Abstract
Sepsis is a severe infection-induced systemic inflammatory syndrome. Inhibition of downstream inflammatory mediators of sepsis, e.g., TNF-α, has failed in clinical trials. The aim of this study was to investigate the effects of inhibiting CD14, a key upstream innate immunity molecule, on the early inflammatory and hemostatic responses in a pig model of gram-negative sepsis. The study comprised two arms, whole live Escherichia coli bacteria and E. coli lipopolysaccharide (LPS) (n=25 and n=9 animals, respectively). The animals were allocated into treatment (anti-CD14) and control (IgG isotype or saline) groups. Inflammatory, hemostatic, physiological, and microbiological parameters were measured. The proinflammatory cytokines TNF-α, IL-1β, IL-6, and IL-8, but not the anti-inflammatory cytokine IL-10, were efficiently inhibited by anti-CD14. Furthermore, anti-CD14 preserved the leukocyte count and significantly reduced granulocyte enzyme matrix metalloproteinase-9 release and expression of the granulocyte membrane activation molecule wCD11R3 (pig CD11b). The hemostatic markers thrombin-antithrombin III complexes and plasminogen activator inhibitor-1 were significantly attenuated. Anti-CD14 did not affect LPS or E. coli DNA levels. This study documents that CD14 inhibition efficiently attenuates the proinflammatory cytokine response and granulocyte activation and reverses the procoagulant state but does not interfere with LPS levels or bacterial counts in E. coli-induced sepsis.—Thorgersen, E. B., Hellerud, B. C., Nielsen, E. W., Barratt-Due, A., Fure, H., Lindstad, J. K., Pharo, A., Fosse, E., Tønnessen, T. I., Johansen, H. T., Castellheim, A., Mollnes, T. E. CD14 inhibition efficiently attenuates early inflammatory and hemostatic responses in Escherichia coli sepsis in pigs.
Keywords: gram-negative bacteria, inflammation, innate immunity, lipopolysaccharide
Sepsis is a serious and life-threatening syndrome that affects body homeostasis, including the innate immune system (1) and the hemostatic systems (2), and may lead to multiple organ failure. Gram-negative pathogens including Escherichia coli account for 25–40% of all cases of sepsis (3, 4).
The inflammatory response in sepsis may become excessive and dysregulated, implying that the innate immune mechanisms that are normally concerned with defense against infections might cause tissue damage and organ failure and potentially lead to death. Danger-associated molecular patterns (DAMPs) of exogenous microbial products called pathogen-associated molecular patterns and of endogenous damaged tissues, often referred to as alarmins, will activate the innate immune system in sepsis (5,6,7,8). This may lead to an early, overwhelming inflammatory response. The Toll-like receptor (TLR) 4 complex, consisting of CD14, MD-2, and TLR4 itself, is a pattern-recognition receptor (PRR) binding DAMPs of both exogenous and endogenous origin. It acts as the receptor for the exogenous lipopolysaccharide (LPS) of gram-negative bacteria (9, 10) and as the receptor for a number of endogenous ligands, including heparan sulfate, which is shed from damaged endothelia (11). Recently, it was shown that TLR4-knockout mice were protected against E. coli sepsis (12). The TLR4 complex is thereby a central upstream exogenous and endogenous “danger sensor” (13, 14). CD14 is crucial for LPS recognition by TLR4 and, in addition, cooperates with other TLRs, including TLR2 and TLR3 (14, 15). Thus, inhibition of CD14 might be an appropriate approach for reduction of a broad range of inflammatory mediators released in sepsis.
Pig models are well suited for investigation of human diseases, and, compared with rodents, pigs have obvious advantages with respect to the relevance for human pathophysiology. The size of pigs allows the use of instrumentation and monitoring devices similar to those used in humans, and the amount of blood allows for repeated blood sampling and comprehensive analyses. Furthermore, the LPS sensitivity of pigs is similar to that of humans (16), in contrast with that of mice (17).
The aim of this study was to investigate the effects of inhibiting CD14, a key upstream innate immunity molecule, on the early inflammatory and hemostatic responses in a recently described pig model of gram-negative sepsis (18).
MATERIALS AND METHODS
Animals and experimental groups
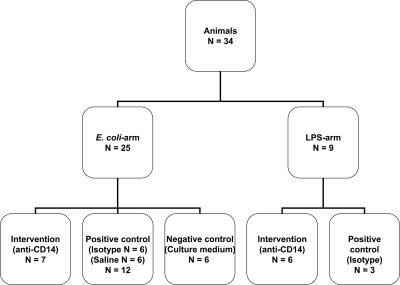
Pigs (Sus scrofa domesticus, outbred stock) of either sex, weighing 15 kg (range 14.5–17 kg) were used. Clinically healthy pigs were allocated into one of two arms (Fig. 1). The E. coli arm consisted of subgroups receiving E. coli and anti-CD14 (n=7), E. coli and isotype control antibody (n=6), E. coli only (n=6), or culture medium without bacteria (n=6). The LPS arm consisted of subgroups receiving LPS and anti-CD14 (n=6) or LPS and isotype control antibody (n=3). Exclusion criteria were hemoglobin (Hb) < 5 g/dl during the stabilization phase or arterial oxygen saturation <90% with fraction of inspired oxygen of 0.30 in the stabilization phase. No animals were excluded.
Figure 1.
Overview of the experimental setup and pigs included.
Anesthesia and surgery
Animals were anesthetized and surgery was performed according to procedures described in detail previously (18).
Monoclonal antibodies for treatment
Mouse anti-porcine CD14 monoclonal antibody clone MIL-2, isotype IgG2b, was purchased from AbD Serotec (Oxford, UK). A mouse anti-human IgG2b (clone BH1) was purchased from Diatec Monoclonals AS (Oslo, Norway) and used as an isotype-matched control. Anti-CD14 or the IgG2b control antibody was infused intravenously at a dose of 5 mg/kg over 10 min before infusion of bacteria or LPS.
Bacteria
Live E. coli (strain LE392; ATCC 33572) was obtained from American Type Culture Collection (Manassas, VA, USA). E. coli was infused intravenously at an increasing rate: 3.75 × 107 bacteria/h from 0 to 60 min, 1.5 × 108 bacteria/h from 60 to 90 min, and 6.0 × 108 bacteria/h from 90 to 240 min. Infusions of bacteria started immediately after anti-CD14 or control treatment was given (T10). A total amount of 1.075 × 108 bacteria/kg, corresponding to 1.1 × 106 bacteria/ml blood, was infused to each animal in the E. coli arm.
LPS
Ultrapure E. coli LPS (strain 0111:B4) was purchased from InvivoGen (San Diego, CA, USA). The LPS was dissolved in sterile water. A total amount of 0.03 mg LPS/kg was infused intravenously over 30 min, immediately after anti-CD14 or isotype matched control antibody was given (T10).
Data registration, blood sampling, and blood processing
Hemodynamic values, respirator settings, and blood samples including blood gases were obtained at regular registration time points starting with time point 0 (T0), immediately after surgery. Blood for serum and plasma analyses was immediately processed and stored at −70°C.
