Abstract
The extracellular matrix (ECM) consists of numerous macromolecules classified traditionally into collagens, elastin, and microfibrillar proteins, proteoglycans including hyaluronan, and noncollagenous glycoproteins. In addition to being necessary structural components, ECM molecules exhibit important functional roles in the control of key cellular events such as adhesion, migration, proliferation, differentiation, and survival. Any structural inherited or acquired defect and/or metabolic disturbance in the ECM may cause cellular and tissue alterations that can lead to the development or progression of disease. Consequently, ECM molecules are important targets for pharmacotherapy. Specific agents that prevent theexcess accumulation of ECM molecules in the vascular system, liver, kidney, skin, and lung; alternatively, agents that inhibit the degradation of the ECM in degenerative diseases such as osteoarthritis would be clinically beneficial. Unfortunately, until recently, the ECM in drug discovery has been largely ignored. However, several of today's drugs that act on various primary targets affect the ECM as a byproduct of the drugs' actions, and this activity may in part be beneficial to the drugs' disease-modifying properties. In the future, agents and compounds targeting directly the ECM will significantly advance the treatment of various human diseases, even those for which efficient therapies are not yet available.
I. Introduction
The extracellular matrix (ECM1) is composed of collagens, elastin, proteoglycans (including hyaluronan), and noncollagenous glycoproteins and forms a complex, three-dimensional network among the cells of different tissues in an organ-specific manner. The ECM was initially considered an inert, space-filling material that provided only mechanical strength to tissues and organs. Today we understand that the ECM is a dynamic structure that interacts with cells and generates signals through feedback loops to control the behavior of cells. Thus, ECM macromolecules are bioactive and modulate cellular events such as adhesion, migration, proliferation, differentiation, and survival (Daley et al., 2008). It is important to realize that structurally very different ECM components possess these activities. It is also important to understand that the ECM molecules are strictly organized and that this organization determines the bioactivity of the ECM. Even minor alterations such as a single amino acid substitution in a single ECM component can lead not only to altered physicochemical properties of the tissues but also to changes in the cellular phenotype and in cell-matrix interactions. These changes in tissue function ultimately lead to development of a disease. There is presumably no disease without quantitative and/or qualitative changes in the ECM. However, it is necessary to distinguish between ECM changes that cause the disease and ECM changes that result from the disease because therapeutic strategies will differ depending on primary or secondary causation.
II. Extracellular Matrix Molecules, Their Functions and Related Diseases
In general, ECM components are classified as fiber-forming and non–fiber-forming (interfibrillar) molecules. Certain collagen species and elastin are typical fiber-forming ECM molecules, whereas the proteoglycans and glycoproteins are generally considered interfibrillary ECM molecules. Quite recently, the term “matricellular proteins” has been applied to a group of ECM molecules, including thrombospondin-1 and -2, SPARC (secreted protein, acidic and rich in cysteine), tenascin-C, and osteopontin, that do not function as structural elements but modulate cell-matrix interactions and cell functions such as in tissue repair (Bornstein and Sage, 2002; Kyriakides and Bornstein, 2003).
During the last 2 decades, the number of individually characterized ECM molecules has expanded markedly. Today, nearly 30 different collagen types involving more than 40 distinct polypeptide chains (α chains) are known in humans, and more than 20 other proteins contain collagen-like domains (Myllyharju and Kivirikko, 2004; Ricard-Blum and Ruggiero, 2005). There are also more than 30 different proteoglycans, most of which reside in the ECM (Järveläinen and Wight, 2002; Schaefer and Iozzo, 2008). The molecular multiplicity is true for matrix glycoproteins as well. For example, in mammals at least 15 different laminins have been detected (Sasaki et al., 2004; Miner, 2008) and, in the case of fibronectin, alternative splicing of the V-region has been shown to generate up to 20 fibronectin isoforms in humans (White et al., 2008). The individual ECM molecules, their isoforms, and even some of their proteolytic fragments, such as endostatin, a 20 kDa C-terminal cleavage product of collagen type XVIII (O'Reilly et al., 1997) and related polypeptides from other basement membrane associated collagens, mediate specific functional effects to control and regulate cell behaviors including those required for angiogenesis (Ingber and Folkman, 1989). It can be expected that all ECM molecules have some role in the normal functions in cell biology. The ECM molecules must act in concert in a finely regulated manner to maintain proper cellular function within tissues and organs (Lukashev and Werb, 1998). In this respect, it is interesting to note that the synthesis of ECM molecules is controlled by specific growth factors, among which the transforming growth factor-βs (TGF-βs) are the most prominent (Border and Noble, 1994; Verrecchia and Mauviel, 2007). Furthermore, the life of the ECM molecules is determined by proteases, especially matrix metalloproteases (MMPs) (Visse and Nagase, 2003). Thus, growth factor signaling and control of ECM turnover by proteases are potential targets for new pharmacotherapies.
A long list of human diseases exhibit disturbances in the ECM. Changes in the ECM are especially distinct in the monogenic disorders of the connective tissue, such as osteogenesis imperfecta (Glorieux, 2008), Ehlers-Danlos syndrome (Mao and Bristow, 2001; Parapia and Jackson, 2008), Marfan's syndrome (Dietz et al., 1994; Keane and Pyeritz, 2008), and other genetic disorders of the ECM. Changes in the ECM are also prominent in a large variety of more common, acquired human diseases or in combination with polygenic inheritance, such as coronary heart disease, which is the number one cause of death in the Western world (Katsuda et al., 1992; Nakashima et al., 2008; Wight, 2008). Here, components of the ECM bind and trap lipoproteins, causing lipid build-up and atherosclerotic plaque formation (Williams and Tabas, 1995). Maintaining the integrity of the ECM in the vessel wall seems critical to either preventing or treating cardiovascular disease (Wight and Merrilees, 2004). Another common cardiovascular disease, hypertension, is characterized by stiffer blood vessels, and heart failure by altered cardiac muscle microenvironments that do not support cardiomyocyte survival (Tayebjee et al., 2003; Fedak et al., 2005; Berk et al., 2007). Pulmonary diseases such as asthma and chronic obstructive pulmonary disease are characterized by ECM changes that lead to loss of tissue elasticity and compliance and eventually to fibrosis (Postma and Timens, 2006; Merrilees et al., 2008). Furthermore, hepatic diseases, in particular liver cirrhosis, involve progressive accumulation of scarring proteins (fibrosis) in response to subtle or overt inflammatory reactions (Wallace et al., 2008). Inflammatory bowel diseases (IBDs) ulcerative colitis, and Crohn's disease also exhibit marked organ-specific changes in the composition and organization of the ECM. An interesting link between the ECM and IBDs and inflammation is highlighted by a series of studies that show that hyaluronan accumulates in IBDs and promotes the adhesion and activation of inflammatory cells such as monocytes (de La Motte et al., 1999, 2003). Chronic kidney diseases, including glomerulosclerosis and tubulointerstitial fibrosis, mainly manifest as abnormal build-up of ECM components leading to loss of function (Liu, 2006). Rheumatoid arthritis and osteoarthritis are manifested by breakdown of the joint ECM components (Roach et al., 2007; Melrose et al., 2008). In neurodegenerative diseases such as Alzheimer's disease, heparan sulfate proteoglycans, especially perlecan, are involved in the formation and stabilization of amyloid fibrils (Castillo et al., 1997). Furthermore, in malignancies, alterations of ECM composition have occasionally been claimed to be the main promoter of carcinogenesis (Marastoni et al., 2008). For example, hyaluronan and versican are increased in a number of human tumors and are believed to contribute to a permissive microenvironment for the growth and metastasis of the tumor cells (Toole, 2002; Theocharis, 2008; Ricciardelli et al., 2009). In a recent study, versican was identified as a factor promoting metastasis in a Lewis lung carcinoma model (Kim et al., 2009). In addition, there is a striking difference in the expression of a small proteoglycan decorin between human malignant and benign vascular tumors. Within Kaposi's sarcoma and angiosarcoma, the expression of decorin lacks completely, whereas within hemangiomas, decorin is expressed in abundant amounts (Salomäki et al., 2008). Such results suggest that decorin is likely to possess a suppressive effect on human tumor angiogenesis. Similar results have been obtained using experimental models (Grant et al., 2002). In addition to its role in disease, the ECM can variously function as a mediator for innate and acquired drug resistance (Vincent and Mechti, 2005; Bonacci et al., 2006). Also the side-effects of drugs and diagnostic agents are often due to alterations in the ECM. For example, a recently discovered side effect of the use of gadolinium-based contrast agents leads to scleroderma-like fibrotic lesions in the kidneys (Grobner, 2006).
A summary of the main ECM molecules, some of their known functions, and examples of genetic and other diseases related to them are presented in Table 1. Although the table is not comprehensive, it illustrates the vast molecular multiplicity and functional complexity of the ECM as well as the diversity of diseases related to ECM molecules.
TABLE 1.
Main ECM molecules, some of their functional roles, and selected genetic and other diseases related to individual molecules
| Group | Distribution/Function | Genetic Disease(s) | Other disease(s) |
|---|---|---|---|
| Collagens | |||
| Fibrillar collagens | |||
| I | Structural component of all tissues except cartilage; e.g. bone, dermis (Gelse et al., 2003), vessel wall (Katsuda et al., 1992; Rauterberg et al., 1993), and heart (Marijianowski et al., 1994) | Osteogenesis imperfecta (Bonadio and Byers, 1985); classic type Ehlers-Danlos syndrome (Nuytinck et al., 2000) | Atherosclerosis (Katsuda et al., 1992; Rauterberg et al., 1993); hypertensive heart disease (López et al., 2001; Díez et al., 2005); fibrotic diseases including chronic kidney diseases (Alexakis et al., 2006) |
| II | Predominant component of cartilage (Bruckner and van der Rest, 1994); mediates interactions with proteoglycans (Mayne, 1989) | Stickler syndrome type I (Maumenee, 1979; Ahmad et al., 1991); spondyloepiphyseal dysplasia (Tiller et al., 1995); achondrogenesis (Eyre et al., 1986); Kniest dysplasia (Winterpacht et al., 1993) | Osteoarthritis (Nelson et al., 1998) |
| III | Dominant collagen type of granulation tissue, and also, e.g., muscles and artery wall (Katsuda et al., 1992; Linehan et al., 2001); produced by young fibroblasts before type I collagen (Voermans et al., 2008) | Vascular Ehlers-Danlos syndrome (Pope et al., 1975; Pepin et al., 2000) | Atherosclerosis (Katsuda et al., 1992); hypertensive heart disease (Díez et al., 2005); fibrotic diseases, e.g. chronic kidney diseases (Alexakis et al., 2006) |
| V | Structural component of basement membrane (Graham et al., 2008); interacts with type I collagen (Birk, 2001); inhibits endothelial cell adhesion, proliferation (Fukuda et al., 1988), and adhesion (Hashimoto et al., 1991) | Classic type Ehlers-Danlos syndrome (Nicholls et al., 1996; Richards et al., 1998) | Atherosclerosis (Katsuda et al., 1992); collagenofibrotic glomerulopathy (Morita et al., 2003); bronchiolitis obliterans syndrome (Burlingham et al., 2007) |
| XI | Structural component of articular cartilage (Mayne, 1989) and ear (Nerlich, 1995); modulates cartilage matrix homeostasis (Eyre, 2004) | Stickler syndrome type II (Richards et al., 1996) and type III (Brunner et al., 1994); Marshall syndrome (Majava et al., 2007b); otospondylomegaepiphyseal dysplasia (Melkoniemi et al., 2000) | Disc herniation (Mio et al., 2007) |
| Nonfibrillar collagens | |||
| IV | Structural component of basement membrane (LeBleu et al., 2007); e.g., in kidney (Smith et al., 1989); associated with angiogenesis (Xu et al., 2001) | Alport syndrome (Barker et al., 1990); hereditary angiopathy (Breedveld et al., 2006) | Diabetic nephropathy (Cohen et al., 2001); organ or tumor fibrosis (Kalluri, 2003); cancer progression (Tanjore and Kalluri, 2006) |
| VIII | Structural component of extracellular matrices, e.g. in sclera (Shuttleworth, 1997) and vasculature (Plenz et al., 2003); involved in stabilization of membranes, angiogenesis and interacts with ECM molecules (Sutmuller et al., 1997; Plenz et al., 2003) | Corneal endothelial dystrophy, e.g., Fuchs' endothelial dystrophy (Biswas et al., 2001) | Atherosclerosis (Plenz et al., 2003); tumor progression (Koon et al., 2004) |
| X | Structural component of hypertrophic cartilage (Gelse et al., 2003); regulates matrix mineralization and compartmentalization of ECM components (Shen, 2005) | Schmid-type metaphyseal chondroplasia (Warman et al., 1993) | Osteochondritis dissecans (Aurich et al., 2006) |
| Association collagens | |||
| VI | Dominant structural component of connective tissue; e.g., in vessels, liver (von der Mark et al., 1984), and muscle (Engvall et al., 1986) | Muscle disorders, e.g., myosclerosis myopathy (Merlini et al., 2008b) | Atherosclerosis (Katsuda et al., 1992) |
| VII | Structural component of basement membrane (Sakai et al., 1986); interacts with other ECM components (Keene et al., 1987) | Dystrophic epidermolysis bullosa (Hovnanian et al., 1992) | Epidermolysis bullosa acquisita (Chen et al., 2007) |
| IX | Structural component of cartilage (Olsen, 1997); interacts with collagen type II (Eyre, 2004) | Stickler syndrome (Van Camp et al., 2006); multiple epiphyseal dysplasia (Bönnemann et al., 2000); Scheuermann disease (Karppinen et al., 2003) | |
| XII | Structural component of connective tissue, e.g., in skin (Wälchli et al., 1994); interacts with other matrix components (Voermans et al., 2008) | Diabetic retinopathy (Ljubimov et al., 1996); keratoconus (Cheng et al., 2001) | |
| XIV | Structural component of connective tissue, e.g., in blood vessels (Castagnola et al., 1992); interacts with other ECM components (Ehnis et al., 1997) | Diabetic retinopathy (Ljubimov et al., 1996) | |
| XIX | Structural component of basement membrane (Myers et al., 1997); associated with muscle cells (Myers et al., 1999) | Rhabdomyosarcomas (Myers et al., 1999); breast cancer (Amenta et al., 2003) | |
| Transmembrane collagens | |||
| XIII | Structural component of, e.g., skin (Peltonen et al., 1999); interacts with other ECM components, e.g., proteoglycans (Michelacci, 2003) | Tumor development (Väisänen et al., 2005); thyroid-associated ophthalmopathy (Yamada et al., 2000) | |
| XVII | Structural component of cutaneous basement membrane (Uitto and Pulkkinen, 1996); involved in cell-matrix adhesion (Van den Bergh and Giudice, 2003) | Non-Herlitz junctional epidermolysis bullosa (Bauer and Lanschuetzer, 2003) | |
| Multiplexins | |||
| XV | Structural component of basement membrane (Myers et al., 1996); acts as structural organizer of ECM (Amenta et al., 2005) | Tumorigenesis (Amenta et al., 2003, Fukushige et al., 2005) | |
| XVIII | Structural component of basement membrane (Saarela et al., 1998); inhibition angiogenesis and tumor growth (Myllyharju and Kivirikko, 2001) | Knobloch syndrome (Suzuki et al., 2002) | Fibrotic diseases, e.g., liver fibrosis (Musso et al., 1998) |
| Elastin and microfibrillar proteins | |||
| Elastin | Structural component of elastic fibers (Rosenbloom et al., 1993); provides elasticity to different tissues, e.g., lungs, large blood vessels, and dermis (Ross, 1973) | Supravalvular aortic stenosis (Ewart et al., 1993); cutis laxa (Tassabehji et al., 1998); Williams-Beuren syndrome (Milewicz et al., 2000) | Hypertension (Martyn and Greenwald, 1997); pulmonary emphysema (Snider et al., 1991); arterial aneurysms (Alberto et al., 2008) |
| Fibrillin 1 | Major structural component of microfibrils together with fibrillin-2 (Ramirez and Pereira, 1999); involved in tissue homeostasis (Zhang et al., 1995) | Marfan's syndrome (Robinson and Booms, 2001); Shprintzen-Goldberg syndrome (Sood et al., 1996); Weill-Marchesani syndrome (Faivre et al., 2003) | Atherosclerosis (Seyama and Wachi, 2004); pulmonary emphysema (Robbesom et al., 2008) |
| Fibrillin 2 | Major structural component of microfibrils together with fibrillin-1 (Ramirez and Pereira, 1999) | Congenital contractural arachnodactyly (Beals and Hecht, 1971) | |
| Fibulin 1 | Structural component of basement membranes (Pan et al., 1993a); interacts with other ECM molecules, e.g., laminins (Timpl and Brown, 1994); modulates platelet adhesion (Godyna et al., 1996) | Synpolydactyly (Debeer et al., 2002); autosomal recessively inherited vitreoretinal dystrophy (Weigell-Weber et al., 2003) | Prostate cancer (Wlazlinski et al., 2007); gastric cancer (Cheng et al., 2008) |
| Fibulin 2 | Structural component of basement membranes (Pan et al., 1993b); involved in vasculogenesis and angiogenesis (Tsuda et al., 2001) | Breast cancer progression (Yi et al., 2007) | |
| Fibulin 3 | Structural component of blood vessels (Giltay et al., 1999) | Macular degenerative disease (Klenotic et al., 2004) | |
| Fibulin 4 | Structural component of blood vessels (Giltay et al., 1999) | Cutis laxa (Hucthagowder et al., 2006) | Colon tumorigenesis (Gallagher et al., 2001); prostate cancer (Wlazlinski et al., 2007) |
| Fibulin 5 | Organizer of elastic fibers (Yanagisawa et al., 2002); regulator of angiogenesis (Kowal et al., 1999); associated with suppression of tumorigenesis (Albig and Schiemann, 2005) | Cutis laxa (Loeys et al., 2002); age-related macular degeneration (Stone et al., 2004) | Prostate cancer (Wlazlinski et al., 2007) |
| Fibulin 6 | Organizer in cell-cell and cell-matrix interactions (Vogel and Hedgecock, 2001) | Macular degeneration (Schultz et al., 2003) | |
| Fibulin 7 | Cell adhesion molecule, associated with, e.g., dentin formation (de Vega et al., 2007) | ||
| Emilin 1 | Microfibril-associated protein (Mongiat et al., 2000); involved in vascular cell morphology and elastogenesis (Zanetti et al., 2004) | Hypertension (Raman and Cobb, 2006) | |
| Proteoglycans and glycosaminoglycans | |||
| Hyaluronan | Major structural carbohydrate component of the ECM (Chen and Abatangelo, 1999); involved in angiogenesis, cell motility, wound healing and cell adhesion (Stuhlmeier, 2006) | Tumorigenesis (Auvinen et al., 2000; Hiltunen et al., 2002; Toole, 2002; Heldin et al., 2008); atherosclerosis and restenosis (Wight, 2008); inflammatory bowel disease (de La Motte et al., 1999; de la Motte et al., 2003) | |
| Hyalectans | |||
| Aggrecan | Structural component of articular cartilage (Dudhia, 2005); interacts with hyaluronan (Kiani et al., 2002) | Intervertebral disc degeneration (Solovieva et al., 2007); spondyloepiphyseal dysplasia (Gleghorn et al., 2005) | Osteoarthritis (Fosang and Little, 2008) |
| Brevican | Abundant proteoglycan in adult brain (Yamaguchi, 1996) | Tumor progression (Hu et al., 2008) | |
| Neurocan | Structural component of the ECM of brain (Grumet et al., 1996); interacts with other ECM molecules and cell surface receptors (Rauch et al., 2001) | ||
| Versican | Structural component of the ECM, e.g., in blood vessels (Kenagy et al., 2006); mediates cell adhesion and migration (Ang et al., 1999) | Erosive vitreoretinopathy and Wagner disease (Mukhopadhyay et al., 2006) | Atherosclerosis and restenosis (Wight and Merrilees, 2004); tumorigenesis (Nara et al., 1997; Suwiwat et al., 2004) and metastasis (Kim et al., 2009) |
| Small leucine-rich proteoglycans (SLRPs) | |||
| Asporin | Structural component of articular cartilage (Lorenzo et al., 2001) | Osteoarthritis (Kizawa et al., 2005) | Bone and joint diseases (Ikegawa, 2008) |
| Biglycan | Structural component of, e.g., vascular wall (Williams, 2001); binds growth factors such as TGF-β and regulates its activity (Hildebrand et al., 1994) | Abdominal aortic aneurysms (Tamarina et al., 1998) | |
| Decorin | Structural component of, e.g., skin (Reed and Iozzo, 2002) and vascular wall (Williams, 2001); regulates collagen fibrillogenesis (Zhang et al., 2006); binds growth factors such as TGF-β and neutralizes its activity (Yamaguchi et al., 1990); involved in angiogenesis (Järveläinen et al., 1992 and 2006) | Congenital stromal dystrophy (Bredrup et al., 2005) | Tumorigenesis (Grant et al., 2002; Goldoni et al., 2008; Salomäki et al., 2008); osteoarthritis (Melrose et al., 2008); renal disease (Schaefer et al., 1998 and 2001) |
| Fibromodulin | Binds to specific collagen types (Hedbom and Heinegård, 1989) and regulates their fibril formation (Svensson et al., 1999); binds growth factors such as TGF-β and regulates its activity (Hildebrand et al., 1994) | Myopia (Majava et al., 2007a) | Osteoarthritis (Melrose et al., 2008) |
| Keratocan | Structural component of e.g. cornea (Kao and Liu, 2002) | Cornea plana (Pellegata et al., 2000) | Osteoarthritis (Melrose et al., 2008) |
| Lumican | Structural component of e.g. cornea (Kao and Liu, 2002) | Myopia (Wang et al., 2006; Majava et al., 2007a) | Fibrosis and tumor growth (Naito, 2005); atherosclerosis (Onda et al., 2002) |
| Osteoadherin | Promotes αv/β3-integrin mediated cell binding (Wendel et al., 1998); regulation of complement activation (Sjöberg et al., 2009) | ||
| PRELP | Anchors basement membranes to the underlying connective tissue (Bengtsson et al., 2002) | Myopia (Majava et al., 2007a) | |
| Epiphycan | Involved in chondrogenesis (Knudson and Knudson, 2001) | ||
| Opticin | Component of the vitreous humour of the eye (Reardon et al., 2000) and cartilage (Monfort et al., 2008) | Myopia (Majava et al., 2007a); primary open angle glaucoma (Acharya et al., 2007) | |
| Osteoglycin/Mimecan | Component of the vascular wall (Shanahan et al., 1997); regulation of collagen fibrillogenesis (Tasheva et al., 2002) | ||
| Chondroadherin | Component of the cornea, brain, and skeletal muscle, retina and lens (Tasheva et al., 2004); regulates chondrocyte growth and proliferation (Shen et al., 1998) | ||
| Nyctalopin | Essential for retinal synaptic transmission (Bahadori et al., 2006) | Congenital stationary night blindness (Bech-Hansen et al., 2000; Pusch et al., 2000) | |
| Tsukushi | Bone morphogenic protein antagonist (Ohta et al., 2004) | ||
| Podocan | Component of glomeruli (Ross et al., 2003) | Glomerulosclerosis (Ross et al., 2003) | |
| Podocan like protein 1 | Facilitates nephrin signaling (Huber et al., 2001) | ||
| Basement membrane proteoglycans | |||
| Agrin | Modulator of synaptogenesis (Bezakova and Ruegg, 2003; Williams et al., 2008) | Cirrhosis and hepatocellular carcinoma (Tátrai et al., 2006); Alzheimer's disease (Berzin et al., 2000) | |
| Bamacan | Structural component of basement membrane (Couchman et al., 1996) | Diabetic retinopathy (Ljubimov et al., 1996) | |
| Perlecan | Structural component of basement membrane (Iozzo, 1994); regulates, e.g., cell migration and proliferation (Whitelock et al., 2008); involved in angiogenesis (Bix and Iozzo, 2008) | Dyssegmental dysplasia (Silverman-Handmaker type) (Arikawa-Hirasawa et al., 2001); Schwartz-Jampel syndrome (Arikawa-Hirasawa et al., 2002) | Diabetic retinopathy (Ljubimov et al., 1996); tumor growth (Mathiak et al., 1997); Alzheimer's disease (Castillo et al., 1997) |
| Other extracellular proteoglycans | |||
| PG-100 | Proteoglycan form of colony-stimulating factor-1 (Partenheimer et al., 1995) | Hepatitis and liver fibrosis (Högemann et al., 1997) | |
| Testican-1, -2, and -3 | Inhibition of different proteases (Nakada et al., 2003; Edgell et al., 2004) | Testican-3: T-cell leukemia (Kamioka et al., 2009) | |
| Noncollagenous ECM glycoproteins | |||
| Fibronectins (>20 different isoforms) | Involved in cellular adhesion (Akiyama et al., 1981), fibril (Kadler et al., 2008) and ECM assembly (Couchman et al., 1990), tissue injury and inflammation (Satoi et al., 1999; Okamura et al., 2001); regulation of vascular morphogenesis including angiogenesis (Astrof and Hynes, 2009) | Glomerulopathy (Castelletti et al., 2008) | Idiopathic pulmonary fibrosis (Kuhn and McDonald, 1991); cancer (Korc, 2007; Astrof and Hynes, 2009); tendinopathy (Riley, 2007) |
| Laminins | Involved in cell adhesion, migration and differentiation (Colognato and Yurchenco, 2000; Patarroyo et al., 2002) | Congenital muscular dystrophy (Hall et al., 2007); epidermolysis bullosa (Uitto et al., 1994); Pierson syndrome (Zenker et al., 2004) | Tumor progression (Ziober et al., 1996; Ono et al., 1999, Rabinovitz et al., 2001; Nelson et al., 2008) |
| SPARC (secreted protein, acidic and rich in cysteine) | Inhibition of cell proliferation (Yan and Sage, 1999) and angiogenesis (Chlenski et al., 2006) | Tumor progression and metastasis (Porter et al., 1995; Watkins et al., 2005; Clark and Sage, 2008; Podhajcer et al., 2008); idiopathic osteoporosis (Delany et al., 2008) | |
| Tenascins (tenascin-C, -R, -X, and -W) | Involved in cell adhesion, migration and growth (Chiquet-Ehrismann and Chiquet, 2003), wound healing, and neovascularization (Hsia and Schwarzbauer, 2005; Trebaul et al., 2007) | Ehlers-Danlos syndrome (Burch et al., 1997; Mao and Bristow, 2001) | Fibrotic diseases, inflammation, tumorigenesis (Chiquet-Ehrismann and Chiquet, 2003) |
| Thrombospondins (1–5) | Involved in cell migration (Yabkowitz et al., 1993), platelet aggregation, inflammatory response, and wound healing (Adams and Lawler, 2004) | Thrombospondin-5; pseudoachondroplasia and multiple epiphyseal dysplasia (Posey et al., 2008) | Tumor progression (Lawler and Detmar, 2004; Kazerounian et al., 2008); atherosclerosis (Roth et al., 1998; Takahashi et al., 2008) |
| Nidogen/entactin (nidogen 1 and nidogen 2) | Involved in cell adhesion (Yi et al., 1998; Grimpe et al., 1999), wound healing (Sephel et al., 1996) and basement membrane stabilization (Ho et al., 2008) | Osteoarthritis (Kruegel et al., 2008); cancer and tumor progression (Oivula et al., 1999; Ulazzi et al., 2007; Douglas et al., 2008) |
In the next parts of this review, we discuss the effects of current drugs on the ECM and thereafter focus on the potential future ECM-targeted pharmacotherapies and the challenges that ECM-targeted agents place to drug discovery and development processes.
