Abstract
Cytokine-NAg fusion proteins represent an emerging platform for specific targeting of self-antigen to particular APC subsets as a means to achieve antigen-specific immunological tolerance. This study focused on cytokine-NAg fusion proteins that targeted NAg to myeloid APC. Fusion proteins contained GM-CSF or the soluble extracellular domain of M-CSF as the N-terminal domain and the encephalitogenic 69–87 peptide of MBP as the C-terminal domain. GMCSF-NAg and MCSF-NAg fusion proteins were ∼1000-fold and 32-fold more potent than NAg in stimulating antigenic proliferation of MBP-specific T cells, respectively. The potentiated antigenic responses required cytokine-NAg covalent linkage and receptor-mediated uptake. That is, the respective cytokines did not potentiate antigenic responses when cytokine and NAg were added as separate molecules, and the potentiated responses were inhibited specifically by the respective free cytokine. Cytokine-dependent targeting of NAg was specific for particular subsets of APC. GMCSF-NAg and MCSF-NAg targeted NAg to DC and macrophages; conversely, IL4-NAg and IL2-NAg fusion proteins, respectively, induced an ∼1000-fold enhancement in NAg reactivity in the presence of B cell and T cell APC. GMCSF-NAg significantly attenuated severity of EAE when treatment was completed before encephalitogenic challenge or alternatively, when treatment was initiated after onset of EAE. MCSF-NAg also had significant tolerogenic activity, but GMCSF-NAg was substantially more efficacious as a tolerogen. Covalent GMCSF-NAg linkage was required for prevention and treatment of EAE. In conclusion, GMCSF-NAg was highly effective for targeting NAg to myeloid APC and was a potent, antigen-specific tolerogen in EAE.
Keywords: rodent, T cells, cytokines, tolerogenic vaccine, DC
Introduction
Therapeutic advancements for treatment of multiple sclerosis are often based on nonantigen-specific approaches that may inhibit pathogenic and adaptive immunity [1,2,3]. Therapies, such as those based on IFN-β, mAb, or anti-metabolic/proliferative drugs, have a generalized effect on the immune system. The uncomfortable paradigm is that efficacy of a drug for multiple sclerosis may be commensurate with the severity of side-effects, such that the degree of benefit for multiple sclerosis may be directly due to the ability of the drug to compromise the immune system.
Antigen-specific therapy represents an important alternative [4,5,6]. Antigen-specific approaches are based on the concept of using immunological tolerance to alleviate an autoimmune disease state. Several antigen-specific strategies have been developed by use of EAE as an immune-regulatory model of multiple sclerosis. These therapies include oral tolerance [7, 8], high dose tolerance [9, 10], deletional tolerance [11], or immune deviation [12, 13] and are based on the use of altered peptide ligands [14, 15], antibody-antigen fusion proteins [16, 17], synthetic peptides, recombinant self-antigens [18, 19], and DNA vaccines [20, 21], among others [22, 23]. Disadvantages of antigen-specific approaches include the possibility of anaphylaxis, inadvertent sensitization, and worsening disease [24, 25]. These disadvantages are coupled with current uncertainty of which self-antigens should be targeted to alleviate multiple sclerosis. Nonetheless, antigen-specific therapies offer the best hope of inhibiting pathogenic autoimmune responses without compromising adaptive immunity. Antigen-specific therapies that reliably re-establish or re-enforce tolerance to self-antigens may prove to be a safe, effective means to transition from a strategy of disease management to strategies that have a curative potential.
Cytokine-NAg fusion proteins represent an alternative antigen-specific approach for induction of tolerance [26,27,28,29]. Fusion proteins comprised of IL-2 fused to the encephalitogenic determinant of MBP (IL2-NAg) were effective for treatment and prevention of EAE [28]. Likewise, a fusion protein consisting of the encephalitogenic peptide fused to the cytokine domain of IL-16 (NAg-IL16) was also highly effective for prophylaxis and treatment of EAE [27].
The postulate was that the cytokine domain would target the covalently tethered NAg to a particular APC subset to achieve substantially enhanced presentation of NAg by that APC subset. Indeed, the IL2-NAg fusion protein was 1000-fold more potent as an antigen than NAg in the presence of rat CD25+MHCII+ T cell blasts [28]. For IL2-NAg and NAg-IL16 fusion proteins, the covalent cytokine-NAg link was critical for effectiveness in modulating EAE. An IFNβ-NAg fusion protein was also tolerogenic [26]. Pretreatment with IFNβ-NAg attenuated a subsequent bout of EAE, but in contrast to IL2-NAg and NAg-IL16, the covalent linkage between IFN-β and the NAg was not necessary for prevention of EAE. Rather, separate injections of IFN-β and NAg at adjacent sites were as effective as the IFNβ-NAg fusion protein, whereas administration of IFN-β alone or NAg alone was without effect. Thus, tolerogenic mechanisms associated with cytokine-NAg fusion proteins correlated with a strict cytokine-mediated targeting of particular APC subsets (IL2-NAg) or appeared to involve a more generalized action on APC (IFN-β).
The purpose of the current study was to test cytokine-NAg fusion proteins that targeted NAg to DC and macrophage APC. These APC have been implicated as critical APC for induction and maintenance of self-tolerance [30,31,32,33,34,35,36,37,38,39]. For example, DC have pivotal roles in negative thymic selection, expansion of Treg cells, and elimination of autoimmune effector T cells. The main question was whether the tolerogenic activity of DC and perhaps other myeloid APC could be harnessed by cytokine-NAg fusion proteins to specifically regulate pathogenic T cells in vivo. GM-CSF and M-CSF were chosen for this study because the two cytokines represent the key cytokines driving the differentiation, survival, and growth of myeloid-derived APC [40, 41]. GM-CSF and M-CSF exhibit substantial overlap in biological function, but each also has a unique spectrum of activity. GM-CSF production is largely activation-dependent, and GM-CSF in turn activates key immunogenic and regulatory pathways of the immune system. In contrast, M-CSF is constitutively produced by many cell types, is normally in the circulation, and is necessary for the homeostatic maintenance of nonactivated, quiescent macrophages. This study revealed that GMCSF-NAg and MCSF-NAg fusion proteins targeted NAg for preferential antigen presentation by APC such as DC and macrophages rather than T cell or B cell APC. GMCSF-NAg, and to a lesser extent, MCSF-NAg were also highly effective tolerogens that could prevent or treat severe EAE effectively.
MATERIALS AND METHODS
Structure and purification of recombinant proteins
Two GM-CSF-based fusion proteins (GMCSF-NAg and GM-CSF) were used in this study. The GMCSF-NAg fusion protein contained the mature rat GM-CSF domain as the N terminus and the major encephalitogenic 69–87 determinant of GPMBP (Y-G-S-L-P-Q-K-S-Q-R-S-Q-D-E-N-P-V-V-H; i.e., the NAg) together with six additional H residues as the C terminus. The numbering system for GPMBP was based on Accession Number P25188 (www.ncbi.nlm.nih.gov). We also expressed a GM-CSF fusion protein that lacked the encephalitogenic peptide but was otherwise identical to GMCSF-NAg. This fusion protein was comprised of the mature rat GM-CSF domain as the N terminus fused directly without a linker to a seven his-tag C terminus. The rat GM-CSF domain of both fusion proteins was based on a partial rat mRNA sequence (NCBI U00620; www.ncbi.nlm.nih.gov), which encoded the mature rat cytokine. The signal sequence of mouse GM-CSF was inserted by standard PCR cloning procedures to ensure processing and secretion of the fusion protein. Thus, both GM-CSF fusion genes contained an AGC-CTC sequence encoding a S15–L16 sequence of mouse GM-CSF, whereas the rat GM-CSF gene had an AGT-TTC sequence encoding a S15-F16 sequence. This 1 aa sequence difference in the signal sequence was of no consequence, as the mouse GM-CSF signal sequence supported efficient expression of rat GM-CSF fusion proteins in baculovirus expression systems.
Two M-CSF fusion proteins (MCSF-NAg and M-CSF) were also used in this study based on the rat M-CSF sequence (NCBI NP_076471; www.ncbi.nlm.nih.gov). The N-terminal domain of both fusion proteins contained the native rat 33-aa M-CSF signal sequence and the 220-aa N-terminal domain that forms a secreted, biologically active homodimer. These proteins lacked the pro-peptide, transmembrane, and cytoplasmic domains of full-length M-CSF. The MCSF-NAg and M-CSF fusion proteins had the same C-terminal domains as the GMCSF-NAg and GM-CSF fusion proteins, respectively.
Several additional cytokine-NAg fusion proteins were used in this study, including IL1RA-NAg, IL2-NAg, IL4-NAg, IL10-NAg, IL13-NAg [27,28,29], IFNβ-NAg [26], and IL6-NAg. These fusion proteins contained the respective rat cytokine as the N-terminal domain linked to a C-terminal domain that included the 73–87 encephalitogenic peptide (P-Q-K-S-Q-R-S-Q-D-E-N-P-V-V-H). All of these fusion proteins had a C-terminal his-tag to facilitate purification. An additional fusion protein, NAgIL-16, was comprised of an N-terminal his-tag, the 69–87 encephalitogenic peptide of GPMBP, and the rat 118-aa IL-16 cytokine C terminus [27].
Recombinant proteins expressed by use of baculovirus expression systems were purified by two consecutive affinity chromatography steps [29, 42]. Expression supernatants containing the GM-CSF- or M-CSF-based fusion proteins were concentrated by ultrafiltration and were purified initially by affinity chromatography based on binding to a single-chain (scFv) anti-6his antibody immobilized on a chitin resin. After elution, the fusion proteins were subjected to the final affinity chromatography step on Ni-NTA agarose columns (Qiagen, Valencia, CA, USA). Proteins were then concentrated and diafiltrated in Amicon Ultra-15 centrifugal filter devices. Protein quantity was assessed by the bicinchoninic acid protein assay (Pierce, Rockford, IL, USA) and by absorbance at 280 nm. Purity was assessed by SDS-PAGE.
