Abstract
BTLA, a recently cloned coreceptor expressed on lymphocytes, negatively regulates cell activation by recruiting SHP-1/SHP-2. However, the mechanisms that regulate the intracellular localization of BTLA and its trafficking to the cell surface in T cells are still unknown. To determine the mechanisms that regulate the expression of BTLA on the surface of T cells, we examined the subcellular localization of BTLA in mouse T cells in a steady state, as well as upon activation by using a confocal laser-scanning microscopy. We found that BTLA was localized mainly in the Golgi apparatus and secretory lysosomes in resting CD4+ T cells. We also found that intracellular BTLA was translocated to the cell surface and accumulated at the immunological synapse upon TCR stimulation. Furthermore, we found that the BTLA-HVEM interaction was required for the association of BTLA with lipid rafts. These results indicate that the surface expression of BTLA and its accumulation at the immunological synapse are tightly regulated by TCR and HVEM stimulation to deliver efficient inhibitory signals in the regulation of CD4+ T cell activation.
Keywords: T cells, costimulation, cell-surface molecules, trafficking
Introduction
T cell activation is regulated positively and negatively by stimulatory and inhibitory receptors to maintain immunological homeostasis. The CTLA-4−B7-1/B7-2 (CD80/CD86) pathway is the best-characterized inhibitory pathway for the maintenance of T cell homeostasis and peripheral tolerance [1,2,3]. Another inhibitory pathway involves PD-1, which interacts with PD-L1 (B7-H1) and PD-L2 (B7-DC) and induces and maintains peripheral tolerance [1, 3, 4]. We have identified a third coinhibitory molecule, BTLA, which is a lymphoid-specific cell-surface molecule with similarities to CTLA-4 [5]. BTLA interacts with HVEM, a costimulatory TNFR family protein, and the binding of HVEM to BTLA attenuates T cell activation [6,7,8,9]. As these coinhibitory molecules inhibit proliferation and cytokine production of T cells in vitro and in vivo, they are thought to play important roles in regulating immune responses.
The surface expression of coinhibitory molecules such as CTLA-4 is strictly regulated to serve as an efficient negative-feedback mechanism for down-regulating T cell activation. CTLA-4 is retained intracellularly in resting T cells and is expressed on the surface of T cells upon activation [10]. Intracellular CTLA-4 is found predominantly in the Golgi apparatus [11, 12] and vesicles [13, 14] in resting T cells, and CTLA-4 is internalized continuously from the cell surface unless T cells are activated [10, 13, 15]. Upon T cell activation, CTLA-4 is exported rapidly to the cell surface, recruited to lipid rafts [16,17,18], and concentrated at the immunological synapse [17, 19, 20]. As a result of the recruitment to the immunological synapse, CTLA-4 transduces inhibitory signals to T cells in the proximity of TCR.
BTLA is expressed on B cells, T cells, and dendritic cells in all strains of mice, whereas its expression on CD11b+ macrophages and NK cells is limited to C57BL/6 mice [21]. Analyses using mAb against BTLA reveal that BTLA is expressed in thymocytes during positive selection [22] and that in the peripheral CD4+ T cells, its expression is low in naïve CD4+ T cells, transiently up-regulated in activated CD4+ T cells, and sustained on differentiated Th1 cells but not on differentiated Th2 cells [5, 21, 22]. Moreover, it has been demonstrated recently that BTLA is highly expressed in follicular B helper T cells [23].
BTLA contains two ITIM sequences in the intracellular domain [5]. Co-cross-linking of TCR and BTLA induces tyrosine phosphorylation of ITIM sequences, SHP-1/SHP-2 association, attenuation of IL-2 production, and proliferation of T cells [5, 22, 24]. Interestingly, the surface expression of full-length BTLA on T cells is much lower than that of BTLA without the cytoplasmic domain (tail-less BTLA) [5]. This suggests that the expression levels of BTLA on the surface of T cells are strictly regulated to exert its inhibitory function. However, the mechanisms that regulate the intracellular localization of BTLA in T cells, its trafficking to the cell surface, and the distribution in the plasma membrane upon activation are still unknown.
We therefore attempted to identify the intracellular localization of BTLA in resting T cells and also investigate the trafficking of BTLA to the cell surface and the formation of immunological synapse upon T cell activation. We found that BTLA was localized mainly in the Golgi apparatus and secretory lysosomes in resting T cells. We also found that intracellular BTLA was translocated to the cell surface and accumulated at the immunological synapse upon TCR stimulation. Furthermore, we found that although a small portion of BTLA was distributed in lipid rafts in resting T cells, BTLA accumulated in lipid rafts upon TCR and HVEM stimulation. Our results indicate that the surface expression of BTLA and its accumulation at the immunological synapse are tightly regulated by TCR and HVEM stimulation to deliver an efficient inhibitory signal during T cell activation.
MATERIALS AND METHODS
Plasmids
BTLA-GFP RV was generated by PCR as follows. First, the open-reading frame of murine BTLA with 3′ overhang homologous to the N-terminal portion of GFP was amplified by primers “J10-RV1-Bgl2” (5′-AGCTCTGAAGATCTCTAGGGAGGAAG-3′) and “3′-J10+10” (5′-CCTTGCTCACACTTCTCACACAAATGGATGC-3′). Second, the open-reading frame of GFP with 5′ overhang homologous to the C-terminal portion of BTLA was amplified from internal ribosomal entry site-GFP RV [25] by primers “5′-GFP+10” (5′-TGTGAGAAGTGTGAGCAAGGGCGAGGAGC-3′) and “3′-GFP+Sal1” (5′-ACGCGTCGACTTACTTGTACAGCTCGTCCATG-3′). These PCR products were annealed and then amplified using primers J10-RV1-Bgl2 and 3′-GFP+Sal1. The PCR product was digested with BglII and SalI and cloned at BglII and XhoI sites of the RV construct. The construction was confirmed by DNA sequencing. Construction of N-terminal Myc3-mBTLA-RV was described previously [5].