Inflammatory mediators
Cytokines were analyzed using commercially available kits. Quantikine Porcine Immunoassay Kits from R&D Systems (Minneapolis, MN, USA) were used for analyses of TNF-α, IL-1β, IL-6, IL-8, and IL-12/IL-23 p40. IL-10 was analyzed by a Swine IL-10 ELISA kit from BioSource Invitrogen (Carlsbad, CA, USA). Vascular endothelial growth factor (VEGF) was analyzed by a Quantikine human immunoassay kit, known to cross-react with porcine VEGF (19), from R&D Systems. Matrix metalloproteinase-9 (MMP-9) in plasma was analyzed by gelatin zymography (20) in 10% polyacrylamide gels. Gelatinase standards from human capillary blood were prepared as described previously (21).
Flow cytometry
At 0, 10, and 180 min, blood anticoagulated with EDTA was drawn and stained with the mouse anti-porcine wCD11R3 IgG1-FITC clone 2F4/11 or the isotype matched IgG1-FITC control antibody clone W3/25 (AbD Serotec) or with FITC-conjugated anti-CD14 monoclonal antibody clone MIL-2 or its isotype-matched control. The samples were incubated for 15 min at room temperature in the dark. The red cells were lysed, and the samples were centrifuged at 300 g for 5 min at 4°C. The pellets were resuspended and washed with PBS. Finally, the cells were resuspended with PBS with 0.1% albumin. The samples were analyzed on a flow cytometer (FACScan; Becton Dickinson, Franklin Lakes, NJ, USA).
Coagulation and fibrinolysis
Thrombin activation in citrate plasma was measured by thrombin-antithrombin (TAT) complexes with a human enzyme immunoassay kit (Dade Behring, Marburg, Germany), proven to be applicable in porcine plasma (22). Plasminogen activator inhibitor-1 (PAI-1) was measured by a porcine PAI-1 activity assay kit (Molecular Innovations, Novi, MI, USA).
Hematology
Hematological parameters were obtained from the hospital’s routine hematology laboratory (Cell-Dyn 4000; Abbott Diagnostics, Abbott Park, IL, USA). The leukocyte differential count fully discriminated neutrophils in porcine blood, but monocytes and lymphocytes were not able to be separated and were therefore considered as one cell population.
E. coli DNA analysis
For the bacteriological DNA analysis, arterial EDTA blood was obtained from the animals at each time point, frozen immediately, and thawed before analysis. E. coli DNA from 200 μl of whole blood was isolated using robotized equipment (MagNA Pure LC instrument; Roche Applied Science, Mannheim, Germany). DNA was isolated based on magnetic bead technology according to the manufacturer’s instruction (MagNA Pure LC DNA Isolation Kit I; Roche Applied Science), eluted into 100 μl of elution buffer, and stored at −70°C. Genomic DNA E. coli O157, strain EDL 933 (Institute for Reference Materials and Measurements, Geel, Belgium) was diluted with water according to the instructions, quantified by optical density measurement, and used as a standard in the assay. The standard was diluted 10-fold with whole blood anticoagulated with lepirudin (from 108 to 103/ml of E. coli DNA copies). Then 200 μl of each dilution was subjected to robotic DNA isolation, and 5 μl of standard DNA was used for real-time PCR. Controls were DNA extracted from lepirudin whole blood mixed with DNA from E. coli strain B (Sigma-Aldrich Corp., St. Louis, MO, USA) or PBS only.
Quantification of E. coli DNA was performed using real-time PCR (AB 7500; Applied Biosystems, Warrington, UK). Optimized specific PCR primers (SeqA-forward 5′-GAACTTCTGCTTTCGGATGAATACG-3′ and SeqA-reverse 5′-ACAACAGCAGCATAAAGCGATTG-3′) and the FAM-labeled probe (5′-ACCGCTCGCTTTTG-3′) were custom designed by Applied Biosystems using a sequence from E. coli strain 536 (O6:K15:H31) from the SeqA gene, generating a PCR product from nucleotide positions 220–286. The primer/probe set detects E. coli, but also Shigella and Salmonella enterica (National Center for Biotechnology Institute gene BLAST). For our purpose, the specificity of the assay was acceptable. The PCR amplification was performed in a total volume of 20 μl, using 5 μl of DNA, 1× TaqMan Universal Master Mix (Applied Biosystems), and amplification temperatures according to the supplier’s instructions. The sensitivity of the method allowed detection of 7000 E. coli DNA copies for these samples. The positive control showed a within-run variation coefficient of <10% (n=3), and the between-run coefficient of variation was <15% (n=5).
LPS detection
Endotoxin levels were quantified in a Pyrochrome Limulus Amoebocyte Lysate assay by an endpoint chromogenic method using a diazo-coupling assay kit (Associates of Cape Cod, Inc., Falmouth, MA, USA). Serum samples were diluted in depyrogenated Pyrotube-D tubes with LAL Reagent water (both Associates of Cape Cod, Inc., Liverpool, UK). The diluted samples were heat-treated at 75°C for 10 min, mixed with Pyrochrome dissolved in a Glucashield β-glucan inhibiting buffer, and incubated in a 96-well Pyroplate (both Associates of Cape Cod, Inc., Falmouth, MA, USA) on a dry block incubator. After incubation, the procedure was followed according to the instructions from the manufacturer.
Immunofluorescence histology
For the immunofluorescence histology experiments, E. coli were stained with Alexa Fluor 488 purchased from Invitrogen Molecular Probes (Eugene, OR, USA). The bacteria were washed once with PBS, centrifuged (3220 g for 20 min), resuspended in PBS, and counted by flow cytometry. Then 3 × 1011 bacteria were spun (3220 g, 20 min), the supernatant was discarded, and 30 ml of sterile-filtered and heat-inactivated (60°C for 60 min) NaHCO3 (0.2 M, pH 8.35) was added together with 300 μl of Alexa Fluor 488 carboxylic acid succinimidyl ester (10 mg/ml) in DMSO. The tube was packed in tinfoil and rotated for 1 h at room temperature. Bacteria were washed 3 times and centrifuged (3220 g for 20 min) after each wash. The bacteria were resuspended in PBS and counted again as described above. The Alexa Fluor-stained bacteria were then infused intravenously into two separate animals not included in the protocol depicted in Fig. 1. The animals were observed for 240 min. Organ biopsy samples were obtained after euthanasia of the animals and frozen immediately.
Immunofluorescence histological analysis was performed on cryosections obtained from the lung and the liver. Cryosections (5 μm thick) were cut from tissue embedded and snap-frozen in optimal cutting temperature (OCT) compound (Tissue-Tek; BDH, Lutterworth, UK). Sections were air dried and fixed for 10 min in ice-cold acetone. Fc receptors were blocked by incubating the sections for 30 min with PBS containing 5% pig serum and 5% goat serum. To identify tissue macrophages, a pretitrated anti-porcine CD45 monoclonal antibody (a kind gift from Karin Haverson, University of Bristol, Bristol, UK) was applied and incubated for 2 h. Slides were washed thoroughly 3 times with PBS for 5 minutes. An isotype-specific goat anti-mouse antiserum (Southern Biotechnology, Birmingham, AL, USA) conjugated to Texas Red was then applied and incubated for 1 h. The slides were washed three more times, and the nuclear dye DAPI was applied and incubated for 10 min. After a final wash, the sections were mounted in Fluoromount (Vector Laboratories, Burlingame, CA, USA) and sealed with nail varnish. Stained slides were examined using a Nikon Eclipse E800 microscope (Nikon, Tokyo, Japan) fitted with a combined excitation and emission filter block specific for the applied fluorescence staining.
