Abstract
Neovascularization is critical to destabilization of atheroma. We previously reported that the angiogenic growth factor pleiotrophin (PTN) coaxes monocytes to assume the phenotype of functional endothelial cells in vitro and in vivo. In this study we show that PTN expression is colocalized with capillaries of human atherosclerotic plaques. Among the various reagents that are critical to the pathogenesis of atherosclerosis, interferon (IFN)-γ was found to markedly induce PTN mRNA expression in a dose-dependent manner in macrophages. Mechanistic studies revealed that the Janus kinase inhibitors, WHI-P154 and ATA, efficiently blocked STAT1 phosphorylation in a concentration- and time-dependent manner. Notably, the level of phosphorylated STAT1 was found to correlate directly with the PTN mRNA levels. In addition, STAT1/STAT3/p44/42 signaling molecules were found to be phosphorylated by IFN-γ in macrophages, and they were translocated into the nucleus. Further, PTN promoter analysis showed that a gamma-activated sequence (GAS) located at −2086 to −2078 bp is essential for IFN-γ-regulated promoter activity. Moreover, electrophoretic mobility shift, supershift, and chromatin immunoprecipitation analyses revealed that both STAT1 and STAT3 bind to the GAS at the chromatin level in the IFN-γ stimulated cells. Finally, to test whether the combined effect of STAT1/STAT3/p44/42 signaling is required for the expression of PTN in macrophages, gene knockdowns of these transcription factors were performed using siRNA. Cells lacking STAT1, but not STAT3 or p42, have markedly reduced PTN mRNA levels. These data suggest that PTN expression in the human plaques may be in part regulated by IFN-γ and that PTN is involved in the adaptive immunity.—Li, F., Tian, F., Wang, L., Williamson, I. K., Sharifi, B. G., Shah, P. K. Pleiotrophin (PTN) is expressed in vascularized human atherosclerotic plaques: IFN-γ/JAK/STAT1 signaling is critical for the expression of PTN in macrophages
Keywords: plaque angiogenesis, neovascularization, vasculogenic mimicry
Atherosclerosis is a complex inflammatory disease of the arterial wall, in which both the innate and adaptive immune system play a significant role (1). Initiation and progression of vascular inflammation involves a complex cellular network, with macrophages as major contributors. Activated macrophages produce proinflammatory mediators, bridge innate and adaptive immunity, regulate lipid retention, and participate directly in vascular repair and remodeling.The activation of monocytes is believed to play a major role in angiogenesis and collateral artery growth (2,3,4). The mechanisms responsible for the formation of intraplaque microvessels are not understood.
Interferon-γ (IFN-γ) is the signature gene that is produced by the proinflammatory T-helper 1 cell subset. Immunohistochemical and in situ hybridization studies indicate that IFN-γ is present in human atherosclerotic plaques (5, 6). Animal studies underscore a central role of IFN-γ in atherosclerosis. Deletion of the IFN-γ gene or IFN-γ receptor expression in mice significantly reduces atherosclerosis (7, 8), whereas administration of exogenous IFN-γ potentiates atherosclerosis (9). IFN-γ has many different biological effects that contribute to the production of proinflammatory cytokines, chemokines, and reactive oxygen species by macrophages (10). The underlying mechanism of IFN-γ involvement in atherosclerosis is unknown.
Pleiotrophin (PTN) is a cytokine named so because it signals diverse functions, including those of a differentiation factor/growth factor/angiogenic factor for various cell types (for review, see ref. 11). In the heart, PTN promotes cardiac vascularization following ischemia (12) and an increase in bromodeoxyuridine incorporation into mouse heart cells in vivo (13). In ischemic brain, PTN mRNA was found to be up-regulated in macrophages within an area of exuberant neovasculature that formed at the margins of the infarct and in endothelial cells of the newly formed vessels (14). Based on these data, it was suggested that PTN signaling is an important determinant of neovascularization in postischemic brain (14). In the ischemic tissues, PTN is expressed by endothelial cells and macrophages. Although PTN expression and function by endothelial cells has been extensively investigated, nothing is known about the mechanism that is responsible for the expression of this angiogenic factor in macrophages.
We previously reported that PTN is expressed in the atheroprone coronary artery and not in the atheroresistant internal mammary artery (15), suggesting a role for this factor in atherosclerosis. We also showed that PTN coaxes monocytes to alter their phenotype and assume characteristics of functional endothelial cells in vitro and in vivo (16). Based on this ability of PTN, we hypothesized that PTN may play a role in the pathogenesis of atherosclerosis in general and in intraplaque neovascularization in particular. To explore this, we examined expression of PTN in highly vascularized human plaques. We noted that PTN is expressed in human atherosclerotic plaques, and this expression is colocalized with microvessels. Mechanistic studies revealed that IFN-γ specifically regulates expression of PTN in macrophages through the JAK-STAT1 pathway and that STAT1 signaling, but not that mediated by STAT3/p44/42, is critical to PTN expression in macrophages. Thus, PTN lies downstream of IFN-γ signaling. The implications of these findings are discussed in relation to atherosclerotic pathogenesis theory and neovascularization of inflamed tissues.
MATERIALS AND METHODS
Mouse peritoneal macrophages were obtained from 12-wk-old C57 BL/6 mice (Jackson Laboratory, Bar Harbor, ME, USA) using a standard method and cultured as previously described (16). Recombinant human and mouse IFN-γ were purchased from PeproTech Inc. (Rocky Hill, NJ, USA). JAK inhibitors, including WHI-P154 and ATA, were purchased from Calbiochem (San Diego, CA, USA). Mice received an intraperitoneal injection of a 3% thioglycolate solution (2 ml), and 3 d later the peritoneal cells were harvested by washing out the peritoneal cavity with culture medium. The harvested cells were washed and plated in RPMI 1640 medium (supplemented with 10% fetal bovine serum and antibiotics; Invitrogen, Carlsbad, CA, USA) for 36 h; the unattached cells were removed by washing with warm medium. The attached macrophages were treated with mouse IFN-γ (10 ng/ml) for different time periods.
Cell culture
The THP-1 cell line (American Type Culture Collection, Manassas, VA, USA) was cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotics (100 IU/ml penicillin and 100 μg/ml streptomycin). Cells were routinely maintained in culture at 37°C in 5% CO2/95% air. All the cells used in the experiments were within 20 passages.
Immunohistochemistry
For immunohistochemical staining, paraffin-embedded sections were stained with a primary anti-PTN antibody (clone ab14025 at 1:500 dilution; Abcam, Cambridge, MA, USA) and a secondary peroxidase-labeled anti-goat IgG (1:250 in PBS) essentially as described previously (17). Briefly, paraffin sections from coronary arteries were deparaffinized in xylol (3 times for 10 min), organic solvent was removed with decreasing concentrations of ethanol, and then sections were immersed in double-distilled water for 10 s. Movat staining was used to analyze all connective tissue elements in a single section (SMCs, red; elastic lamina, dark blue-black; collage, yellow; proteoglycan, blue-green; red blood cells, red). Intraplaque hemorrhage is clearly visible with a very narrow lumen. For immunohistochemistry, sections were incubated overnight at 4°C with anti-PTN antibody (1:500 dilution in PBS; Abcam). Sections were then washed 3 times with TBS, incubated with peroxidase-labeled anti-goat IgG (1:250 in PBS), washed another 3 times with TBS, stained for 7 min at room temperature with 3-amino-9- ethylcarbazole/H2O2 (Universal Peroxidase Detection Kit; Coulter-Immunotech, Fullerton, CA, USA).