Cells
Mouse DO11.10 T cell hybridomas (DO11.10 T cells) [26] and A20 cells were cultured in RPMI 1640 (Sigma Chemical Co., St. Louis, MO, USA), supplemented with FBS (10%), penicillin (100 U/ml), streptomycin (100 mg/ml), and 2-ME (2 μM, Sigma Chemical Co.). To prepare a Th1 cell line that lacks BTLA, BTLA−/− mice [5] were crossed with DO11.10 TCR-transgenic mice (DO11.10 mice) [27], and then splenocytes from DO11.10 BTLA−/− mice were stimulated with antigenic peptide OVA323–339 (0.3 μM) in the presence of IL-12 (10 U/ml, PeproTech, Rocky Hill, NJ, USA) and anti-IL-4 mAb (20 μg/ml, 11B11, BD PharMingen, San Diego, CA, USA). After 7 days of culture, lymphocytes were harvested by lympholyte M (Cedarlane, Ontario, Canada). DO11.10 BTLA−/− Th1 cells were stimulated with OVA323–339 peptide presented by irradiated BALB/c splenocytes in the presence of IL-12 and anti-IL-4 mAb every 2 weeks. DO11.10 T cells and DO11.10 BTLA−/− Th1 cells were infected with RV of BTLA-GFP RV or GFP RV and used for confocal microscopic analysis. DO11.10 T cells infected with Myc3-mBTLA-RV [5] were used for the flow cytometric and biochemical analysis. CHO cells expressing I-Ad or I-Ad and HVEM, a ligand for BTLA [7], were maintained in DMEM (Sigma Chemical Co.), supplemented with FBS (10%), penicillin, streptomycin, and 2-ME.
Antibodies
Antibodies and staining reagents used in this study included the following. Anti-calnexin polyclonal antibody was purchased from Stressgen Biotechnologies (Victoria, Canada). Anti-Golgi 58K protein mAb (clone 58K-9) was purchased from Sigma Chemical Co. Cy3-conjugated goat anti-mouse IgG, Cy3-conjugated goat anti-rabbit IgG, and Cy3-conjugated streptoavidin were from Jackson ImmunoResearch (West Grove, PA, USA). Mouse anti-Myc mAb (9E10), rabbit polyclonal anti-Myc antibody (A14), and biotin-conjugated anti-Myc mAb (9E10) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse IgG1 (15H6) was from Southern Biotechnology Associates (Birmingham, AL, USA). Anti-CD4 (L3T4) mAb, biotin-conjugated anti-CD11a (LFA-1) antibody, biotin-conjugated mouse IgG1 (isotype control), and allophycocyanin-conjugated streptavidin were from BD PharMingen. Anti-BTLA mAb (6F7) was purchased from eBioscience (San Diego, CA, USA). Alexa Flour 647-conjugated KJ1-26 mAb was from Caltag Laboratories (Burlingame, CA, USA). Anti-LAT antibody was from Upstate Biotechnology (Lake Placid, NY, USA). HRP-conjugated goat anti-rabbit IgG antibody was from Amersham Biosciences (Uppsala, Sweden).
Quantification of surface expression of BTLA by flow cytometry
DO11.10 T cells were infected with Myc3-mBTLA-RV. DO11.10 T cells expressing Myc-BTLA were stimulated with PMA (50 ng/ml, Sigma Chemical Co.) and calcium ionophore A23187 (1 μM, Sigma Chemical Co.) at 37ºC for indicated time periods. Where indicated, cells were preincubated with CHX (10 μg/ml, Sigma Chemical Co.) for 1 h to prevent new protein synthesis. Cells were stained with anti-Myc antibody for 45 min, followed by staining with a secondary antibody for 45 min, and then analyzed by FACSCalibur (Becton Dickinson, San Jose, CA, USA) using the CellQuest Pro software.
Immunostaining and confocal laser-scanning microscopy
Cells were cultured on poly-L-lysine-coated 12 mm round coverslips in 24-well plates, fixed with 4% paraformaldehyde in PBS for 15 min at room temperature, and permeabilized with 0.1% Triton-X 100 in PBS for 10 min. Cells were incubated with anti-BTLA mAb (6F7) for 1 h at room temperature and visualized with a secondary antibody. For ER and Golgi apparatus staining, cells were incubated with anti-calnexin polyclonal antibody and anti-Golgi 58K protein mAb, respectively, for 1 h at room temperature and visualized with secondary antibodies. For lysosome staining, cells were stained with LysoTracker Red DND-99 (Molecular Probes, Eugene, OR, USA), according to the manufacturer’s instruction. Coverslips were washed and mounted onto slides with SlowFade antifade kit (Molecular Probes). Images were obtained using a multi-track mode of a LSM5 Pascal confocal laser-scanning microscope (Carl Zeiss, Jena, Germany).
Conjugate formation assay
DO11.10 T cells and DO11.10 BTLA−/− Th1 cells were infected with BTLA-GFP RV, and cells that express GFP were isolated by FACSAria (Becton Dickinson). DO11.10 T cells expressing BTLA-GFP or DO11.10 BTLA−/− Th1 cells expressing BTLA-GFP were mixed with OVA323–339-pulsed A20 cells, centrifuged at 500 g for 1 min, and incubated for 15, 30, or 60 min at 37ºC. Cells were adhered to poly-L-lysine-coated 12 mm round coverslips in 24-well plates on ice and fixed with 500 μl 4% paraformaldehyde for 15 min at room temperature.
