Abstract
Docosahexaenoic acid (DHA) protects neural cells from stress-induced apoptosis. On the contrary, DHA exerts anticancer effects, and we have shown that DHA induces apoptosis in neuroblastoma, an embryonal tumor of the sympathetic nervous system. We now investigate the DHA metabolome in neuroblastoma using a targeted lipidomic approach in order to elucidate the mechanisms behind the DHA-induced cytotoxicity. LC-MS/MS analysis was used to identify DHA-derived lipid mediators in neuroblastoma cells. Presence of the 15-lipoxygenase enzyme was investigated using immunoblotting, and cytotoxic potency of DHA and DHA-derived compounds was compared using the MTT cell viability assay. Neuroblastoma cells metabolized DHA to 17-hydroxydocosahexaenoic acid (17-HDHA) via 17-hydroperoxydocosahexaenoic acid (17-HpDHA) through 15-lipoxygenase and autoxidation. In contrast to normal neural cells, neuroblastoma cells did not produce the anti-inflammatory and protective lipid mediators, resolvins and protectins. 17-HpDHA had significant cytotoxic potency, with an IC50 of 3–6 μM at 72 h, compared to 12–15 μM for DHA. α-Tocopherol protected cells from 17-HpDHA-induced cytotoxicity. DHA inhibited secretion of prostaglandin-E2 and augmented the cytotoxic potency of the cyclooxygenase-2-inhibitor celecoxib. The cytotoxic effect of DHA in neuroblastoma is mediated through production of hydroperoxy fatty acids that accumulate to toxic intracellular levels with restricted production of its products, resolvins and protectins.—Gleissman, H., Yang, R., Martinod, K., Lindskog, M., Serhan, C. N., Johnsen, J. I., Kogner, P. Docosahexaenoic acid metabolome in neural tumors: identification of cytotoxic intermediates.
Keywords: neuroblastoma, docosanoids, lipidomics, omega-3 fatty acids, cancer
Docosahexaenoic acid (22:6n-3, DHA) is the longest-chain and most unsaturated ω-3 fatty acid, and is mainly acquired in humans through consumption of cold-water fish products. Epidemiological studies support the argument that DHA is important in the prevention of several types of cancer (1,2,3). Moreover, we and others have published several reports showing that DHA exhibits cytotoxic effects on cancer cells in vitro and in vivo (4,5,6,7,8). Studies suggest a number of biological effects that could contribute to the mechanisms of DHA-induced cytotoxicity in cancer cells. These include inhibition of proliferation (9) and migration of tumor cells (10), and induction of apoptosis (7), as well as DHA-mediated effects on the tumor microenvironment by inhibition of inflammation and angiogenesis (11, 12) and by induction of immune responses (13, 14).
The mechanisms of DHA-induced tumor cell toxicity are not fully determined in molecular terms. On the one hand, DHA appears to exert pleiotropic mechanisms of action by forming intracellular reactive oxygen species (ROS), modulating protein expression important for cell cycle regulation, altering membrane fluidity and function of membrane-associated proteins, inhibiting eicosanoid synthesis, and mobilizing intracellular Ca2+ (1, 2, 15). On the other hand, DHA has been shown to protect normal cells from oxidative stress-induced apoptosis (16, 17). DHA accumulates primarily as phosphatidylethanolamine and phosphatidylserine in cellular membranes, but it can also be oxygenated to produce bioactive lipids. This oxygenation is thought to proceed through two main pathways: a lipoxygenase (LOX)-mediated pathway, which converts DHA to resolvins of the D series (RvD1–6), protectins/neuroprotectins (PD/NPD1) (18, 19), and a free radical-mediated peroxidation pathway that gives rise to neuroprostanes (20, 21).
In humans, high levels of DHA are found in membranes of neural cells, and DHA is the most abundant fatty acid in the brain (15). Enrichment of DHA in the human nervous system begins in early fetal life, and the level of this enrichment largely depends on the dietary habits of the pregnant mother (22). Deficiency of DHA will lead to delayed and incomplete neural development, and its importance is demonstrated by the fact that the placenta can actively and selectively transport DHA from mother to fetus against a concentration gradient (23). Interestingly, compared to normal nervous tissue, neuroblastoma, an embryonic tumor of the sympathetic nervous system, is profoundly deficient in DHA (24). Similar findings have been reported for glioma and meningioma (25, 26), which suggests that DHA depletion may serve as an adaptation mechanism in nervous system tumors.
In normal nervous tissue, the active lipid mediators of DHA, resolvins and neuroprotectins, control the duration and magnitude of inflammation partly through the inhibition of oxidative stress and apoptotic processes (17). In fact, NPD1 has been shown to up-regulate the antiapoptotic proteins Bcl-2 and Bcl-xL and to decrease the expression of the proapoptotic proteins Bax and Bad in neurons and human retinal pigment epithelial cells (17, 27). Conversely, we have shown that DHA induces cytotoxicity of neuroblastoma cells by a mechanism involving oxidative stress-mediated apoptosis (4). Our discovery that resolvins and protectins show potent anti-inflammatory and neuroprotective actions in vivo may explain the different modes of action of DHA in cell survival (28, 29).
To investigate the different functions and conversion of DHA in tumorigenic neural cells, we used a targeted LC-MS/MS-based lipidomic approach (18, 19). By this, we established the DHA metabolome in neuroblastoma cells, confirmed the presence of 15-LOX, performed chiral analysis of the metabolic lipid intermediates formed to determine the level of enzymatic vs. nonenzymatic conversion, and analyzed the cytotoxic potency of the different DHA-derived lipid mediators.
MATERIALS AND METHODS
Cell lines and culture conditions
Human neuroblastoma cell lines were grown in Eagle’s MEM (SH-SY5Y) or RPMI 1640 [SK-N-BE(2)], supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 IU/ml penicillin G, and 100 μM streptomycin (Gibco BRL, Sundbyberg, Sweden) at 37°C in a humidified atmosphere of 5% CO2.
Chemicals and compounds
DHA (22:6, n-3) was purchased from Nu-ChekPrep (Elysian, MN, USA) and Cayman Chemicals (Ann Arbor, MI, USA) and dissolved in 99.5% ethanol to make a 0.5 M stock solution. To avoid lipid hydroperoxides present in the starting substrate, DHA was purified by RP-HPLC before cell incubation. Nordihydroguaiaretic acid (NDGA) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in 99.5% ethanol to make a 0.331 M stock solution. α-Tocopherol was purchased from Sigma-Aldrich and further dissolved in 99.5% ethanol to make a 1 M stock solution. 15-Lipoxygenase (15-LOX) type 1 antibody along with the recombinant enzyme used as positive control were kindly provided by Dr. Hans-Erik Claesson (Karolinska Institutet, Stockholm, Sweden). The calcium ionophore A23187 was purchased from Sigma-Aldrich and dissolved in ethanol to make a 5 mg/ml stock solution. RvD1 (7S,8R,17S-trihydroxydocosa-4Z,9E,11E,13Z,15E,19Z-hexaenoic acid) and PD1 (10R,17S-dihydroxydocosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid) were prepared from total organic synthesis as described previously (30). 17-HpDHA (17-hydroperoxydocosa-4Z,7Z,10Z,13Z,15E,19Z-hexaenoic acid) was prepared from incubation of DHA with soybean 15-LOX (Sigma-Aldrich). 17-HpDHA was then reduced with NaBH4 to yield 17-HDHA (17-hydroxydocosa-4Z,7Z,10Z,13Z,15E,19Z-hexaenoic acid), as described previously (31). Each reagent from 99.5% ethanol stocks was freshly prepared in PBS prior to incubations.