Data presentation and statistical analysis
The two positive control groups in the E. coli arm (E. coli and isotype control antibody and E. coli only) showed identical results and were therefore merged into one group. Overall differences between the treatment group and the positive control group in the E. coli arm were analyzed using 2-way ANOVA with repeated measures unless otherwise stated. Differences in wCD11R3 and CD14 saturation between the treatment group and the positive control group in the E. coli arm were analyzed using a 2-sample t test at T180. The difference in MMP-9 between the treatment group and the positive control group in the E. coli arm was analyzed using a two-sample t test at T120. Differences in E. coli DNA and LPS levels between the treatment group and the positive control group in the E. coli arm were analyzed using a Mann-Whitney U test at T180 and T240, respectively. The LPS arm is presented without statistical analysis, because the positive control group was limited to three animals. For all of the statistical analysis, P < 0.05 was considered statistically significant. Results were analyzed using SPSS for Windows 15.0 (SPSS Inc., Chicago, IL, USA).
Ethics
The study was approved by The Norwegian Animal Experimental Board, and animals were treated according to the Norwegian laboratory animal regulations.
RESULTS
Saturation of CD14
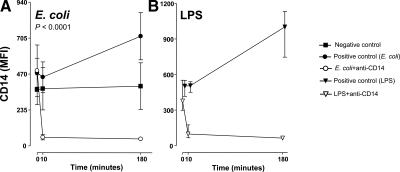
In both the E. coli and the LPS arms of the study, an increase in granulocyte CD14 was seen from 0 to 180 min in the positive control groups (Fig. 2). An immediate and complete saturation of CD14 was seen after injection of anti-CD14 in both arms. The saturation was sustained throughout the observation period.
Figure 2.
Saturation of CD14 on granulocytes. Expression of CD14 on granulocytes was measured by flow cytometry using fluorescence-labeled anti-CD14 of the same clone as that used for treatment of the animals. A) Saturation of CD14 in the anti-CD14 group of the E. coli arm compared with the positive and negative control groups. B) Saturation of CD14 in the anti-CD14 group of the LPS arm compared with the positive control group. Data are presented as mean and 95% confidence intervals in the E. coli arm and mean and range in the LPS arm. P value is indicated in the E. coli arm.
Inflammatory markers
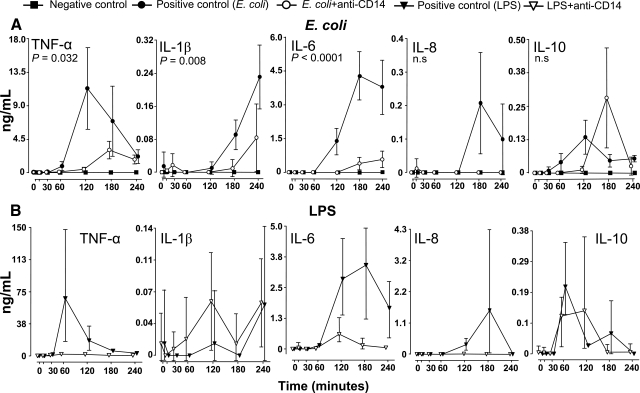
Cytokines in the E. coli arm
TNF-α increased from 60 min, peaked after 120 min, and thereafter declined in the positive control group (Fig. 3A). Anti-CD14 virtually abolished the TNF-α release (P=0.032).
Figure 3.
Pro- and anti-inflammatory cytokines. Plasma concentrations of TNF-α, IL-1β, IL-6, IL-8, and IL-10 in all the groups in the E. coli arm (A) and in the LPS arm (B) are shown. Data are presented as mean and 95% confidence intervals in the E. coli arm and mean and range in the LPS arm. P values are indicated in the E. coli arm.
IL-1β increased from 120 min and throughout the observation period in the positive control group (Fig. 3A). Anti-CD14 markedly reduced IL-1β release almost completely at 180 min and to half the level at 240 min (P=0.008).
IL-6 increased from 60 min and peaked at 180 min in the positive control group (Fig. 3A). Anti-CD14 virtually abolished the IL-6 release (P<0.0001).
IL-8 increased from 120 min and peaked at 180 min in the positive control group (Fig. 3A). IL-8 did not increase at all in the anti-CD14 group, but the difference did not reach significance consistent with the interanimal variation in the positive control group.
IL-10 increased from 30 min, peaked after 120 min, and then declined in the positive control group (Fig. 3A). Anti-CD14 apparently delayed the increase, but peaked to higher levels at 180 min. The difference between the groups was not statistically significant.
IL-12 showed some fluctuation in all groups without significant differences throughout the observation period (data not shown). VEGF increased in both the positive control group and the anti-CD14 group from 60 min without significant differences between the groups. Even the negative control group increased slightly from 120 min and throughout the experiment (data not shown).
None of the cytokines in the E. coli arm, except for VEGF, increased in the negative control group (Fig. 3A).
Cytokines in the LPS arm
TNF-α peaked after 60 min and then declined for the rest of the observation period (Fig. 3B). TNF-α did not increase in the anti-CD14 group.
IL-1β responded marginally in the LPS-group, and fluctuated around the lower detection limit of the assay. The data, therefore, should be interpreted with caution (Fig. 3B).
IL-6 increased from 60 min and peaked after 180 min in the positive control group (Fig. 3B). IL-6 was virtually abolished in the anti-CD14 group.
IL-8 increased at 120 min, peaked at 180 min, and thereafter declined to baseline values in the positive control group (Fig. 3B). IL-8 did not increase in the anti-CD14 group.
IL-10 increased similarly in the positive control group and in the anti-CD14 group from 30 min, peaked after 60 to 120 min, and thereafter declined (Fig. 3B).
IL-12 increased from 60 min and peaked at 120 min in the positive control group, whereas no increase was seen in the anti-CD14 group (data not shown). VEGF did not increase in the positive control group, whereas an increase was seen in the anti-CD14 group as described for the negative control group in the E. coli arm (data not shown).
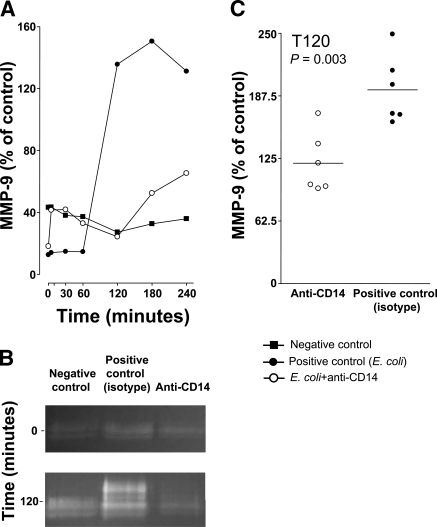
MMP-9 in the E. coli arm
MMP-9 increased from 60 to 120 min in the positive control group. Anti-CD14 markedly reduced and delayed the increase (Fig. 4). All samples in the isotype control group and in the anti-CD14 group were quantified at 120 min, and the difference between the groups was highly significant (P=0.003) (Fig. 4C). MMP-9 did not increase in the negative control group.
