Abstract
Ectodomain shedding is a proteolytic mechanism by which a transmembrane protein is converted into a secreted form. Pmel17/gp100 is a melanocyte-specific membrane-bound glycoprotein that has amyloid characteristics and forms fibrillar structures in melanosomes after a complex sequence of post-translational processing and trafficking events, including cleavage by a furin-like proprotein convertase (PC). A secreted form of Pmel17 (termed sPmel17) was also thought to be released due to cleavage by a PC. We used multidisciplinary approaches to demonstrate that sPmel17 is released by ectodomain shedding at the juxtamembrane and/or intramembrane motif and to show that this is independent of cleavage by a PC. We further show that sPmel17 consists of 2 fragments linked by disulfide bonds and that the shedding is inhibited at low temperature but not by metalloproteinase inhibitors. Moreover, treatment with a phorbol ester or a calmodulin inhibitor induces Pmel17 shedding. We also refine the reactivity of HMB50 and NKI/beteb, 2 monoclonal antibodies commonly used as melanoma-specific markers. The fact that those antibodies require physically separated domains of Pmel17 sheds interesting light on its 3-dimensional conformation. We conclude that sPmel17 is released by regulated proteolytic ectodomain shedding.—Hoashi, T., Tamaki, K., Hearing, V. J. The secreted form of a melanocyte membrane-bound glycoprotein (Pmel17/gp100) is released by ectodomain shedding.
Keywords: melanosome, glycosylation, proprotein convertase
Melanoma is a life-threatening tumor that originates from transformed melanocytes (1). Several different research groups have raised monoclonal antibodies (HMB45, HMB50, and NKI/beteb) that recognize melanoma-specific targets and those were later shown to react with a protein termed Pmel17/gp100/SILV/ME20 (hereafter termed Pmel17) (2,3,4,5,6), reviewed recently by Theos et al. (7). However, the reactivities between HMB45, HMB50, and NKI/beteb are distinct (8,9,10,11). Pmel17 has since been shown to play an important role in the structural organization of pigment granules (termed melanosomes) produced by melanocytic cells. Melanosomes are a type of lysosome-related organelle that have the unique capacity to produce melanin pigment (12) and that progress through 4 sequential morphological stages as they mature (13). Stage I melanosomes are amorphous, round, membrane-bound, and electron-lucent vesicles. Stage II melanosomes result from the elongation of those vesicles and the appearance within of distinct fibrillar structures, which depend on the presence of correctly processed “mature” Pmel17 (10, 11, 14,15,16). Melanins are subsequently synthesized and deposited on those melanosomal fibers, at which time the organelles are termed stage III melanosomes. In highly pigmented tissues, melanin synthesis and deposition continue until nearly all structural details are obscured, at which time they are termed stage IV melanosomes.
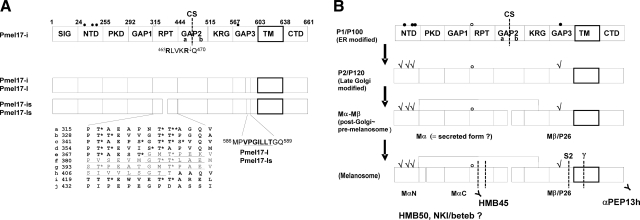
Pmel17 is a type I membrane protein consisting of several domains predicted by homology modeling, as shown in Fig. 1A (http://www.uniprot.org/uniprot/P40967), with details as previously reported (10). SIG is the signal peptide thought to determine the entry of Pmel17 into the secretory pathway (10,17), while PKD is a polycystic kidney disease-like domain bearing an immunoglobulin-like folding structure (18). RPT is an imperfect 10 regions of repeats of 13 proline, serine, and threonine-rich amino acids (10, 19,20,21). The RPT domain has been shown to be crucial for fibrillogenesis (10, 15, 22), and it contains 26 potential O-glycosylation sites, at least some of which are utilized (10, 11, 23). KRG is a kringle-like domain (although not a true kringle domain), which is a triple disulfide-linked autonomous structural domain found throughout blood clotting and fibrinolytic proteins (24) that is generally considered to play a role in binding interactions with other proteins necessary for their regulation (25). TM is a predicted transmembrane domain (26). The remainder of the domains of Pmel17 are annotated as NTD (N-terminal domain), GAP1, GAP2, GAP3, and CTD (C-terminal domain). Pmel17 has 5 potential N-glycosylation sites; however, the N321 site in the RPT domain is thought not to be glycosylated (7, 17). Three of the N-glycosylation sites are located in the NTD domain, and another is in GAP3. Four isoforms of Pmel17 that are generated by alternative splicing have been identified to date (5, 19), and Pmel17-is and Pmel17-ls have truncated RPT domains. Pmel17-l and Pmel17-ls have an additional 7-aa insert in the GAP3 region immediately following the N-glycosylation site. Pmel17-i has been reported to be the most abundant form produced in melanocytic cells (19) (unpublished results).