Sample preparation and solid-phase extraction
Cells were harvested, washed in PBS with Ca2+ and Mg2+, and counted using trypan blue exclusion. Cells (1–2×107) were suspended in 1 ml PBS with Mg2+ and Ca2+ for short incubations (30 min), or in phenol red indicator-free RPMI medium for longer incubations (4 h). The following concentrations were used for the different compounds: DHA, 10 μM; RvD1, 1–100 nM; NDGA, 25 and 100 μM; and ionophore, 5 μM. Cells were incubated with designated compounds at 37°C and shielded from light for 30 min (DHA, NDGA, ionophore) or 4 h (RvD1 and PD1). A cell-free negative control was always used with each run. Reaction was stopped with 2 vol of ice-cold methanol, and samples were stored at −80°C for ≥2 h to allow protein precipitation. Samples were then centrifuged at 2000 rpm for 10 min to remove any precipitation and cellular material, after which internal standards 5(S)-HETE-d8 (2 ng) or PGE2-d4 (2 ng) were added (Cayman Chemical, Ann Arbor, MI, USA). Samples were then concentrated (SpeedVac AES1010; Savant, Ramsey, MN, USA), and 4 ml of water and 200 μl methanol were added to the concentrate. The resulting clear supernatants were acidified with 2 M hydrochloric acid to pH 3.5 and immediately applied to C18 SPE cartridges that had been preconditioned with 20 ml methanol followed by 20 ml water (Waters Corp, Milford, MA, USA). The cartridges were then washed with 4 ml water and 10 ml hexane in succession. Finally, the hydroxy fatty acids were eluted with 8 ml methyl formate. The extraction procedure was performed using a vacuum manifold (Waters Corp, Milford, MA, USA). The vacuum was adjusted so that individual drops could be seen from each cartridge. The organic solvent was evaporated under a fine stream of nitrogen and resuspended in 100 μl methanol, flushed with nitrogen, and stored at −80°C awaiting LC-MS/MS analysis.
LC-MS/MS analysis
LC-MS/MS analysis was performed with an Agilent 1100 series HPLC (Agilent Technologies, Santa Clara, CA, USA) paired with an ABI Sciex Instruments 3200 Qtrap linear ion trap quadrupole mass spectrometer (Applied Biosystems, Foster City, CA, USA) equipped with an Agilent Eclipse Plus C18 column (4.6 mm×50 mm×1.8 μm). Instrument control and data acquisition were performed using Analyst™ 1.4.2 software (Applied Biosystems). The mobile phase consisted of methanol/water/acetic acid (60/40/0.01; v/v/v) and was ramped to 80/20/0.01 (v/v/v) after 5 min, 95/5/0.01 (v/v/v) after 8 min, and 100/0/0.01 (v/v/v) after 14 min to wash the column. Ion pairs from previously reported multiple reaction monitoring (MRM) methods were used for profiling and quantitation of various lipid mediators, including 17-HDHA, resolvins, protectins, PGE2, and internal standards. The MRM transitions were 17-HDHA (343.2/245), 14-HDHA (343.2/205), 7-HDHA (343.2/141), 4-HDHA (343.2/101), PGE2 (351.2/189), RvD1 (375.2/215), PD1 (359.2/153), d8-5-HETE (327.5/116), and d4-PGE2 (355.2/193). The criteria used for positive identification of compounds of interest were matching retention time and ≥6 diagnostic ions to synthetic standards. Quantitation was performed using standard calibration curves for each compound, and recovery was calculated using deuterated internal standards (PGE2-d4 or 5(S)-HETE-d8).
Direct transesterification
To analyze the DHA-uptake of neuroblastoma cells, SK-N-BE(2) cells (1×107) were incubated with 100 μM of DHA (32.84 μg/ml). Cells were harvested, washed, and resuspended in PBS. An aliquot of the suspension was extracted using a direct transesterification method described previously (32). Briefly, 100 μl of cell suspension was added to 2 ml of a methanol/toluene solution (4:1) containing internal standard (methyl pentacosanoate, 25 μg/ml) and BHT (10 μg/ml) in a glass tube. Acetyl chloride (200 μl) was then slowly added during magnetic stirring. Before closing the lid tightly, nitrogen gas was blown into the tube for 30 s to remove remaining oxygen. The tubes were then incubated in 100°C during 60 min. After allowing the tubes to cool, 5 ml 6% K2CO3 was added slowly, followed by 1 ml heptane. The solution was mixed thoroughly, and the tubes were centrifuged 10 min at 3000 rpm. The methanol/toluene phase was collected and analyzed for DHA content by GLC.
GLC and GC-MS analysis
Identification of methyl-esterified fatty acids in cell suspension was performed by GC-MS using a Hewlett-Packard model 5970B mass selective detector connected to a Hewlett-Packard model 5890 gas chromatograph (Hewlett-Packard, Palo Alto, CA, USA) fitted with a capillary column of 5% phenylmethylsiloxane (length, 12 m; film thickness, 0.33 μm). Helium at a flow rate of 30 cm/s was used as the carrier gas. The oven temperature was raised from 120 to 300°C at a rate of 10°C/min. A Hewlett-Packard model 5890 gas chromatograph equipped with a flame ionization detector and a capillary column of Equity-1701 (Supelco, Bellefonte, PA, USA; length, 30 m; film thickness, 0.25 μm) was used for quantitative determination of DHA. Helium at a flow rate of 25 cm/s was used as the carrier gas. The oven temperature was raised from 210 to 280°C at a rate of 4°C/min. Peaks corresponding to the methyl esters of DHA and pentacosanoic acid (internal standard) were integrated with respect to peak height using a Hewlett-Packard model 3396 integrator. Standard curves obtained by injection of known mixtures of DHA and pentacosanoic acid methyl esters gave average response factors of 0.97 from DHA relative to pentacosanoic acid.
Cytotoxicity assay
Toxicity of DHA, DPA, EPA, 17-HpDHA, 17-HDHA, RvD1, and PD1 on neuroblastoma cells was determined using a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoleum (MTT) assay (Sigma-Aldrich). Briefly, cells were seeded in 96-well plates, allowed to attach overnight, and incubated with compounds as indicated (1 nM–100 μM) for 24, 48, or 72 h. Medium was then replaced by 50 μl serum-free medium containing 5 mg/ml MTT. Cells were incubated for 3 h, and 150 μl acidified isopropanol was then added to dissolve crystals. The plates were then shaken overnight at 4°C, and absorbance was measured using a microplate reader at 570 nm.