Figure 4.
MMP-9. MMP-9 was measured in selected samples from the E. coli arm of the study. A) Samples at all time points from one animal in each group were measured. B) Representative samples from 0 and 120 min showed a marked increase in MMP-9 in the isotype-matched positive control group after 120 min compared with baseline values. A modest increase in the anti-CD14 group and no increase in the negative control group were seen. C) Samples from 120 min were quantified, and the anti-CD14 group (n=6) was compared with the isotype-matched positive control (n=6) (mean, individual values, and P values are shown). The y axes of A and C are not comparable, because of different runs.
MMP-9 in the LPS arm
MMP-9 increased after 30–60 min and peaked after 60–120 min in the positive control group. No inhibitory effect of anti-CD14 was seen (data not shown).
wCD11R3 in the E. coli arm
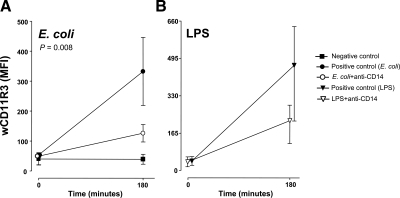
wCD11R3, the pig analog to the human CD11b, on granulocytes increased from 0 to 180 min in the positive control group (Fig. 5A). Anti-CD14 reduced this increase significantly (P=0.008).
Figure 5.
Up-regulation of wCD11R3 on granulocytes. Granulocyte expression of wCD11R3, as measured by flow cytometry, is shown for the E. coli arm (P value is indicated) (A) and the LPS arm (B). Data are presented as mean and 95% confidence intervals in the E. coli arm and as mean and range in the LPS arm.
wCD11R3 in the LPS arm
A similar pattern was found for wCD11R3 on granulocytes in the LPS arm (Fig. 5B).
Hemostatic markers
TAT complexes in the E. coli arm
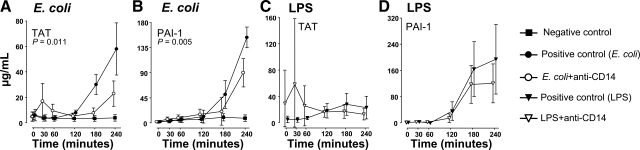
TAT increased markedly from 120 to 240 min in the positive control group (Fig. 6A). In the anti-CD14 group, TAT increased slightly after injection but thereafter stayed low until the end of the experiment. The TAT level was overall significantly lower in the anti-CD14 group than in the positive control group (P=0.011). TAT did not increase in the negative control group.
Figure 6.
Coagulation markers. TAT complexes (A, C) and PAI-1 (B, D) are shown for the E. coli arm (A, B) and the LPS arm (C, D). Data are presented as mean and 95% confidence intervals in the E. coli arm and mean and range in the LPS arm. P values are indicated in the E. coli arm.
TAT complexes in the LPS arm
TAT increased modestly during the observation period in the positive control group (Fig. 6C). In the anti-CD14 group, an initial increase with broad interanimal variation was observed, but during the late course the level was slightly lower in the anti-CD14 group.
PAI-1 in the E. coli arm
PAI-1 increased after 120 min and reached the highest level after 240 min in the positive control group (Fig. 6B). In the anti-CD14 group, there was a delayed and reduced PAI-1 increase compared with that for the positive control group (P=0.005). PAI-1 did not increase in the negative control group.
PAI-1 in the LPS arm
PAI-1 increased after 60 min in both the positive control group and the anti-CD14 group, reaching higher final values in the positive control group (Fig. 6D).
Microbiological analyses
Bacteria and LPS analyses in the E. coli arm
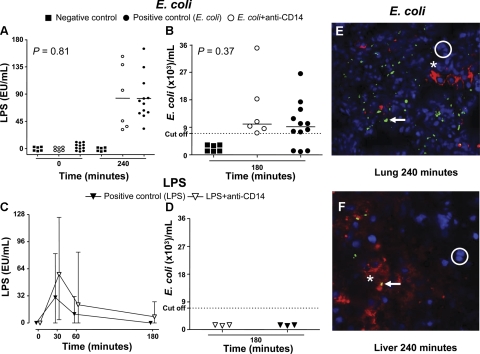
LPS levels in blood increased during the time course and reached the highest level after 240 min in both the positive control group and the anti-CD14 group (Fig. 7A). There were no difference between the groups (P=0.81). No increase in LPS levels was seen in the negative control group.
Figure 7.
Microbiological analyses. A–D) LPS values (A, C) and E. coli DNA (B, D) measured in the E. coli arm (A, B) and in the LPS arm (C, D). Data are presented as median and measured values (A, B), mean and range (C), and measured values (D). P values are indicated in the E. coli arm. E, F) Example of Alexa Fluor 488-stained bacteria in lung (E) and liver biopsies (F) obtained after euthanasia of two pigs challenged with E. coli only. White circles, DAPI-stained nuclei; white asterisk, Texas Red-stained macrophages; white arrows, Alexa Fluor 488-stained E. coli.
E. coli DNA levels increased during the time course up to 180 min in both the positive control group and the anti-CD14 group without any differences between the groups (P=0.31) (Fig. 7B). No increase in E. coli DNA levels was seen in the negative control group.
Immunofluorescence histological analysis of organ biopsy samples after 240 min revealed E. coli bacteria trapped in organs, in particular in the lung and the liver (Fig. 7E, F).
Bacteria and LPS analyses in the LPS arm
LPS levels in blood peaked after 30 min and thereafter decreased in both the positive control group and in the anti-CD14 group (Fig. 7C). E. coli DNA was not detected in either of the groups (Fig. 7D).
Hematology
E. coli arm
Hb increased during the experiment in both the positive control group and the anti-CD14 group, but the increase was significantly lower in the anti-CD14 group (P=0.047) (Table 1). The total amount of white blood cells (WBCs) and neutrophils fell steadily throughout the experiment in the positive control group, in contrast with the anti-CD14 group, in which they both peaked early and remained above baseline levels during the experiment (Table 1). The differences between the two groups were significant (P=0.047 and 0.009, respectively). Hb and the WBC count remained stable in the negative control group, whereas a slight increase in neutrophils was seen.
TABLE 1.
