Abstract
Androgens are functionally required for the normal growth of the prostate gland and in prostate tumor development and progression. Epithelial-mesenchymal-transition (EMT) is an important process during normal development and in cancer cell metastasis induced by factors within the microenvironment, such as transforming growth factor-β (TGF-β). This study examined the ability of androgens to influence EMT of prostate cancer epithelial cells. The EMT pattern was evaluated on the basis of expression of the epithelial markers E-cadherin/β-catenin, and the mesenchymal markers N-cadherin, as well as cytoskeleton reorganization in response to 5α-dihydrotestosterone (DHT; 1 nM) and/or TGF-β (5 ng/ml). Overexpressing and silencing approaches to regulate androgen receptor (AR) expression were conducted to determine the involvement of AR in EMT in the presence or absence of an AR antagonist. Our results demonstrate that androgens induce the EMT pattern in prostate tumor epithelial cell with Snail activation and lead to significant changes in prostate cancer cell migration and invasion potential. Expression levels of AR inversely correlated with androgen-mediated EMT in prostate tumor epithelial cells, pointing to a low AR content required for the EMT phenotype. These findings indicate the ability of androgens to induce EMT by potentially bypassing the functional involvement of TGF-β, thus contributing to metastatic behavior of prostate cancer cells.—Zhum, M.-L., Kyprianou, N. Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells.
Keywords: cytoskeleton reorganization, actin, talin, cofilin, metastasis, TGF-β signaling
Prostate cancer is the most frequently diagnosed nonskin cancer and the third leading cause of cancer mortality among men in the United States (1). Prostate cancer mortality is primarily due to failure to cure patients with metastatic disease. In the initial stages, prostate cancer is dependent on androgens for growth and can be suppressed by androgen deprivation therapy (ADT) (2). Prostate tumors, however, eventually recur due to a transition from androgen-dependent to an androgen-independent state leading to highly metastatic disease for which there is no effective therapy available. Androgen action proceeds via an axis involving testicular synthesis of testosterone, its transport to target tissues, and its conversion by 5α-reductase to the active metabolite 5α-dihydrotestosterone (DHT). Androgens exert their biological effects by binding to the androgen receptor (AR) and inducing its transcriptional activity. The 5α-reductase enzyme is present in the urogenital sinus before and during prostate development (3, 4), and its inhibition during fetal development results in partial prostate development (5). In adult males, androgens promote secretory epithelial cell survival, the cells primarily transformed in tumor development (6). Androgen deprivation is the only clinically effective therapy for advanced prostate cancer; however, because of the relapse of castration-resistant androgen-independent tumors, the long-term benefit of androgen deprivation in patients with metastatic disease has been debated (7,8,9).
The process of epithelial-mesenchymal transition (EMT) is a critical event during embryonic development, required for morphogenetic movements during parietal endoderm formation, gastrulation, and formation of organs and tissues (e.g., neural crest, heart, and craniofacial structures) (10). A growing body of recent evidence links EMT to tumor progression and metastasis. Loss of epithelial-cell markers (e.g., E-cadherin and β-catenin) and gain of mesenchymal-cell markers (e.g., N-cadherin and vimentin), particularly at the leading edge or invasive front of solid tumors, has been reported in human tumor specimens and is associated with tumor progression to metastasis (11). Epithelial tumor cells lose cell polarity and cell-junction proteins and at the same time acquire protein mesenchymal-cell markers (e.g., N-cadherin and vimentin) and signaling activities associated with mesenchymal cells facilitating migration and survival in an anchorage-independent environment and ultimately metastasis (11, 12). Pathological EMT in tumor cells results from transcriptional reprogramming of abnormal survival signals via receptors, such as platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), transform growth factor-β receptor (TGF-βR), and insulin-like growth factor-1 receptor (IGF-1R); and regulatory kinases, such as PI3K, AKT, and mTOR (13, 14). TGF-β is a potent EMT inducer in normal development and organ homeostasis, as well as during tumor progression (15). TGF-β induces EMT via Smad-dependent and Smad-independent transcriptional pathways (16). Thus Smad-mediated induction of Snail, Slug, and Twist via HMGA2 (high-motility group A2) and Smad-independent phosphorylation of Par6 contribute to dissolution of cell-junction complexes (17, 18). Furthermore, EMT recruits the cooperation between oncogenic Ras and receptor tyrosine kinases (RTKs) to induce downstream Raf/MAPK signaling associated with tumor progression and poor clinical diagnosis (19).
We previously demonstrated a functional interplay between androgens and TGF-β signaling toward enhanced apoptosis in androgen-sensitive prostate cancer cells (20). Because tumor epithelial cells gain the ability to migrate and invade by dedifferentiating through activation of biological pathways associated with EMT, the present study investigated the involvement of the androgen signaling axis in EMT and invasive phenotype of prostate cancer cells. We report that androgens induce changes characteristic of EMT and cytoskeleton reorganization, involved in the metastatic behavior of castration-resistant prostate cancer cells.