Isolation of lipid rafts by sucrose density gradient centrifugation
HVEM (–) CHO cells and HVEM (+) CHO cells [7] were cultured in a six-well plate and pulsed with OVA323–339 (5 μM) for 60 min. DO11.10 T cells expressing Myc-BTLA were added to the wells coated with the HVEM (–) or HVEM (+) CHO cells, centrifuged for 5 min at 500 g, and incubated for 30 min at 37°C. DO11.10 T cells were harvested, washed once in cold PBS, and lysed in 0.5 ml ice-cold MES-buffered saline [25 mM MES, 150 mM NaCl, 1% Triton X-100, 5 mM Na3VO4, 5 mM EDTA, 1 mM PMSF, and protease inhibitor cocktail (Sigma Chemical Co.)]. Sucrose density gradient centrifugation was performed as described previously [28].
Endo H sensitivity assay
Lysates from DO11.10 T cells expressing Myc-BTLA were immunoprecipitated with anti-Myc mAb (9E10). An aliquot of immumoprecipitated Myc-BTLA was treated with or without Endo H (New England Biolabs, Ipswich, MA, USA), according to the manufacturer’s instructions at 37°C for 60 min and then subjected to immunoblotting with rabbit anti-Myc antibody (A-14). As controls, 10 μg RNase B (New England Biolabs), a well-known endoglycosidase substrate, was treated with or without Endo H and was subjected to SDS-PAGE.
Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting were performed as described previously [5]. The intensity of the blot was measured by using NIH ImageJ 1.41. Data are shown as the percentages of the signal intensity of the raft fractions.
Statistical analysis
Data are summarized as mean ± sd. The statistical analysis of the results was performed by the unpaired t-test. P values <0.05 were considered significant.
RESULTS
BTLA is localized mainly in the cytoplasm in resting T cells
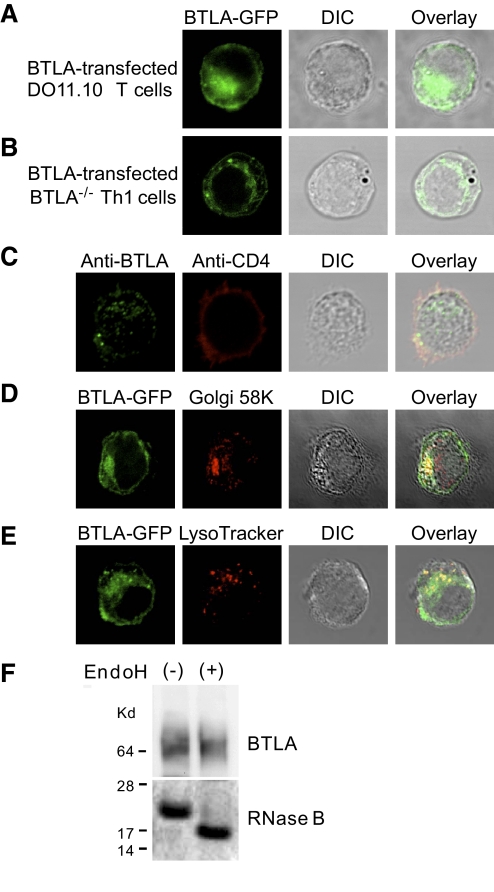
We first investigated the subcellular localization of BTLA in T cells. DO11.10 T cell hybridomas (DO11.10 T cells) and Th1 cells derived from DO11.10 TCR-transgenic BTLA−/−mice (DO11.10 BTLA−/− Th1 cells) were infected with the BTLA-GFP RV, and then the subcellular localization of BTLA-GFP was examined by confocal microscopy. Although BTLA was thought to be a cell-surface receptor, BTLA-GFP was localized mainly in the perinuclear region and vesicles in DO11.10 T cells (Fig. 1A) as well as in DO11.10 BTLA−/− Th1 cells (Fig. 1B). To examine the subcellular localization of endogenously expressed BTLA, we stained DO11.10 Th1 cells with anti-mouse BTLA antibody (6F7). As shown in Figure 1C, endogenous BTLA was also localized in the perinuclear region and vesicles.
Figure 1.
BTLA is localized in the Golgi apparatus and lysosomes in T cells. (A and B) BTLA is localized mainly in the cytoplasm. DO11.10 T cells (A) and Th1 cells derived from DO11.10 TCR-transgenic BTLA−/− mice (DO11.10 BTLA−/− Th1 cells; B) were expressed with BTLA-GFP fusion protein using RV, and the distribution of BTLA-GFP fusion protein was analyzed by confocal microscopy. DIC images were taken simultaneously and merged with the color fields. Shown are representative images from five independent experiments. (C) Endogenous BTLA is localized mainly in the cytoplasm. Th1 cells derived from DO11.10 TCR-transgenic mice were fixed, permeabilized, stained with anti-mouse BTLA mAb (6F7) and anti-CD4 mAb, and analyzed by confocal microscopy. Shown are representative images from five independent experiments. (D and E) BTLA is localized in the Golgi apparatus and lysosomes. DO11.10 T cells expressing BTLA-GFP fusion protein were stained with anti-Golgi 58K protein mAb (D) or LysoTracker (E) and analyzed by confocal microscopy. Yellow indicates the overlay of red and green signals. Shown are representative images from five independent experiments. (F) BTLA molecules in CD4+ T cells are resistant to Endo H. Lysates from DO11.10 T cells expressing Myc-BTLA were immunoprecipitated with anti-Myc mAb (9E10). An aliquot of immunoprecipitated Myc-BTLA was treated with or without Endo H at 37°C for 60 min and subjected to immunoblotting. As controls, RNase B was treated with or without Endo H and subjected to SDS-PAGE.