Animals and reagents
Lewis rats were housed at East Carolina University Brody School of Medicine (Greenville, NC, USA). Animal care and use were performed in accordance with approved animal use protocols and institutional guidelines of the East Carolina University Institutional Animal Care and Use Committee. Injections were administered to Lewis rats anesthetized by isoflurane (Abbott Laboratories, Chicago, IL, USA). GPMBP was purified from spinal cords (Rockland, Gilbertsville, PA, USA). Synthetic GP69-88 peptide (Y-G-S-L-P-Q-K-S-Q-R-S-Q-D-E-N-P-V-V-H-F) was obtained from Quality Controlled Biologicals, Inc. (Hopkinton, MA, USA). B cell hybridoma supernatants containing the OX-6 anti-I-A (RT1B) IgG1, OX-33 anti-CD45 (B cell form) IgG1, OX-8 anti-CD8-α IgG1, OX-1 anti-CD45 (rat leukocytes) IgG1, and W3/25 anti-CD4 IgG1 were concentrated by ultrafiltration on Amicon (Millipore, Billerica, MA, USA) spiral wound membranes (100 kd exclusion). Hybridomas were obtained from the European Collection of Cell Cultures (Health Protection Agency Culture Collections, Salisbury, UK). Mouse anti-rat CD11c MCA1441 IgG2a was purchased from AbD Serotec (Raleigh, NC, USA), and anti-B7.1 3H5 IgG1 and anti-B7.2 24F IgG1 were purchased from PharMingen (San Diego, CA, USA). FITC-conjugated goat anti-mouse IgG1 and PE-conjugated goat anti-mouse IgG2a were used as secondary staining reagents to detect primary antibody labeling of cell-surface markers (Southern Biotechnology Associates, Birmingham, AL, USA). PE-conjugated goat anti-rat IgG(H+L)/IgM(H+L) was used to label B cells for sorting by FACS, and FITC-goat anti-mouse IgG1 was used as a secondary staining reagent to stain OX33-labeled B cells (Southern Biotechnology Associates).
Cell lines and culture conditions
The RsL.11 MBP-specific T cell clone was a primary, IL-2-dependent line derived from Lewis rats sensitized with rat MBP in CFA [43]. The R1T T cell clone was a blastogenic, IL-2-dependent clone derived from Lewis rats that constitutively expressed high levels of the IL-2R CD25, MHC class II glycoproteins (MHCII), B7.1, and B7.2 [43, 44]. Assays were performed in complete RPMI medium [10% heat-inactivated FBS, 2 mM glutamine, 100 μg/ml streptomycin and 100 U/ml penicillin (Whittaker Bioproducts, Walkersville, MD, USA), and 50 μM 2-ME (Fisher Scientific, Pittsburgh, PA, USA)]. T cell lines were propagated in complete RPMI supplemented with rat rIL-2 (0.4% v/v Sf9 supernatant) [45].
Derivation and analysis of myeloid and B cell APC
To isolate myeloid-derived DC or macrophages, bone marrow cells were obtained from the tibias and femurs of Lewis rats and were cultured in complete RPMI in the presence or absence of GM-CSF or M-CSF baculovirus supernatant (0.1% v/v) or purified GM-CSF or M-CSF (50 nM) for 7–10 days. Plastic adherent cell populations were used as a source of DC or macrophages. Adherent populations were detached from plastic surfaces by incubation in 3 mM EDTA-HBSS and were used as APC in bioassays or were analyzed by flow cytometry. Cells were stained with anti-CDllc, OX-1, OX-6, OX-8, W3/25, anti-B7.1, or anti-B7.2 mAb for 45 min, were washed twice in HBSS-1% FBS, and were incubated with a FITC- or PE-conjugated secondary antibody for 45 min. mAb were used at a concentration of 2.5 μg/ml or 1/20 dilution of a concentrated hybridoma supernatant. FcRs were blocked with 5% heat-inactivated normal rat serum for 20 min before the addition of antibody reagents. Washes and incubations were performed at 4°C. Dead cells were excluded by forward- versus side-scatter profiles. Data were acquired by use of a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) and were analyzed with the CELLQuest software program.
To purify B cell APC, OX33+ [46] or OX33+Ig+ splenocytes were purified by FACS. Cells were labeled with OX-33 (mouse anti-rat B220, IgG1) for 1 h. Cells were next washed two times in HBSS-1% FBS and were incubated with 2.5 μg/ml FITC-conjugated goat anti-mouse IgG1 (Southern Biotechnology Associates) and/or 2.5 μg/ml R-PE-conjugated goat anti-rat IgG/M (H+L) (Southern Biotechnology Associates). Purified B cells were >95% pure. Cells were sorted with a Becton Dickinson FACSVantage flow cytometer and were analyzed with the CELLQuest software program.
Measurement of antigen-specific responses
To measure antigen-specific proliferation, RsL.11 T cell responders (2.5×104/well) were cultured with irradiated splenocytes (3000 rads, 2.5×105/well), myeloid APC (1.5×104/well), or purified B cells (2.5×105/well) in the presence of designated antigen concentrations. Cultures were pulsed with 1 μCi [3H]thymidine (6.7 Ci/mmol, New England Nuclear, Perkin Elmer, Waltham, MA, USA) during the last 24 h of a 72-h culture. Cultures were harvested onto filters by use of a Tomtec Mach III harvester (Hamden, CT, USA). [3H]Thymidine incorporation into DNA was measured by use of a Wallac 1450 Microbeta Plus liquid scintillation counter (Perkin Elmer). Error bars routinely represented sd of triplicate sets of wells. One-way ANOVA and Bonferroni post-hoc tests were used to analyze these data.
To measure T cell killing, the MHCII+ clone of R1T cells (2.5×104/well) was cultured with irradiated (1000 rads; 5.0×104/well) RsL.11 (killer) cells in the presence of designated antigen concentrations. Rat rIL-2 was added as a 0.4% v/v IL-2 baculovirus supernatant 4 h after the addition of fusion proteins to the T cell killing assays to drive R1T growth. In this assay, R1T cells grew in response to IL-2 unless killed during MHCII-dependent antigen presentation to irradiated RsL.11 responders [47].
Induction and assessment of EAE
EAE was induced in Lewis rats by the s.c. injection of an emulsion comprised of 50 μg DHFR-NAg fusion protein and 200 μg Mycobacterium tuberculosis in a total volume of 0.1 ml CFA. Two injections of 50 μl were administered on either side at the base of the tail. DHFR-NAg consists of an N-terminal mouse dihydrofolate reductase domain and a C-terminal encephalitogenic peptide (GP69-87) domain [27,28,29]. The GM-CSF-based and M-CSF-based fusion proteins were tested for tolerogenic activity by s.c. injection in saline without adjuvant, according to designated schedules and doses. The following scale was used to score clinical signs of EAE: distal limp tail (0.25), limp tail (0.5), ataxia (1.0), partial hind-limb paralysis (2.0), and full hind-limb paralysis (3.0). Partial hind-limb paralysis was defined as the retention of some voluntary ambulatory movement in the hind limbs without the ability to ambulate upright. Full hind-limb paralysis manifested as complete flaccid hind-limb paralysis.
The mean cumulative score was calculated by summing the daily scores for each rat and then averaging the cumulative scores within a group to obtain the mean cumulative score for the group. The mean maximal score was calculated by averaging the most severe score of EAE for each rat within each group. Weight loss was calculated as a percent of each daily weight compared with the maximal initial weight for each animal prior to EAE onset. Each mean value was reported with the sd. Severe EAE was defined as the incidence of ataxia hind-leg paresis, or full hind-limb paralysis. Compiled data from three replicate experiments (Tables 1 and 2) were used to assess differences in the mean cumulative score, mean maximal score, and the mean number of days with severe EAE, as analyzed by parametric two-way ANOVA (experiment vs. treatment group). Median cumulative score and median maximal score were listed as the median values for all rats in each group and were analyzed by nonparametric two-way ANOVA based on ranked data. Differences among groups for daily scores of mean EAE severity and percent weight loss (see Fig. 4, A and B) of Experiment 1 (Table 1) were, respectively, analyzed by nonparametric and parametric one-way ANOVA. Likewise, differences in daily scores of mean EAE severity and mean percent weight loss compiled from Experiments 2 and 3 of Table 1 (see Fig. 4, C and D) and Experiments 1–3 of Table 2 (see Fig. 5, A and B) were analyzed by two-way ANOVA, which was analyzed with the Bonferroni post-hoc test. The incidence of severe EAE was analyzed pair-wise with Fisher’s exact test.
TABLE 1.
GMCSF-NAg Is a Potent Tolerogen: Requirement for Covalent Cytokine-NAg Linkage
| Exp. | Pretreatmenta | Incidence | Mean cum. score | Median cum. score | Mean max. score | Median max. score | % Weight loss | Incidence of severe EAE | Mean # days with severe EAE |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NAg | 8 of 8 | 16.9 ± 5.8 | 17.3 | 2.8 ± 0.7 | 3.0 | 18.8 ± 5.5 | 8 of 8 | 2.9 ± 1.0 |
| MCSF-NAg | 5 of 5 | 4.3 ± 0.9 | 4.0 | 0.8 ± 0.7 | 0.5 | 10.1 ± 2.9 | 1 of 5 | 0.2 ± 0.5 | |
| GMCSF-NAg | 5 of 5 | 1.8 ± 0.8 | 1.5 | 0.3 ± 0.0 | 0.3 | 0.6 ± 1.9 | 0 of 5 | 0.0 ± 0.0 | |
| 2 | NAg | 7 of 7 | 15.4 ± 5.2 | 16.0 | 2.9 ± 1.0 | 3.0 | 17.2 ± 2.8 | 7 of 7 | 2.7 ± 0.9 |
| GM-CSF + NAg | 4 of 4 | 14.9 ± 4.4 | 16.4 | 2.9 ± 0.3 | 3.0 | 15.9 ± 1.6 | 4 of 4 | 2.8 ± 0.3 | |
| M-CSF + NAg | 4 of 4 | 10.6 ± 4.0 | 11.6 | 2.5 ± 1.0 | 3.0 | 14.9 ± 1.7 | 4 of 4 | 2.3 ± 0.5 | |
| MCSF-NAg | 4 of 4 | 6.7 ± 3.7 | 7.8 | 2.1 ± 1.3 | 2.5 | 13.4 ± 2.1 | 3 of 4 | 1.4 ± 1.0 | |
| GMCSF-NAg | 4 of 4 | 1.3 ± 0.5 | 1.1 | 0.3 ± 0.1 | 0.3 | 5.3 ± 3.2 | 0 of 4 | 0.0 ± 0.0 | |
| 3 | NAg | 6 of 6 | 12.5 ± 8.2 | 12.9 | 2.1 ± 1.0 | 2.5 | 18.0 ± 2.0 | 6 of 6 | 2.2 ± 1.2 |
| GM-CSF + NAg | 6 of 6 | 14.9 ± 5.8 | 12.9 | 3.0 ± 0.0 | 3.0 | 17.7 ± 2.0 | 6 of 6 | 2.8 ± 0.9 | |
| M-CSF + NAg | 6 of 6 | 12.4 ± 7.3 | 13.9 | 2.3 ± 1.2 | 3.0 | 19.7 ± 1.6 | 5 of 6 | 2.3 ± 1.3 | |
| MCSF-NAg | 5 of 5 | 6.0 ± 7.0 | 2.7 | 1.2 ± 1.2 | 0.5 | 14.5 ± 1.3 | 2 of 5 | 1.0 ± 1.4 | |
| GMCSF-NAg | 2 of 6 | 0.5 ± 0.8 | 0.0 | 0.1 ± 0.1 | 0.0 | 7.8 ± 4.4 | 0 of 6 | 0.0 ± 0.0 | |
| 1–3b | NAg | 21 of 21 | 15.1 ± 6.3 | 17.3 | 2.6 ± 0.7 | 3.0 | 17.9 ± 3.8 | 21 of 21 | 2.6 ± 1.0 |
| GM-CSF + NAg | 10 of 10 | 14.9 ± 5.0 | 14.3 | 3.0 ± 0.2 | 3.0 | 17.0 ± 2.0 | 10 of 10 | 2.8 ± 0.7 | |
| M-CSF + NAg | 10 of 10 | 11.7 ± 6.0 | 12.8 | 2.4 ± 1.1 | 3.0 | 17.8 ± 2.9 | 9 of 10 | 2.3 ± 1.0 | |
| MCSF-NAg | 14 of 14 | 5.6 ± 4.4 | 4.1 | 1.3 ± 1.1 | 0.5 | 12.6 ± 2.8 | 6 of 14 | 0.8 ± 1.1 | |
| GMCSF-NAg | 11 of 15 | 1.2 ± 0.9 | 1.3 | 0.2 ± 0.1 | 0.3 | 5.0 ± 4.3 | 0 of 15 | 0.0 ± 0.0 |
Rats were pretreated with NAg (synthetic peptide 69–88 of GPMBP), a mixture of GM-CSF and NAg (GM-CSF + NAg), a mixture of M-CSF and NAg (M-CSF + NAg), MCSF-NAg, or GMCSF-NAg (4 nmoles each) 21, 14, and 7 days before challenge with 50 μg DHFR-NAg in CFA on Day 0. Rats were scored twice/day at approximate 12 h intervals. Severe EAE was defined as the incidence of ataxia or partial or full hind-limb paralysis (disease scores of 1.0–3.0).