III. Do Currently Used Drugs Target the Extracellular Matrix?
To date, only limited progress has been taken place in the specific targeting of ECM components. Perhaps the best known examples are the drugs that target the major cellular receptors for ECM components, the integrins. These drugs include abciximab (used to treat acute coronary syndrome), efalizumab (used to treat psoriasis), and natalizumab (used to treat multiple sclerosis and Crohn's disease) (Rosove, 2004; Baker, 2007; Schön, 2008). Except for the above protein drugs, practically no others are primarily targeted at the ECM. Nevertheless, several of today's drugs have been shown to influence ECM metabolism and subsequently modulate the composition and organization of the ECM. Table 2 lists examples of currently available drugs affecting the ECM beyond their primary targets. The effects of these drugs on the ECM are often, albeit not always, beneficial in the treatment of various human diseases.
TABLE 2.
Examples of currently available drugs modulating ECM metabolism beyond primary target
| Drug | Primary Mechanism of Action | Effect on ECM metabolism |
|---|---|---|
| Anti-inflammatory and immunomodulatory drugs | ||
| Corticosteroids | ||
| e.g., hydrocortisone, prednisone, dexamethasone | Various genomic and nongenomic mechanisms of action (Stahn et al., 2007) | Modulation of the expression of various types of collagen (Oikarinen et al., 1987; Russell et al., 1989; Oishi et al., 2002), elastin (Del Monaco et al., 1997), proteoglycans decorin and biglycan (Kähäri et al., 1995), HA (Saarni and Hopsu-Havu, 1978), and various noncollagenous matrix glycoproteins such as fibronectin, laminin, and tenascin (Dean et al., 1988; Simo et al., 1992; Ekblom et al., 1993); suppression of MMP synthesis and activity (DiBattista et al., 1991; Sadowski and Steinmeyer, 2001); influence on TIMP expression and activity (Sadowski and Steinmeyer, 2001) |
| NSAIDs | ||
| e.g., indomethacin, diclofenac, naproxen, and -coxibs such as celecoxib | Inhibition of cyclooxygenase enzymes -1 and -2 (Gasparini et al., 2003) | Modulation of the synthesis of fibronectin (Ernst et al., 1995), various types of collagen (Ernst et al., 1995; Liu et al., 2007), large chondroitin sulfate proteoglycans (Okada et al., 1994), and HA (Sussmann et al., 2004); suppression of MMP expression, synthesis, and activity (Sadowski and Steinmeyer, 2001; Pavlovic et al., 2006) |
| Other immunosuppressive drugs | ||
| Cyclosporine A | Calcineurin inhibitor (Ho et al., 1996) | Differential effect on collagen synthesis and fibrosis (Eickelberg et al., 2001; Fornoni et al., 2001; Waller et al., 2004); inhibition of MMP activity (Fornoni et al., 2001; Chiu et al., 2009; Saygili et al., 2009) |
| Leflunomide | Pyrimidine synthesis inhibitor (Fukushima et al., 2007) | Inhibitory effect on fibrogenesis (Yao et al., 2004; Si et al., 2007) |
| Methotrexate | Inhibitor of dihydrofolate reductase (Warren et al., 2008) | Reduction of type IV collagen synthesis and ECM expansion (Yozai et al., 2005); influence on MMP and TIMP production (Seitz and Dayer, 2000; Fiedorczyk et al., 2006). |
| Sirolimus/ rapamycin | Mammalian target of rapamycin (mTOR) inhibitor (Sehgal, 2003; Granville et al., 2006) | Blocking of the synthesis of fibrillin-1 (Schaefer et al., 2007); blocking of HA accumulation (Gouëffic et al., 2007); inhibition of growth factor induced collagen synthesis (Park et al., 2005) |
| Tacrolimus | Calcineurin inhibitor (Nghiem et al., 2002) | Suppression of cytokine stimulated MMP synthesis (Migita et al., 2005); stimulation of collagen synthesis in vascular smooth muscle cells via TGF-β signaling (Giordano et al., 2008) |
| Microbial drugs | ||
| Doxycycline | Antimicrobial drug | Inhibition of specific MMPs (Golup et al., 1998; Ahuja, 2003; Clark et al., 2008) |
| Minocycline | Antimicrobial drug | Inhibition of specific MMPs (Sutton et al., 2005; Machado et al., 2006) |
| Cytokine modulators | ||
| Etanercept | Inhibition of TNF-α receptor (Moreland, 1999) | Modulation of type II collagen synthesis and degradation (Briot et al., 2008) |
| Infliximab, adalimumab | Inhibition of TNF-α (Moreland, 1999) | Inhibition of fibrosis (Sorrentino et al., 2007); downregulation of tissue degrading MMPs (Wendling et al, 2008; Di Sabatino et al., 2009) |
| Thalidomide | Inhibitory action on TNF-α (Moreira et al., 1993) | Attenuation of fibrosis (Chong et al., 2006; Yndestad et al., 2006); influence on endostatin level (Aydoğan et al., 2007) |
| Other drugs | ||
| ACE-inhibitors | ||
| e.g., Captopril, enalapril and numerous other ones | Blocking of angiotensin converting enzyme (Johnston and Jackson, 1983) | Modulation of collagen and proteoglycan synthesis and fibrosis (Kaneto et al., 1994; Cruz et al., 2000; Wapstra et al., 2001); influence on MMPs (Rizzoni et al., 2004; Aoki et al., 2007; Okada et al., 2008) |
| ATR-blockers | ||
| e.g., Losartan and numerous other ones | Blocking of angiotensin II receptor (Gavras and Salerno, 1996) | Upregulation of thrombospondin-1 expression (Fischer et al., 2001); downregulation of fibronectin expression (Fischer et al., 2001); inhibition of CTGF and fibrosis (Rupérez et al., 2003); inhibition of specific MMPs and TIMPs (Masutomo et al., 2001; Saygili et al., 2009) |
| Calcium channel blockers | ||
| e.g., Amlodipine, felodipine, diltiazem, and verapamil | Blocking of the L-type calcium channel (Mason et al., 2003) | Inhibition of collagen expression and accumulation (Roth et al., 1996; Sugiura et al., 2000); modulation of MMP activity (Roth et al., 1996; Wada et al., 2001) |
| Calcium sensitizing drugs | ||
| e.g., Levosimendan | Calcium-sensitizer (Ng, 2004) | Reduction of MMP-2 (Tziakas et al., 2005) |
| Endothelin receptor antagonists | ||
| e.g., Bosentan | Endothelin-1 inhibitor (Motte et al., 2006) | Upregulation of MMP-9 (Giannelli et al., 2005); downregulation of fibronectin (Siddiqui et al., 2006) |
| Statins | ||
| e.g., Simvastatin, atorvastatin, and numerous other ones | Inhibition of HMG-CoA reductase (Vaughan and Gotto, 2004) | Inhibition of collagen and CTGF production (Martin et al., 2005; Rupérez et al., 2007); reduction of HA accumulation (Sakr et al., 2008); reduction of MMP-9 synthesis (Kalyanasundaram et al., 2006) |
| Tranilast | Histamine H1 receptor antagonist (Namazi and Soma, 2005) | Inhibition of collagen synthesis (Yamada et al., 1994) and accumulation (Isaji et al., 1987) |
| Tyrosine kinase inhibitors | ||
| e.g., Imatinib | Specific tyrosine kinase inhibitor (Lydon and Druker, 2004) | Blocking of the stimulation of TGF-β induced collagen gene expression (Bhattacharyya et al., 2009) |
HA, hyaluronan
A. Current Anti-Inflammatory and Immunomodulatory Drugs with Beneficial Disease-Modifying Properties on the Extracellular Matrix
Evidence is available indicating that many of the current drugs exert their beneficial disease-modifying properties, at least in part, via their pleiotropic effects on the ECM. This is true for glucocorticoids and certain other anti-inflammatory and immunomodulatory drugs, such as infliximab, a chimeric monoclonal antibody that blocks the action of tumor necrosis factor-α (TNF-α). Such drugs have been shown to effectively modulate the ECM when used in the treatment of specific fibrotic diseases exhibiting overt inflammation, such as Riedel's thyroiditis and Crohn's disease (Moulik et al., 2004; Sorrentino et al., 2007). The effect of glucocorticoids on the ECM dates back almost 20 years, when it was shown that the inhibitory effect of these drugs was not related to their anti-inflammatory activity (Cutroneo et al., 1990). Glucocorticoids are now known to decrease the synthesis of type I collagen and prevent scarring by decreasing the binding of the TGF-β activator protein complex to TGF-β element in the distal promoter of the proa1 type I collagen gene (Cutroneo and Sterling Jr, 2004). Glucocorticoids may also affect collagen synthesis by reducing prolyl hydroxylase activity (Oikarinen and Hannuksela, 1980), an important rate-limiting enzyme of collagen synthesis (Kivirikko and Risteli, 1976; Myllyharju, 2008). Furthermore, glucocorticoids have been shown to repress collagen synthesis by increasing the degradation of collagen mRNAs (Nuutinen et al., 2001). As summarized in Table 2, besides collagens, several other ECM molecules are also targeted by glucocorticoids. These include elastin (Del Monaco et al., 1997); proteoglycans decorin and biglycan (Kähäri et al., 1995); hyaluronan (Saarni and Hopsu-Havu, 1978); noncollagenous glycoproteins, particularly fibronectin, laminin, and tenascin (Dean et al., 1988; Simo et al., 1992; Ekblom et al., 1993); and certain ECM-degrading MMPs (DiBattista et al., 1991; Sadowski and Steinmeyer, 2001) as well as their inhibitors [i.e., tissue inhibitors of MMPs (TIMPs)] (Sadowski and Steinmeyer, 2001). The effects of glucocorticoids on these molecules are also likely to play a role in their antifibrotic property (Schoepe et al., 2006), although the mechanisms whereby glucocorticoids regulate these ECM genes are far less understood. However, certain MMPs are regulated by glucocorticoids via interference with the binding of the transcription factor activator protein-1, the major enhancer factor of the collagenase promoter (Jonat et al., 1990). Infliximab and other TNF-α antagonists can also act on the ECM by stimulating MMP activity and, therefore, ECM degradation (Wendling et al., 2008; Di Sabatino et al., 2009).
Nonsteroidal anti-inflammatory drugs, which primarily inhibit cyclooxygenase enzymes and thereby prevent the synthesis of prostanoids, are able to ameliorate fibrotic processes via influencing TGF-β synthesis (Liu et al., 2007). Furthermore, nonsteroidal anti-inflammatory drugs have been shown to affect the ECM through suppression of MMP expression, synthesis, and activity (Sadowski and Steinmeyer, 2001; Pavlovic et al., 2006). This mechanism of action has also been described for antimicrobial tetracycline analogs such as doxycycline (Golup et al., 1998; Ahuja, 2003; Clark et al., 2008) and minocycline (Sutton et al., 2005; Machado et al., 2006) when used in the treatment of abdominal aortic aneurysm and neurodegenerative diseases, respectively (Abdul-Hussien et al., 2009; Kim and Suh, 2009).
It has recently been discovered that cyclosporine A, a widely used immunosuppressant and an inhibitor of the mitochondrial permeability pore, is a curative agent for congenital collagen type VI myopathies (i.e., Ullrich congenital muscular dystrophy and Bethlem myopathy) (Merlini et al., 2008a). That is, cyclosporine A corrects mitochondrial dysfunction and muscle apoptosis that are characteristic features in these patients (Merlini et al., 2008a). This discovery is of great importance, because it demonstrates that an unexpected collagen type VI/mitochondrial connection forms the basis of the pathogenesis of collagen type VI myopathies (Maraldi et al., 2009). Furthermore, it also represents a proof of the principle that hereditary diseases, including hereditary ECM diseases, can be treated with proper drugs downstream of the genetic lesion, if the pathogenetic mechanism is understood (Olsen, 2008). Besides being a curative agent for Ullrich congenital muscular dystrophy and Bethlem myopathy, cyclosporine A can beneficially contribute to ECM remodelling by regulating the activity of specific MMPs (Chiu et al., 2009; Saygili et al., 2009). These examples highlight the point that the mechanisms driving fibrogenesis are not necessarily the same as those regulating inflammation (Wynn, 2007, 2008; Sivakumar and Das, 2008) and that there is a specific need to develop drugs targeting the ECM besides developing better drugs for various inflammatory diseases.
B. Other Current Drugs with Beneficial Disease-Modifying Properties on the Extracellular Matrix
In addition to anti-inflammatory and immunomodulatory drugs, there are several other drug classes that have been shown to influence the ECM in a beneficial way beyond their primary targets. This is true for anti-hypertensive agents, particularly angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, and calcium channel blockers, which all possess marked antifibrotic properties via their ability to reduce collagen production (Roth et al., 1996; Sugiura et al., 2000; Tayebjee et al., 2003; Duprez, 2006). ACE inhibitors such as captopril primarily block angiotensin II-converting enzyme (Johnston and Jackson, 1983) and limit ECM expansion in numerous models of acute and chronic renal disease (Taal and Brenner, 2000). Angiotensin receptor blockers, including losartan, inhibit the effects of angiotensin by selectively blocking angiotensin II type 1 receptor (Gavras and Salerno, 1996). As a result, antifibrotic action is also evident. Both of the angiotensin II-blocking drugs act on the ECM by reducing sustained overexpression of TGF-β (Peters et al., 1999). The combination of angiotensin II blockade and straight inhibition of TGF-β with antibodies has proven to be a promising therapeutic strategy to slow down fibrotic diseases (Yu et al., 2002, 2004). Angiotensin II blockade by angiotensin II type 1 receptor blockers in patients with Marfan's syndrome has been found to significantly slow the rate of progressive aortic-root dilation (Brooke et al., 2008). In addition, calcium channel blockers such as nifedipine and amlodipine, which block the L-type calcium channel (Mason et al., 2003), have been proposed to act on the ECM via suppressing TGF-β, thereby resulting in reduced matrix accumulation (Sugiura et al., 2000). However, this finding has been disputed in some published work (Campistol et al., 2001). On the other hand, calcium channel blockers have also been reported to modulate the activity/expression of MMPs and TIMPs, but the mechanisms of action are still unclear (Roth et al., 1996; Wada et al., 2001). Cholesterol-lowering drugs, the statins, can reduce tissue fibrosis (Louneva et al., 2006) by their ability to inhibit angiotensin-induced connective tissue growth factor production (CTGF) (Rupérez et al., 2007). Statins inhibit the conversion of HMG-CoA to mevalonate, which is required for the post-translational modification of Rho family and Ras GTPases (Jackson et al., 1997). Rho family GTPases Rac1 and Cdc42 have been shown to be the principal mediators of the TGF-β-stimulated expression of CTGF in human gingival fibroblasts, and CTGF expression can be reduced significantly with lovastatin (Black and Trackman, 2008). The use of pravastatin has also been reported to inhibit the Rho/CTGF/ECM cascade in human fibrosis explants (Haydont et al., 2007).
Other examples of current drugs that are able to modulate ECM metabolism in a way that can be regarded beneficial for disease-modifying processes include several newer agents, such as levosimendan, a calcium sensitizer used for patients with decompensated heart failure (Ng, 2004; Tziakas et al., 2005). Levosimendan use reduces the serum level of MMP-2 in these patients (Tziakas et al., 2005). In contrast, bosentan, an endothelin ETA- and ETB-receptor antagonist, increases the serum MMP-9 without affecting TIMPs when used in patients with pulmonary arterial hypertension (Giannelli et al., 2005, 2006). Thus, bosentan favors proteolytic imbalance by increasing the turnover of ECM proteins. Sirolimus, an effective antimitotic agent used in stent restenosis therapy, blocks the accumulation of hyaluronan and the adhesion of monocytes to ECM produced by vascular smooth muscle cells (Gouëffic et al., 2007). Of course, numerous other examples of drugs and pharmaceutical agents influencing the ECM in a potentially beneficial way exist. However, the problem with all the mentioned drugs is that their effects on the ECM are rather weak and unspecific.
C. Deleterious Effects of Current Drugs on the Extracellular Matrix
The effects of current drugs on the ECM are not always desired. A classic example is peritoneal fibrosis and a wide variety of skin lesions after the use of the β-adrenergic-blocking drug practolol (Brown et al., 1974), although the mechanism whereby practolol causes peritoneal fibrosis and cutaneous lesions is, unfortunately, not known. Methysergide, a receptor antagonist for the 5-hydroxytryptamine (5HT) receptor 2C, earlier prescribed for prophylaxis of migraine, has also caused retroperitoneal fibrosis (Utz et al., 1965). It is noteworthy that the antiobesity drugs dexfenfluramine and fenfluramine, which act by releasing 5HT and also partially via conversion to the active metabolite norfenfluramine, directly stimulate 5HT2B-receptors and have been associated with hyperplastic valvular and endocardial lesions with increased ECM via TGF-β-mediated mechanisms (Gardin et al., 2000; Jian et al., 2002; Rothman and Baumann, 2002). Because of this side effect, these agents were voluntarily withdrawn from the market in 1997. Furthermore, dopamine receptor agonists of the ergot-derivative class used in the treatment of Parkinson's disease and prolactin-producing pituitary gland tumors have caused valvular diseases, possibly via their effects on 5HT2B-receptors (Antonini and Poewe, 2007). Antineoplastic agents bleomycin and cyclophosphamide represent additional well known drugs with side effects on the ECM that include fibrosis (Lazo and Hoyt, 1990). Bleomycin seems to mediate fibrosis by increasing the expression and synthesis of TGF-β1 (Cutroneo et al., 2007). Methotrexate, a first-line antirheumatic drug by the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR), primarily inhibits the metabolism of folic acid. Because of its first-pass metabolism in the liver, it may cause liver fibrosis (Salliot and van der Heijde, 2008; Lindsay et al., 2009). The underlying mechanisms for methotrexate-induced liver cell damage are still incompletely understood. However, the activation of complement pathway within the liver tissue has been suggested to play an important role (Belinsky et al., 2007). Gingival enlargement due to an increase in the ECM of the gingival connective tissue has been traditionally recognized as an adverse effect of phenytoin and L-type calcium channel blocker therapies as well as cyclosporin use (Bonnaure-Mallet et al., 1995; Brunet et al., 1996). Evidence is available indicating that in the case of phenytoin, TGF-β1 and also platelet-derived growth factor-BB are closely associated with this aesthetic side effect (i.e., gingival enlargements) (Kuru et al., 2004). The underlying mechanisms whereby L-type calcium channel blockers cause gingival hyperplasia remains to be fully understood. The proposed mechanisms include defective collagenase activity that was due to decreased uptake of folic acid (Brown et al., 1990) as well as up-regulation of TGF-β1 and other factors responsible for the production of a fibrous scaffold in the gingiva (Lafzi et al., 2006).
Recently discovered deleterious effects of drugs on the ECM are tendinopathies associated with the use of statins (Marie et al., 2008) and fluoroquinolones (Conforti et al., 2007). Furthermore, skeletal consequences (particularly increased risk of bone fractures in the appendicular skeleton in women with type 2 diabetes, caused by thiazolidinediones, an antidiabetic drug class) has been noticed (Grey, 2008). Statin and fluoroquinolone-induced tendinopathies have mainly been attributed to the ability of these two drug classes to modulate the activity of specific MMPs in tendon cells (Corps et al., 2002, 2005; Pullatt et al., 2007). The side effects of thiazolidinediones on bones are thought to be mainly due to the proadipocytic and antiosteoblastogenic effects of these drugs on pluripotent mesenchymal stem cells (Lecka-Czernik et al., 2002; Shockley et al., 2009). However, reduction in ECM production via inhibited TGF-β1 or CTGF expression may be of equal importance (Peng et al., 2006; Gao et al., 2007) in defining the efficacy of these drugs. The new biological anti-inflammatory and immunomodulatory drugs, such as tumor necrosis factor inhibitors and the interleukin-1 inhibitor anakinra, may also cause adverse side-effects on the ECM, particularly in the skin (Deng et al., 2006; Regula et al., 2008). In clinical practice, glucocorticoids are probably the most well known drugs that cause marked side-effects via their influence on the ECM. Systemic use of glucocorticoids for a longer period of time in the treatments of chronic inflammatory disease processes is almost inevitably harmful to healthy tissues (McDonough et al., 2008), particularly bones, by causing premature or exaggerated osteoporosis (Ton et al., 2005; Canalis et al., 2007), and skin, by inducing its thinning (Schoepe et al., 2006; Zöller et al., 2008). Recent developments in bone biology have markedly advanced our understanding of how glucocorticoids affect the receptor activator of the nuclear factor-κB–ligand–osteoprotegerin system and the Wnt-catenin signaling pathway (Berris et al., 2007; Canalis et al., 2007) and via these effects favor osteoclas-togenesis instead of osteoblastogenesis. Glucocorticoids can also inhibit osteoblast-driven synthesis of type I collagen, the major component of the bone ECM, with a consequent decrease in bone matrix available for mineralization (Canalis, 2005). Additional mechanisms whereby glucocorticoids induce osteoporosis include enhanced expression of selected MMPs by osteoblasts (Delany et al., 1995) and effects on the synthesis or receptor binding of growth factors (Canalis et al., 2007). However, the exact mechanisms of glucocorticoid-induced osteoporosis are still incompletely understood. The molecular mechanisms of glucocorticoid-induced skin atrophy are similarly complicated. Glucocorticoids are known to regulate the gene expression of a number of ECM molecules and other molecules in cutaneous cells via many and diverse genomic and nongenomic mechanisms, including TGF-β signaling pathway as described in section III.A (Cutroneo and Sterling Jr, 2004; Schoepe et al., 2006). To avoid the harmful effects of glucocorticoids on healthy tissues, site-specific targeting using modified glucocorticoids is desired (Joner et al., 2008). Therefore, the involvement of ECM proteins should be more often considered as possible targets in the mechanistic toxicology during drug development when possible side-effects are sought to be avoided.