Immunoblotting
SH-SY5Y and SK-N-BE(2) cells were pelleted, and proteins were extracted in RIPA buffer (Cell Signaling, Beverly, MA, USA) containing protease inhibitor (Roche Diagnostics, Stockholm, Sweden). Protein concentrations were measured using Bradford reagent (Bio-Rad, Sundbyberg, Sweden). Equal quantities (50–150 μg) were separated by SDS-PAGE, transferred to nylon membranes (Millipore Inc, Solna, Sweden), and probed with antibody against 15-LOX type 1 diluted 1:2000 in 1% BSA in TBS-T, or against PARP (Cell Signaling, Danvers, MA, USA) diluted 1:1000 in 5% dry milk in TBS-T. Anti-rabbit IgG conjugated with horseradish peroxidase (Cell Signaling) diluted 1:2000 in 5% dry milk in TBS-T was used as the secondary antibody, and Pierce Super Signal (Pierce, Rockford, IL, USA) was used for chemiluminescent detection.
Fluorescence-activated cell sorting
Cells were seeded in 6-well plates, allowed to attach overnight, and incubated with DHA, 17-HpDHA, or 17-HDHA (1–100 μM) for 24 h. Cells were then harvested, washed in PBS, and stained with annexin V according to the manufacturer’s protocol (Sigma-Aldrich). FACS analysis was then performed using the F1 channel with the CellQuest software (Phoenix Flow Systems, San Diego, CA, USA).
Steric analysis of 17-HDHA
Incubates of 100 μM DHA with cells suspended in 10 ml of medium were extracted with diethyl ether, and the product was methyl-esterified by treatment with diazomethane. Analysis by normal-phase HPLC using a column of Nucleosil 50–5 [Machery-Nagel, Düren, Germany; 250×4 mm, eluted with 2-propanol-hexane (0.6:99.4, v/v) at a flow rate of 2 ml/min] revealed a peak of material absorbing at 234 nm (effluent volume, 15 ml). This material was collected and further analyzed by chiral-phase HPLC using a Chiralcel OB-H column [Daicel, Osaka, Japan; 250×4.6 mm, eluted with 2-propanol-hexane (3:97, v/v) at a flow rate of 0.5 ml/min. As references, the methyl esters of 17(S)- and 17(R)-HDHA were used; the elution volumes were 10.3 ml [17(S) enantiomer] and 11.5 ml [17(R) enantiomer].
RESULTS
Neuroblastoma cells convert DHA to monohydroxy fatty acids but not to resolvins or protectins
To determine whether neuroblastoma cells have the capability to convert DHA into various downstream bioactive lipid mediators, we used two human neuroblastoma cell lines, SK-N-BE(2) and SH-SY5Y. SK-N-BE(2) cells were isolated from a relapsed tumor and are p53 mutated, MYCN amplified, and multiresistant; whereas SH-SY5Y cells exhibit no MYCN amplification or p53 mutation and are less resistant to chemotherapeutic drugs. Lipids in supernatants from cells incubated with DHA or vehicle, in the absence or presence of the calcium ionophore A23187, were extracted by solid-phase extraction followed by LC-MS/MS analysis. In the first wide screening of above-mentioned cell lines, the spectra showed that both neuroblastoma cell lines synthesize the intermediates 17-HDHA, 14-HDHA, 7-HDHA, and 4-HDHA, but no production of resolvins, and very low production of protectins, were detected (Fig. 1A). In addition, no production of the dihydroxy compounds 4,17- and 7,17-di-HDHA was detected (data not shown). In the absence of exogenously supplied DHA, its further metabolites were not detected in the neuroblastoma cell lines. The response was not altered in cells treated with a calcium ionophore, which suggests that neuroblastoma cells efficiently metabolize DHA independently of external stimulation in the form of increased Ca2+ influx. The DHA uptake was calculated to be 24% of the DHA added to the culture medium (data not shown).
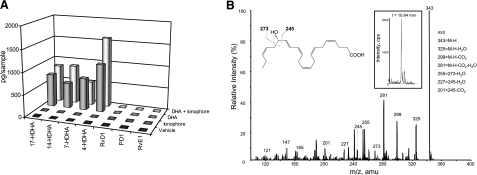
Figure 1.
Neuroblastoma cells convert DHA to hydroxy fatty acids but not to resolvins or protectins. Two different neuroblastoma cell lines were incubated at 37°C for 30 min with 10 μM DHA alone (gray), DHA and 5 μM A23187 ionophore (light gray), ionophore only (dark gray), or vehicle (black). Methyl formate fraction was collected from solid-phase extraction, and the sample was analyzed using LC-MS/MS for lipid mediators generated from DHA. A) Neuroblastoma cells converted DHA to the monohydroxy products 17-HDHA, 14-HDHA, 7-HDHA, and 4-HDHA but not to resolvins or protectins, as represented by the SK-N-BE(2) cell line. B) Representative MS2 spectrum of 17-HDHA of m/z 343 from the SK-N-BE(2) cell line incubated with DHA and ionophore. Inset: chromatogram of the compound (retention time: 10.840 to 10.922 min).
Neuroblastoma cells express the enzymes required for metabolic conversion of DHA, and inhibition of these enzymes partly blocks the production of 4-, 7-, 14-, and 17-HDHA
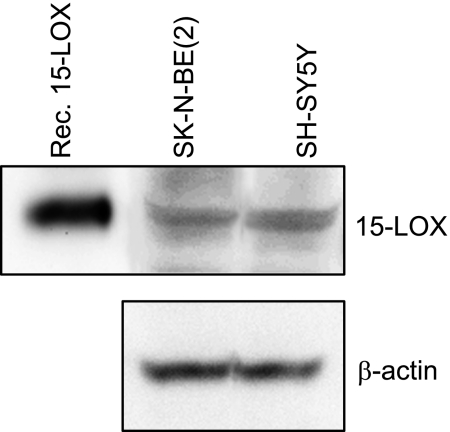
DHA is converted through a series of enzymatic steps to D-series resolvins and protectins via the intermediates 17-HpDHA and 17-HDHA (18). This conversion is catalyzed by enzymes of the lipoxygenase family, which oxygenate the carbon chain. 15-LOX catalyzes the first conversion of DHA to 17-HpDHA, whereas 5-LOX later forms the bioactive products, resolvins and protectins. We have recently shown that neuroblastoma cells express 5-LOX (33). To determine whether neuroblastoma cells also express 15-LOX, we performed Western blotting and detected a specific protein band of 75 kDa corresponding to 15-LOX in neuroblastoma cells (Fig. 2). Hence, neuroblastoma cells contain both of the enzymes necessary for converting DHA to resolvins and protectins, but as shown above, this conversion does not take place.
Figure 2.
Neuroblastoma cells express 15-LOX type 1. Protein from the cell lines SH-SY5Y and SK-N-BE(2) was extracted, quantified, and separated by SDS-PAGE, transferred to nylon membranes, and probed with antibody against 15-LOX type 1, followed by anti-rabbit IgG conjugated with horseradish peroxidase as secondary antibody. A recombinant enzyme was used as positive control. A 75-kDa band, which corresponds to 15-LOX type 1, was detected in both cell lines. β-Actin was used to ensure equal protein loading.