Combined experiments 1–3: Values for the mean and median cumulative scores, mean or median maximal scores, percent weight loss, the incidence of severe EAE, and mean number of days with severe EAE differed significantly among groups as designated below. No significant differences were noted among treatment groups regarding day of onset. GMCSF-NAg vs. NAg, GM-CSF + NAg, or M-CSF + NAg: P < 0.001 for all comparisons. GMCSF-NAg vs. MCSF-NAg: P ≤ 0.013 for median cumulative score, mean or median maximal score, and weight loss. MCSF-NAg vs. NAg or GM-CSF + NAg: P ≤ 0.008 for all comparison. MCSF-NAg vs. M-CSF + NAg: P ≤ 0.033 for median cumulative score, mean or median maximal score, weight loss, incidence of severe EAE, and mean number of days with severe EAE.
TABLE 2.
The GMCSF-NAg Fusion Protein Halts Progression of EAE
| Exp. | Treatmenta | Incidence | Mean cum. score | Median cum. score | Mean max. score | Median max. score | % Weight loss | Incidence of severe EAE | Mean # days with severe EAE |
|---|---|---|---|---|---|---|---|---|---|
| 1b | NAg | 7 of 7 | 9.4 ± 3.3 | 8.3 | 2.4 ± 0.8 | 3.0 | 20.3 ± 3.0 | 7 of 7 | 2.1 ± 0.5 |
| MCSF-NAg | 8 of 8 | 3.6 ± 2.4 | 2.6 | 1.1 ± 1.2 | 0.4 | 13.7 ± 4.5 | 3 of 8 | 0.4 ± 0.7 | |
| GM-CSF+ NAg | 8 of 8 | 4.4 ± 2.2 | 3.5 | 1.0 ± 1.0 | 0.5 | 20.9 ± 2.4 | 3 of 8 | 0.5 ± 0.8 | |
| GMCSF-NAg | 9 of 9 | 2.8 ± 0.8 | 2.5 | 0.6 ± 0.6 | 0.5 | 12.7 ± 3.8 | 2 of 9 | 0.1 ± 0.2 | |
| 2 | NAg | 9 of 9 | 6.4 ± 5.1 | 4.5 | 1.6 ± 1.1 | 1.0 | 18.2 ± 2.6 | 7 of 9 | 1.7 ± 1.5 |
| GM-CSF+ NAg | 7 of 7 | 9.0 ± 5.7 | 11.0 | 2.1 ± 1.2 | 3.0 | 21.3 ± 3.6 | 5 of 7 | 1.6 ± 1.5 | |
| GMCSF-NAg | 8 of 8 | 2.2 ± 0.5 | 2.3 | 0.4 ± 0.1 | 0.4 | 13.6 ± 2.2 | 0 of 8 | 0.0 ± 0.0 | |
| 3 | NAg | 6 of 6 | 9.2 ± 6.9 | 6.4 | 1.9 ± 1.2 | 2.0 | 26.2 ± 2.9 | 5 of 6 | 1.8 ± 1.3 |
| GM-CSF+ NAg | 7 of 7 | 10.6 ± 5.3 | 10.8 | 2.4 ± 1.0 | 3.0 | 25.1 ± 1.7 | 7 of 7 | 1.9 ± 0.8 | |
| GMCSF-NAg | 7 of 7 | 5.1 ± 3.6 | 3.5 | 1.4 ± 1.2 | 1.0 | 19.8 ± 4.7 | 5 of 7 | 0.6 ± 0.7 | |
| 1–3c | NAg | 22 of 22 | 8.1 ± 5.1 | 6.6 | 2.0 ± 1.1 | 2.0 | 21.1 ± 4.3 | 19 of 22 | 1.8 ± 1.2 |
| GM-CSF+ NAg | 22 of 22 | 7.8 ± 5.2 | 6.1 | 1.8 ± 1.2 | 2.0 | 22.3 ± 3.2 | 15 of 22 | 1.3 ± 1.2 | |
| GMCSF-NAg | 24 of 24 | 3.3 ± 2.3 | 2.6 | 0.8 ± 0.8 | 0.5 | 15.1 ± 4.7 | 7 of 24 | 0.2 ± 0.5 |
Rats were challenged with 50 μg DHFR-NAg in CFA on Day 0 (Experiments 1–3) and also with pertussis toxin (400 ng i.p.) on Days 0 and 1 (Experiment 3). During disease onset, rats were matched for clinical signs of EAE. The range of average clinical scores per group for Experiments 1–3 respectively, was 0.36–0.39, 0.28–0.29, and 0.29 on the day of the initial treatment. Rats were treated with 1 nmole of designated proteins on Days 9, 10, 12, and 14 (Experiment 1), Days 10, 11, and 13 (Experiment 2), or 4 nmoles on Day 8 and 1 nmole on Day 11 (Experiment 3). For the GM-CSF + NAg group, GM-CSF and NAg were administered as a mixture in equal molar doses.
Experiment 1: (MCSF-NAg vs. NAg): Mean and median cumulative scores (P≤0.003), mean or median maximal scores (P<0.05), percent weight loss (P<0.001), the incidence of severe EAE (P=0.0256), and mean number of days with severe EAE (P<0.001).
Combined Experiments 1–3: Values for the mean and median cumulative scores, mean or median maximal scores, percent weight loss, the incidence of severe EAE, and mean number of days with severe EAE differed significantly among groups. GMCSF-NAg versus NAg: P ≤ 0.004 for all comparisons. GMCSF-NAg versus GM-CSF + NAg: P ≤ 0.020 for all comparisons.
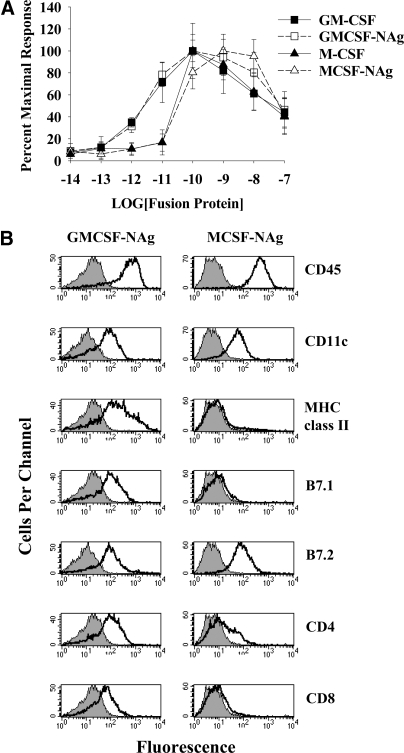
Figure 1.
The cytokine domains of GMCSF-NAg and MCSF-NAg had full biological activity and supported differentiation of distinct APC subsets. (A) Lewis rat bone marrow cells (105/well) were cultured with designated concentrations of purified fusion proteins. Cultures were pulsed with [3H]thymidine during the last day of a 3-day culture. These data are representative of three experiments. (B) Bone marrow cells were cultured with 50 nM GMCSF-NAg (left panels) or 50 nM MCSF-NAg (right panels) for 10 days. Surface markers of adherent APC were analyzed by flow cytometry. These data are representative of three experiments.
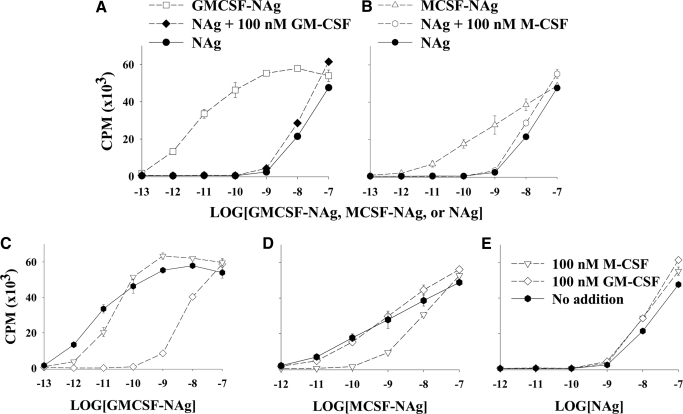
Figure 2.
The cytokine domains of GMCSF-NAg and MCSF-NAg strongly promoted reactivity to the covalently linked NAg by a mechanism that was blocked competitively and specifically by the respective cytokine. (A and B) Irradiated splenic APC and MBP-specific RsL.11 T cells were cultured with designated concentrations of GMCSF-NAg, MCSF-NAg, or NAg (x-axis) in the presence or absence of 100 nM GM-CSF or 100 nM M-CSF. (C–E) Irradiated, splenic APC and RsL.11 T cells were cultured with 100 nM GM-CSF, 100 nM M-CSF, or no cytokine for 4 h before addition of designated concentrations (x-axis) of GMCSF-NAg (C), MCSF-NAg (D), or NAg (E). (C and D) See the legend for E. Cultures were pulsed with [3H]thymidine during the last day of a 3-day culture. These data are representative of three experiments. (A) GMCSF-NAg versus NAg or versus NAg + GM-CSF (P<0.001 for 1 pM–10 nM). (B) MCSF-NAg versus NAg or versus NAg + M-CSF (P<0.001 for 100 pM–1 nM). (C) GM-CSF versus M-CSF or versus “no addition” (P<0.001 for 10 pM–10 nM). (D) M-CSF versus GM-CSF or versus “no addition” (P≤0.001 for 100 pM–1 nM). (E) No significant difference was noted among the three groups.
