot. Single or double bands of appropriate molecular weight were found for mTOR, P-mTOR, 4E-BP1/2, P-4E-BP1/2 and S6 and P-S6 protein.
Cell counting
To count the number of c-Fos-labeled and P-S6-labeled cells, the superficial dorsal horn was divided into three ipsilateral domains: the superficial laminae I-II, laminae III-IV and the deep laminae V-VII. For each rat, Fos- or P-S6 immunoreactive (Fos-IR or P-S6-IR) neurons counted in the 3–5 most labeled sections were averaged, and the mean was used for further statistical analysis.
Image analysis and quantification of immunofluorescence
All images of double stained tissue were acquired by confocal microscopy using a laser scanning microscope (Leica TCS NT SP) as described before (Jiménez-Díaz et al., 2008). Postacquisition processing was performed with Adobe Photoshop and Adobe Illustrator.
Tissue collection and immunoblotting
For fresh tissue collection, animals were terminally anesthetized with CO2. The L4, L5 and L6 dorsal roots ganglia (DRGs), their central processes proximal to the spinal cord and the superficial dorsal horn L4-L6 were dissected out and frozen on dry ice in three separate collecting tubes. Samples were then stored at – 80°C until further processing. For protein extraction, one dorsal horn sample was homogenized in 500 μl of lysis buffer, DRGs (6 DRGs per sample) in 200 μl of lysis buffer as well as the corresponding dorsal roots (six 0.5 cm root sections corresponding to L4, L5 and L6, proximal to the cord) and incubated on ice for 2 h. Samples were then centrifuged at 13 000 rpm for 15 min and supernatants collected. Total protein concentration was assessed using a bicinchoninic acid (BCA) protein assay kit (Pierce Biotechnology) before each preparation of protein samples. Samples (20 μg of proteins per well for spinal cord and 30 μg of proteins per well for DRGs and roots) were run on 10% Bis-Tris gels (Biorad Laboratories) for detection of P-4E-BP1/2, 4E-BP1/2, P-S6 and S6 protein. Proteins were transferred onto a PVDF membrane (Biorad). Membranes were blocked and incubated. HRP activity was visualized by applying a chemiluminescent substrate (ECL; GE Healthcare Pharmacia Biotech) and using Chemi Doc XRS from Biorad. Membranes were then washed and incubated with Gapdh antibody (1:1000) for 45 min, and further processed as described above. Signal intensity was measured using Quantity One software (Biorad). For each sample and each membrane signal for P-4E-BP1/2, 4E-BP1/2, P-S6 and S6 protein was normalized with the intensity of the corresponding Gapdh signal. The signal for each phosphorylated antibody was then normalized with that of the corresponding antibody for the total protein (except for the roots samples where the signal for S6 protein was too weak). The mean value obtained for the vehicle treatment at 30 min was arbitrarily set at 100%. For an extra confirmation of equal loading of proteins, membranes were stained with Coomassie dye and staining of the wells visually compared.
Statistical data analysis
All statistical tests were performed in SPSS PC+. Repeated-measures ANOVA followed by Tukey or Bonferroni post hoc analysis where appropriate was used to analyze all behavioral data, including EMG studies. The Greenhouse–Geisser ‘ε’ correction was applied to compensate for any violation of sphericity. If the Levene's test for normal distribution was significant then data were normalized by logarithmic (log2) transformation. Data were always analyzed as presented in the figures (raw data or log2 transformed). For the compound action potential experiments, threshold values for the activation of fibers were compared by Student's t test within each fiber group. For cell counting, the number of labeled cells was analyzed by univariate analysis. For Western blots, normalized signals were compared in vehicle- and rapamycin-treated animals by a Student's t test (4E-BP1/2, P-4E-BP1/2) or multivariate analysis followed by appropriate post hoc tests (S6, P-S6 protein).
Results
Acute nociceptive thresholds and primary sensitization are not influenced by intrathecal injection of rapamycin
Injection of capsaicin produces peripheral sensitization of C- and some Aδ-nociceptors and drives the central sensitization of dorsal horn neurons. Here we show that sensory neurons response to capsaicin stimulation is unaffected by rapamycin pretreatment.
First, thermal and mechanical thresholds were monitored 1–24 h after intrathecal administration of rapamycin (10 μl of 250 μm, i.e., 2.3 μg) into the lumbar spinal cord (Fig. 1). Rapamycin (or vehicle) did not modify thermal (Fig. 1A1) and mechanical (Fig. 1B1, C1) thresholds at any time point.
Figure 1.
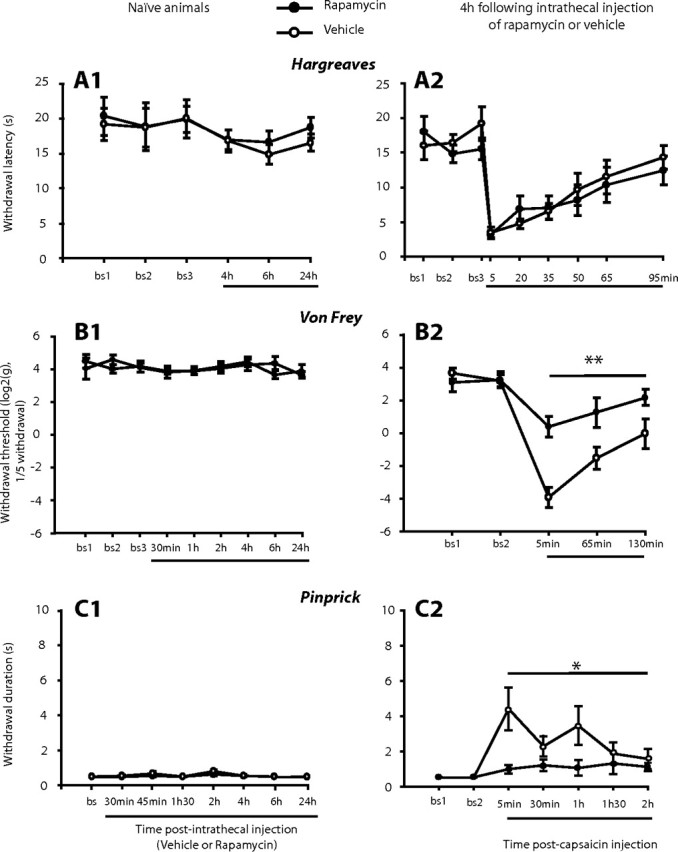
Intrathecal administration of rapamycin does not affect acute pain but reduces capsaicin-induced secondary but not primary hyperalgesia. A1, B1, C1, Effects of intrathecal injection of rapamycin (or vehicle) in naive rats on the following: withdrawal latency to heat using the Hargreaves test (A1; N = 6 in each group), mechanical withdrawal threshold measured with Von Frey hairs (B1; N = 8 in each group), and withdrawal response duration after nociceptive mechanical stimulation (pinprick stimulus) of the plantar surface of the paw (C1; N = 4 in each group). A2, B2, C2, Rapamycin (or vehicle) was delivered 4 h before the administration of capsaicin in the center of the plantar surface of the paw. Effects of intrathecal injection of rapamycin (or vehicle) on the following: withdrawal latency to heat after capsaicin (A2, N = 6 in each group), lateral mechanical withdrawal threshold after capsaicin injection measured with Von Frey hairs (B2, N = 7 in each group), withdrawal response duration to lateral pinprick stimulation after capsaicin (C2, N = 9–10). Mean ± SEM for the injected (left) hindpaw is illustrated in each panel. *p < 0.05, **p < 0.01.