MATERIALS AND METHODS
Cell lines and transfections
The androgen-sensitive and TGF-β-responsive human prostate cancer LNCaP TβRII cells (generated in our laboratory) (21, 22) and the parental LNCaP, CW22, and PC-3 prostate cancer cell lines were used. The human breast cancer MCF-7 cells and the human renal cancer 786-0 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). To determine the effects of DHT (Sigma-Aldrich, St. Louis, MO, USA) and TGF-β (R&D Systems, Minneapolis, MN, USA), cells were grown in DMEM or RPMI 1640 with 10% FBS (without phenol red) and transferred to medium 5% charcoal strip serum (CSS) before treatment. Casodex was a generous gift from Dr. Chendil Damodaran (University of Kentucky College of Health Sciences). Subconfluent cultures of PC-3 or LNCaP cells were transfected with the pCDNA-Zeo AR vector or AR shRNA vector (Open Biosystems, Huntsville, AL, USA), using the Lipofectamine 2000 reagent (Invitrogen; Carlsbad, CA, USA). pCDNA-zeo AR construct was prepared by cloning the full AR fragment from pCMV5-AR vector (BamHI/XhoI). pCMV5-AR was a generous gift from Dr. Donald Tindall (Mayo Clinic, Rochester, MN, USA). After transfection (exposure to plasmid DNA for 6 h at 37°C, 5% CO2), the growth medium was changed to 10% FCS for 48 h, prior to selection in antibiotic-containing medium (25 μg/ml zeosin/puromycin; Invitrogen, Grand Island, NY, USA). Individual colonies were selected, cloned, and grown in 10% FCS-containing medium. Protein expression of transfected AR was examined by Western blot analysis.
Wounding assay
Cell cultures (80% confluency) were subjected to wounding as described previously (23). The number of cells migrating to the wounding area was counted at the end points indicated.
Flow cytometry analysis
Prostate cancer cells (1×106) were labeled using a specific primary antibody and stained by fluorescent-conjugated secondary antibody. Samples were subjected to fluorescence analysis using the Partec system (Partec GmbH, Münster, Germany).
Western blot analyses
Total cellular protein was extracted from the cell pellets by homogenization in RIPA buffer. Protein samples (20–60 μg) were loaded on 4%/12% SDS-PAGE gels and subjected to electrophoretic analysis and subsequent blocking. Membranes were incubated with the primary antibody (overnight at 4°C) and the relevant secondary antibodies (1 h at room temperature). The E-cadherin, β-catenin, vimentin, and Parp antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA); The AR, tubulin, and N-cadherin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA); The cofilin and α-actin antibodies were purchased from Sigma-Aldrich. The antibody against talin1 was a generous gift from Dr. R. McCann (Mercer College, Macon, GA, USA); The GAPDH antibody was purchased from Novus Biologicals (Littleton, CO, USA).
Invasion assay
The invasion ability of prostate cancer cells was determined using the transwell chamber assay. Matrigel (1 mg/ml) in serum-free cold cell culture medium was placed in the upper chamber of a 24-well transwell and incubated for 5 h at 37°C. Cells were harvested, and cell suspensions (100 μl) were placed on the matrigel, and the lower chamber of the transwell was filled with culture medium in the presence of 5 μg/ml fibronectin, as an adhesive substrate. DHT (1 nM) was added in both upper and lower chambers. Following 48 h of incubation at 37°C, transwells were removed and stained with Giemsa solution. Noninvading cells on top of transwells were removed, and invading cells were counted under the microscope.
Immunofluorescence staining
Cells were plated (1×105 cells/well) in chamber slides, and after 24 h, cells were incubated with RPMI 1640 + 10% CSS supplemented with either DHT (1 nM), TGF-β (5 ng/ml), or the combination of DHT (1 nM)-TGF-β (5 ng/ml), as indicated. Following treatment, cells were fixed in 2% paraformaldehyde in phosphate-buffered saline (PBS) and permeabilized in 0.1% Triton X-100 in PBS. Cells were stained by incubation with the primary antibody (overnight at 4°C), followed by exposure to the secondary immunofluorescence antibody and FITC-phalloidin, (1 h at room temperature). FITC-phalloidin was purchased from Sigma-Aldrich. Slides were mounted by Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA).
RNA extraction and real-time RT-PCR
RNA samples extracted with TRIzol reagent were treated with RNase-free DNase I and reverse transcript to cDNA (Bio-Rad, Hercules, CA, USA). Taqman real-time RT-PCR analysis of the cDNA samples was conducted in an ABI 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) with the specific primers of E-cadherin and Snail (Applied Biosystems).
Statistical analysis
One-way analysis of variance (ANOVA) was performed using the StatView statistical program (SAS Institute, Cary, NC, USA) to determine the statistical significance between values. All numerical data are presented as mean ± se values. A value of P < 0.05 was considered statistically significant.
RESULTS
Effect of androgens on EMT pattern of prostate cancer cells
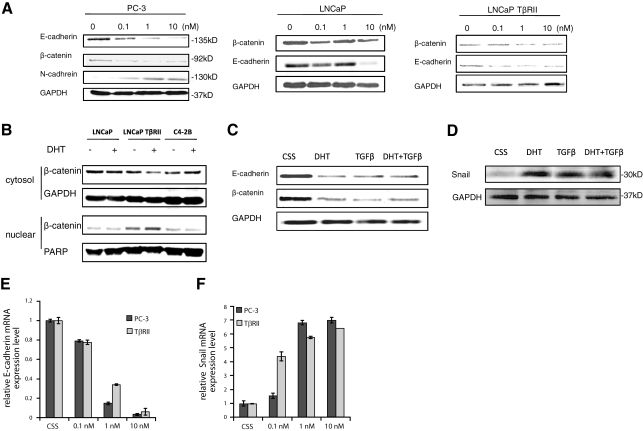
Exposure of PC-3 prostate cancer cells to DHT results in reduced expression of the epithelial markers, E-cadherin and β-catenin, and induction of the mesenchymal marker, N-cadherin expression (Fig. 1A), changes characteristic of EMT. The androgen-sensitive LNCaP cells did not exhibit the same sensitivity as PC-3 cells to DHT-induced EMT; a significant reduction in E-cadherin and β-catenin was detected only after exposure to high doses of DHT (10 nM) (Fig. 1A). However, the presence of TGF-β receptor II (TGF-βRII) sensitizes LNCaP prostate cancer cells to the androgenic effect on EMT (0.1 nM DHT) (Fig. 1A). Since nuclear translocation of β-catenin has been established as a significant event in EMT (24, 25), we subsequently analyzed the cytosolic and nuclear fractions of three different cell lines, LNCaP, LNCaP TβRII, and C4–2B; we found that DHT triggered a marked nuclear translocation of β-catenin only in LNCaP TβRII cells. Consistent with the E-cadherin expression pattern (Fig. 1A), DHT (1 nM) failed to trigger β-catenin nuclear translocation in either the LNCaP or the C4–2 cells (Fig. 1B). To determine the transcriptional modulation of E-cadherin by DHT, quantitative PCR analysis was performed, and down-regulation of E-cadherin mRNA levels was detected in both PC-3 and LNCaP TβRII cells (Fig. 1E).