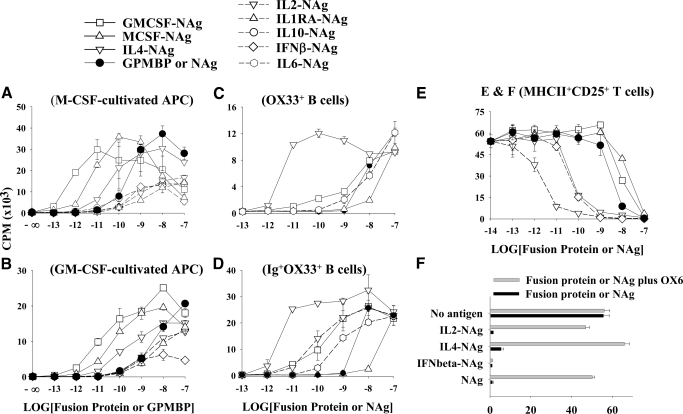
Figure 3.
The cytokine domains of GMCSF-NAg and MCSF-NAg efficiently targeted NAg to myeloid APC subsets. Bone marrow cells were cultured with M-CSF (A) or GM-CSF (B) baculovirus supernatants (0.1% v/v) for 7 days and were used as APC. Bone marrow-derived APC (15×103/well) were cultured with RsL.11 T cells and 2.5 mM aminoguanidine in the presence of designated concentrations of purified fusion protein (x-axis). (C and D) Splenic OX33+ B cells (C) and OX33+Ig+ B cells (D) were sorted by FACS and were cultured (2.5×104/well) with RsL.11 T cells and designated concentrations of fusion protein or NAg (x-axis). (E and F) R1T cells were cultured for 24 h in complete RPMI without IL-2 to allow clearance of IL-2 from cell surface receptors. R1T cells were then cultured with irradiated RsL.11 T responders in the presence of designated concentrations of fusion protein or NAg (x-axis) in the presence or absence of the anti-RT1B mAb (OX6; anti-I-A mAb). After 4 h, IL-2 (0.4% v/v IL-2 baculovirus supernatant) was added to all wells. This assay measured IL-2-dependent proliferation of R1T cells unless these T cell APC were killed upon antigen presentation to irradiated RsL.11 responders. Cultures were pulsed with [3H]thymidine during the last day of a 3-day culture. Not all fusion proteins were used in each experiment. These data are representative of three experiments.
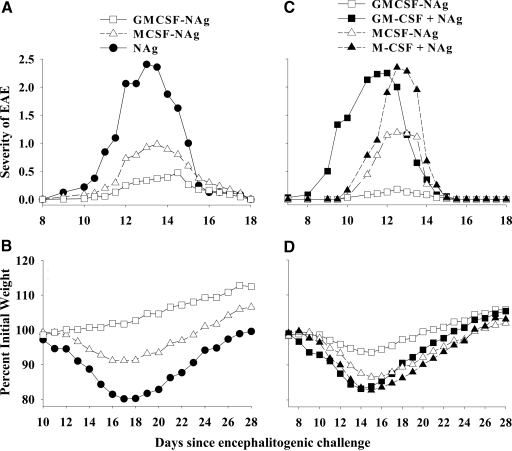
Figure 4.
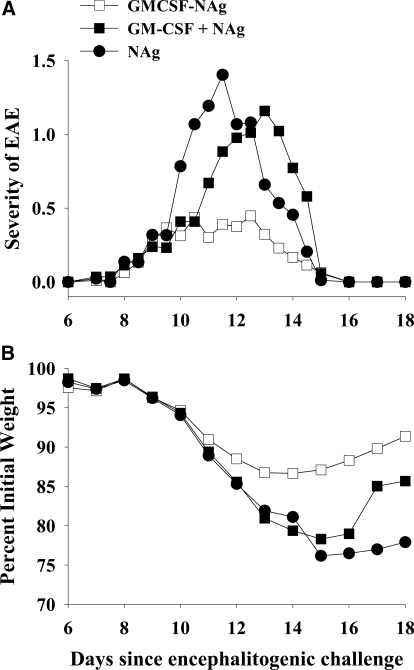
GMCSF-NAg was a potent tolerogen that prevented subsequent induction of EAE by a mechanism that required covalent linkage of GM-CSF and NAg. Rats were pretreated with 4 nmoles GMCSF-NAg, MCSF-NAg, NAg, a mixture of GM-CSF and NAg (GM-CSF+NAg; 4 nmoles each), and a mixture of M-CSF and NAg (M-CSF+NAg; 4 nmoles each) on Days –21, –14, and –7 before challenge, with 50 μg DHFR-NAg in CFA on Day 0. Rats were scored twice/day at approximate 12-h intervals, and weights were recorded daily. Shown are the time courses of clinical signs and weight loss for Experiment 1 of Table 1 (A and B, respectively) as well as the compiled time courses of clinical signs and weight loss for Experiments 2 and 3 (C and D, respectively) of Table 1. Analysis was performed with one-way or two-way ANOVA and Bonferroni post-hoc tests. (A and B) GMCSF-NAg versus NAg (EAE severity): P ≤ 0.037 for Days 12–14; (weight loss): P ≤ 0.035 for Days 11–28; MCSF-NAg versus NAg (EAE severity): P ≤ 0.017 for Days 10.5 and 12–13; (weight loss): P ≤ 0.042 for Days 15–22; GMCSF-NAg versus MCSF-NAg (weight loss): P ≤ 0.029 for Days 16–23. GMCSF-NAg versus MCSF-NAg (EAE severity): P = 0.029 for Day 14. (C and D) GMCSF-NAg versus GM-CSF + NAg (EAE severity): P ≤ 0.002 for Days 9–12; (weight loss): P ≤ 0.029 for Days 10–19; GMCSF-NAg versus MCSF-NAg (EAE severity): P ≤ 0.02 for Days 13.5 and 14.5; (weight loss): P ≤ 0.012 for Days 14–20; GMCSF-NAg versus M-CSF + NAg (EAE severity): P ≤ 0.003 for Days 9.5 and 11–13.5; (weight loss): P ≤ 0.012 for Days 12–21.
Figure 5.
GMCSF-NAg blocked the progression of EAE when treatment was initiated after disease onset. Rats were challenged with 50 μg DHFR-NAg in CFA on Day 0 (Experiments 1–3) and also with pertussis toxin (400 ng i.p.) on Days 0 and 1 (Experiment 3). On the day of initial treatment, rats were matched into treatment groups based on clinical signs of EAE as described in Table 2. Rats were treated with 1 nmole of the designated proteins on Days 9, 10, 12, and 14 (Experiment 1) and Days 10, 11, and 13 (Experiment 2) or 4 nmoles on Day 8 and 1 nmole on Day 11 (Experiment 3). Shown are the compiled time-course data for clinical EAE (A) and weight loss (B) for Experiments 1, 2, and 3 of Table 2. Analysis was performed with two-way ANOVA and Bonferroni post-hoc tests. GMCSF-NAg versus NAg (EAE severity): P ≤ 0.015 for Days 10–12.5; (weight loss): P ≤ 0.007 for Days 12, 13, and 15–18; GMCSF-NAg versus GMCSF + NAg (EAE severity): P ≤ 0.022 for Days 11–13.5; (weight loss): P ≤ 0.022 for Days 12, 13, and 15–18.
RESULTS
Biological activity of the cytokine domain
GMCSF-NAg, MCSF-NAg, GM-CSF, and M-CSF were expressed in baculovirus expression systems and were purified by a two-step affinity chromatography strategy. The cytokine domain of GMCSF-NAg was biologically active and was not affected by addition of the C-terminal NAg domain (Fig. 1A). Likewise, MCSF-NAg and M-CSF were also equipotent in stimulating growth of bone marrow cells. These data support the conclusion that fusion of the cytokine and NAg domains had no effect on the interaction of the cytokine with the respective receptor. The GM-CSF fusion proteins were approximately tenfold more potent than the M-CSF fusion proteins for stimulation of bone marrow cells. The GM-CSF fusion proteins exhibited half-maximal stimulation in the 1- to 10-picomolar range, whereas M-CSF had half-maximal activity in the 10- to 100-picomolar range. These results are consistent with the role of GM-CSF and M-CSF as survival and growth factors for hematopoietic progenitors. Neither fusion protein stimulated proliferation of rat splenocytes, although both fusion proteins facilitated survival and differentiation of adherent splenic APC (not shown).
GMCSF-NAg and MCSF-NAg facilitated the differentiation of adherent APC bearing CD11c (Fig. 1B). Bone marrow-derived APC cultured for 8–10 days with GMCSF-NAg had substantially higher surface concentrations of MHC class II glycoproteins, B7.1, CD4, and CD8, as compared to APC cultured with MCSF-NAg. The presence or absence of the NAg domain had no effect on the ability of the GMCSF-derived or MCSF-derived fusion proteins to drive differentiation of bone marrow cells (data not shown). Furthermore, the NAg domain alone did not facilitate survival or differentiation of bone marrow cells (data not shown). These observations are consistent with the prediction that GM-CSF and M-CSF would, respectively, guide distinct differentiation pathways to generate the DC and macrophage lineages. Hence, the superior efficacy of GM-CSF versus M-CSF as cytokine fusion partners may be related to superior APC activities of DC versus macrophage APC subsets.
Biological activity of the NAg domain
The NAg domain of GMCSF-NAg had extremely potent antigenic activity in assays measuring in vitro proliferation of a MBP-specific T cell clone (RsL.11) cultured with irradiated, splenic APC. The antigenic activity of GMCSF-NAg was at least 1000-fold more potent than NAg (Fig. 2A). GMCSF-NAg had half-maximal NAg activity in the 1- to 10-picomolar range, which was similar to the half-maximal stimulatory concentrations noted for the GM-CSF domain in the cytokine bioassay. MCSF-NAg was also more potent than NAg by at least tenfold in these assays (Fig. 2B). MCSF-NAg exhibited significant antigenic activity in the 10- to 100-picomolar range, which was similar to the potency exhibited by the M-CSF domain in the cytokine bioassay. These observations suggested that the affinity of the cytokine/receptor interaction may facilitate the rate-limiting step of antigen capture by APC. The rank order of activity (GMCSF-NAg>MCSF-NAg>NAg) that was noted in the RsL.11 T cell-proliferative assay closely paralleled the rank order in assays measuring NAg-stimulated IL-2 production by RsL.11 T cells (not shown). These data revealed that the GM-CSF and M-CSF cytokine domains substantially augmented antigen presentation by a mechanism that appeared to involve cytokine-mediated targeting of NAg to APC.