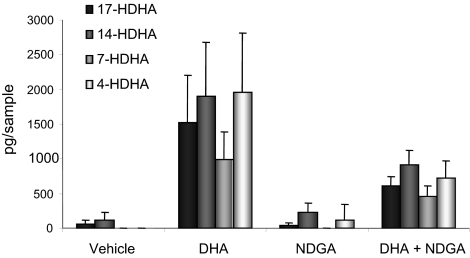
To further determine the involvement of LOX enzymes in the conversion of DHA by neuroblastoma cells, cells were coincubated with DHA and NDGA, a pan-LOX inhibitor in two different concentrations (25 and 100 μM). Analysis of cell culture supernatants by LC-MS/MS showed that the lower concentration resulted in an 12–18% decrease in levels of 14- and 17-HDHA content (data not shown), and the higher concentration caused a >50% decrease of 4-, 7-, 14-, and 17-HDHA in supernatants isolated from NDGA-treated cells compared to cells treated with DHA alone (Fig. 3).
Figure 3.
Coincubation with DHA and a pan-LOX-inhibitor reduces levels of DHA-derived hydroxy fatty acids in neuroblastoma cells. SK-N-BE(2) cells (1×107) were preincubated at 37°C with 100 μM NDGA or PBS. After 30 min, DHA was added to a final concentration of 10 μM, and the samples were incubated another 30 min. Reaction was stopped with ice-cold methanol, and the methyl formate fraction was collected from solid-phase extraction. Samples were analyzed using LC-MS/MS targeted on DHA-hydroxides. Untreated cells and cells treated with NDGA only showed no or low production of DHA-derived hydroxy fatty acids. Cells treated with DHA alone showed high production of 17-, 14-, 7-, and 4-HDHA, and this production was reduced by 60, 52, 54, and 63%, respectively, in cells coincubated with DHA and the pan-LOX inhibitor NDGA; P = 0.0286. Graph shows means ± sd of 3 separate experiments. A cell-free negative control was used with each run.
DHA is converted to its products in both a 15-LOX-dependent and 15-LOX-independent manner
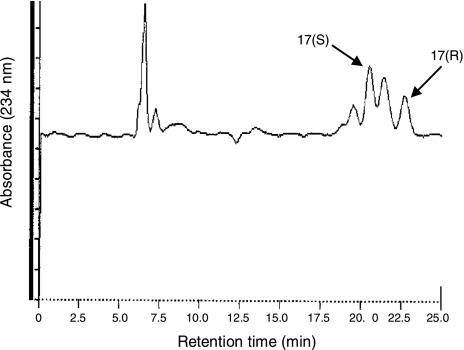
Intracellular oxygenation of DHA to monohydroxy fatty acids can be mediated enzymatically, i.e., via 15-LOX, or by autoxidation. We performed chiral analysis by HPLC in order to evaluate whether the conversion of DHA to hydroxy-DHA in neuroblastoma cells is caused independently of 15-LOX, which would produce a racemic hydroxy fatty acid mixture (50% each of the R and the S enantiomer), or by 15-LOX-mediated conversion, which would yield an end product predominantly in S configuration. We analyzed the configuration of 17-HDHA, one abundant hydroxy fatty acid found in DHA-treated neuroblastoma cells (Fig. 1). Biologically derived 17-HDHA separated into two peaks due to the 17(S) enantiomer (64%) and the 17(R) enantiomer (36%) as matched to authentic standards, which suggests that hydroxy fatty acids are both produced by 15-LOX mediated enzymatic conversion and autoxidation (Fig. 4). The blank samples without cells showed a very low degree of DHA conversion (data not shown), which indicates that autoxidation occurs to a limited extent, and that the presence of neuroblastoma cells, their intracellular environment and the enzymes that they express, are required for conversion of DHA to substantial amounts of 17-HDHA.
Figure 4.
Steric analysis of 17-HDHA generated by neuroblastoma cells after exogenous supply of DHA. SK-N-BE(2) cells were incubated in PBS at 37°C for 30 min with 10 μM DHA. Reaction was stopped with methanol, extracted, and analyzed with normal- and then chiral-phase HPLC. Enzymatic conversion of DHA to 17-HDHA by 15-LOX would produce a product in S configuration only, whereas nonenzymatic conversion would yield a racemic mixture. Biologically derived 17-HDHA separated into two peaks due to the 17(S) enantiomer (64%) and the 17(R) enantiomer (36%) and hence suggests a combination of 15-LOX-dependent and independent conversion of DHA to 17-HDHA in neuroblastoma cells. Two additional peaks are seen in the chromatogram. Retention times of the peaks labeled 17(S) and 17(R) exactly matched the retention times of authentic 17(S)- and 17(R)-hydroxy-DHA, respectively, and rechromatography of the collected peaks did not show any sign of heterogenity. However, the presence of other hydroxy-DHA isomers in these peaks cannot be ruled out. The earlier- and later-eluting peaks are likely to be due to other hydroxy-DHA regio/stereoisomers, since the detection was set to a wavelength (234 nm) where such isomers show strong absorption.
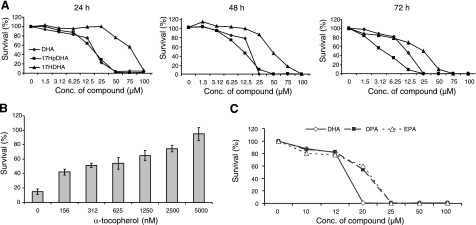
DHA-derived hydroperoxy fatty acids show higher cytotoxic potency than their precursors, and α-tocopherol rescues cells from this cytotoxicity dose-dependently
To analyze the effect of DHA and its metabolites on neuroblastoma cell survival, SH-SY5Y and SK-N-BE(2) cells were incubated with DHA, 17-HpDHA, or 17-HDHA, and cell survival was measured using MTT assay. 17-HpDHA had significant cytotoxic potency, with an IC50 of 3–6 μM at 72 h, compared to 12–15 μM for DHA. 17-HDHA was less toxic, with an IC50 of 25 μM. All compounds induced cytotoxity in a time- and dose-dependent manner (Fig. 5A). Hence, some of the DHA-mediated cytotoxic effects observed in neuroblastoma cells can be explained by the conversion of DHA to the more cytotoxic metabolite 17-HpDHA. Reduction of this compound to 17-HDHA reduces this effect. RvD1 and PD1 did not affect neuroblastoma cell survival (data not shown). Addition of the antioxidant α-tocopherol (0.0–5.0 μM) to growth medium, in combination with 17-HpDHA, protected cells from the 17-HpDHA-induced cytotoxicity in a dose-dependent manner (Fig. 5B). This finding agrees with our previously published data, where we show that α-tocopherol abolishes DHA-induced cytotoxicity in neuroblastoma cells (4). To evaluate the specificity of DHA in comparison to other long-chain fatty acids, we also performed viability assays using eicosapentaenoic acid (EPA) and docosapentaenoic acid (DPA). Their effects on neuroblastoma cells were similar to DHA, however DHA seemed to be the most toxic (Fig. 5C). Unspecific lipid peroxidation must then be a main trigger of cell toxicity. In addition, increased number of double bonds was associated with increased cytotoxicity, which has been shown previously (34).