We then measured the effects of rapamycin on the development of thermal primary hyperalgesia that follows capsaicin injections into the paw. Rapamycin was given 4 h before subsequent treatments. Capsaicin on its own increased thermal sensitivity (i.e., induced primary hyperalgesia) for up to 35 min after intraplantar injection. No significant changes in withdrawal latency were seen in the contralateral hindpaw (Jiménez-Díaz et al., 2008). Intrathecal pretreatment with rapamycin or vehicle 4 h before capsaicin did not change the increased thermal sensitivity that follows capsaicin injection (Fig. 1A2).
The effects of intrathecally administered rapamycin on the C-fibers activation by capsaicin injection were studied with c-Fos immunoreactivity (c-Fos-IR). Fos expression has been widely used to map neuronal activity within nociceptive pathways (Hunt et al., 1987). c-Fos-IR was analyzed 2 h after capsaicin injection which occurred 4 h after the intrathecal administration of rapamycin or vehicle. Capsaicin injection induced the expected pattern of c-Fos expression in the dorsal horn but there was no difference between rapamycin and vehicle pretreated animals in any of the three ipsilateral lamina domains studied (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). Together, these results suggest that the capsaicin induced stimulation of C-fiber nociceptors is not modulated by rapamycin.
Intrathecally administered rapamycin blocks the spread of hypersensitivity into undamaged cutaneous areas (secondary mechanical hyperalgesia)
The increased mechanical sensitivity that occurs around the area of capsaicin injection (secondary mechanical hyperalgesia) is mainly mediated by a subset of capsaicin-insensitive A-nociceptors, the response of which is amplified by sensitized dorsal horn neurons (Magerl et al., 2001; Jiménez-Díaz et al., 2008). Here we show that rapamycin specifically reduces this secondary hyperalgesia.
To determine response thresholds, both Von Frey hairs, which cover the spectrum of both A- and C-fiber mechanical response thresholds, and pinprick test, a more specific stimulus for A-fiber nociceptors, were used.
Von Frey hair testing
Intrathecal injection of rapamycin 4 h before capsaicin injection reduced the capsaicin induced secondary hyperalgesia from 5 min to 2 h after capsaicin (F(1,12) = 14.4, p < 0.01 vs vehicle pretreatment) (Fig. 1B2).
Response to pinprick
To confirm that secondary mechanical hyperalgesia can be largely abolished by rapamycin pretreatment we examined the response to pinprick, a more specific stimulus for A-fiber nociceptors (Magerl et al., 2001). Capsaicin alone increased withdrawal duration to the pinprick stimulus in the area of secondary hyperalgesia for up to 2 h after intraplantar injection. Intrathecal administration of rapamycin 4 h before capsaicin injection greatly reduced capsaicin-induced secondary hyperalgesia (Fig. 1C2) (F(1,17) = 7.5, p < 0.05 vs vehicle pretreatment).
Rapamycin reduces mechanical sensitivity in a rat model of chronic pain
We next show that neuropathic pain, that shares many features with secondary hyperalgesia (Pertovaara, 1998; Urban and Gebhart, 1999), is also alleviated by central rapamycin treatment.
Following SNI, rats showed an enhanced response to Von Frey and pinprick stimulation in the lateral part of the hindpaw, the sural territory, from day 2 to day 6 after surgery (Fig. 2A,B). On day 6, animals received an intrathecal injection of 2.3 μg of rapamycin or vehicle.
Figure 2.
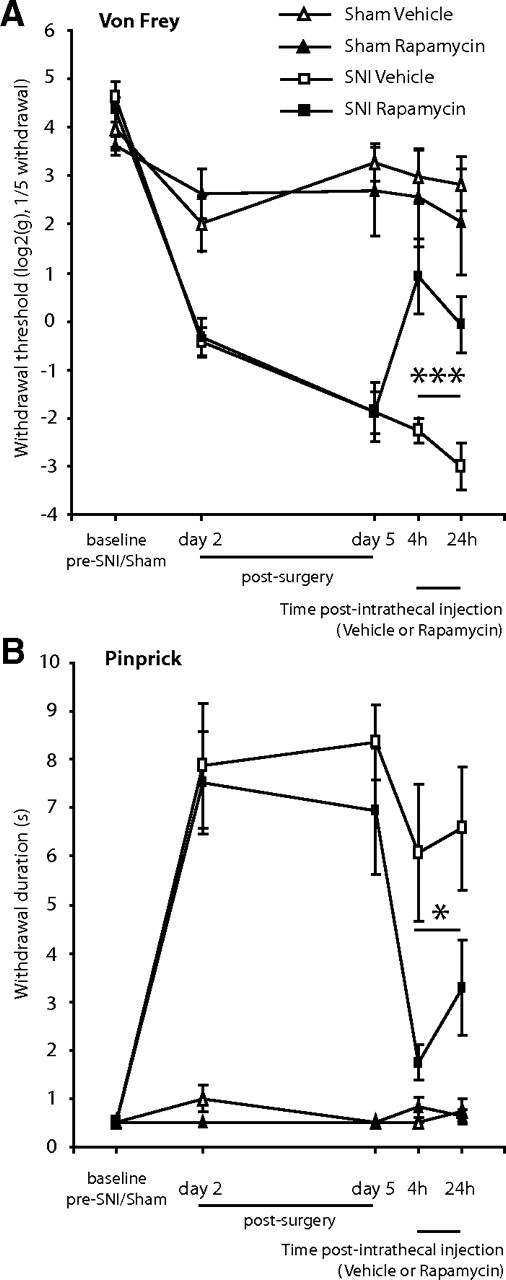
Intrathecal administration of rapamycin attenuates mechanical hyperalgesia in the SNI model. Effect of intrathecal injection of rapamycin, or its vehicle on, A, mechanical withdrawal threshold measured with Von Frey hairs, and B, withdrawal response duration (s) after nociceptive mechanical stimulation (pinprick stimulus) of the lateral plantar surface of the paw of SNI animals, or sham animals. Mean ± SEM is illustrated. N = 3–4 in sham groups, N = 7–8 in SNI groups. *p < 0.05, ***p < 0.001.
Von Frey hair testing
Rapamycin treatment resulted in a substantial increase in mechanical thresholds 4–24 h postinjection when compared with vehicle treatment (post hoc SNI rapamycin vs SNI vehicle F(1,13) = 17.5; p < 0.001) (Fig. 2A), with a maximum reduction seen at 4 h. With a single time point post hoc analysis, the effects of rapamycin were significant at both the 4 and 24 h time points (p < 0.01 at both time points).
Response to pinprick
Rapamycin treatment resulted in a decrease in the amount of time the animals were holding up their paw following pricking stimulation, 4–24 h postinjection, when compared with vehicle treatment (post hoc SNI rapamycin vs SNI vehicle F(1,13) = 7.8; p < 0.05) (Fig. 2B), with a maximum reduction compared to vehicle of 61% seen at 4 h. With a single time point post hoc analysis, the effects of rapamycin were significant at both the 4 and 24 h time points (p < 0.05 at both time points).
Rapamycin increases the thermal and electrical threshold of activation of Aδ-fibers
Our results show that intrathecally delivered rapamycin can reduce the mechanical sensitivity seen both in undamaged tissue around the site of injury and in a model of neuropathic pain. We next investigated the effects of intrathecal administration of rapamycin on the thermal sensitivity of A- and C-fibers in experiments that preferentially activate A- and C-fiber heat nociceptors. We also measured the effect of rapamycin on the electrical activation threshold of C-, Aδ-, and Aβ-fibers in vitro. We show that the sensitivity of a subset of A-fibers is reduced by intrathecal rapamycin.