RNA isolation and real-time PCR analysis
Total RNA was isolated from mouse peritoneal macrophages and THP-1 cells using TRIzol reagent (Invitrogen) following the manufacturer’s instructions. First-strand cDNA was synthesized using the Omniscript Reverse Transcription Kit (Qiagen, Valencia, CA, USA). The cDNA was used for either semiquantitative or real-time quantitative PCR analysis using an iQ5 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). For real-time PCR, all reactions were performed in triplicate, in a total reaction volume of 25 μl. The human primer sequences for Ptn were 5′-ATGCAGGCTCAACAGTACCAGCA-3′ and 5′-ACTCCACTGCCATTCTCCAC-3′, and for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), primer sequences were 5′-TTGGTATCGTGGAAGGACTCA-3′ and 5′-TGTCATCATATTTGGCAGGTTT-3′. The mouse primer sequences for Ptn were 5′-TGTCGTCCCAGCAATATCAG-3′ and 5′-ACTCCACTGCCATTCTCCAC-3′, and for GAPDH, primer sequences were 5′-ATCACTGCCACCCAGAAGAC-3′ and 5′-CACATTGGGGGTAGGAACAC-3′. The PCR parameters were 95°C for 3 min, followed by 40 cycles of 95°C for 10 s, 55°C for 30 s, 72°C for 30 s, and final extension at 72°C for 10 min. The relative Ptn mRNA levels were quantified against GAPDH, using iQ5 Optical System Software analysis (Bio-Rad).
Western blotting
After treatment with IFN-γ or inhibitors (AG 490, WHI-154, and ATA) for different time periods, THP-1 cells were collected and lysed by the addition of cold RIPA buffer containing protease inhibitors for 10 min, on ice, with occasional mixing, and the lysate was clarified by centrifugation.
Samples for PTN protein detection in conditioned medium were prepared following the method of Hatziapostolou et al. (18). After THP-1 cells were treated without or with IFN- γ (50 ng/ml) for 48 h, conditioned medium was incubated with heparin-agarose (Sigma-Aldrich, St. Louis, MO, USA) at 4°C overnight with rotation. The heparin agarose was washed twice with 50 mM Tris-HCl containing 0.5M NaCl, pH 7.5. Bound proteins were eluted with 50 μl of Laemmli sample buffer.
The samples were first separated by SDS-PAGE, using a 12% polyacrylamide gel under reducing conditions, and then transferred onto a nitrocellulose membrane (Bio-Rad). After blocking with PBS containing 5% nonfat dry milk (Santa Cruz Biotechnology, Santa Cruz, CA, USA), membranes were probed with either anti-phospho-STAT1 (Tyr701; Santa Cruz Biotechnology) or STAT1 (9H2) mouse mAb; phospho-STAT3 (Tyr705) mouse mAb or STAT3 (124H6) mouse mAb; phospho-STAT6 (Tyr641) Ab or STAT6 Ab; or phospho-p44/42 MAPK (Thr202/Tyr204) (E10) mouse mAb or p44/42 MAP kinase (3A7) mouse mAb (Cell Signaling Technology, Danvers, MA, USA). For PTN protein detection, goat anti-PTN antibody (R&D Systems, Minneapolis, MN, USA) was used. Following washing, the membranes were incubated with peroxidase-conjugated secondary antibodies; the Western Blotting Luminol Reagent (Santa Cruz Biotechnology) was used for detection.
Immunofluorescence
Approximately 1 × 105 THP-1 cells were seeded and grown onto 8-well chamber slides (BD Biosciences, San Jose, CA, USA) overnight. After treatment with IFN-γ (50 ng/ml) for 5 min (STAT1, STAT3) or 15 min (p44/42), the slides were fixed with freshly prepared 4% paraformaldehyde at room temperature for 15 min and permeabilized with ice-cold 100% methanol for 10 min in a freezer, then blocked with 5% normal donkey serum in PBS and 0.3% Triton ×-100 (PBS/Triton) at room temperature for 1 h. The mouse anti-STAT1 (BD Biosciences), rabbit anti-STAT3, and mouse monoclonal anti- p44/42 MAPK (3A7) primary antibodies diluted in PBS/Triton (1:50) were incubated overnight at 4°C. The Cy™3 conjugated donkey anti-mouse IgG (H+L) secondary antibody or donkey anti-rabbit IgG (715-166-150, 711-096-152; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) was incubated (1:100 dilution) at room temperature for 2 h. After mounting with MAXfluor™ DAPI Mounting Medium (Active Motif, Carlsbad, CA, USA), the coverslips were sealed with nail polish. Immunofluorescence was detected with a Zeiss Axioplan microscope (Carl Zeiss, Thornwood, NY, USA) with an ×63 oil-immersion objective.
Plasmid constructs
Genomic DNA was isolated from THP-1 cells by using the FlexiGene DNA Kit (Qiagen), and the PTN promoter regions from −3290 to +309, −2738 to +309, and −1407 to +309 bp were PCR amplified with the following primers (unless otherwise stated, the incorporated BglII restriction site is underscored): PTN-3290, 5′-GAGTCGAGATCTCTGTCAAATATCATCCCAAGGA-3′; PTN-2738, 5′-GAGTCGAGATCTTGACTATGCCGTAGGATGAAGT-3′; PTN-1407, 5′-GAGTCGGATATCTTTTTTGGTAAAGGGACTGGGA-3′ (Eco RV restriction site underscored); and a common reverse primer, 5′-GATGGAAGATCTGAGGTTGCTACCGCTGAGTC-3′ (BglII site underscored). After digestion with BglII (−3290 to +309 and −2738 to +309 bp fragments) or Eco RV plus BglII (−1407 to +309 bp fragment), PTN promoters of different sizes were inserted into pGL4.19 [luc2CP/Neo] luciferase reporter vector (Promega Corp., Madison, WI, USA), which was digested with either BglII or Eco RV/BglII. The resultant clones were analyzed by restriction digestion, confirmed by DNA sequencing, and designated as PTN-3290-Luc, PTN-2738-Luc, and PTN-1407-Luc, respectively.
Transient transfection assay
Transient transfection experiments were performed with THP-1 cells using the Nucleofector kit V (Lonza, Walkersville, MD, USA), following the manufacturer’s protocol. Briefly, 1.0 μg of each of the prepared luciferase constructs expressing firefly luciferase and 2.0 ng of pRL-β-actin, a plasmid expressing Renilla luciferase under the control of β-actin promoter (kindly provided by Dr. Rivkah Gonsky, Cedars-Sinai Medical Center), were mixed with 100 μl Nucleofector solution V, and cotransfected into 1 × 106 THP-1 cells using the V-001 program (high viability). After transfection, cells were transferred to complete culture medium and treated with IFN-γ (50 ng/ml) immediately for 3 h. Cells were then harvested and lysed with 60 μl passive lysis buffer (Promega). Luciferase activity was assayed using Dual Luciferase Reporter Assay System (Promega). All the transfection experiments were repeated ≥3 times, in triplicate.
siRNA transfection
siRNAs targeting human STAT1 (siRNA ID s277), STAT3 (s743), ERK2 (s11137) and Silencer® Cy3-labeled Negative Control #1 siRNA were purchased from Applied Biosystems (Foster City, CA, USA). Transfection into THP-1 cells was accomplished by using the Nucleofector kit V (Lonza) according to the manufacture’s protocol. Briefly, 1 × 106 THP-1 cells in 100 μl Nucleofector solution V were mixed with 200 pmol siRNA in the supplied cuvette. Electroporation was performed using program V-001 (high transfection efficiency). After electroporation, 500 μl prewarmed complete medium was added immediately to the cells, and the cells were transferred into a 1.5-ml microcentrifuge tube and incubated at 37°C in a water bath for 10 min. Then the cells were transferred to each well of 6-well plates. At 24 h post-transfection, cells were treated with IFN-γ in fresh complete medium for 3 h, then cells were harvested and lysed; gene knockdown at the protein level was confirmed by Western blotting. Total RNA was extracted for real-time PCR analysis of PTN mRNA expression.
