Abstract
Mechanisms behind carcinogenesis and resistance of tumor cells to treatment regimes remain elusive. The major stress proteins Hsp72, Hsp90, and Hsp27 are credible candidates to provide this resistance, as their overexpression in many cancer types is well documented. In addition to being present inside tumor cells, where they confer resistance to apoptosis, Hsp72, in particular, is presented externally, embedded in the cell membrane of cancer cells. This study aimed to investigate the localization of Hsp72, Hsp90, and Hsp27 in leukocytes from patients with CLL and age-matched control subjects. CLL patients were found to express significantly higher levels of iHsp90 (CLL=2463 MFI; control=748 MFI) and iHsp27 (CLL=2190 MFI; control=1031 MFI) in lymphocytes than that expressed by lymphocytes from control subjects. Furthermore, expression of iHsp90 was shown to be related to stage of disease, and expression of iHsp27 correlated with levels of active caspase-3. Patients were found to express very high levels or very low levels of sHsp72 and iHsp72 in CD5+/CD19+ cells, although surface and intracellular datasets did not correlate. Levels of extracellular Hsp72 circulating in the serum were found to correlate with internal levels of Hsp72 and were also found to be significantly lower in patients receiving corticosteroid treatment than in patients not receiving corticosteroid treatment. Finally, analysis of the number of circulating Tregs revealed significantly elevated numbers in CLL patients compared with control subjects.
Keywords: Hsp72, Hsp90, Hsp27, CLL, Tregs
Introduction
CLL is the most common hematological malignancy in adults [1]. Approximately 50% of patients are asymptomatic at time of diagnosis, but the condition may progress to a symptomatic form, characterized by accumulation of CD5+ B lymphocytes within the blood, bone marrow, and secondary lymphoid organs [2]. Median age at diagnosis is ∼70 years, and so, CLL can be considered to be an age-related disease. The multistep progression to carcinogenesis may take several decades, and CLL is likely to become more frequent as a result of an increasingly aging population. The difficulties involved in treating these patients and the frequent occurrence of drug resistance warrant investigation into the possible mechanisms involved in CLL progression.
Hsps are overexpressed in many types of cancer, and as a result, several studies have investigated their prognostic and therapeutic potential [3,4,5]. Hsp72 (HSPA1A) increases have been observed in breast cancer [6], colorectal cancer [7, 8], kidney cancer [9], and leukemia [10, 11]. Elevated Hsp72 has also been associated with resistance to cancer therapy and/or poor prognosis for the patient [11, 12]. Mechanistic investigations have demonstrated that Hsp72 is involved in carcinogenesis at different levels, from enhancement of the activity of many oncogenes and inhibition of tumor suppressor genes such as p53 [13] to promoting the survival of tumor cells through inhibition of apoptosis [14, 15]. Conversely, cell membrane-embedded (sHsp72) has been shown to act as a target for activated NK cells [16], suggesting that its cellular location influences tumor cell survival. The stimulation of NK cells using a peptide region of the Hsp72 protein, termed the TKD peptide, and low dose IL-2 results in up-regulation of CD94/CD56 on NK cells and initiates NK cell killing of sHsp72+ tumor cells [17,18,19]. Similar stimulation of NK cells was also observed following incubation with sHsp72+ tumor cell-derived exosomes [20]. The initiation of NK cell responses toward sHsp72+ tumor cells has been used to explain observed differences in prognoses between different cancer types. Expression of sHsp72 in gastric and colon cancers, which metastasize via the liver, was associated with an increased overall survival. In contrast, sHsp72+ in lower rectal and squamous cell carcinomas correlates with poor prognosis, as these do not metastasize via the liver and are therefore not subject to hepatic-NK cell responses [21]. Hsp72 has also been shown to be coexpressed with PS on the surface of hypoxic tumor cell lines [22]. Furthermore, exogenously added Hsp72 was found to bind to PS on the surface of these cells and was shown to enhance the response to radiation. These results indicate that the radiotherapy resistance observed in many hypoxic tumors may be overcome by prior treatment with Hsp72.
Hsp90 is also elevated in several types of cancer, including human breast cancer [23], melanoma metastases [24], and in Reed-Sternberg cells of Hodgkin’s disease [25]. Hsp90 is present in tumor cells within multi-chaperone complexes with proteins such as p23, mutant p53, and Hop, and these Hsp90 complexes predominate over the free form of Hsp90 found in normal cells [26]. Hsp90 has also been described as an extracellular protein [24, 27, 28], where it is essential for tumor invasiveness through its interaction with matrix metalloprotein 2 [28]. Pharmacological targeting of Hsp90 using analogs of geldanamycin has resulted in the suppression of tumor cell growth and apoptosis [29, 30]. Hsp27 has also been found to be up-regulated in various types of cancer, including those of breast, liver, prostate, and larynx [12, 31,32,33]. Hsp27 promotes tumor survival through antiapoptotic and proangiogenesis activities [34,35,36]. Although higher levels of extracellular Hsp27 in serum have been observed in cancer patients compared with healthy controls [37, 38], the significance of this is still unknown. Inhibition of Hsp72, Hsp90, or Hsp27 proves lethal to tumor cells, in contrast to normal cells [14, 29, 39]. All three proteins, therefore, have therapeutic potential, but the importance of their cellular location has not been fully investigated.
In addition to the abnormal expression of Hsps, elevated levels of naturally occurring immunosuppressive Tregs with a CD4+/CD25+/FoxP3+ phenotype, could facilitate evasion of immune responses by cancer cells. Indeed, an increase in the numbers of Tregs has been demonstrated in several cancer cell types [40, 41] and has been associated directly with risk of relapse [42] and reduced survival [43]. Furthermore, a study by Beyer et al. [44] found increased numbers of circulating Tregs in CLL and discovered a correlation between Treg frequency and stage of disease. Interestingly, Tregs have been found to be stimulated by Hsp60 [45], as demonstrated by the inhibition of IFN-γ and TNF-α cytokine secretion and proliferation in target CD4+/CD25− and CD8+ T cells. Furthermore, Hsp60-stimulated Tregs were shown to stimulate IL-10 secretion from CD4+/CD25− cells. Although a stimulatory effect on Treg proliferation by other Hsps has not been demonstrated, the results observed by Zanin-Zhorov et al. [45] suggest that an association may exist between the expression of Hsps and the presence of Tregs in cancer patients.
Therefore, this study aimed to explore the distribution of Hsp72, Hsp90, and Hsp27 in CLL to assess whether manipulation of these Hsps would be beneficial.
MATERIALS AND METHODS
Collection and processing of blood
Local research ethics committee approval (Center of Research Ethical Campaign–05/QI506/103) was obtained for this study, and each patient completed consent forms. Blood samples were collected from patients affected by CLL (n=40) and normal, age-matched control subjects (n=18). Characteristics of the patients involved in this study can be seen in Table 1. Blood was obtained from CLL patients at various stages of disease. Some patients donated blood before receiving treatment, and blood was obtained from other patients following various treatment regimes, including chlorambucil, methylprednisolone, a combination of fludarabine and cyclophosphamide, or a combination of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. When considering patients with stable disease (not requiring treatment) or progressive disease (requiring treatment), all patients receiving different treatment regimes were considered as a group.
TABLE 1.