Hematological analysis of both arms of the study
| Parameter | Time (min)
|
|||||
|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 120 | 180 | 240 | |
| E. coli | ||||||
| Hb (g/L)* | ||||||
| Positive control | 7.8 (7.4–8.3) | 7.9 (7.4–8.4) | 7.9 (7.4–8.4) | 8.5 (8.0–9.0) | 8.9 (8.4–9.4) | 9.0 (8.3–9.7) |
| aCD14 | 7.5 (6.5–8.4) | 8.0 (7.0–9.0) | 7.9 (6.9–8.9) | 7.7 (6.8–8.6) | 8.0 (7.0–8.9) | 8.2 (7.2–9.2) |
| Negative control | 8.5 (7.3–9.6) | 8.1 (7.1–9.1) | 8.0 (6.9–9.0) | 8.0 (7.0–9.0) | 8.0 (7.0–9.0) | 8.3 (7.3–9.2) |
| WBC (×109/L)* | ||||||
| Positive control | 15.3 (12.6–18) | 13.6 (11.5–15.6) | 10.8 (8.8–12.8) | 8.3 (5.4–11.1) | 7.1 (3.8–10.4) | 7.5 (4.7–10.3) |
| aCD14 | 17.3 (13.4–21.2) | 23.0 (14.7–31.3) | 23.8 (14.9–32.8) | 19.6 (12.5–26.8) | 13.9 (9.6–18.2) | 21.5 (11.5–31.4) |
| Negative control | 19.2 (15.3–23.1) | 16.3 (12.2–20.4) | 15.4 (10.0–20.7) | 16.1 (10.6–21.6) | 15.9 (10.9–20.9) | 18.0 (13.1–22.9) |
| Neutrophils (×109/L)* | ||||||
| Positive control | 6.2 (4.0–8.3) | 6.3 (4.5–8.0) | 4.6 (3.2–6.0) | 4.4 (1.9–6.9) | 3.9 (0.9–6.9) | 4.4 (2.0–6.9) |
| aCD14 | 7.6 (5.1–10.2) | 14.7 (8.3–21.0) | 17.0 (9.7–24.3) | 14.3 (8.3–20.3) | 10.4 (6.6–14.1) | 18.0 (8.9–27.0) |
| Negative control | 8.8 (5.1–12.4) | 8.7 (5.1–12.2) | 8.5 (3.0–14.0) | 9.6 (3.6–15.6) | 9.8 (4.9–14.6) | 11.3 (6.2–16.3) |
| LPS | ||||||
| Hb (g/L) | ||||||
| Positive control | 7.6 (6.8–8.1) | 9.3 (8.1–10.4) | 8.2 (7.1–8.9) | 8.7 (7.6–9.3) | 9.4 (7.8–10.5) | 9.5 (7.9–10.3) |
| aCD14 | 7.9 (7.6–8.5) | 9.0 (8.4–9.9) | 9.0 (8.5–10.3) | 9.0 (8.5–10.8) | 9.1 (8.3–10.8) | 8.6 (7.2–10.1) |
| WBC (×109/L) | ||||||
| Positive control | 18.1 (13.4–23.5) | 9.7 (7.3–13.7) | 5.6 (3.8–8.6) | 4.1 (2.4–7.3) | 5.9 (3.1–11.3) | 6.8 (3.7–12.9) |
| aCD14 | 16.3 (12.2–21.1) | 19.5 (14.2–22.9) | 11.0 (7.2–18.4) | 12.2 (10.6–14.2) | 16.5 (14.0–21.3) | 17.6 (13.0–22.6) |
| Neutrophils (×109/L) | ||||||
| Positive control | 8.0 (4.9–13.2) | 5.4 (3.4–9.3) | 2.0 (0.9–3.4) | 1.6 (0.2–4.0) | 3.4 (0.9–7.9) | 4.3 (1.3–9.5) |
| aCD14 | 6.2 (3.5–8.9) | 11.0 (4.9–15.3) | 5.6 (2.3–10.3) | 7.6 (6.1–9.3) | 11.9 (9.5–15.8) | 13.3 |
Data in the E. coli arm are presented as mean and 95% CI; data in the LPS arm are presented as mean and range.
P < 0.05 for anti-CD14 vs. positive control group.
LPS arm
Hb, the WBC count, and neutrophils showed the same pattern in the LPS arm as described above for the E. coli arm (Table 1).
Biochemical markers and hemodynamics
Biochemical and hemodynamic data for the E. coli and the LPS arm are presented in Supplemental Tables S1 and S2, respectively. The pigs were ventilated to keep pH at ∼7.40. No differences in pH, base excess, or lactate were seen between the anti-CD14 groups and the positive control groups. The lactate levels were, in general, low in all groups. There was a small nonsignificant reduction in albumin in both the anti-CD14 groups and in the positive control groups in both the arms of the study. The pigs were resuscitated with volume (Ringer’s acetate) and norepinephrine to keep mean arterial pressure (MAP) > 50 mmHg in all groups receiving E. coli or LPS. No significant difference between the anti-CD14 groups and the positive control groups was seen. Changes in heart rate, MAP, and central venous pressure were also similar in both groups without significant differences.
DISCUSSION
This study documents for the first time the fact that CD14 inhibition efficiently attenuates central inflammatory and hemostatic processes during the early phase of E. coli sepsis in a pig model based on whole live bacteria. Although inhibition of CD14 was postulated as a treatment regimen for sepsis many years ago (23), studies of large animal models testing this hypothesis by using whole bacteria have not been published. Several studies have, however, investigated the effect of CD14 inhibition in LPS models in various species including humans (24,25,26,27,28). With respect to relevance for clinical sepsis, LPS models clearly have their limitations compared with whole bacteria models, but to facilitate comparison with published LPS studies, we included an LPS arm in addition to the main whole bacteria arm in the present study.
The anti-porcine CD14 antibody used in the present study has been shown previously to bind to the pig CD14 molecule in vitro in a dose-dependent manner and efficiently inhibited LPS-induced cytokine production (29). To ensure that the dose given was sufficient, the saturation of granulocyte CD14 in vivo was investigated after bolus injection of the antibody. The CD14 molecules were instantly and completely saturated, and the saturation lasted for at least 180 min, when the last sample for investigating saturation was obtained. This was the case even under challenge with whole E. coli bacteria or LPS, which induced an increase in CD14 expression in the absence of anti-CD14. Repeated injections were therefore not necessary.
A striking effect of anti-CD14 treatment was seen on the proinflammatory cytokines TNF-α and IL-6 in both arms of the study, consistent with the well-known potency of LPS to induce these cytokines. Both TNF-α and IL-6 are shown to be important prognostic markers depicting the outcome in sepsis (30,31,32), but clinical trials inhibiting proinflammatory cytokines, in particular TNF-α, have been disappointing (33). Subgroups in a few studies have shown some promise though, in particular, TNF-α inhibition in IL-6-positive patients (34). As the present study has shown, inhibition of the upstream PRR CD14 reduces the proinflammatory cytokines collectively and might therefore be a better treatment regimen than single-mediator inhibition.
IL-1β differed between the two arms because LPS, in contrast to whole E. coli, failed to induce the cytokine in substantial amounts. Failure of LPS to induce an IL-1β response in vivo has also been shown in humans (35). Thus, there is an important difference between LPS models and whole bacteria models with respect to generation of this important cytokine, emphasizing whole bacteria models as being more relevant for human sepsis. Notably, IL-β was markedly delayed and inhibited by anti-CD14. The inhibition was not complete, however, suggesting that other mechanisms such as complement activation by non-LPS structures may be responsible for some IL-β production.