BTLA is localized in the Golgi apparatus and lysosomes in resting T cells
To identify the compartments where BTLA localizes in resting T cells, we stained BTLA-GFP-expressing DO11.10 T cells with markers for cytoplasmic organelles. As the perinuclear accumulation of BTLA-GFP suggests that BTLA is localized in the ER or Golgi apparatus, we stained the BTLA-GFP-expressing DO11.10 T cells with anti-calnexin antibody as a marker for ER and anti-Golgi 58K protein mAb as a marker for the Golgi apparatus. A fraction of BTLA-GFP was localized in the ER, presumably as a newly synthesized protein (data not shown), but the majority of BTLA-GFP in the perinuclear region was overlapped with the staining of anti-Golgi 58K protein mAb (Fig. 1D). These results suggest that a large portion of the intracellular BTLA localizes in the Golgi apparatus.
As the cytoplasmic region of BTLA contains two tyrosine-containing sequences YXXΦ (an amino acid with a bulky hydrophobic side-chain such as leucine, isoleucine, phenylalanine, methionine, or valine) [5, 24] that can be recognized by clathrin-associated adaptor complexes [29, 30], we speculated that BTLA was transported to the endosomal and lysosomal compartments. We then stained BTLA-GFP-expressing DO11.10 T cells with LysoTracker that accumulates in the lysosomal compartment [31]. As shown in Figure 1E, vesicular BTLA-GFP colocalized with LysoTracker staining.
To further address the compartments where BTLA localizes in resting T cells by a biochemical approach, we examined the sensitivity of BTLA molecules to Endo H, an enzyme that cleaves immature glycoproteins not subjected to post-translational modification in the Golgi apparatus. As shown in Figure 1F, most of BTLA molecules in DO11.10 T cells were resistant to Endo H treatment, whereas RNase B, a well-known Endo H substrate, was cleaved by Endo H treatment. These data suggest that BTLA is localized mainly in the Golgi apparatus and lysosomes, and then the surface expression of BTLA is maintained at low levels in a steady state.
BTLA is translocated to the cell surface upon PMA and calcium ionophore stimulation
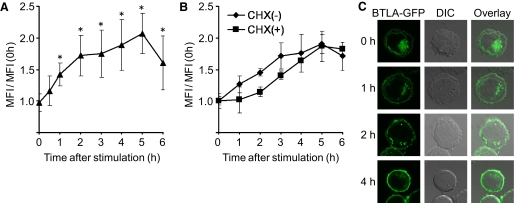
To determine whether BTLA is translocated to the cell surface upon activation, we investigated the expression levels of surface BTLA on activated T cells by flow cytometry. We stimulated DO11.10 T cells expressing Myc-BTLA with PMA and calcium ionophore and examined the levels of surface BTLA using anti-Myc antibody. As shown in Figure 2A, the expression levels of surface BTLA started to increase after stimulation and kept increasing until 5 h after stimulation. The increase of surface BTLA expression was still observed in Myc-BTLA-expressing DO11.10 T cells that were pretreated with CHX (Fig. 2B), suggesting that prestored BTLA but not newly synthesized BTLA is involved in the increase of surface BTLA expression. In addition, the increase of surface BTLA expression was prevented partly by monensin (data not shown), an inhibitor of protein transport through the Golgi apparatus, suggesting that the translocation from the Golgi apparatus participates in the activation-induced increase of surface BTLA expression. We also found that BTLA-GFP in the perinuclear region was decreased gradually, but BTLA-GFP on the cell surface was increased by PMA and calcium ionophore stimulation in DO11.10 T cells (Fig. 2C). Moreover, the number of the intracellular vesicles containing BTLA-GFP was decreased at 4 h after stimulation (Fig. 2C). Taken together, these results suggest that prestored BTLA is translocated from the Golgi apparatus and other intracellular compartments such as secretory lysosomes to the cell surface upon activation.
Figure 2.
BTLA is translocated to the cell surface upon activation. (A) Up-regulation of cell-surface BTLA by PMA and calcium ionophore stimulation. DO11.10 T cells expressing BTLA were stimulated with PMA (50 ng/ml) and calcium ionophore (1 μM) for the indicated time periods and analyzed by FACS using anti-Myc mAb. Data were shown as mean ± sd of the ratio of MFI of anti-Myc staining of stimulated DO11.10 T cells to that of prestimulated DO11.10 T cells [MFI/MFI (0 h)]; n = 5; *, P < 0.05. (B) Up-regulation of cell-surface BTLA by PMA and calcium ionophore stimulation is independent of new protein synthesis. DO11.10 T cells expressing Myc-BTLA were pretreated with CHX (10 μg/ml) for 1 h, stimulated with PMA and calcium ionophore for the indicated time periods, and analyzed by FACS. Data were mean ± sd of MFI/MFI (0 h); n = 5. (C) Translocation of BTLA to the cell surface by PMA and calcium ionophore stimulation. DO11.10 T cells expressing BTLA-GFP were stimulated with PMA and calcium ionophore for the indicated time periods and analyzed by confocal microscopy. Shown are representative images from five independent experiments.