Electrophoretic mobility shift assay (EMSA)
For EMSA, 21-mer oligonucleotide probes encoding the γ-activated sequence (GAS) were synthesized and designated wtGAS; the sequences were 5′-ACCCCTTTCCAGGAAAATTCA-3′ (sense strand, −2092 to −2072 bp) and 5′-TGAATTTTCCTGGAAAGGGGT-3′ (antisense strand). Underscored is the GAS located from −2086 to −2078 bp in the PTN promoter. The sequences were 3′ end labeled separately using the Biotin 3′ End DNA Labeling Kit (Pierce Biotechnology, Rockford, IL, USA). Briefly, 100 nM single-stranded oligo was incubated with 0.2 U/μl of terminal deoxynucleotidyl transferase (TdT) and 0.5 μM Biotin-11-dUTP in TdT reaction buffer at 37°C for 30 min; and the reaction was stopped by the addition of 1 μl of 0.5 M EDTA (pH 8.0). The labeled oligo was purified with an equal volume of cholorform:isoamyl alcohol (24:1) (Sigma-Aldrich). The biotin-labeled double-stranded probe was prepared using an equal molar ratio of purified 3′-end-labeled complementary oligos in the presence of salt (50 mM NaCl). Oligos were annealed by PCR: 1 cycle of 95°C for 5 min, 70 cycles of 95 °C for 1 min (temperature decrease 1°C/cycle). The annealed double- stranded DNA probe was portioned into aliquots and stored at −20°C. A mutated probe (mtGAS), which had 4 base changes (underscored), was also prepared. The sequences were 5′-ACCCCTTCACAGCGAAATTCA-3′ (sense strand) and 5′- TGAATTTCGCTGTGAAGGGGT-3′ (antisense strand).
Nuclear extracts were prepared from THP-1 cells treated with IFN-γ (50 ng/ml) for 5, 10, 20, 30, and 60 min, as well as untreated cells, using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce). EMSA was performed using the LightShift™ Chemiluminescent EMSA Kit (Pierce) following the manufacturer’s instructions. Briefly, 5 μg of nuclear extract protein was incubated with 50 fmol of biotin-labeled probe in 1× EMSA Binding Buffer (10 mM Tris, 50 mM KCl, and 1 mM DDT, pH 7.5) containing 50 ng/μl poly(dI·dC). For the competition assay, a 50- and 100-fold molar excess of unlabeled wtGAS and mtGAS probes was added to the binding reactions. For the antibody supershift EMSA, 5 μg of nuclear extracts was preincubated with 1 and 2 μg of anti-STAT1 9H2 monoclonal antibody (Cell Signaling Technology) and 1 μg of an unrelated control mouse monoclonal antibody for 1 h, on ice, prior to the addition of the labeled probe. After incubation for 20 min at room temperature, protein-DNA complexes were resolved on a 6% nondenaturing polyacrylamide gel and transferred onto nylon membrane (Hybond N+; GE Healthcare, Piscataway, NJ, USA) using a Trans-Blot SD Semi-Dry Electrophoretic Transfer Cell (Bio-Rad). The transferred DNA was cross- linked by UV onto the membrane. Detection of biotin-labeled probes was accomplished by using the LightShift Chemiluminescent EMSA Kit (Pierce).
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed using the EZ-Magna ChIP™ G Kit (Millipore, Billerica, MA, USA) following the manufacturer’s protocol. Briefly, 2 × 107 THP-1 cells were treated with IFN-γ (50 ng/ml) for 10 min, and chromatins were cross-linked by adding freshly prepared formaldehyde to the medium (final concentration of 1%) for 10 min. Cells were harvested and lysed with the cell lysis buffer, and subsequently with nuclear lysis buffer. The resulting nuclear lysate was sonicated on ice for 140 s, with 20% amplitude to shear DNA to an average length between 200 and 1000 bp. Following centrifugation, the supernatant (chromatin) was used for multiple immunoprecipitations with anti-STAT1 9H2 monoclonal antibody, anti-STAT3 antibody, anti-p44/42 MAP kinase antibody, and normal mouse IgG. The reaction components for each of the immunoprecipitations included an equal volume of chromatin, 20 μl fully suspended protein G magnetic beads, antibody, and ChIP dilution buffer added to a total volume of 500 μl. One percent (5 μl) of the reaction was removed as input chromatin from each.
Immunoprecipitation was performed with constant rotation at 4°C, overnight. Using the magnetic separator, the protein G beads were pelleted, and the complexes were washed with low salt, high salt, LiCl wash buffer, and TE buffer, once each. The protein/DNA complexes were eluted from the beads with ChIP elution buffer, and the cross-links were reversed by a proteinase K incubation at 62°C for 2 h with constant shaking. The freed DNA was purified by using spin columns (Qiagen). PCR detection was performed using the primer sets specific for the STAT1 binding site in the PTN promoter region. The sequences of the primer sets were 5′-CCATGGCAGTGTCAGGAAGT-3′ and 5′-TCTGGCTGTCCATCTGTTTG-3′, 5′-AAAAGGGGAAGAACCCTCAG-3′ and 5′-AATGCTGGCTTGATTGAGGT-3′, which amplify segments from −2163 to −2009 and −2172 to −1543 bp, encompassing the STAT1 binding sites in the PTN promoter.
RESULTS
PTN is expressed in highly vascularized human atherosclerotic plaques
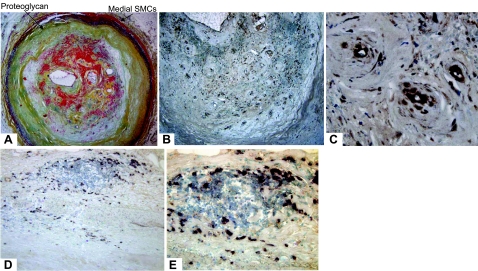
To elucidate the role of PTN in atherosclerosis, we first asked whether PTN is expressed in human plaque, and if so, which cell type expresses the gene. A representative Movat staining of a vascularized human plaque is shown in Fig. 1A. The medial SMCs (red) are visible. The inner layer of intima is primarily hypocellular, containing proteoglycan (greenish-yellowish) with a hypercellular outer layer that contains intraplaque hemorrhage (red in the intima). Staining of serial sections with anti-PTN antibody revealed that PTN is expressed in the lesion, and its expression is associated with the intraplaque capillaries (Fig. 1B, C; ×20 and × 40, respectively), suggesting that microvessel endothelial cells may express PTN.
Figure 1.
Representative photomicrographs of PTN expression in advanced human atherosclerotic plaques. A) Advanced human plaques were stained with Movat using standard protocol. B) Brown PTN staining (immunocytochemistry) is associated with microvessels in the intima and at the plaque base (×10). C) Higher magnification (×40) of the intimal area, showing expression of PTN associated with the microvessels and with the inflammatory cells in the plaque. D) Immunostaining using an anti-CD68 antibody (for macrophages; ×20). E) In situ hybridization using an antisense PTN riboprobe (×40).
We also investigated whether plaque macrophages express PTN, because this cytokine is reported to be expressed by macrophages in a highly vascularized region of ischemic brain (14). To explore this idea, a combination of immunostaining and in situ hybridization approaches were employed, because PTN is a secreted cytokine that could potentially diffuse out from its cellular site of expression. The immunostaining of serial section with anti-CD68 antibody revealed that macrophages are present in the plaque, and they are primarily concentrated in the region with intraplaque hemorrhage (Fig. 1D). The in situ hybridization was performed using previously published PTN riboprobes and according to the protocol (15). We found that the expression of PTN mRNA colocalizes with macrophages located in the intraplaque hemorrhage area. The ability of plaque macrophages to express PTN is further supported by cultured macrophages (see below). The expression pattern of PTN in the atherosclerotic plaques appears to be similar to that of infarcted brain (14).