IV. Potential Future Pharmacotherapies Targeting the Extracellular Matrix
As discussed above, several of the currently available drugs can modulate ECM metabolism and assembly beyond their primary targets. However, their effects on the ECM are often neither potent nor specific enough. Furthermore, several drugs can have deleterious effects on the ECM. Therefore, novel drugs influencing the ECM in a more direct way are still desired. In recent years, the number of drug development projects aiming to primarily target the ECM has increased (Huxley-Jones et al., 2008). In these projects, cell- and gene-based strategies, particularly gene and antisense therapies as well as RNA interference and recombinant technologies, are used (Huxley-Jones et al., 2008). Although these approaches offer fascinating new ways to treat various human diseases (Isner et al., 1996; Ye at al., 2007), they have not yet been put into clinical practice. The multiplicity and functional complexity of the ECM molecules and the diversity of diseases related to the ECM offer significant challenges for future targeting strategies. However, the overwhelming evidence, some of which has been presented in this review, indicates a need to target specific components of the ECM to prevent or treat a variety of diseases. On the other hand, the molecular complexity of diseases suggests that agents targeting not only a single ECM molecule but concomitantly several aspects of the ECM will also have an important position in future drug development. The widespread targeting has already been successfully applied in the development of the second-generation tyrosine kinase inhibitors used for the treatment of cancers (Baselga, 2006). In the following chapters, potential future pharmacotherapies targeting primarily the synthesis, degradation, or signaling of the ECM will be discussed. A summary of these drugs is presented in Table 3. Furthermore, a schematic illustration of the ECM and its outside-in signaling pathways as well as the sites of potential future pharmacotherapeutics targeting the ECM are shown in Fig. 1.
TABLE 3.
Examples of potential future pharmacotherapies targeting the synthesis, degradation, or signaling of the ECM
| Drug | Mechanism of action |
|---|---|
| Drugs targeting the synthesis of the ECM | |
| Cytokine inhibitors | |
| Lerdelimumab (Cordeiro, 2003) | Antibody to TGF-β2 |
| Metelimumab (Denton et al., 2007) | Antibody to TGF-β1 |
| GC-1008 (Grütter et al., 2008) | Antibody to TGF-β1 |
| SB431542 (Mori et al., 2004) | Inhibits TGF-β signaling through selective interference with ALK5-mediated Smad activation |
| SD-208 (Uhl et al., 2004) | TGF-β receptor I kinase inhibitor |
| SM305 (Ishida et al., 2006) | Inhibits TGF-β signaling through selective interference with ALK5-mediated Smad activation |
| FG-3019 (Aikawa et al., 2006) | Antibody to CTGF |
| Cytokines | Recombinant TGF-β3 |
| Avotermin (Durani et al., 2008) | |
| Ilodecakin (Marshall, 1999) | Recombinant interleukin-10 |
| Drugs targeting the degradation of the ECM | |
| Tanomastat (Hirte et al., 2006) | MMP inhibitor |
| Cipemastat (Trocade) (Ishikawa et al., 2005) | Collagenase and MMP-14 inhibitor |
| FR255031 (Ishikawa et al., 2005) | Broad-spectrum MMP inhibitor |
| Balicatib (Desmarais et al., 2008) | Selective cathepsin K inhibitor |
| Odanacatib (Gauthier et al., 2008) | Selective cathepsin K inhibitor |
| PI-88 (McKenzie, 2007) | Inhibits heparanase activity |
| AA-4500 (Occleston et al., 2008) | Collagenase stimulator |
| Drugs targeting the signaling of the ECM | |
| Etaracizumab (Delbaldo et al., 2008) | Antibody to αv/β3 integrin |
| Vedolizumab (MLN0002) (Feagan et al., 2008; Behm and Bickston, 2009) | Antibody to α4/β7 integrin |
| Volociximab (Ricart et al., 2008) | Antibody to α5/β1 integrin |
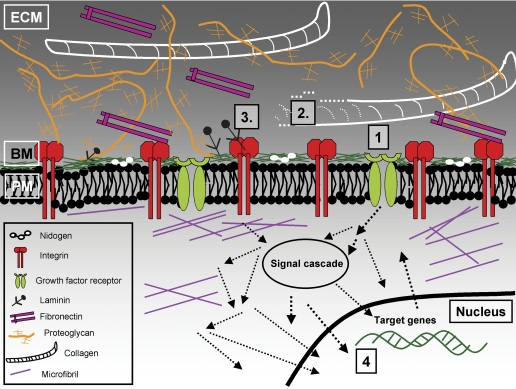
Fig. 1.
Schematic illustration of the ECM and potential targets in pharmacotherapy in the future. 1, targeting the synthesis of the ECM by blocking of specific growth factors such as TGF-β, their receptor molecules, or intracellular signal transduction. 2, controlling of the degradation of ECM by interfering with enzymes involved in ECM remodelling (e.g., MMPs, ADAMTS, cathepsins) and/or their inhibitors. 3, interfering with the ECM signaling pathways (e.g., via integrins) either by blocking the ECM and integrin interactions or subsequent signal transduction. 4, influencing the transcription of specific ECM molecules (e.g., by TFOs). BM, basement membrane; PM, plasma membrane.
A. Targeting the Synthesis of the Extracellular Matrix
Fibrosis, characterized as a relative increase in the amount of fibrous connective tissue, is a hallmark of numerous human diseases, most of which exhibit an inflammatory component in their pathogenesis. However, as discussed above, current anti-inflammatory and immunomodulatory drugs as well as certain other drugs have only a limited efficacy in preventing fibrotic processes. Much evidence is available indicating that the molecular family of transforming growth factor-βs (here grouped as TGF-β) are essential mediators of fibrosis. Indeed, TGF-β has been implicated in the fibrotic disorders of heart, kidney, liver, lung, skin, and several other organs (Gordon and Blobe, 2008). Therefore, TGF-β is a highly potential target for antifibrotic therapy. Current understanding of the signaling pathways of TGF-β provides three main strategies for blocking pathological TGF-β responses in the treatment of fibrotic processes: 1) blocking the TGF-β ligand by neutralizing antibodies [such as metelimumab (Denton et al., 2007) and lerdelimumab (Cordeiro, 2003)], soluble TGF-β receptors, receptor mimetics, or natural TGF-β-binding proteins such as decorin; 2) blocking TGF-β receptor activation and downstream signaling by orally active small-molecule TGF-β receptor kinase inhibitors such as SB431542 (Mori et al., 2004), SD-208 (Uhl et al., 2004), and SM305 (Ishida et al., 2006); and 3) selective inhibition of intracellular signal transduction by interfering with Smads by physiological endogenous inhibitor Smad7 or with coactivators by aptamers (thioredoxin A-Smad anchor for receptor activation) (Varga and Pasche, 2008). However, because TGF-β blockade has not yet translated into an effective and safe therapy in human patients, additional growth factors or cytokines involved in fibrotic processes have also been explored as potential targets for the treatment of fibrosis. These molecules include interleukin-6, interleukin-13, CC and CXC family of chemokines, bone morphogenetic protein-7 and CTGF (Nguyen and Goldschmeding, 2008; Sivakumar and Das, 2008). For example, FG-3019 (Aikawa et al., 2006) is an IgG1 antibody that can bind specifically to domain 2 of CTGF and block angiotensin II and advanced glycation end product-induced fibronectin production by vascular smooth muscle cells (Occleston et al., 2008). This drug reduces or inhibits fibrosis in lung, liver, and kidney in vivo. As such, it has already been used in phase I/II clinical trials for treatment of idiopathic pulmonary fibrosis, and trials for treatment of focal segmental glomerulosclerosis and other fibrotic diseases are planned (Occleston et al., 2008). On the other hand, TGF-β3, which is present in high levels in developing embryonic skin and thus also in embryonic wounds, is known to be involved in wound healing with no scar. Therefore, recombinant human TGF-β3 called avotermin has been developed and tested for scar formation in adults (Durani et al., 2008; Ferguson et al., 2009). Avotermin has been postulated to promote the regeneration of normal skin and to improve scar appearance by reducing the deposition of ECM components such as collagen and fibronectin and by influencing the organization of the newly deposited ECM in the wounded dermis (Occleston et al., 2008). Ilodecakin, a recombinant interleukin-10 (Marshall, 1999), is another example of a cytokine drug modulating ECM metabolism in a way that creates an environment conducive for degenerative wound healing (Moroguchi et al., 2004; Peranteau et al., 2008). Which of the above agents will finally translate into clinical practice remains to be seen. However, recent results with avotermin are encouraging: in three double-blinded, placebo controlled studies, avotermin administered intradermally to both margins of skin incisions, before wounding and 24 h later, has shown an accelerated and permanent improvement in scarring without any substantial adverse events (Ferguson et al., 2009).
Besides interfering with the activity of profibrotic growth factors and cytokines, the synthesis of ECM molecules can be regulated more directly by targeting their promoters and enhancers. A series of triplex-forming oligonucleotides (TFOs) have been developed for inhibiting the transcription of α1(I) collagen gene (Ye et al., 2005). Furthermore, bioconjugation of oligonucleotides with other molecules such as lipids, sugars, or peptides makes the site-specific delivery of TFOs possible (Ye et al., 2007). This kind of targeted delivery of TFOs provides a whole new area for antifibrotic drugs in pharmacotherapy. On the other hand, it is also possible to influence the synthesis of ECM molecules at the post-translational level. For example, inhibition of type I collagen prolyl-4-hydroxylase generates scurvy-like unstable collagen fibrils and results in reduced collagen production (Rocnik et al., 1998). In contrast, overexpression of the α subunit of type I collagen prolyl-4-hydroxylase is associated with excess collagen production (John et al., 1999). Thus, collagen prolyl-4-hydroxylases, and especially their α subunits, can be regarded as attractive targets for pharmacological inhibition to control excessive collagen accumulation in fibrotic diseases and severe scarring (Myllyharju, 2008). Here, organ-specific targeting is still an unresolved problem. Collagen is the main component of all fibrous connective tissues from skin to bone or tubular organs. When type I collagen synthesis is targeted, side effects are expected to be noted in all organs synthesizing collagen.
Although the inhibition of ECM synthesis is desired in the treatment of fibrotic diseases, there are also numerous situations in which drugs capable of increasing ECM production are useful. These situations include degenerative diseases of intervertebral disc and articular cartilage, impaired wound healing, and nerve basement membrane regeneration (Wang et al., 2004; Roughley et al., 2006; Armstrong et al., 2007). Nutraceuticals, in particular glucosamine, have been used as a treatment option in degenerative diseases of cartilage. It has been proposed that glucosamine leads to an increased glycosaminoglycan production by chondrocytes, because it is the basic building block of glycosaminoglycan molecules. However, the results have been controversial (Bassleer et al., 1998; Mroz and Silbert, 2004). Nevertheless, glucosamine, when used in combination with chondroitin sulfate, has been claimed to be effective in the subgroup of arthrotic patients with moderate-to-severe knee pain (Clegg et al., 2006). A plausible explanation as to why these agents reduce pain is that they possess anti-inflammatory activities rather than anabolic properties to replace joint fluid (Sakai et al., 2006). However, approaches to develop nutraceuticals for the treatment of degenerative cartilage diseases may still be worthwhile, even though targeted delivery of TFOs, as well as growth factors stimulating ECM production or molecules inhibiting matrix degradation, is likely to provide a more practical and potent remedy for various degenerative diseases including those of the cartilage.
B. Targeting the Degradation of the Extracellular Matrix
Proteolytic degradation of ECM components is a pathognomic feature, not only of well known degenerative diseases such as osteoarthritis (Smith, 1999; Kobayashi et al., 2005) but also in cardiovascular pathologies (Raffetto and Khalil, 2008), malignancies (Ingber, 2008), and several other diseases. Numerous enzymes are able to degrade individual ECM components. However, ECM degradation is primarily under the control of specific MMPs and their inhibitors (i.e., TIMPs) (Visse and Nagase, 2003), disintegrin and metalloproteinase with thrombopondin motifs (ADAMTS) family of proteinases (Tang, 2001), and cysteine protease cathepsins (Chapman et al., 1997). Thus, strategies for the prevention of proteolytic matrix degradation have mainly focused on these enzymes. Synthetic broad-spectrum MMP inhibitors such as batimastat, ilomastat, and marimastat, as well as more selective MMP inhibitors, particularly tanomastat and trocade, have been developed and tested in clinical trials, foremost to treat cancer (Overall and Kleifeld, 2006). Unfortunately, the results from these trials have been disappointing (Coussens et al., 2002; Hirte et al., 2006). The reasons for the failures of the above synthetic MMP inhibitors are not exactly understood, but it has been thought that they cover too wide a spectrum and so more selective MMP inhibitors are still required (Hu et al., 2007). In line with this idea, evidence is available suggesting that efforts to inhibit MMP activity should be directed at therapies exploiting endogenous MMP inhibitors, TIMPs (Ramirez-Correa et al., 2004; Zacchigna et al., 2004), or monoclonal antibodies against individual MMPs (Martens et al., 2007). Experiences from animal studies indicate that ADAMTS inhibitors are likely to have a position as future drugs. For example, inhibition of specific ADAMTS, namely AD-AMTS-4 and -5, by a flavonoid called nobiletin, has been found to decrease aggrecan degradation in cartilage and subsequently to prevent cartilage destruction (Imada et al., 2008). The most promising results of the potential utility of targeting matrix degradation in pharmacotherapy come from studies using cathepsin inhibitors, particularly cathepsin K inhibitors. There are two cathepsin K inhibitors, odanacatib (Gauthier et al., 2008) and balicatib (Desmarais et al., 2008), both of which have been demonstrated to inhibit bone resorption and to increase bone mass in patients with osteoporosis (Stoch and Wagner, 2008). It can be expected that cathepsin K inhibitors will be the first targeted agents getting approval to be used for ECM therapy, especially for osteoporosis. Cathepsin K inhibitors also are promising strategies for targeting proteolytic degradation of the ECM for use in pharmacotherapy. It remains to be seen whether in the future there will be specific drugs that inhibit ECM degradation within vascular wall and through this mechanism prevent acute coronary syndromes and other aneurysmatic processes in the vasculature (Kim et al., 2005; Senzaki, 2006; Hu et al., 2007).
In addition to targeting the enzymes that degrade the ECM, a number of other potential candidate enzymes and their inhibitors have to be evaluated for drug discovery. For example, inhibition of hyaluronidases, the enzymes that degrade hyaluronan, provides an attractive pharmacological tool to be used in physiological processes ranging from fertilization to aging and in pathological processes such as malignancies (Girish and Kemparaju, 2007). Furthermore, the finding that heparanase is elevated in a wide variety of tumor types and is subsequently linked to the development of pathological processes has led to an explosion of therapeutic strategies to inhibit its enzyme activity. So far at least one compound, the sulfated oligosaccharide PI-88, which both inhibits heparanase activity and has effects on growth factor binding, has reached clinical trials, where it has shown promising efficacy (McKenzie, 2007). A molecule named AA-4500 that can stimulate collagenase activity has demonstrated efficacy in phase III trial when administered locally to treat Dupuytren's contracture (Occleston et al., 2008). On the other hand, putrescine, inhibitor of tissue transglutaminase (required for crosslinking of collagen with other ECM proteins), has shown no greater efficacy than placebo in phase II trial when evaluated as treatment for hypertrophic scarring (Occleston et al., 2008).
C. Targeting the Signaling of the Extracellular Matrix
The altered assembly of connective tissue, whether it is a result of the synthesis or degradation of the matrix components, can markedly modify cell-matrix interactions by activating various signaling pathways that regulate cell behavior particularly growth, differentiation, motility and viability of the cells (Lukashev and Werb, 1998; Larsen et al., 2006; Grzesiak et al., 2007; Marastoni et al., 2008). The effects of individual matrix molecules on the cells are primarily transmitted through specific cell-surface receptors called integrins that are transmembrane heterodimeric glycoproteins composed of one α and one β subunit (Hynes, 2002). So far, 18 α and 8 β subunits have been identified in mammals, and these subunits can form at least 24 different combinations, each able to specifically bind one or several ECM molecules. Because integrins usually recognize only relatively short peptide motifs in a molecule (Ruoslahti and Pierschbacher, 1987; Hynes, 2002), it is possible to target the integrin recognition peptide motifs of the ECM molecules by using specific blocking antibodies and through this mechanism influence interactions between the remodelled matrix and the integrins. This approach has successfully been used with cytokines such as TNF-α, whose receptor binding and activity can be blocked [e.g., with a monoclonal chimeric antibody infliximab, a fully human monoclonal antibody adalimumab, or a PEGylated Fab′ fragment of a humanized TNF-α inhibitor monoclonal antibody certolizumab (Bourne et al., 2008)]. However, strategies for generating antibodies against ECM molecules and using them for therapies have not gained much favor (Huxley-Jones et al., 2008). Instead, strategies for generating monoclonal antibodies against integrins have been under intensive investigation. As mentioned earlier, there are already a few anti-integrin antibodies in clinical practice used for acute coronary syndrome, psoriasis, multiple sclerosis, and Crohn's disease (Rosove, 2004; Baker, 2007; Schön, 2008). Although the use of these anti-integrin antibodies as drugs has not always been completely safe (Baker, 2007; Schön, 2008; Tamhane and Gurm, 2008), each of them has clearly verified that this approach is very useful in drug discovery. For example, vedolizumab (MLN0002), a humanized antibody targeting the α4β7 integrin, has exhibited a beneficial effect on active Crohn's disease (Feagan et al., 2008) and ulcerative colitis (Behm and Bickston, 2009) by virtue of its highly selective capacity to block lymphocyte migration to inflamed areas of the gut. Vedolizumab specifically interferes with the interaction between the α4β7 integrin on lymphocytes and its principal ligand, mucosal addressin cell adhesion molecule-1, on endothelial cells (Berlin et al., 1993). It is noteworthy that α4β7 integrin can also bind fibronectin (Rüegg et al., 1992), suggesting that, in the future, vedolizumab may have a wide spectrum of application in the treatment of pathological conditions such as atherosclerosis, cardiac hypertrophy, and tumorigenesis (Astrof and Hynes, 2009). Volociximab, a chimeric humanized monoclonal antibody that is a high-affinity function inhibitor of the α5β1 integrin, has been applied in clinical phase II trials for solid tumors in renal cell carcinoma, metastatic melanoma, and pancreatic cancer (Huveneers et al., 2007). The rationale of using volociximab in cancer therapy can be based on the fact that integrin α5β1, which binds fibronectin, is expressed mainly on vascular endothelial cells and up-regulated together with fibronectin in tumor vasculature (Astrof and Hynes, 2009). As such, volociximab has a potential to inhibit tumor angiogenesis, without which tumors cannot grow. It remains to be seen whether volociximab might also provide a cure for a large number of nonmalignant human diseases that are dependent on angiogenesis (Folkman, 2007). On the other hand, as mentioned earlier, angiogenesis is also under regulation of a number of other integrins, particularly the αv integrin subfamily, several ECM molecules (including some of their proteolytically released cleavage products such as endostatin), and different soluble factors (i.e., growth factors and cytokines) (Ingber and Folkman, 1989; Hynes, 2007). Therefore, it is likely that in most situations, volociximab alone is not potent enough to repress angiogenesis, and developing antiangiogenic therapies based on the other molecules mentioned above would be vital.
Besides generating anti-integrin antibodies, it is possible to generate specific small-molecule compounds, peptidomimetics, to block integrin signaling pathways that are activated by the remodelled ECM. Indeed, this approach has been recognized to be an important area in drug discovery (Huveneers et al., 2007; Huxley-Jones et al., 2008). Eptifibatide, a synthetic cyclic peptide with a Lys-Gly-ASP (KGD) sequence that antagonizes αvβ3 integrin, is an example of the small-molecule integrin antagonists. Eptifibatide acts at the final common step of the platelet aggregation pathway and has already been in use for years for patients with acute coronary syndrome and/or undergoing percutaneous coronary intervention (Curran and Keating, 2005). A number of other small-molecule integrin antagonists, both peptide and nonpeptide peptidomimetics, inhibiting different integrins have been developed for use in treating various human diseases such as cancer, rheumatoid arthritis, osteoporosis, asthma, and ulcerative colitis (Huveneers et al., 2007; Woodside and Vanderslice, 2008). Targeting nonintegrin ECM receptors may also have therapeutic value. For example, blocking antibodies to CD44, which is the main receptor for hyaluronan but interacts with a number of other ECM components, induces resistance to developing type 1 diabetes in a mouse model (Weiss et al., 2000). Administration of hyaluronan oligosaccharides, which interfere with CD44 polyvalent binding to hyaluronan, are effective inhibitors of several tumor types in vivo, such as mammary and lung carcinomas and melanoma (Zeng et al., 1998; Toole et al., 2008). Similar to other future drugs discussed above, it remains to be seen whether and when these compounds are ready for clinical use. Currently, a major drawback with the above potential future therapies may be the lack of their oral use. In addition, there are huge difficulties in developing therapies that are not only site-specific but also have few side effects.
V. Conclusions
Our aim has been to convince the reader that many, and perhaps most, human diseases have some connection to the remodelling of the ECM and tissue pathology. However, currently available drugs are neither specific nor potent enough to target ECM molecules and prevent tissue destruction due to diseases. The approach that we have reviewed is both complex and complicated. Success has been achieved at targeting the receptors for many of the ECM components. Targeting the cytokines, enzymes, and signaling molecules involved in the synthesis, accumulation, and degradation of the ECM is another approach that is showing encouraging results. Less attention has been given to targeting specific individual ECM components as a means of interfering with specific diseases. However, a growing number of examples show that specific ECM components have a dramatic effect on several disease phenotypes, so this approach may be a fruitful area for drug development in the future. What is certain is that the ECM has been a neglected target for drug pharmacotherapy in the past but promises to be an important target to consider in the future.
Acknowledgments
This work was funded by Finnish Foundation for Cardiovascular Research, Medical Research Fund (EVO) of Turku University Hospital, Paavo Nurmi Foundation, Turku University Foundation, Finnish Cultural Foundation and Foundation for Diabetes Research (all to H.J.), by National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL18645 and R24-HL64387-06A1 (to T.N.W.); and by a pilot grant from the Washington Research Foundation. We thank all the people and sources of financial support that have made this article possible.
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.109.001289.
Footnotes
Abbreviations: 5HT, 5-hydroxytryptamine; AA-4500, clostridial collagenase; ACE, angiotensin-converting enzyme; ADAMTS, disintegrin and metalloproteinase with thrombospondin motifs; CTGF, connective tissue growth factor; ECM, extracellular matrix; FG-3019, a human CTGF-specific monoclonal antibody; MMP, matrix metalloprotease; PI-88, phosphomannopentose sulfate; SB431542, 4-(5-benzo(1,3)dioxol-5-yl-4-pyridin-2-yl-1H-imidazol-2-yl)benzamide; SD-208, 2-(5-chloro-2-fluorophenyl)-4-[(4-pyridyl)amino]p-teridine; SM305, an activin-like receptor kinase inhibitor 5 (ALK5); SPARC, secreted protein, acidic and rich in cysteine; TFO, triplex-forming oligonucleotide; TGF, transforming growth factor; TIMP, tissue inhibitors of MMP; TNF, tumor necrosis factor.