Cytokine-mediated targeting of the NAg
Two observations verified the hypothesis that potentiated recognition of the NAg domain in GMCSF-NAg or MCSF-NAg reflected a mechanism of antigen targeting. First, potentiated antigen recognition required covalent linkage of the cytokine and NAg domains. As shown in Figure 2, A and B, the T cell-proliferative response to NAg at concentrations from 1 pM to 100 nM was not substantially altered in the presence or absence of 100 nM GM-CSF or 100 nM M-CSF. Thus, the antigenic response to the fusion protein could not be duplicated by addition of cytokine and NAg as separate molecules. Therefore, the role of the cytokine domain could not be ascribed to an independent cytokine-mediated activity. For example, the ability of GM-CSF or M-CSF to promote costimulatory activity, MHC class II expression, or APC differentiation was not responsible for augmented antigen recognition because the cytokine domain would mediate these activities independently of the covalent linkage with NAg. Rather, these data show that the covalent linkage of cytokine to NAg was necessary for the potentiated recognition of the antigenic domain in the fusion proteins.
Second, the potentiated antigenic recognition of GMCSF-NAg and MCSF-NAg was competitively blocked by preincubation of APC with the respective GM-CSF or M-CSF cytokine. As shown in Figure 2, C–E, preincubation of APC with M-CSF blocked the potentiated recognition of MCSF-NAg without modifying the response to GMCSF-NAg or NAg. Likewise, preincubation of APC with GM-CSF blocked the potentiated recognition of GMCSF-NAg without affecting responses to MCSF-NAg or NAg. In both cases, the presence of M-CSF or GM-CSF blocked the response to the respective fusion protein to the extent that the response was essentially the same as that achieved by NAg alone. These data indicated that potentiated antigenic activity of MCSF-NAg or GMCSF-NAg required unoccupied M-CSF receptors or GM-CSF receptors.
Cytokine-mediated targeting of NAg to particular APC subsets
A central prediction of this model was that distinct cytokine-NAg fusion proteins would selectively target NAg to different APC subsets, given that DC, macrophages, B cells, and T cells have distinct expression profiles of cytokine receptors. DC and macrophages were derived by culture of bone marrow cells with GM-CSF or M-CSF for 7 days (Fig. 3, A and B). Addition of aminoguanidine to macrophage and DC cultures was necessary to prevent antigen-stimulated NO production. Otherwise, accumulation of NO abrogated antigen-driven T cell proliferation (not shown). These APC supported a rank order for antigen presentation in which GMCSF-NAg was the most potent fusion protein (GMCSF-NAg>MCSF-NAg>IL4-NAg>NAg). Other fusion proteins, such as IL1RA-NAg, IL2-NAg, IFNβ-NAg, or IL10-NAg, were less active than NAg (Fig. 3, A and B). The efficiency of a cytokine-NAg fusion protein most likely depended on the quantitative expression of the respective cytokine receptor and the efficiency of ligation-dependent internalization into the MHCII antigen-processing compartment.
In contrast to DC and macrophages, B cells exhibited a distinct profile. Sorted OX33+ B cells supported the following rank order for antigen presentation (IL4-NAg>>NAg=GMCSF-NAg=IL6-NAg>MCSF-NAg; Fig. 3C). Sorted OX33+ B cells were compared with sorted Ig+OX33+ B cells, as the former was largely resting, quiescent B cells, whereas the latter received activation signals as a result of cross-linking of surface Ig during purification. Ig+OX33+ B cell APC exhibited a similar rank order of antigen presentation (IL4-NAg>GMCSF-NAg=IL2-NAg>IL6-NAg>NAg>MCSF-NAg; Fig. 3D). Thus, Ig+OX33+ B cells, purified by ligation of surface Ig, exhibited enhanced presentation of GMCSF-NAg and IL6-NAg compared with OX33+ B cells. Other fusion proteins, including IL1RA-NAg, IL10-NAg, and IL13-NAg, were substantially less active than NAg (not shown).
T cells exhibited yet another distinct profile (IL2-NAg>IL4-NAg>NAg>GMCSF-NAg, MCSF-NAg; Fig. 3E). Other fusion proteins, such as IL1RA-NAg, IL10-NAg, or IL13-NAg, were less active than NAg (not shown). R1T cells were used as T cell APC, as these T cells had a blastogenic phenotype marked by high expression of MHC class II and IL-2R. The potentiated recognition of IL2-NAg and IL4-NAg was consistent with the observation that R1T cells were responsive to IL-2 and IL-4 [45]. To assay antigen presentation by R1T cells, a cytotoxicity assay was used in which IL-2 was used to stimulate the growth of R1T cells, which were killed upon antigen presentation to irradiated RsL.11 responders [47]. That is, cytotoxicity was measured by decreased IL-2-dependent growth of R1T. As the assay required the presence of IL-2 in all wells, IL-2 was added to these cultures 4 h after addition of fusion proteins. Otherwise, when IL-2 and IL2-NAg were added at the same time, IL-2 blocked the capture of IL2-NAg for antigen presentation [28]. Also, the anti-RT1B mAb (OX6) was used to verify that cytotoxicity was a result of antigen recognition rather than an inhibitory effect of the cytokine (Fig. 3F). The OX6 mAb completely reversed the inhibitory activity of 100 nM IL2-NAg, IL4-NAg, or NAg but did not block the inhibitory activity of 100 nM IFNβ-NAg. These data indicated that the inhibitory activity of IL2-NAg, IL4-NAg, and NAg was contingent on antigen presentation, whereas the inhibitory activity of IFNβ-NAg was a direct cytotoxicity mediated by the IFNβ cytokine domain.
Overall, these data indicated that GMCSF-NAg was most efficient for targeting NAg to DC and macrophages, IL4-NAg was the most efficient for targeting NAg to B cell APC, and IL2-NAg was the most efficient for targeting NAg to blastogenic T cells.
Linkage of GM-CSF and NAg was required to prevent and treat EAE
Administration of GMCSF-NAg or MCSF-NAg to Lewis rats significantly attenuated the subsequent induction of EAE (Table 1, Experiment 1, and Fig. 4A). GMCSF-NAg, MCSF-NAg, or NAg (4 nmoles/injection) was administered s.c. in saline on Days –21, –14, and –7 before encephalitogenic challenge (total of three injections/rat). Rats were challenged with DHFR-NAg in CFA on Day 0 and were monitored for clinical signs of EAE and for weight loss. Rats pretreated with GMCSF-NAg or MCSF-NAg exhibited significantly reduced cumulative scores and maximal scores together with a lower incidence and duration of severe EAE. Compared with MCSF-NAg, GMCSF-NAg was a more profound inhibitor of EAE (Fig. 4A). GMCSF-NAg was also more effective than MCSF-NAg for prevention of the weight loss associated with disease (Table 1 and Fig. 4B).
To address whether antigen targeting was also required for prevention of EAE, rats were pretreated on Days –21, –14, and –7 with 4 nmoles GMCSF-NAg or a mixture of GM-CSF and NAg (4 nmoles each) as separate molecules (Table 1, Experiments 2–3, and Fig. 4, C and D). Likewise, rats were pretreated with MCSF-NAg or a mixture of M-CSF and NAg as separate molecules. Rats pretreated with GMCSF-NAg or MCSF-NAg showed resistance to a subsequently induced bout of EAE, whereas rats pretreated with the combination of cytokine and NAg exhibited severe EAE. Previous research indicated that rats pretreated with NAg or saline exhibited equivalent disease intensity [26,27,28]. These data indicated that the tolerogenic effectiveness of GMCSF-NAg and MCSF-NAg required physical linkage of the cytokine and NAg domains. The requirement for cytokine-NAg linkage indicated that the cytokine domain was important for targeting NAg to APC. Overall, these data reveal that GMCSF-NAg is a highly effective, antigen-specific tolerogen.
A central question was whether GMCSF-NAg retained tolerogenic activity when introduced into an inflammatory environment or whether tolerogenic activity was confined to “steady-state” environments. To assess this question, rats were immunized with DHFR-NAg in CFA and were allowed to develop the initial paralytic signs of EAE (Table 2, Experiments 1–3, and Fig. 5, A and B). After the initial onset of EAE, rats were matched into groups that expressed essentially equivalent disease scores. Groups matched for EAE severity were then given designated doses of GMCSF-NAg, a mixture of GM-CSF and NAg, or NAg. Treatment with the GMCSF-NAg fusion protein inhibited the progression of EAE in Lewis rats, such that afflicted rats did not progress to more severe stages of EAE, whereas rats treated with NAg progressed to paralytic disease (Table 2 and Fig. 5A). Disease progression of rats treated with NAg did not differ significantly from rats treated with saline [26]. After initiation of treatment, administration of GMCSF-NAg halted disease progression, as measured by the cumulative and maximal disease scores or the incidence and duration of severe EAE (Table 1 and Fig. 5A). Treatment with GMCSF-NAg also prevented any additional weight loss (Fig. 5B). Although rats treated with the combination of GM-CSF and NAg had mild disease in Experiment 1, the same rats had severe weight loss (Table 2). Rats treated with GM-CSF and NAg in Experiments 2 and 3 had severe EAE together with severe weight loss (Table 2, Experiments 2 and 3). The GMCSF-NAg fusion protein blocked progression of EAE in all three experiments. These data showed that GMCSF-NAg, when used as a treatment for ongoing EAE, required covalent linkage of the cytokine and NAg domains for inhibitory activity. Thus, GMCSF-NAg was not only highly effective for prevention of EAE, this fusion protein was also highly effective for halting progression of EAE by a mechanism that was not contingent on a steady-state, noninflammatory environment.
A group of rats was also treated with MCSF-NAg (Table 2). Treatment with MCSF-NAg inhibited EAE measured by the cumulative and maximal disease scores, percent weight loss, incidence of severe EAE, and number of days afflicted with severe EAE. However, the GMCSF-NAg fusion protein was more effective than MCSF-NAg, as measured by the cumulative disease score and weight loss.
DISCUSSION
Cytokine-NAg fusion proteins may mediate unique biological activity by induction of two synergistic, concurrent events. First, these fusion proteins interact with cytokine receptors on APC to initiate cytokine-dependent activation or differentiation programs. Second, cytokine-NAg fusion proteins deliver the antigenic domain in relatively high concentrations to the surface of the APC to potentiate the efficiency of antigen presentation by several orders of magnitude. The result is that selected subsets of cytokine-conditioned APC present substantial quantities of target antigen to antigen-specific T cells. The utility is that injection of soluble cytokine-NAg fusion proteins can therefore drive antigen-specific interactions among a given APC subset and antigen-specific T cells in vivo. Although cytokine-antigen fusion proteins have been investigated as vaccines against cancer and infectious disease, cytokine-NAg fusion proteins also represent an important, emerging tool for induction of antigen-specific immunological tolerance (Tables 1 and 2 and Figs. 4 and 5) [26,27,28].