Characteristics of the CLL and Control Subjects Involved in This Study
| CLL patients | Control patients | |
|---|---|---|
| Number of patients included | 40 | 18 |
| Sex (male/female) | 28/12 | 10/8 |
| Mean age of patients (Range) | 66 (45–80) | 66 (42–88) |
| White blood cell count at time of sample (×109/l) | 66.99 (1.2–326.5) | 6.87 (3.7–10.3) |
| Hemoglobin at time of sample | 12.62 (8–15.3) | 13.1 (10–14.4) |
| Platelet count at time of sample | 183.03 (33–381) | 257.16 (128–402) |
Data are presented as mean (range).
Total leukocyte isolation
Blood was collected by venepuncture into 7 ml EDTA tubes (BD Biosciences, San Jose, CA, USA; 367873). The whole blood was then washed with 2 vol DPBS (Lonza, Switzerland; BE17-513F) and centrifuged at 500 g for 5 min at RT. The supernatant was discarded, and the RBCs were lysed using lysing buffer (BD Biosciences; 349202). The lysed whole blood was then centrifuged at 500 g for 5 min at RT and the supernatant discarded. The cell pellet was washed with DPBS and the cells counted using Trypan blue exclusion on a hemocytometer. Experiments were only performed when the percentage of dead cells was <1%. Concentration of viable cells was adjusted to 1 × 106 cells/ml, and the cells were centrifuged at 500 g for 5 min at RT. Supernatant was discarded, and cells were washed with DPBS. The remaining cell pellet was kept on ice until analysis by flow cytometry.
PBMC purification
Blood was collected by venepuncture into 7 ml EDTA tubes. The blood was then added to an equal volume of DPBS before addition of an equal volume of Histopaque® (Sigma Chemical Co., St. Louis, MO, USA; 10771) to the bottom of the tube. This was then centrifuged at 400 g for 30 min at RT. The plasma was discarded and the buffy coat added to 10 ml DPBS. After centrifugation at 250 g for 10 min, the supernatant was discarded, and the cell pellet was washed twice in 5 ml DPBS. The cell pellet was resuspended in 1 ml DPBS, and the cells were counted using Trypan blue exclusion on a hemocytometer. The cells were then analyzed for the presence of Tregs or were centrifuged at 200 g for 3 min before being frozen at −80°C pending Western blot analysis.
Flow cytometry analysis of sHsps in conjunction with specific CD markers
For CLL patients, 20 μl of the two surface CD markers—anti-human CD5-APC (BD Biosciences; 555355) and anti-human CD19-PE (BD Biosciences; 555413)—was added to the cell pellet to distinguish the malignant B cells from the normal B lymphocyte populations, having, respectively, a CD5+/CD19+ and CD5−/CD19+ profile. The surface labeling was completed by adding, simultaneously, an anti-Hsp72-FITC (Multimmune Ltd., Germany; cmHsp70.1), which recognizes membrane-embedded Hsp72 (sHsp72). In the first part of the study, the detection of Hsp72, using the cmHsp70.1 antibody, was compared with a mouse monoclonal anti-Hsp72-FITC (Assay Designs, Ann Arbor, MI, USA; SPA-810, clone number C92F3A-5) in a number of control subjects. In these samples, cells were stained with these antibodies in isolation. For sHsp90 and sHsp27, cells were single-stained with a mouse monoclonal anti-Hsp90-PE (Assay Designs; SPA-830PEE, clone number AC88) or an anti-human Hsp27-FITC (Assay Designs; SPA-800FI, clone number G3.1). The antibodies were diluted 1:5 (for cmHsp70.1) and 1:50 (for Assay Designs antibodies) in binding buffer (5% FBS in DPBS), and the cells were incubated for 30 min at 4°C in the dark. Any unbound antibodies were then washed by centrifugation at 500 g for 5 min at RT, supernatant removed, and the cell pellet resuspended and analyzed immediately by flow cytometry (FACScanto, Becton Dickinson, San Diego, CA, USA). sHsp72 analysis, using the cmHsp70.1 antibody, was carried out on a total of 17 CLL patients and seven control subjects.
iHsp72, iHsp90, iHsp27 detection and active caspase-3 analysis
The cell pellet from lysed whole blood was first labeled with surface markers as described before and then fixed and permeabilized with Fix/Perm solution (BD Biosciences; 554722) for 20 min at 4°C in the dark. The cells were then washed with binding buffer, centrifuged at 500 g for 5 min at RT, and the supernatant removed. The cell pellet was then incubated with a FITC-conjugated mouse monoclonal anti-Hsp72 (Assay Designs; SPA-810), a PE-conjugated mouse monoclonal anti-Hsp90 (Assay Designs; 830-PEE), a FITC-conjugated mouse monoclonal anti-Hsp27 (Assay Designs; SPA-800FI), or a FITC-conjugated rabbit monoclonal anti-active caspase-3 antibody (BD Biosciences; 559341, clone number C92-605). Each antibody was diluted in binding buffer and incubated with the cells for 60 min at 4°C in the dark. The cell pellet was then washed with binding buffer by centrifugation at 500 g for 5 min at RT, the supernatant was removed, and the cells were then analyzed by flow cytometry.
Isotype controls were performed using isotypic mAb [Dako, Denmark; mouse IgG1/FITC (X0927) and mouse IgG1/PE (X0928)] as additional controls in all experiments to evaluate the background fluorescence. Cell populations were gated to discard debris, and 10,000 events were recorded. Analysis of iHsp72 was carried out on a total of 40 CLL patients and 18 control subjects, iHsp90 analysis was carried out on 21 CLL patients and eight control patients, and iHsp27 analysis was carried out on a total of 24 CLL patients and 7 control subjects. Caspase-3 analysis was carried out on 40 CLL patients and 11 control subjects.
Treg detection
Tregs were analyzed in PBMCs. This allowed clearer identification of Treg populations that only make up a small percentage of total lymphocytes. Following PBMC purification, the concentration of cells was adjusted to 1 × 106cells/well. Cells were washed with 250 μl/well binding buffer and then double-stained with 20 μl anti-human CD25-PE (BD Biosciences; 555432) and 4 μl anti-human CD4-PE-Cy7 (BD Biosciences; 557852). Cells were incubated for 30 min at 4°C in the dark. Cells were washed again with binding buffer before being fixed and permeabilized with Fix/Perm solution for 20 min at 4°C in the dark. After a further wash with binding buffer, the cells were stained with 20 μl anti-human FoxP3-Alexa Fluor 488 (BD Biosciences; 560047) and incubated for 1 h in the dark at 4°C. Finally, the cells were washed again in binding buffer before being resuspended in 100 μl/well DPBS and analyzed. Tregs were analyzed in 22 CLL patients and 11 control subjects.
Western blot analysis
Western blots were performed on cell extracts from PBMCs, purified from whole blood using methods described previously [46]. Hsp72 was detected using a biotinylated anti-Hsp72 (Assay Designs; SPA-810BF) at 1:2000, followed by the addition of 1:2000 dilution of Extravidin® HRP (Sigma Chemical Co.; E2886), washed with TBS-Tween 20, and then incubated for 5 min in chemiluminescent substrate (SuperSignal® West Pico, Pierce Biotechnology Inc., Rockford, IL, USA). Chemiluminescence was detected using the ChemiDoc XRS detection system (Bio-Rad, Hercules, CA, USA).
Hsp72 ELISA
Hsp72 ELISAs were performed on serum from CLL and control subjects.