IFN-γ up-regulates PTN expression in macrophages
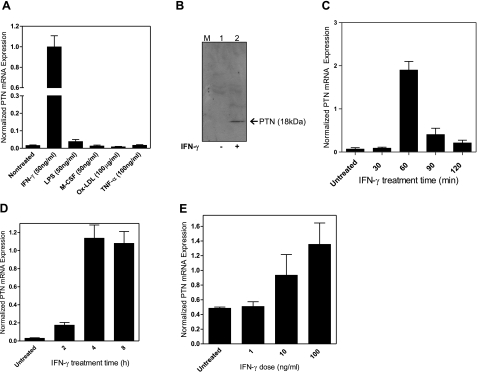
PTN is known to be expressed by endothelial cells and macrophages in the highly vascularized region of ischemic tissue (14, 19). Although the expression of PTN in endothelial cells has been demonstrated, nothing is known about the expression of this cytokine by macrophages, a cell type that is central to the pathogenesis of atherosclerosis. To understand the underlying mechanism that is responsible for PTN expression in macrophages, THP-1 cells were treated with reagents known to be important in atherosclerosis. Real-time PCR analysis revealed that a low level of PTN mRNA was expressed by resting THP-1 cells, and the addition of human IFN-γ (50 ng/ml) markedly up-regulated PTN mRNA transcript. Other factors that are important in atherosclerosis such as LPS, M-CSF, ox-LDL, and TNF-α had little effect in regulating PTN expression in macrophages (Fig. 2A). IFN-γ was also found to stimulate PTN expression at the protein level, as the conditioned medium from IFN-γ stimulated macrophages showed marked increased in the secreted PTN protein (Fig. 2B, lane 2) when compared to unstimulated cells (Fig. 2B, lane 1). This regulation pattern of PTN expression by IFN-γ seems to be a conserved event in humans and mice, because when mouse peritoneal-derived macrophages were treated with mouse recombinant IFN-γ (10 ng/ml), the PTN mRNA was increased by ∼20 fold as normalized against GAPDH mRNA (Fig. 2C). Also, we found that the expression of PTN by IFN-γ is time dependent, as the expression level was increased by 4- and 35-fold 2 and 4 h after treatment of macrophages by IFN-γ, respectively (Fig. 2D). The expression is also dose dependent, as 10 and 100 ng/ml of IFN-γ markedly increased PTN mRNA levels in a dose-dependent manner (Fig. 2E). These results suggest that IFN-γ is an important regulator of PTN expression in human monocytes/macrophages.
Figure 2.
Up-regulation of PTN by IFN-γ in THP-1 cells and mouse peritoneal macrophages. A) THP-1 cells were treated with IFN-γ, LPS, M-CSF, and TNF-α for 48 h, and PTN mRNA expression was quantified by real-time PCR. B) Western blotting analysis of PTN protein secreted in the culture medium of IFN-γ-treated THP-1 cells (50 ng/ml for 48 h). C) Real-time PCR analysis of PTN mRNA induction in mouse peritoneal macrophages by IFN-γ (10 ng/ml). Values were normalized against GAPDH mRNA. D) Time course of IFN-γ induction of PTN mRNA expression by THP-1 cells. E) Dose-dependent IFN-γ induction of PTN mRNA expression by THP-1 cells. Cells were treated for 3 h. In all experiments, untreated cells were used as a control.
IFN-γ up-regulates PTN mRNA expression by activating JAK/STAT signaling pathway
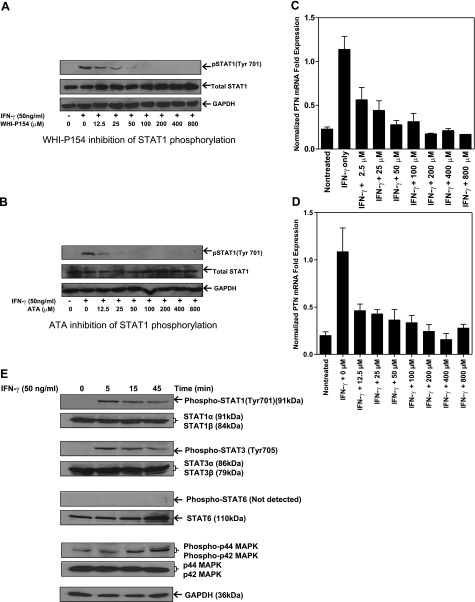
To further explore the mechanism of PTN expression in macrophages, we examined the role of JAK/STAT signaling in PTN expression. The phosphorylation of STAT1 was blocked by known pharmacological inhibitors, such as WHI-P154 and ATA, and the effect of this inhibition on IFN-γ-induced PTN expression was determined. THP-1 cells were pretreated with increasing concentrations of each of the two inhibitors for 1 h, followed by addition of IFN-γ for 10 min. The cell extracts were prepared and analyzed by immunoblot using tyrosine-specific antibody. The results revealed that although the levels of total STAT1 and GAPDH proteins were not affected by the treatments of cells with the inhibitors, the phosphorylation of STAT1 was completely blocked by the JAK inhibitors in a dose-dependent manner (Fig. 3A, B). The STAT1 phosphorylation was reduced by ∼50% in the presence of 12.5 μM ATA or WHI-P154 inhibitors (Fig. 3A, B). Real-time PCR analysis of total RNA extracted from the inhibitor-treated cells showed a good correlation between STAT1 phosphorylation and PTN mRNA expression. In the absence of the inhibitor, IFN-γ markedly up-regulated PTN mRNA, and addition of WHI-P154 (Fig. 3C) or ATA (Fig. 3D) reduced the transcript levels in a dose-dependent manner. These results suggest that phosphorylation of STAT1 is critical to the expression of PTN by IFN-γ in macrophages.
Figure 3.
IFN-γ up-regulates PTN expression by activating JAK/STAT signal pathway. A, B) THP-1 cells preincubated with the indicated concentrations of JAK inhibitors, WHI-P154 (A) or ATA (B), for 1 h, followed by IFN-γ treatment for 10 min in the presence of each inhibitor. Cell lysates were probed with anti-STAT1 (Tyr 701) antibody by Western blotting. Untreated THP-1 cells and those preincubated with increasing concentrations of the inhibitors for 1 h were further treated with IFN-γ for 2 h. C, D) PTN mRNA levels were quantified by real-time RT-PCR and normalized against GAPDH mRNA. E) THP-1 cells were treated with IFN-γ (50 ng/ml) for the indicated times, and phosphorylation levels of STAT1, STAT3, STAT6, and p44/42 were determined by Western blot.
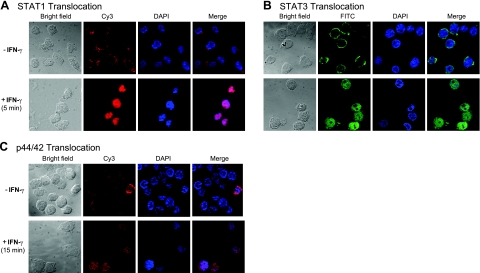
In addition to STAT1, IFN-γ is known to phosphorylate other signaling molecules, such as STAT3, STAT6, and p44/42 MAPK (ERK1/2), in different cell types. We therefore determined phosphorylation of the signaling molecules by IFN-γ in macrophages. Time-course analysis of the cell extract revealed that STAT1 and STAT3 were phosphorylated as early as 5 min after exposure of the cells to IFN-γ (Fig. 3E). In contrast, STAT6 was not phosphorylated under identical experimental condition. The phosphorylation pattern of p44/42 was, however, different from those of STAT proteins. The p44/42 was weakly phosphorylated in the untreated macrophages, and the phosphorylation level was markedly increased 15 min after addition of IFN-γ to the macrophages (Fig. 3E). This phosphorylation was associated with translocation of these transcription factors into the nuclei (Fig. 4). Thus, in addition to STAT1, STAT3 and p44/42 are activated by IFN-γ in macrophages.