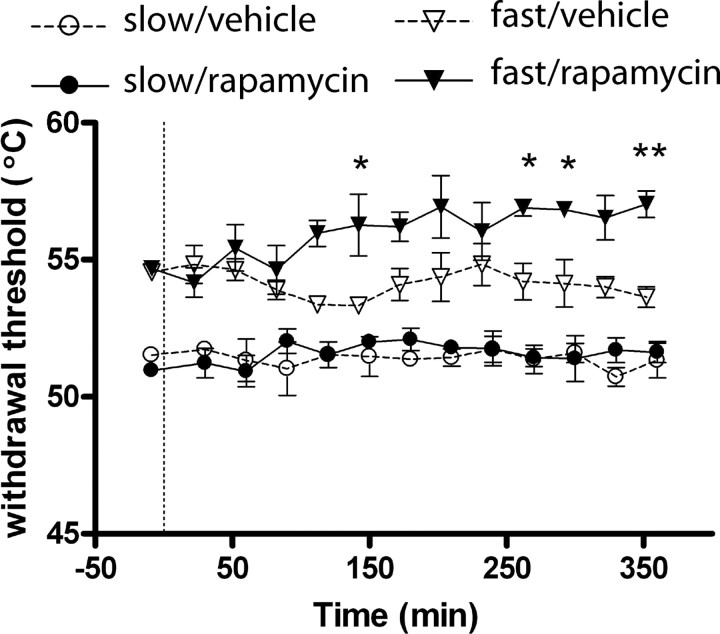
Heat-responsive and capsaicin-insensitive A-nociceptors (that mediate secondary hyperalgesia, i.e., the spread of pain sensitivity around the site of injury) are preferentially activated by a fast heat ramp, whereas C-fibers respond only to a slower heat ramp (McMullan et al., 2004; Leith et al., 2007). Intrathecal injection of rapamycin significantly increased threshold temperatures for paw withdrawal evoked by fast heat ramps (activating A-fiber nociceptors) from 150 min postinjection, compared to control injections of vehicle (Fig. 3) (p < 0.05, Bonferroni post hoc test). In contrast, paw withdrawal thresholds to slow heat ramps (activating C-fiber nociceptors) remained unchanged after rapamycin (Fig. 3).
Figure 3.
Intrathecal administration of rapamycin increases A- but not C-nociceptor-evoked paw withdrawal thresholds. Time course effects of intrathecal injection of rapamycin or vehicle on paw withdrawal thresholds to fast and slow heat ramps that preferentially activate A- and C-nociceptors respectively. N = 3 in each group. Mean ± SEM heat withdrawal threshold (°C) is illustrated in each panel. Vertical dashed line indicates the drug injection time. *p < 0.05; **p < 0.01.
Primary afferents could be divided into three distinct groups, corresponding to Aα/β-, Aδ-, and C-fibers, on the basis of threshold and conduction velocity of compound action potentials recorded extracellularly from the dorsal root (Fig. 4). Rapamycin treatment of isolated dorsal roots (incubation in 200 nm solution) significantly increased the threshold stimulation intensity required to activate the Aδ-component, compared to vehicle-treated roots (vehicle: 47.1 ± 5.7 μA, rapamycin: 67.5 ± 5.3 μA; p < 0.05, unpaired t test). In contrast, there was no significant difference in the threshold values for activation of the Aα/β-component (vehicle: 4.4 ± 0.2 μA, rapamycin: 4.8 ± 0.4 μA) or the C-component (vehicle: 171.4 ± 10.10, rapamycin: 187.5 ± 15.7 μA). The conduction velocity of the Aα/β- (vehicle: 4.4 ± 0.3 m/s, rapamycin: 4.6 ± 0.4 m/s), the Aδ- (vehicle: 0.5 ± 0.08, rapamycin: 0.4 ± 0.01), and the C- (vehicle: 0.2 ± 0.004, rapamycin: 0.2 ± 0.008) fiber components were not significantly altered by rapamycin treatment, and are similar to comparable in vitro recordings (Labrakakis et al., 2003). The amplitudes of the Aα/β- (vehicle: 4.3 ± 1.2, rapamycin: 4.3 ± 1.0), the Aδ- (vehicle: 0.1 ± 0.01, rapamycin 0.2 ± 1.0), and the C- (vehicle: 0.3 ± 0.07, rapamycin 0.3 ± 0.04) fiber components were also unaltered by rapamycin treatment.
Figure 4.
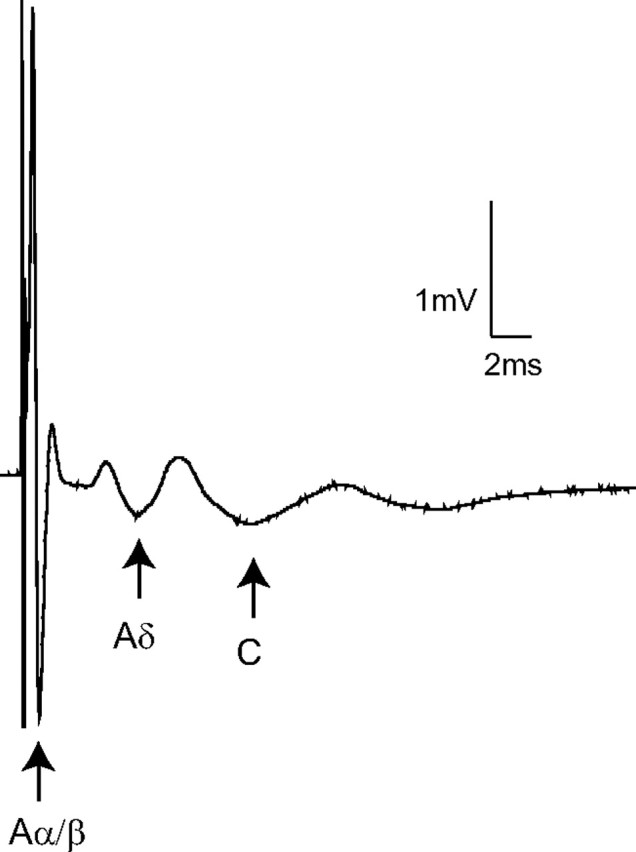
Extracellular compound action potential recordings. Representative compound action potentials recorded from an isolated rapamycin-treated dorsal root illustrating the fast (Aα/β-), medium (Aδ-) and slow (C-) conducting components evoked by 500 μA stimulation (average of 10 traces shown). Arrows indicate the negative peak of each triphasic (positive-negative-positive) profile. In this example the last positive peak of the Aδ-component overlaps the first positive peak of the C-component. The threshold stimulation intensities for the Aα/β-, Aδ-, and C-fiber components were 5, 60, and 200 μA, respectively.
mTOR is present in primary afferent fibers in dorsal roots but not in primary afferent central terminals
Together, our results strongly suggest that local mTORC1 activity regulates the sensitivity of a subset of Aδ-fibers within dorsal roots. We therefore used immunochemical techniques to localize mTOR within dorsal roots and measured the effects of intrathecally administered rapamycin on mTORC1 activity in the roots, spinal cord, and DRG cells.
Immunohistochemical staining of sections of adult rat dorsal roots showed that mTOR and P-mTOR were extensively expressed in subsets of primary afferent sensory fibers as well as in non-neuronal cells of surrounding tissue (Fig. 5). Sensory fibers were identified from costaining with PGP, a general marker for sensory afferents, N52, a specific marker for myelinated fibers and peripherin, a marker for small fibers. The majority of P-mTOR labeled fibers were N52 positive, implying that P-mTOR was largely restricted to myelinated A-fibers. However, a small number of peripherin-positive fibers (<5% of peripherin-positive fibers) were also seen. This may imply expression of mTOR in some C-fibers (Fig. 5D,E), although peripherin also labels small myelinated fibers (Ferri et al., 1990). P-mTOR labeling was found within ∼40% of myelinated axons and was continuous for hundreds of micrometers or could also appear as accumulations along the nerve fibers. Costaining with the neurofascin antibody Nfc2, which labels both axonal (nodal) and glial (paranodal) neurofascins [Nfasc186 and Nfasc155, respectively (Sherman et al., 2005)] demonstrated that in some cases these P-mTOR accumulations were associated with nodes of Ranvier but were also occasionally localized adjacent to the Schmidt-Lanterman incisura of the surrounding Schwann cells (Fig. 5F). Generally, the cytoplasm of Schwann cells stained positive for P-mTOR. Finally, P-mTOR staining in sensory axons could not be traced beyond the dorsal root entry zone (Fig. 5G). Therefore, not surprisingly, double staining spinal cord sections for P-mTOR and synaptophysin, synapsin, or VGLUT1 did not show localization of mTOR in axon terminals (results not shown).