Figure 1.
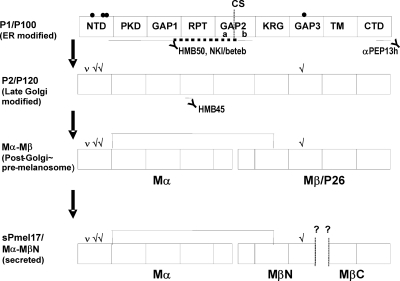
Domain mapping and processing of Pmel17. A) Pmel17-i is subdivided into the following 10 domains: SIG, signal peptide; NTD, N-terminal domain; PKD, polycystic kidney disease-like domain; GAP1, undefined domain between PKD and RPT; RPT, proline, serine, threonine-rich repeat domain; GAP2, undefined domain between RPT and KRG; KRG, kringle-like domain; GAP3, undefined domain between KRG and TM; TM, transmembrane domain; CTD, C-terminal domain. Numbers represent amino acid count based on Pmel17-i. Five potential N-glycosylation sites are indicated with circles; however, one is not likely used (open circle). Furin-mediated cleavage site (CS) in GAP2 is shown with a dashed line and is subdivided into GAP2a and GAP2b by the CS. RPT domain is further subdivided into 10 imperfect series of 13 aa each. Each series is annotated from a to j. Asterisks indicate potential O-glycosylation sites. Four isoforms of Pmel17 are currently identified. RPT domains of Pmel17-is and Pmel17-ls lack the underscored 42 aa. Pmel17-l and Pmel17-ls contain the underscored 7 aa in GAP3. B) Schematics of the processing of Pmel17. P1/P100 is the partially glycosylated form of Pmel17. P2/P120 is the fully glycosylated form of Pmel17; check marks indicate mature N-linked glycans. Mα and Mβ/P26 are the cleaved products P2/P120, which may be disulfide-bond linked. Mα is also believed to be the secreted form of Pmel17 (sPmel17). Scheme at bottom shows the further processed Pmel17 forms found in stage II melanosomes. Mα is further cleaved into MαN and MαC, then MαC is subject to multiple shedding. Mβ is also further cleaved at S2 by the metalloproteinases and at γ by γ-secretases. αPEP13h reacts with the carboxyl terminus of Pmel17. HMB50 and NKI/beteb are believed to react with the lumenal domain, but the precise epitopes recognized by HMB50 or NKI/beteb are unknown. HMB45 has been proven to react with sialylated RPT domain. This schematic is based on previous publications (10, 11, 14, 15, 17, 19, 27,28,29, 34). Nomenclatures P1/P100, P2/P120, Mα, Mβ/P26, MαN, and MαC were taken from refs. 10, 14, 34.
Figure 1B shows a schematic of the complex pattern of maturation and processing of Pmel17 that occurs in melanocytic cells (7, 10, 11, 14, 15, 27,28,29). P1/P100 is the major partially glycosylated form, which is endoglycosidase H (EndoH)-sensitive. Some P1 then undergoes further glycosylation to generate P2/120, the fully glycosylated form, which is EndoH resistant (14, 28). P2 is then cleaved at a furin-sensitive cleavage site (CS) between R469 and Q470, probably by a proprotein convertase (PC) within the post-Golgi and/or the premelanosomal compartments into Mα and Mβ/P26 fragments. Those two fragments are thought to remain linked via a disulfide bond in melanosome precursors. GAP2 is subdivided into GAP2a and GAP2b at the CS. Mα is further processed into MαN and MαC to generate the striated fibrils seen in stage II melanosomes, although the putative CSs in the Mα fragment are unknown (note that N and C indicate the N-terminal and C-terminal sides, respectively). Very recently, other CSs have been reported: Mβ is also processed intracellularly at a metalloproteinase-sensitive CS (S2) between Q583 and L584 in GAP3 and then undergoes intramembrane cleavage by γ-secretases (29). Moreover, Mα is thought to be secreted (14, 17) and to contain the epitopes recognized by HMB50 and NKI/beteb (11, 14, 27). HMB50 and NKI/beteb have also been reported to react with Mβ (28).
HMB45, HMB50, and NKI/beteb are monoclonal antibodies that are widely used for ultrastructural studies of melanosomes, as well as for melanoma detection (14, 16, 30, 31). Several other groups have reported that HMB45 specifically reacts with the fibrillar matrix in stage II melanosomes (14, 16, 30) and that the reactive epitope is sialylated (32, 33). Recently, HMB45 was shown to react with the sialylated RPT domain (10, 11, 15). In contrast, HMB50 reacts with the lumenal structure of stage I, II, III, and IV melanosomes, although it preferentially reacts with stage II melanosomes (14, 16, 23). Recently, ΔPKD (aa 235–292) was shown to completely lose immunoreactivity with HMB50 and with NKI/beteb (11). By characterizing the epitopes recognized by HMB50 and by NKI/beteb in more detail, the further processing, and thus the maturation of melanosomes, will be unveiled at the molecular level.