Figure 1.
Effect of androgens on EMT of prostate cancer cells. A) Prostate cancer cells (PC-3, LNCaP, and LNCaP TβRII) were treated with DHT (0.1–10 nM) as shown for 72 h. Total cell lysates were analyzed by Western blotting to determine the expression of E-cadherin, β-catenin, and N-cadherin. B) LNCaP, LNCaP TβRII, and C4–2B cells were treated with DHT for 72 h and subjected to subcellular fractionation as described in Materials and Methods. Western blot analysis was performed in cytosolic and nuclear fractions to determine β-catenin levels. GAPDH and PARP served as an internal control for cytosolic and nuclear fractions, respectively. C, D) Combined effect of androgens and TGF-β on EMT markers E-cadherin and β-catenin (C) and Snail protein expression (D). E, F) Results from the real-time PCR analysis of E-cadherin and Snail mRNA levels. LNCaP TβRII and PC-3 cells were treated with DHT (0.1–10 nM) for 24 h, and relative mRNA expression levels of E-cadherin (E) and Snail (F) were evaluated as described in Materials and Methods.
In view of the widely acknowledged role of TGF-β as a potent EMT inducer, the effect of TGF-β on prostate cancer cell EMT was examined as a positive/reference control. Exposure to DHT alone or in combination with TGF-β led to comparable reduction in E-cadherin and β-catenin levels in LNCaP TβRII cells (Fig. 1C). Because EMT is driven by the transcriptional factor Snail, which is up-regulated by TGF-β (16, 26), we subsequently investigated the effect of DHT on Snail expression. As shown in Fig. 1D, treatment of LNCaP TβRII cells with DHT alone or in combination with TGF-β led to a significant increase in Snail expression. Furthermore, a marked induction in Snail expression by DHT was detected at the mRNA level in both LNCaP TβRII and PC-3 cells (Fig. 1F).
Androgens affect cytoskeleton reorganization in prostate cancer cells
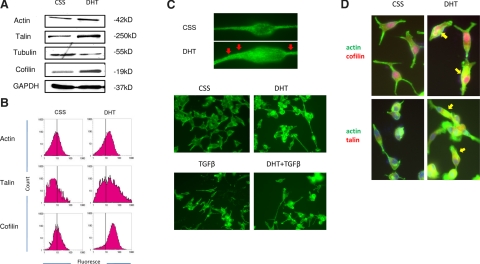
The process of cytoskeleton reorganization, which directly affects cell migration and metastatic ability, is a characteristic phenomenon in EMT. One of the critical proteins that promotes actin polymerization and defines the direction of cell motility is cofilin. Cofilin is a ubiquitous actin-binding factor required for the reorganization of actin filaments by causing depolymerization at the end of filaments and preventing their reassembly (27, 28). Talin is another actin-binding protein that links integrins to the actin cytoskeleton in focal adhesion complexes and plays a role in cell adhesion and cell motility (29, 30). To study changes in cytoskeleton organization responses to androgens, the expression of key cytoskeleton components was evaluated by Western blot analysis and immunofluorescence staining. DHT treatment of LNCaP TβRII cells led to up-regulation of β-actin and its partner cofilin, as well as the major focal adhesion effector, talin (Fig. 2A). Expression of α-tubulin was also significantly down-regulated (Fig. 2A). Flow cytometric analysis revealed a significant increase in actin, talin, and cofilin fluorescence density in cells after DHT treatment, compared to CSS-control cells (Fig. 2B). In addition, DHT exposure led to changes in actin cytoskeleton reorganization: prostate cancer cells exhibit more cytopodia and microvilli (Fig. 2C, red arrowheads) and share similar features with TGF-β-treated cells. In addition, a large number of cells acquire a more round morphology in response to DHT and TGF-β treatment (Fig. 2C). Exposure to DHT for 3 d enhanced the association of actin with both cofilin and talin (Fig. 2D, arrowheads). A similar association was detected after short-term exposure (10 min) to DHT (data not shown). These observations implicate an association between the actin microfilaments with cell motility and migration in response to androgens, possibly facilitating interaction with the ECM.
Figure 2.