ELISA plates were coated with 100 μl/well 2 μg/ml polyclonal affinity-purified sheep anti-Hsp72 (Hsp72-DEG-EI, produced in-house), which was raised against a peptide sequence from Hsp72, HSPA1A. The antibody was diluted in 0.1 M carbonate buffer and incubated overnight at 4°C. The plate was washed three times with 300 μl/well ELISA wash buffer (0.05% Tween 20 in PBS) and blotted dry. The wells were then blocked with 300 μl/well blocking solution (0.5% BSA in PBS) and incubated for 1 h at 25°C. The plate was washed as before, followed by the addition of 100 μl/well standards, ranging from 100 ng/ml to 3.125 ng/ml, diluted in blocking solution, or samples. The plate was incubated for 2 h at 37°C. The plate was washed again as before, and then 100 μl/well mouse monoclonal anti-Hsp72 raised against bovine Hsp72 (a kind gift from Dr. Nygård, Arhus University, Denmark), diluted 1:1000 in antibody diluent (0.5% BSA in wash buffer), was added. The plate was then incubated at 37°C for 1 h. After a further wash, 100 μl/well anti-mouse IgG HRP conjugate (Sigma Chemical Co.; A5278) was added, and the plate was diluted 1:2500 in antibody diluent. The plate was then incubated at 37°C for 1 h. After a final wash, 100 μl/well tetramethylbenzidine substrate (Cheshire Sciences, UK; UP664781) was added, and the plate was incubated at 25°C on a plate shaker for 30 min. Finally, 100 μl/well 1 M orthophosphoric acid was added to stop the reaction, and the plate was read on a Bio-tek Synergy HT plate reader at 450 nm. Extracellular Hsp72 was analyzed in serum from 23 CLL patients and 10 control subjects. Four CLL patients donated blood on six occasions during treatment with methylprednisolone, and the levels of extracellular Hsp72 were analyzed. Data from these repeated samples are represented (see in Fig. 5C only) and are not used in any other analysis.
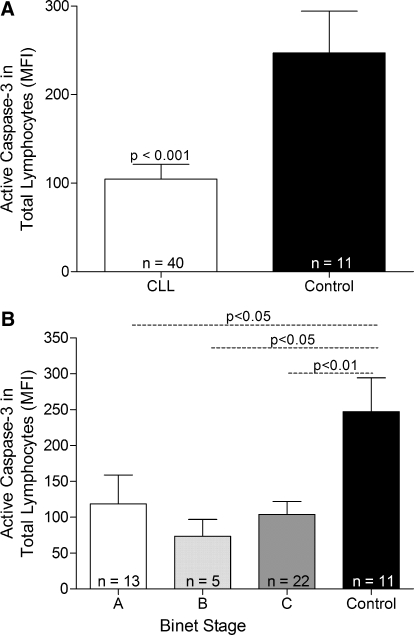
Figure 1.
Active caspase-3 expression in total lymphocytes (CD5+/CD19+ and CD5–/CD19+ cells) from CLL patients and lymphocytes from control subjects. (A) CLL patients were grouped as a whole, and statistical analysis was performed using the unpaired t-test. (B) CLL patients were grouped according to Binet stage, and statistical analysis was performed using the one-way ANOVA with Bonferroni’s multiple comparison test. Each group of patients is represented as mean ± sem.
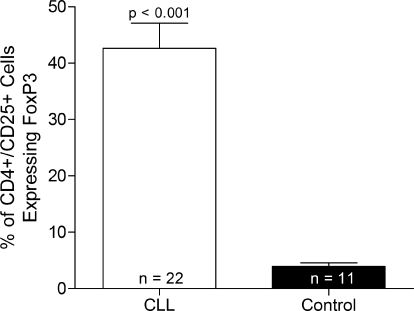
Figure 2.
Numbers of Tregs in PBMCs from CLL and control subjects.Numbers represent the percentage of CD4+/CD25+ cells positive for FoxP3. Data are represented as mean + sem. Statistical analysis was performed using the unpaired t-test.
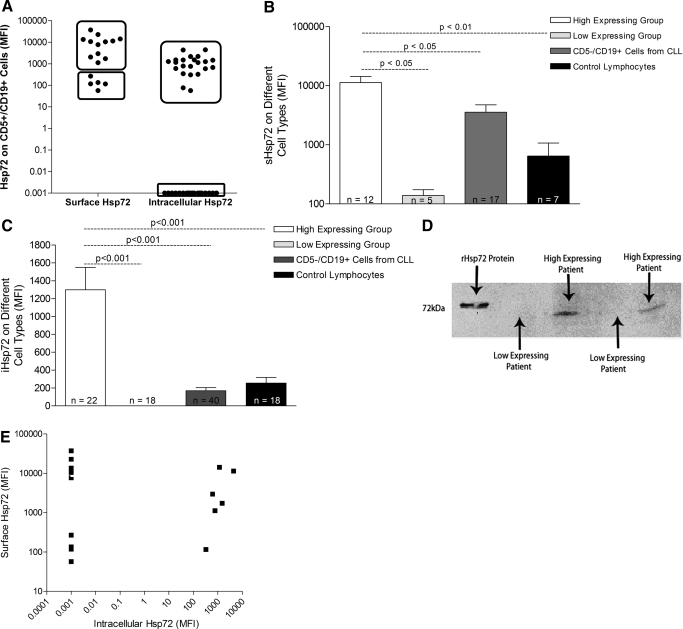
Figure 3.
sHsp72 and iHsp72 expression on malignant and nonmalignant cells from CLL and control subjects. (A) sHsp72 and iHsp72 in CD5+/CD19+ cells from CLL patients were analyzed by flow cytometry. Values are expressed as absolute MFI and plotted on a log scale. (B) sHsp72 expression and (C) iHsp72 expression on the malignant (CD5+/CD19+) and normal B lymphocytes (CD5–/CD19+) from CLL patients and lymphocytes from control subjects. Each group of patients is represented as a mean + sem and plotted on a log scale. Statistical analysis was performed using the one-way ANOVA with Bonferroni’s multiple comparison test. sHsp72 analysis was performed on 17 CLL patients, and iHsp72 analysis was performed in 40 CLL patients. (D) Western blot of Hsp72 expression in high- and low-expressing CLL patients. Cell extracts were prepared from PBMCs purified from whole blood and are representative of 22 high-expressing patients and 18 low-expressing patients. (E) Correlation analysis between iHsp72 and sHsp72 in CD5+/CD19+ cells from CLL patients. Correlation analysis was performed using Spearman’s correlation coefficient, and P = 0.8864.
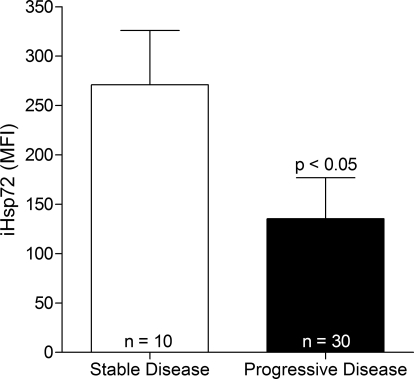
Figure 4.
iHsp72 expression in CD5+/CD19+ cells from patients with stable (not requiring treatment) and progressive (requiring treatment) disease was analyzed by flow cytometry. Each group of patients is represented as mean + sem. Statistical analysis was performed using the unpaired t-test.
Figure 5.