Cytokine-antigen fusion proteins regulate the balance of tolerance and immunity
Several fusion proteins showed pronounced efficacy as antigen-specific tolerogens in EAE. As shown in this and other studies, GMCSF-NAg, IL2-NAg, and NAg-IL16 fusion proteins inhibited EAE effectively when administered before encephalitogenic challenge or conversely, when administration was initiated during disease onset (Tables 1 and 2) [27, 28]. For all three proteins, physical linkage of cytokine and NAg was necessary in prevention and treatment protocols. Linkage was also required for antigenic targeting to APC in vitro. GMCSF-NAg facilitated presentation by DC and macrophage APC by over 1000-fold (Fig. 3). IL2-NAg facilitated presentation of NAg by blastogenic T cells by ∼1000-fold [28]. NAg-IL16 potentiated presentation by Con A-activated splenocytes by approximately ten-fold [27]. The targeted APC subset was an important consideration. IL4-NAg facilitated B cell-mediated presentation of NAg by ∼1000-fold, yet this fusion protein was relatively inefficient for tolerance induction (Fig. 3) [28]. Additional Th2 cytokines IL-10 and IL-13, fused to NAg, were also not efficient for tolerance induction [27]. IL-4 and IL-13 are key cytokines for B cell activation, and activated B cells may drive humoral immunity more so than tolerance. In contrast, the covalent cytokine-NAg link in the IFNβ-NAg fusion protein was not needed for protection against the subsequent induction of EAE, although the covalent link appeared important when IFNβ-NAg administration was initiated after disease onset [26].
Cytokine-antigen fusions have also been used to elicit immunity rather than tolerance. For example, IL-2 and GM-CSF cytokines were used as fusion partners to potentiate effector immune responses [48,49,50,51,52,53]. The paradox of using GM-CSF as a fusion partner in one experimental system to induce immunity and in another experimental system to induce tolerance is suggestive that the cytokine domain is not the sole consideration. The antigenic domain may also play a major role. Self-antigen is known to facilitate differentiation of Treg cells [54, 55]. In contrast, foreign antigens usually have a much more transient exposure to the immune system and therefore, do not drive Treg differentiation efficiently. In a GMCSF-antigen fusion protein, if the antigenic domain contains multiple foreign epitopes, then the antigen may be recognized by super-threshold frequencies of conventional T cells and a paucity of Treg cells. Conversely, if the antigenic domain is largely self in origin, then the relative proportion of cognate T cells may strongly favor the Treg subset. The relative balance of conventional and Treg subsets that interact with a given APC may in turn control the activation of that APC and thereby, set the balance of tolerogenic versus immunogenic signals during antigen presentation. These considerations support the possibility that cytokine and antigen domains may be of importance in determining the balance of immunogenic versus tolerogenic activities of a fusion protein.
The self-antigen domain of cytokine-NAg fusion proteins
A central question in autoimmune disease is whether self-antigen is an etiological initiator of autoimmunity or whether the self-antigen is primarily a target, at least in the early phases of disease. For example, human autoimmune disease may result from a combination of two conditions. First, a chronic or latent infectious agent may drive heteroclitic immunity against a cross-reactive self-antigen by a mechanism of molecular mimicry. Second, this foreign antigen mimic may not cross-react efficiently with the TCR repertoire of Treg subsets. If the foreign heteroclitic antigen drives autoreactive populations of cross-reactive, conventional T cells without engaging autoantigen-specific Treg cells, regulatory control would not be sufficient. In this case, the foreign antigen mimic would be mainly responsible for autoimmune etiology, whereas the cross-reactive self-antigen would be the autoimmune target. The implication is that regulatory networks specific for the targeted self-antigen may be intact rather than eroded (as is commonly assumed), in that these regulatory networks may not be engaged by the heteroclitic foreign antigen. These regulatory networks, however, could be engaged by administration of self-antigen as part of a tolerogenic vaccine.
As a case in point, immunity to certain streptococcal M proteins causes autoimmunity in rheumatic fever, but the autoimmune response is not self-sustaining despite the continued presence of the relevant self-antigen [56, 57]. Rather, the autoimmune response subsides after elimination of foreign bacterial antigen and clearance of autoantibody. As another example, many cases of PAP are a result of autoantibody production against the GM-CSF cytokine [58]. In a subset of patients, PAP can be treated effectively by administration of exogenous GM-CSF. Likewise, as shown in this study, the GMCSF-NAg fusion protein reversed EAE, even when the fusion protein was administered after disease onset (Table 2 and Fig. 5). Thus, peripheral s.c. injection of GMCSF-NAg controlled EAE, even after clonally expanded autoimmune effector cells had infiltrated the CNS. When exogenous GM-CSF is used for treatment of PAP or when GMCSF-NAg is used to treat EAE, epitopes within GM-CSF or contiguous with GM-CSF have the capacity to restore homeostatic regulation and halt progression of an ongoing autoimmune assault. In these cases, the suggestion is that self-antigen retains beneficial regulatory activity that can be enhanced by administration of exogenous self-antigen, even as endogenous self-antigen is the target of ongoing autoimmune inflammation. The possibility is that self-antigens, even as they are being targeted in an ongoing immune response, may be the key to beneficial regulatory responses.
The effective use of self-antigen to reverse ongoing autoimmunity is not entirely consistent with the contemporary hypothesis that “inflammatory” versus “steady-state” environments control the balance of immunity and tolerance. A paradox of autoimmune disease is that healthy and afflicted persons harbor similar frequencies of T cells bearing autoimmune specificities [59]. Sustained injury or infection seldom leads to induction of autoimmune disease in otherwise healthy people, although autoreactive T cells, in all probability, migrate into these inflammatory environments. Recognition of self-antigen within these inflammatory environments does not typically lead to autoimmunity, although these environments are replete with “danger” and costimulatory signals and contain activated APC of the innate immune system. As shown in this study, administration of appropriate cytokine-NAg fusion proteins in a “steady-state” or an “inflammatory” environment was conducive for maintenance or restoration of homeostasis (Tables 1 and 2). These considerations lend doubt as to whether the balance of tolerance versus immunity simply depends on the degree of antecedent inflammation.
The GM-CSF domain of cytokine-NAg fusion proteins
This study showed that GMCSF-NAg targeted NAg to GM-CSF receptor-bearing APC for enhanced antigen presentation. The antigen-targeting activity of GMCSF-NAg was also important for tolerance induction in pretreatment and treatment models. The identity of the APC subset that was targeted by GMCSF-NAg in vivo and that was responsible for tolerance induction remains unknown. The GM-CSF receptor is distributed widely and is present on virtually all myeloid lineages, vascular endothelial cells, and CD34+ hematopoietic progenitors. GMCSF-NAg thereby may target NAg to a wide variety of cell types, including immature and mature myeloid or lymphoid DC, macrophages, monocytes, and NK cells. The main biological effects of GMCSF-NAg may involve any one or a combination of these cell types.
GM-CSF has strong proinflammatory and anti-inflammatory activities that may underlie the immunoregulatory actions of a GMCSF-NAg fusion protein. Several observations revealed key proinflammatory activities of GM-CSF in EAE. GM-CSF-deficient mice are resistant to EAE, and reconstitution of GM-CSF by administration of exogenous GM-CSF restores susceptibility to EAE [60]. GM-CSF facilitates clonal expansion of encephalitogenic T cells, is needed for sustained parenchymal infiltration into the CNS, promotes T cell activation in the CNS by enhancing the stimulatory capacity and phagocytic activity of microglial cells, and facilitates recruitment of peripheral macrophages into the CNS [60,61,62,63]. Enforced expression of GM-CSF by transgenic, MBP-specific T cells precludes spontaneous recovery and instead causes a lethal course of severe, chronic, progressive EAE [64]. GM-CSF appears important in the IL-23-mediated pathway of EAE induction in association with mobilization of the Th17 lineage of effector T cells [65]. Hence, a GMCSF-NAg fusion protein could be used as “bait” to attract and engage pathogenic T cell subsets at peripheral sites of inoculation.
GM-CSF also has important anti-inflammatory activity. GM-CSF promotes differentiation of DC subsets, which in turn, facilitate Treg differentiation [66,67,68,69,70]. Also, GM-CSF promotes differentiation of myeloid-derived suppressor cells or DCreg subsets that elaborate anti-inflammatory cytokines and mediators such as NO to down-regulate T cell responses [35, 71,72,73,74,75]. Our study supported these findings. Rat bone marrow cells cultured with GM-CSF or M-CSF were strong APC for T cell activation, but NO production was pronounced to the extent that the presence of aminoguanidine, an inducible NO synthase inhibitor, was required to detect antigen-stimulated proliferation (not shown). The early activation event of IL-2 production was unimpaired by NO production, whereas the subsequent activation-dependent event of proliferation was abrogated. One possibility is that proinflammatory activities of GM-CSF attract encephalitogenic T cells to NAg-bearing DCreg at peripheral sites of inoculation, whereby these effector T cells are silenced by these cytotoxic APC.
The balance of tolerogenic and immunogenic activities of antigen-targeted DC
The results of this study are in accordance with other strategies of targeting antigen to DC. Recombinant antibody-antigen fusion proteins specific for DEC-205 (CD205) also targeted covalently tethered peptide antigen to DC for enhanced presentation by a mechanism that resulted in antigen-specific tolerance [76]. DEC-205 is expressed abundantly by a DC subset but is also expressed by other cell types including bone marrow stromal cells, follicular B cells, and thymic, pulmonary, and intestinal epithelia [77]. When antigen was targeted to DC, transgenic T cells initially exhibited a burst of antigen-specific proliferation but the response collapsed as the activated T cells disappeared or became anergic. However, coinjection of an agonistic anti-CD40 antibody resulted in sustained T cell activation and immunity [78]. The use of the agonistic anti-CD40 antibody controlled the balance of tolerance and immunity for class II MHC antigen presentation and for cross-presented antigen on class I MHC. Polyinosinic:polycytidylic acid could also be used as a maturation factor to promote immunity to the anti-DEC-205-tethered antigen [79]. A similar antibody-antigen fusion protein was also used to prevent the induction of EAE [80]. A recombinant anti-DEC-205 antibody was expressed that contained the encephalitogenic MOG peptide as the C terminus. When administered 7 days before encephalitogenic challenge, this anti-DEC-MOG fusion protein blocked the subsequent development of EAE. These studies provide evidence that targeting the encephalitogenic peptide to DC in the form of an anti-DEC-205-MOG fusion protein [80] can lessen susceptibility to EAE.
Conclusion
This study showed that the GMCSF-NAg fusion protein is a potent tolerogen that is highly effective for targeting antigen to myeloid APC including DC and macrophages. Administration of GMCSF-NAg before encephalitogenic challenge ameliorated the subsequent episode of EAE. When administered after disease onset, GMCSF-NAg prevented progression of disease. To our knowledge, this study provided the first example of a DC-targeting, antigen-specific therapeutic capable of inhibiting an ongoing inflammatory autoimmune disease. Overall, GMCSF-“self antigen” fusion proteins may represent an important tool for manipulating the immune response and causing antigen-specific immunological tolerance.