Figure 5.
17-HpDHA shows high dose- and time-dependent cytotoxic potency, and can be inhibited through the antioxidant α-tocopherol. A) SK-N-BE(2) cells were seeded in 96-well plates and allowed to attach overnight. Medium was then changed to fresh medium containing DHA, 17-HpDHA, or 17-HDHA (1.5–100 μM). Cell survival was measured at 24, 48, and 72 h, as indicated by MTT proliferation assay. 17-HpDHA showed the highest cytotoxic potency, whereas 17-HDHA was less cytotoxic than DHA. IC50 values: DHA, 12–15 μM; 17-HpDHA, 3–6 μM; 17-HDHA, 25 μM. B) SK-N-BE(2) cells were seeded in 96-well plates and allowed to attach overnight. Medium was then changed to fresh medium containing 17-HpDHA (12 μM) combined with increasing concentrations of α-tocopherol (0.0–5.0 μM). Cell survival was measured at 48 h by MTT proliferation assay. α-Tocopherol rescued cells from 17-HpDHA-induced cytotoxicity in a dose-dependent manner. C) SK-N-BE(2) cells were seeded in 96-well plates and allowed to attach overnight. Medium was then changed to fresh medium containing the three different long-chain fatty acids DHA (22:6), EPA (20:5), or DPA (22:5) in concentrations between 0 and 100 μM. Cell survival was measured at 72 h by MTT proliferation assay. Increased number of double bonds was associated with increased cytotoxicity, as DHA was more toxic to EPA and DPA, which exerted similar cytotoxicity.
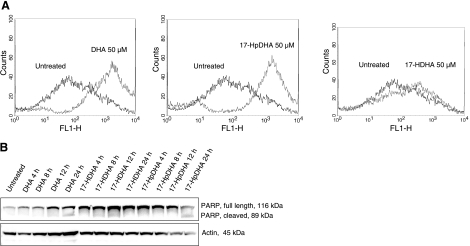
DHA and 17-HpDHA, but not 17-HDHA, cause apoptosis in neuroblastoma cells
To study the mechanisms of DHA and its metabolic intermediates on neuroblastoma cell toxicity, we evaluated the effect of DHA, 17-HpDHA, and 17-HDHA on the induction of apoptosis. Flow cytometric analysis of neuroblastoma cells incubated with DHA, 17-HpDHA, or 17-HDHA showed early signs of apoptosis using Annexin V staining in DHA- and 17-HpDHA-treated cells, whereas no positive staining for Annexin V was detected in cells treated with 17-HDHA (Fig. 6A). Similarly, late apoptotic events were detected by cleavage of the effector caspase substrate poly (ADP-ribose) polymerase (PARP) in neuroblastoma cells treated with DHA and 17-HpDHA, but not 17-HDHA (Fig. 6B).
Figure 6.
DHA and 17-HpDHA, but not 17-HDHA, induce apoptosis in neuroblastoma cells. A) SK-N-BE(2) cells were treated with 50 μM DHA, 17-HpDHA, or 17-HDHA for 24 h. Cells were then harvested, stained with annexin V, and analyzed with flow cytometry in the F1 channel to detect early apoptosis. Black lines represent untreated cells; gray lines represent cells treated with compound as indicated. A shift to the right in the histogram indicates apoptotic cells. DHA and 17-HpDHA, but not 17-HDHA, stained positive for annexin V. B) SK-N-BE(2) cells were treated with 50 μM DHA, 17-HpDHA, or 17-HDHA and harvested at different time points (4–24 h). Protein was extracted, quantified, and separated by SDS-PAGE, transferred to nylon membranes, and probed with antibody against full length (116-kDa) and cleaved (89-kDa) PARP, followed by anti-rabbit IgG conjugated with horseradish peroxidase as secondary antibody. DHA and 17-HpDHA, but not 17-HDHA, caused PARP cleavage after 24 h of incubation. β-Actin was used to ensure equal protein loading.
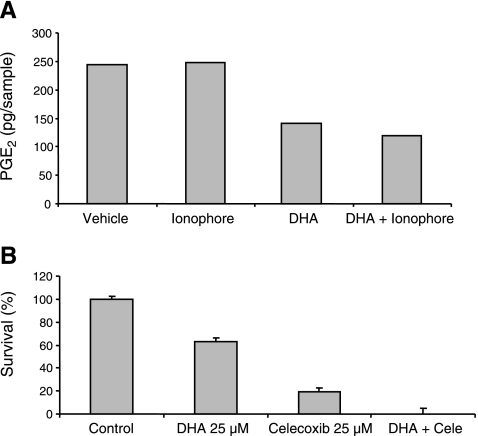
DHA decreases PGE2-production in neuroblastoma cells and augments the cytotoxic effect of the cyclooxygenase-2 inhibitor, celecoxib
Elevated PGE2 has been reported in neural tumor tissues and is suggested to contribute to tumor development (35). We therefore analyzed the effect of DHA and its metabolites on PGE2 secretion in neuroblastoma cells. SK-N-BE(2) cells were incubated with DHA, 17-HDHA, RvD1, or PD1, and the amount of PGE2 secretion was analyzed by LC-MS/MS. DHA reduced the PGE2 concentration by >50%, as could be calculated after quantification of PGE2 using deuterium-labeled PGE2 as internal standard in LC-MS/MS (Fig. 7A). 17-HDHA, RvD1, and PD1 did not affect PGE2 production in these incubations (data not shown). We have previously shown that cyclooxygenase-2 (COX-2) and microsomal PGE synthase 1 (mPGES-1), the enzymes that convert arachidonic acid to PGE2, are highly expressed in neural tumors and that inhibition of these enzymes has profound effects on the survival of these tumors (35,36,37). We therefore investigated the effect of combining DHA with the COX-2 specific inhibitor celecoxib in neuroblastoma cells and show that these compounds induced synergistic cytotoxicity (Fig. 7B).
Figure 7.
Incubation with DHA decreases the levels of PGE2 in neuroblastoma cells, and enhances cytotoxicity of the cycloxygenase-2 inhibitor celecoxib. A) SK-N-BE(2) cells were incubated at 37°C for 30 min with 10 μM DHA alone, DHA and 5 μM ionophore, ionophore only, or vehicle. Methyl formate fraction was collected from solid-phase extraction and was analyzed using LC-MS/MS. PGE2 levels were reduced by >50% in the samples treated with DHA. B) SK-N-BE(2) cells were seeded in 96-well plates and incubated with DHA, the COX-2 inhibitor celecoxib, or a combination of the two for 72 h. Cell survival was then measured with the MTT assay. DHA and celecoxib acted in synergy in terms of neuroblastoma cytotoxicity.