Figure 5.
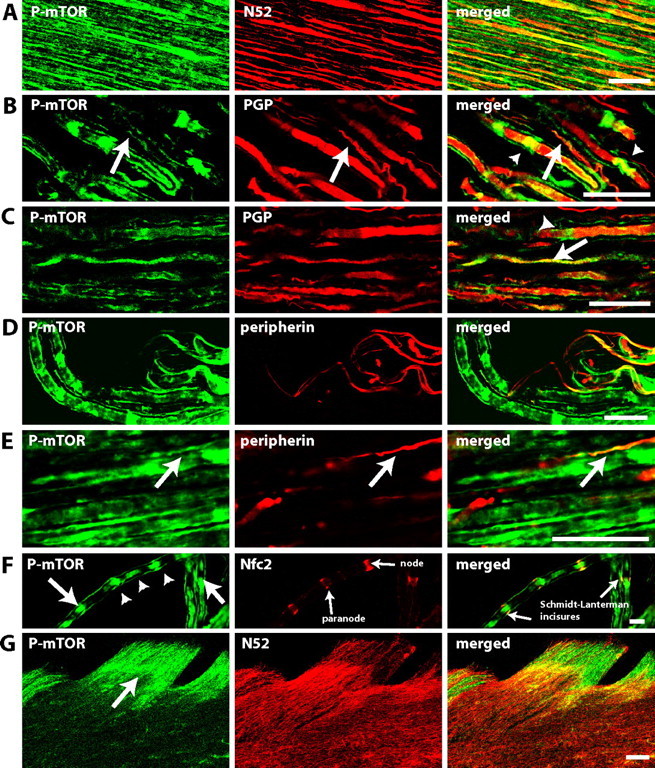
P-mTOR immunoreactivity in dorsal roots is largely found within myelinated fibers. A–F, Confocal images of longitudinal sections of dorsal roots. A, Colocalization of P-mTOR (green) and myelinated fiber marker N52 (red). B, C, Colocalization of P-mTOR (green) and nerve fibers marker PGP (red), a general marker of nerve fibers. P-mTOR staining can be seen along the nerve fibers (arrows) but also in accumulations along the fibers (arrow heads). D, E, Colocalization of P-mTOR (green) and peripherin (red), a marker for small fibers. F, Colocalization of P-mTOR (green) and neurofascin (red), a marker for nodes and paranodes. Axonal accumulations of P-mTOR (arrow head) can be seen in association with nodes of Ranvier but in some cases also localized adjacent to the Schmidt-Lanterman incisures (arrow) of the surrounding Schwann cells. G, Confocal images of horizontal sections of dorsal horn. Pictures show P-mTOR (green) and myelinated fiber marker N52 (red). P-mTOR staining in sensory axons does not extend beyond the dorsal root entry zone (arrow). In A–G the single staining for each antibody and the merged image are shown from left to right and double staining appears in yellow. A–F, Single plane picture. Scale bars: A–E, 25 μm; F, 50 μm; G, 100 μm.
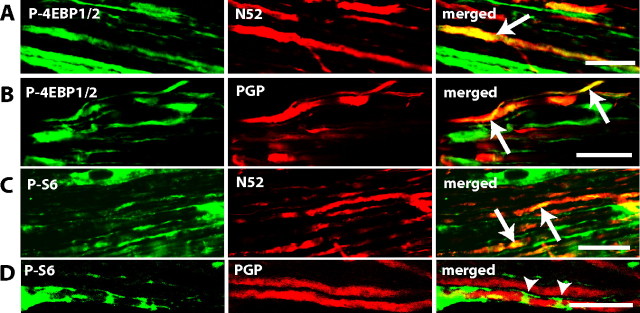
Immunohistochemical staining of sections of adult rat dorsal roots indicated that P-S6 and P-4EBP1/2 followed the same pattern of expression as P-mTOR (Fig. 6).
Figure 6.
P-4EBP1/2 and P-S6 immunoreactivity in dorsal roots is largely found within myelinated fibers. A–D, Confocal images of longitudinal sections of dorsal roots. A, Colocalization of P-4EBP1/2 (green) and myelinated fiber marker N52 (red). B, Colocalization of P-4EBP1/2 (green) and nerve fibers marker PGP (red). C, Colocalization of P-S6 (green) and myelinated fiber marker N52 (red). D, Colocalization of P-S6 (green) and nerve fibers marker PGP (red). P-4EBP1/2 and P-S6 staining can be seen along the nerve fibers but also in accumulations along the fibers. In A–D, the single staining for each antibody and the merged image are shown from left to right and double staining appears in yellow. A–D, Single plane picture. Scale bars: A–D, 25 μm.
Intrathecal administration of rapamycin blocks mTORC1 activity in the dorsal roots but not in DRGs
Activity of mTORC1 was assessed by quantifying phosphorylation of the downstream target S6 protein. Animals received an intrathecal injection of the mTORC1 inhibitor rapamycin or vehicle. Tissue was taken 2 h after the intrathecal injection. Using Western blotting, we found that intrathecal injections of rapamycin reduced phosphorylation of S6 in dorsal roots (supplemental Fig. S2A, available at www.jneurosci.org as supplemental material) (100 ± 29% vs 23 ± 12%, vehicle vs rapamycin; p < 0.05) but not in DRGs (supplemental Fig. S2B, available at www.jneurosci.org as supplemental material) (100 ± 8% vs 98 ± 23%, vehicle vs rapamycin), indicating that mTORC1 activity was inhibited by rapamycin only in the dorsal roots.
P-mTOR, P-S6, and P-4EBP1/2 are strongly expressed in lamina I/III projection neurons
Having established that rapamycin has a profound effect on the sensitivity of subsets of dorsal root fibers, we next looked at the expression of mTOR in the dorsal horn, since this compartment is reached by rapamycin injected intrathecally. P-mTOR, P-S6, and P-4EBP1/2 staining was observed in neuronal and glial cells (Fig. 7). A striking feature of the expression of these three phosphorylated proteins in the superficial dorsal horn was their dense expression within lamina I/III neurons, which lead us to investigate their expression specifically in lamina I/III projection neurons. Indeed, these projection neurons have been shown to be key to the initiation and maintenance of many pain states (Nichols et al., 1999).
Figure 7.