Androgens regulate cytoskeleton reorganization of prostate cancer cells. A) LNCaP TβRII cells were exposed to DHT (1 nM) and/or TGF-β (5 ng/ml). Expression of actin, talin, cofilin, and tubulin was determined by Western blot analysis. GAPDH served as internal loading control. B) LNCaP TβRII cells were exposed to DHT and subjected to immunofluorescence for actin, talin, and cofilin detection. Level of cytoskeleton proteins was assessed by FACS. C) LNCaP TβRII cells were treated with DHT and/or TGF-β, and F-actin was detected using FITC-phalloidin under fluorescent microscopy. Red arrowheads indicate microvilli formation. D) Actin colocalization of talin and cofilin in response to androgens (yellow arrowheads); after treatment with DHT, LNCaP TβRII cells were subjected to immunofluorescence: red indicates cofilin (top panels) and talin (bottom panels), respectively; green indicates actin; blue indicates nuclear staining; and yellow indicates colocalization.
Androgens and TGF-β promote prostate cancer cell migration and invasion
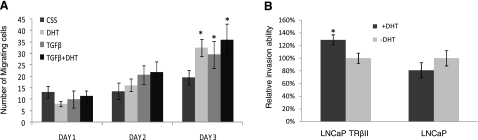
Exposure of LNCAP TβRII cells to either DHT or TGF-β (as single treatments) significantly enhanced cell migration. Interestingly, the DHT/TGF-β combination did not lead to a synergistic increase in prostate cancer cell migration ability after 3 d treatment (Fig. 3A), consistent with our observation in EMT pattern (Fig. 1C, D). We subsequently examined the effect of DHT on prostate cancer cell invasion using the Boyden chamber invasion assay. As shown in Fig. 3B, DHT enhances the invasion ability of LNCaP TβRII cells, but has no significant effect on the parental LNCaP cells, consistent with our observation that low androgen levels (1 nM DHT) failed to induce EMT in LNCaP cells, indicating that intact TGF-β signaling is required for the manifestation of the androgenic effect.
Figure 3.
Effect of androgens and TGF-β on prostate cancer cell invasive behavior. A) LNCaP TβRII cells were treated with DHT (1 nM) and/or TGF-β (5 ng/ml) for 24, 48, and 72 h, and cell migration was determined. B) Effect of androgens on prostate cancer cell invasion. LNCaP and LNCaP TβRII cells were exposed to DHT for 48 h, and cell invasion was assessed as described in Materials and Methods.
High AR content suppresses androgen-induced EMT phenotype
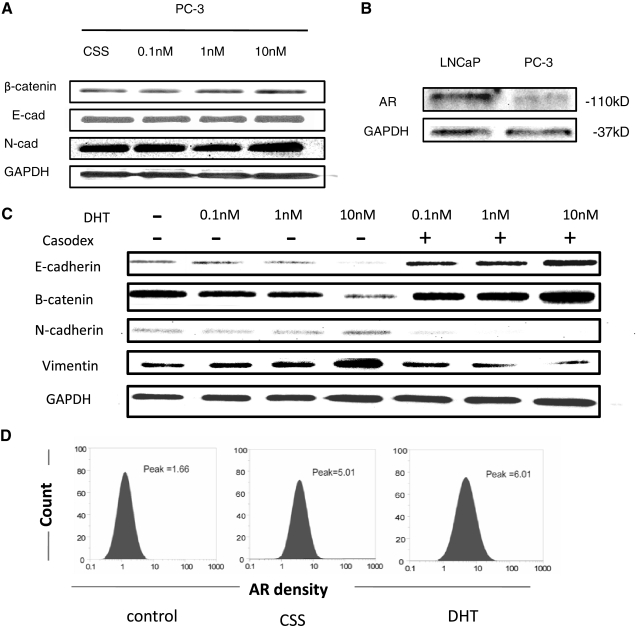
PC-3 cells exhibited a strong sensitivity to the EMT effect by DHT (Fig. 1A). To determine the role of AR in androgen-induced EMT, we initially evaluated AR expression in PC-3 cells using Western blot analysis and flow cytometric analysis. In accordance with the recent reports that AR is expressed in PC-3 cells at low levels (31, 32), we found that prolonged exposure of Western blots revealed detectable AR levels (Fig. 4B). This was confirmed by FACS that revealed a marked peak shift in AR immunofluorescence, compared to the isotope IgG staining control, and AR expression could be induced by the DHT treatment (Fig. 4D). Considering the evidence that membrane-located, nonclassical AR could be activated by androgens to elicit multiple downstream effects (33), we pursued the significance of membrane-associated AR in signaling the EMT effect. Figure 4A reveals that in PC-3 cells, treatment with BSA-conjugated testosterone (unable to go through cell membrane) failed to induce the EMT phenotype. However, exposure of both LNCAP TβRII and PC-3 cells to BSA-conjugated testosterone induces critical downstream signaling events, including MAPK and Src activation (Supplemental Fig. 1), implicating that nongenomic AR signaling might be involved in dictating EMT.
Figure 4.
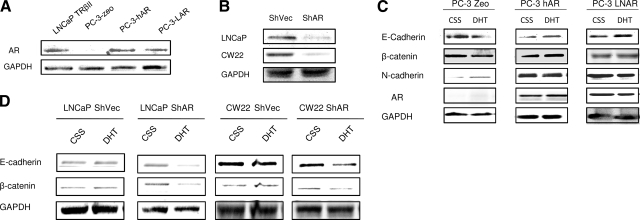
Detection of AR in PC-3 Cells. A) PC-3 cells were treated with increasing doses (0.1, 1, and 10 nM) of BSA-testosterone. Expression patterns of β-catenin, E-cadherin, and N-cadherin were evaluated by Western blotting. GAPDH was used as a loading control. B) AR expression in PC-3 cells after DHT treatment was detected by Western blot analysis. C) PC-3 cells were treated with DHT (0.1–10 nM) and Casodex (10 μM). Immunoblotting was used to assess expression of E-cadherin, β-catenin, N-cadherin, and vimentin. D) AR expression in PC-3 cells after DHT treatment, as determined by immunofluorescence followed by FACS analysis.