DISCUSSION
Resolvins and neuroprotectins can be produced by intra- and transcellular metabolism of DHA. These second messengers, commonly called docosanoids, are potent endogenous anti-inflammatory and proresolving chemical mediators (14, 18). Growing evidence suggests that the production of these bioactive products may be an internal anti-inflammatory mechanism for preventing brain damage (19, 38). In fact, infusion of PD1 following brain ischemic reperfusion injury or during oxidative stress in neural cell cultures down-regulates oxidative stress and apoptotic DNA damage (17, 27).
These protecting properties of DHA-derived mediators such as PD1 and RvD1 on neural tissue are opposed to the cytotoxic effects of DHA observed in neuroblastoma (4), a heterogenic embryonal tumor that is derived from the neural crest (39). DHA-mediated cytotoxicity is not restricted to neuroblastoma cells. A number of reports have shown that DHA induces cytotoxicity in a wide spectrum of cancer cells, which has led to the inclusion of DHA as a treatment option in several clinical cancer trials (1, 40).
The Fat-1 transgenic mouse model provides evidence that DHA and DHA-derived compounds may have significance in cancer development (41). These mice carry a gene that encodes a desaturase that catalyzes conversion of omega-6 to omega-3 fatty acids, a feature that is lacking in most mammals, including humans. In this mouse model, melanoma formation and growth (41), colitis-associated colon cancer growth (42), and prostate cancer growth (43) were all inhibited compared to tumor growth in nontransgenic animals. These data, together with our current findings, further support the implementation of DHA in treatment of various human malignancies including childhood tumors.
In mammals, DHA is predominantly enriched in neural tissue, where it may constitute up to 25–35% of the total membrane acyl chains (15, 44). In contrast, tumors of the nervous system are profoundly deficient in DHA (24,25,26). Although not proven, it is becoming perceptible that DHA provides a protective function in cells with a high concentration of DHA embedded in their membranes, whereas cells with a low concentration of DHA are susceptible to DHA-mediated cytotoxicity (45).
To investigate the opposing effect of DHA on neural tissue and its tumors, we used a lipidomic approach to study the DHA metabolome in human neuroblastoma cells. We found that exogenous DHA is oxygenated to the monohydroxy fatty acids 17-HDHA, 14-HDHA, 7-HDHA, and 4-HDHA, but no or low production of RvD1 and PD1 was detected (Fig. 1). In contrast, in normal neural cells, DHA is converted through sequential lipoxygenation by 15- and 5-LOX enzymes to resolvins and protectins (30, 38). Both neuroblastoma cell lines used in this study expressed substantial amounts of 15-LOX type 1 (Fig. 3). We have also recently shown that these cells express 5-LOX (33).
The absence of resolvin and/or protectin production, despite presence of the enzymes required for this conversion, may be a survival strategy for neuroblastoma cells. Cellular effectors and mediators of inflammation such as cytokines, chemokines, and eicosanoids are important constituents of the tumor microenviroment. These substances support the growth and survival of malignant cells, promote angiogenesis and metastasis, affect adaptive immune responses, and alter the effect of hormones and chemotherapeutic drugs (46). Since resolvins and protectins are powerful inducers of resolution of inflammation, they dampen the stimuli of these effectors and mediators, making the microenviroment less adapted to tumor growth, which may result in restricted malignant cell growth.
Apart from enzymatic conversion via LOX, DHA can also be converted to monohydroxy fatty acids through autoxidation (20). After performing steric analysis of 17-HDHA in neuroblastoma cells incubated with DHA, we detected a mixture of 17(S) and 17(R) enantiomers, with a higher abundance of the 17(S) enantiomer. This result suggests that oxygenation of DHA is mediated through LOX activity to a significant degree (Fig. 4), since autoxidation would produce a racemic mixture, and pure enzymatic conversion would produce an end product predominantly in S configuration. We also showed that NDGA, a pan-LOX inhibitor, reduces the production of 4-, 7-, 14-, and 17-HDHA, further suggesting that LOX is involved in the oxygenation of DHA in neuroblastoma cells. However, a substantial amount of the DHA is converted to hydroperoxy fatty acids, most likely via nonenzymatic mechanisms.
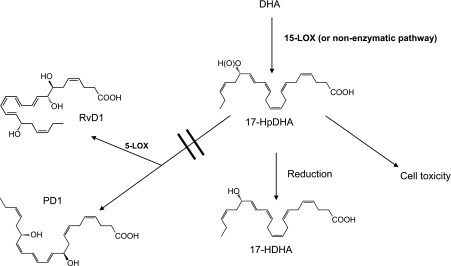
We have shown previously that DHA induces cytotoxicity of neuroblastoma cells through mechanisms involving accumulation of ROS and mitochondrial-dependent apoptosis (4). Since lipid peroxidation also has been shown to induce ROS, we investigated the cytotoxic potential of the hydroperoxy and hydroxy fatty acids in neuroblastoma cells and found that the 17-HpDHA intermediate has higher cytotoxic potential compared to DHA itself. Apart from induction of ROS, hydroperoxides exert their toxic effects by disintegration of cellular membranes and DNA damage (47). This occurrence leads to several apoptotic events, including nuclei condensation, DNA fragmentation, poly (ADP-ribose) polymerase cleavage, and increased activity of caspase-3. In addition, hydroperoxides cause signaling changes in mitochondria-mediated apoptosis, such as cytochrome c release, increased expression of Bcl-2, as well as a dose-dependent attenuation of mitochondrial membrane potential (48). 17-HDHA was less toxic to neuroblastoma cells than DHA, and resolvins or protectins had no effect on neuroblastoma. Hence, some of the DHA-mediated cytotoxic effects observed in neuroblastoma cells can be explained by the conversion of DHA to the more cytotoxic metabolite 17-HpDHA, but reduction of this compound to 17-HDHA reduces this effect. Figure 8 shows the proposed fate of DHA in neuroblastoma cells.Addition of an antioxidant (α-tocopherol) rescued neuroblastoma cells from 17-HpDHA-mediated cytotoxicity (Fig. 5B), which further supports lipid peroxidation involvement in the DHA-mediated growth inhibition and cell death described by us and others (4, 34, 49).
Figure 8.
Proposed DHA metabolome in neuroblastoma cells. In neuroblastoma cells, DHA is converted by 15-LOX and by other nonenzymatic pathways to the cytotoxic intermediate 17-HpDHA, which is reduced to 17-HDHA. 17-HpDHA has direct cytotoxic actions on neuroblastoma cells by inducing apoptosis and decreasing proliferation. Neuroblastoma cells express the enzyme 5-LOX, which is required to transform DHA-derived compounds to resolvins and protectins. However, this step takes place to a limited extent in neuroblastoma cells, which might be seen as a survival strategy.
PGE2 is the major eicosanoid produced from arachidonic acid by the sequential action of COXs and PGESs. Aberrant metabolism of arachidonic acid by the inducible COX-2 has been widely implicated in tumorigenesis (50). We recently showed that COX-2 is highly expressed in neuroblastoma and that inhibition of COX-2 has profound effects on neuroblastoma growth (37). PGE2 promotes tumor growth by stimulating EP receptor signaling, with subsequent enhancement of cellular proliferation, promotion of angiogenesis, inhibition of apoptosis, stimulation of invasion, and suppression of immune responses (51). By LC-MS/MS analyses we here show that exogenous addition of DHA to neuroblastoma cells results in decreased PGE2 production (Fig. 7A). Moreover, DHA synergistically augmented the cytotoxic effect of the COX-2 inhibitor celecoxib (Fig. 7B), which agrees with previously published results (52). Hence, DHA can induce cytotoxicity in neuroblastoma cells through the intracellular formation of hydroperoxy fatty acids, as well as by competition with arachidonic acid for incorporation into the membrane phospholipids and binding to catalytic sites of elongases, desaturases, and COX-2 (2).