In this study, we focused on characterizing the epitopes recognized by HMB50 and NKI/beteb and analyzing what that reveals about the nature of Pmel17 at the molecular and biochemical levels. Surprisingly, HMB50 and NKI/beteb require part of Mβ, as well as part of Mα for immune reactivity. We report that the secreted form of Pmel17 (termed sPmel17), which is produced by shedding the membrane spanning or juxtamembrane domain, consists of Mα and MβN and that the ectodomain shedding of Pmel17 is independent of cleavage by a PC. The shedding of sPmel17 can be induced by phorbol myristate acetate (PMA) or by calmodulin (CaM) inhibition, whereas it is metalloproteinase independent. This study provides important advances in understanding the characteristics of the secreted form of the melanocyte membrane-bound glycoprotein, Pmel17/gp100.
MATERIALS AND METHODS
Cell cultures
Highly pigmented MNT-1 melanoma cells and HeLa cells were obtained and were cultured as described previously (10, 34). Furin-deficient adenocarcinoma (LoVo) cells were purchased from the American Type Culture Collection (Manassas, VA, USA). Normal human melanocytes (NHMs) were purchased from Cascade Biologics (Portland, OR, USA). Melanocyte cultures were grown in melanocyte growth medium, consisting of medium 154 and human melanocyte growth supplement (HMGS; Cascade Biologics). Melanocytes from the third to fifth passage were used in these experiments.
Plasmids and transfection
The pCI mammalian expression vector was purchased from Promega (Madison, WI, USA). pCI-Pmel17-i, pCI-Pmel17-l, and pCI-Pmel17-s, which contain Pmel17-i, Pmel17-l, and Pmel17-ls, respectively, in the pCI vector were kind gifts from Dr. Michael S. Marks (University of Pennsylvania, Philadelphia, PA, USA). pCI-Pmel17-is and the ΔCS mutation constructs (K468Q and R469Q) in pCI-Pmel17-i, pCI-Pmel17-l, pCI-Pmel17-is, and pCI-Pmel17-ls were reported previously (10, 27). The ΔS2 mutation construct (Q583L) in pCI-Pmel17-i was introduced as previously reported (29). The ΔSIG, ΔPKD, ΔGAP1, ΔRPT, ΔGAP2, ΔKRG, ΔGAP3, ΔTM, and ΔCTD mutations in pCI-Pmel17-i were also reported previously (10). ΔNTD, ΔGAP2a, and ΔGAP2b were constructed using pCI-Pmel17-i as the template. Note that a hemagglutinin (HA)-tag was artificially inserted in the ΔNTD construct between the SIG and PKD domains. Enhanced green fluorescent protein (EGFP)-NP1 was constructed by inserting the PCR-amplified NTD, PKD, and GAP1 domains of ΔRPT into the XhoI-EcoRI site of EGFP-C3 (Clontech, Mountain View, CA, USA). Similarly, EGFP-NP12 was constructed by inserting the PCR-amplified NTD, PKD, GAP1, and GAP2 domains of ΔRPT. EGFP-NP12a and EGFP-NP12b were also constructed by site-directed mutagenesis using EGFP-NP12 as the template (10). EGFP-N196–254P12b was constructed by inserting the PCR-amplified NTD (aa 196–254), PKD, GAP1, and GAP2b domains using EGFP-NP12b as a template into the XhoI-EcoRI site of EGFP-C3. S83A, T108A, S113A, S83A/T108A/S113A, and S570A were constructed using pCI-Pmel17-i as the template by site-directed mutagenesis (10). hATM-pcDNA3.1 containing human α1-antitrypsin was a kind gift from Richard N. Sifers (Baylor College of Medicine, Houston, TX, USA) (35). The FLAG tag insertion after the N-terminal signal sequence and the α1-antitrypsin Portland (α1-PDX) mutation (A379R, M382R) (36) were introduced by site-directed mutagenesis (10). The KpnI-NotI fragment was inserted into the pCI vector and is designated as pCI-FLAG-α1-PDX. pCMV-furin containing human furin was a kind gift from Dr. Donald F. Steiner (University of Chicago, Chicago, IL, USA) (37). Insertion of the HA tag after the C terminus was performed by conventional PCR, and the EcoRI-NotI fragment was inserted into the pCI vector and is designated as pCI-furin-HA. All constructs were sequence verified and were transfected into HeLa cells with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), as described previously (10, 34). Transfection efficiencies were ∼70–80% assayed by monitoring the fluorescent signals using the EGFP-C3 vector.