BTLA accumulates at the immunological synapse upon TCR stimulation by APCs
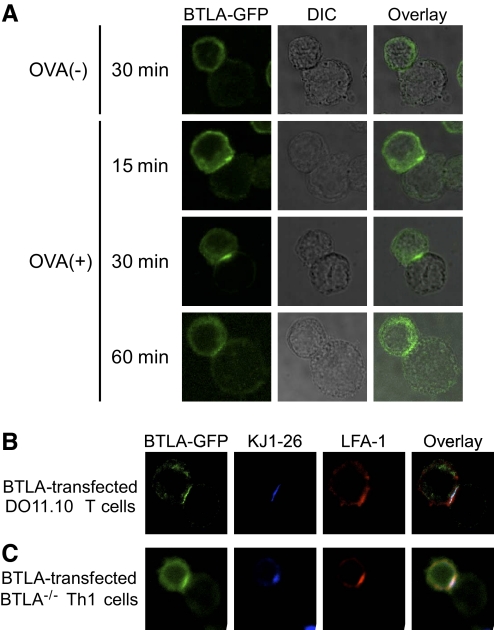
We next analyzed the distribution of BTLA-GFP on the cell surface of T cells upon TCR stimulation. DO11.10 T cells expressing BTLA-GFP were cocultured with OVA323–339-pulsed APCs, and the distribution of BTLA-GFP was determined by confocal microscopy. As we found that A20 cells express HVEM, a ligand for BTLA [7, 8], by RT-PCR (data not shown), we used A20 cells as I-Ad (+) HVEM (+) APCs in the experiments. As shown in Figure 3A, BTLA-GFP was accumulated at the interface between DO11.10 T cells and OVA323–339-pulsed A20 cells at 30 min after incubation, and the accumulation was sustained at least until 60 min. Without OVA323–339 peptide, BTLA-GFP was not accumulated at the interface between DO11.10 T cells and A20 cells (Fig. 3A), suggesting that the signaling via TCR is required for the accumulation of BTLA at the interface to APCs. Interestingly, the accumulation of BTLA on the cell surface of DO11.10 T cells is much faster with the stimulation with antigenic peptide presented by APCs (Fig. 3A) than with the stimulation with PMA and calcium ionophore (Fig. 2C), suggesting that additional signals provided by APCs may promote the rapid accumulation of BTLA on the cell surface of DO11.10 T cells. As expected, TCR (KJ1-26) as well as LFA-1 were accumulated at the interface between DO11.10 T cells and OVA323–339-pulsed A20 cells (Fig. 3B), indicating that a typical immunological synapse is formed at the interface. The colocalization of BTLA with TCR and LFA-1 at the interface was also observed when DO11.10 BTLA−/− Th1 cells expressing BTLA-GFP were stimulated with OVA323–339-pulsed A20 cells (Fig. 3C). These results indicate that BTLA accumulates at the immunological synapse upon TCR stimulation by antigen-pulsed HVEM (+) APCs.
Figure 3.
BTLA accumulates at the interface between T cells and APCs. (A) DO11.10 T cells expressing BTLA-GFP (cell in upper left of each panel) were incubated with OVA323–339-pulsed [OVA (+)] or unpulsed [OVA (–)] A20 cells (cell in lower right of each panel) for the indicated time period. A20 cells were identified based on the lack of BTLA-GFP. DIC images were taken simultaneously and merged with the color fields. Shown are representative images from five independent experiments. (B and C) Similar to A, DO11.10 T cells expressing BTLA-GFP (B) or DO11.10 BTLA−/− Th1 cells expressing BTLA-GFP (C) were incubated with OVA323–339-pulsed A20 cells for 30 min. Cells were stained with anti-TCR antibody (KJ1-26) and anti-LFA-1 antibody and analyzed by confocal microscopy. Shown are representative images from five independent experiments.
BTLA is enriched in lipid rafts by HVEM stimulation
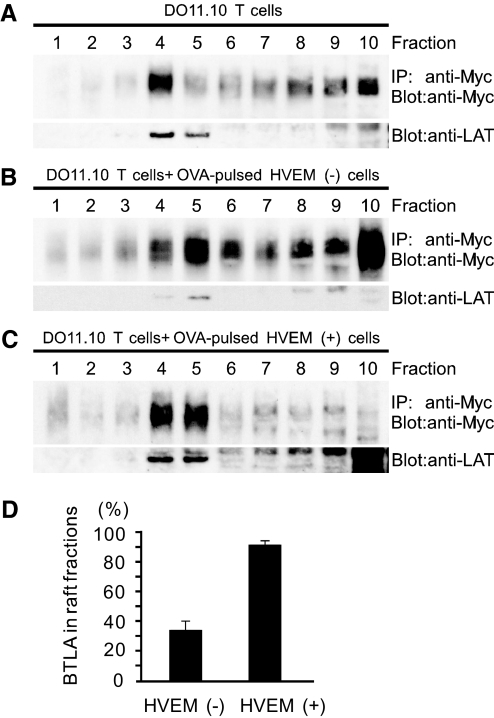
As BTLA accumulates at the immunological synapse upon TCR stimulation (Fig. 3), we next investigated whether BTLA is enriched in lipid rafts. We examined first whether BTLA is localized in lipid rafts in resting DO11.10 T cells. Cell lysates of DO11.10 T cells expressing Myc-BTLA were subjected to sucrose density gradient fractionation, and BTLA in each fraction was immunoprecipitated with anti-Myc mAb and then detected by immunoblotting with polyclonal anti-Myc antibody. Each fraction was also subjected to immunoblotting with anti-LAT antibody to identify the raft fractions [28]. As shown in Figure 4A, a considerable amount of BTLA was detected in raft fractions and non-raft fractions in resting DO11.10 T cells. Densitometric analysis showed that ∼30% of BTLA existed in the raft fractions (Fractions 4 and 5, Fig. 4A).
Figure 4.