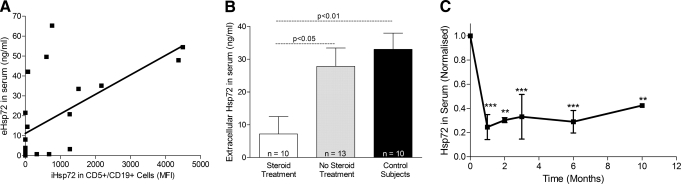
Extracellular Hsp72 in serum. (A) Correlation analysis between iHsp72 expression in CD5+/CD19+ cells and extracellular Hsp72 (eHsp72) in serum from CLL patients. Correlation analysis was performed using Spearman’s correlation coefficient, and P = 0.0009. (B) Levels of extracellular Hsp72 in control subjects and CLL patients, treated or not treated with steroids. Each group of patients is represented as a mean + sem. Statistical analysis was performed using the unpaired t-test. (C) Normalized extracellular Hsp72 in serum from four representative CLL patients undergoing treatment with corticosteroids. Statistical analysis was performed using one-way ANOVA with Bonferroni’s multiple comparison test. ** and ***, P < 0.01 and P < 0.001, respectively.
Interassay variability analysis gave an r value of 0.9949, and intra-assay variability analysis gave an r2 value of 0.9960. The CV values from the intra-assay variability analysis are shown in Table 2.
TABLE 2.
Intra-Assay Variability Data
| Standard sample ng/ml | CV value % |
|---|---|
| 100 | 2.57 |
| 50 | 1.72 |
| 25 | 1.03 |
| 12.5 | 2.61 |
| 6.25 | 1.5 |
| 3.125 | 5.82 |
| 1.5625 | 8.17 |
| 0.78125 | 2.6 |
| 0 | 1.79 |
Binet stage classification
CLL patients were all classified with the standard Binet system, which groups patients according to the number of affected lymphoid tissue groups and degree of anemia and thrombocytopenia.
Statistical analysis
Statistical analysis was performed using the t-test or one-way ANOVA with Bonferroni’s post-hoc test depending on the data analyzed: P values <0.05 were considered to be significant. Correlation analysis was performed using the Spearman’s correlation coefficient: P values <0.05 were considered to be significant.
RESULTS
Active caspase-3 expression in CLL patients
Expression of active caspase-3 in total lymphocytes of CLL patients was compared with the expression in lymphocytes from control subjects to determine if there was any difference in the degree of apoptosis between the two patient groups. The majority of leukocytes in CLL patients were negative for the active form of caspse-3. Moreover, CLL patients had a significantly decreased level of active caspase-3 when compared with control subjects (P<0.001; Fig. 1A). When patients were grouped according to Binet stage, there was no significant difference in caspase-3 levels among the three groups; however, patients in all three Binet stages displayed caspase-3 levels lower than those observed in control subjects (Fig. 1B).
Treg numbers in CLL patients
Numbers of Tregs were analyzed in PBMCs from CLL and control subjects. Cells were labeled with antibodies to CD4 and CD25 to detect a population containing activated T cells and Tregs and then labeled further with an antibody to FoxP3 to detect the number of Tregs within the mixed population. CLL patients were found to possess a significantly higher number of circulating Tregs than control subjects (Fig. 2). No correlation was found between numbers of circulating Tregs and expression of any Hsps (data not shown).
Detection of sHsp72, sHsp90, and sHsp27
Prior to the main study, confirmation of the detection of the membrane-embedded form of Hsp72 was carried out using two antibodies on a number of control subjects. Analysis was performed using the cmHsp70.1 (Multimmune Ltd.) antibody, which recognizes the TKD peptide specifically in the N-terminal region of the Hsp72 protein (epitope located between aa residues 450 and 463). This peptide has been demonstrated to be presented outside of the cell membrane when the protein is embedded and is a target for NK cell anti-tumor responses. Although the epitope for the second antibody (anti-human Hsp72-FITC, Assay Designs; SPA-810) overlaps the TKD region, it contains more aa residues (location between the aa residues 436 and 503), which become hidden in the membrane when the protein is embedded, making binding to embedded Hsp72 impossible. Detection of sHsp72 protein was significantly higher following staining with the cmHsp70.1 antibody than after staining with the SPA-810 antibody (Supplemental Data S1). Subsequent analysis of sHsp72 in CLL and control subjects was performed using the cmHsp70.1 antibody. At no stage was Hsp27 or Hsp90 detectable on the surface of any cells (data not shown).
Hsp72 in CLL patients
A subset of CLL patients and control subjects was analyzed for the expression of sHsp72. CLL patients demonstrated very high or very low expression of sHsp72 on malignant cells CD5+/CD19+ (Supplemental Data S2). These two distinct groups of patients could also be seen when the percentage of sHsp72+ cells was analyzed as an alternative to the intensity of expression (Supplemental Data S3). This expression pattern was also apparent when analyzing iHsp72 in a larger group of CLL patients (Supplemental Data S2). Further analysis of sHsp72 and iHsp72 in CD5+/CD19+ cells revealed that the patients formed two distinct groups, based on the level of sHsp72 and iHsp72 (Fig. 3A). The high sHsp72-expressing group was not only significantly different (100-fold higher) from the low-expressing group (P<0.05) but also from normal CD5−/CD19+ B lymphocytes (P<0.05) from CLL patients and total lymphocytes from control subjects (P<0.01; Fig. 3B). The low-expressing sHsp72 group was not significantly different either from the CD5−/CD19+ lymphocytes or the control lymphocytes. Comparison of iHsp72 expression in CD5+/CD19+ cells with expression in CD5−/CD19+ cells and normal lymphocytes from control subjects presented a similar pattern: The high iHsp72 group was significantly different from the low-expressing CD5+/CD19+ group (P<0.001) and from CD5−/CD19+ lymphocytes (P<0.001) and control lymphocytes (P<0.001; Fig. 3C). The low-expressing iHsp72 group was not significantly different either from the CD5−/CD19+ lymphocytes or the lymphocytes from age-matched control subjects. These two distinct groups of patients could not be separated by other factors such as sex, age, or cytogenetic abnormalities (data not shown). The difference in Hsp72 expression between different patients was confirmed by Western blot analysis of PBMCs from CLL patients belonging to the two different groups (Fig. 3D). Interestingly, there was a lack of correlation between surface and intracellular datasets, and so, patients expressing high sHsp72 did not necessarily express high iHsp72 (Fig. 3E). There was no correlation between sHsp72 or iHsp72 with levels of caspase-3 activity in CD5+/CD19+ cells (data not shown).
The relevance of Hsp72 expression to disease progression was analyzed in two ways. First, patients were divided into two groups based on those with stable disease (not requiring treatment) and those with progressive disease (requiring treatment). For this specific analysis, patients receiving different treatment regimes were grouped together. iHsp72 expression in CD5+/CD19+ was found to be significantly higher in CLL patients, who were considered stable, than those who were considered to have progressive disease (Fig. 4). Second, when CLL patients were grouped according to Binet stage, neither sHsp72 nor iHsp72 appeared to be dependent on stage of disease (Supplemental Data S4). However, patients in Binet stage C did have a greater range of expression of sHsp72 than those patients in Binet stages A and B.