Interplay of pro- and anti-inflammatory stimuli exists in the continuum of sepsis, and IL-10 seems to be essential on the anti-inflammatory side. The effect of anti-CD14 on IL-10 clearly differed from that of the proinflammatory cytokines. In the E. coli arm the increase was delayed but reached levels higher than that in the positive control group and the total amount released was not inhibited by anti-CD14. This effect could be beneficial in sepsis as IL-10 dampens the inflammatory response and may enhance the effect of antibiotics (36). There is a close relationship between IL-10, the CD14/MD-2/TLR4 complex, and proinflammatory cytokines. IL-10 has been shown to increase membrane expression of CD14 (37) and to induce release of soluble MD-2 and CD14 from monocytes (38). Whereas in vitro studies have shown a reduction in whole gram-negative bacteria-induced IL-10 by anti-CD14 antibodies (39, 40), the present study suggests a different situation in vivo. It has been shown that the inductive signaling pathways for production of proinflammatory cytokines such as TNF-α differ from the signaling pathways of anti-inflammatory cytokines such as IL-10 in leukocytes from patients with systemic inflammation (41). Accordingly, our in vivo findings indicate that the proinflammatory response relied mainly on CD14, whereas the IL-10 response was largely CD14-independent.
There is an inverse relation between the severity of sepsis and the peripheral WBC count, and a very low WBC count is a prognostic marker for a fatal outcome in sepsis (42, 43). Accordingly, we found a marked decrease in WBC counts and in neutrophil counts in the E. coli arm, which was markedly and significantly inhibited by anti-CD14. In fact, the leukocyte counts increased at the start in the anti-CD14 group and at the end were comparable to the baseline levels. Whether this leukocyte profile would be beneficial or not in sepsis remains to be shown, but it is reasonable to suggest that anti-CD14 attenuates leukocyte activation and might prevent dysregulation, leading to the known hypofunction (also called “reprogramming”) seen in sepsis (44).
MMP-9 (gelatinase B) was recently shown to be markedly increased in pig E. coli sepsis (18). The enzyme is stored in granules and is rapidly released by exocytosis when monocytes or neutrophils are activated (45). It cleaves extracellular matrix proteins such as collagen and thereby probably facilitates extravasation of these leukocytes (46). MMP-9-deficient mice were shown to be resistant to LPS-induced shock and death (47). However, a certain level of MMP-9 is probably important because MMP-9-deficient mice in another study displayed an impaired host defense against E. coli-induced peritonitis (48). In the E. coli arm, anti-CD14 significantly inhibited the release of MMP-9 during the observation period. Both LPS and IL-8 are known to be potent triggers of neutrophil MMP-9 release (45, 49); accordingly, blocking anti-CD14 might directly or indirectly via reduction of IL-8 inhibit this release.
wCD11R3 in pigs, the analog to CD11b in humans, together with CD18 forms the important phagocytosis receptor CR3. Up-regulation of wCD11R3 and CD11b on granulocytes in response to E. coli challenge in vitro in pigs and humans was shown to be inhibited by complement inhibitors but not by anti-CD14 alone (29, 50). However, a combination of complement inhibition and anti-CD14 inhibited human CD11b up-regulation synergistically (50). Here, we show that wCD11R3 on granulocytes was significantly inhibited in the E. coli arm by anti-CD14, most likely owing to an LPS effect because the same trend was seen in the LPS arm. This assumption is supported by the finding that E. coli LPS was able to induce clustering of a large receptor complex, including CD11b/CD18, in the cell membrane around the LPS (CD14/MD-2/TLR4) receptor, suggested to be important for the magnitude and type of signal seen in response to the pathogen (51).
In sepsis, both increased coagulability and decreased fibrinolysis contribute to a procoagulant state, which can ultimately lead to disseminated intravascular coagulation (52). An increased level of TAT complexes is a marker for coagulability, and the level is shown to increase during endotoxemia in pigs and humans (35, 53). Fibrinolysis regulates degradation of blood clots by formation of plasmin, which is activated by plasminogen activators. PAI-1 regulates plasmin formation under normal conditions. In sepsis there is an increase in the levels of PAI-1, which suppresses fibrinolysis and, along with increased coagulability, drives the patient toward the dangerous procoagulant state (54). In the present study, both TAT complexes and PAI-1 were reduced by anti-CD14, suggesting that anti-CD14 protects against procoagulant activity through both coagulation and fibrinolysis mechanisms. The reduction seen may be a direct effect of anti-CD14 on TAT complexes or PAI-I and/or an indirect effect by the pronounced reduction in IL-6 and TNF-α, as these cytokines are postulated to contribute to the hemostatic dysregulation (55). Our data are in accordance with previous observations on coagulation and fibrinolysis by anti-CD14 in LPS-induced endotoxemia in humans (28). In the present study we extend these findings to include whole E. coli bacteria as well.
Many bacteria were found trapped in the lungs and liver, a finding that correlates well with the relatively lower number of bacteria found in the circulation after 180 min (∼104/ml of whole blood) compared with the accumulated number of infused bacteria per milliliter at this time point (8.44×105/ml). Notably, no effect of anti-CD14 was seen on LPS levels or E. coli DNA levels in the study, confirming our previous in vitro finding that anti-CD14 did not affect clearance of E. coli in porcine whole blood (29). In contrast, anti-CD14 treatment of E. coli sepsis in rabbits was found to reduce the clearance of the bacteria (56, 57). Species differences may explain this difference. Proper antibiotic therapy is, in any case, mandatory in the treatment of sepsis.
Capillary leak is an important feature of sepsis, and the pig is known to be sensitive to sepsis-induced capillary leak (16). Hemoconcentration as an indication of capillary leak was observed in both arms. Interestingly, the anti-CD14 group had significantly lower Hb than the positive control group, despite an insignificant difference in the volume infused, suggesting that anti-CD14 may reduce the capillary leak.
Treatment of sepsis is a great challenge. The fact that neutralization of single, downstream mediators such as cytokines has mostly failed as a treatment strategy is not surprising, taking into account the complex interplay between the many potential pathogenic mediators in the inflammatory reaction. Failure to inhibit LPS in bacterial sepsis may be explained by principal differences between LPS and whole gram-negative bacteria. Upstream inhibition of central effector pathways, such as CD14 and complement, which recognize DAMPs and thereby initiate inflammation, is an alternative treatment regimen (58, 59). Thus, in the present study inhibition of CD14 reduced the up-regulation of a broad range of proinflammatory mediators as well as activation of coagulation and granulocytes. Not only changes induced by LPS but also largely those induced by E. coli were inhibited. Provided that these biomarkers, induced by the host’s response to the DAMPs of invading pathogens and of damaged tissues, are harmful and may contribute to the development of severe sepsis and septic shock, we suggest that neutralization of CD14 should be regarded as an early treatment approach in sepsis.
Supplementary Material
Acknowledgments
The authors thank Kjersti Wendt, Hilde Nilsen, and Carmen Louwerens for excellent technical assistance and Dorte Christiansen for growing and preparing the bacteria. This work was supported by U.S. National Institutes of Health grant R01 EB003968-01A1, Helse Sor-Ost grant 2008036, the Research Council of Norway, the Norwegian Council on Cardiovascular Disease, the Odd Fellows Foundation, and the Family Blix Foundation.