Adenovirus construction
pShuttle-CMV-FLAG-α1-PDX, pShuttle-CMV-Pmel17-i, and pShuttle-CMV-furin-HA were constructed by insertion of the NheI-NotI fragment of pCI-α1-PDX, pCI-Pmel17-i, and pCI-furin-HA, respectively, into the pShuttle-CMV (Stratagene, La Jolla, CA, USA). Ad-FLAG-α1-PDX, Ad-Pmel17-i, and Ad-furin-HA adenoviruses containing α1-PDX with a FLAG tag, Pmel17-i, and furin with an HA tag, respectively, were produced using AdEasy (Stratagene), according to the manufacturer’s instructions. Ad-LacZ or Ad-EGFP adenoviruses containing LacZ or EGFP were also produced and were quantified using an adenovirus ELISA kit (Cell Biolabs, San Diego, CA, USA). These adenoviruses were used at a multiplicity of infection (MOI) 30, and transfectants were harvested 4 d after transduction. Transduction efficiencies into MNT-1 cells and LoVo cells were ∼60–80% and 40–70%, respectively, assayed by monitoring the fluorescent signals. Ad-LacZ was used as a negative control.
Antibodies and reagents
The αPEP13h polyclonal antibody was generated in rabbits against a synthetic peptide corresponding to the carboxyl terminus of human Pmel17 (10, 28). HMB45 (Dako, Carpentaria, CA, USA), HMB50 (NeoMarkers, Fremont, CA, USA), and NKI/beteb (NeoMarkers) were also used to detect Pmel17. The cell-permeable PC inhibitor, dec-RVKR-CMK, ethylene glycol tetraacetic acid (EGTA), and tunicamycin (Tun) were purchased from Calbiochem (San Diego, CA, USA). Dimethyl sulfoxide (DMSO), brefeldin-A (BFA), ethylenediamine-tetraacetic acid (EDTA), PMA, sodium azide (NaN3), N-(-6-aminohexyl)-5-chloro-1-napthalenesulfonamide (W-7), and 1-deoxymannojirimycin (DMM) were purchased from Sigma (St. Louis, MO, USA). Endoglycosidase H (EndoH) and protein glycanase F (PNGaseF) were purchased from New England Biolabs (Ipswich, MA, USA).
Immunofluorescence microscopy
Cells cultured in 2-well Lab-Tek chamber slides (Nalge Nunc, Rochester, NY, USA) were immunostained as described previously (10, 34). The reactivities of Alexa Fluor 488, 594, and 647 (Molecular Probes, Eugene, OR, USA) were visualized as green, red, and blue signals, respectively. All preparations were examined with a confocal microscope (LSM 510; Zeiss, Jena, Germany), equipped with HeNe (543 and 633 nm), argon, and krypton laser sources.
Protein extraction and immunoblotting
Cell extracts were prepared using the M-PER mammalian protein extraction reagent (Pierce, Rockford, IL, USA). After centrifugation at 20,000 g for 30 min, the supernatants were harvested. Protein concentrations were measured using the BCA protein assay (Pierce). Immunoblotting was performed as described previously (10, 34).
Metabolic labeling and immunoprecipitation
Transfected HeLa cells, LoVo cells, MNT-1 cells, or NHMs were pulsed for 30 min with [35S]Met/Cys (GE Healthcare Bio-Sciences, Piscataway, NJ, USA), then chased for the periods indicated in the figure legends. Supernatants were harvested after low-speed centrifugation. Cells were solubilized with lysis buffer containing 1% Triton-X100 (TX). Twenty millimolar N-ethylmaleimide was added to each sample to inhibit the artificial formation of disulfide bonds where indicated. Immunopurified samples were electrophoresed and visualized by fluorography, as described previously (10, 34). Quantitations were done using Scion Image software (Scion, Frederick, MD, USA) (34).
Glycosidase digestion
Immunopurified samples were split into 3 aliquots and were digested with or without EndoH (750 U) or PNGaseF (750 U) (10). Digestion reactions were performed for 12 h at 37°C. Samples were electrophoresed and visualized by fluorography as described above.
Statistical analysis
Data are presented as means ± sd. Student’s t test was used to assess statistical significance of differences, and a value of P < 0.05 was considered significant.