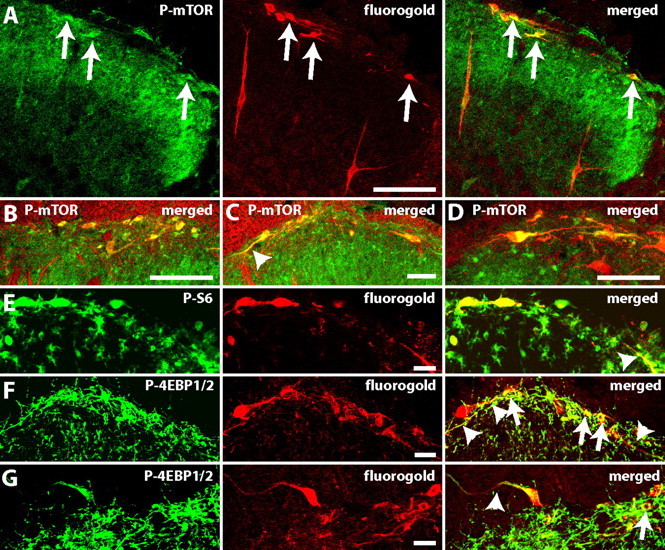
P-mTOR, P-S6 protein, and P-4EBP1/2 are strongly expressed in lamina I/III projection neurons. Colocalization of projection neurons labeled with Fluorogold and an antibody against Fluorogold (red) and (green): A–D, P-mTOR; E, P-S6; and F, G, P-4EBP1/2. In A, E–G, the single staining for each antibody and the merged image are shown from left to right and double staining appears in yellow. Notice the extensive staining of dendrites (arrow heads) marked on merged pictures. The percentage of projections neurons labeled with P-mTOR and P-S6 was, respectively: 77 ± 3% and 80 ± 1%; B–D show the merged pictures. Arrows show double labeled neurons. Scale bars: A–C, 100 μm; D–G, 25 μm.
Animals were injected with a retrograde label (FG) in the parabrachial area and perfused 3 d later. When spinal cord sections were double-labeled with an antibody against P-mTOR, P-S6, or P-4EBP1/2 and an antibody against FG, we found that P-mTOR and P-S6 were strongly expressed in lamina I/III projections neurons (77 ± 3% and 80 ± 1%, respectively; N = 3) and their dendrites (Fig. 7A–E). P-4EBP1/2 staining was intense in dendrites but at lower levels in cell bodies (Fig. 7F,G).
Intrathecal administration of rapamycin blocks mTORC1 activity in the dorsal horn
Activity of mTORC1 in the spinal cord was assessed by quantifying phosphorylation of the downstream targets 4E-BP1/2 and S6 protein. As above, animals received an intrathecal injection of the mTORC1 inhibitor rapamycin or vehicle. Using Western blotting, we found that intrathecal injections of rapamycin reduced phosphorylation of downstream targets of mTORC1. Specifically, Western blot analysis showed a significant decrease in 4E-BP1/2 phosphorylation in the dorsal horn 30 min after injection of rapamycin compared to vehicle (supplemental Fig. S3, available at www.jneurosci.org as supplemental material) (p < 0.05). There was also a significant decrease in S6 phosphorylation in the dorsal horn in the rapamycin-treated group when compared to vehicle at 2 h after treatment but not at 30 min (Fig. 8) (p < 0.05).
Figure 8.
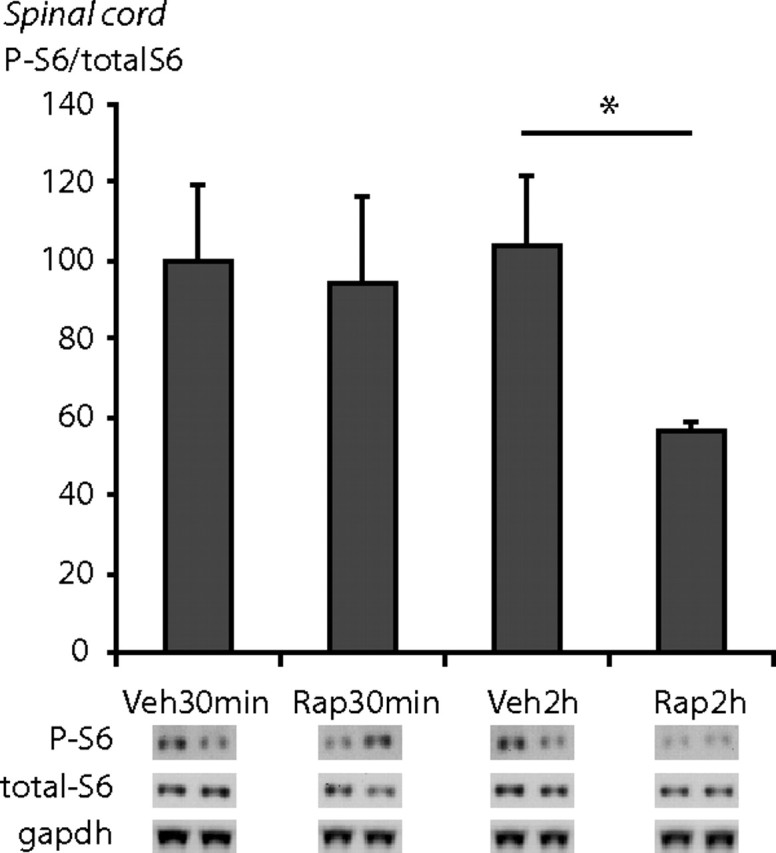
Intrathecal administration of rapamycin decreases phosphorylation of the downstream target of mTORC1 S6 protein (S6) in the spinal cord. Immunoblots probed with anti-P-S6 antibody and anti-S6 antibody after gel electrophoresis of lysates from spinal cord tissue. Animals received an intrathecal injection of rapamycin or vehicle 30 min or 2 h before killing. There was a significant reduction in S6 phosphorylation 2 h after rapamycin injection in spinal cord tissue. N = 3–4 in each condition. Mean ± SEM is illustrated. *p < 0.05.
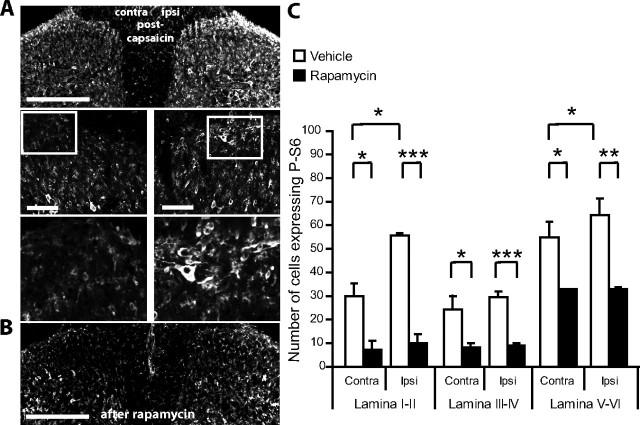
The number of P-S6 protein expressing cells increases in the superficial dorsal horn after capsaicin administration, especially in lamina I, and rapamycin can attenuate this effect
To investigate the influence of noxious stimulation on mTORC1 activity in dorsal horn neurons, we measured the effect of intraplantar injection of capsaicin on the phosphorylation of mTORC1 downstream target S6. Two hours after capsaicin administration there was a significant increase in the number of neurons expressing P-S6 protein, specifically in lamina I/II (176.8% increase, ipsi vs contra) and lamina V-VI (136.5% increase, ipsi vs contra) (P < 0.01 in both areas) (supplemental Fig. S4, available at www.jneurosci.org as supplemental material, and Fig. 9A).
Figure 9.
Intrathecal administration of rapamycin reduces the capsaicin-induced increase in phosphorylation of S6 protein in the spinal cord. A, Confocal images of P-S6 immunostaining in the superficial dorsal horn 2 h after intraplantar injection of capsaicin in the center of the plantar surface of the hindpaw. Scale bar, 100 μm. B, Confocal images of P-S6 immunostaining in the superficial dorsal horn 4 h after intrathecal administration of rapamycin. Scale bar, 100 μm. C, Effects of intrathecal administration of rapamycin (or vehicle) before capsaicin injection in the hindpaw. Rapamycin (or vehicle) was administered 4 h before capsaicin and animals perfused 2 h following capsaicin injection. Rapamycin significantly reduced P-S6 protein levels in the three spinal cord domains. Furthermore, following rapamycin treatment, capsaicin did not increase P-S6 protein expression. *p < 0.05, **p < 0.01, ***p < 0.001. N = 3 in each group.