Our study elucidates a mechanism for DHA-mediated cytotoxicity of neuroblastoma cells contrasting the known protecting effects of DHA in healthy neural cells. This potential of DHA both to inhibit tumor-supporting inflammation through inhibition of eicosanoid production and to act directly as a cytotoxic agent through the formation of toxic hydroperoxy fatty acids makes DHA a highly interesting agent for future clinical application in children with neuroblastoma.
Acknowledgments
The authors thank Prof. Mats Hamberg (Karolinska Institutet, Stockholm, Sweden) for critical scientific input and help with the steric analyses and Prof. Hans-Erik Claesson (Karolinska Institutet, Stockholm, Sweden) for kindly providing the 15-LOX type 1 antibody. This project was supported by the Swedish Childhood Cancer Foundation, the Swedish Research Council, the Swedish Cancer Society, the Cystic Fibrosis Foundation, Erik and Edith Fernström′s Foundation for Medical Research, and U.S. National Institutes of Health grants GM38765, DK074448, and P50-DE016191 (C.N.S.). The authors also thank P. Pillai, S. Oh, S. Krishnamoorthy, A. Recchiuti, and L. Elfman for excellent technical assistance.
References
- Berquin I M, Edwards I J, Chen Y Q. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269:363–377. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson S C, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- Wolk A, Larsson S C, Johansson J E, Ekman P. Long-term fatty fish consumption and renal cell carcinoma incidence in women. JAMA. 2006;296:1371–1376. doi: 10.1001/jama.296.11.1371. [DOI] [PubMed] [Google Scholar]
- Lindskog M, Gleissman H, Ponthan F, Castro J, Kogner P, Johnsen J I. Neuroblastoma cell death in response to docosahexaenoic acid: sensitization to chemotherapy and arsenic-induced oxidative stress. Int J Cancer. 2006;118:2584–2593. doi: 10.1002/ijc.21555. [DOI] [PubMed] [Google Scholar]
- Sun H, Berquin I M, Owens R T, O'Flaherty J T, Edwards I J. Peroxisome proliferator-activated receptor gamma-mediated up-regulation of syndecan-1 by n-3 fatty acids promotes apoptosis of human breast cancer cells. Cancer Res. 2008;68:2912–2919. doi: 10.1158/0008-5472.CAN-07-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N A, Nishimura K, Aires V, Yamashita T, Oaxaca-Castillo D, Kashiwagi K, Igarashi K. Docosahexaenoic acid inhibits cancer cell growth via p27Kip1, CDK2, ERK1/ERK2, and retinoblastoma phosphorylation. J Lipid Res. 2006;47:2306–2313. doi: 10.1194/jlr.M600269-JLR200. [DOI] [PubMed] [Google Scholar]
- Serini S, Trombino S, Oliva F, Piccioni E, Monego G, Resci F, Boninsegna A, Picci N, Ranelletti F O, Calviello G. Docosahexaenoic acid induces apoptosis in lung cancer cells by increasing MKP-1 and down-regulating p-ERK1/2 and p-p38 expression. Apoptosis. 2008;13:1172–1183. doi: 10.1007/s10495-008-0246-1. [DOI] [PubMed] [Google Scholar]
- Moyad M A. An introduction to dietary/supplemental omega-3 fatty acids for general health and prevention: part II. Urol Oncol. 2005;23:36–48. doi: 10.1016/j.urolonc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Hossain Z, Hosokawa M, Takahashi K. Growth inhibition and induction of apoptosis of colon cancer cell lines by applying marine phospholipid. Nutr Cancer. 2009;61:123–130. doi: 10.1080/01635580802395725. [DOI] [PubMed] [Google Scholar]
- Brown M D, Hart C A, Gazi E, Bagley S, Clarke N W. Promotion of prostatic metastatic migration towards human bone marrow stoma by omega 6 and its inhibition by omega 3 PUFAs. Br J Cancer. 2006;94:842–853. doi: 10.1038/sj.bjc.6603030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D P, Connolly J M. Antiangiogenicity of docosahexaenoic acid and its role in the suppression of breast cancer cell growth in nude mice. Int J Oncol. 1999;15:1011–1015. doi: 10.3892/ijo.15.5.1011. [DOI] [PubMed] [Google Scholar]
- Mukutmoni-Norris M, Hubbard N E, Erickson K L. Modulation of murine mammary tumor vasculature by dietary n-3 fatty acids in fish oil. Cancer Lett. 2000;150:101–109. doi: 10.1016/s0304-3835(99)00380-8. [DOI] [PubMed] [Google Scholar]
- Calder P C. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77:327–335. doi: 10.1016/j.plefa.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Serhan C N, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D, Astarita G, Rapaka R. A neuroscientist’s guide to lipidomics. Nat Rev Neurosci. 2007;8:743–754. doi: 10.1038/nrn2233. [DOI] [PubMed] [Google Scholar]
- Akbar M, Kim H Y. Protective effects of docosahexaenoic acid in staurosporine-induced apoptosis: involvement of phosphatidylinositol-3 kinase pathway. J Neurochem. 2002;82:655–665. doi: 10.1046/j.1471-4159.2002.01015.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee P K, Marcheselli V L, Serhan C N, Bazan N G. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C N, Arita M, Hong S, Gotlinger K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004;39:1125–1132. doi: 10.1007/s11745-004-1339-7. [DOI] [PubMed] [Google Scholar]
- Hong S, Lu Y, Yang R, Gotlinger K H, Petasis N A, Serhan C N. Resolvin D1, protectin D1, and related docosahexaenoic acid-derived products: analysis via electrospray/low energy tandem mass spectrometry based on spectra and fragmentation mechanisms. J Am Soc Mass Spectrom. 2007;18:128–144. doi: 10.1016/j.jasms.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Musiek E S, Gao L, Porter N A, Morrow J D. Regiochemistry of neuroprostanes generated from the peroxidation of docosahexaenoic acid in vitro and in vivo. J Biol Chem. 2005;280:26600–26611. doi: 10.1074/jbc.M503088200. [DOI] [PubMed] [Google Scholar]
- Jahn U, Galano J M, Durand T. Beyond prostaglandins–chemistry and biology of cyclic oxygenated metabolites formed by free-radical pathways from polyunsaturated fatty acids. Angew Chem Int Ed Engl. 2008;47:5894–5955. doi: 10.1002/anie.200705122. [DOI] [PubMed] [Google Scholar]
- Koletzko B, Cetin I, Brenna J T. Dietary fat intakes for pregnant and lactating women. Br J Nutr. 2007;98:873–877. doi: 10.1017/S0007114507764747. [DOI] [PubMed] [Google Scholar]