Figure 4.
Immunofluorescence analysis of nuclear translocation of activated STAT1, STAT3, and p44/42. Immunofluorescence staining of THP-1 cells was performed using a mouse monoclonal anti-STAT1 (A), a rabbit anti-STAT3 (B), and a mouse monoclonal anti-p44/42 MAPK (C). Activated STAT1, STAT3, and p44/42 translocated to the nuclei on IFN-γ stimulation. Red fluorescence shows STAT1 and p44/42 localization (A, C); pink in the merged images shows colocalization of activated STAT1, p44/42 in the nuclei (A, C); green fluorescence shows STAT3 localization (B); blue shows DAPI nuclear stain (all panels).
Human PTN gene promoter contains IFN-γ responsive element
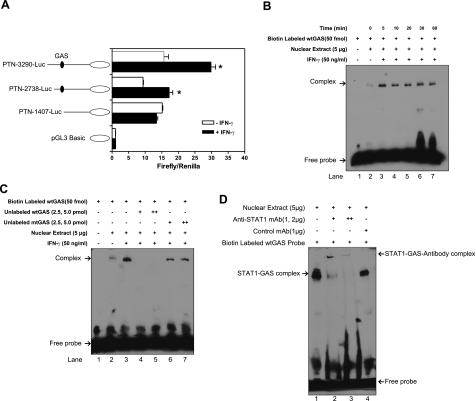
To map the cis-acting GAS element that is responsible for the expression of PTN by IFN-γ, we examined PTN promoter activity in response to IFN-γ treatment in macrophages. Three different fragments of PTN promoter were constructed by PCR, cloned into pGL4 luciferase reporter vector, and transiently transfected into THP-1 cells. Statistical analysis (Student’s t test) of the 3 independent experiments performed in triplicate showed that means ± se for the pGL4-PTN and pGL4-PTN-3290 + IFNγ constructs were 15.64 ± 1.362 and 29.88 ± 1.315, respectively (P=0.0072). Similarly, means ± se for the pGL4-PTN-2738 and pGL4-PTN-2738 + IFNγ constructs were 9.336 ± 0.1400 and 17.20 ± 1.100, respectively (P=0.0193). No statistically significant difference was found in the PTN-1407-Luc constructs. Thus, the two constructs containing the GAS element exhibited strong activity when cells were treated with IFN-γ, whereas the relative luciferase activity of the short PTN-1407-Luc construct was low, comparable to the activity of the promoter in the untreated cells. To identify the cis-acting element of the PTN promoter that is modulated by IFN-γ, we examined transcriptional factor binding sites in the PTN promoter using TFSEARCH software (http://www.cbrc.jp/research/db/TFSEARCH.html). We found a putative IFN-γ cis-acting element in the PTN promoter sequence that is located between −2086 and −2078 bp. This region is covered by the two PTN-fragment constructs that exhibited luciferase activity on IFN-γ stimulation, the PTN-3290-Luc and PTN-2738-Luc constructs, whereas the short inactive construct was devoid of this region. These results demonstrate that the putative cis element in the PTN promoter is essential for up-regulation by IFN-γ in macrophages.
We further extended these observations by investigating the interaction between STAT1 transcription factor and its putative binding element in macrophages in response to treatment with IFN-γ. It is known that on activation, STAT1 forms homodimers and translocates into the cell nucleus, where it interacts with the cis-acting element, leading to activation of gene expression (20). To explore this, nuclear extracts from THP-1 treated with or without IFN-γ were analyzed by gel-shift assay. The nuclear extracts were mixed with the 3′-end biotin-labeled probe encoding the GAS sequence, and the binding activity was analyzed by a 6% nondenaturing polyacrylamide gel. The protein-DNA complex was clearly detectable in the IFN-γ stimulated cells. The nuclear extract prepared from IFN-γ treated THP-1 cells (5 min) strongly reacted with the specific probe (Fig. 5B, lane 3), whereas the nuclear extract from untreated cells showed a faint band (Fig. 5B, lane 2). This interaction is specific, as addition of 50- and 100-fold molar excess nonlabeled probe completely blocked the complex formation (Fig. 5C, lanes 4 and 5). The binding specificity was further verified by using a mutant probe, where we observed that the addition of 50- and 100-fold molar excess inactive mutant probe had little, if any, effect on the interaction between the nuclear cell extract components and the STAT1 probe (Fig. 5C, lanes 6 and 7). The specificity of the complex formation was further supported by the supershift assay using anti-STAT1 monoclonal antibody. The position of the primary complex moved up to a new “supershifted” position (Fig. 5D, lanes 2 and 3) as compared to the control monoclonal antibody (Fig. 5D, lane 4).
Figure 5.
Human PTN promoter contains an IFN-γ activated element that interacts with STAT1. A) Schematic representation of human PTN promoter luciferase reporter constructs used in the transient transfection of THP-1 cells, showing position of the IFN-γ activated element GAS. PTN promoter luciferase constructs were cotransfected with an internal control vector, pRL-β-actin. Results are means ± sd of 3 independent experiments performed in triplicates. Asterisk indicates statistically significant difference in luciferase activity. B) EMSA assay showing the binding of THP-1 nuclear extracts to wtGAS following 5-, 10-, 20-, 30-, and 60-min IFN-γ treatments. C) Specificity of binding; 50 (+)- and 100 (++)-fold molar excess of unlabeled probe wtGAS (lane 4 and 5), but not mutant probe mtGAS (lanes 6 and 7), inhibited formation of the nuclear extract/probe complex in the presence of biotin-labeled wtGAS (50 fmol) D) Supershift assay with 1.0 and 2.0 μg (lanes 2 and3, respectively) of an anti-STAT1 monoclonal antibody; unrelated monoclonal antibody had no effect on the nuclear extract/probe complex (lane 4).
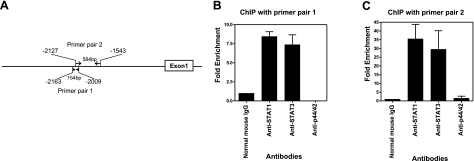
To determine whether the specificity of STAT1 binding to GAS element can be demonstrated at the chromatin level, we performed ChIP assay using specific antibodies to STAT1. Chromatin fragments from IFN-γ treated THP-1 cells were immunoprecipitated with anti-STAT1 monoclonal antibody, and the presence of cis-acting element was determined by PCR using two different primers that detect 154- and 584-bp regions that span the GAS element in the PTN promoter (Fig. 6A). Strong 154- and 584-bp amplified bands were detected in the chromatin preparation that was immunoprecipited with anti-STAT1 monoclonal antibody (Fig. 6B, C).
Figure 6.