We repeated the experiment but this time animals were separated in two groups receiving an intrathecal injection of rapamycin or vehicle 4 h before the injection of capsaicin. Rapamycin significantly reduced the expression of P-S6 protein in the dorsal horn across all superficial dorsal horn domains. Moreover, the level of P-S6 protein on the ipsilateral side was comparable to the contralateral side (Fig. 9B,C).
This experiment demonstrates that capsaicin stimulation activates mTORC1 signaling in the dorsal horn and that this is sensitive to rapamycin inhibition.
mTOR activity in the dorsal roots and in the superficial dorsal horn does not change 7 d after SNI surgery
We assessed mTOR activity after SNI by measuring P-S6 expression in the dorsal roots (by Western blot analysis, N = 4) and in the dorsal horn (by immunohistochemistry, N = 3, and by Western blot analysis, N = 6), 7 d following SNI (or sham) surgery. There was no difference in P-S6 expression in the dorsal roots or spinal cord, compared to sham or the contralateral side (supplemental Fig. S5, available at www.jneurosci.org as supplemental material). Finally, our observations of dorsal roots 7 d after SNI surgery showed that P-mTOR expression remained largely restricted to A-fibers and there was no new expression of the translational machinery within small fibers; indeed, the percentage of peripherin-labeled fibers expressing P-mTOR did not change after SNI (3.8 ± 0.13 and 3.9 ± 0.08, ipsi vs contra respectively). We interpret this to mean that following SNI surgery, increased pain sensitivity is not driven by a maintained increase in axonal or dorsal horn neuron mTOR activity.
Discussion
Chronic pain, particularly neuropathic pain in humans, is difficult to manage with current therapeutic approaches (Breivik et al., 2006). Here, we show that central application of rapamycin, which inhibits mTORC1-regulated protein synthesis, attenuates persistent pain states in rats. We demonstrate that this effect is mediated by blocking mTORC1 activity in peripheral A-fibers and central neurons, specifically a subset of primary afferent A-nociceptors and lamina I/III dorsal horn projection neurons that convey nociceptive information to the brain. Rapamycin, at this concentration, had no obvious effect on the physiology of C-fibers or other capsaicin-sensitive sensory afferents. These findings suggest a new pharmacological route for therapeutic intervention in chronic pain.
mTOR in sensory nerve fibers
We have shown that P-mTOR is expressed in a subset of dorsal root fibers, the majority of which are A-fibers, although we also noted a small number of peripherin-positive fibers, some of which may be C-fibers (Fig. 5D,E). Indeed, peripherin labels the majority of C-fibers but some coexpression with small diameter N52-positive neurons has been reported (Ferri et al., 1990). This extends previous observations that P-eIF-4E, ribosomal protein P0, and components of the degradative machinery, 20S proteosome core, ubiquitin, and ubiquinated proteins are also found in myelinated dorsal root axons (Koenig et al., 2000; Verma et al., 2005). By monitoring the activation state of one of mTORC1 downstream target proteins, S6, we show that intrathecally administered rapamycin will reach the dorsal roots but that within the time scale used here does not affect phosphorylation of S6 in the DRGs. We have previously observed a similar reduction in S6 phosphorylation in myelinated axons following rapamycin injections into cutaneous tissue. Our in vitro studies of dorsal roots incubated in low-dose rapamycin indicate that while amplitude and conduction velocity are not changed, rapamycin increases the activation threshold of Aδ-fibers. Finally, we show that the thermal sensitivity of capsaicin-insensitive Aδ-fibers decrease following rapamycin, while C-fiber responses remain unaffected by rapamycin at this concentration. These data support previous evidence that the sensitivity of a subset of A-nociceptors is maintained by tonic activity in the mTORC1 signaling pathway. Rapamycin treatment inhibits mTOR signaling and reduces the response of A-nociceptors resulting in both blunted secondary hyperalgesia and decreased mechanical sensitivity in a model of neuropathic pain (Jiménez-Díaz et al., 2008).
The present data show that mTORC1 signaling pathway maintains the sensitivity of a subset of A-nociceptors. Rapamycin treatment selectively increased the stimulation intensity required to elicit the Aδ-component of extracellularly recorded compound action potentials from isolated dorsal roots. In myelinated axons, voltage-gated sodium channels, which are essential for action potential generation and propagation, are clustered in high densities at nodes of Ranvier, whereas voltage-gated potassium channels are found both at the node of Ranvier and at juxtaparanodes (Rasband and Trimmer, 2001; Vacher et al., 2008). The “threshold” or amount of current required to drive the membrane potential to the threshold for eliciting an action potential will be regulated by the proportions and different types of these voltage-gated channels present. Therefore, it would seem likely that mTORC1-mediated local translation regulates the function, expression, or localization of particular voltage-gated channels, which maintain the sensitivity of Aδ-fibers under normal conditions. Interestingly, activation of mTOR has been shown to suppress potassium channel Kv1.1 expression in dendrites (Raab-Graham et al., 2006), which would in turn increase excitability, and this channel is expressed in peripheral myelinated axons at juxtaparanodes (Rasband et al., 1998). Other voltage-gated channels present in peripheral myelinated axons include Nav1.6 (Caldwell et al., 2000), Kv7.2 (Devaux et al., 2004), and Kv1.2 (Rasband et al., 1998) although it is unclear whether mRNAs for these different channel proteins are transported in sensory nerves.
Unexpectedly, while we were able to localize P-mTOR to dorsal root fibers, we were unable to trace these labeled fibers into the dorsal horn. Close examination and double labeling with a range of synaptic markers revealed that P-mTOR immunoreactivity in axons did not extend beyond the dorsal root entry zone and raises the possibility that local translation in sensory axons requires an environment unique to the peripheral nervous system. One suggestion is that local translation is modulated by Schwann cells, glial cells found only in peripheral nervous system. In this respect it has recently been suggested that ribosomes and associated mRNA may be transferred from Schwann cells to axons (Court et al., 2008). The route for this transfer is unknown but may be through Schmidt-Lanterman incisura (SLI), which are formed from strands of Schwann cell cytoplasm and pass through the compact myelin to the adaxonal surface of the axon. The SLI have high levels of P-mTOR, suggesting that ribosomal subunits may be synthesized in the incisura adjacent to local accumulations of axonal mTOR.
Rapamycin effects on the dorsal horn
Our results clearly indicate that intrathecally administered rapamycin can influence pain response by acting on A-fibers in dorsal roots. However, there have also been several reports that late phase of the formalin response is attenuated by intrathecal rapamycin (Price et al., 2007; Asante et al., 2009), with the implication that this reflects a direct effect of rapamycin on spinal neurons. Although our understanding of the formalin response is far from clear, with both peripheral and central mechanisms being invoked to explain the second phase of the response (Puig and Sorkin, 1996; De Felipe et al., 1998; McNamara et al., 2007), dorsal horn mechanisms are likely to contribute to the changes in persistent pain thresholds seen after intrathecal administration of rapamycin. Indeed, targets of mTORC1 signaling such as S6 are rapidly phosphorylated in neurons throughout the dorsal horn, following peripheral capsaicin stimulation that activates largely small diameter C-fibers, and this is inhibited by intrathecal rapamycin.