Considering that elevation of AR in PC-3 cells suppressed the EMT phenotype, we subsequently determined whether the EMT effect requires AR function (ligand induced). To further confirm the AR involvement in DHT-induced EMT, the EMT phenotype was profiled in the presence of the androgen receptor antagonist Casodex (10 μM) in PC-3 cells. As shown in Fig. 4C, DHT-induced down-regulation of E-cadherin and β-catenin (epithelial markers), and up-regulation of N-cadherin (mesenchymal marker) and vimentin (EMT marker) was abolished (Fig. 4C). The tissue-type specificity of androgen-induced EMT was investigated in AR-bearing MCF-7 human breast cancer cells and 786-0 renal carcinoma cells. In response to DHT, the EMT phenotype was evident in MCF-7 breast cancer cells but not in renal cancer cells (Supplemental Fig. 3).
The potential role of AR in mediating the EMT effect was investigated by introducing the wild-type (wt) AR and the mutant (mt) AR (877A mutation; with higher androgen affinity), in PC-3 cells (low endogenous AR) (Fig. 5A). Overexpression of either the wtAR or mtAR (LNCaP-harbored AR mutation), significantly suppressed the growth of prostate cancer PC-3 cells (Supplemental Fig. 2). The expression pattern of E-cadherin, β-catenin, and N-cadherin was evaluated after exposure of cells to DHT (1 nM). As shown in Fig. 5C, DHT led to decreased expression of E-cadherin and β-catenin, and up-regulation of N-cadherin, changes characteristic of EMT, in the parental PC-3 cells, but not in AR-overexpressing cells.
Figure 5.
Relationship between AR status and EMT. A) Results of AR overexpression in prostate cancer cells. Expression vectors encoding the wild-type AR and mutant AR (harboring the LNCaP AR mutation) were transfected in PC-3 cells. Expression of AR was detected in stable clones by Western blot analysis. B) ShAR RNA was transfected into LNCaP cells and CW22 cells, and stable transfectants were generated. Reduction/loss of AR protein was examined by Western blotting. C) PC-3 Zeo, PC-3-hAR, and PC-3-LAR cells were treated with DHT (1 nM) and/or TGF-β (5 ng/ml). Expression of E-cadherin, β-catenin, and N-cadherin were determined by Western blot analysis. GAPDH served as internal control. D) Expression profile of E-cadherin and β-catenin in LNCaP-null vector control and LNCaP ShAR cells; CW22-null vector control and CW22 ShAR cells after treatment with DHT and TGF-β.
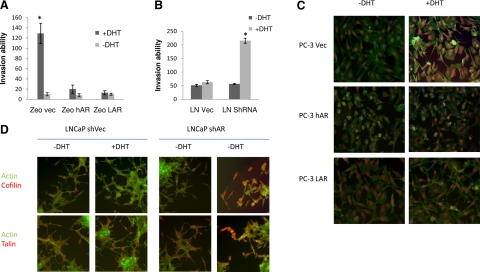
Subsequent experiments examined the effect of DHT on cell migration and invasion in PC-3 AR-overexpressing cells. DHT enhanced the invasion ability in PC-3 parental cells, while there was no effect on either the wt or the mtAR-overexpressing cells (Fig. 6A). To trace androgen-regulated changes in the cytoskeleton reorganization in prostate cancer cells, the intracellular localization and distribution of cofilin and β-actin were determined in response to DHT. As shown on Fig. 6C, in response to DHT, parental PC-3 cells exhibited marked changes in the actin cytoskeleton organization and cofilin/actin colocalization, resembling the EMT characteristics, while AR-overexpressing PC-3 cells failed to exhibit any such changes.
Figure 6.
AR involvement in EMT-related cytoskeleton reorganization and cell invasion. A) PC-3 Zeo, PC-3-hAR, and PC-3-LAR cells were treated with DHT, and their invasion ability was assessed. B) Effect of AR loss on the invasion ability of prostate cancer cells. LNCaP-null vector control cells and LNCaP ARSh cells; CW22-null vector control cells and CW22 ShAR-silenced cells were exposed to androgens, and their invasion potential was determined. C) PC-3 Zeo, PC-3-hAR, and PC-3-LAR cells were treated with DHT and subjected to immunofluorescence analysis as described in Materials and Methods. Red indicates cofilin; green indicates actin microfilaments; blue indicates nuclei. D) LNCaP-null vector control cells and LNCaP AR sh cells; CW22-null vector control cells and CW22 ShAR cells were treated with DHT, and immunofluorescence analysis for actin (green), cofilin (red, top panels), and talin (red, bottom panels) was conducted.
Low AR content sensitizes prostate cancer cells to androgen-induced EMT
The AR requirement in androgen-induced EMT was examined by loss-of-expression studies. AR expression was effectively suppressed in LNCaP and CWR22 cells using the shRNA approach (Fig. 5B). The expression pattern of E-cadherin and β-catenin was used to evaluate the EMT effect in both LNCaP and CWR22 cells. DHT (1 nM) failed to induce EMT in the parental LNCaP or CWR22 cells (Fig. 5D). In cells harboring low AR content, DHT induced down-regulation of E-cadherin and β-catenin (Fig. 5D). Immunofluorescence analysis revealed the actin cytoskeleton reorganization and the enhancement of colocalization of actin filament and cofilin/talin in the AR-silenced cells, but not in parental control cells (Fig. 6D). In addition, DHT increased the invasion potential of LNCaP AR-silenced cells, while there was no significant change in the LNCaP parental cell invasion in response to DHT (Fig. 6B). Thus, low intracellular AR levels sensitize prostate cancer cells to androgen-induced EMT.