AUTHORSHIP
J. L. B. and M. D. M. contributed to the conception, design, performance, and composition of this article.
ACKNOWLEDGMENTS
This study was supported by research grants from the National Multiple Sclerosis Society and the Brody Brothers Endowment Fund.
Footnotes
Abbreviations: DC=dendritic cell, DCreg=regulatory DC, DHFR=dihydrofolate reductase, EAE=experimental autoimmune encephalomyelitis, GPMBP= guinea pig myelin basic protein, IL1RA-NAg=IL-1R antagonist-NAg, MBP=myelin basic protein, MOG=myelin oligodendrocyte glycoprotein, NAg=neuroantigen (the dominant encephalitogenic region of GPMBP and the GP69-88 synthetic peptide), PAP=pulmonary alveolar proteinosis, Treg cell=regulatory T cell
References
- Goodin D S, Cohen B A, O'Connor P, Kappos L, Stevens J C. Assessment: the use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;71:766–773. doi: 10.1212/01.wnl.0000320512.21919.d2. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Meuth S G, Wiendl P H. The trials and errors in MS therapy. Int MS J. 2008;15:79–90. [PubMed] [Google Scholar]
- Linker R A, Kieseier B C, Gold R. Identification and development of new therapeutics for multiple sclerosis. Trends Pharmacol Sci. 2008;29:558–565. doi: 10.1016/j.tips.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Lutterotti A, Sospedra M, Martin R. Antigen-specific therapies in MS—current concepts and novel approaches. J Neurol Sci. 2008;274:18–22. doi: 10.1016/j.jns.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Miller S D, Turley D M, Podojil J R. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- Steinman L. Antigen-specific therapy of multiple sclerosis: the long-sought magic bullet. Neurotherapeutics. 2007;4:661–665. doi: 10.1016/j.nurt.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Kingsley C I, Zhang X, Jabs C, Izikson L, Sobel R A, Weiner H L, Kuchroo V K, Sharpe A H. The ICOS molecule plays a crucial role in the development of mucosal tolerance. J Immunol. 2005;175:7341–7347. doi: 10.4049/jimmunol.175.11.7341. [DOI] [PubMed] [Google Scholar]
- Song F, Guan Z, Gienapp I E, Shawler T, Benson J, Whitacre C C. The thymus plays a role in oral tolerance in experimental autoimmune encephalomyelitis. J Immunol. 2006;177:1500–1509. doi: 10.4049/jimmunol.177.3.1500. [DOI] [PubMed] [Google Scholar]
- Weishaupt A, Jander S, Bruck W, Kuhlmann T, Stienekemeier M, Hartung T, Toyka K V, Stoll G, Gold R. Molecular mechanisms of high-dose antigen therapy in experimental autoimmune encephalomyelitis: rapid induction of Th1-type cytokines and inducible nitric oxide synthase. J Immunol. 2000;165:7157–7163. doi: 10.4049/jimmunol.165.12.7157. [DOI] [PubMed] [Google Scholar]
- Minguela A, Pastor S, Mi W, Richardson J A, Ward E S. Feedback regulation of murine autoimmunity via dominant anti-inflammatory effects of interferon γ. J Immunol. 2007;178:134–144. doi: 10.4049/jimmunol.178.1.134. [DOI] [PubMed] [Google Scholar]
- Chan J, Ban E J, Chun K H, Wang S, Backstrom B T, Bernard C C, Toh B H, Alderuccio F. Transplantation of bone marrow transduced to express self-antigen establishes deletional tolerance and permanently remits autoimmune disease. J Immunol. 2008;181:7571–7580. doi: 10.4049/jimmunol.181.11.7571. [DOI] [PubMed] [Google Scholar]
- Sewell D, Qing Z, Reinke E, Elliot D, Weinstock J, Sandor M, Fabry Z. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol. 2003;15:59–69. doi: 10.1093/intimm/dxg012. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hancock W W, Marks R, Gonnella P, Weiner H L. Mechanisms of recovery from experimental autoimmune encephalomyelitis: T cell deletion and immune deviation in myelin basic protein T cell receptor transgenic mice. J Neuroimmunol. 1998;82:149–159. doi: 10.1016/s0165-5728(97)00193-8. [DOI] [PubMed] [Google Scholar]
- Nicholson L B, Greer J M, Sobel R A, Lees M B, Kuchroo V K. An altered peptide ligand mediates immune deviation and prevents autoimmune encephalomyelitis. Immunity. 1995;3:397–405. doi: 10.1016/1074-7613(95)90169-8. [DOI] [PubMed] [Google Scholar]
- Young D A, Lowe L D, Booth S S, Whitters M J, Nicholson L, Kuchroo V K, Collins M. IL-4, IL-10, IL-13, and TGF-β from an altered peptide ligand-specific Th2 cell clone down-regulate adoptive transfer of experimental autoimmune encephalomyelitis. J Immunol. 2000;164:3563–3572. doi: 10.4049/jimmunol.164.7.3563. [DOI] [PubMed] [Google Scholar]
- Saoudi A, Simmonds S, Huitinga I, Mason D. Prevention of experimental allergic encephalomyelitis in rats by targeting autoantigen to B cells: evidence that the protective mechanism depends on changes in the cytokine response and migratory properties of the autoantigen-specific T cells. J Exp Med. 1995;182:335–344. doi: 10.1084/jem.182.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Gregg R K, Bell J J, Ellis J S, Divekar R, Lee H H, Jain R, Waldner H, Hardaway J C, Collins M, Kuchroo V K, Zaghouani H. Specific T regulatory cells display broad suppressive functions against experimental allergic encephalomyelitis upon activation with cognate antigen. J Immunol. 2005;174:6772–6780. doi: 10.4049/jimmunol.174.11.6772. [DOI] [PubMed] [Google Scholar]
- Zhong M C, Kerlero de Rosbo N, Ben-Nun A. Multiantigen/multiepitope-directed immune-specific suppression of “complex autoimmune encephalomyelitis” by a novel protein product of a synthetic gene. J Clin Invest. 2002;110:81–90. doi: 10.1172/JCI15692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E A, Cofiell R, Wilkins J A, Raine C S, Matis L A, Mueller J P. Immune tolerance mediated by recombinant proteolipid protein prevents experimental autoimmune encephalomyelitis. J Neuroimmunol. 1997;79:1–11. doi: 10.1016/s0165-5728(97)00093-3. [DOI] [PubMed] [Google Scholar]
- Ho P P, Fontoura P, Platten M, Sobel R A, DeVoss J J, Lee L Y, Kidd B A, Tomooka B H, Capers J, Agrawal A, Gupta R, Zernik J, Yee M K, Lee B J, Garren H, Robinson W H, Steinman L. A suppressive oligodeoxynucleotide enhances the efficacy of myelin cocktail/IL-4-tolerizing DNA vaccination and treats autoimmune disease. J Immunol. 2005;175:6226–6234. doi: 10.4049/jimmunol.175.9.6226. [DOI] [PubMed] [Google Scholar]
- Garren H, Ruiz P J, Watkins T A, Fontoura P, Nguyen L T, Estline E R, Hirschberg D L, Steinman L. Combination of gene delivery and DNA vaccination to protect from and reverse Th1 autoimmune disease via deviation to the Th2 pathway. Immunity. 2001;15:15–22. doi: 10.1016/s1074-7613(01)00171-6. [DOI] [PubMed] [Google Scholar]
- Margot C D, Ford M L, Evavold B D. Amelioration of established experimental autoimmune encephalomyelitis by an MHC anchor-substituted variant of proteolipid protein 139–151. J Immunol. 2005;174:3352–3358. doi: 10.4049/jimmunol.174.6.3352. [DOI] [PubMed] [Google Scholar]
- Turley D M, Miller S D. Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:2212–2220. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- McDevitt H. Specific antigen vaccination to treat autoimmune disease. Proc Natl Acad Sci USA. 2004;101:14627–14630. doi: 10.1073/pnas.0405235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedotti R, Mitchell D, Wedemeyer J, Karpuj M, Chabas D, Hattab E M, Tsai M, Galli S J, Steinman L. An unexpected version of horror autotoxicus: anaphylactic shock to a self-peptide. Nat Immunol. 2001;2:216–222. doi: 10.1038/85266. [DOI] [PubMed] [Google Scholar]
- Mannie M D, Abbott D J, Blanchfield J L. Experimental autoimmune encephalomyelitis in Lewis rats: IFN-β acts as a tolerogenic adjuvant for induction of neuroantigen-dependent tolerance. J Immunol. 2009;182:5331–5341. doi: 10.4049/jimmunol.0803756. [DOI] [PubMed] [Google Scholar]
- Mannie M D, Abbott D J. A fusion protein consisting of IL-16 and the encephalitogenic peptide of myelin basic protein constitutes an antigen-specific tolerogenic vaccine that inhibits experimental autoimmune encephalomyelitis. J Immunol. 2007;179:1458–1465. doi: 10.4049/jimmunol.179.3.1458. [DOI] [PubMed] [Google Scholar]
- Mannie M D, Clayson B A, Buskirk E J, DeVine J L, Hernandez J J, Abbott D J. IL-2/neuroantigen fusion proteins as antigen-specific tolerogens in experimental autoimmune encephalomyelitis (EAE): correlation of T cell-mediated antigen presentation and tolerance induction. J Immunol. 2007;178:2835–2843. doi: 10.4049/jimmunol.178.5.2835. [DOI] [PubMed] [Google Scholar]
- Mannie M D, Devine J L, Clayson B A, Lewis L T, Abbott D J. Cytokine-neuroantigen fusion proteins: new tools for modulation of myelin basic protein (MBP)-specific T cell responses in experimental autoimmune encephalomyelitis. J Immunol Methods. 2007;319:118–132. doi: 10.1016/j.jim.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Yu S, Zhao Z, Ciric B, Zhang G X, Rostami A. Antigen presenting cells treated in vitro by macrophage colony-stimulating factor and autoantigen protect mice from autoimmunity. J Neuroimmunol. 2007;192:68–78. doi: 10.1016/j.jneuroim.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y M, Yang J S, Xu L Y, Link H, Xiao B G. Autoantigen-pulsed dendritic cells induce tolerance to experimental allergic encephalomyelitis (EAE) in Lewis rats. Clin Exp Immunol. 2000;122:437–444. doi: 10.1046/j.1365-2249.2000.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury S J, Gallon L, Chen W, Betres K, Russell M E, Hancock W W, Carpenter C B, Sayegh M H, Weiner H L. Mechanisms of acquired thymic tolerance in experimental autoimmune encephalomyelitis: thymic dendritic-enriched cells induce specific peripheral T cell unresponsiveness in vivo. J Exp Med. 1995;182:357–366. doi: 10.1084/jem.182.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang G X, Chen Y, Xu H, Fitzgerald D C, Zhao Z, Rostami A. CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008;181:2483–2493. doi: 10.4049/jimmunol.181.4.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117:2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools N, Ponsaerts P, Van Tendeloo V F, Berneman Z N. Balancing between immunity and tolerance: an interplay between dendritic cells, regulatory T cells, and effector T cells. J Leukoc Biol. 2007;82:1365–1374. doi: 10.1189/jlb.0307166. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Dudziak D, Heidkamp G F, Fiorese C, Bonito A J, Inaba K, Nussenzweig M C, Steinman R M. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Steinman R M. Dendritic cells as controllers of antigen-specific Foxp3+ regulatory T cells. J Dermatol Sci. 2009;54:69–75. doi: 10.1016/j.jdermsci.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J A. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- Conti L, Gessani S. GM-CSF in the generation of dendritic cells from human blood monocyte precursors: recent advances. Immunobiology. 2008;213:859–870. doi: 10.1016/j.imbio.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Blank K, Lindner P, Diefenbach B, Pluckthun A. Self-immobilizing recombinant antibody fragments for immunoaffinity chromatography: generic, parallel, and scalable protein purification. Protein Expr Purif. 2002;24:313–322. doi: 10.1006/prep.2001.1575. [DOI] [PubMed] [Google Scholar]