Immunohistochemically, we noted that lamina I projection neurons and their dendrites were strongly P-mTOR, P-S6 and P-4EBP1/2 positive. Lamina I projection neurons receive inputs from A-nociceptors (Torsney and MacDermott, 2006) and are essential for the development and maintenance of both neuropathic and inflammatory pain states (Mantyh et al., 1997; Nichols et al., 1999). It seems likely that some of these A-nociceptors may indeed include those in which mTOR-regulated control of sensitivity is present. mTOR has been shown to be essential for local translation in hippocampal neuron dendrites and for the generation of late long-term potentiation (L-LTP). Indeed, hippocampal late (but not early) LTP requires activation of downstream targets of mTORC1, 4E-BP1/2, and S6K, and this is correlated with an increase in TOP mRNAs expression. mTOR has also been shown to be essential for the formation and stability of learning and memory in the amygdala and the hippocampus (Tang et al., 2002; Parsons et al., 2006; Costa-Mattioli et al., 2009). Lamina I projection neurons also support LTP following low-frequency, high-intensity stimulation of the peripheral nerve (Ikeda et al., 2003; Ikeda et al., 2006). Therefore, it appears likely that the attenuation by rapamycin of the increased mechanical sensitivity that follows capsaicin injection could partly be a reflection of the attenuation of LTP-like events in lamina I neurons. However, this is unlikely to be the case after peripheral nerve damage. Indeed, electrophysiological studies have shown that rapamycin is only effective at inhibiting hippocampal L-LTP when present during the induction phase of LTP and that later application of rapamycin is without effect (Cammalleri et al., 2003). If the effect of rapamycin on dorsal horn contributes to the inhibition of the increased mechanical sensitivity seen after SNI, this would indicate that the effect of rapamycin on lamina I projection neurons is independent of LTP- like phenomena or that the mechanism of LTP in lamina I is different from that in the hippocampus. Moreover, we also investigated mTOR activity in the superficial dorsal horn 7 d following SNI surgery: there was no difference in P-S6 expression in the spinal cord or dorsal roots, compared to both sham or the contralateral side (supplemental Fig. S5, available at www.jneurosci.org as supplemental material). Together with a previous report showing that the dorsal roots A-fiber thresholds and conduction velocities are unchanged after SNI surgery (Kohno et al., 2003), we interpret this to mean that increased pain sensitivity in SNI is not driven by a maintained increase in axonal or dorsal horn neuron mTOR activity.
Lamina I projection neurons are thought to control spinal excitability through the engagement of descending pathways at brainstem levels (Hunt, 2000; Suzuki et al., 2002). Of particular relevance to the present study is the suggestion that these descending controls largely regulate secondary hyperalgesia (Pertovaara, 1998; Urban and Gebhart, 1999; Sanoja et al., 2009), that is, the pain sensitivity that develops in the undamaged skin around an injury and which can be alleviated by rapamycin given peripherally or centrally. Moreover, the increased mechanical sensitivity that defines secondary hyperalgesia is also characteristic of neuropathic pain and specific lesions of lamina I neurons alleviate both experimentally induced pain states underlining the mechanistic similarities between the two conditions (Mantyh et al., 1997; Nichols et al., 1999). However, complete loss of lamina I projection neurons does have a more widespread influence on pain processing than rapamycin application. Indeed, injection of capsaicin into the rat hindpaw results in prolonged thermal and mechanical hyperalgesia, both of which are reduced by lesions of lamina I neurons (Mantyh et al., 1997), while rapamycin had no effect on thermal thresholds after capsaicin treatment. This suggests a more specific effect of rapamycin on mechanical sensitivity.
The substantial reduction in neuropathic pain sensitivity by rapamycin may also reflect an action on non-neuronal cells that hypertrophy following peripheral nerve damage. Signaling of the mTORC1 pathway has been shown to be upregulated in astrocytes following direct damage to the spinal cord (Codeluppi et al., 2009). Moreover, astrocytic hypertrophy could be inhibited by rapamycin in vitro, while in vivo rapamycin reduced reactive gliosis suggesting that this drug could be used to reduce astrocytic response (Codeluppi et al., 2009). Astrocytic response during neuropathic pain has been shown to contribute to increased sensitivity (Gao et al., 2009; Kim et al., 2009) and activated microglia release factors that maintain neuropathic sensitivity (Inoue and Tsuda, 2009). P-mTOR, P-S6, and P-4EBP1/2 were expressed in astrocytes on the side ipsilateral to peripheral nerve damage but also on the contralateral side and in naive tissue (supplemental Fig. S6, available at www.jneurosci.org as supplemental material). However, we did not find any evidence for increased activation of mTOR in dorsal horn astrocytes or microglia 7 d after SNI. Thus, there was no increase in glial expression of phoshorylated translational markers following peripheral nerve damage. This was supported by our Western and immunohistochemical analysis of mTOR activity 7 d after SNI (supplemental Fig. S5, available at www.jneurosci.org as supplemental material). Nevertheless, we cannot rule out the possibility that intrathecally administered rapamycin may influence astrocytes in the superficial dorsal horn and contribute to the reduction in neuropathic pain sensitivity described in the present study.
In summary, our results suggest that increased sensitivity around the site of injury, which is essential for the prevention of further damage and healing, is communicated by a dedicated subset of mTOR-positive A-nociceptors that may synapse with lamina I projection neurons, which are also characterized by high levels of mTORC1 activity. We present evidence to show that rapamycin, an inhibitor of mTORC1 signaling, generates a profound anti-nociceptive effect specifically on the spread of pain outside of the area of injury and on neuropathic pain sensitivity. We show that this is mediated by a direct action on dorsal root fibers and possibly on dorsal horn neurons and suggest that rapamycin treatment may offer hope for the treatment of intractable pain states.
Footnotes
This work was supported by grants from the Wellcome Trust (080506 and 085566), the Medical Research Council (G0801381), the Caledonian Research Foundation (R39726), the Biotechnology and Biological Sciences Research Council (G012717), and the Spanish Ministerio de Educación y Ciencia (JCI-2005-1775-25). We thank Peter Brophy for the neurofascin antibody and for helpful discussion during the preparation of this manuscript.