DISCUSSION
The precise role of the androgen axis and the effect of androgen-deprivation therapy in prostate cancer metastasis are still unclear. EMT is a process during which polarized epithelial cells acquire a migratory fibroblastoid phenotype and a critical event during cancer metastasis (11, 34). The hallmark of EMT is loss of expression of the cell adhesion molecule E-cadherin. E-cadherin is a cell-cell adhesion molecule that participates in calcium-dependent interactions to form epithelial adherent junctions. Prostate epithelial cells undergo EMT in response to an array of soluble factors, including TGF-β1 plus EGF, IGF-1, and β2-microglobulin (β2-m), or exposure to a bone microenvironment (35). The present findings demonstrate that androgens suppress E-cadherin expression and induce mesenchymal marker expression in prostate cancer epithelial cells. One could argue that this might facilitate escape of prostate cancer cells from the primary site and migration to distant sites, an important concept considering that activation of EMT may result in increased bone turnover, implicated in prostate cancer bone colonization in metastatic disease. Furthermore, alterations in cytoskeleton reorganization induced by androgens may enable cell migration and metastasis of the escaped prostate tumor cells. Changes in actin microfilament network organization in androgen-treated cells could provide active movement assisting cell migration and the dynamics of interaction with adherent molecules in the ECM. Considering that the reactive prostate stroma has been assigned a critical role in the context of the tumor microenvironment in prostate cancer progression to metastasis, AR signaling in prostate fibroblasts may function as a promoter of prostate epithelial cell proliferation (36), as well as a mediator of a functional exchange between prostate epithelial and stromal cells, thus contributing to the EMT effect during cancer metastasis (37).
The existence of crosstalk between androgen and TGF-β signaling has been established (37). Interaction of Smad4, alone or together with Smad3, with the AR in the DNA-binding and ligand-binding domains, may result in the modulation of DHT-induced AR transactivation. In human prostate cancer PC-3 and LNCaP cells, Smad3 enhances AR transactivation, while cotransfection of Smad3 and Smad4 repress AR transactivation (20, 38, 39). The interaction between the androgen axis and TGF-β signaling could be the determining factor for EMT manifestation. Nuclear translocation of β-catenin has been reported in the invasive front of colorectal carcinoma (40). Moreover, β-catenin activates DNA binding protein LEF-1/TCFs to induce several signaling pathways toward mesenchymal marker expression (24). A functional exchange between AR and β-catenin results in increased nuclear colocalization and interaction of AR with β-catenin in castrate-resistant prostate tumors (41, 42). The present study suggests that activation of β-catenin by androgen signaling could serve as an alternative mechanism of androgen-induced EMT in prostate tumor epithelial cells. The involvement of several transcriptional factors (e.g., zinc-finger factors Snail and Slug, 2-handed zinc-finger factors ZEB1 and SIP1, and basic helix-loop-helix factors Twist and E12/E47) in the EMT process by repressing E-cadherin expression and consequently inducing migration and metastasis has recently been documented (43, 44). Downstream activation of Snail by the TGF-β/Smad pathway represses E-cadherin expression in several cancer cell types (26, 45). Our results demonstrate that DHT alone or in combination with TGF-β leads to a significant increase in Snail expression at both the mRNA and protein level in the androgen-sensitive, TGF-β-responsive LNCaP TβRII cells, suggesting that androgens can independently induce EMT, potentially bypassing the effect elicited by TGF-β. The ability of DHT to induce Snail expression in prostate cancer cells by engaging a crosstalk between the androgen axis and TGF-β signaling on Snail transcriptional activation is currently being investigated. Further studies focus on the recruitment of β-catenin by Snail in EMT under conditions of androgen deprivation.
The functional outcome of EMT in prostate cancer progression to castration-resistant disease is likely to be complex, given the uncertainty surrounding the contribution of the androgen axis to prostate cancer metastasis. Indeed, the impact of androgen suppression to metastatic dissemination of prostate cancer cells is still a subject of debate, with the notion that androgen deprivation therapy may down-regulate AR in prostate tumors. One could speculate that a threshold low AR level may promote EMT, ultimately facilitating metastatic spread of prostate tumor epithelial cells. The inhibition of EMT response to androgens by AR overexpression points to an inverse relationship between AR content and EMT induction and a potential biochemical basis for the metastatic behavior of prostate cancer cells from recurrent castration-resistant tumors. Since long-term androgen deprivation may down-regulate AR expression, this threshold of “low” AR status facilitates DHT-induced EMT, thus promoting cancer metastasis. This is in accord with our observations that the AR antagonist reverses the EMT changes triggered by androgens in prostate cancer cells, thus providing proof of principle as to the ability of elevated AR to prevent DHT-induced E-cadherin reduction and N-cadherin induction. The concept gains indirect support from the clinical evidence that intermittent androgen deprivation therapy benefits patients in prostate cancer progression (46). Emerging data from an ongoing clinical trial show intermittent androgen deprivation therapy to be a promising option for patients with locally advanced and metastatic prostate cancer, in accord with preclinical evidence, suggesting that androgen deprivation therapy (on the basis of intermittent administration) delays androgen independence (47, 48). Pulse administration can effectively target AR regulation, providing proof of principle that low AR levels induced by androgen deprivation therapy might be responsible for the more aggressive behavior of recurring prostate tumors and supporting the requirement of a threshold AR level to maintain prostate tumor growth. Gain-of-function studies have shown that activated AR (via mutational activation or ligand independent activation) promotes proliferation of prostate cancer cells (49, 50). In a “double-sword” twist, the present data suggest that loss of AR can actually promote prostate cancer cell metastatic ability by regulating EMT. This study provides a novel insight into the androgen-mediated EMT effect, as a biological process significantly contributing to castration-resistant prostate cancer metastasis.