References
- Abdul-Hussien H, Hanemaaijer R, Verheijen JH, van Bockel JH, Geelkerken RH, and Lindeman JHN (2009) Doxycycline therapy for abdominal aneurysm: Improved proteolytic balance through reduced neutrophil content. J Vasc Surg 49 741-749. [DOI] [PubMed] [Google Scholar]
- Acharya M, Mookherjee S, Bhattacharjee A, Thakur SK, Bandyopadhyay AK, Sen A, Chakrabarti S, and Ray K (2007) Evaluation of the OPTC gene in primary open angle glaucoma: functional significance of a silent change. BMC Mol Biol 8 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC and Lawler J (2004) The thrombospondins. Int J Biochem Cell Biol 36 961-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad NN, Ala-Kokko L, Knowlton RG, Jimenez SA, Weaver EJ, Maguire JI, Tasman W, and Prockop DJ (1991) Stop codon in the procollagen II gene (COL2A1) in a family with the Stickler syndrome (arthro-ophthalmopathy). Proc Natl Acad Sci U S A 88 6624-6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja TS (2003) Doxycycline decreases proteinuria in glomerulonephritis. Am J Kidney Dis 42 376-380. [DOI] [PubMed] [Google Scholar]
- Aikawa T, Gunn J, Spong SM, Klaus SJ, and Korc M (2006) Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Mol Cancer Ther 5 1108-1116. [DOI] [PubMed] [Google Scholar]
- Akiyama SK, Yamada KM, and Hayashi M (1981) The structure of fibronectin and its role in cellular adhesion. J Supramol Struct Cell Biochem 16 345-348. [DOI] [PubMed] [Google Scholar]
- Alberto P, Maddalena G, Giuseppe G, Ilaria Q, Franci B, Campagna MS, Eugenio N, Antonio B, Carlo S, and Ranuccio N (2008) Prevalence of risk factors, coronary and systemic atherosclerosis in abdominal aortic aneurysm: comparison with high cardiovascular risk population. Vasc Health Risk Manag 4 877-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albig AR and Schiemann WP (2005) Fibulin-5 function during tumorigenesis. Future Oncol 1 23-35. [DOI] [PubMed] [Google Scholar]
- Alexakis C, Maxwell P, and Bou-Gharios G (2006) Organ-specific collagen expression: implications for renal disease. Nephron Exp Nephrol 102 e71-e75. [DOI] [PubMed] [Google Scholar]
- Amenta PS, Hadad S, Lee MT, Barnard N, Li D, and Myers JC (2003) Loss of types XV and XIX collagen precedes basement membrane invasion in ductal carcinoma of the female breast. J Pathol 199 298-308. [DOI] [PubMed] [Google Scholar]
- Amenta PS, Scivoletti NA, Newman MD, Sciancalepore JP, Li D, and Myers JC (2005) Proteoglycan-collagen XV in human tissues is seen linking banded collagen fibers subjacent to the basement membrane. J Histochem Cytochem 53 165-176. [DOI] [PubMed] [Google Scholar]
- Ang LC, Zhang Y, Cao L, Yang BL, Young B, Kiani C, Lee V, Allan K, and Yang BB (1999) Versican enhances locomotion of astrocytoma cells and reduces cell adhesion through its G1 domain. J Neuropathol Exp Neurol 58 597-605. [DOI] [PubMed] [Google Scholar]
- Antonini A and Poewe W (2007) Fibrotic heart-valve reactions to dopamine-agonist treatment in Parkinson's disease. Lancet Neurol 6 826-829. [DOI] [PubMed] [Google Scholar]
- Aoki H, Yoshimura K, and Matsuzaki M (2007) Turning back the clock: regression of abdominal aortic aneurysms via pharmacotherapy. J Mol Med 85 1077-1088. [DOI] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Le AH, Nishino I, Nonaka I, Ho NC, Francomano CA, Govindraj P, Hassell JR, Devaney JM, Spranger J, et al. (2002) Structural and functional mutations of the perlecan gene cause Schwartz-Jampel syndrome, with myotonic myopathy and chondrodysplasia. Am J Hum Genet 70 1368-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Wilcox WR, Le AH, Silverman N, Govindraj P, Hassell JR, and Yamada Y (2001) Dyssegmental dysplasia, Silverman-Handmaker type, is caused by functional null mutations of the perlecan gene. Nat Genet 27 431-434. [DOI] [PubMed] [Google Scholar]
- Armstrong SJ, Wiberg M, Terenghi G, and Kingham PJ (2007) ECM molecules mediate both Schwann cell proliferation and activation to enhance neurite outgrowth. Tissue Eng 13 2863-2870. [DOI] [PubMed] [Google Scholar]
- Astrof S and Hynes RO (2009) Fibronectins in vascular morphogenesis. Angiogenesis doi: 10.1007/s10456-009-9136-6. [DOI] [PMC free article] [PubMed]
- Aurich M, Anders J, Trommer T, Liesaus E, Seifert M, Schömburg J, Rolauffs B, Wagner A, and Mollenhauer J (2006) Histological and cell biological characterization of dissected cartilage fragments in human osteochondritis dissecans of the femoral condyle. Arch Orthop Trauma Surg 126 606-614. [DOI] [PubMed] [Google Scholar]
- Auvinen P, Tammi R, Parkkinen J, Tammi M, Agren U, Johansson R, Hirvikoski P, Eskelinen M, and Kosma VM (2000) Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol 156 529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydoðan S, Türkçüoðlu P, Celiker U, and Ilhan N (2007) Effect of thalidomide on endostatin levels in retinal ischemia/reperfusion injury. Ophthalmologica 221 418-420. [DOI] [PubMed] [Google Scholar]
- Bahadori R, Biehlmaier O, Zeitz C, Labhart T, Makhankov YV, Forster U, Gesemann M, Berger W, and Neuhauss SC (2006) Nyctalopin is essential for synaptic transmission in the cone dominated zebrafish retina. Eur J Neurosci 24 1664-1674. [DOI] [PubMed] [Google Scholar]
- Baker DE (2007) Natalizumab: overview of its pharmacology and safety. Rev Gastroenterol Disord 7 38-46. [PubMed] [Google Scholar]
- Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, and Tryggvason K (1990) Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248 1224-1227. [DOI] [PubMed] [Google Scholar]
- Baselga J (2006) Targeting tyrosine kinases in cancer: the second wave. Science 312 1175-1178. [DOI] [PubMed] [Google Scholar]
- Bassleer C, Rovati L, and Franchimont P (1998) Stimulation of proteoglycan production by glucosamine sulfate in chondrocytes isolated from human osteoarthritic articular cartilage in vitro. Osteoarthritis Cartilage 6 427-434. [DOI] [PubMed] [Google Scholar]
- Bauer JW and Lanschuetzer C (2003) Type XVII collagen gene mutations in junctional epidermolysis bullosa and prospects for gene therapy. Clin Exp Dermatol 28 53-60. [DOI] [PubMed] [Google Scholar]
- Beals RK and Hecht F (1971) Congenital contractural arachnodactyly. A heritable disorder of connective tissue. J Bone Joint Surg Am 53 987-993. [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Sparkes RL, Koop B, Birch DG, Bergen AA, Prinsen CF, Polomeno RC, Gal A, et al. (2000) Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet 26 319-323. [DOI] [PubMed] [Google Scholar]
- Behm BW and Bickston SJ (2009) Humanized antibody to the alpha4beta7 integrin for induction of remission in ulcerative colitis. Cochrane Database Syst Rev doi: 10.1002/14651858.CD007571. [DOI] [PubMed]
- Belinsky GS, Parke AL, Huang Q, Blanchard K, Jayadev S, Stoll R, Rothe M, Achenie LE, Gupta RR, Wu GY, et al. (2007) The contribution of methotrexate exposure and host factors on transcriptional variance in human liver. Toxicol Sci 97 582-594. [DOI] [PubMed] [Google Scholar]
- Bengtsson E, Mörgelin M, Sasaki T, Timpl R, Heinegård D, and Aspberg A (2002) The leucine-rich repeat protein PRELP binds perlecan and collagens and may function as a basement membrane anchor. J Biol Chem 277 15061-15068. [DOI] [PubMed] [Google Scholar]
- Berk BC, Fujiwara K, and Lehoux S (2007) ECM remodeling in hypertensive heart disease. J Clin Invest 117 568-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, and Butcher EC (1993) Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74 185-195. [DOI] [PubMed] [Google Scholar]
- Berris KK, Repp AL, and Kleerekoper M (2007) Glucocorticoid-induced osteoporosis. Curr Opin Endocrinol Diabetes Obes 14 446-450. [DOI] [PubMed] [Google Scholar]
- Berzin TM, Zipser BD, Rafii MS, Kuo-Leblanc V, Yancopoulos GD, Glass DJ, Fallon JR, and Stopa EG (2000) Agrin and microvascular damage in Alzheimer's disease. Neurobiol Aging 21 349-355. [DOI] [PubMed] [Google Scholar]
- Bezakova G and Ruegg MA (2003) New insights into the roles of agrin. Nat Rev Mol Cell Biol 4 295-308. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Ishida W, Wu M, Wilkes M, Mori Y, Hinchcliff M, Leof E, and Varga J (2009) A non-Smad mechanism of fibroblast activation by transforming growth factor-beta via c-Abl and Egr-1: selective modulation by imatinib mesylate. Oncogene 28 1285-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk DE (2001) Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron 32 223-237. [DOI] [PubMed] [Google Scholar]
- Biswas S, Munier FL, Yardley J, Hart-Holden N, Perveen R, Cousin P, Sutphin JE, Noble B, Batterbury M, Kielty C, et al. (2001) Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Genet 10 2415-2423. [DOI] [PubMed] [Google Scholar]
- Bix G and Iozzo RV (2008) Novel interactions of perlecan: unraveling perlecan's role in angiogenesis. Microsc Res Tech 71 339-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black SA Jr and Trackman PC (2008) Transforming growth factor-beta1 (TGFbeta1) stimulates connective tissue growth factor (CCN2/CTGF) expression in human gingival fibroblasts through a RhoA-independent, Rac1/Cdc42-dependent mechanism: statins with forskolin block TGFbeta1-induced CCN2/CTGF expression. J Biol Chem 283 10835-10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacci JV, Schuliga M, Harris T, and Stewart AG (2006) Collagen impairs glucocorticoid actions in airway smooth muscle through integrin signalling. Br J Pharmacol 149 365-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadio J and Byers PH (1985) Subtle structural alterations in the chains of type I procollagen produce osteogenesis imperfecta type II. Nature 316: 363-366. [DOI] [PubMed] [Google Scholar]
- Bonnaure-Mallet M, Tricot-Doleux S, and Godeau GJ (1995) Changes in extracellular matrix macromolecules in human gingiva after treatment with drugs inducing gingival overgrowth. Arch Oral Biol 40 393-400. [DOI] [PubMed] [Google Scholar]
- Border WA and Noble NA (1994) Transforming growth factor beta in tissue fibrosis. N Engl J Med 331 1286-1292. [DOI] [PubMed] [Google Scholar]
- Bornstein P and Sage EH (2002) Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 14 608-616. [DOI] [PubMed] [Google Scholar]
- Bourne T, Fossati G, and Nesbitt A (2008) A PEGylated Fab' fragment against tumor necrosis factor for the treatment of Crohn disease: exploring a new mechanism of action. BioDrugs 22 331-337. [DOI] [PubMed] [Google Scholar]
- Bredrup C, Knappskog PM, Majewski J, Rødahl E, and Boman H (2005) Congenital stromal dystrophy of the cornea caused by a mutation in the decorin gene. Invest Ophthalmol Vis Sci 46 420-426. [DOI] [PubMed] [Google Scholar]
- Breedveld G, de Coo IF, Lequin MH, Arts WF, Heutink P, Gould DB, John SW, Oostra B, and Mancini GM (2006) Novel mutations in three families confirm a major role of COL4A1 in hereditary porencephaly. J Med Genet 43 490-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briot K, Roux C, Gossec L, Charni N, Kolta S, Dougados M, and Garnero P (2008) Effects of etanercept on serum biochemical markers of cartilage metabolism in patients with spondyloarthropathy. J Rheumatol 35 310-314. [PubMed] [Google Scholar]
- Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, and Dietz HC 3rd (2008) Angiotensin II blockade and aortic-root dilation in Marfan's syndrome. N Engl J Med 358 2787-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Baddeley H, Read AE, Davies JD, and McGarry J (1974) Sclerosing peritonitis, an unusual reaction to a beta-adrenergic-blocking drug (practolol). Lancet 2 1477-1481. [DOI] [PubMed] [Google Scholar]
- Brown RS, Sein P, Corio R, and Bottomley WK (1990) Nitrendipine-induced gingival hyperplasia. First case report. Oral Surg Oral Med Oral Pathol 70 593-596. [DOI] [PubMed] [Google Scholar]
- Bruckner P and van der Rest M (1994) Structure and function of cartilage collagens. Microsc Res Tech 28 378-384. [DOI] [PubMed] [Google Scholar]
- Brunet L, Miranda J, Farré M, Berini L, and Mendieta C (1996) Gingival enlargement induced by drugs. Drug Saf 15 219-231. [DOI] [PubMed] [Google Scholar]
- Brunner HG, van Beersum SE, Warman ML, Olsen BR, Ropers HH, and Mariman EC (1994) A Stickler syndrome gene is linked to chromosome 6 near the COL11A2 gene. Hum Molec Genet 3 1561-1564. [DOI] [PubMed] [Google Scholar]
- Burch GH, Gong Y, Liu W, Dettman RW, Curry CJ, Smith L, Miller WL, and Bristow J (1997) Tenascin-X deficiency is associated with Ehlers-Danlos syndrome. Nat Genet 17 104-108. [DOI] [PubMed] [Google Scholar]
- Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, et al. (2007) IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest 117 3498-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bönnemann CG, Cox GF, Shapiro F, Wu JJ, Feener CA, Thompson TG, Anthony DC, Eyre DR, Darras BT, and Kunkel LM (2000) A mutation in the alpha 3 chain of type IX collagen causes autosomal dominant multiple epiphyseal dysplasia with mild myopathy. Proc Natl Acad Sci U S A 97 1212-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campistol JM, Iñigo P, Larios S, Bescos M, and Oppenheimer F (2001) Role of transforming growth factor-β1 in the progression of chronic allograft nephropathy. Nephrol Dial Transplant 16 114-116. [DOI] [PubMed] [Google Scholar]
- Canalis E (2005) Mechanisms of glucocorticoid action in bone. Curr Osteoporos Rep 3 98-102. [DOI] [PubMed] [Google Scholar]
- Canalis E, Mazziotti G, Giustina A, and Bilezikian JP (2007) Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 18 1319-1328. [DOI] [PubMed] [Google Scholar]
- Castagnola P, Tavella S, Gerecke DR, Dublet B, Gordon MK, Seyer J, Cancedda R, van der Rest M, and Olsen BR (1992) Tissue-specific expression of type XIV collagen–a member of the FACIT class of collagens. Eur J Cell Biol 59 340-347. [PubMed] [Google Scholar]
- Castelletti F, Donadelli R, Banterla F, Hildebrandt F, Zipfel PF, Bresin E, Otto E, Skerka C, Renieri A, Todeschini M, et al. (2008) Mutations in FN1 cause glomerulopathy with fibronectin deposits. Proc Natl Acad Sci U S A 105 2538-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo GM, Ngo C, Cummings J, Wight TN, and Snow AD (1997) Perlecan binds to the beta-amyloid proteins (A beta) of Alzheimer's disease, accelerates A beta fibril formation, and maintains A beta fibril stability. J Neurochem 69 2452-2465. [DOI] [PubMed] [Google Scholar]
- Chapman HA, Riese RJ, and Shi GP (1997) Emerging roles for cysteine proteases in human biology. Annu Rev Physiol 59 63-88. [DOI] [PubMed] [Google Scholar]
- Chen WY and Abatangelo G (1999) Functions of hyaluronan in wound repair. Wound Repair Regen 7 79-89. [DOI] [PubMed] [Google Scholar]
- Chen M, Doostan A, Bandyopadhyay P, Remington J, Wang X, Hou Y, Liu Z, and Woodley DT (2007) The cartilage matrix protein subdomain of type VII collagen is pathogenic for epidermolysis bullosa acquisita. Am J Pathol 170 2009-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YY, Jin H, Liu X, Siu JM, Wong YP, Ng EK, Yu J, Leung WK, Sung JJ, and Chan FK (2008) Fibulin 1 is downregulated through promoter hypermethylation in gastric cancer. Br J Cancer 99 2083-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EL, Maruyama I, SundarRaj N, Sugar J, Feder RS, and Yue BY (2001) Expression of type XII collagen and hemidesmosome-associated proteins in keratoconus corneas. Curr Eye Res 22 333-340. [DOI] [PubMed] [Google Scholar]
- Chlenski A, Liu S, Guerrero LJ, Yang Q, Tian Y, Salwen HR, Zage P, and Cohn SL (2006) SPARC expression is associated with impaired tumor growth, inhibited angiogenesis and changes in the extracellular matrix. Int J Cancer 118 310-316. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R and Chiquet M (2003) Tenascins: regulation and putative functions during pathological stress. J Pathol 200 488-499. [DOI] [PubMed] [Google Scholar]
- Chiu HC, Lu YT, Chin YT, Tu HP, Chiang CY, Gau CH, Nieh S, and Fu E (2009) Cyclosporine A inhibits the expression of membrane type-I matrix metalloproteinase in gingiva. J Periodontal Res 44 338-347. [DOI] [PubMed] [Google Scholar]
- Chong LW, Hsu YC, Chiu YT, Yang KC, and Huang YT (2006) Anti-fibrotic effects of thalidomide on hepatic stellate cells and dimethylnitrosamine-intoxicated rats. J Biomed Sci 13 403-418. [DOI] [PubMed] [Google Scholar]
- Clark CJ and Sage EH (2008) A prototypic matricellular protein in the tumor microenvironment–where there's SPARC, there's fire. J Cell Biochem 104 721-732. [DOI] [PubMed] [Google Scholar]
- Clark IM, Swingler TE, Sampieri CL, and Edwards DR (2008) The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol 40 1362-1378. [DOI] [PubMed] [Google Scholar]
- Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM, Bradley JD, Bingham CO 3rd, Weisman MH, Jackson CG, et al. (2006) Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 354 795-808. [DOI] [PubMed] [Google Scholar]
- Cohen MP, Shearman CW, and Lautenslager GT (2001) Serum type IV collagen in diabetic patients at risk for nephropathy. Diabetes Care 24 1324-1327. [DOI] [PubMed] [Google Scholar]
- Colognato H and Yurchenco PD (2000) Form and function: the laminin family of heterotrimers. Dev Dyn 218 213-234. [DOI] [PubMed] [Google Scholar]
- Conforti A, Chiamulera C, Moretti U, Colcera S, Fumagalli G, and Leone R (2007) Musculoskeletal adverse drug reactions: a review of literature and data from ADR spontaneous reporting databases. Curr Drug Saf 2 47-63. [DOI] [PubMed] [Google Scholar]
- Cordeiro MF (2003) Technology evaluation: lerdelimumab, Cambridge Antibody Technology. Curr Opin Mol Ther 5 199-203. [PubMed] [Google Scholar]
- Corps AN, Harrall RL, Curry VA, Fenwick SA, Hazleman BL, and Riley GP (2002) Ciprofloxacin enhances the stimulation of matrix metalloproteinase 3 expression by interleukin-1beta in human tendon-derived cells. A potential mechanism of fluoroquinolone-induced tendinopathy. Arthritis Rheum 46 3034-3040. [DOI] [PubMed] [Google Scholar]
- Corps AN, Harrall RL, Curry VA, Hazleman BL, and Riley GP (2005) Contrasting effects of fluoroquinolone antibiotics on the expression of the collagenases, matrix metalloproteinases (MMP)-1 and -13, in human tendon-derived cells. Rheumatology 44 1514-1517. [DOI] [PubMed] [Google Scholar]
- Couchman JR, Austria MR, and Woods A (1990) Fibronectin-cell interactions. J Invest Dermatol 94 (6 Suppl): 7S-14S. [DOI] [PubMed] [Google Scholar]
- Couchman JR, Kapoor R, Sthanam M, and Wu RR (1996) Perlecan and basement membrane-chondroitin sulfate proteoglycan (bamacan) are two basement membrane chondroitin/dermatan sulfate proteoglycans in the Engelbreth-Holm-Swarm tumor matrix. J Biol Chem 271 9595-9602. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, and Matrisian LM (2002) Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295 2387-2392. [DOI] [PubMed] [Google Scholar]
- Cruz CI, Ruiz-Torres P, del Moral RG, Rodríguez-Puyol M, and Rodríguez-Puyol D (2000) Age-related progressive renal fibrosis in rats and its prevention with ACE inhibitors and taurine. Am J Physiol Renal Physiol 278 F122-F129. [DOI] [PubMed] [Google Scholar]
- Curran MP and Keating GM (2005) Eptifibatide: a review of its use in patients with acute coronary syndromes and/or undergoing percutaneous coronary intervention. Drugs 65 2009-2035. [DOI] [PubMed] [Google Scholar]
- Cutroneo KR, DiPetrillo TA, and Cutroneo KR Jr (1990) Variation of corticosteroid-induced inhibition of collagen synthesis at equivalent anti-inflammatory doses. J Am Acad Dermatol 22 1007-1010. [DOI] [PubMed] [Google Scholar]
- Cutroneo KR and Sterling KM Jr (2004) How do glucocorticoids compare to oligo decoys as inhibitors of collagen synthesis and potential toxicity of these therapeutics? J Cell Biochem 92 6-15. [DOI] [PubMed] [Google Scholar]
- Cutroneo KR, White SL, Phan SH, and Ehrlich HP (2007) Therapies for bleomycin induced lung fibrosis through regulation of TGF-beta1 induced collagen gene expression. J Cell Physiol 211 585-589. [DOI] [PubMed] [Google Scholar]
- Daley WP, Peters SB, and Larsen M (2008) Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci 121 255-264. [DOI] [PubMed] [Google Scholar]
- de La Motte CA, Hascall VC, Calabro A, Yen-Lieberman B, and Strong SA (1999) Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(I·C). J Biol Chem 274 30747-30755. [DOI] [PubMed] [Google Scholar]
- de la Motte CA, Hascall VC, Drazba J, Bandyopadhyay SK, and Strong SA (2003) Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-alphatrypsin inhibitor is crucial to structure and function. Am J Pathol 163 121-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vega S, Iwamoto T, Nakamura T, Hozumi K, McKnight DA, Fisher LW, Fukumoto S, and Yamada Y (2007) TM14 is a new member of the fibulin family (fibulin-7) that interacts with extracellular matrix molecules and is active for cell binding. J Biol Chem 282 30878-30888. [DOI] [PubMed] [Google Scholar]
- Dean DC, Newby RF, and Bourgeois S (1988) Regulation of fibronectin biosynthesis by dexamethasone, transforming growth factor beta, and cAMP in human cell lines. J Cell Biol 106 2159-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeer P, Schoenmakers EF, Twal WO, Argraves WS, De Smet L, Fryns JP, and Van De Ven WJ (2002) The fibulin-1 gene (FBLN1) is disrupted in a t(12;22) associated with a complex type of synpolydactyly. J Med Genet 39 98-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delany AM, Jeffrey JJ, Rydziel S, and Canalis E (1995) Cortisol increases interstitial collagenase expression in osteoblasts by post-transcriptional mechanisms. J Biol Chem 270 26607-26612. [DOI] [PubMed] [Google Scholar]
- Delany AM, McMahon DJ, Powell JS, Greenberg DA, and Kurland ES (2008) Osteonectin/SPARC polymorphisms in Caucasian men with idiopathic osteoporosis. Osteoporos Int 19 969-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbaldo C, Raymond E, Vera K, Hammershaimb L, Kaucic K, Lozahic S, Marty M, and Faivre S (2008) Phase I and pharmacokinetic study of etaracizumab (Abegrin), a humanized monoclonal antibody against alphavbeta3 integrin receptor, in patients with advanced solid tumors. Invest New Drugs 26 35-43. [DOI] [PubMed] [Google Scholar]