- Hanebutt F L, Demmelmair H, Schiessl B, Larque E, Koletzko B. Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clin Nutr. 2008;27:685–693. doi: 10.1016/j.clnu.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Reynolds L M, Dalton C F, Reynolds G P. Phospholipid fatty acids and neurotoxicity in human neuroblastoma SH-SY5Y cells. Neurosci Lett. 2001;309:193–196. doi: 10.1016/s0304-3940(01)02071-7. [DOI] [PubMed] [Google Scholar]
- Martin D D, Robbins M E, Spector A A, Wen B C, Hussey D H. The fatty acid composition of human gliomas differs from that found in nonmalignant brain tissue. Lipids. 1996;31:1283–1288. doi: 10.1007/BF02587914. [DOI] [PubMed] [Google Scholar]
- Kokoglu E, Tuter Y, Yazici Z, Sandikci K S, Sonmez H, Ulakoglu E Z, Ozyurt E. Profiles of the fatty acids in the plasma membrane of human brain tumors. Cancer Biochem Biophys. 1998;16:301–312. [PubMed] [Google Scholar]
- Bazan N G. Lipid signaling in neural plasticity, brain repair, and neuroprotection. Mol Neurobiol. 2005;32:89–103. doi: 10.1385/MN:32:1:089. [DOI] [PubMed] [Google Scholar]
- Serhan C N, Hong S, Gronert K, Colgan S P, Devchand P R, Mirick G, Moussignac R L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C N, Chiang N, Van Dyke T E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y P, Oh S F, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan S P, Petasis N A, Serhan C N. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- Serhan C N, Hong S, Lu Y. Lipid mediator informatics-lipidomics: novel pathways in mapping resolution. AAPS J. 2006;8:E284–E297. doi: 10.1007/BF02854899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage G, Roy C C. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- Sveinbjornsson B, Rasmuson A, Baryawno N, Wan M, Pettersen I, Ponthan F, Orrego A, Haeggstrom J Z, Johnsen J I, Kogner P. Expression of enzymes and receptors of the leukotriene pathway in human neuroblastoma promotes tumor survival and provides a target for therapy. FASEB J. 2008;22:3525–3536. doi: 10.1096/fj.07-103457. [DOI] [PubMed] [Google Scholar]
- Dommels Y E, Haring M M, Keestra N G, Alink G M, van Bladeren P J, van Ommen B. The role of cyclooxygenase in n-6 and n-3 polyunsaturated fatty acid mediated effects on cell proliferation, PGE(2) synthesis and cytotoxicity in human colorectal carcinoma cell lines. Carcinogenesis. 2003;24:385–392. doi: 10.1093/carcin/24.3.385. [DOI] [PubMed] [Google Scholar]
- Baryawno N, Sveinbjornsson B, Eksborg S, Orrego A, Segerstrom L, Oqvist C O, Holm S, Gustavsson B, Kagedal B, Kogner P, Johnsen J I. Tumor-growth-promoting cyclooxygenase-2 prostaglandin E2 pathway provides medulloblastoma therapeutic targets. Neuro Oncol. 2008;10:661–674. doi: 10.1215/15228517-2008-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponthan F, Wickstrom M, Gleissman H, Fuskevag O M, Segerstrom L, Sveinbjornsson B, Redfern C P, Eksborg S, Kogner P, Johnsen J I. Celecoxib prevents neuroblastoma tumor development and potentiates the effect of chemotherapeutic drugs in vitro and in vivo. Clin Cancer Res. 2007;13:1036–1044. doi: 10.1158/1078-0432.CCR-06-1908. [DOI] [PubMed] [Google Scholar]
- Johnsen J I, Lindskog M, Ponthan F, Pettersen I, Elfman L, Orrego A, Sveinbjornsson B, Kogner P. Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Res. 2004;64:7210–7215. doi: 10.1158/0008-5472.CAN-04-1795. [DOI] [PubMed] [Google Scholar]
- Marcheselli V L, Hong S, Lukiw W J, Tian X H, Gronert K, Musto A, Hardy M, Gimenez J M, Chiang N, Serhan C N, Bazan N G. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- Johnsen J I, Kogner P, Albihn A, Henriksson M A. Embryonal neural tumours and cell death. Apoptosis. 2009;14:424–438. doi: 10.1007/s10495-009-0325-y. [DOI] [PubMed] [Google Scholar]
- Jones G R, Singer P P. An unusual trichloroethanol fatality attributed to sniffing trichloroethylene. J Anal Toxicol. 2008;32:183–186. doi: 10.1093/jat/32.2.183. [DOI] [PubMed] [Google Scholar]
- Xia S, Lu Y, Wang J, He C, Hong S, Serhan C N, Kang J X. Melanoma growth is reduced in fat-1 transgenic mice: impact of omega-6/omega-3 essential fatty acids. Proc Natl Acad Sci U S A. 2006;103:12499–12504. doi: 10.1073/pnas.0605394103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q, Lupton J R, Smith R, Weeks B R, Callaway E, Davidson L A, Kim W, Fan Y Y, Yang P, Newman R A, Kang J X, McMurray D N, Chapkin R S. Reduced colitis-associated colon cancer in Fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res. 2008;68:3985–3991. doi: 10.1158/0008-5472.CAN-07-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Nie D, Witt W T, Chen Q, Shen M, Xie H, Lai L, Dai Y, Zhang J. Expression of the fat-1 gene diminishes prostate cancer growth in vivo through enhancing apoptosis and inhibiting GSK-3 beta phosphorylation. Mol Cancer Ther. 2008;7:3203–3211. doi: 10.1158/1535-7163.MCT-08-0494. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui R A, Harvey K, Stillwell W. Anticancer properties of oxidation products of docosahexaenoic acid. Chem Phys Lipids. 2008;153:47–56. doi: 10.1016/j.chemphyslip.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Crean C, Shao J, Yun B H, Geacintov N E, Shafirovich V. The role of one-electron reduction of lipid hydroperoxides in causing DNA damage. Chemistry. 2009;15:10634–10640. doi: 10.1002/chem.200900500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shibata T, Hisaka S, Kawai Y, Osawa T. DHA hydroperoxides as a potential inducer of neuronal cell death: a mitochondrial dysfunction-mediated pathway. J Clin Biochem Nutr. 2008;43:26–33. doi: 10.3164/jcbn.2008040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti A W. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- Wang M T, Honn K V, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 2007;26:525–534. doi: 10.1007/s10555-007-9096-5. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy S, Honn K V. Eicosanoids in tumor progression and metastasis. Subcell Biochem. 2008;49:145–168. doi: 10.1007/978-1-4020-8831-5_6. [DOI] [PubMed] [Google Scholar]
- Swamy M V, Cooma I, Patlolla J M, Simi B, Reddy B S, Rao C V. Modulation of cyclooxygenase-2 activities by the combined action of celecoxib and decosahexaenoic acid: novel strategies for colon cancer prevention and treatment. Mol Cancer Ther. 2004;3:215–221. [PubMed] [Google Scholar]