PCR analysis of chromatin immunoprecipitation. Chromatin from IFN-γ treated THP-1 cells was immunoprecipited with antibodies against STAT1, STAT3, p44/42, and normal mouse IgG. After elution of protein-DNA complexes from protein G magnetic beads and reversal of the cross-link, purified DNA was used for PCR analysis and quantification using a densitometer. A) Schematic diagram of PTN promoter. Arrows indicate locations of the primer pairs that cover the GAS motif. B, C) Fold enrichment of PCR products from each antibody, as quantified by densitometery from independent experiments. Data are expressed as the ratio of each antibody signal to IgG signal, calculated by normalization against input chromatin. Anti-STAT1 and anti-STAT3 antibodies markedly enriched PTN promoter DNA with both primer pairs [8.4- and 7.3-fold with primer pair 1 (B); 35- and 29.5-fold with primer pair 2 (C)], but substantially less was detected in the anti-p44/42 and normal IgG control.
As STAT3 and p44/42 are phosphorylated (Fig. 3) and are translocated into the nucleus (Fig. 4) in response to IFN-γ treatment, we asked whether these signaling molecules interact with the chromatin. The anti-STAT-3 and anti-p44/42 immunoprecipitated the chromatin fragments, but less intense 154- and 584-bp bands were observed with anti-STAT3 antibody (Fig. 6B, C). The amount of PCR product was significantly reduced when anti-p44/42 antibody or normal IgG control antibody was used, specifically with primer 2, which amplified the 584-bp band (Fig. 6C). These results suggest that both STAT1 and STAT3 strongly interact with the GAS region within the PTN promoter.
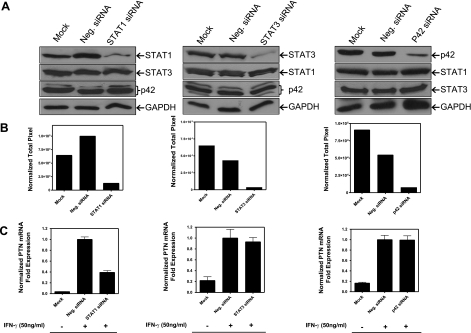
Although IFN-γ activates STAT1/STAT3/p44/42 signaling in macrophages, it is unclear whether their combined activation is required for regulation of PTN expression. To explore this, we used a gene-knockdown approach to determine the effect of this manipulation on PTN expression. THP-1 cells were transfected with specific siRNAs to STAT1, STAT3, and p42, and 24 h after transfection the cells were treated with IFN-γ for 3 h, and cell lysates and RNA were prepared. Western blot analysis of cell lysates revealed that each siRNA efficiently reduced targeted gene expression as compared to control siRNA (Fig. 7A). The relative band intensities calculated by densitometry showed 87, 92, and 87% reduction of STAT1, STAT3, and p42 when normalized to the GAPDH loading control, respectively (Fig. 7B). The specificity of the knockdown experiment was demonstrated by the selectivity of the siRNAs to down-regulate specific genes: the STAT1 siRNA had no effect on the protein expression level of STAT3, p42, and GAPDH (Fig. 7A). Similarly, STAT3 and p42 siRNAs suppressed only their targeted genes (Fig. 7A). Real-time PCR analysis revealed that STAT1 knockdown reduced PTN mRNA level by 61%, whereas STAT3 and P42 had no effect (Fig. 7C). These results underscore the importance of STAT1 signaling in the expression of PTN in macrophages.
Figure 7.
Analysis of siRNA knockdown of STAT1, STAT3, and p42 gene at protein levels and their effects on IFN-γ induced up-regulation of PTN mRNA expression. A) THP-1 cells were transfected with STAT1, STAT3, and p42 siRNAs. At 24 h posttransfection, cells were treated with IFN-γ (50 ng/ml for 3 h), and Western blotting confirmed the knockdown of the expression level of each protein. B) Densitometry of STAT1, STAT3 and p42 levels, normalized against GAPDH. C) Real-time PCR analysis of the effect of STAT1, STAT3, and p42 knockdown on IFN-γ induced PTN mRNA expression.
DISCUSSION
Although neovascularization is thought to be an important contributor to atherogenesis and plaque instability (21), the underlying mechanism that regulates this process is still not fully understood. It is thought that the wall thickness-induced hypoxia activates hypoxia-inducible transcription factor HIF-1α, which stimulates expression of VEGF and other angiogenic regulators (22). Plaque thickness, however, seems to be unrelated to intraplaque neovascularization, because the number of capillaries in thick fibrous plaques is significantly lower than lipid-rich plaques. In such lesions the number of capillaries strongly correlates with the number of macrophages (23). Similarly, neovessel density was found to be strongly correlated with the severity of plaque inflammation (24). In the murine animal model, the frequency of microvessels is unrelated to plaque thickness (25). Hence, inflammation seems to be the critical determinant of plaque angiogenesis. The mechanism by which inflammation induces neovascularization is poorly defined.
We found that PTN, a cytokine/growth factor that is induced by proinflammatory IFN-γ, is expressed in human plaques. This expression most likely affects plaque pathology through at least 3 mechanisms. First, PTN can promote intraplaque vascularization by stimulating migration/proliferation of endothelial cells that are recruited into the intima from the vasa vasorum. Second, PTN can amplify the severity of plaque inflammation as this factor is known to stimulate replication of peripheral-derived monocytes and tissue macrophages (26, 27). This cell type is known to replicate in the rabbit lesions (28) and human plaques (29), and that the proliferating macrophages, rather than endothelial cells, are associated with intimal microvessels in primary lesions (30). We noted that the expression of PTN in monocytes/macrophages is regulated by IFN-γ. Hence, inflammation may establish an autocrine loop that promotes proliferation of monocytes/macrophages through expression of PTN. Third, PTN can promote intraplaque neovascularization by coaxing monocytes/macrophages to assume an endothelial cell phenotype. We previously reported that the expression of PTN by monocytes/macrophages led to down-regulation of cell type-specific markers (c-fms/CD68/CD14) and up-regulation of endothelial cell markers (VE cadherin, vWF, eNOS, Tie-2, and αvβ3. These phenotypically modulated cells formed tubelike structures in vitro, similar to endothelial cells (16). More important, PTN-expressing monocytes, but not control monocytes, incorporated into the blood vessels of the quail chorioallantoic membrane and intracardial injection of cells into chicken embryos led to the integration of cells into the developing vasculature (16). Moreover, injection of the PTN-expressing monocytes into mouse ischemic hind limb significantly improved perfusion of ischemic tissue (16). Our results have since been confirmed by other groups. The exposure of macrophages to the conditioned medium from PTN-expressing breast cancer MDA-MB-231 cells induced VEGFR2 expression, whereas PTN-nonexpressing MCF-7 cells conditioned medium had no effect (31). In another study, a strong correlation was reported between PTN expression and neovascularization of bone marrow in patients with multiple myeloma (32). When human peripheral blood monocytes were exposed to serum or cultured with bone marrow from these patients, monocytes expressed endothelial cell markers and formed tubelike structures, effects that were specifically blocked by anti-PTN antibodies (33). Notably, when GFP-tagged human monocytes were coinjected with human multiple myeloma cells into severe combined immunodeficient mice, GFP-labeled monocytes lined the lumen of tumor blood vessels and expressed human endothelial cell markers, effects that were also blocked by an anti-PTN antibody (32, 33). Thus, in addition to their secondary role as a source of angiogenic factors, monocytes/macrophages may directly promote intimal neovascularization by acting like functional endothelial cells through expression of PTN.
Although the recruitment of new vessels into a plaque from the preexisting vasa vasorum has been described (18, 34), nothing is known about the role of nonendothelial cells in the neovascularization of atherosclerotic plaques. Some tumor cells mimic the activities of endothelial cells such as neovascularization and the formation of a fluid-conducting meshwork. This type of tumor perfusion, referred to as vasculogenic mimicry, points to de novo generation of blood vessels without the participation of endothelial cells and angiogenesis (35, 36). For example, in xenografted and spontaneous human colon carcinomas (37) and in the highly invasive melanoma (35), vascular channels are lined by tumor cells themselves that express endothelial cell markers (38) and facilitate tumor perfusion, independent of angiogenesis. Interestingly, these tumors express a high level of PTN (39,40,41,42). The nonendothelial cell-originated microvessels are leaky and fragile and are prone to rupture (36), similar to those of plaque capillaries (21, 43). Thus, reminiscent of a cancerous lesion, the intraplaque capillaries may in part originate from macrophages in the presence of PTN.