- Del Monaco M, Covello SP, Kennedy SH, Gilinger G, Litwack G, and Uitto J (1997) Identification of novel glucocorticoid-response elements in human elastin promoter and demonstration of nucleotide sequence specificity of the receptor binding. J Invest Dermatol 108 938-942. [DOI] [PubMed] [Google Scholar]
- Deng A, Harvey V, Sina B, Strobel D, Badros A, Junkins-Hopkins JM, Samuels A, Oghilikhan M, and Gaspari A (2006) Interstitial granulomatous dermatitis associated with the use of tumor necrosis factor alpha inhibitors. Arch Dermatol 142 198-202. [DOI] [PubMed] [Google Scholar]
- Denton CP, Merkel PA, Furst DE, Khanna D, Emery P, Hsu VM, Silliman N, Streisand J, Powell J, Akesson A, et al. (2007) Recombinant human antitransforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum 56 323-333. [DOI] [PubMed] [Google Scholar]
- Desmarais S, Black WC, Oballa R, Lamontagne S, Riendeau D, Tawa P, Duong le T, Pickarski M, and Percival MD (2008) Effect of cathepsin k inhibitor basicity on in vivo off-target activities. Mol Pharmacol 73 147-156. [DOI] [PubMed] [Google Scholar]
- Díez J, González A, López B, and Querejeta R (2005) Mechanisms of disease: pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat Clin Pract Cardiovasc Med 2 209-216. [DOI] [PubMed] [Google Scholar]
- Dietz HC, Ramirez F, and Sakai LY (1994) Marfan's syndrome and other microfibrillar diseases. Adv Hum Genet 22 153-186. [DOI] [PubMed] [Google Scholar]
- DiBattista JA, Martel-Pelletier J, Wosu LO, Sandor T, Antakly T, and Pelletier JP (1991) Glucocorticoid receptor mediated inhibition of interleukin-1 stimulated neutral metalloprotease synthesis in normal human chondrocytes. J Clin Endocrinol Metab 72 316-326. [DOI] [PubMed] [Google Scholar]
- Di Sabatino A, Saarialho-Kere U, Buckley MG, Gordon JN, Biancheri P, Rovedatti L, Corazza GR, Macdonald TT, and Pender SL (2009) Stromelysin-1 and macrophage metalloelastase expression in the intestinal mucosa of Crohn's disease patients treated with infliximab. Eur J Gastroenterol Hepatol doi: 10.1097/MEG.0b013e3283293d0f. [DOI] [PubMed]
- Douglas D, Hsu JH, Hung L, Cooper A, Abdueva D, van Doorninck J, Peng G, Shimada H, Triche TJ, and Lawlor ER (2008) BMI-1 promotes ewing sarcoma tumorigenicity independent of CDKN2A repression. Cancer Res 68 6507-6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudhia J (2005) Aggrecan, aging and assembly in articular cartilage. Cell Mol Life Sci 62 2241-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez DA (2006) Role of the renin-angiotensin-aldosterone system in vascular remodeling and inflammation: a clinical review. J Hypertens 24 983-991. [DOI] [PubMed] [Google Scholar]
- Durani P, Occleston N, O'Kane S, and Ferguson MW (2008) Avotermin: a novel antiscarring agent. Int J Low Extrem Wounds 7 160-168. [DOI] [PubMed] [Google Scholar]
- Edgell CJ, BaSalamah MA, and Marr HS (2004) Testican-1: a differentially expressed proteoglycan with protease inhibiting activities. Int Rev Cytol 236 101-122. [DOI] [PubMed] [Google Scholar]
- Ehnis T, Dieterich W, Bauer M, Kresse H, and Schuppan D (1997) Localization of a binding site for the proteoglycan decorin on collagen XIV (undulin). J Biol Chem 272 20414-20419. [DOI] [PubMed] [Google Scholar]
- Eickelberg O, Pansky A, Koehler E, Bihl M, Tamm M, Hildebrand P, Perruchoud AP, Kashgarian M, and Roth M (2001) Molecular mechanisms of TGF-(beta) antagonism by interferon (gamma) and cyclosporine A in lung fibroblasts. FASEB J 15 797-806. [DOI] [PubMed] [Google Scholar]
- Ekblom M, Fässler R, Tomasini-Johansson B, Nilsson K, and Ekblom P (1993) Downregulation of tenascin expression by glucocorticoids in bone marrow stromal cells and in fibroblasts. J Cell Biol 123 1037-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E, Hessle H, and Klier G (1986) Molecular assembly, secretion, and matrix deposition of type VI collagen. J Cell Biol 102 703-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst H, Grunert S, Schneider HT, Beck WS, Brune K, and Hahn EG (1995) Distribution of extracellular matrix proteins in indomethacin-induced lesions in the rat stomach. Scand J Gastroenterol 30: 847-853. [DOI] [PubMed] [Google Scholar]
- Ewart AK, Morris CA, Atkinson D, Jin W, Sternes K, Spallone P, Stock AD, Leppert M, and Keating MT (1993) Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nat Genet 5 11-16. [DOI] [PubMed] [Google Scholar]
- Eyre DR (2004) Collagens and cartilage matrix homeostasis. Clin Orthop Relat Res 427 S118-S822. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Upton MP, Shapiro FD, Wilkinson RH, and Vawter GF (1986) Nonexpression of cartilage type II collagen in a case of Langer-Saldino achondrogenesis. Am J Hum Genet 39 52-67. [PMC free article] [PubMed] [Google Scholar]
- Faivre L, Gorlin RJ, Wirtz MK, Godfrey M, Dagoneau N, Samples JR, Le Merrer M, Collod-Beroud G, Boileau C, Munnich A, et al. (2003) In frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani syndrome. J Med Genet 40 34-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, Cohen A, Bitton A, Baker J, Dubé R, et al. (2008) Treatment of active Crohn's disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol 6 1370-1377. [DOI] [PubMed] [Google Scholar]
- Fedak PW, Verma S, Weisel RD, and Li RK (2005) Cardiac remodeling and failure. From molecules to man (part II). Cardiovasc Pathol 14 49-60. [DOI] [PubMed] [Google Scholar]
- Ferguson MW, Duncan J, Bond J, Bush J, Durani P, So K, Taylor L, Chantrey J, Mason T, James G, et al. (2009) Prophylactic administration of avotermin for improvement of skin scarring: three double-blind, placebo-controlled, phase I/II studies. Lancet 373 1264-1274. [DOI] [PubMed] [Google Scholar]
- Fiedorczyk M, Klimiuk PA, Sierakowski S, Gindzienska-Sieskiewicz E, and Chwiecko J (2006) Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with early rheumatoid arthritis. J Rheumatol 33 1523-1529. [PubMed] [Google Scholar]
- Fischer JW, Stoll M, Hahn AW, and Unger T (2001) Differential regulation of thrombospondin-1 and fibronectin by angiotensin II receptor subtypes in cultured endothelial cells. Cardiovasc Res 51 784-791. [DOI] [PubMed] [Google Scholar]
- Folkman J (2007) Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 6 273-286. [DOI] [PubMed] [Google Scholar]
- Fornoni A, Cornacchia F, Howard GA, Roos BA, Striker GE, and Striker LJ (2001) Cyclosporin A affects extracellular matrix synthesis and degradation by mouse MC3T3–E1 osteoblasts in vitro. Nephrol Dial Transplant 16 500-505. [DOI] [PubMed] [Google Scholar]
- Fosang AJ and Little CB (2008) Drug insight: aggrecanases as therapeutic targets for osteoarthritis. Nat Clin Pract Rheumatol 4 420-427. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Koshihara Y, Oda H, Ohyama M, and Ooyama T (1988) Type V collagen selectively inhibits human endothelial cell proliferation. Biochem Biophys Res Commun 151 1060-1068. [DOI] [PubMed] [Google Scholar]
- Fukushige T, Kanekura T, Ohuchi E, Shinya T, and Kanzaki T (2005) Immunohistochemical studies comparing the localization of type XV collagen in normal human skin and skin tumors with that of type IV collagen. J Dermatol 32 74-83. [PubMed] [Google Scholar]
- Fukushima R, Kanamori S, Hirashiba M, Hishikawa A, Muranaka RI, Kaneto M, Nakamura K, and Kato I (2007) Teratogenicity study of the dihydroorotate-dehydrogenase inhibitor and protein tyrosine kinase inhibitor Leflunomide in mice. Reprod Toxicol 24 310-316. [DOI] [PubMed] [Google Scholar]
- Gallagher WM, Greene LM, Ryan MP, Sierra V, Berger A, Laurent-Puig P, and Conseiller E (2001) Human fibulin-4: analysis of its biosynthetic processing and mRNA expression in normal and tumour tissues. FEBS Lett 489 59-66. [DOI] [PubMed] [Google Scholar]
- Gao DF, Niu XL, Hao GH, Peng N, Wei J, Ning N, and Wang NP (2007) Rosiglitazone inhibits angiotensin II-induced CTGF expression in vascular smooth muscle cells–role of PPAR-gamma in vascular fibrosis. Biochem Pharmacol 73 185-197. [DOI] [PubMed] [Google Scholar]
- Gardin JM, Schumacher D, Constantine G, Davis KD, Leung C, and Reid CL (2000) Valvular abnormalities and cardiovascular status following exposure to dexfenfluramine or phentermine/fenfluramine. JAMA 283 1703-1709. [DOI] [PubMed] [Google Scholar]
- Gasparini G, Longo R, Sarmiento R, and Morabito A (2003) Inhibitors of cyclooxygenase 2: a new class of anticancer agents? Lancet Oncol 4 605-615. [DOI] [PubMed] [Google Scholar]
- Gauthier JY, Chauret N, Cromlish W, Desmarais S, Duong le T, Falgueyret JP, Kimmel DB, Lamontagne S, Léger S, LeRiche T, et al. (2008) The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg Med Chem Lett 18 923-928. [DOI] [PubMed] [Google Scholar]
- Gavras HP and Salerno CM (1996) The angiotensin II type 1 receptor blocker losartan in clinical practice: a review. Clin Ther 18 1058-1067. [DOI] [PubMed] [Google Scholar]
- Gelse K, Pöschl E, and Aigner T (2003) Collagens–structure, function, and biosynthesis. Adv Drug Deliv Rev 55 1531-1546. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Iannone F, Marinosci F, Lapadula G, and Antonaci S (2005) The effect of bosentan on matrix metalloproteinase-9 levels in patients with systemic sclerosis-induced pulmonary hypertension. Curr Med Res Opin 21 327-332. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Iannone F, Marinosci F, Lapadula G, and Antonaci S (2006) Clinical outcomes of bosentan in pulmonary arterial hypertension do not correlate with levels of TIMPs. Eur J Clin Invest 36 (Suppl 3): 73-77. [DOI] [PubMed] [Google Scholar]
- Giltay R, Timpl R, and Kostka G (1999) Sequence, recombinant expression and tissue localization of two novel extracellular matrix proteins, fibulin-3 and fibulin-4. Matrix Biol 18 469-480. [DOI] [PubMed] [Google Scholar]
- Giordano A, Romano S, Mallardo M, D'Angelillo A, Calì G, Corcione N, Ferraro P, and Romano MF (2008) FK506 can activate transforming growth factor-beta signalling in vascular smooth muscle cells and promote proliferation. Cardiovasc Res 79 519-526. [DOI] [PubMed] [Google Scholar]
- Girish KS and Kemparaju K (2007) The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci 80 1921-1943. [DOI] [PubMed] [Google Scholar]
- Gleghorn L, Ramesar R, Beighton P, and Wallis G (2005) A mutation in the variable repeat region of the aggrecan gene (AGC1) causes a form of spondyloepiphyseal dysplasia associated with severe, premature osteoarthritis. Am J Hum Genet 77 484-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorieux FH (2008) Osteogenesis imperfecta. Best Pract Res Clin Rheumatol 22 85-100. [DOI] [PubMed] [Google Scholar]
- Godyna S, Diaz-Ricart M, and Argraves WS (1996) Fibulin-1 mediates platelet adhesion via a bridge of fibrinogen. Blood 88 2569-2577. [PubMed] [Google Scholar]
- Goldoni S, Seidler DG, Heath J, Fassan M, Baffa R, Thakur ML, Owens RT, McQuillan DJ, and Iozzo RV (2008) An antimetastatic role for decorin in breast cancer. Am J Pathol 173 844-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, and Sorsa T (1998) Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res 12 12-26. [DOI] [PubMed] [Google Scholar]
- Gordon KJ and Blobe GC (2008) Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta 1782 197-228. [DOI] [PubMed] [Google Scholar]
- Gouëffic Y, Potter-Perigo S, Chan CK, Johnson PY, Braun K, Evanko SP, and Wight TN (2007) Sirolimus blocks the accumulation of hyaluronan (HA) by arterial smooth muscle cells and reduces monocyte adhesion to the ECM. Atherosclerosis 195 23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham HK, Horn M, and Trafford AW (2008) Extracellular matrix profiles in the progression of heart failure. European Young Physiologists Symposium Keynote Lecture-Bratislava 2007. Acta Physiol (Oxf) 194 3-21. [DOI] [PubMed] [Google Scholar]
- Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, and Iozzo RV (2002) Decorin suppresses tumor cell-mediated angiogenesis. Oncogene 21 4765-4777. [DOI] [PubMed] [Google Scholar]
- Granville CA, Memmott RM, Gills JJ, and Dennis PA (2006) Handicapping the race to develop inhibitors of the phosphoinositide 3-kinase/Akt/mammalian target of rapamycin pathway. Clin Cancer Res 12 679-689. [DOI] [PubMed] [Google Scholar]
- Grey A (2008) Skeletal consequences of thiazolidinedione therapy. Osteoporos Int 19 129-137. [DOI] [PubMed] [Google Scholar]
- Grimpe B, Probst JC, and Hager G (1999) Suppression of nidogen-1 translation by antisense targeting affects the adhesive properties of cultured astrocytes. Glia 28 138-149. [DOI] [PubMed] [Google Scholar]
- Grobner T (2006) Gadolinium–a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 21 1104-1108. [DOI] [PubMed] [Google Scholar]
- Grumet M, Friedlander DR, and Sakurai T (1996) Functions of brain chondroitin sulfate proteoglycans during developments: interactions with adhesion molecules. Perspect Dev Neurobiol 3 319-330. [PubMed] [Google Scholar]
- Grütter C, Wilkinson T, Turner R, Podichetty S, Finch D, McCourt M, Loning S, Jermutus L, and Grütter MG (2008) A cytokine-neutralizing antibody as a structural mimetic of 2 receptor interactions. Proc Natl Acad Sci U S A 105 20251-20256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiak JJ, Ho JC, Moossa AR, and Bouvet M (2007) The integrin-extracellular matrix axis in pancreatic cancer. Pancreas 35 293-301. [DOI] [PubMed] [Google Scholar]
- Hall TE, Bryson-Richardson RJ, Berger S, Jacoby AS, Cole NJ, Hollway GE, Berger J, and Currie PD (2007) The zebrafish candyfloss mutant implicates extracellular matrix adhesion failure in laminin alpha2-deficient congenital muscular dystrophy. Proc Natl Acad Sci U S A 104 7092-7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Hatai M, and Yaoi Y (1991) Inhibition of cell adhesion by type V collagen. Cell Struct Funct 16 391-397. [DOI] [PubMed] [Google Scholar]
- Haydont V, Bourgier C, Pocard M, Lusinchi A, Aigueperse J, Mathé D, Bourhis J, and Vozenin-Brotons MC (2007) Pravastatin inhibits the Rho/CCN2/extracellular matrix cascade in human fibrosis explants and improves radiation-induced intestinal fibrosis in rats. Clin Cancer Res 13 5331-5340. [DOI] [PubMed] [Google Scholar]
- Hedbom E and Heinegård D (1989) Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J Biol Chem 264 6898-6905. [PubMed] [Google Scholar]
- Heldin P, Karousou E, Bernert B, Porsch H, Nishitsuka K, and Skandalis SS (2008) Importance of hyaluronan-CD44 interactions in inflammation and tumorigenesis. Connect Tissue Res 49 215-218. [DOI] [PubMed] [Google Scholar]
- Hildebrand A, Romarís M, Rasmussen LM, Heinegård D, Twardzik DR, Border WA, and Ruoslahti E (1994) Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 302 527-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen EL, Anttila M, Kultti A, Ropponen K, Penttinen J, Yliskoski M, Kuronen AT, Juhola M, Tammi R, Tammi M, et al. (2002) Elevated hyaluronan concentration without hyaluronidase activation in malignant epithelial ovarian tumors. Cancer Res 62 6410-6413. [PubMed] [Google Scholar]
- Hirte H, Vergote IB, Jeffrey JR, Grimshaw RN, Coppieters S, Schwartz B, Tu D, Sadura A, Brundage M, and Seymour L (2006) A phase III randomized trial of BAY 12–9566 (tanomastat) as maintenance therapy in patients with advanced ovarian cancer responsive to primary surgery and paclitaxel/platinum containing chemotherapy: a National Cancer Institute of Canada Clinical Trials Group Study. Gynecol Oncol 102 300-308. [DOI] [PubMed] [Google Scholar]
- Ho MS, Böse K, Mokkapati S, Nischt R, and Smyth N (2008) Nidogens–extracellular matrix linker molecules. Microsc Res Tech 71 387-395. [DOI] [PubMed] [Google Scholar]
- Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, Nourse J, and Crabtree GR (1996) The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol 80 S40-S45. [DOI] [PubMed] [Google Scholar]
- Hovnanian A, Duquesnoy P, Blanchet-Bardon C, Knowlton RG, Amselem S, Lathrop M, Dubertret L, Uitto J, and Goossens M (1992) Genetic linkage of recessive dystrophic epidermolysis bullosa to the type VII collagen gene. J Clin Invest 90 1032-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia HC and Schwarzbauer JE (2005) Meet the tenascins: multifunctional and mysterious. J Biol Chem 280 26641-26644. [DOI] [PubMed] [Google Scholar]
- Hu B, Kong LL, Matthews RT, and Viapiano MS (2008) The proteoglycan brevican binds to fibronectin after proteolytic cleavage and promotes glioma cell motility. J Biol Chem 283 24848-24859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Van den Steen PE, Sang QX, and Opdenakker G (2007) Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov 6 480-498. [DOI] [PubMed] [Google Scholar]
- Huber TB, Kottgen M, Schilling B, Walz G, and Benzing T (2001) Interaction with podocin facilitates nephrin signaling. J Biol Chem 276 41543-41546. [DOI] [PubMed] [Google Scholar]
- Hucthagowder V, Sausgruber N, Kim KH, Angle B, Marmorstein LY, and Urban Z (2006) Fibulin-4: a novel gene for an autosomal recessive cutis laxa syndrome. Am J Hum Genet 78 1075-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers S, Truong H, and Danen HJ (2007) Integrins: signaling, disease, and therapy. Int J Radiat Biol 83 743-751. [DOI] [PubMed] [Google Scholar]
- Huxley-Jones J, Foord SM, and Barnes MR (2008) Drug discovery in the extracellular matrix. Drug Discov Today 13 685-694. [DOI] [PubMed] [Google Scholar]
- Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110 673-687. [DOI] [PubMed] [Google Scholar]
- Hynes RO (2007) Cell-matrix adhesion in vascular development. J Thromb Haemost 5 32-40. [DOI] [PubMed] [Google Scholar]
- Högemann B, Edel G, Schwarz K, Krech R, and Kresse H (1997) Expression of biglycan, decorin and proteoglycan-100/CSF-1 in normal and fibrotic human liver. Pathol Res Pract 193 747-751. [DOI] [PubMed] [Google Scholar]
- Ikegawa S (2008) Expression, regulation and function of asporin, a susceptibility gene in common bone and joint diseases. Curr Med Chem 15 724-728. [DOI] [PubMed] [Google Scholar]
- Imada K, Lin N, Liu C, Lu A, Chen W, Yano M, Sato T, and Ito A (2008) Nobiletin, a citrus polymethoxy flavonoid, suppresses gene expression and production of aggrecanases-1 and -2 in collagen-induced arthritic mice. Biochem Biophys Res Commun 373 181-185. [DOI] [PubMed] [Google Scholar]
- Ingber DE (2008) Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol 18 356-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE and Folkman J (1989) How does extracellular matrix control capillary morphogenesis? Cell 58 803-805. [DOI] [PubMed] [Google Scholar]
- Iozzo RV (1994) Perlecan: a gem of a proteoglycan. Matrix Biol 14 203-208. [DOI] [PubMed] [Google Scholar]
- Isaji M, Nakajoh M, and Naito J (1987) Selective inhibition of collagen accumulation by N-(3,4-dimethoxycinnamoyl)anthranilic acid (N-5′) in granulation tissue. Biochem Pharmacol 36 469-474. [DOI] [PubMed] [Google Scholar]
- Ishida W, Mori Y, Lakos G, Sun L, Shan F, Bowes S, Josiah S, Lee WC, Singh J, Ling LE, et al. (2006) Intracellular TGF-beta receptor blockade abrogates Smad-dependent fibroblast activation in vitro and in vivo. J Invest Dermatol 126 1733-1744. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Nishigaki F, Miyata S, Hirayama Y, Minoura K, Imanishi J, Neya M, Mizutani T, Imamura Y, Naritomi Y, et al. (2005) Prevention of progressive joint destruction in collagen-induced arthritis in rats by a novel matrix metalloproteinase inhibitor, FR255031. Br J Pharmacol 144 133-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, Rosenfield K, Razvi S, Walsh K, and Symes JF (1996) Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet 348 370-374. [DOI] [PubMed] [Google Scholar]
- Jackson SM, Ericsson J, and Edwards PA (1997) Signaling molecules derived from the cholesterol biosynthetic pathway. Subcell Biochem 28 1-21. [DOI] [PubMed] [Google Scholar]
- Jian B, Xu J, Connolly J, Savani RC, Narula N, Liang B, and Levy RJ (2002) Serotonin mechanisms in heart valve disease I: serotonin-induced up-regulation of transforming growth factor-beta1 via G-protein signal transduction in aortic valve interstitial cells. Am J Pathol 161 2111-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John DC, Watson R, Kind AJ, Scott AR, Kadler KE, and Bulleid NJ (1999) Expression of an engineered form of recombinant procollagen in mouse milk. Nat Biotechnol 17 385-389. [DOI] [PubMed] [Google Scholar]
- Johnston CI and Jackson B (1983) Pharmacology of agents acting on the renin-angiotensin system. Anaesth Intensive Care 11 377-383. [DOI] [PubMed] [Google Scholar]
- Jonat C, Rahmsdorf HJ, Park KK, Cato AC, Gebel S, Ponta H, and Herrlich P (1990) Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell 62 1189-1204. [DOI] [PubMed] [Google Scholar]
- Joner M, Morimoto K, Kasukawa H, Steigerwald K, Merl S, Nakazawa G, John MC, Finn AV, Acampado E, Kolodgie FD, et al. (2008) Site-specific targeting of nanoparticle prednisolone reduces in-stent restenosis in a rabbit model of established atheroma. Arterioscler Thromb Vasc Biol 28 1960-1966. [DOI] [PubMed] [Google Scholar]
- Järveläinen HT, Iruela-Arispe ML, Kinsella MG, Sandell LJ, Sage EH, and Wight TN (1992) Expression of decorin by sprouting bovine aortic endothelial cells exhibiting angiogenesis in vitro. Exp Cell Res 203 395-401. [DOI] [PubMed] [Google Scholar]
- Järveläinen H, Puolakkainen P, Pakkanen S, Brown EL, Höök M, Iozzo RV, Sage EH, and Wight TN (2006) A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen 14 443-452. [DOI] [PubMed] [Google Scholar]
- Järveläinen H and Wight TN (2002) Vascular proteoglycans, in Proteoglycans in lung disease (Garg HG, Roughley PJ, and Hales CA eds) pp 291-321, Marcel Dekker Inc., New York, Basel.