References
- Sriskandan S, Altmann D M. The immunology of sepsis. J Pathol. 2008;214:211–223. doi: 10.1002/path.2274. [DOI] [PubMed] [Google Scholar]
- Levi M, Opal S M. Coagulation abnormalities in critically ill patients. Crit Care. 2006;10:222. doi: 10.1186/cc4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annane D, Bellissant E, Cavaillon J M. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- Martin G S, Mannino D M, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Bianchi M E. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Janeway C A., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu M Y, Van H C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Chow J C, Young D W, Golenbock D T, Christ W J, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Tang A H, Brunn G J, Cascalho M, Platt J L. Pivotal advance: Endogenous pathway to SIRS, sepsis, and related conditions. J Leukoc Biol. 2007;82:282–285. doi: 10.1189/jlb.1206752. [DOI] [PubMed] [Google Scholar]
- Roger T, Froidevaux C, Le R D, Reymond M K, Chanson A L, Mauri D, Burns K, Riederer B M, Akira S, Calandra T. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci U S A. 2009;106:2348–2352. doi: 10.1073/pnas.0808146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Miyake K. Roles for accessory molecules in microbial recognition by Toll-like receptors. J Endotoxin Res. 2006;12:195–204. doi: 10.1179/096805106X118807. [DOI] [PubMed] [Google Scholar]
- Akashi-Takamura S, Miyake K. TLR accessory molecules. Curr Opin Immunol. 2008;20:420–425. doi: 10.1016/j.coi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Goldfarb R D, Dellinger R P, Parrillo J E. Porcine models of severe sepsis: emphasis on porcine peritonitis. Shock. 2005;24:75–81. doi: 10.1097/01.shk.0000191337.01036.b7. [DOI] [PubMed] [Google Scholar]
- Michie H R. The value of animal models in the development of new drugs for the treatment of the sepsis syndrome. J Antimicrob Chemother. 1998;41:47–49. doi: 10.1093/jac/41.suppl_1.47. [DOI] [PubMed] [Google Scholar]
- Castellheim A, Thorgersen E B, Hellerud B C, Pharo A, Johansen H T, Brosstad F, Gaustad P, Brun H, Fosse E, Tonnessen T I, Nielsen E W, Mollnes T E. New biomarkers in an acute model of live Escherichia coli-induced sepsis in pigs. Scand J Immunol. 2008;68:75–84. doi: 10.1111/j.1365-3083.2008.02122.x. [DOI] [PubMed] [Google Scholar]
- Barboni B, Turriani M, Galeati G, Spinaci M, Bacci M L, Forni M, Mattioli M. Vascular endothelial growth factor production in growing pig antral follicles. Biol Reprod. 2000;63:858–864. doi: 10.1095/biolreprod63.3.858. [DOI] [PubMed] [Google Scholar]
- Heussen C, Dowdle E B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Makowski G S, Ramsby M L. Calibrating gelatin zymograms with human gelatinase standards. Anal Biochem. 1996;236:353–356. doi: 10.1006/abio.1996.0179. [DOI] [PubMed] [Google Scholar]
- Saetre T, Lindgaard A K, Lyberg T. Systemic activation of coagulation and fibrinolysis in a porcine model of serogroup A streptococcal shock. Blood Coagul Fibrinolysis. 2000;11:433–438. doi: 10.1097/00001721-200007000-00006. [DOI] [PubMed] [Google Scholar]
- Ulevitch R J. New therapeutic targets revealed through investigations of innate immunity. Crit Care Med. 2001;29:S8–S12. doi: 10.1097/00003246-200107001-00004. [DOI] [PubMed] [Google Scholar]
- Leturcq D J, Moriarty A M, Talbott G, Winn R K, Martin T R, Ulevitch R J. Antibodies against CD14 protect primates from endotoxin-induced shock. J Clin Invest. 1996;98:1533–1538. doi: 10.1172/JCI118945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbon A, Dekkers P E, ten Hove T, Hack C E, Pribble J P, Turner T, Souza S, Axtelle T, Hoek F J, van Deventer S J, van der Poll T. IC14, an anti-CD14 antibody, inhibits endotoxin-mediated symptoms and inflammatory responses in humans. J Immunol. 2001;166:3599–3605. doi: 10.4049/jimmunol.166.5.3599. [DOI] [PubMed] [Google Scholar]
- Schimke J, Mathison J, Morgiewicz J, Ulevitch R J. Anti-CD14 mAb treatment provides therapeutic benefit after in vivo exposure to endotoxin. Proc Natl Acad Sci U S A. 1998;95:13875–13880. doi: 10.1073/pnas.95.23.13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszyna D P, Verbon A, Pribble J P, Turner T, Axtelle T, van Deventer S J, van der Poll T. Effect of IC14, an anti-CD14 antibody, on plasma and cell-associated chemokines during human endotoxemia. Eur Cytokine Netw. 2003;14:158–162. [PubMed] [Google Scholar]
- Verbon A, Meijers J C, Spek C A, Hack C E, Pribble J P, Turner T, Dekkers P E, Axtelle T, Levi M, van Deventer S J, Reitsma P H, van der Poll T. Effects of IC14, an anti-CD14 antibody, on coagulation and fibrinolysis during low-grade endotoxemia in humans. J Infect Dis. 2003;187:55–61. doi: 10.1086/346043. [DOI] [PubMed] [Google Scholar]
- Thorgersen E B, Pharo A, Haverson K, Axelsen A K, Gaustad P, Kotwal G J, Sfyroera G, Mollnes T E. Complement- and CD14-inhibition attenuate Escherichia coli-induced inflammatory response in porcine whole blood. Infect Immun. 2008;77:725–732. doi: 10.1128/IAI.01305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A, Halstensen A, Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987;1:355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Patel R T, Deen K I, Youngs D, Warwick J, Keighley M R. Interleukin 6 is a prognostic indicator of outcome in severe intra-abdominal sepsis. Br J Surg. 1994;81:1306–1308. doi: 10.1002/bjs.1800810914. [DOI] [PubMed] [Google Scholar]
- Oberholzer A, Souza S M, Tschoeke S K, Oberholzer C, Abouhamze A, Pribble J P, Moldawer L L. Plasma cytokine measurements augment prognostic scores as indicators of outcome in patients with severe sepsis. Shock. 2005;23:488–493. [PubMed] [Google Scholar]
- Marshall J C. Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nat Rev Drug Discov. 2003;2:391–405. doi: 10.1038/nrd1084. [DOI] [PubMed] [Google Scholar]
- Panacek E A, Marshall J C, Albertson T E, Johnson D H, Johnson S, MacArthur R D, Miller M, Barchuk W T, Fischkoff S, Kaul M, Teoh L, Van Meter L, Daum L, Lemeshow S, Hicklin G, Doig C. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab′)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med. 2004;32:2173–2182. doi: 10.1097/01.ccm.0000145229.59014.6c. [DOI] [PubMed] [Google Scholar]