Extracellular Hsp72 was analyzed in serum samples from CLL and control subjects by ELISA. Although a wide range in levels of this protein was observed amongst CLL patients, correlation analysis between levels of internal Hsp72 in CLL cells and levels of extracellular Hsp72 in the same patients revealed a positive relationship (P=0.0011). Patients expressing low levels of iHsp72 were also found to express low levels of extracellular Hsp72 in serum (Fig. 5A). Furthermore, CLL patients receiving corticosteroid treatment were found to be releasing significantly lower levels of Hsp72 than patients not receiving corticosteroid treatment and control subjects (Fig. 5B). Analyzing the release of Hsp72 in individual patients at different time-points revealed that on commencement of corticosteroid treatment, Hsp72 levels in serum decrease dramatically and remain low for the duration of the treatment (Fig. 5C). Patients show ∼70% reduction in Hsp72 release 1 month after initiation of corticosteroid treatment.
iHsp90 and iHsp27 in CLL patients
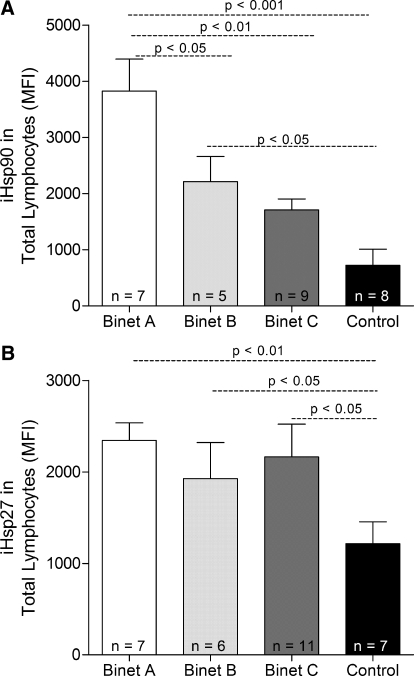
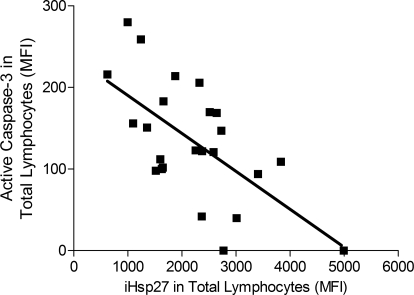
CLL patients, considered as a group, were found to express significantly higher levels of iHsp90 (P<0.01) and iHsp27 (P<0.001) in lymphocytes when compared with that expressed by control subjects (Fig. 6, A and B). Furthermore, iHsp90, but not iHsp27, expression was found to correlate to stage of disease, as patients in Binet stage A appeared to have significantly higher levels of iHsp90 than those in Binet stage B (P<0.05) and Binet stage C (P<0.01). Expression of iHsp27, but not iHsp90, was found to correlate with levels of active caspase-3 in CLL patients (P=0.0009). Patients displaying high levels of iHsp27 were found to express low levels of active caspase-3 (Fig. 7).
Figure 6.
(A) iHsp90 and (B) iHsp27 expression in total lymphocytes from CLL patients grouped according to Binet stage and in lymphocytes from control subjects.Each group of patients is represented as mean + sem. Statistical analysis was performed using one-way ANOVA with Bonferroni’s post-hoc test.
Figure 7.
Correlation analysis between iHsp27 expression and levels of active caspase-3 in total lymphocytes (CD5+/CD19+ and CD5–/CD19+ cells) from CLL patients. Correlation analysis was performed using Spearman’s correlation coefficient, and P = 0.0031.
DISCUSSION
The up-regulated appearance of Hsps in cancer cells is well documented and has been shown, in many cases, to be unfavorable for the patient. iHsp72 is expressed in AML, MDS, and CML patients and is associated with poor prognosis and resistance to treatment [11, 47,48,49]. Expression of Hsps has also been correlated with French-American-British subtype, Bcl-2-related proteins, CD34 expression, and bone marrow blast percentage in MDS patients [47]. iHsp90 and iHsp27 have been shown to be up-regulated in a number of cancer types in comparison with normal tissues and have been associated with poor prognoses and resistance to therapy [12, 23, 24, 31, 33]. However, much of the literature about Hsp expression in cancer focuses on the intracellular levels of these proteins. Hsp72 is present embedded in the surface of numerous cancer cell types [21] and has been shown to act as a recognition structure for activated NK cells [16,17,18]. Furthermore, the transfection of mouse mastocytoma cells with membrane-bound Hsp72 results in tumor regression mediated by NK cells and T cells [50], and the induced release of Hsp72 from murine tumor cells also results in tumor rejection [51]. These results have led to studies into the development of immunotherapies, in which the immunostimulatory properties of sHsp72 are exploited [52, 53]. However, to date, the small number of studies investigating the presence of sHsp72 in primary cells from leukemic patients suggests that its presence may in fact be detrimental for the patient. Although work by Gehrmann et al. [16] provides evidence that sHsp72 acts as a recognition structure for NK cells, this study also demonstrates that the presence of sHsp72 can be correlated with unfavorable or intermediate cytogenetics. AML patients with treatment-refractory AML were also shown to have higher sHsp72 levels than patients in complete remission, suggesting that presentation of this protein on the membrane is associated with a poor prognosis. Work by Steiner et al. [48] also found that AML patients in complete remission displayed lower levels of sHsp72 than patients with active disease. Furthermore, this study also showed that patients who went into remission but continued to express moderately high levels of sHsp72 had a shorter relapse-free survival time than remission patients with lower levels of sHsp72. It was proposed that this may be a result of sHsp72+ blasts being more resistant to apoptosis. However, no significant differences in sHsp72 expression could be found between patients responding to chemotherapy and patients not responding to chemotherapy. Similarly to the work presented here on CLL, Steiner et al. [48] showed that the expression of sHsp72 could not be correlated with stage of disease in AML. The lack of association among the presence of sHsp72, stage of disease, and resistance to chemotherapy suggests that surface localization of this protein cannot be investigated in isolation. Several studies have investigated iHsps or sHsps in cancer; at present, no single study has explored the roles of sHsps, iHsps, and extracellular Hsps in CLL and their associations with disease stage and levels of apoptosis.
Our data, on first consideration, are consistent with the hypothesis that elevated iHsp leads to tumor cells being resistant to apoptosis [11, 12, 14, 15]. Levels of iHsp27 and iHsp90 were higher in CLL patients, and the marker of apoptosis, activated caspase-3, was lower compared with age-matched control subjects. Furthermore, levels of iHsp27 and caspase-3 negatively correlated. The difference in caspase-3 levels between CLL and control subjects is to be expected, as the underlying basis of CLL is an inability for B lymphocytes to commit to apoptosis. There was, however, no difference in active caspase-3 between patients at different stages of the disease, which demonstrated that the progression of the disease is more likely to be a result of an increased cellular, clonal replication rather than an increased resistance to apoptosis from the same cells. However, this analysis of the data may be over-simplistic, as it ignores the presence of sHsp72.
sHsp72 and iHsp72 were found to be expressed at very high or very low levels in CD5+/CD19+ cells. The iHsp72 data showed a 1000-fold difference in expression between the two patient groups, although the difference in expression of sHsp72 was less pronounced. This variation between patients was demonstrated by flow cytometry and Western blot. Despite this huge variation in expression, neither sHsp72 nor iHsp72 could be correlated with stage of disease or numbers of circulating Tregs. Nevertheless, patients expressing high levels of sHsp72 or iHsp72 were found to be significantly different, not only from patients expressing low levels of Hsp72 but also from levels expressed by nonmalignant lymphocytes from CLL patients and lymphocytes from control subjects. Expression of iHsp72 was shown to be higher in stable patients compared with patients with progressive disease. The presence of high levels of iHsp72 in these less-severe patients could be interpreted as a stress response during the early stages of CLL, which then allows cancer cells to survive attempts by the immune system to restore normality. As the disease progresses, cells replicate uncontrollably, and iHsp72 levels return to control values. Therefore, the decrease of iHsp72 with the severity of the disease may be indicative of progression to a more aggressive phase and be a useful marker, complementing Binet staging.