References
- Alvarez J. The autonomous axon: a model based on local synthesis of proteins. Biol Res. 2001;34:103–109. doi: 10.4067/s0716-97602001000200014. [DOI] [PubMed] [Google Scholar]
- Antion MD, Merhav M, Hoeffer CA, Reis G, Kozma SC, Thomas G, Schuman EM, Rosenblum K, Klann E. Removal of S6K1 and S6K2 leads to divergent alterations in learning, memory, and synaptic plasticity. Learn Mem. 2008;15:29–38. doi: 10.1101/lm.661908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante CO, Wallace VC, Dickenson AH. Formalin-induced behavioural hypersensitivity and neuronal hyperexcitability are mediated by rapid protein synthesis at the spinal level. Mol Pain. 2009;5:27. doi: 10.1186/1744-8069-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Poulin F, Hou L, DeMaria CT, Sonenberg N, Klann E. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J Neurosci. 2005;25:9581–9590. doi: 10.1523/JNEUROSCI.2423-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc Natl Acad Sci U S A. 2000;97:5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammalleri M, Lütjens R, Berton F, King AR, Simpson C, Francesconi W, Sanna PP. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci U S A. 2003;100:14368–14373. doi: 10.1073/pnas.2336098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codeluppi S, Svensson CI, Hefferan MP, Valencia F, Silldorff MD, Oshiro M, Marsala M, Pasquale EB. The Rheb-mTOR pathway is upregulated in reactive astrocytes of the injured spinal cord. J Neurosci. 2009;29:1093–1104. doi: 10.1523/JNEUROSCI.4103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court FA, Hendriks WT, Macgillavry HD, Alvarez J, van Minnen J. Schwann cell to axon transfer of ribosomes: toward a novel understanding of the role of glia in the nervous system. J Neurosci. 2008;28:11024–11029. doi: 10.1523/JNEUROSCI.2429-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- De Felipe C, Herrero JF, O'Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F, Hunt SP. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. J Neurosci. 2004;24:1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri GL, Sabani A, Abelli L, Polak JM, Dahl D, Portier MM. Neuronal intermediate filaments in rat dorsal root ganglia: differential distribution of peripherin and neurofilament protein immunoreactivity and effect of capsaicin. Brain Res. 1990;515:331–335. doi: 10.1016/0006-8993(90)90618-l. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Géranton SM, Morenilla-Palao C, Hunt SP. A role for transcriptional repressor methyl-CpG-binding protein 2 and plasticity-related gene serum- and glucocorticoid-inducible kinase 1 in the induction of inflammatory pain states. J Neurosci. 2007;27:6163–6173. doi: 10.1523/JNEUROSCI.1306-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hengst U, Cox LJ, Macosko EZ, Jaffrey SR. Functional and selective RNA interference in developing axons and growth cones. J Neurosci. 2006;26:5727–5732. doi: 10.1523/JNEUROSCI.5229-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SP. Pain control: breaking the circuit. Trends Pharmacol Sci. 2000;21:284–287. doi: 10.1016/s0165-6147(00)01496-6. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Heinke B, Ruscheweyh R, Sandkühler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299:1237–1240. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jäger T, Sandkühler J. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- Inoue K, Tsuda M. Microglia and neuropathic pain. Glia. 2009;57:1469–1479. doi: 10.1002/glia.20871. [DOI] [PubMed] [Google Scholar]
- Jiménez-Díaz L, Géranton SM, Passmore GM, Leith JL, Fisher AS, Berliocchi L, Sivasubramaniam AK, Sheasby A, Lumb BM, Hunt SP. Local translation in primary afferent fibers regulates nociception. PLoS One. 2008;3:e1961. doi: 10.1371/journal.pone.0001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Figueroa KW, Li KW, Boroujerdi A, Yolo T, David Luo Z. Profiling of dynamically changed gene expression in dorsal root ganglia postperipheral nerve injury and a critical role of injury-induced glial fibrillary acetic protein in maintenance of pain behaviors. Pain. 2009;143:114–122. doi: 10.1016/j.pain.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Thomas KS, Calejesan AA, Zhuo M. Macromolecular synthesis contributes to nociceptive response to subcutaneous formalin injection in mice. Neuropharmacology. 1998;37:1091–1093. doi: 10.1016/s0028-3908(98)00099-9. [DOI] [PubMed] [Google Scholar]
- Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5:931–942. doi: 10.1038/nrn1557. [DOI] [PubMed] [Google Scholar]
- Koenig E. Organized ribosome-containing structural domains in axons. Results Probl Cell Differ. 2009;48:173–191. doi: 10.1007/400_2008_29. [DOI] [PubMed] [Google Scholar]
- Koenig E, Giuditta A. Protein-synthesizing machinery in the axon compartment. Neuroscience. 1999;89:5–15. doi: 10.1016/s0306-4522(98)00282-6. [DOI] [PubMed] [Google Scholar]
- Koenig E, Martin R, Titmus M, Sotelo-Silveira JR. Cryptic peripheral ribosomal domains distributed intermittently along mammalian myelinated axons. J Neurosci. 2000;20:8390–8400. doi: 10.1523/JNEUROSCI.20-22-08390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T, Moore KA, Baba H, Woolf CJ. Peripheral nerve injury alters excitatory synaptic transmission in lamina II of the rat dorsal horn. J Physiol. 2003;548:131–138. doi: 10.1113/jphysiol.2002.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrakakis C, Tong CK, Weissman T, Torsney C, MacDermott AB. Localization and function of ATP and GABAA receptors expressed by nociceptors and other postnatal sensory neurons in rat. J Physiol. 2003;549:131–142. doi: 10.1113/jphysiol.2002.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leith JL, Wilson AW, Donaldson LF, Lumb BM. Cyclooxygenase-1-derived prostaglandins in the periaqueductal gray differentially control C-versus A-fiber-evoked spinal nociception. J Neurosci. 2007;27:11296–11305. doi: 10.1523/JNEUROSCI.2586-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magerl W, Fuchs PN, Meyer RA, Treede RD. Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain. 2001;124:1754–1764. doi: 10.1093/brain/124.9.1754. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Martin KC. Local protein synthesis during axon guidance and synaptic plasticity. Curr Opin Neurobiol. 2004;14:305–310. doi: 10.1016/j.conb.2004.05.009. [DOI] [PubMed] [Google Scholar]
- McMullan S, Simpson DA, Lumb BM. A reliable method for the preferential activation of C- or A-fibre heat nociceptors. J Neurosci Methods. 2004;138:133–139. doi: 10.1016/j.jneumeth.2004.03.020. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashov AK, Chintalgattu V, Islamov RR, Lever TE, Pak ES, Sierpinski PL, Katwa LC, Van Scott MR. RNAi pathway is functional in peripheral nerve axons. FASEB J. 2007;21:656–670. doi: 10.1096/fj.06-6155com. [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Ataka T, Kumamoto E, Tamaki T, Yoshimura M. Alteration in synaptic inputs through C-afferent fibers to substantia gelatinosa neurons of the rat spinal dorsal horn during postnatal development. Neuroscience. 2000;99:549–556. doi: 10.1016/s0306-4522(00)00224-4. [DOI] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Pertovaara A. A neuronal correlate of secondary hyperalgesia in the rat spinal dorsal horn is submodality selective and facilitated by supraspinal influence. Exp Neurol. 1998;149:193–202. doi: 10.1006/exnr.1997.6688. [DOI] [PubMed] [Google Scholar]
- Price TJ, Géranton SM. Translating nociceptor sensitivity: the role of axonal protein synthesis in nociceptor physiology. Eur J Neurosci. 2009;29:2253–2263. doi: 10.1111/j.1460-9568.2009.06786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, Cervero F. Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J Neurosci. 2007;27:13958–13967. doi: 10.1523/JNEUROSCI.4383-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Raab-Graham KF, Haddick PC, Jan YN, Jan LY. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science. 2006;314:144–148. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS. Developmental clustering of ion channels at and near the node of Ranvier. Dev Biol. 2001;236:5–16. doi: 10.1006/dbio.2001.0326. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS, Schwarz TL, Levinson SR, Ellisman MH, Schachner M, Shrager P. Potassium channel distribution, clustering, and function in remyelinating rat axons. J Neurosci. 1998;18:36–47. doi: 10.1523/JNEUROSCI.18-01-00036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Sanoja R, Tortorici V, Fernandez C, Price TJ, Cervero F. Role of RVM neurons in capsaicin-evoked visceral nociception and referred hyperalgesia. Eur J Pain. 2009 doi: 10.1016/j.ejpain.2009.04.006. Advance online publication. Retrieved August 31, 2009. doi: 10.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, Macklin WB, Meek S, Smith AJ, Cottrell DF, Brophy PJ. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48:737–742. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakor DK, Lin A, Matsuka Y, Meyer EM, Ruangsri S, Nishimura I, Spigelman I. Increased peripheral nerve excitability and local NaV1.8 mRNA up-regulation in painful neuropathy. Mol Pain. 2009;5:14. doi: 10.1186/1744-8069-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006;26:1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth CC, Willis D, Twiss JL, Walsh S, Martinez JA, Liu WQ, Midha R, Zochodne DW. Locally synthesized calcitonin gene-related Peptide has a critical role in peripheral nerve regeneration. J Neuropathol Exp Neurol. 2009;68:326–337. doi: 10.1097/NEN.0b013e31819ac71b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci U S A. 1999;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P, Chierzi S, Codd AM, Campbell DS, Meyer RL, Holt CE, Fawcett JW. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25:331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D, Li KW, Zheng JQ, Chang JH, Smit A, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ, Kelly TK, Chang B, Ryazantsev S, Rajasekaran AK, Martin KC, Twiss JL. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J Neurosci. 2001;21:9291–9303. doi: 10.1523/JNEUROSCI.21-23-09291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]