- Kadler KE, Hill A, and Canty-Laird EG (2008) Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol 20 495-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R (2003) Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 3 422-433. [DOI] [PubMed] [Google Scholar]
- Kalyanasundaram A, Elmore JR, Manazer JR, Golden A, Franklin DP, Galt SW, Zakhary EM, and Carey DJ (2006) Simvastatin suppresses experimental aortic aneurysm expansion. J Vasc Surg 43 117-124. [DOI] [PubMed] [Google Scholar]
- Kamioka M, Imamura J, Komatsu N, Daibata M, and Sugiura T (2009) Testican 3 expression in adult T-cell leukemia. Leuk Res 33 913-918. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Morrissey J, McCracken R, Reyes A, and Klahr S (1994) Enalapril reduces collagen type IV synthesis and expansion of the interstitium in the obstructed rat kidney. Kidney Int 45 1637-1647. [DOI] [PubMed] [Google Scholar]
- Kao WW and Liu CY (2002) Roles of lumican and keratocan on corneal transparency. Glycoconj J 19 275-285. [DOI] [PubMed] [Google Scholar]
- Karppinen J, Pääkkö E, Paassilta P, Lohiniva J, Kurunlahti M, Tervonen O, Nieminen P, Göring HH, Malmivaara A, Vanharanta H, et al. (2003) Radiologic phenotypes in lumbar MR imaging for a gene defect in the COL9A3 gene of type IX collagen. Radiology 227 143-148. [DOI] [PubMed] [Google Scholar]
- Katsuda S, Okada Y, Minamoto T, Oda Y, Matsui Y, and Nakanishi I (1992) Collagens in human atherosclerosis. Immunohistochemical analysis using collagen type-specific antibodies. Arterioscler Thromb 12 494-502. [DOI] [PubMed] [Google Scholar]
- Kazerounian S, Yee KO, and Lawler J (2008) Thrombospondins in cancer. Cell Mol Life Sci 65 700-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane MG and Pyeritz RE (2008) Medical management of Marfan syndrome. Circulation 117 2802-2813. [DOI] [PubMed] [Google Scholar]
- Keene DR, Sakai LY, Lunstrum GP, Morris NP, and Burgeson RE (1987) Type VII collagen forms an extended network of anchoring fibrils. J Cell Biol 104 611-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenagy RD, Plaas AH, and Wight TN (2006) Versican degradation and vascular disease. Trends Cardiovasc Med 16 209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani C, Chen L, Wu YJ, Yee AJ, and Yang BB (2002) Structure and function of aggrecan. Cell Res 12 19-32. [DOI] [PubMed] [Google Scholar]
- Kim HS and Suh YH (2009) Minocycline and neurodegenerative diseases. Behav Brain Res 196 168-179. [DOI] [PubMed] [Google Scholar]
- Kim MP, Zhou M, and Wahl LM (2005) Angiotensin II increases human monocyte matrix metalloproteinase-1 through the AT2 receptor and prostaglandin E2: implications for atherosclerotic plaque rupture. J Leukoc Biol 78 195-201. [DOI] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, and Karin M (2009) Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457: 102-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivirikko KI and Risteli L (1976) Biosynthesis of collagen and its alterations in pathological states. Med Biol 54 159-186. [PubMed] [Google Scholar]
- Kizawa H, Kou I, Iida A, Sudo A, Miyamoto Y, Fukuda A, Mabuchi A, Kotani A, Kawakami A, Yamamoto S, et al. (2005) An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat Genet 37 138-144. [DOI] [PubMed] [Google Scholar]
- Klenotic PA, Munier FL, Marmorstein LY, and Anand-Apte B (2004) Tissue inhibitor of metalloproteinases-3 (TIMP-3) is a binding partner of epithelial growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1). Implications for macular degenerations. J Biol Chem 279 30469-30473. [DOI] [PubMed] [Google Scholar]
- Knudson CB and Knudson W (2001) Cartilage proteoglycans. Semin Cell Dev Biol 12 69-78. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, and Poole AR (2005) Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum 52 128-135. [DOI] [PubMed] [Google Scholar]
- Koon N, Schneider-Stock R, Sarlomo-Rikala M, Lasota J, Smolkin M, Petroni G, Zaika A, Boltze C, Meyer F, Andersson L, et al. (2004) Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut 53 235-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korc M (2007) Pancreatic cancer-associated stroma production. Am J Surg 194 S84-S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal RC, Richardson JA, Miano JM, and Olson EN (1999) EVEC, a novel epidermal growth factor-like repeat-containing protein upregulated in embryonic and diseased adult vasculature. Circ Res 84 1166-1176. [DOI] [PubMed] [Google Scholar]
- Kruegel J, Sadowski B, and Miosge N (2008) Nidogen-1 and nidogen-2 in healthy human cartilage and in late-stage osteoarthritis cartilage. Arthritis Rheum 58 1422-1432. [DOI] [PubMed] [Google Scholar]
- Kuhn C and McDonald JA (1991) The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol 138 1257-1265. [PMC free article] [PubMed] [Google Scholar]
- Kyriakides TR and Bornstein P (2003) Matricellular proteins as modulators of wound healing and the foreign body response. Thromb Haemost 90 986-992. [DOI] [PubMed] [Google Scholar]
- Kuru L, Yilmaz S, Kuru B, Köse KN, and Noyan U (2004) Expression of growth factors in the gingival crevice fluid of patients with phenytoin-induced gingival enlargement. Arch Oral Biol 49 945-950. [DOI] [PubMed] [Google Scholar]
- Kähäri VM, Häkkinen L, Westermarck J, and Larjava H (1995) Differential regulation of decorin and biglycan gene expression by dexamethasone and retinoic acid in cultured human skin fibroblasts. J Invest Dermatol 104 503-508. [DOI] [PubMed] [Google Scholar]
- Lafzi A, Farahani RM, and Shoja MA (2006) Amlodipine-induced gingival hyperplasia. Med Oral Patol Oral Cir Bucal 11 E480-E482. [PubMed] [Google Scholar]
- Larsen M, Artym VV, Green JA, and Yamada KM (2006) The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol 18 463-471. [DOI] [PubMed] [Google Scholar]
- Lawler J and Detmar M (2004) Tumor progression: the effects of thrombospondin-1 and -2. Int J Biochem Cell Biol 36 1038-1045. [DOI] [PubMed] [Google Scholar]
- Lazo JS and Hoyt DG (1990) The molecular basis of interstitial pulmonary fibrosis caused by antineoplastic agents. Cancer Treat Rev 17 165-167. [DOI] [PubMed] [Google Scholar]
- LeBleu VS, Macdonald B, and Kalluri R (2007) Structure and function of basement membranes. Exp Biol Med (Maywood) 232 1121-1129. [DOI] [PubMed] [Google Scholar]
- Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, and Jilka RL (2002) Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology 143 2376-2384. [DOI] [PubMed] [Google Scholar]
- Lindsay K, Fraser AD, Layton A, Goodfield M, Gruss H, and Gough A (2009) Liver fibrosis in patients with psoriasis and psoriatic arthritis on long-term, high cumulative dose methotrexate therapy. Rheumatology 48 569-572. [DOI] [PubMed] [Google Scholar]
- Linehan KA, Seymour AM, and Williams PE (2001) Semiquantitative analysis of collagen types in the hypertrophied left ventricle. J Anat 198 83-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Peng Y, Liu F, Li J, Chen X, Liu Y, and Zhang H (2007) A selective cyclooxygenase-2 inhibitor decreases transforming growth factor-beta1 synthesis and matrix production in human peritoneal mesothelial cells. Cell Biol Int 31 508-515. [DOI] [PubMed] [Google Scholar]
- Liu Y (2006) Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 69 213-217. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Zardi L, Ninomiya Y, Sado Y, Huang ZS, Nesburn AB, and Kenney MC (1996) Basement membrane abnormalities in human eyes with diabetic retinopathy. J Histochem Cytochem 44 1469-1479. [DOI] [PubMed] [Google Scholar]
- Loeys B, Van Maldergem L, Mortier G, Coucke P, Gerniers S, Naeyaert JM, and De Paepe A (2002) Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum Mol Genet 11 2113-2118. [DOI] [PubMed] [Google Scholar]
- López B, González A, Varo N, Laviades C, Querejeta R, and Díez J (2001) Biochemical assessment of myocardial fibrosis in hypertensive heart disease. Hypertension 38 1222-1226. [DOI] [PubMed] [Google Scholar]
- Lorenzo P, Aspberg A, Onnerfjord P, Bayliss MT, Neame PJ, and Heinegard D (2001) Identification and characterization of asporin. a novel member of the leucine-rich repeat protein family closely related to decorin and biglycan. J Biol Chem 276 12201-12211. [DOI] [PubMed] [Google Scholar]
- Louneva N, Huaman G, Fertala J, and Jiménez SA (2006) Inhibition of systemic sclerosis dermal fibroblast type I collagen production and gene expression by simvastatin. Arthritis Rheum 54 1298-1308. [DOI] [PubMed] [Google Scholar]
- Lukashev ME and Werb Z (1998) ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol 8 437-441. [DOI] [PubMed] [Google Scholar]
- Lydon NB and Druker BJ (2004) Lessons learned from the development of imatinib. Leuk Res 28 S29-38. [DOI] [PubMed] [Google Scholar]
- Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, and Fagan SC (2006) Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci 7 56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majava M, Bishop PN, Hägg P, Scott PG, Rice A, Inglehearn C, Hammond CJ, Spector TD, Ala-Kokko L, and Männikkö M (2007a) Novel mutations in the small leucine-rich repeat protein/proteoglycan (SLRP) genes in high myopia. Hum Mutat 28 336-344. [DOI] [PubMed] [Google Scholar]
- Majava M, Hoornaert KP, Bartholdi D, Bouma MC, Bouman K, Carrera M, Devriendt K, Hurst J, Kitsos G, Niedrist D, et al. (2007b) A report on 10 new patients with heterozygous mutations in the COL11A1 gene and a review of genotype-phenotype correlations in type XI collagenopathies. Am J Med Genet A 143 258-264. [DOI] [PubMed] [Google Scholar]
- Mao JR and Bristow J (2001) The Ehlers-Danlos syndrome: on beyond collagens. J Clin Invest 107 1063-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraldi NM, Sabatelli P, Columbaro M, Zamparelli A, Manzoli FA, Bernardi P, Bonaldo P, and Merlini L (2009) Collagen VI myopathies: from the animal model to the clinical trial. Adv Enzyme Regul doi: 10.1016/j.advenzreg.2008.12.009. [DOI] [PubMed]
- Marastoni S, Ligresti G, Lorenzon E, Colombatti A, and Mongiat M (2008) Extracellular matrix: a matter of life and death. Connect Tissue Res 49 203-206. [DOI] [PubMed] [Google Scholar]
- Marie I, Delafenêtre H, Massy N, Thuillez C, and Noblet C (2008) Tendinous disorders attributed to statins: a study on ninety-six spontaneous reports in the period 1990–2005 and review of the literature. Arthritis Rheum 59 367-372. [DOI] [PubMed] [Google Scholar]
- Marijianowski MM, van der Loos CM, Mohrschladt MF, and Becker AE (1994) The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. J Am Coll Cardiol 23 1204-1208. [DOI] [PubMed] [Google Scholar]
- Marshall JK (1999) Ilodecakin. Schering-Plough Corp. IDrugs 2 1045-1058. [PubMed] [Google Scholar]
- Martens E, Leyssen A, Van Aelst I, Fiten P, Piccard H, Hu J, Descamps FJ, Van den Steen PE, Proost P, Van Damme J, et al. (2007) A monoclonal antibody inhibits gelatinase B/MMP-9 by selective binding to part of the catalytic domain and not to the fibronectin or zinc binding domains. Biochim Biophys Acta 1770 178-186. [DOI] [PubMed] [Google Scholar]
- Martin J, Denver R, Bailey M, and Krum H (2005) In vitro inhibitory effects of atorvastatin on cardiac fibroblasts: implications for ventricular remodelling. Clin Exp Pharmacol Physiol 32 697-701. [DOI] [PubMed] [Google Scholar]
- Martyn CN and Greenwald SE (1997) Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet 350 953-955. [DOI] [PubMed] [Google Scholar]
- Mason RP, Marche P, and Hintze TH (2003) Novel vascular biology of third-generation L-type calcium channel antagonists: ancillary actions of amlodipine. Arterioscler Thromb Vasc Biol 23 2155-2163. [DOI] [PubMed] [Google Scholar]
- Masutomo K, Makino N, and Fushiki MS (2001) Effects of losartan on the collagen degradative enzymes in hypertrophic and congestive types of cardiomyopathic hamsters. Mol Cell Biochem 224 19-27. [DOI] [PubMed] [Google Scholar]
- Mathiak M, Yenisey C, Grant DS, Sharma B, and Iozzo RV (1997) A role for perlecan in the suppression of growth and invasion in fibrosarcoma cells. Cancer Res 57 2130-2136. [PubMed] [Google Scholar]
- Maumenee IH (1979) Vitreoretinal degeneration as a sign of generalized connective tissue diseases. Am J Ophthalmol 88 432-449. [DOI] [PubMed] [Google Scholar]
- Mayne R (1989) Cartilage collagens. What is their function, and are they involved in articular disease? Arthritis Rheum 32 241-246. [DOI] [PubMed] [Google Scholar]
- McDonough AK, Curtis JR, and Saag KG (2008) The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol 20 131-137. [DOI] [PubMed] [Google Scholar]
- McKenzie EA (2007) Heparanase: a target for drug discovery in cancer and inflammation. Br J Pharmacol 151 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkoniemi M, Brunner HG, Manouvrier S, Hennekam R, Superti-Furga A, Kääriäinen H, Pauli RM, van Essen T, Warman ML, Bonaventure J, et al. (2000) Autosomal recessive disorder otospondylomegaepiphyseal dysplasia is associated with loss-of-function mutations in the COL11A2 gene. Am J Hum Genet 66 368-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose J, Fuller ES, Roughley PJ, Smith MM, Kerr B, Hughes CE, Caterson B, and Little CB (2008) Fragmentation of decorin, biglycan, lumican and keratocan is elevated in degenerate human meniscus, knee and hip articular cartilages compared with age-matched macroscopically normal and control tissues. Arthritis Res Ther 10 R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlini L, Angelin A, Tiepolo T, Braghetta P, Sabatelli P, Zamparelli A, Ferlini A, Maraldi NM, Bonaldo P, and Bernardi P (2008a) Cyclosporin A corrects mitochondrial dysfunction and muscle apoptosis in patients with collagen VI myopathies. Proc Natl Acad Sci U S A 105 5225-5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlini L, Martoni E, Grumati P, Sabatelli P, Squarzoni S, Urciuolo A, Ferlini A, Gualandi F, and Bonaldo P (2008b) Autosomal recessive myosclerosis myopathy is a collagen VI disorder. Neurology 71 1245-1253. [DOI] [PubMed] [Google Scholar]
- Merrilees MJ, Ching PS, Beaumont B, Hinek A, Wight TN, and Black PN (2008) Changes in elastin, elastin binding protein and versican in alveoli in chronic obstructive pulmonary disease. Respir Res 9 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelacci YM (2003) Collagens and proteoglycans of the corneal extracellular matrix. Braz J Med Biol Res 36 1037-1046. [DOI] [PubMed] [Google Scholar]
- Migita K, Miyashita T, Maeda Y, Aoyagi T, Kawabe Y, Nakamura M, Yatsuhashi H, Ishibashi H, and Eguchi K (2005) FK506 suppresses the stimulation of matrix metalloproteinase 13 synthesis by interleukin-1beta in rheumatoid synovial fibroblasts. Immunol Lett 98 194-199. [DOI] [PubMed] [Google Scholar]
- Milewicz DM, Urbán Z, and Boyd C (2000) Genetic disorders of the elastic fiber system. Matrix Biol 19 471-480. [DOI] [PubMed] [Google Scholar]
- Miner JH (2008) Laminins and their roles in mammals. Microsc Res Tech 71 349-356. [DOI] [PubMed] [Google Scholar]
- Mio F, Chiba K, Hirose Y, Kawaguchi Y, Mikami Y, Oya T, Mori M, Kamata M, Matsumoto M, Ozaki K, et al. (2007) A functional polymorphism in COL11A1, which encodes the alpha 1 chain of type XI collagen, is associated with susceptibility to lumbar disc herniation. Am J Hum Genet 81 1271-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfort J, Tardif G, Roughley P, Reboul P, Boileau C, Bishop PN, Pelletier JP, and Martel-Pelletier J (2008) Identification of opticin, a member of the small leucine-rich repeat proteoglycan family, in human articular tissues: a novel target for MMP-13 in osteoarthritis. Osteoarthritis Cartilage 16 749-755. [DOI] [PubMed] [Google Scholar]
- Mongiat M, Mungiguerra G, Bot S, Mucignat MT, Giacomello E, Doliana R, and Colombatti A (2000) Self-assembly and supramolecular organization of EMILIN. J Biol Chem 275 25471-25480. [DOI] [PubMed] [Google Scholar]
- Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, and Kaplan G (1993) Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med 177 1675-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland LW (1999) Inhibitors of tumor necrosis factor: new treatment options for rheumatoid arthritis. Cleve Clin J Med 66 367-374. [DOI] [PubMed] [Google Scholar]
- Mori Y, Ishida W, Bhattacharyya S, Li Y, Platanias LC, and Varga J (2004) Selective inhibition of activin receptor-like kinase 5 signaling blocks profibrotic transforming growth factor beta responses in skin fibroblasts. Arthritis Rheum 50 4008-4021. [DOI] [PubMed] [Google Scholar]
- Morita H, Hasegawa T, Minamoto T, Oda Y, Inui K, Tayama H, Nakao N, Nakamoto Y, Ideura T, and Yoshimura A (2003) Collagenofibrotic glomerulopathy with a widespread expression of type-V collagen. Virchows Arch 442 163-168. [DOI] [PubMed] [Google Scholar]
- Moroguchi A, Ishimura K, Okano K, Wakabayashi H, Maeba T, and Maeta H (2004) Interleukin-10 suppresses proliferation and remodeling of extracellular matrix of cultured human skin fibroblasts. Eur Surg Res 36 39-44. [DOI] [PubMed] [Google Scholar]
- Motte S, McEntee K, and Naeije R (2006) Endothelin receptor antagonists. Pharmacol Ther 110 386-414. [DOI] [PubMed] [Google Scholar]
- Moulik PK, Al-Jafari MS, and Khaleeli AA (2004) Steroid responsiveness in a case of Riedel's thyroiditis and retroperitoneal fibrosis. Int J Clin Pract 58 312-315. [DOI] [PubMed] [Google Scholar]
- Mroz PJ and Silbert JE (2004) Use of 3H-glucosamine and 35S-sulfate with cultured human chondrocytes to determine the effect of glucosamine concentration on formation of chondroitin sulfate. Arthritis Rheum 50 3574-3579. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A, Nikopoulos K, Maugeri A, de Brouwer AP, van Nouhuys CE, Boon CJ, Perveen R, Zegers HA, Wittebol-Post D, van den Biesen PR, et al. (2006) Erosive vitreoretinopathy and wagner disease are caused by intronic mutations in CSPG2/Versican that result in an imbalance of splice variants. Invest Ophthalmol Vis Sci 47 3565-3572. [DOI] [PubMed] [Google Scholar]
- Musso O, Rehn M, Saarela J, Théret N, Liétard J, Hintikka, Lotrian D, Campion JP, Pihlajaniemi T, and Clément B (1998) Collagen XVIII is localized in sinusoids and basement membrane zones and expressed by hepatocytes and activated stellate cells in fibrotic human liver. Hepatology 28 98-107. [DOI] [PubMed] [Google Scholar]
- Myers JC, Dion AS, Abraham V, and Amenta PS (1996) Type XV collagen exhibits a widespread distribution in human tissues but a distinct localization in basement membrane zones. Cell Tissue Res 286 493-505. [DOI] [PubMed] [Google Scholar]
- Myers JC, Li D, Bageris A, Abraham V, Dion AS, and Amenta PS (1997) Biochemical and immunohistochemical characterization of human type XIX defines a novel class of basement membrane zone collagens. Am J Pathol 151 1729-1740. [PMC free article] [PubMed] [Google Scholar]
- Myers JC, Li D, Rubinstein NA, and Clark CC (1999) Up-regulation of type XIX collagen in rhabdomyosarcoma cells accompanies myogenic differentiation. Exp Cell Res 253 587-598. [DOI] [PubMed] [Google Scholar]
- Myllyharju J (2008) Prolyl 4-hydroxylases, key enzymes in the synthesis of collagens and regulation of the response to hypoxia, and their roles as treatment targets. Ann Med 40 402-417. [DOI] [PubMed] [Google Scholar]
- Myllyharju J and Kivirikko KI (2001) Collagens and collagen-related diseases. Ann Med 33 7-21. [DOI] [PubMed] [Google Scholar]
- Myllyharju J and Kivirikko KI (2004) Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet 20 33-43. [DOI] [PubMed] [Google Scholar]
- Naito Z (2005) Role of the small leucine-rich proteoglycan (SLRP) family in pathological lesions and cancer cell growth. J Nippon Med Sch 72 137-145. [DOI] [PubMed] [Google Scholar]
- Nakada M, Miyamori H, Yamashita J, and Sato H (2003) Testican 2 abrogates inhibition of membrane-type matrix metalloproteinases by other testican family proteins. Cancer Res 63 3364-3369. [PubMed] [Google Scholar]
- Nakashima Y, Wight TN, and Sueishi K (2008) Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc Res 79 14-23. [DOI] [PubMed] [Google Scholar]
- Namazi MR and Soma J (2005) Tranilast: a novel weapon against insulin resistance. Med Hypotheses 64 1135-1137. [DOI] [PubMed] [Google Scholar]
- Nara Y, Kato Y, Torii Y, Tsuji Y, Nakagaki S, Goto S, Isobe H, Nakashima N, and Takeuchi J (1997) Immunohistochemical localization of extracellular matrix components in human breast tumours with special reference to PG-M/versican. Histochem J 29 21-30. [DOI] [PubMed] [Google Scholar]
- Nelson F, Dahlberg L, Laverty S, Reiner A, Pidoux I, Ionescu M, Fraser GL, Brooks E, Tanzer M, Rosenberg LC, et al. (1998) Evidence for altered synthesis of type II collagen in patients with osteoarthritis. J Clin Invest 102 2115-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J, McFerran NV, Pivato G, Chambers E, Doherty C, Steele D, and Timson DJ (2008) The 67 kDa laminin receptor: structure, function and role in disease. Biosci Rep 28 33-48. [DOI] [PubMed] [Google Scholar]
- Nerlich AG (1995) Collagen types in the middle ear mucosa. Eur Arch Otorhinolaryngol 252 443-449. [DOI] [PubMed] [Google Scholar]
- Ng TM (2004) Levosimendan, a new calcium-sensitizing inotrope for heart failure. Pharmacotherapy 24 1366-1884. [DOI] [PubMed] [Google Scholar]
- Nghiem P, Pearson G, and Langley RG (2002) Tacrolimus and pimecrolimus: from clever prokaryotes to inhibiting calcineurin and treating atopic dermatitis. JAm Acad Dermatol 46 228-241. [DOI] [PubMed] [Google Scholar]
- Nguyen TQ and Goldschmeding R (2008) Bone morphogenetic protein-7 and connective tissue growth factor: novel targets for treatment of renal fibrosis? Pharm Res 25 2416-2426. [DOI] [PubMed] [Google Scholar]
- Nicholls AC, Oliver JE, McCarron S, Harrison JB, Greenspan DS, and Pope FM (1996) An exon skipping mutation of a type V collagen gene (COL5A1) in Ehlers-Danlos syndrome. J Med Genet 33 940-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuutinen P, Autio P, Hurskainen T, and Oikarinen A (2001) Glucocorticoid action on skin collagen: overview on clinical significance and consequences. J Eur Acad Dermatol Venereol 15 361-362. [PubMed] [Google Scholar]