When levels of extracellular Hsp72 were analyzed in serum from CLL patients and control subjects, there was considerable variation between patients. Grouping CLL patients as a whole and comparing with control subjects showed no significant difference in extracellular Hsp72. However, further examination of the results revealed a correlation between the levels of extracellular and iHsp72 in CLL patients. Furthermore, patients receiving corticosteroid treatment displayed significantly lower levels of Hsp72 in serum when compared with patients not receiving corticosteroid treatment. Indeed, levels of Hsp72 in serum from a number of steroid-treated patients were only just detectable by the ELISA, suggesting that steroid treatment may be totally inhibiting Hsp72 secretion. Examination of extracellular Hsp72 in individual CLL patients over time revealed a dramatic decrease in released Hsp72 after initiation of steroid treatment, and serum Hsp72 levels remained low throughout the treatment period. The source of this extracellular Hsp72 in CLL patients and control subjects was not identified in this study. The mechanism of action of corticosteroids on the inhibition of Hsp72 release has not been determined. The mode of action of corticosteroids is multifarious and includes decreased lymphocyte proliferation, decreased T cell activation, impaired NK cell function, and decreased IL-2 production. At high doses, as in those used in various combination regimes, corticosteroids result in steroid-induced apoptosis, the mechanism of which is still poorly understood. Interestingly, corticosteroids regulate transcription factors, and the fact that extracellular Hsp72 levels are lower in steroid-treated patients, and extracellular Hsp72 levels correlate with iHsp72 levels in the same patients indicate that transcription of Hsp72 has been inhibited.
Although the Hsp27, Hsp72, and Hsp90 proteins have all been identified as having antiapoptotic properties in tumor cells [11, 12, 14, 15], no studies have investigated the relative expression of each in tumor cells. Hsp90 is essential for the stabilization, activation, and consequent function of a vast number of client proteins, including many oncoproteins required for tumor cell survival [24, 26]. As a result, several Hsp90 inhibitors, such as geldanamycin and 17-AAG, have been used with some success [29, 30]. However, these compounds are toxic to cells, and they also activate heat shock factor-1, which is usually bound to Hsp90. The result is an increase in Hsp27 and Hsp72 with consequent resistance to apoptosis [54]. Therefore, it is important to establish if there is a concomitant Hsp72 and Hsp90 overexpression to determine if inhibition of both proteins is required to induce tumor-specific apoptosis. Recent work has highlighted the effectiveness of this strategy in a CML cell line, where Hsp72 is overexpressed. The combination of the Hsp72 inhibitor resveratrol and Hsp90 inhibitor 17-AAG increased the number of apoptotic tumor cells [55].
Resistance to corticosteroid therapy is displayed frequently by CLL patients and is attributed to the imbalanced expression of GR isoforms. Indeed, higher expression of the transcriptionally inactive GR-β, in relation to the hormone-activated transcription factor GR-α, has been observed in CLL cells [56]. However, another mechanism of glucocorticoid resistance may be defective ligand binding as a result of variations in the concentration of Hsp90 [57]. In a study about steroid resistance, it was shown that the ratio of Hsp90 to GR expression was significantly higher in steroid-resistant compared with steroid-sensitive patients with multiple sclerosis [58]. This suggests that elevated Hsp90 expression in the GR complex results in a reduced sensitivity to the steroid, and the possibility that CLL patients in Binet stage A, who have very high levels of Hsp90, would have reduced sensitivity to steroids.
In addition to the aberrant levels of Hsps observed in CLL patients, numbers of Tregs were also found to be significantly higher in these patients when compared with control. This supports the work of Beyer et al. [44], who not only showed elevated levels of Tregs in CLL but also found a relationship between Treg numbers and stage of disease. However, it is unclear whether this is a cause or effect: Does the observation of high numbers of Tregs in later stage disease indicate a commandeering of these naturally occurring immunosupressors by the tumor, or does the presence of Tregs at an earlier stage of disease allow the tumor to evade immune responses and progress further? No association between numbers of circulating Tregs and any of the Hsps analyzed was determined.
In summary, Hsp72 was shown to be localized inside and on the surface of leukocytes from CLL and control subjects, and expression of Hsp90 and Hsp27 was shown to be restricted to the inside of these leukocytes. Levels of iHsp90 and iHsp27 were significantly higher in CLL patients when compared with controls, and iHsp72 was found to be significantly higher in patients with stable disease. Furthermore, release of Hsp72 into serum was shown to be inhibited by corticosteroids, as up to 70% reduction in release was seen in patients on commencement of treatment. However, the fact that no correlations existed between sHsp72 and iHsp72 or Hsps, and the numbers of Tregs and the fact that iHsp27 but not iHsp90 or Hsp72 correlated with levels of caspase-3 indicate that these Hsps have a dynamic relationship during the development of CLL, and the consequences for prognosis and treatment require further investigation.
AUTHORSHIP
C. H. and J. H. H. W. were responsible for conception of the study and all authors for experimental design. C. H. was responsible for sample collection and N. C. D., H. E. I., and F. L. for sample processing and analysis. N. C. D. and F. L. wrote the first draft of the paper, and all authors were involved in subsequent editing.