RESULTS
Multiple distant domains are required for immunoreactivity with HMB50 or NKI/beteb
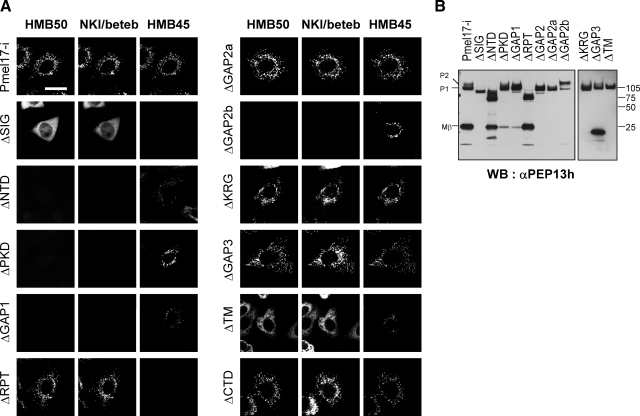
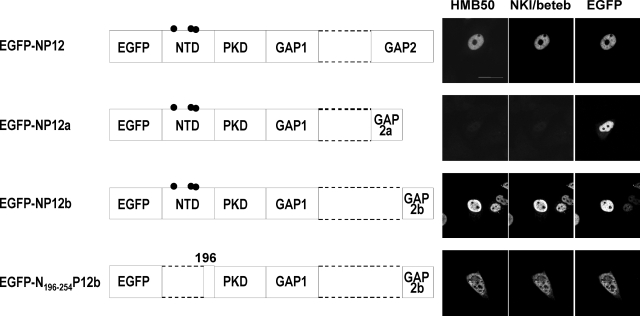
To identify which forms of Pmel17 are recognized by HMB50 and/or NKI/beteb, we characterized the HMB50- and NKI/beteb-reactive domains of Pmel17. We designed a series of 10 deletion mutants of Pmel17-ΔSIG (deletion of SIG), ΔNTD, ΔPKD, ΔGAP1, ΔRPT, ΔGAP2, ΔGAP2a, ΔGAP2b, ΔKRG, ΔGAP3, ΔTM, and ΔCTD. Those expression constructs were transfected into HeLa cells, which have no endogenous expression of Pmel17, and immunoreactivities with HMB50, NKI/beteb, and/or HMB45 were assessed by triple immunocytochemistry (Fig. 2A). Expression of the mutant Pmel17 was confirmed in each case by immunoblotting using the αPEP13h antibody, which reacts with the C terminus of Pmel17 (Fig. 2B), with the exception of ΔCTD, which lacks that epitope (Fig. 1B). ΔNTD, ΔPKD, ΔGAP1, and ΔGAP2b lost immunoreactivity with HMB50 and with NKI/beteb, although ΔGAP2a retained immunoreactivity with both of those antibodies. HMB45 did not react with ΔRPT, as expected since we have previously shown that HMB45 reacts with the sialylated RPT domain (10). From these results, we conclude that NTD, PKD, GAP1, and GAP2b are required for immunoreactivity with HMB50 and with NKI/beteb, but the question remains whether those domains are sufficient for that immunoreactivity? HMB45 requires sialylation (10, 32, 33). The NTD contains 3 potential N-glycosylation sites (Fig. 1B), raising the question of whether HMB50 and/or NKI/beteb requires N-glycosylation in that domain. To answer those questions, we designed several fusion constructs: EGFP-NP1 (containing EGFP, NTD, PKD, and GAP1), EGFP-NP12 (containing EGFP, NTD, PKD, GAP1, and GAP2), EGFP-NP12a (containing EGFP, NTD, PKD, GAP1, and GAP2a), EGFP-NP12b (containing EGFP, NTD, PKD, GAP1, and GAP2b), and EGFP-N196–254P12b (containing EGFP, aa 196–254 of NTD, PKD, GAP1, and GAP2b) (Fig. 3). We transfected HeLa cells with those constructs and then analyzed them by immunocytochemistry using HMB50 and NKI/beteb. EGFP-NP12 and EGFP-NP12b were immunoreactive with HMB50 and NKI/beteb, but EGFP-NP1 (data not shown) or EGFP-NP12a was not. Moreover, EGFP-N196–254P12b, which does not have any potential N-glycosylation sites, was immunoreactive with HMB50 and with NKI/beteb. EGFP-NP12, EGFP-NP12a, and EGFP-NP12b seem to localize to the nuclei, while EGFP-N196–254P12b also seems to localize to the cytoplasm probably because they have no signal sequence or transmembrane domains. These results strongly suggest that NTD, PKD, GAP1, and GAP2b are required for immunoreactivity with HMB50 or NKI/beteb and that N-glycans are not required.
Figure 2.
Mutants of Pmel17 analyzed with HMB50 and NKI/beteb. A) HeLa cells transfected with mutants of Pmel17, including ΔSIG (SIG domain is artificially deleted), ΔNTD, ΔPKD, ΔGAP1, ΔRPT, ΔGAP2a, ΔGAP2b, ΔKRG, ΔGAP3, ΔTM, and ΔCTD were immunocytochemically stained with HMB50, HMB45, and NKI/beteb and then reacted with Alexa Fluor 594, 488, and 647, respectively. Scale bars = 20 μm. B) HeLa cells transfected with mutants of Pmel17, including ΔSIG, ΔNTD, ΔPKD, ΔGAP1, ΔRPT, ΔGAP2, ΔGAP2a, ΔGAP2b, ΔKRG, ΔGAP3, ΔTM, and ΔCTD, analyzed by immunoblotting using αPEP13h.