We have identified a new signaling pathway that specifically regulates expression of PTN in inflamed tissues. This regulation is mediated by the putative cis-acting element that is located between −2086 and −2078 of the PTN promoter region and is responsible for the expression of PTN by IFN-γ in macrophages. This gene expression is specific to IFN-γ and is not due to the general activation of macrophages because other activators of macrophages such as LPS, M-CSF, ox-LDL, or TNF-α were ineffective in up-regulating PTN gene expression. Past studies have shown that PDGF stimulates PTN mRNA expression in NIH/3T3 cells through serum response element (44). HOXA5 transcriptional factor was found to regulate PTN expression in Hs578T cells (45). Hydrogen peroxide induction of PTN in prostate cancer cells was found to be mediated by AP-1 element in the PTN promoter (46). The identification of IFN-γ/STAT1/GAS axis as a regulator of PTN gene expression may indicate that this signaling pathway links inflammation to angiogenesis.
Mechanistic studies revealed that STAT1 plays a key role in the expression of PTN in macrophages. We noted that STAT1 is rapidly phosphorylated at Tyr-701 in response to IFN-γ. Western blot analysis demonstrated that STAT1 was phosphorylated within 5 min after treatment of macrophages with IFN-γ, and the phosphorylation was completely blocked by JAK/STAT inhibitors. Notably, inhibition of STAT1 phosphorylation blocked PTN mRNA expression, suggesting a direct link between STAT1 Tyr-701 phosphorylation and IFN-γ-mediated PTN expression. In addition to STAT1, we found that both STAT3 and p44/42, but not STAT6, are phosphorylated by IFN-γ, translocated into the nuclei and interacted with the chromatin. However, the combined activities of these IFN-γ-activated factors are not required for the expression of PTN because knockdown of STAT1, but not STAT3 or p44/42, blocked PTN expression in macrophages. The significance of activation of STAT3/p44/42 signaling molecules by IFN-γ in the expression of PTN in macrophages is not immediately apparent. It is reported that STAT1 and STAT3 activation by IFN-γ leads to the formation of a STAT1/STAT3 heterodimer, in addition to STAT1 and STAT3 homodimers (20). STAT3 appears to play a critical negative role in controlling inflammation, as shown in mice with STAT3 deletion in specific cell types, including T cells (47), macrophages/neutrophils (48), or endothelial cells (49). STAT3 deletion in mice within the macrophage/neutrophil lineage results in chronic inflammation due to the enhancement of the Th1 response by a blockade of IL-10 signaling (48). Similarly, removal of STAT3 from hematopoietic progenitors increases proinflammatory cytokine production and an expanded macrophage population (50). Thus, formation of STAT1/STAT3 heterodimers by IFN-γ appears to counterbalance the proinflammatory effect of STAT1 homodimers. These data highlight the importance of the STAT1/STAT3 heterodimer signaling as a regulator of PTN expression in macrophages.
CONCLUSIONS
To develop a therapeutic strategy that is based on neovascular regression, it is important to gain critical knowledge about the mechanisms that control intraplaque neovascularization. We found that PTN expression is strongly associated with the highly vascularized region of human plaques and that IFN-γ/STAT1 signaling is an important determinant of PTN expression in monocytes/macrophages. Although it is thought that intraplaque microvessels originate from the ingrowth of vasa vasorum capillaries, the ability of PTN to promote the proliferation of monocytes/macrophages and its ability to coax these cells to adopt a phenotype of functional endothelial cells raises an intriguing possibility that intimal capillaries may in part originate from monocytes/macrophages. The out-growth (toward adventitia) of these microvessels and their connection to vasa vasorum capillaries could significantly contribute to the disease process.
Acknowledgments
This work was supported by the U.S. National Institutes of Health (grants RO1 HL050566 and RO1 HL090653), the Spielberg Research Fund, the Heart Foundation, the Corday Foundation, the Feintech Foundation, the Shapell and Guerin Foundation, and United Hostesses.
References
- Hansson G K. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002;283:H2411–H2419. doi: 10.1152/ajpheart.01098.2001. [DOI] [PubMed] [Google Scholar]
- Arras M, Ito W D, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy P E. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP) J Biol Chem. 2008;283:14542–14551. doi: 10.1074/jbc.M802139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y J, Holm J, Nygren S, Bruzelius M, Stemme S, Hansson G K. Expression of the macrophage scavenger receptor in atheroma: relationship to immune activation and the T-cell cytokine interferon-γ. Arterioscler Thromb Vasc Biol. 1995;15:1995–2002. doi: 10.1161/01.atv.15.11.1995. [DOI] [PubMed] [Google Scholar]
- Stemme S, Faber B, Holm J, Wiklund O, Witztum J L, Hansson G K. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono C, Come C E, Stavrakis G, Maguire G F, Connelly P W, Lichtman A H. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- Gupta S, Pablo A M, Jiang X, Wang N, Tall A R, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman S C, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-γ enhances atherosclerosis in apolipoprotein e−/− mice. Am J Pathol. 2000;157:1819–1824. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey E J, Ramji D P. Interferon-γ and atherosclerosis: pro- or anti-atherogenic? Cardiovasc Res. 2005;67:11–20. doi: 10.1016/j.cardiores.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem (Tokyo) 2002;132:359–371. doi: 10.1093/oxfordjournals.jbchem.a003231. [DOI] [PubMed] [Google Scholar]
- Christman K L, Fang Q, Yee M S, Johnson K R, Sievers R E, Lee R J. Enhanced neovasculature formation in ischemic myocardium following delivery of pleiotrophin plasmid in a biopolymer. Biomaterials. 2005;26:1139–1144. doi: 10.1016/j.biomaterials.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Chen H W, Yu S L, Chen W J, Yang P C, Chien C T, Chou H Y, Li H N, Peck K, Huang C H, Lin F Y, Chen J J W, Lee Y T. Dynamic changes of gene expression profiles during postnatal development of the heart in mice. Heart. 2004;90:927–934. doi: 10.1136/hrt.2002.006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H J, He Y Y, Xu J, Hsu C Y, Deuel T F. Upregulation of pleiotrophin gene expression in developing microvasculature, macrophages, and astrocytes after acute ischemic brain injury. J Neurosci. 1998;18:3699–3707. doi: 10.1523/JNEUROSCI.18-10-03699.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Zeng Z, Zheng J, Shah P K, Schwartz S M, Adams L D, Sharifi B G. Suppression subtractive hybridization identifies distinctive expression markers for coronary and internal mammary arteries. Arterioscler Thromb Vasc Biol. 2003;23:425–433. doi: 10.1161/01.ATV.0000059303.94760.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi B G, Zeng Z, Wang L, Song L, Chen H, Qin M, Sierra-Honigmann M R, Wachsmann-Hogiu S, Shah P K. Pleiotrophin induces transdifferentiation of monocytes into functional endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1273–1280. doi: 10.1161/01.ATV.0000222017.05085.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner K, Li C, Shah P K, Fishbein M C, Forrester J S, Kaul S, Sharifi B G. Tenascin-C is expressed in macrophage-rich human coronary atherosclerotic plaque. Circulation. 1999;99:1284–1289. doi: 10.1161/01.cir.99.10.1284. [DOI] [PubMed] [Google Scholar]