BTLA associates with lipid rafts by HVEM stimulation. (A) BTLA is localized in raft and non-raft fractions in resting T cells. DO11.10 T cells expressing Myc-BTLA were lysed and fractionated by sucrose density gradient centrifugation. Each fraction was immunoprecipitated (IP) with anti-Myc mAb (9E10) and immunoblotted with anti-Myc antibody (A14). Each fraction was also blotted with anti-LAT antibody to identify raft fractions. (B–D) The ligation of BTLA with HVEM induces the accumulation of BTLA in lipid rafts. DO11.10 T cells expressing Myc-BTLA were stimulated with OVA323–339-pulsed I-Ad (+) HVEM (–) CHO cells (B) or I-Ad (+) HVEM (+) CHO cells (C) for 30 min and then were harvested and analyzed as described in A. Shown are representative blots (B and C) and mean ± sd of the percent density of BTLA in raft fractions (Fractions 4 and 5) by densitometric analysis (D); n = 5.
We finally examined whether T cell activation induces the accumulation of BTLA in the raft fractions and whether the ligation of BTLA with its ligand HVEM is required for the accumulation of BTLA in the raft fractions. To achieve this purpose, we used HVEM-expressing or nonexpressing OVA323–339-pulsed I-Ad (+) CHO cells as APCs. When DO11.10 T cells expressing Myc-BTLA were stimulated with OVA323–339-pulsed HVEM (–) CHO cells, the distribution of BTLA in each fraction was not affected significantly (Fig. 4B). In contrast, when these cells were stimulated with OVA323–339-pulsed HVEM (+) CHO cells for 30 min, most of BTLA was accumulated in the raft fractions (Fig. 4C). Densitometric analysis showed that ∼90% of BTLA was accumulated in the raft fractions after stimulation with OVA323–339-pulsed HVEM (+) CHO cells, whereas only 30% of BTLA was distributed in raft fractions after stimulation with OVA323–339-pulsed HVEM (–) CHO cells (Fig. 4D). These results suggest that the ligation of TCR with APCs is not sufficient, and the ligation of BTLA with HVEM is required to enhance accumulation of BTLA in lipid rafts.
DISCUSSION
In this study, we show that the surface expression of BTLA and its accumulation at the immunological synapse are tightly regulated by TCR and HVEM stimulation to provide its inhibitory function in the regulation of T cell activation. We found that BTLA was localized mainly in the Golgi apparatus and secretory lysosomes in resting T cells (Fig. 1). We also found that intracellular BTLA was translocated to the cell surface and accumulated at the immunological synapse upon stimulation by antigen-loaded APCs (Figs. 2 and 3). Furthermore, we found that the BTLA-HVEM interaction was required for the accumulation of BTLA in lipid rafts (Fig. 4). As co-cross-linking of TCR and BTLA has been shown to result in the association of BTLA with SHP-1 and SHP-2 [5, 22, 24], our results indicate that BTLA accumulates at the immunological synapse upon TCR and HVEM stimulation to deliver an efficient inhibitory co-signal in the proximity of TCR.
We show that BTLA is expressed weakly on the cell surface but stored abundantly in the cytoplasm, mainly in the Golgi apparatus and lysosomes, in resting CD4+ T cells (Fig. 1, C–E). In addition to analyses by confocal microscope, the Endo H sensitivity assay indicates that most of BTLA molecules have already been subjected to post-translational modifications in the Golgi apparatus (Fig. 1F). This intracellular localization of BTLA may be explained by AP complex-mediated trafficking, as there are two potential AP-binding consensus sequences—YASL and YASI—in the cytoplasmic region of BTLA [5]. AP complexes selectively recognize and bind directly to the YXXΦ motif in the proteins to form clathrin-coated vesicles and convey to their destination [30, 32, 33]. For example, it has been demonstrated that CTLA-4 expressed on the cell surface interacts with the AP-2 complex by its YVKM sequence in the cytoplasmic region, resulting in rapid internalization under resting conditions [14, 15, 34,35,36]. AP-1 also associates with intracellular CTLA-4, which is found primarily in the Golgi apparatus [11, 12, 37], and mediates shuttling between the Golgi apparatus and lysosomes [12]. Therefore, AP-1 and/or AP-2 complexes may associate with YASL or YASI sequence of BTLA and then may regulate the intracellular localization and surface expression of BTLA.
We found that BTLA also accumulated in lysosomes in CD4+ T cells. Recently, it has been reported that lysosomes can function as conveyers to transport proteins to the cell surface [38]. Most of hematopoietic cells such as lymphocytes, mast cells, eosinophils, and basophils have secretory lysosomes that contain not only secretory proteins, such as perforin, granzyme A, and histamine, but also transmembrane proteins [39], and the cells secrete these molecules to carry out their specific effector functions. Secretory lysosomes in T cells are known to contain CTLA-4 [14] and Fas ligand [40] and regulate the surface expression of these molecules. As the increase of surface BTLA and the disappearance of vesicles containing BTLA occur simultaneously (Fig. 2), it is suggested that surface BTLA is up-regulated by exocytosis of secretory lysosomes upon activation.
We show that BTLA accumulates at the immunological synapse concurrently with its recruitment to lipid rafts by TCR and HVEM stimulation (Figs. 3 and 4). An immunological synapse is formed at the T cell-APC interface by the recognition of peptide-MHC complexes by TCR [41,42,43,44]. In a mature immunological synapse, which is formed 5–30 min after an initial cell contact, key ligands and receptors, such as the peptide-MHC-TCR-CD3 complex and CD80/CD86-CD28/CTLA-4, concentrate to the central supramolecular activation cluster. Our findings of the kinetics of BTLA accumulation to the T cell-APC interface are consistent with the kinetics of the formation of a mature immunological synapse. Thus, as a result of the recruitment to the immunological synapse, BTLA can effectively transduce inhibitory signals to T cells in the proximity of TCR.