Figure 3.
NTD, PKD, GAP1, and GAP2b domains are required for the immunoreactivity of HMB50 or NKI/beteb. EGFP constructs used in this study are shown. EGFP-NP12 contains EGFP and the NTD, PKD, GAP1, and GAP2 domains. EGFP-NP12a contains EGFP and the NTD, PKD, GAP1, and GAP2a domains. EGFP-NP12b contains EGFP and the NTD, PKD, GAP1, and GAP2b domains. EGFP-N196–254P12b contains part of the NTD (aa 196–254), PKD, GAP1, and GAP2b domains. HeLa cells overexpressing EGFP-NP12, EGFP-NP12a, EGFP-NP12b, and EGFP-N196–254P12b were fixed and stained with HMB50 or NKI/beteb and then were reacted with Alexa Fluor 594 or 647, respectively. Scale bars = 20 μm.
Recently, HMB50 and NKI/beteb were reported to react with the PKD domain of Pmel17 (aa 215–292) because immunoreactivity with ΔPKD (aa 235–292) was completely lost (11). Moreover, ΔΝTR (Ν-terminal region; aa 27–201) largely but not completely lost immunoreactivity, probably because the NTR deletion compromises the accessibility of those antibodies. In this study, we defined PKD (aa 255–292) differently (cf. Fig. 1A), and thus PKD (aa 215–292) involves part of the NTD (aa 196–254) and PKD (aa 255–292), which are required for immunoreactivity (Fig. 3). That is also the reason why immunoreactivity with ΔNTD (aa 24–254) was completely lost (Fig. 2A). Taking those differences of the domain mapping into account, our result is fully consistent with that reported previously (11). The most important new finding in this study is the requirement for GAP1 and GAP2b for immunoreactivity, which that earlier study did not take into consideration. Theoretically, neither HMB50 nor NKI/beteb can react with Mα in the absence of Mβ or MβN.
Secreted form of Pmel17 requires the lower-molecular-weight fragment, MβN
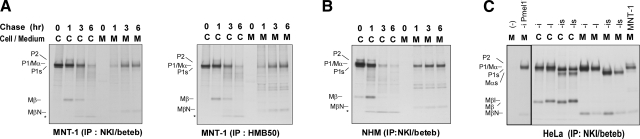
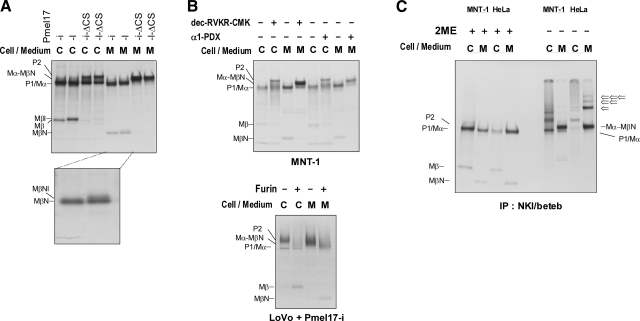
sPmel17 can be immunoprecipitated using HMB50 (3, 14) but that form of Pmel17 has been reported to be the same as Mα (14, 17), which should not react with HMB50 or NKI/beteb (based on the results above). What is the explanation for this discrepancy? To resolve that question, we radiolabeled pigmented MNT-1 melanoma cells with [35S] for 30 min and chased them for up to 6 h. The cell lysates and medium were then immunoprecipitated using NKI/beteb or HMB50 (Fig. 4A). There was no apparent difference between the bands immunoprecipitated by NKI/beteb and by HMB50 as expected. In the cell lysates, the ER-modified P1 form was further glycosylated to P2 at 1 h and then was cleaved to the Mα and Mβ fragments, as reported previously (14, 28). Even after the production of Mβ, Mα, and Mβ remain linked, probably via disulfide bonds (14). We proved that neither NKI/beteb nor HMB50 would detect Mα only. Surprisingly, these results also suggest that HMB50 and NKI/beteb can react with Mα-Mβ, as far as they remain linked and that part of Mα (NTD, PKD, and GAP1) and part of Mβ might be spatially close in the folded protein. Might a similar situation also be true for sPmel17? We hypothesized that Mα and the part of Mβ that contains GAP2b remain linked by disulfide bonds in the medium, since if that did not occur, neither NKI/beteb nor HMB50 would detect Mα. Figure 4A shows that in the lanes with medium, Mα gradually accumulates over time. According to this analysis, fast-migrating small fragments became detectable at 3 and 6 h, and we tentatively annotated that band as MβN. We also examined this sequence of processing of Pmel17 using NHMs (Fig. 4B). Mβ could be identified at 0 h in the cell lysate, and MβN fragments could be identified in the medium, even as early as at 1 h. Fast-migrating fragments were also noticed in earlier studies but were not further characterized (3, 14).