- Nuytinck L, Freund M, Lagae L, Pierard GE, Hermanns-Le T, and De Paepe A (2000) Classical Ehlers-Danlos syndrome caused by a mutation in type I collagen. Am J Hum Genet 66 1398-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occleston NL, O'Kane S, Goldspink N, and Ferguson MWJ (2008) New therapeutics for the prevention and reduction of scarring. Drug Discov Today 13 973-981. [DOI] [PubMed] [Google Scholar]
- Ohta K, Lupo G, Kuriyama S, Keynes R, Holt CE, Harris WA, Tanaka H, and Ohnuma S (2004) Tsukushi functions as an organizer inducer by inhibition of BMP activity in cooperation with chordin. Dev Cell 7 347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikarinen A and Hannuksela M (1980) Effect of hydrocortisone-17-butyrate, hydrocortisone, and clobetasol-17-propionate on prolyl hydroxylase activity in human skin. Arch Dermatol Res 267 79-82. [DOI] [PubMed] [Google Scholar]
- Oikarinen A, Salo T, Ala-Kokko L, and Tryggvason K (1987) Dexamethasone modulates the metabolism of type IV collagen and fibronectin in human basement-membrane-forming fibrosarcoma (HT-1080) cells. Biochem J 245 235-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y, Fu ZW, Ohnuki Y, Kato H, and Noguchi T (2002) Molecular basis of the alteration in skin collagen metabolism in response to in vivo dexamethasone treatment: effects on the synthesis of collagen type I and III, collagenase, and tissue inhibitors of metalloproteinases. Br J Dermatol 147 859-868. [DOI] [PubMed] [Google Scholar]
- Oivula J, Lohi J, Tani T, Kangas L, Kiviluoto T, Kivilaakso E, Butkowski R, and Virtanen I (1999) Renal cell carcinomas and pancreatic adenocarcinomas produce nidogen in vitro and in vivo. J Pathol 187 455-461. [DOI] [PubMed] [Google Scholar]
- Okada M, Kikuzuki R, Harada T, Hori Y, Yamawaki H, and Hara Y (2008) Captopril attenuates matrix metalloproteinase-2 and -9 in monocrotaline-induced right ventricular hypertrophy in rats. J Pharmacol Sci 108 487-494. [DOI] [PubMed] [Google Scholar]
- Okada M, Takeuchi J, Kimura H, Yoshijima K, Itoh K, and Furuhashi T (1994) Histochemistry and immunohistochemistry of extracellular matrix of rat fetal Ductus arteriosus during the indomethacin-induced constriction. Jikken Dobutsu 43 551-558. [DOI] [PubMed] [Google Scholar]
- Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, and Strauss JF 3rd (2001) The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 276 10229-10233. [DOI] [PubMed] [Google Scholar]
- Olsen BR (1997) Collagen IX. Int J Biochem Cell Biol 29 555-558. [DOI] [PubMed] [Google Scholar]
- Olsen BR (2008) Treatment of mitochondrial dysfunction in patients with collagen VI mutations. Matrix Biol 27 273. [DOI] [PubMed] [Google Scholar]
- Onda M, Ishiwata T, Kawahara K, Wang R, Naito Z, and Sugisaki Y (2002) Expression of lumican in thickened intima and smooth muscle cells in human coronary atherosclerosis. Exp Mol Pathol 72 142-149. [DOI] [PubMed] [Google Scholar]
- Ono Y, Nakanishi Y, Ino Y, Niki T, Yamada T, Yoshimura K, Saikawa M, Nakajima T, and Hirohashi S (1999) Clinocopathologic significance of laminin-5 gamma2 chain expression in squamous cell carcinoma of the tongue: immunohistochemical analysis of 67 lesions. Cancer 85 2315-2321. [PubMed] [Google Scholar]
- O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, and Folkman J (1997) Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88 277-285. [DOI] [PubMed] [Google Scholar]
- Overall CM and Kleifeld O (2006) Tumour microenvirnment–opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer 6 227-239. [DOI] [PubMed] [Google Scholar]
- Pan TC, Kluge M, Zhang RZ, Mayer U, Timpl R, and Chu ML (1993a) Sequence of extracellular mouse protein BM-90/fibulin and its calcium-dependent binding to other basement-membrane ligands. Eur J Biochem 215 733-740. [DOI] [PubMed] [Google Scholar]
- Pan TC, Sasaki T, Zhang RZ, Fässler R, Timpl R, and Chu ML (1993b) Structure and expression of fibulin-2, a novel extracellular matrix protein with multiple EGF-like repeats and consensus motifs for calcium binding. J Cell Biol 123 1269-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parapia LA and Jackson C (2008) Ehlers-Danlos syndrome–a historical review. Br J Haematol 141 32-35. [DOI] [PubMed] [Google Scholar]
- Park J, Ha H, Ahn HJ, Kang SW, Kim YS, Seo JY, and Kim MS (2005) Sirolimus inhibits platelet-derived growth factor-induced collagen synthesis in rat vascular smooth muscle cells. Transplant Proc 37 3459-3462. [DOI] [PubMed] [Google Scholar]
- Partenheimer A, Schwarz K, Wrocklage C, Kölsch E, and Kresse H (1995) Proteoglycan form of colony-stimulating factor-1 (proteoglycan-100). Stimulation of activity by glycosaminoglycan removal and proteolytic processing. J Immunol 155 5557-5565. [PubMed] [Google Scholar]
- Patarroyo M, Tryggvason K, and Virtanen I (2002) Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin Cancer Biol 12 197-207. [DOI] [PubMed] [Google Scholar]
- Pavlovic S, Du B, Sakamoto K, Khan KM, Natarajan C, Breyer RM, Dannenberg AJ, and Falcone DJ (2006) Targeting prostaglandin E2 receptors as an alternative strategy to block cyclooxygenase-2-dependent extracellular matrix-induced matrix metalloproteinase-9 expression by macrophages. J Biol Chem 281 3321-3328. [DOI] [PubMed] [Google Scholar]
- Pellegata NS, Dieguez-Lucena JL, Joensuu T, Lau S, Montgomery KT, Krahe R, Kivelä T, Kucherlapati R, Forsius H, and de la Chapelle A (2000) Mutations in KERA, encoding keratocan, cause cornea plana. Nat Genet 25 91-95. [DOI] [PubMed] [Google Scholar]
- Peltonen S, Hentula M, Hägg P, Ylä-Outinen H, Tuukkanen J, Lakkakorpi J, Rehn M, Pihlajaniemi T, and Peltonen J (1999) A novel component of epidermal cell-matrix and cell-cell contacts: transmembrane protein type XIII collagen. J Invest Dermatol 113 635-642. [DOI] [PubMed] [Google Scholar]
- Peng Y, Liu H, Liu F, Liu Y, Li J, and Chen X (2006) Troglitazone inhibits synthesis of transforming growth factor-beta1 and reduces matrix production in human peritoneal mesothelial cells. Nephrology 11 516-523. [DOI] [PubMed] [Google Scholar]
- Pepin M, Schwarze U, Superti-Furga A, and Byers PH (2000) Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med 342 673-680. [DOI] [PubMed] [Google Scholar]
- Peranteau WH, Zhang L, Muvarak N, Badillo AT, Radu A, Zoltick PW, and Liechty KW (2008) IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol 128 1852-1860. [DOI] [PubMed] [Google Scholar]
- Peters H, Border WA, and Noble NA (1999) Targeting TGF-beta overexpression: maximizing the antifibrotic actions of angiotensin II blockade in anti-Thy1 glomerulonephritis. Nephrol Dial Transplant 14 (Suppl 4): 22-23. [DOI] [PubMed] [Google Scholar]
- Plenz GA, Deng MC, Robenek H, and Völker W (2003) Vascular collagens: spotlight on the role of type VIII collagen in atherogenesis. Atherosclerosis 166 1-11. [DOI] [PubMed] [Google Scholar]
- Podhajcer OL, Benedetti L, Girotti MR, Prada F, Salvatierra E, and Llera AS (2008) The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev 27 523-537. [DOI] [PubMed] [Google Scholar]
- Pope FM, Martin GR, Lichtenstein JR, Penttinen R, Gerson B, Rowe DW, and McKusick VA (1975) Patients with Ehlers-Danslos syndrome type IV lack type III collagen. Proc Natl Acad Sci U S A 72 1314-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter PL, Sage EH, Lane TF, Funk SE, and Gown AM (1995) Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem 43 791-800. [DOI] [PubMed] [Google Scholar]
- Posey KL, Yang Y, Veerisetty AC, Sharan SK, and Hecht JT (2008) Model systems for studying skeletal dysplasias caused by TSP-5/COMP mutations. Cell Mol Life Sci 65 687-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma DS and Timens W (2006) Remodeling in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 3 434-439. [DOI] [PubMed] [Google Scholar]
- Pullatt RC, Gadarla MR, Karas RH, Alsheikh-Ali AA, and Thompson PD (2007) Tendon rupture associated with simvastatin/ezetimibe therapy. Am J Cardiol 100 152-153. [DOI] [PubMed] [Google Scholar]
- Pusch CM, Zeitz C, Brandau O, Pesch K, Achatz H, Feil S, Scharfe C, Maurer J, Jacobi FK, Pinckers A, et al. (2000) The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nat Genet 26 324-327. [DOI] [PubMed] [Google Scholar]
- Rabinovitz I, Gipson IK, and Mercurio AM (2001) Traction forces mediated by alpha6beta4 integrin: implications for basement membrane organization and tumor invasion. Mol Biol Cell 12 4030-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffetto JD and Khalil RA (2008) Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharm 75 346-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman M and Cobb MH (2006) TGF-beta regulation by Emilin1: new links in the etiology of hypertension. Cell 124 893-895. [DOI] [PubMed] [Google Scholar]
- Ramirez F and Pereira L (1999) The fibrillins. Int J Biochem Cell Biol 31 255-259. [DOI] [PubMed] [Google Scholar]
- Ramirez Correa GA, Zacchigna S, Arsic N, Zentilin L, Salvi A, Sinagra G, and Giacca M (2004) Potent inhibition of arterial intimal hyperplasia by TIMP1 gene transfer using AAV vectors. Mol Ther 9 876-884. [DOI] [PubMed] [Google Scholar]
- Rauch U, Feng K, and Zhou XH (2001) Neurocan: a brain chondroitin sulfate proteoglycan. Cell Mol Life Sci 58 1842-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauterberg J, Jaeger E, and Althaus M (1993) Collagens in atherosclerotic vessel wall lesions. Curr Top Pathol 87 163-192. [DOI] [PubMed] [Google Scholar]
- Reardon AJ, Le Goff M, Briggs MD, McLeod D, Sheehan JK, Thornton DJ, and Bishop PN (2000) Identification in vitreous and molecular cloning of opticin, a novel member of the family of leucine-rich repeat proteins of the extracellular matrix. J Biol Chem 275 2123-2129. [DOI] [PubMed] [Google Scholar]
- Reed CC and Iozzo RV (2002) The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J 19 249-255. [DOI] [PubMed] [Google Scholar]
- Regula CG, Hennessy J, Clarke LE, Adams DR, Ioffreda MD, Graber EM, and Helm KF (2008) Interstitial granulomatous drug reaction to anakinra. J Am Acad Dermatol 59 S25-S27. [DOI] [PubMed] [Google Scholar]
- Ricard-Blum S and Ruggiero F (2005) The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol 53 430-442. [DOI] [PubMed] [Google Scholar]
- Ricart AD, Tolcher AW, Liu G, Holen K, Schwartz G, Albertini M, Weiss G, Yazji S, Ng C, and Wilding G (2008) Volociximab, a chimeric monoclonal antibody that specifically binds alpha5beta1 integrin: a phase I, pharmacokinetic, and biological correlative study. Clin Cancer Res 14 7924-7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardelli C, Sakko AJ, Ween MP, Russell DL, and Horsfall DJ (2009) The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev 28 233-245. [DOI] [PubMed] [Google Scholar]
- Richards AJ, Martin S, Nicholls AC, Harrison JB, Pope FM, and Burrows NP (1998) A single base mutation in COL5A2 causes Ehlers-Danlos syndrome type II. J Med Genet 35 846-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards AJ, Yates JR, Williams R, Payne SJ, Pope FM, Scott JD, and Snead MP (1996) A family with Stickler syndrome type 2 has a mutation in the COL11A1 gene resulting in the substitution of glycine 97 by valine in alpha 1 (XI) collagen. Hum Molec Genet 5 1339-1343. [DOI] [PubMed] [Google Scholar]
- Riley G (2007) Tendinopathy–from basic science to treatment. Nat Clin Pract Rheumatol 4 82-89. [DOI] [PubMed] [Google Scholar]
- Rizzoni D, Rossi GP, Porteri E, Sticchi D, Rodella L, Rezzani R, Sleiman I, De Ciuceis C, Paiardi S, Bianchi R, et al. (2004) Bradykinin and matrix metalloproteinases are involved the structural alterations of rat small resistance arteries with inhibition of ACE and NEP. J Hypertens 22 759-766. [DOI] [PubMed] [Google Scholar]
- Roach HI, Aigner T, Soder S, Haag J, and Welkerling H (2007) Pathobiology of osteoarthritis: pathomechanisms and potential therapeutic targets. Curr Drug Targets 8 271-282. [DOI] [PubMed] [Google Scholar]
- Robbesom AA, Koenders MM, Smits NC, Hafmans T, Versteeg EM, Bulten J, Veerkamp JH, Dekhuijzen PN, and van Kuppevelt TH (2008) Aberrant fibrillin-1 expression in early emphysematous human lung: a proposed predisposition for emphysema. Mod Pathol 21 297-307. [DOI] [PubMed] [Google Scholar]
- Robinson PN and Booms P (2001) The molecular pathogenesis of the Marfan syndrome. Cell Mol Life Sci 58 1698-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocnik EF, Chan BM, and Pickering JG (1998) Evidence for a role of collagen synthesis in arterial smooth muscle cell migration. J Clin Invest 101 1889-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom J, Abrams WR, and Mecham R (1993) Extracellular matrix 4: the elastic fiber. FASEB J 7 1208-1218. [PubMed] [Google Scholar]
- Rosove MH (2004) Platelet glycoprotein IIb/IIIa inhibitors. Best Pract Res Clin Haematol 17 65-76. [DOI] [PubMed] [Google Scholar]
- Ross R (1973) The elastic fiber. J Histochem Cytochem 21 199-208. [DOI] [PubMed] [Google Scholar]
- Ross MD, Bruggeman LA, Hanss B, Sunamoto M, Marras D, Klotman ME, and Klotman PE (2003) Podocan, a novel small leucine-rich repeat protein expressed in the sclerotic glomerular lesion of experimental HIV-associated nephropathy. J Biol Chem 278 33248-33255. [DOI] [PubMed] [Google Scholar]
- Roth JJ, Gahtan V, Brown JL, Gerhard C, Swami VK, Rothman VL, Tulenko TN, and Tuszynski GP (1998) Thrombospondin-1 is elevated with both intimal hyperplasia and hypercholesterolemia. J Surg Res 74 11-16. [DOI] [PubMed] [Google Scholar]
- Roth M, Eickelberg O, Kohler E, Erne P, and Block LH (1996) Ca2+ channel blockers modulate metabolism of collagens within the extracellular matrix. Proc Natl Acad Sci U S A 93 5478-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB and Baumann MH (2002) Serotonin releasing agents. Neurochemical, therapeutic and adverse effects. Pharmacol Biochem Behav 71 825-836. [DOI] [PubMed] [Google Scholar]
- Roughley P, Martens D, Rantakokko J, Alini M, Mwale F, and Antoniou J (2006) The involvement of aggrecan polymorphism in degeneration of human intervertebral disc and articular cartilage. Eur Cell Mater 11 1-7. [PubMed] [Google Scholar]
- Rupérez M, Lorenzo O, Blanco-Colio LM, Esteban V, Egido J, and Ruiz-Ortega M (2003) Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation 108 1499-1505. [DOI] [PubMed] [Google Scholar]
- Rupérez M, Rodrigues-Díez R, Blanco-Colio LM, Sánchez-López E, Rodríguez-Vita J, Esteban V, Carvajal G, Plaza JJ, Egido J, and Ruiz-Ortega M (2007) HMG-CoA reductase inhibitors decrease angiotensin II-induced vascular fibrosis: role of RhoA/ROCK and MAPK pathways. Hypertension 50 377-383. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E and Pierschbacher MD (1987) New perspectives in cell adhesion: RGD and integrins. Science 238 491-497. [DOI] [PubMed] [Google Scholar]
- Russell SB, Trupin JS, Myers JC, Broquist AH, Smith JC, Myles ME, and Russell JD (1989) Differential glucocorticoid regulation of collagen mRNAs in human dermal fibroblasts. Keloid-derived and fetal fibroblasts are refractory to down-regulation. J Biol Chem 264 13730-13735. [PubMed] [Google Scholar]
- Rüegg C, Postigo AA, Sikorski EE, Butcher EC, Pytela R, and Erle DJ (1992) Role of integrin alpha 4 beta 7/alpha 4 beta P in lymphocyte adherence to fibronectin and VCAM-1 and in homotypic cell clustering. J Cell Biol 117 179-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarela J, Rehn M, Oikarinen A, Autio-Harmainen H, and Pihlajaniemi T (1998) The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am J Pathol 153 611-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarni H and Hopsu-Havu VK (1978) The decrease of hyaluronate synthesis by anti-inflammatory steroids in vitro. Br J Dermatol 98 445-449. [DOI] [PubMed] [Google Scholar]
- Sadowski T and Steinmeyer J (2001) Effects of non-steroidal antiinflammatory drugs and dexamethasone on the activity and expression of matrix metalloproteinase-1, matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 by bovine articular chondrocytes. Osteoarthritis Cartilage 9 407-415. [DOI] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Morris NP, and Burgeson RE (1986) Type VII collagen is a major structural component of anchoring fibrils. J Cell Biol 103 1577-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S, Akiyama H, Sato Y, Yoshioka Y, Linhardt RJ, Goda Y, Maitani T, and Toida T (2006) Chondroitin sulfate intake inhibits the IgE-mediated allergic response by down-regulating Th2 responses in mice. J Biol Chem 281 19872-19880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr SW, Potter-Perigo S, Kinsella MG, Johnson PY, Braun KR, Goueffic Y, Rosenfeld ME, and Wight TN (2008) Hyaluronan accumulation is elevated in cultures of low density lipoprotein receptor-deficient cells and is altered by manipulation of cell cholesterol content. J Biol Chem 283 36195-36204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salliot C and van der Heijde D (2008) Long term safety of methotrexate monotherapy in rheumatoid arthritis patients: a systematic literature research. Ann Rheum Dis doi: 10.1136/ard.2008.093690. [DOI] [PMC free article] [PubMed]
- Salomäki HH, Sainio AO, Söderström M, Pakkanen S, Laine J, and Järveläinen HT (2008) Differential expression of decorin by human malignant and benign vascular tumors. J Histochem Cytochem 56 639-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Fässler R, and Hohenester E (2004) Laminin: the crux of basement membrane assembly. J Cell Biol 164 959-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoi S, Hiramatsu Y, Kitade H, Kwon AH, Matsui K, Miyashita K, Sakashita E, Sekiguchi K, Takahashi H, and Kamiyama Y (1999) Different responses to surgical stress between extra domain A+ and plasma fibronectins. Clin Exp Pharmacol Physiol 26 225-229. [DOI] [PubMed] [Google Scholar]
- Saygili E, Rana OR, Meyer C, Gemein C, Andrzejewski MG, Ludwig A, Weber C, Schotten U, Krüttgen A, Weis J, et al. (2009) The angiotensin-calcineurin-NFAT pathway mediates stretch-induced up-regulation of matrix metalloproteinases-2/-9 in atrial myocytes. Basic Res Cardiol 104 435-448. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Hausser H, Altenburger M, Ugorcakova J, August C, Fisher LW, Schaefer RM, and Kresse H (1998) Decorin, biglycan and their endocytosis receptor in rat renal cortex. Kidney Int 54 1529-1541. [DOI] [PubMed] [Google Scholar]
- Schaefer L and Iozzo RV (2008) Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem 283 21305-21309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, Raslik I, Grone HJ, Schonherr E, Macakova K, Ugorcakova J, Budny S, Schaefer RM, and Kresse H (2001) Small proteoglycans in human diabetic nephropathy: discrepancy between glomerular expression and protein accumulation of decorin, biglycan, lumican, and fibromodulin. FASEB J 15 559-561. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Tsalastra W, Babelova A, Baliova M, Minnerup J, Sorokin L, Gröne HJ, Reinhardt DP, Pfeilschifter J, Iozzo RV, et al. (2007) Decorin-mediated regulation of fibrillin-1 in the kidney involves the insulin-like growth factor-I receptor and Mammalian target of rapamycin. Am J Pathol 170 301-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepe S, Schäcke H, May E, and Asadullah K (2006) Glucocorticoid therapy-induced skin atrophy. Exp Dermatol 15 406-420. [DOI] [PubMed] [Google Scholar]
- Schön MP (2008) Efalizumab in the treatment of psoriasis: mode of action, clinical indications, efficacy, and safety. Clin Dermatol 26 509-514. [DOI] [PubMed] [Google Scholar]
- Schultz DW, Klein ML, Humpert AJ, Luzier CW, Persun V, Schain M, Mahan A, Runckel C, Cassera M, Vittal V, et al. (2003) Analysis of the ARMD1 locus: evidence that a mutation in HEMICENTIN-1 is associated with age-related macular degeneration in a large family. Hum Mol Genet 12 3315-3323. [DOI] [PubMed] [Google Scholar]
- Sehgal SN (2003) Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc 35 7S-14S. [DOI] [PubMed] [Google Scholar]
- Seitz M and Dayer JM (2000) Enhanced production of tissue inhibitor of metalloproteinases by peripheral blood mononuclear cells of rheumatoid arthritis patients responding to methotrexate treatment. Rheumatology 39 637-645. [DOI] [PubMed] [Google Scholar]
- Senzaki H (2006) The pathophysiology of coronary artery aneurysms in Kawasaki disease: role of matrix metalloproteinases. Arch Dis Child 91 847-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephel GC, Kennedy R, and Kudravi S (1996) Expression of capillary basement membrane components during sequential phases of wound angiogenesis. Matrix Biol 15 263-279. [DOI] [PubMed] [Google Scholar]
- Seyama Y and Wachi H (2004) Atherosclerosis and matrix dystrophy. J Atheroscler Thromb 11 236-245. [DOI] [PubMed] [Google Scholar]
- Shanahan CM, Cary NR, Osbourn JK, and Weissberg PL (1997) Identification of osteoglycin as a component of the vascular matrix. Differential expression by vascular smooth muscle cells during neointima formation and in atherosclerotic plaques. Arterioscler Thromb Vasc Biol 17 2437-2447. [DOI] [PubMed] [Google Scholar]
- Shen G (2005) The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod Craniofac Res 8 11-17. [DOI] [PubMed] [Google Scholar]
- Shen Z, Gantcheva S, Mânsson B, Heinegârd D, and Sommarin Y (1998) Chondroadherin expression changes in skeletal development. Biochem J 330 549-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockley KR, Lazarenko OP, Czernik PJ, Rosen CJ, Churchill GA, and Lecka-Czernik B (2009) PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem 106 232-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth CA (1997) Type VIII collagen. Int J Biochem Cell Biol 29 1145-1148. [DOI] [PubMed] [Google Scholar]
- Si HF, Li J, Lü XW, and Jin Y (2007) Suppressive effects of leflunomide on leptin-induced collagen I production involved in hepatic stellate cell production. Exp Biol Med (Maywood) 232 427-436. [PubMed] [Google Scholar]