Supplementary Material
Footnotes
Abbreviations: 17-AAG=17-N-allylamino-17-demethoxygeldanamycin, AML=acute myelogenous leukemia, CLL=chronic lymphocytic leukemia, CML=chronic myelogenous leukemia CV=coefficient of variation, DPBS=Dulbecco’s PBS, FoxP3=forkhead box P3, GR=glucocorticoid receptor, Hsp=heat shock protein, iHsp=intracellular Hsp, MDS= myelodysplasia, MFI=mean fluorescence intensity, PS=phosphatidylserine, RT=room temperature, sHsp=surface-embedded Hsp, TKD peptide= TKDNNLLGRFELSG, Treg=regulatory T cell
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
References
- Yee K W L, O'Brien S M. Chronic lymphocytic leukemia: diagnosis and treatment. Mayo Clin Proc. 2006;81:1105–1129. doi: 10.4065/81.8.1105. [DOI] [PubMed] [Google Scholar]
- Falt S, Merup M, Gahton G, Lambert B, Wenboorg A. Identification of progression markers in B-CLL by gene expression profiling. Exp Hematol. 2005;33:883–893. doi: 10.1016/j.exphem.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Ciocca D R, Calderwood S K. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S K, Khaleque M A, Sawyer D B, Ciocca D R. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Sherman M, Multhoff G. Heat shock proteins in cancer. Ann N Y Acad Sci. 2007;1113:192–201. doi: 10.1196/annals.1391.030. [DOI] [PubMed] [Google Scholar]
- Tauchi K, Tsutsumi Y, Hori S, Yoshimura S, Osamura R Y, Watanabe K. Expression of heat shock protein 70 and c-myc protein in human breast cancer: an immunohistochemical study. Jpn J Clin Oncol. 1991;21:256–263. [PubMed] [Google Scholar]
- Shotar A M. p53 and heat shock protein 70 expressions in colorectal adenocarcinoma. Saudi Med J. 2005;26:1602–1606. [PubMed] [Google Scholar]
- Milicevic Z T, Petkovic M Z, Drndarevic N C, Pavlovic M D, Todorovic V N. Expression of heat shock protein 70 (HSP70) in patients with colorectal adenocarcinoma—immunohistochemistry and Western blot analysis. Neoplasma. 2007;54:37–45. [PubMed] [Google Scholar]
- Ramp U, Mahotka C, Heikaus S, Shibata T, Grimm M O, Willers R, Gabbert H E. Expression of heat shock protein 70 in renal cell carcinoma and its relation to tumor progression and prognosis. Histol Histopathol. 2007;22:1099–1107. doi: 10.14670/HH-22.1099. [DOI] [PubMed] [Google Scholar]
- Chant I D, Rose P E, Morris A G. Analysis of heat-shock protein expression in myeloid-leukemia cells by flow-cytometry. Br J Haematol. 1995;90:163–168. doi: 10.1111/j.1365-2141.1995.tb03395.x. [DOI] [PubMed] [Google Scholar]
- Thomas X, Campos L, Mounier C, Cornillon J, Flandrin P, Le Q H, Piselli S, Guyotat D. Expression of heat-shock proteins is associated with major adverse prognostic factors in acute myeloid leukemia. Leuk Res. 2005;29:1049–1058. doi: 10.1016/j.leukres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Vargas-Roig L M, Gago F E, Tello O, Anzar J C, Ciocca D R. Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer. 1998;79:468–475. doi: 10.1002/(sici)1097-0215(19981023)79:5<468::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Yaglom J A, Gabai V L, Sherman M Y. High levels of heat shock protein Hsp72 in cancer cells suppress default senescence pathways. Cancer Res. 2007;67:2373–2381. doi: 10.1158/0008-5472.CAN-06-3796. [DOI] [PubMed] [Google Scholar]
- Nylandsted J, Brand K, Jaattela M. Heat shock protein 70 is required for the survival of cancer cells. Ann N Y Acad Sci. 2000;926:122–125. doi: 10.1111/j.1749-6632.2000.tb05605.x. [DOI] [PubMed] [Google Scholar]
- Khaleque M A, Bharti A, Sawyer D, Gong J, Benjamin I J, Stevenson M A, Calderwood S K. Induction of heat shock proteins by heregulin β1 leads to protection from apoptosis and anchorage-independent growth. Oncogene. 2005;24:6564–6573. doi: 10.1038/sj.onc.1208798. [DOI] [PubMed] [Google Scholar]
- Gehrmann M, Schmetzer H, Eissner G, Haferlach T, Hiddemann W, Multhoff G. Membrane-bound heat shock protein 70 in acute myeloid leukemia: a tumor-specific recognition structure for the cytolytic activity of autologous natural killer cells. Haematologica. 2003;88:474–476. [PubMed] [Google Scholar]
- Multhoff G, Mizzen L, Winchester C C, Milner C M, Wenk S, Eissner G, Kampinga H H, Laumbacher B, Johnson J. Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol. 1999;27:1627–1636. doi: 10.1016/s0301-472x(99)00104-6. [DOI] [PubMed] [Google Scholar]
- Gross C, Schmidt-Wolf IG, Nagaraj S, Gastpar R, Ellwart J, Kunz-Schughart L A, Multhoff G. Heat shock protein 70-reactivity is associated with increased cell surface density of CD94/CD56 on primary natural killer cells. Cell Stress Chaperones. 2003;8:348–360. doi: 10.1379/1466-1268(2003)008<0348:hspria>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Holler E, Stangl S, Dickinson A, Pockley A G, Asea A A, Mallappa N, Multhoff G. An Hsp70 peptide initiates NK cell killing of leukemic blasts after stem cell transplantation. Leuk Res. 2008;32:527–534. doi: 10.1016/j.leukres.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Gastpar R, Gehrmann M, Bausero M A, Asea A, Gross C, Schroeder J A, Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K, Radons J, Busch R, Tidball J, Pfeifer M, Freitag L, Feldman H J, Milani V, Issels R, Multhoff G. Patient survival by Hsp70 membrane-phenotype: association with different routes of metastasis. Cancer. 2007;110:926–935. doi: 10.1002/cncr.22864. [DOI] [PubMed] [Google Scholar]
- Schilling D, Gehrmann M, Steinem C, De Maio A, Pockley A G, Abend M, Molls M, Multhoff G. Binding of heat shock protein 70 to extracellular phosphatidylserine promotes killing of normoxic and hypoxic tumor cells. FASEB J. 2009;23:2467–2477. doi: 10.1096/fj.08-125229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Naito Z, Yokoyama M, Shiraki Y, Ishiwata T, Inokuchi M, Asano G. Expression of hsp90 and cyclin D1 in human breast cancer. Cancer Lett. 1999;137:45–51. doi: 10.1016/s0304-3835(98)00338-3. [DOI] [PubMed] [Google Scholar]
- Becker B, Multhoff G, Farkas B, Wild P J, Landthaler M, Stolz W, Vogt T. Induction of Hsp90 protein expression in malignant melanomas and melanoma metastases. Exp Dermatol. 2004;13:27–32. doi: 10.1111/j.0906-6705.2004.00114.x. [DOI] [PubMed] [Google Scholar]
- Hsu P L, Hsu S M. Abundance of heat shock proteins (hsp89, hsp60, and hsp27) in malignant cells of Hodgkin’s disease. Cancer Res. 1998;58:5507–5513. [PubMed] [Google Scholar]
- Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm M F, Fritz L C, Burrows F J. A high-affinity conformation of Hsp90 confers tumor selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- Ferrarini M, Heltai S, Zocchi M R, Rugarli C. Unusual expression and localization of heat-shock proteins in human tumor-cells. Int J Cancer. 1992;51:613–619. doi: 10.1002/ijc.2910510418. [DOI] [PubMed] [Google Scholar]
- Eustace B K, Sakurai T, Stewart J K, Yimlamai D, Unger C, Zehetmeier C, Lain B, Torella C, Henning S W, Beste G, Scroggins B T, Neckers L, Ilag L L, Jay D G. Functional proteomic screens reveal an essential extracellular role for hsp90 α in cancer cell invasiveness. Nat Cell Biol. 2004;6:507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- Lin K, Rockliffe N, Johnson G G, Sherrington P D, Pettitt A R. Hsp90 inhibition has opposing effects on wild-type and mutant p53 and induces p21 expression and cytotoxicity irrespective of p53/ATM status in chronic lymphocytic leukemia cells. Oncogene. 2007;27:2445–2455. doi: 10.1038/sj.onc.1210893. [DOI] [PubMed] [Google Scholar]