- Mannie M D, Norris M S. MHC class-II-restricted antigen presentation by myelin basic protein-specific CD4+ T cells causes prolonged desensitization and outgrowth of CD4– responders. Cell Immunol. 2001;212:51–62. doi: 10.1006/cimm.2001.1843. [DOI] [PubMed] [Google Scholar]
- Patel D M, Arnold P Y, White G A, Nardella J P, Mannie M D. Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition. J Immunol. 1999;163:5201–5210. [PubMed] [Google Scholar]
- Mannie M D, Fraser D J, McConnell T J. IL-4 responsive CD4+ T cells specific for myelin basic protein: IL-2 confers a prolonged postactivation refractory phase. Immunol Cell Biol. 2003;81:8–19. doi: 10.1046/j.1440-1711.2003.01131.x. [DOI] [PubMed] [Google Scholar]
- Woollett G R, Barclay A N, Puklavec M, Williams A F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985;15:168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]
- Patel D M, Dudek R W, Mannie M D. Intercellular exchange of class II MHC complexes: ultrastructural localization and functional presentation of adsorbed I-A/peptide complexes. Cell Immunol. 2001;214:21–34. doi: 10.1006/cimm.2002.1887. [DOI] [PubMed] [Google Scholar]
- Chen T T, Tao M H, Levy R. Idiotype-cytokine fusion proteins as cancer vaccines. Relative efficacy of IL-2, IL-4, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1994;153:4775–4787. [PubMed] [Google Scholar]
- Tao M H, Levy R. Idiotype/granulocyte-macrophage colony-stimulating factor fusion protein as a vaccine for B-cell lymphoma. Nature. 1993;362:755–758. doi: 10.1038/362755a0. [DOI] [PubMed] [Google Scholar]
- Schwegler C, Dorn-Beineke A, Nittka S, Stocking C, Neumaier M. Monoclonal anti-idiotype antibody 6G6.C4 fused to GM-CSF is capable of breaking tolerance to carcinoembryonic antigen (CEA) in CEA-transgenic mice. Cancer Res. 2005;65:1925–1933. doi: 10.1158/0008-5472.CAN-04-3591. [DOI] [PubMed] [Google Scholar]
- Wortham C, Grinberg L, Kaslow D C, Briles D E, McDaniel L S, Lees A, Flora M, Snapper C M, Mond J J. Enhanced protective antibody responses to PspA after intranasal or subcutaneous injections of PspA genetically fused to granulocyte-macrophage colony-stimulating factor or interleukin-2. Infect Immun. 1998;66:1513–1520. doi: 10.1128/iai.66.4.1513-1520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso C L, Zisman A, Pantuck A, Calilliw R, Hernandez J M, Paik S, Nguyen D, Gitlitz B, Shintaku P I, de Kernion J, Figlin R, Belldegrun A. Induction of G250-targeted and T-cell-mediated antitumor activity against renal cell carcinoma using a chimeric fusion protein consisting of G250 and granulocyte/monocyte-colony stimulating factor. Cancer Res. 2001;61:7925–7933. [PubMed] [Google Scholar]
- Rodriguez D, Rodriguez J R, Llorente M, Vazquez I, Lucas P, Esteban M, Martinez A C, del Real G. A human immunodeficiency virus type 1 Env-granulocyte-macrophage colony-stimulating factor fusion protein enhances the cellular immune response to Env in a vaccinia virus-based vaccine. J Gen Virol. 1999;80:217–223. doi: 10.1099/0022-1317-80-1-217. [DOI] [PubMed] [Google Scholar]
- Picca C C, Larkin J, III, Boesteanu A, Lerman M A, Rankin A L, Caton A J. Role of TCR specificity in CD4+ CD25+ regulatory T-cell selection. Immunol Rev. 2006;212:74–85. doi: 10.1111/j.0105-2896.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- Larkin J, III, Rankin A L, Picca C C, Riley M P, Jenks S A, Sant A J, Caton A J. CD4+CD25+ regulatory T cell repertoire formation shaped by differential presentation of peptides from a self-antigen. J Immunol. 2008;180:2149–2157. doi: 10.4049/jimmunol.180.4.2149. [DOI] [PubMed] [Google Scholar]
- Cunningham M W. Autoimmunity and molecular mimicry in the pathogenesis of post-streptococcal heart disease. Front Biosci. 2003;8:s533–s543. doi: 10.2741/1067. [DOI] [PubMed] [Google Scholar]
- Guilherme L, Ramasawmy R, Kalil J. Rheumatic fever and rheumatic heart disease: genetics and pathogenesis. Scand J Immunol. 2007;66:199–207. doi: 10.1111/j.1365-3083.2007.01974.x. [DOI] [PubMed] [Google Scholar]
- Ioachimescu O C, Kavuru M S. Pulmonary alveolar proteinosis. Chron Respir Dis. 2006;3:149–159. doi: 10.1191/1479972306cd101rs. [DOI] [PubMed] [Google Scholar]
- Pette M, Fujita K, Kitze B, Whitaker J N, Albert E, Kappos L, Wekerle H. Myelin basic protein-specific T lymphocyte lines from MS patients and healthy individuals. Neurology. 1990;40:1770–1776. doi: 10.1212/wnl.40.11.1770. [DOI] [PubMed] [Google Scholar]
- McQualter J L, Darwiche R, Ewing C, Onuki M, Kay T W, Hamilton J A, Reid H H, Bernard C C. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194:873–882. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev E D, Shriver L P, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel B N. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- Aloisi F, De Simone R, Columba-Cabezas S, Penna G, Adorini L. Functional maturation of adult mouse resting microglia into an APC is promoted by granulocyte-macrophage colony-stimulating factor and interaction with Th1 cells. J Immunol. 2000;164:1705–1712. doi: 10.4049/jimmunol.164.4.1705. [DOI] [PubMed] [Google Scholar]
- King I L, Dickendesher T L, Segal B M. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusic S, Miyashiro J S, Douhan J, III, Konz R F, Xuan D, Pelker J W, Ling V, Leonard J P, Jacobs K A. Local delivery of granulocyte macrophage colony-stimulating factor by retrovirally transduced antigen-specific T cells leads to severe, chronic experimental autoimmune encephalomyelitis in mice. Neurosci Lett. 2002;332:185–189. doi: 10.1016/s0304-3940(02)00947-3. [DOI] [PubMed] [Google Scholar]
- Kroenke M A, Carlson T J, Andjelkovic A V, Segal B M. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh B B, Cheatem D M, Sheng J R, Vasu C, Prabhakar B S. GM-CSF-induced CD11c+CD8a–dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol. 2009;21:269–282. doi: 10.1093/intimm/dxn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G, Amrani A. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:3638–3647. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]
- Sheng J R, Li L, Ganesh B B, Vasu C, Prabhakar B S, Meriggioli M N. Suppression of experimental autoimmune myasthenia gravis by granulocyte-macrophage colony-stimulating factor is associated with an expansion of FoxP3+ regulatory T cells. J Immunol. 2006;177:5296–5306. doi: 10.4049/jimmunol.177.8.5296. [DOI] [PubMed] [Google Scholar]
- Sheng J R, Li L C, Ganesh B B, Prabhakar B S, Meriggioli M N. Regulatory T cells induced by GM-CSF suppress ongoing experimental myasthenia gravis. Clin Immunol. 2008;128:172–180. doi: 10.1016/j.clim.2008.03.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kared H, Leforban B, Montandon R, Renand A, Layseca Espinosa E, Chatenoud L, Rosenstein Y, Schneider E, Dy M, Zavala F. Role of GM-CSF in tolerance induction by mobilized hematopoietic progenitors. Blood. 2008;112:2575–2578. doi: 10.1182/blood-2008-02-140681. [DOI] [PubMed] [Google Scholar]
- Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- Serafini P, Carbley R, Noonan K A, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- Sotomayor E M, Fu Y X, Lopez-Cepero M, Herbert L, Jimenez J J, Albarracin C, Lopez D M. Role of tumor-derived cytokines on the immune system of mice bearing a mammary adenocarcinoma. II. Down-regulation of macrophage-mediated cytotoxicity by tumor-derived granulocyte-macrophage colony-stimulating factor. J Immunol. 1991;147:2816–2823. [PubMed] [Google Scholar]
- Tang H, Guo Z, Zhang M, Wang J, Chen G, Cao X. Endothelial stroma programs hematopoietic stem cells to differentiate into regulatory dendritic cells through IL-10. Blood. 2006;108:1189–1197. doi: 10.1182/blood-2006-01-007187. [DOI] [PubMed] [Google Scholar]
- Zhang M, Tang H, Guo Z, An H, Zhu X, Song W, Guo J, Huang X, Chen T, Wang J, Cao X. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch J V, Steinman R M, Nussenzweig M C. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer-Pack M D, Swiggard W J, Mirza A, Inaba K, Steinman R M. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. II. Expression in situ in lymphoid and nonlymphoid tissues. Cell Immunol. 1995;163:157–162. doi: 10.1006/cimm.1995.1110. [DOI] [PubMed] [Google Scholar]
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig M C, Steinman R M. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpfheller C, Finke J S, Lopez C B, Moran T M, Moltedo B, Soares H, Huang Y, Schlesinger S J, Park C G, Nussenzweig M C, Granelli-Piperno A, Steinman R M. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger D, Masilamani R F, Bettelli E, Kuchroo V K, Nussenzweig M C. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695–705. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]