Figure 4.
sPmel17 consists of Mα and lower-molecular-weight fragment, MβN. A) Highly pigmented MNT-1 cells were metabolically radiolabeled, then chased for specific periods as noted. Cell lysates and medium were immunoprecipitated with HMB50 or NKI/beteb, separated by electrophoresis, and visualized by autoradiography. Faster-migrating bands in the medium are annotated as MβN. Asterisk indicates the nonspecific band. B) NHMs were radiolabeled and chased as described above. C) HeLa cells overexpressing an empty vector, Pmel17-i, Pmel17-l, Pmel17-is, or Pmel17-ls were radiolabeled, then chased for 3 h. Cell lysates and medium were immunoprecipitated with NKI/beteb, separated by electrophoresis, and visualized by autoradiography.
We then tried to characterize the MβN fragment. Four isoforms of Pmel17 are derived via alternative splicing (19) (shown schematically in Fig. 1A). We overexpressed Pmel17-i, Pmel17-l, Pmel17-is, and Pmel17-ls in HeLa cells, and then we radiolabeled them and chased them for 3 h. Cell lysates and medium were immunoprecipitated and analyzed as described above. Mα (or Mαs) and Mβ (or Mβl) were clearly detected in those cell lysates (Fig. 4C). Mα (or Mαs) and MβN were also clearly detected in the medium. We also used an empty vector overexpressed in HeLa cells as a negative control, and MβN could not be detected. The sum of these results demonstrate that the MβN fragment detected by NKI/beteb and by HMB50 is not nonspecific but is derived from Pmel17.
PC cleavage is not associated with Pmel17 secretion but with MβN production
The cleavage of P2 to generate Mα-Mβ has been reported to be mediated by a furin-like PC (27). If sPmel17 is only Mα (3, 14, 17), secretion might be inhibited for Pmel17-i-ΔCS or Pmel17-l-ΔCS (whose furin consensus dibasic amino acid sequence is mutated from 465RLVKR to 465RLVQQ) (27). We overexpressed Pmel17-i-ΔCS and Pmel17-l-ΔCS in HeLa cells, radiolabeled them, and then chased them for 3 h. In the cell lysates, the production of Mβ was inhibited, and the accumulation of P2 was observed as reported previously (Fig. 5A) (27). Surprisingly, Pmel17-i-ΔCS or Pmel17-l-ΔCS was still secreted into the medium, but with molecular weights between Mα and P2. Accordingly, MβN was not observed. These results suggest that the molecular weights of the secreted forms of Pmel17-i-ΔCS or Pmel17-l-ΔCS correspond to Mα-MβN, which is greater than Mα but less than P2. Note that these results also suggest that MβN is not nonspecific but is derived from Pmel17. Lysates of cells with overexpressed Pmel17-i and Pmel17-l show a slower-migrating band (P2), and P2 is more clearly identified in cells expressing Pmel17-i-ΔCS or Pmel17-l-ΔCS (Fig. 5A). Similarly, the medium from cells expressing Pmel17-i or Pmel17-l shows trace amounts of the slower-migrating band (Mα-MβN), and Mα-MβN is also more clearly identified in cells expressing Pmel17-i-ΔCS or Pmel17-l-ΔCS (Fig. 5A). Mβl shows a different molecular weight from Mβ due to the existence of the additional 7 aa (Figs. 1A and 4C). However, there seems to be no difference between MβN fragments derived from cells expressing Pmel17-i or Pmel17-l (Fig. 5A). Are the 7 aa acids involved in production of the MβN fragment? We exposed the gels for a longer time to answer that question. The majority of MβN fragments derived from cells expressing Pmel17-i or Pmel17-l have the same molecular weight. Interestingly, MβN derived from Pmel17-l was accompanied by a slower-migrating band (inset, Fig. 5A), which we tentatively annotate as MβNl. The difference between MβN and MβNl probably reflects the existence of the additional 7 aa in Pmel17-l. The reason why both Pmel17-i and Pmel17-l produce MβN is described below.
Figure 5.