- Van Deventer S J, Buller H R, ten Cate J W, Aarden L A, Hack C E, Sturk A. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood. 1990;76:2520–2526. [PubMed] [Google Scholar]
- Wang E, Simard M, Ouellet N, Bergeron Y, Beauchamp D, Bergeron M G. Modulation of cytokines and chemokines, limited pulmonary vascular bed permeability, and prevention of septicemia and death with ceftriaxone and interleukin-10 in pneumococcal pneumonia. J Infect Dis. 2000;182:1255–1259. doi: 10.1086/315811. [DOI] [PubMed] [Google Scholar]
- Rahimi A A, Gee K, Mishra S, Lim W, Kumar A. STAT-1 mediates the stimulatory effect of IL-10 on CD14 expression in human monocytic cells. J Immunol. 2005;174:7823–7832. doi: 10.4049/jimmunol.174.12.7823. [DOI] [PubMed] [Google Scholar]
- Sandanger O, Ryan L, Bohnhorst J, Iversen A C, Husebye H, Halaas O, Landro L, Aukrust P, Froland S S, Elson G, Visintin A, Oktedalen O, Damas J K, Sundan A, Golenbock D, Espevik T. IL-10 enhances MD-2 and CD14 expression in monocytes and the proteins are increased and correlated in HIV-infected patients. J Immunol. 2009;182:588–595. doi: 10.4049/jimmunol.182.1.588. [DOI] [PubMed] [Google Scholar]
- Mollnes T E, Brekke O L, Fung M, Fure H, Christiansen D, Bergseth G, Videm V, Lappegård K T, Köhl J, Lambris J D. Essential role of the C5a receptor in E. coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood. 2002;100:1869–1877. [PubMed] [Google Scholar]
- Van Furth A M, Verhard-Seijmonsbergen E M, Langermans J A, van Dissel J T, van Furth R. Anti-CD14 monoclonal antibodies inhibit the production of tumor necrosis factor α and interleukin-10 by human monocytes stimulated with killed and live Haemophilus influenzae or Streptococcus pneumoniae organisms. Infect Immun. 1999;67:3714–3718. doi: 10.1128/iai.67.8.3714-3718.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adib-Conquy M, Moine P, Asehnoune K, Edouard A, Espevik T, Miyake K, Werts C, Cavaillon J M. Toll-like receptor-mediated tumor necrosis factor and interleukin-10 production differ during systemic inflammation. Am J Respir Crit Care Med. 2003;168:158–164. doi: 10.1164/rccm.200209-1077OC. [DOI] [PubMed] [Google Scholar]
- Stephens D S, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369:2196–2210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- Leibovici L, Drucker M, Samra Z, Konisberger H, Pitlik S D. Prognostic significance of the neutrophil count in immunocompetent patients with bacteremia. Q J Med. 1995;88:181–189. [PubMed] [Google Scholar]
- Cavaillon J M, Adrie C, Fitting C, Adib-Conquy M. Reprogramming of circulatory cells in sepsis and SIRS. J Endotoxin Res. 2005;11:311–320. doi: 10.1179/096805105X58733. [DOI] [PubMed] [Google Scholar]
- Masure S, Proost P, Van Damme J, Opdenakker G. Purification and identification of 91-kDa neutrophil gelatinase: release by the activating peptide interleukin-8. Eur J Biochem. 1991;198:391–398. doi: 10.1111/j.1432-1033.1991.tb16027.x. [DOI] [PubMed] [Google Scholar]
- Opdenakker G, Van den Steen P E, Dubois B, Nelissen I, Van Coillie E, Masure S, Proost P, Van Damme J. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001;69:851–859. [PubMed] [Google Scholar]
- Dubois B, Starckx S, Pagenstecher A, Oord J, Arnold B, Opdenakker G. Gelatinase B deficiency protects against endotoxin shock. Eur J Immunol. 2002;32:2163–2171. doi: 10.1002/1521-4141(200208)32:8<2163::AID-IMMU2163>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Renckens R, Roelofs J J, Florquin S, de Vos A F, Lijnen H R, Van't V C, van der Poll T. Matrix metalloproteinase-9 deficiency impairs host defense against abdominal sepsis. J Immunol. 2006;176:3735–3741. doi: 10.4049/jimmunol.176.6.3735. [DOI] [PubMed] [Google Scholar]
- Hu J, Van den Steen P E, Dillen C, Opdenakker G. Targeting neutrophil collagenase/matrix metalloproteinase-8 and gelatinase B/matrix metalloproteinase-9 with a peptidomimetic inhibitor protects against endotoxin shock. Biochem Pharmacol. 2005;70:535–544. doi: 10.1016/j.bcp.2005.04.047. [DOI] [PubMed] [Google Scholar]
- Brekke O L, Christiansen D, Fure H, Fung M, Mollnes T E. The role of complement C3 opsonization, C5a receptor, and CD14 in E. coli-induced up-regulation of granulocyte and monocyte CD11b/CD18 (CR3), phagocytosis, and oxidative burst in human whole blood. J Leukoc Biol. 2007;81:1404–1413. doi: 10.1189/jlb.0806538. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Brandenburg K, Kusumoto S, Fukase K, Mackie A, Seydel U, Triantafilou K. Combinational clustering of receptors following stimulation by bacterial products determines lipopolysaccharide responses. Biochem J. 2004;381:527–536. doi: 10.1042/BJ20040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M, Ten C H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586–592. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- Kutzsche S, Schlichting E, Aspelin T, Lyberg T. Hemodynamic changes and systemic activation of coagulation and fibrinolysis during controlled endotoxemia in pigs. Thromb Res. 2000;98:517–529. doi: 10.1016/s0049-3848(00)00189-4. [DOI] [PubMed] [Google Scholar]
- Zeerleder S, Schroeder V, Hack C E, Kohler H P, Wuillemin W A. TAFI and PAI-1 levels in human sepsis. Thromb Res. 2006;118:205–212. doi: 10.1016/j.thromres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Levi M, van der Poll T, ten Cate H, van Deventer S J. The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur J Clin Invest. 1997;27:3–9. doi: 10.1046/j.1365-2362.1997.570614.x. [DOI] [PubMed] [Google Scholar]
- Frevert C W, Matute-Bello G, Skerrett S J, Goodman R B, Kajikawa O, Sittipunt C, Martin T R. Effect of CD14 blockade in rabbits with Escherichia coli pneumonia and sepsis. J Immunol. 2000;164:5439–5445. doi: 10.4049/jimmunol.164.10.5439. [DOI] [PubMed] [Google Scholar]
- Opal S M, Palardy J E, Parejo N, Jasman R L. Effect of anti-CD14 monoclonal antibody on clearance of Escherichia coli bacteremia and endotoxemia. Crit Care Med. 2003;31:929–932. doi: 10.1097/01.CCM.0000054870.25767.EE. [DOI] [PubMed] [Google Scholar]
- Marshall J C. Sepsis: rethinking the approach to clinical research. J Leukoc Biol. 2008;83:471–482. doi: 10.1189/jlb.0607380. [DOI] [PubMed] [Google Scholar]
- Mollnes T E, Christiansen D, Brekke O L, Espevik T. Hypothesis: combined inhibition of complement and CD14 as treatment regimen to attenuate the inflammatory response. Adv Exp Med Biol. 2008;632:253–263. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.