- Hatziapostolou M, Delbe J, Katsoris P, Polytarchou C, Courty J, Papadimitriou E. Heparin affin regulatory peptide is a key player in prostate cancer cell growth and angiogenicity. Prostate. 2005;65:151–158. doi: 10.1002/pros.20270. [DOI] [PubMed] [Google Scholar]
- Takeda A, Onodera H, Sugimoto A, Itoyama Y, Kogure K, Rauvala H, Shibahara S. Induction of heparin-binding growth-associated molecule expression in reactive astrocytes following hippocampal neuronal injury. Neuroscience. 1995;68:57–64. doi: 10.1016/0306-4522(95)00110-5. [DOI] [PubMed] [Google Scholar]
- Darnell J E., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Virmani R, Kolodgie F D, Burke A P, Finn A V, Gold H K, Tulenko T N, Wrenn S P, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- Pugh C W, Ratcliffe P J. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- De Boer O J, van der Wal A C, Teeling P, Becker A E. Leucocyte recruitment in rupture prone regions of lipid-rich plaques: a prominent role for neovascularization? Cardiovasc Res. 1999;41:443–449. doi: 10.1016/s0008-6363(98)00255-7. [DOI] [PubMed] [Google Scholar]
- Moreno P R, Purushothaman K R, Fuster V, Echeverri D, Truszczynska H, Sharma S K, Badimon J J, O'Connor W N. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–2038. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- Moulton K S, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo K M, Gillies S, Javaherian K, Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achour A, Laaroubi D, Caruelle D, Barritault D, Courty J. The angiogenic factor heparin affin regulatory peptide (HARP) induces proliferation of human peripheral blood mononuclear cells. [Online] Cell Mol Biol. 2001;47:OL73–OL77. [PubMed] [Google Scholar]
- Pufe T, Bartscher M, Petersen W, Tillmann B, Mentlein R. Expression of pleiotrophin, an embryonic growth and differentiation factor, in rheumatoid arthritis. Arthritis Rheum. 2003;48:660–667. doi: 10.1002/art.10839. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M E, Ross R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of WHHL and comparably hypercholesterolemic fat-fed rabbits. Arterioscler Thromb Vasc Biol. 1990;10:680–687. doi: 10.1161/01.atv.10.5.680. [DOI] [PubMed] [Google Scholar]
- Gordon D, Reidy M A, Benditt E P, Schwartz S M. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A. 1990;87:4600–4604. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E R, Garvin M R, Dev R, Stewart D K, Hinohara T, Simpson J B, Schwartz S M. Angiogenesis in human coronary atherosclerotic plaques. Am J Pathol. 1994;145:883–894. [PMC free article] [PubMed] [Google Scholar]
- Dineen S P, Lynn K D, Holloway S E, Miller A F, Sullivan J P, Shames D S, Beck A W, Barnett C C, Fleming J B, Brekken R A. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res. 2008;68:4340–4346. doi: 10.1158/0008-5472.CAN-07-6705. [DOI] [PubMed] [Google Scholar]
- Chen H, Gordon M S, Campbell R A, Li M, Wang C S, Lee H J, Sanchez E, Manyak S J, Gui D, Shalitin D, Said J, Chang Y, Deuel T F, Baritaki S, Bonavida B, Berenson J R. Pleiotrophin is highly expressed by myeloma cells and promotes myeloma tumor growth. Blood. 2007;110:287–295. doi: 10.1182/blood-2006-08-042374. [DOI] [PubMed] [Google Scholar]
- Chen H, Campbell R A, Chang Y, Li M, Wang C S, Li J, Sanchez E, Share M, Steinberg J, Berenson A, Shalitin D, Zeng Z, Gui D, Perez-Pinera P, Berenson R J, Said J, Bonavida B, Deuel T F, Berenson J R. Pleiotrophin produced by multiple myeloma induces transdifferentiation of monocytes into vascular endothelial cells: a novel mechanism of tumor-induced vasculogenesis. Blood. 2009;113:1992–2002. doi: 10.1182/blood-2008-02-133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger A C, Beeuwkes R, 3rd, Lainey L L, Silverman K J. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984;310:175–177. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- Folberg R, Hendrix M J, Maniotis A J. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361–381. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix M J, Seftor E A, Hess A R, Seftor R E. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- Chang Y S, di Tomaso E, McDonald D M, Jones R, Jain R K, Munn L L. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A. 2000;97:14608–14613. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood R, Cai J, Zheng T, Smith D L, Hinton D R, Gill P S. Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF receptor-positive human tumors. Blood. 2001;98:1904–1913. doi: 10.1182/blood.v98.6.1904. [DOI] [PubMed] [Google Scholar]
- Czubayko F, Schulte, Anke M, Berchem, Guy J, Wellstein A. Melanoma angiogenesis and metastasis modulated by ribozyme targeting of the secreted growth factor pleiotrophin. Proc Natl Acad Sci U S A. 1996;93:14753–14758. doi: 10.1073/pnas.93.25.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D, Klomp H J, Czubayko F, Wellstein A, Juhl H. Pleiotrophin can be rate-limiting for pancreatic cancer cell growth. Cancer Res. 2000;60:5284–5288. [PubMed] [Google Scholar]
- Vacherot F, Caruelle D, Chopin D, Gil-Diez S, Barritault D, Caruelle J P, Courty J. Involvement of heparin affin regulatory peptide in human prostate cancer. Prostate. 1999;38:126–136. doi: 10.1002/(sici)1097-0045(19990201)38:2<126::aid-pros6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Riegel A T, Wellstein A. The potential role of the heparin-binding growth factor pleiotrophin in breast cancer. Breast Cancer Res Treat. 1994;31:309–314. doi: 10.1007/BF00666163. [DOI] [PubMed] [Google Scholar]
- Barger A C, Beeuwkes R., 3rd Rupture of coronary vasa vasorum as a trigger of acute myocardial infarction. Am J Cardiol. 1990;66:41G–43G. doi: 10.1016/0002-9149(90)90394-g. [DOI] [PubMed] [Google Scholar]
- Li Y S, Gurrieri M, Deuel T F. Pleiotrophin gene expression is highly restricted and is regulated by platelet-derived growth factor. Biochem Biophys Res Commun. 1992;184:427–432. doi: 10.1016/0006-291x(92)91211-8. [DOI] [PubMed] [Google Scholar]
- Chen H, Rubin E, Zhang H, Chung S, Jie C C, Garrett E, Biswal S, Sukumar S. Identification of transcriptional targets of HOXA5. J Biol Chem. 2005;280:19373–19380. doi: 10.1074/jbc.M413528200. [DOI] [PubMed] [Google Scholar]
- Polytarchou C, Hatziapostolou M, Papadimitriou E. Hydrogen peroxide stimulates proliferation and migration of human prostate cancer cells through activation of activator protein-1 and up-regulation of the heparin affin regulatory peptide gene. J Biol Chem. 2005;280:40428–40435. doi: 10.1074/jbc.M505120200. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- Takeda K, Clausen B E, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- Kano A, Wolfgang M J, Gao Q, Jacoby J, Chai G X, Hansen W, Iwamoto Y, Pober J S, Flavell R A, Fu X Y. Endothelial cells require Stat3 for protection against endotoxin-induced inflammation. J Exp Med. 2003;198:1517–1525. doi: 10.1084/jem.20030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte T, Zhang S S M, Wang T, Zhang Z, Hesslein D G T, Yin Z, Kano A, Iwamoto Y, Li E, Craft J E, Bothwell A L M, Fikrig E, Koni P A, Flavell R A, Fu X Y. Stat3 deletion during hematopoiesis causes Crohn’s disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci U S A. 2003;100:1879–1884. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]