Supplementary Material
Acknowledgments
This work was supported by a U.S. National Institutes of Health R01 CA107575-06 grant (N.K.). The authors acknowledge Dr. Donald Tindall (Mayo Clinic, Rochester, MN, USA) and Dr. Scott Dehm (University of Minnesota, Minneapolis, MN, USA) for the generous gift of the plasmid pCMV5-AR; Dr. Mary Vore (Department of Toxicology, University of Kentucky, Lexington, KY, USA) for useful advice; and Lorie Howard for assistance in the submission of the manuscript.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun M J. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Wang X, Yin L, Rao P, Stein R, Harsch K M, Lee Z, Heston W D. Targeted treatment of prostate cancer. J Cell Biochem. 2007;102:571–579. doi: 10.1002/jcb.21491. [DOI] [PubMed] [Google Scholar]
- Siiteri P K, Wilson J D. Testosterone formation and metabolism during male sexual differentiation in the human embryo. J Clin Endocrinol Metab. 1974;38:113–125. doi: 10.1210/jcem-38-1-113. [DOI] [PubMed] [Google Scholar]
- Heinlein C A, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J, Binienda Z, Arthur A, Mininberg D T, Vaughan E D, Jr, Quimby F W. The development of a male pseudohermaphroditic rat using an inhibitor of the enzyme 5 alpha-reductase. Endocrinology. 1985;116:807–812. doi: 10.1210/endo-116-2-807. [DOI] [PubMed] [Google Scholar]
- De Marzo A M, Nelson W G, Meeker A K, Coffey D S. Stem cell features of benign and malignant prostate epithelial cells. J Urol. 1998;160:2381–2392. doi: 10.1097/00005392-199812020-00004. [DOI] [PubMed] [Google Scholar]
- Shahinian V B, Kuo Y F, Freeman J L, Goodwin J S. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- Makhsida N, Shah J, Yan G, Fisch H, Shabsigh R. Hypogonadism and metabolic syndrome: implications for testosterone therapy. J Urol. 2005;174:827–834. doi: 10.1097/01.ju.0000169490.78443.59. [DOI] [PubMed] [Google Scholar]
- Lu-Yao G L, Albertsen P C, Moore D F, Shih W, Lin Y, DiPaola R S, Yao S L. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–181. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J P. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Thiery J P. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Huber M A, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Xie L, Law B K, Chytil A M, Brown K A, Aakre M E, Moses H L. Activation of the Erk pathway is required for TGF-β1-induced EMT in vitro. Neoplasia. 2004;6:603–610. doi: 10.1593/neo.04241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J P, Sleeman J P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Derynck R, Akhurst R J. Differentiation plasticity regulated by TGF-β family proteins in development and disease. Nat Cell Biol. 2007;9:1000–1004. doi: 10.1038/ncb434. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-β in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin C H, Moustakas A. Transforming growth factor-β employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol. 2006;174:175–183. doi: 10.1083/jcb.200512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang H R, Zhang Y, Wrana J L. Regulation of the polarity protein Par6 by TGF-β receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4:657–665. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- Bruckheimer E M, Kyprianou N. Dihydrotestosterone enhances transforming growth factor-β-induced apoptosis in hormone-sensitive prostate cancer cells. Endocrinology. 2001;142:2419–2426. doi: 10.1210/endo.142.6.8218. [DOI] [PubMed] [Google Scholar]
- Guo Y, Kyprianou N. Overexpression of transforming growth factor (TGF) β1 type II receptor restores TGF-β1 sensitivity and signaling in human prostate cancer cells. Cell Growth Differ. 1998;9:185–193. [PubMed] [Google Scholar]
- Guo Y, Kyprianou N. Restoration of transforming growth factor β signaling pathway in human prostate cancer cells suppresses tumorigenicity via induction of caspase-1-mediated apoptosis. Cancer Res. 1999;59:1366–1371. [PubMed] [Google Scholar]
- Tahmatzopoulos A, Sheng S, Kyprianou N. Maspin sensitizes prostate cancer cells to doxazosin-induced apoptosis. Oncogene. 2005;24:5375–5383. doi: 10.1038/sj.onc.1208684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger A, Stockinger A, Schaffhauser B, Beug H, Foisner R. Epithelial mesenchymal transition by c-Fos estrogen receptor activation involves nuclear translocation of β-catenin and upregulation of β-catenin/lymphoid enhancer binding factor-1 transcriptional activity. J Cell Biol. 2000;148:173–188. doi: 10.1083/jcb.148.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland D J, Cheng H, Reid K, Rennie P S, Nelson C C. The androgen receptor can promote β-catenin nuclear translocation independently of adenomatous polyposis coli. J Biol Chem. 2002;277:17933–17943. doi: 10.1074/jbc.M200135200. [DOI] [PubMed] [Google Scholar]
- Thuault S, Tan E J, Peinado H, Cano A, Heldin C H, Moustakas A. HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition. J Biol Chem. 2008;283:33437–33446. doi: 10.1074/jbc.M802016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Kim B, van Golen C, Feldman E L. Cofilin activity during insulin-like growth factor I-stimulated neuroblastoma cell motility. Cell Mol Life Sci. 2005;62:461–470. doi: 10.1007/s00018-004-4456-6. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Song X, Mouneimne G, Sidani M, Lawrence D S, Condeelis J S. Cofilin promotes actin polymerization and defines the direction of cell motility. Science. 2004;304:743–746. doi: 10.1126/science.1094561. [DOI] [PubMed] [Google Scholar]