It is known that lipid rafts containing various signaling molecules, such as adaptors or kinases, accumulate in the immunological synapse by TCR signaling [44,45,46,47]. As ligation of BTLA by anti-BTLA antibody does not induce phosphorylation of BTLA effectively, but co-cross-linking of TCR and BTLA induces its phosphorylation [5, 24], we speculate that BTLA accumulation at the immunological synapse, where lipid rafts are aggregated, can induce phosphorylation of BTLA by kinases that are activated by TCR stimulation, resulting in efficient attenuation of TCR signals.
We also show that TCR signaling and the ligation of BTLA with HVEM are required for the recruitment of BTLA to lipid rafts (Fig. 4C). We found that the antigen-pulsed APCs that lacked HVEM failed to induce the association of BTLA to lipid rafts (Fig. 4B). In contrast, we found that the antigen-pulsed APCs that expressed HVEM induced the association of BTLA to lipid rafts (Fig. 4, C and D). Similarly, it has been shown that CTLA-4 is recruited to the T cell-APC interface by binding with CD80/CD86 [19, 20]. Therefore, it is suggested that the ligation of TCR is not sufficient for the association of BTLA to lipid rafts but that the binding of BTLA with HVEM and/or signaling through BTLA are required for the recruitment of BTLA to lipid rafts.
In conclusion, we have shown that the surface expression of BTLA and its accumulation at the immunological synapse are tightly regulated by TCR and HVEM stimulation. Our data also give new insight to the molecular behavior of a new coreceptor BTLA in T cells and provide a clue to the understanding of the function of BTLA.
ACKNOWLEDGMENTS
This work was supported in part by Grants-in-Aids for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, the Japanese Government, and Global COE Program (Global Center for Education and Research in Immune System Regulation and Treatment), MEXT, Japan. We thank Dr. T. Yokosuka for technical assistance and Mr. T. Ito for cell sorting.
DISCLOSURE
The authors have no financial conflict of interest.
Footnotes
Abbreviations: AP=adaptor protein, BTLA=B and T lymphocyte attenuator, BTLA−/−=BTLA-deficient, BTLA-GFP RV=retrovirus-mediated expression vector for BTLA-GFP fusion protein, CHO=Chinese hamster ovary, CHX=cycloheximide, DIC=differential interference contrast, Endo H=endoglycosidase H, ER=endoplasmic reticulum, HVEM=herpes virus entry mediator, HVEM (–)=CHO cells expressing I-Ad, HVEM (+)=CHO cells expressing I-Ad and HVEM, LAT=linker for activation of T cells, MES=2-(N-morpholino)ethanesulfonic acid, MFI=mean fluorescence intensity, Myc-BTLA=Myc-tagged BTLA, Myc3=mBTLA-RV=Myc-tagged murine BTLA, PD-1=programmed death-1, RV=retrovirus, SHP-1=Src homology-2-containing tyrosine phosphatase 1
References
- Greenwald R J, Freeman G J, Sharpe A H. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Scalapino K J, Daikh D I. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol Rev. 2008;223:143–155. doi: 10.1111/j.1600-065X.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- Fife B T, Bluestone J A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- Keir M E, Butte M J, Freeman G J, Sharpe A H. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Gavrieli M, Sedy J R, Yang J, Fallarino F, Loftin S K, Hurchla M A, Zimmerman N, Sim J, Zang X, Murphy T L, Russell J H, Allison J P, Murphy K M. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- Watts T H. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Sedy J R, Gavrieli M, Potter K G, Hurchla M A, Lindsley R C, Hildner K, Scheu S, Pfeffer K, Ware C F, Murphy T L, Murphy K M. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- Gonzalez L C, Loyet K M, Calemine-Fenaux J, Chauhan V, Wranik B, Ouyang W, Eaton D L. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci USA. 2005;102:1116–1121. doi: 10.1073/pnas.0409071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K M, Nelson C A, Sedy J R. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol. 2006;6:671–681. doi: 10.1038/nri1917. [DOI] [PubMed] [Google Scholar]
- Alegre M L, Noel P J, Eisfelder B J, Chuang E, Clark M R, Reiner S L, Thompson C B. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762–4770. [PubMed] [Google Scholar]
- Leung H T, Bradshaw J, Cleaveland J S, Linsley P S. Cytotoxic T lymphocyte-associated molecule-4, a high-avidity receptor for CD80 and CD86, contains an intracellular localization motif in its cytoplasmic tail. J Biol Chem. 1995;270:25107–25114. doi: 10.1074/jbc.270.42.25107. [DOI] [PubMed] [Google Scholar]
- Schneider H, Martin M, Agarraberes F A, Yin L, Rapoport I, Kirchhausen T, Rudd C E. Cytolytic T lymphocyte-associated antigen-4 and the TCR ζ/CD3 complex, but not CD28, interact with clathrin adaptor complexes AP-1 and AP-2. J Immunol. 1999;163:1868–1879. [PubMed] [Google Scholar]
- Linsley P S, Bradshaw J, Greene J, Peach R, Bennett K L, Mittler R S. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- Iida T, Ohno H, Nakaseko C, Sakuma M, Takeda-Ezaki M, Arase H, Kominami E, Fujisawa T, Saito T. Regulation of cell surface expression of CTLA-4 by secretion of CTLA-4-containing lysosomes upon activation of CD4+ T cells. J Immunol. 2000;165:5062–5068. doi: 10.4049/jimmunol.165.9.5062. [DOI] [PubMed] [Google Scholar]
- Shiratori T, Miyatake S, Ohno H, Nakaseko C, Isono K, Bonifacino J S, Saito T. Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2. Immunity. 1997;6:583–589. doi: 10.1016/s1074-7613(00)80346-5. [DOI] [PubMed] [Google Scholar]