- Siddiqui I, Khan ZA, Lian D, Jiang J, Zhong R, Garcia B, and Chakrabarti S (2006) Endothelin-mediated oncofetal fibronectin expression in chronic allograft nephropathy. Transplantation 82 406-414. [DOI] [PubMed] [Google Scholar]
- Simo P, Simon-Assmann P, Arnold C, and Kedinger M (1992) Mesenchyme-mediated effect of dexamethasone on laminin in cocultures of embryonic gut epithelial cells and mesenchyme-derived cells. J Cell Sci 101 161-171. [DOI] [PubMed] [Google Scholar]
- Sivakumar P and Das AM (2008) Fibrosis, chronic inflammation and new pathways for drug discovery. Inflamm Res 57 410-418. [DOI] [PubMed] [Google Scholar]
- Sjöberg AP, Manderson GA, Mörgelin M, Day AJ, Heinegård D, and Blom AM (2009) Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol Immunol 46 830-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PS, Fanning JC, and Aarons I (1989) The structure of the normal human glomerular basement membrane. Ultrastructural localization of type IV collagen and laminin. Pathology 21 254-258. [DOI] [PubMed] [Google Scholar]
- Smith RL (1999) Degradative enzymes in osteoarthritis. Front Biosci 4 D704-D712. [DOI] [PubMed] [Google Scholar]
- Snider GL, Ciccolella DE, Morris SM, Stone PJ, and Lucey EC (1991) Putative role of neutrophil elastase in the pathogenesis of emphysema. Ann N Y Acad Sci 624 45-59. [DOI] [PubMed] [Google Scholar]
- Solovieva S, Noponen N, Männikkö M, Leino-Arjas P, Luoma K, Raininko R, Ala-Kokko L, and Riihimäki H (2007) Association between the aggrecan gene variable number of tandem repeats polymorphism and intervertebral disc degeneration. Spine 32 1700-1705. [DOI] [PubMed] [Google Scholar]
- Sood S, Eldadah ZA, Krause WL, McIntosh I, and Dietz HC (1996) Mutation in fibrillin-1 and the Marfanoid-craniosynostosis (Shprintzen-Goldberg) syndrome. Nat Genet 12 209-211. [DOI] [PubMed] [Google Scholar]
- Sorrentino D, Terrosu G, Vadalà S, and Avellini C (2007) Fibrotic strictures and anti-TNF-alpha therapy in Crohn's disease. Digestion 75 22-24. [DOI] [PubMed] [Google Scholar]
- Stahn C, Löwenberg M, Hommes DW, and Buttgereit F (2007) Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol 275 71-78. [DOI] [PubMed] [Google Scholar]
- Stoch SA and Wagner JA (2008) Cathepsin K inhibitors: a novel target for osteoporosis therapy. Clin Pharmacol Ther 83 172-176. [DOI] [PubMed] [Google Scholar]
- Stone EM, Braun TA, Russell SR, Kuehn MH, Lotery AJ, Moore PA, Eastman CG, Casavant TL, and Sheffield VC (2004) Missense variations in the fibulin 5 gene and age-related macular degeneration. N Engl J Med 351 346-353. [DOI] [PubMed] [Google Scholar]
- Stuhlmeier KM (2006) Aspects of the biology of hyaluronan, a largely neglected but extremely versatile molecule. Wien Med Wochenschr 156 563-568. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Imai E, Moriyama T, Horio M, and Hori M (2000) Calcium channel blockers inhibit proliferation and matrix production in rat mesangial cells: possible mechanism of suppression of AP-1 and CREB activities. Nephron 85 71-80. [DOI] [PubMed] [Google Scholar]
- Sussmann M, Sarbia M, Meyer-Kirchrath J, Nüsing RM, Schrör K, and Fischer JW (2004) Induction of hyaluronic acid synthase 2 (HAS2) in human vascular smooth muscle cells by vasodilatory prostaglandins. Circ Res 94 592-600. [DOI] [PubMed] [Google Scholar]
- Sutmuller M, Bruijn JA, and de Heer E (1997) Collagen types VIII and X, two non-fibrillar, short-chain collagens. Structure homologies, functions and involvement in pathology. Histol Histopathol 12 557-566. [PubMed] [Google Scholar]
- Sutton TA, Kelly KJ, Mang HE, Plotkin Z, Sandoval RM, and Dagher PC (2005) Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am J Physiol Renal Physiol 288 F91-97. [DOI] [PubMed] [Google Scholar]
- Suwiwat S, Ricciardelli C, Tammi R, Tammi M, Auvinen P, Kosma VM, LeBaron RG, Raymond WA, Tilley WD, and Horsfall DJ (2004) Expression of extracellular matrix components versican, chondroitin sulfate, tenascin, and hyaluronan, and their association with disease outcome in node-negative breast cancer. Clin Cancer Res 10 2491-2498. [DOI] [PubMed] [Google Scholar]
- Suzuki OT, Sertié AL, Der Kaloustian VM, Kok F, Carpenter M, Murray J, Czeizel AE, Kliemann SE, Rosemberg S, Monteiro M, et al. (2002) Molecular analysis of collagen XVIII reveals novel mutations, presence of a third isoform, and possible genetic heterogeneity in Knobloch syndrome. Am J Hum Genet 71 1320-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L, Aszódi A, Reinholt FP, Fässler R, Heinegård D, and Oldberg A (1999) Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem 274 9636-9647. [DOI] [PubMed] [Google Scholar]
- Taal MW and Brenner BM (2000) Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int 57 1803-1817. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Oka M, Ikeda T, Akiba S, and Sato T (2008) [Role of thrombospondin-1 in hypoxia-induced migration of human vascular smooth muscle cells]. Yakugaku Zasshi 128 377-383. [DOI] [PubMed] [Google Scholar]
- Tamarina NA, Grassi MA, Johnson DA, and Pearce WH (1998) Proteoglycan gene expression is decreased in abdominal aortic aneurysms. J Surg Res 74 76-80. [DOI] [PubMed] [Google Scholar]
- Tamhane UU and Gurm HS (2008) The chimeric monoclonal antibody abciximab: a systematic review of its safety in contemporary practice. Expert Opin Drug Saf 7 809-819. [DOI] [PubMed] [Google Scholar]
- Tang BL (2001) ADAMTS: a novel family of extracellular matrix proteases. Int J Biochem Cell Biol 33 33-44. [DOI] [PubMed] [Google Scholar]
- Tanjore H and Kalluri R (2006) The role of type IV collagen and basement membranes in cancer progression and metastasis. Am J Pathol 168 715-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasheva ES, Ke A, and Conrad GW (2004) Analysis of the expression of chondroadherin in mouse ocular and non-ocular tissues. Mol Vis 10 544-554. [PubMed] [Google Scholar]
- Tasheva ES, Koester A, Paulsen AQ, Garrett AS, Boyle DL, Davidson HJ, Song M, Fox N, and Conrad GW (2002) Mimecan/osteoglycin-deficient mice have collagen fibril abnormalities. Mol Vis 8 407-415. [PubMed] [Google Scholar]
- Tassabehji M, Metcalfe K, Hurst J, Ashcroft GS, Kielty C, Wilmot C, Donnai D, Read AP, and Jones CJP (1998) An elastin gene mutation producing abnormal tropoelastin and abnormal elastic fibres in a patient with autosomal dominant cutis laxa. Hum Mol Genet 7 1021-1028. [DOI] [PubMed] [Google Scholar]
- Tátrai P, Dudás J, Batmunkh E, Máthé M, Zalatnai A, Schaff Z, Ramadori G, and Kovalszky I (2006) Agrin, a novel basement membrane component in human and rat liver, accumulates in cirrhosis and hepatocellular carcinoma. Lab Invest 86 1149-1160. [DOI] [PubMed] [Google Scholar]
- Tayebjee MH, MacFadyen RJ, and Lip GY (2003) Extracellular matrix biology: a new frontier in linking the pathology and therapy of hypertension? J Hypertens 21 2211-2218. [DOI] [PubMed] [Google Scholar]
- Theocharis AD (2008) Versican in health and disease. Connect Tissue Res 49 230-234. [DOI] [PubMed] [Google Scholar]
- Tiller GE, Polumbo PA, Weis MA, Bogaert R, Lachman RS, Cohn DH, Rimoin DL, and Eyre DR (1995) Dominant mutations in the type II collagen gene, COL2A1, produce spondyloepimetaphyseal dysplasia, Strudwick type. Nat Genet 11 87-89. [DOI] [PubMed] [Google Scholar]
- Timpl R and Brown JC (1994) The laminins. Matrix Biol 14 275-281. [DOI] [PubMed] [Google Scholar]
- Ton FN, Gunawardene SC, Lee H, and Neer RM (2005) Effects of low-dose prednisone on bone metabolism. J Bone Miner Res 20 464-470. [DOI] [PubMed] [Google Scholar]
- Toole BP (2002) Hyaluronan promotes the malignant phenotype. Glycobiology 12 37R-42R. [DOI] [PubMed] [Google Scholar]
- Toole BP, Ghatak S, and Misra S (2008) Hyaluronan oligosaccharides as a potential anticancer therapeutic. Curr Pharm Biotechnol 9 249-252. [DOI] [PubMed] [Google Scholar]
- Trebaul A, Chan EK, and Midwood KS (2007) Regulation of fibroblast migration by tenascin-C. Biochem Soc Trans 35 695-697. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Wang H, Timpl R, and Chu ML (2001) Fibulin-2 expression marks transformed mesenchymal cells in developing cardiac valves, aortic arch vessels, and coronary vessels. Dev Dyn 222 89-100. [DOI] [PubMed] [Google Scholar]
- Tziakas DN, Chalikias GK, Hatzinikolaou HI, Stakos DA, Papanas N, Tentes IK, Kortsaris AX, Maltezos E, Hatseras DI, and Kaski JC (2005) Levosimendan use reduces matrix metalloproteinase-2 in patients with decompensated heart failure. Cardiovasc Drugs Ther 19 399-402. [DOI] [PubMed] [Google Scholar]
- Uhl M, Aulwurm S, Wischhusen J, Weiler M, Ma JY, Almirez R, Mangadu R, Liu YW, Platten M, Herrlinger U, et al. (2004) SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res 64 7954-7961. [DOI] [PubMed] [Google Scholar]
- Uitto J and Pulkkinen L (1996) Molecular complexity of the cutaneous basement membrane zone. Mol Biol Rep 23 35-46. [DOI] [PubMed] [Google Scholar]
- Uitto J, Pulkkinen L, and Christiano AM (1994) Molecular basis of the dystrophic and junctional forms of epidermolysis bullosa: mutations in the type VII collagen and kalinin (laminin 5) genes. J Invest Dermatol 103 39S-46S. [DOI] [PubMed] [Google Scholar]
- Ulazzi L, Sabbioni S, Miotto E, Veronese A, Angusti A, Gafà R, Manfredini S, Farinati F, Sasaki T, Lanza G, et al. (2007) Nidogen 1 and 2 gene promoters are aberrantly methylated in human gastrointestinal cancer. Mol Cancer 6 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz DC, Rooke ED, Spittell JA Jr, and Bartholomew LG (1965) Retroperitoneal fibrosis in patients taking methysergide. JAMA 191 983-985. [DOI] [PubMed] [Google Scholar]
- Van Camp G, Snoeckx RL, Hilgert N, van den Ende J, Fukuoka H, Wagatsuma M, Suzuki H, Smets RM, Vanhoenacker F, Declau F, et al. (2006) A new autosomal recessive form of Stickler syndrome is caused by a mutation in the COL9A1 gene. Am J Hum Genet 79 449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh F and Giudice GJ (2003) BP180 (type XVII collagen) and its role in cutaneous biology and disease. Adv Dermatol 19 37-71. [PubMed] [Google Scholar]
- Varga J and Pasche B (2008) Antitransforming growth factor-beta therapy in fibrosis: recent progress and implications for systemic sclerosis. Curr Opin Rheumatol 20 720-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CJ and Gotto AM Jr (2004) Update on statins: 2003. Circulation 110 886-892. [DOI] [PubMed] [Google Scholar]
- Verrecchia F and Mauviel A (2007) Transforming growth factor-beta and fibrosis. World J Gastroenterol 13 3056-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent T and Mechti N (2005) Extracellular matrix in bone marrow can mediate drug resistance in myeloma. Leuk Lymphoma 46 803-811. [DOI] [PubMed] [Google Scholar]
- Visse R and Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92 827-839. [DOI] [PubMed] [Google Scholar]
- Voermans NC, Bönnemann CG, Huijing PA, Hamel BC, van Kuppevelt TH, de Haan A, Schalkwijk J, van Engelen BG, and Jenniskens GJ (2008) Clinical and molecular overlap between myopathies and inherited connective tissue diseases. Neuromuscul Disord 18 843-856. [DOI] [PubMed] [Google Scholar]
- Vogel BE and Hedgecock EM (2001) Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development 128 883-894. [DOI] [PubMed] [Google Scholar]
- von der Mark H, Aumailley M, Wick G, Fleischmajer R, and Timpl R (1984) Immunochemistry, genuine size and tissue localization of collagen VI. Eur J Biochem 142 493-502. [DOI] [PubMed] [Google Scholar]
- Väisänen T, Väisänen MR, Autio-Harmainen H, and Pihlajaniemi T (2005) Type XIII collagen expression is induced during malignant transformation in various epithelial and mesenchymal tumours. J Pathol 207 324-335. [DOI] [PubMed] [Google Scholar]
- Wada Y, Kato S, Okamoto K, Izumaru S, Aoyagi S, and Morimatsu M (2001) Diltiazem, a calcium antagonist, inhibits matrix metalloproteinase-1 (tissue collagenase) production and collagenolytic activity in human vascular smooth muscle cells. Int J Mol Med 8 561-566. [DOI] [PubMed] [Google Scholar]
- Wallace K, Burt AD, and Wright MC (2008) Liver fibrosis. Biochem J 411 1-18. [DOI] [PubMed] [Google Scholar]
- Waller JR, Brook NR, Bicknell GR, and Nicholson ML (2004) Differential effects of modern immunosuppressive agents on the development of intimal hyperplasia. Transpl Int 17 9-14. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Pieper J, Schotel R, van Blitterswijk CA, and Lamme EN (2004) Stimulation of skin repair is dependent on fibroblast source and presence of extracellular matrix. Tissue Eng 10 1054-1064. [DOI] [PubMed] [Google Scholar]
- Wang IJ, Chiang TH, Shih YF, Hsiao CK, Lu SC, Hou YC, and Lin LL (2006) The association of single nucleotide polymorphisms in the 5′-regulatory region of the lumican gene with susceptibility to high myopia in Taiwan. Mol Vis 12 852-857. [PubMed] [Google Scholar]
- Wapstra FH, Navis GJ, van Goor H, van den Born J, Berden JH, de Jong PE, and de Zeeuw D (2001) ACE inhibition preserves heparan sulfate proteoglycans in the glomerular basement membrane of rats with established adriamycin nephropathy. Exp Nephrol 9 21-27. [DOI] [PubMed] [Google Scholar]
- Warman ML, Abbott M, Apte SS, Hefferon T, McIntosh I, Cohn DH, Hecht JT, Olsen BR, and Francomano CA (1993) A type X collagen mutation causes Schmid metaphyseal chondrodysplasia. Nat Genet 5 79-82. [DOI] [PubMed] [Google Scholar]
- Warren RB, Chalmers RJ, Griffiths CE, and Menter A (2008) Methotrexate for psoriasis in the era of biological therapy. Clin Exp Dermatol 33 551-554. [DOI] [PubMed] [Google Scholar]
- Watkins G, Douglas-Jones A, Bryce R, Mansel RE, and Jiang WG (2005) Increased levels of SPARC (osteonectin) in human breast cancer tissues and its association with clinical outcomes. Prostaglandins Leukot Essent Fatty Acids 72 267-272. [DOI] [PubMed] [Google Scholar]
- Weigell-Weber M, Sarra GM, Kotzot D, Sandkuijl L, Messmer E, and Hergersberg M (2003) Genomewide homozygosity mapping and molecular analysis of a candidate gene located on 22q13 (fibulin-1) in a previously undescribed vitreoretinal dystrophy. Arch Ophthalmol 121 1184-1188. [DOI] [PubMed] [Google Scholar]
- Weiss L, Slavin S, Reich S, Cohen P, Shuster S, Stern R, Kaganovsky E, Okon E, Rubinstein AM, and Naor D (2000) Induction of resistance to diabetes in non-obese diabetic mice by targeting CD44 with a specific monoclonal antibody. Proc Natl Acad Sci U S A 97 285-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel M, Sommarin Y, and Heinegård D (1998) Bone matrix proteins: isolation and characterization of a novel cell-binding keratan sulfate proteoglycan (osteoadherin) from bovine bone. J Cell Biol 141 839-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling D, Cedoz JP, and Racadot E (2008) Serum levels of MMP-3 and cathepsin K in patients with ankylosing spondylitis: effect of TNFalpha antagonist therapy. Joint Bone Spine 75 559-562. [DOI] [PubMed] [Google Scholar]
- White ES, Baralle FE, and Muro AF (2008) New insights into form and function of fibronectin splice variants. J Pathol 216 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelock JM, Melrose J, and Iozzo RV (2008) Diverse cell signaling events modulated by perlecan. Biochemistry 47 11174-11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight TN (2008) Arterial remodeling in vascular disease: a key role for hyaluronan and versican. Front Biosci 13 4933-4937. [DOI] [PubMed] [Google Scholar]
- Wight TN and Merrilees MJ (2004) Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res 94 1158-1167. [DOI] [PubMed] [Google Scholar]
- Williams KJ (2001) Arterial wall chondroitin sulfate proteoglycans: diverse molecules with distinct roles in lipoprotein retention and atherogenesis. Curr Opin Lipidol 12 477-487. [DOI] [PubMed] [Google Scholar]
- Williams KJ and Tabas I (1995) The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 15 551-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Ryan C, and Jacobson C (2008) Agrin and neuregulin, expanding roles and implications for therapeutics. Biotechnol Adv 26 187-201. [DOI] [PubMed] [Google Scholar]
- Winterpacht A, Hilbert M, Schwarze U, Mundlos S, Spranger J, and Zabel BU (1993) Kniest and Stickler dysplasia phenotypes caused by collagen type II gene (COL2A1) defect. Nat Genet 3 323-326. [DOI] [PubMed] [Google Scholar]
- Wlazlinski A, Engers R, Hoffmann MJ, Hader C, Jung V, Müller M, and Schulz WA (2007) Downregulation of several fibulin genes in prostate cancer. Prostate 67 1770-1780. [DOI] [PubMed] [Google Scholar]
- Woodside DG and Vanderslice P (2008) Cell adhesion antagonists: therapeutic potential in asthma and chronic obstructive pulmonary disease. BioDrugs 22 85-100. [DOI] [PubMed] [Google Scholar]
- Wynn TA (2007) Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 117 524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214 199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wälchli C, Koch M, Chiquet M, Odermatt BF, and Trueb B (1994) Tissue-specific expression of the fibril-associated collagens XII and XIV. J Cell Sci 107 669-681. [DOI] [PubMed] [Google Scholar]
- Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Moon YS, Davis GE, Brooks PC, and Yuen SM (2001) Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol 154 1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabkowitz R, Mansfield PJ, Ryan US, and Suchard SJ (1993) Thrombospondin mediates migration and potentiates platelet-derived growth factor-dependent migration of calf pulmonary artery smooth muscle cells. J Cell Physiol 157 24-32. [DOI] [PubMed] [Google Scholar]
- Yamada M, Li AW, and Wall JR (2000) Thyroid-associated ophthalmopathy: clinical features, pathogenesis, and management. Crit Rev Clin Lab Sci 37 523-549. [DOI] [PubMed] [Google Scholar]
- Yamada H, Tajima S, Nishikawa T, Murad S, and Pinnell SR (1994) Tranilast, a selective inhibitor of collagen synthesis in human skin fibroblasts. J Biochem 116 892-897. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y (1996) Brevican: a major proteoglycan in adult brain. Perspect Dev Neurobiol 3 307-317. [PubMed] [Google Scholar]
- Yamaguchi Y, Mann DM, and Ruoslahti E (1990) Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 346: 281-284. [DOI] [PubMed] [Google Scholar]
- Yan Q and Sage EH (1999) SPARC, a matricellular glycoprotein with important biological functions. J Histochem Cytochem 47 1495-1506. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, and Olson EN (2002) Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 415: 168-171. [DOI] [PubMed] [Google Scholar]
- Yao HW, Li J, Chen JQ, and Xu SY (2004) Inhibitory effect of leflunomide on hepatic fibrosis induced by CCl4 in rats. Acta Pharmacol Sin 25 915-920. [PubMed] [Google Scholar]
- Ye Z, Cheng K, Guntaka RV, and Mahato RI (2005) Targeted delivery of a triplex-forming oligonucleotide to hepatic stellate cells. Biochemistry 44 4466-4476. [DOI] [PubMed] [Google Scholar]
- Ye Z, Houssein HS, and Mahato RI (2007) Bioconjugation of oligonucleotides for treating liver fibrosis. Oligonucleotides 17 349-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CH, Smith DJ, West WW, and Hollingsworth MA (2007) Loss of fibulin-2 expression is associated with breast cancer progression. Am J Pathol 170 1535-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi XY, Wayner EA, Kim Y, and Fish AJ (1998) Adhesion of cultured human kidney mesangial cells to native entactin: role of integrin receptors. Cell Adhes Commun 5 237-248. [DOI] [PubMed] [Google Scholar]
- Yndestad A, Vinge LE, Bjørnerheim R, Ueland T, Wang JE, Frøland SS, Attramadal H, Aukrust P, and Oie E (2006) Thalidomide attenuates the development of fibrosis during post-infarction myocardial remodelling in rats. Eur J Heart Fail 8 790-796. [DOI] [PubMed] [Google Scholar]
- Yozai K, Shikata K, Sasaki M, Tone A, Ohga S, Usui H, Okada S, Wada J, Nagase R, Ogawa D, et al. (2005) Methotrexate prevents renal injury in experimental diabetic rats via anti-inflammatory actions. J Am Soc Nephrol 16 3326-3338. [DOI] [PubMed] [Google Scholar]
- Yu L, Border WA, Anderson I, McCourt M, Huang Y, and Noble NA (2004) Combining TGF-beta inhibition and angiotensin II blockade results in enhanced antifibrotic effect. Kidney Int 66 1774-1784. [DOI] [PubMed] [Google Scholar]
- Yu L, Noble NA, and Border WA (2002) Therapeutic strategies to halt renal fibrosis. Curr Opin Pharmacol 2 177-181. [DOI] [PubMed] [Google Scholar]
- Zacchigna S, Zentilin L, Morini M, Dell'Eva R, Noonan DM, Albini A, and Giacca M (2004) AAV-mediated gene transfer of tissue inhibitor of metalloproteinases-1 inhibits vascular tumor growth and angiogenesis in vivo. Cancer Gene Ther 11 73-80. [DOI] [PubMed] [Google Scholar]
- Zanetti M, Braghetta P, Sabatelli P, Mura I, Doliana R, Colombatti A, Volpin D, Bonaldo P, and Bressan GM (2004) EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Mol Cell Biol 24 638-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Toole BP, Kinney SD, Kuo JW, and Stamenkovic I (1998) Inhibition of tumor growth in vivo by hyaluronan oligomers. Int J Cancer 77 396-401. [DOI] [PubMed] [Google Scholar]
- Zenker M, Aigner T, Wendler O, Tralau T, Müntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wühl E, et al. (2004) Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13 2625-2632. [DOI] [PubMed] [Google Scholar]
- Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, and Birk DE (2006) Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem 98 1436-1449. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hu W, and Ramirez F (1995) Developmental expression of fibrillin genes suggests heterogeneity of extracellular microfibrils. J Cell Biol 129 1165-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziober BL, Lin CS, and Kramer RH (1996) Laminin-binding integrins in tumor progression and metastasis. Semin Cancer Biol 7 119-128. [DOI] [PubMed] [Google Scholar]
- Zöller NN, Kippenberger S, Thaçi D, Mewes K, Spiegel M, Sättler A, Schultz M, Bereiter-Hahn J, Kaufmann R, and Bernd A (2008) Evaluation of beneficial and adverse effects of glucocorticoids on a newly developed full-thickness skin model. Toxicol In Vitro 22 747-759. [DOI] [PubMed] [Google Scholar]