- Tanentzapf G, Brown N H. An interaction between integrin and the talin FERM domain mediates integrin activation but not linkage to the cytoskeleton. Nat Cell Biol. 2006;8:601–606. doi: 10.1038/ncb1411. [DOI] [PubMed] [Google Scholar]
- Calderwood D A, Yan B, de Pereda J M, Alvarez B G, Fujioka Y, Liddington R C, Ginsberg M H. The phosphotyrosine binding-like domain of talin activates integrins. J Biol Chem. 2002;277:21749–21758. doi: 10.1074/jbc.M111996200. [DOI] [PubMed] [Google Scholar]
- Alimirah F, Chen J, Basrawala Z, Xin H, Choubey D. DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: implications for the androgen receptor functions and regulation. FEBS Lett. 2006;580:2294–2300. doi: 10.1016/j.febslet.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Martinez H D, Jasavala R J, Hinkson I, Fitzgerald L D, Trimmer J S, Kung H J, Wright M E. RNA editing of androgen receptor gene transcripts in prostate cancer cells. J Biol Chem. 2008;283:29938–29949. doi: 10.1074/jbc.M800534200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinar B, Mukhopadhyay N K, Meng G, Freeman M R. Phosphoinositide 3-kinase-independent non-genomic signals transit from the androgen receptor to Akt1 in membrane raft microdomains. J Biol Chem. 2007;282:29584–29593. doi: 10.1074/jbc.M703310200. [DOI] [PubMed] [Google Scholar]
- Fuchs I B, Lichtenegger W, Buehler H, Henrich W, Stein H, Kleine-Tebbe A, Schaller G. The prognostic significance of epithelial-mesenchymal transition in breast cancer. Anticancer Res. 2002;22:3415–3419. [PubMed] [Google Scholar]
- Zhau H E, Odero-Marah V, Lue H W, Nomura T, Wang R, Chu G, Liu Z R, Zhou B P, Huang W C, Chung L W. Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clin Exp Metastasis. 2008;25:601–610. doi: 10.1007/s10585-008-9183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Altuwaijri S, Yeh S, Lai K P, Yu S, Chuang K H, Huang S P, Lardy H, Chang C. Targeting the stromal androgen receptor in primary prostate tumors at earlier stages. Proc Natl Acad Sci U S A. 2008;105:12188–12193. doi: 10.1073/pnas.0804701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M L, Kyprianou N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer. 2008;15:841–849. doi: 10.1677/ERC-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H Y, Huang K E, Chang S Y, Ma W L, Lin W J, Chang C. Differential modulation of androgen receptor-mediated transactivation by Smad3 and tumor suppressor Smad4. J Biol Chem. 2002;277:43749–43756. doi: 10.1074/jbc.M205603200. [DOI] [PubMed] [Google Scholar]
- Kang H Y, Lin H K, Hu Y C, Yeh S, Huang K E, Chang C. From transforming growth factor-beta signaling to androgen action: identification of Smad3 as an androgen receptor coregulator in prostate cancer cells. Proc Natl Acad Sci U S A. 2001;98:3018–3023. doi: 10.1073/pnas.061305498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart L A, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Wang J, Sadar M D. Crosstalk between the androgen receptor and beta-catenin in castrate-resistant prostate cancer. Cancer Res. 2008;68:9918–9927. doi: 10.1158/0008-5472.CAN-08-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronauer M V, Schulz W A, Ackermann R, Burchardt M. Effects of WNT/beta-catenin pathway activation on signaling through T-cell factor and androgen receptor in prostate cancer cell lines. Int J Oncol. 2005;26:1033–1040. doi: 10.3892/ijo.26.4.1033. [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- Horiguchi K, Shirakihara T, Nakano A, Imamura T, Miyazono K, Saitoh M. Role of Ras signaling in the induction of snail by transforming growth factor-beta. J Biol Chem. 2009;284:245–253. doi: 10.1074/jbc.M804777200. [DOI] [PubMed] [Google Scholar]
- Nelson W J, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccon-Gibod L, Hammerer P, Madersbacher S, Mottet N, Prayer-Galetti T, Tunn U. The role of intermittent androgen deprivation in prostate cancer. BJU Int. 2007;100:738–743. doi: 10.1111/j.1464-410X.2007.07053.x. [DOI] [PubMed] [Google Scholar]
- Gleave M E, Hsieh J T, Wu H C, Hong S J, Zhau H E, Guthrie P D, Chung L W. Epidermal growth factor receptor-mediated autocrine and paracrine stimulation of human transitional cell carcinoma. Cancer Res. 1993;53:5300–5307. [PubMed] [Google Scholar]
- Suzuki H, Kamiya N, Imamoto T, Kawamura K, Yano M, Takano M, Utsumi T, Naya Y, Ichikawa T. Current topics and perspectives relating to hormone therapy for prostate cancer. Int J Clin Oncol. 2008;13:401–410. doi: 10.1007/s10147-008-0830-y. [DOI] [PubMed] [Google Scholar]
- Balk S P, Knudsen K E. AR, the cell cycle, and prostate cancer [Online] Nucl Recept Signal. 2008;6:e001. doi: 10.1621/nrs.06001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstein K L. Regulation of androgen receptor levels: implications for prostate cancer progression and therapy. J Cell Biochem. 2005;95:657–669. doi: 10.1002/jcb.20460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.