- Martin M, Schneider H, Azouz A, Rudd C E. Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T cell function. J Exp Med. 2001;194:1675–1681. doi: 10.1084/jem.194.11.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington P J, Baroja M L, Chau T A, Siu E, Ling V, Carreno B M, Madrenas J. Surface cytotoxic T lymphocyte-associated antigen 4 partitions within lipid rafts and relocates to the immunological synapse under conditions of inhibition of T cell activation. J Exp Med. 2002;195:1337–1347. doi: 10.1084/jem.20011868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikuma S, Imboden J B, Bluestone J A. Negative regulation of T cell receptor-lipid raft interaction by cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2003;197:129–135. doi: 10.1084/jem.20021646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egen J G, Allison J P. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- Pentcheva-Hoang T, Egen J G, Wojnoonski K, Allison J P. B7–1 and B7–2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Hurchla M A, Sedy J R, Gavrieli M, Drake C G, Murphy T L, Murphy K M. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly induced in anergic CD4+ T cells. J Immunol. 2005;174:3377–3385. doi: 10.4049/jimmunol.174.6.3377. [DOI] [PubMed] [Google Scholar]
- Han P, Goularte O D, Rufner K, Wilkinson B, Kaye J. An inhibitory Ig superfamily protein expressed by lymphocytes and APCs is also an early marker of thymocyte positive selection. J Immunol. 2004;172:5931–5939. doi: 10.4049/jimmunol.172.10.5931. [DOI] [PubMed] [Google Scholar]
- Nurieva R I, Chung Y, Hwang D, Yang X O, Kang H S, Ma L, Wang Y H, Watowich S S, Jetten A M, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli M, Watanabe N, Loftin S K, Murphy T L, Murphy K M. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of B and T lymphocyte attenuator required for association with protein tyrosine phosphatases SHP-1 and SHP-2. Biochem Biophys Res Commun. 2003;312:1236–1243. doi: 10.1016/j.bbrc.2003.11.070. [DOI] [PubMed] [Google Scholar]
- Afkarian M, Sedy J R, Yang J, Jacobson N G, Cereb N, Yang S Y, Murphy T L, Murphy K M. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- Haskins K, Kubo R, White J, Pigeon M, Kappler J, Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J Exp Med. 1983;157:1149–1169. doi: 10.1084/jem.157.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K M, Heimberger A B, Loh D Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- Zhang W, Trible R P, Samelson L E. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier M C, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino J S. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Bonifacino J S, Traub L M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J S, Dell'Angelica E C. Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol. 1999;145:923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu F, Ohno H. Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct Funct. 2003;28:419–429. doi: 10.1247/csf.28.419. [DOI] [PubMed] [Google Scholar]
- Bradshaw J D, Lu P, Leytze G, Rodgers J, Schieven G L, Bennett K L, Linsley P S, Kurtz S E. Interaction of the cytoplasmic tail of CTLA-4 (CD152) with a clathrin-associated protein is negatively regulated by tyrosine phosphorylation. Biochemistry. 1997;36:15975–15982. doi: 10.1021/bi971762i. [DOI] [PubMed] [Google Scholar]
- Chuang E, Alegre M L, Duckett C S, Noel P J, Vander Heiden M G, Thompson C B. Interaction of CTLA-4 with the clathrin-associated protein AP50 results in ligand-independent endocytosis that limits cell surface expression. J Immunol. 1997;159:144–151. [PubMed] [Google Scholar]
- Zhang Y, Allison J P. Interaction of CTLA-4 with AP50, a clathrin-coated pit adaptor protein. Proc Natl Acad Sci USA. 1997;94:9273–9278. doi: 10.1073/pnas.94.17.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead K I, Zheng Y, Manzotti C N, Perry L C, Liu M K, Burke F, Powner D J, Wakelam M J, Sansom D M. Exocytosis of CTLA-4 is dependent on phospholipase D and ADP ribosylation factor-1 and stimulated during activation of regulatory T cells. J Immunol. 2005;174:4803–4811. doi: 10.4049/jimmunol.174.8.4803. [DOI] [PubMed] [Google Scholar]
- Blott E J, Griffiths G M. Secretory lysosomes. Nat Rev Mol Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- Stinchcombe J C, Griffiths G M. Regulated secretion from hemopoietic cells. J Cell Biol. 1999;147:1–6. doi: 10.1083/jcb.147.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi G, Griffiths G M. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med. 1999;5:90–96. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]
- Monks C R, Freiberg B A, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Cemerski S, Shaw A. Immune synapses in T-cell activation. Curr Opin Immunol. 2006;18:298–304. doi: 10.1016/j.coi.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Saito T, Yokosuka T. Immunological synapse and microclusters: the site for recognition and activation of T cells. Curr Opin Immunol. 2006;18:305–313. doi: 10.1016/j.coi.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Taner S B, Onfelt B, Pirinen N J, McCann F E, Magee A I, Davis D M. Control of immune responses by trafficking cell surface proteins, vesicles and lipid rafts to and from the immunological synapse. Traffic. 2004;5:651–661. doi: 10.1111/j.1600-0854.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- Janes P W, Ley S C, Magee A I. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montixi C, Langlet C, Bernard A M, Thimonier J, Dubois C, Wurbel M A, Chauvin J P, Pierres M, He H T. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 1998;17:5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]