- Pashtan I, Tsutsumi S, Wang S Q, Xu W P, Neckers L. Targeting Hsp90 prevents escape of breast cancer cells from tyrosine kinase inhibition. Cell Cycle. 2008;7:2936–2941. doi: 10.4161/cc.7.18.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornford P A, Dodson A R, Parsons K F, Desmond A D, Woolfenden A, Fordham M, Neoptolemos J P, Ke Y Q, Foster C S. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60:7099–7105. [PubMed] [Google Scholar]
- Lee J H, Sun D, Cho K J, Kim M S, Hong M H, Kim I K, Lee J S, Lee J H. Overexpression of human 27 kDa heat shock protein in laryngeal cancer cells confers chemoresistance associated with cell growth delay. J Cancer Res Clin Oncol. 2007;133:37–46. doi: 10.1007/s00432-006-0143-3. [DOI] [PubMed] [Google Scholar]
- Romani A A, Crafa P, Desenzani S, Graiani G, Lagrasta C, Sianesi M, Soliani P, Borghetti A F. The expression of hsp27 is associated with poor clinical outcome in intrahepatic cholangiocarcinoma. BMC Cancer. 2007;7:232. doi: 10.1186/1471-2407-7-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keezer S M, Ivie S E, Krutzsch H C, Tandle A, Libutti S K, Roberts D D. Angiogenesis inhibitors target the endothelial cell cytoskeleton through altered regulation of heat shock protein 27 and cofilin. Cancer Res. 2003;63:6405–6412. [PubMed] [Google Scholar]
- Schepers H, Geugien M, van der Toorn M, Bryantsev A L, Kampinga H H, Eggen B J L, Vellenga E. Hsp27 protects AML cells against VP-16-induced apoptosis through modulation of p38 and c-Jun. Exp Hematol. 2005;33:660–670. doi: 10.1016/j.exphem.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Wu R, Kausar H, Johnson P, Montoya-Durango D E, Merchant M, Rane M J. Hsp27 regulates Akt activation and polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. J Biol Chem. 2007;282:21598–21608. doi: 10.1074/jbc.M611316200. [DOI] [PubMed] [Google Scholar]
- Fanelli M A, Carrion F D C, Dekker J, Schoemaker J, Ciocca D R. Serological detection of heat shock protein hsp27 in normal and breast cancer patients. Cancer Epidemiol Biomarkers Prev. 1998;7:791–795. [PubMed] [Google Scholar]
- Rui Z, Jian-Guo J, Yuan-Peng T, Hui P, Bing-Gen R. Use of serological proteomic methods to find biomarkers associated with breast cancer. Proteomics. 2003;3:433–439. doi: 10.1002/pmic.200390058. [DOI] [PubMed] [Google Scholar]
- Aloy M T, Hadchity E, Bionda C, Diaz-Latoud C, Claude L, Rousson R, Arrigo A P, Rodriguez-Lafrasse C. Protective role of Hsp27 protein against γ radiation-induced apoptosis and radiosensitization effects of Hsp27 gene silencing in different human tumor cells. Int J Radiat Oncol Biol Phys. 2008;70:543–553. doi: 10.1016/j.ijrobp.2007.08.061. [DOI] [PubMed] [Google Scholar]
- Ohara M, Yamaguchi Y, Matsuura K, Murakami S, Arihiro K, Okada M. Possible involvement of regulatory T cells in tumor onset and progression in primary breast cancer. Cancer Immunol Immunother. 2009;58:441–447. doi: 10.1007/s00262-008-0570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohling S D, Allison K H. Immunosuppressive regulatory T cells are associated with aggressive breast cancer phenotypes: a potential therapeutic target. Mod Pathol. 2008;21:1527–1532. doi: 10.1038/modpathol.2008.160. [DOI] [PubMed] [Google Scholar]
- Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin A C, Durand I, Olive D, Perez S, Pasqual N, Faure C, Coquard I R, Puisieux A, Caux C, Blay J Y, Menetrier-Caux C. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- Curiel T J, Coukos G, Zou L H, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia J R, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis M L, Knutson K L, Chen L P, Zou W P. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, Knolle P A, Thomas R K, von Bergwelt-Baildon M, Debey S, Hallek M, Schultze J L. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen I R. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hunter-Lavin C, Davies E L, Bacelar M M F V G, Marshall M J, Andrew S M, Williams J H H. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- Duval A, Olaru D, Campos L, Flandrin P, Nadal N, Guyotat D. Expression and prognostic significance of heat-shock proteins in myelodysplastic syndromes. Haematologica. 2006;91:713–714. [PubMed] [Google Scholar]
- Steiner K, Graf M, Hecht K, Reif S, Rossbacher L, Pfister K, Kolb H J, Schmetzer H M, Multhoff G. High Hsp70-membrane expression on leukemic cells from patients with acute myeloid leukemia is associated with a worse prognosis. Leukemia. 2006;20:2076–2079. doi: 10.1038/sj.leu.2404391. [DOI] [PubMed] [Google Scholar]
- Pocaly M, Lagarde V, Etienne G, Ribeil J A, Claverol S, Bonneu M, Moreau-Gaudry F, Guyonnet-Duperat V, Hermine O, Melo J V, Dupouy M, Turcq B, Mahon F X, Pasquet J M. Overexpression of the heat-shock protein 70 is associated to imatinib resistance in chronic myeloid leukemia. Leukemia. 2007;21:93–101. doi: 10.1038/sj.leu.2404463. [DOI] [PubMed] [Google Scholar]
- Chen X, Tao Q, Yu H, Zhang L, Cao X. Tumor cell membrane bound Hsp70 elicits antitumor immunity. Immunol Lett. 2002;84:81–87. doi: 10.1016/s0165-2478(02)00042-1. [DOI] [PubMed] [Google Scholar]
- Wang M-H, Grossmann M E, Young C Y F. Forced expression of Hsp70 increases the secretion of Hsp70 and provides protection against tumor growth. Br J Cancer. 2004;90:926–931. doi: 10.1038/sj.bjc.6601583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause S W, Gastpar R, Andreesen R, Gross C, Ullrich H, Thonigs G, Pfister K, Multhoff G. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: a clinical phase I trial. Clin Cancer Res. 2004;10:3699–3707. doi: 10.1158/1078-0432.CCR-03-0683. [DOI] [PubMed] [Google Scholar]
- Gehrmann M, Radons J, Molls M, Multhoff G. The therapeutic implications of clinically applied modifiers of heat shock protein 70 (Hsp70) expression by tumor cells. Cell Stress Chaperones. 2008;13:1–10. doi: 10.1007/s12192-007-0006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravagnan L, Gurbuxani S, Susin S A, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger J M, Garrido C, Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- Chakraborty P K, Mustafi S B, Ganguly S, Chatterjee M, Raha S. Resveratrol induces apoptosis in K562 (chronic myelogenous leukemia) cells by targeting a key survival protein, heat shock protein 70. Cancer Sci. 2008;99:1109–1116. doi: 10.1111/j.1349-7006.2008.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi H, Vottero A, Stratakis C A, Taymans S E, Karl M, Longui C A, Chrousos G P, Daughaday W H, Gregory S A, Plate J M D. Imbalanced expression of the glucocorticoid receptor isoforms in cultured lymphocytes from a patient with systemic glucocorticoid resistance and chronic lymphocytic leukemia. Biochem Biophys Res Commun. 1999;254:559–565. doi: 10.1006/bbrc.1998.9980. [DOI] [PubMed] [Google Scholar]
- Bailey S, Hall A G, Pearson A D J, Redfern C P F. The role of AP-1 in glucocorticoid resistance in leukemia. Leukemia. 2001;15:391–397. doi: 10.1038/sj.leu.2402039. [DOI] [PubMed] [Google Scholar]
- Matysiak M, Makosa B, Walczak A, Selmaj K. Patients with multiple sclerosis resisted to glucocorticoid therapy: abnormal expression of heat-shock protein 90 in glucocorticoid receptor complex. Mult Scler. 2008;14:919–926. doi: 10.1177/1352458508090666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.