Pmel17 secretion is independent from the cleavage by PCs. A) HeLa cells overexpressing Pmel17-i, Pmel17-l, Pmel17-i-ΔCS, or Pmel17-l-ΔCS were radiolabeled, then chased for 3 h. Cell lysates and medium were immunoprecipitated with NKI/beteb, separated by electrophoresis, and visualized by autoradiography. Film was developed after a relatively long exposure time to evaluate faster-migrating bands in the medium of Pmel17-i and Pmel17-l (bottom panel). B) MNT-1 cells with or without dec-RVKR-CMK at 50 μM were radiolabeled, then chased for 3 h. Cell lysates and medium were immunoprecipitated with NKI/beteb, separated by electrophoresis, and visualized by autoradiography. MNT-1 cells transduced with adenovirus containing LacZ (control) or α1-PDX (engineered mutant of α1-antitrypsin) were radiolabeled and chased for 3 h, then analyzed as described previously (top panel). LoVo cells transduced with adenovirus containing Pmel17-i or Pmel17-i + furin were radiolabeled and chased for 3 h, then analyzed as described previously (bottom panel). C) MNT-1 cells and HeLa cells overexpressing Pmel17-i were radiolabeled and chased for 3 h. Cell lysates and medium were immunoprecipitated with NKI/beteb. Immunopurified samples were incubated with or without 2ME, then separated by electrophoresis and visualized by autoradiography.
Next, we tried to inhibit the endogenous PC activity in MNT-1 cells using two independent approaches. We used a cell-permeable PC inhibitor, dec-RVKR-CMK, and we also transduced an adenovirus containing LacZ or α1-PDX (a PC-specific variant of α1-antitrypsin inhibitor) into MNT-1 cells, which were then radiolabeled and chased for 3 h (27, 36). Cell lysates and medium were harvested, and then were immunoprecipitated and analyzed (Fig. 5B). The production of Mβ in the cell lysates was suppressed by the PC inhibitor and by α1-PDX and P2 became a much stronger band, as reported previously (27). Similarly, the production of MβN in the medium was suppressed, and the Mα-MβN bands became much stronger. Conversely, we transduced Pmel17-i with or without exogenous furin to LoVo cells, which lack endogenous furin and several other PCs (38, 39), radiolabeled and chased them, and then immunoprecipitated and analyzed the extracts. The production of Mβ in the cell lysates was enhanced in the presence of exogenously added furin, as previously reported (27). Interestingly, MβN in the medium was also enhanced. Because P2 in LoVo cells was cleaved into Mα-Mβ complex by the exogenous furin intracellularly and the Mα-Mβ complex would be trafficked to the plasma membrane, the Mα-MβN complex was secreted and was separated by electrophoresis, as detailed below. These results convincingly suggest that the PC cleavage is not associated with the ectodomain release of Pmel17 but is closely associated with the production of MβN fragments.
Mα and MβN are linked in sPmel17
As demonstrated above, HMB50 and NKI/beteb each require part of Mα (NTD, PKD, and GAP1) and part of Mβ (GAP2b) for reactivity. Mα and Mβ were previously reported to be covalently coupled (14). We then examined whether Mα and MβN are cross-linked in the medium. MNT-1 cells and HeLa cells overexpressing Pmel17-i were radiolabeled and chased, after which cell lysates and medium were harvested and immunoprecipitated using NKI/beteb. Half of the eluted samples were boiled with sodium dodecyl sulfate (SDS) buffer containing 2-mercaptoethanol (2ME) to reduce disulfide bonds, and the remainder was mixed with SDS buffer but without 2ME to preserve any disulfide bonds. Interestingly, Mβ or MβN was not detected in the absence of 2ME (Fig. 5C). In the medium of HeLa cells, a dimer (Fig. 5C, ⇐) or oligomers (Fig. 5C, ⇐⇐ and ⇐⇐⇐) were clearly detected, but this might be an artifact due to the overexpression of Pmel17. Thus, we conclude that Mα and MβN are covalently linked in sPmel17, the mature secreted form of Pmel17.
ΔKRG or ΔGAP3 does not abrogate the secretion of Pmel17
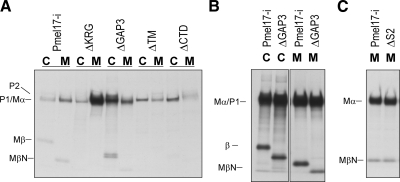
We next assessed which domains of Pmel17 are important for its secretion, especially for Mβ. HeLa cells overexpressing deletion mutants of Pmel17-i were radiolabeled and then chased. Cells and medium were harvested and were immunoprecipitated using NKI/beteb (Fig. 6A). ΔTM produced sPmel17, probably because it lacks the transmembrane domain and might behave as a secreted protein in cell lysates and in the medium. The diffuse cytoplasmic distribution observed for ΔTM suggests this (as seen in Fig. 2A). ΔKRG, ΔGAP3, and ΔCTD showed no impaired intracellular trafficking to LAMP-2-positive organelles (10). Interestingly, ΔCTD produced trace amounts of sPmel17, but ΔKRG and ΔGAP3 produced significant amounts of it (Fig. 6A). ΔKRG did not undergo the PC cleavage, probably because the KRG domain might be necessary for efficient PC-dependent cleavage (10). This also suggests that the secretion is independent of its cleavage by a PC. Moreover, ΔGAP3 produced a faster-migrating band in the medium, so we exposed the radiography film for a longer time to visualize the faster-migrating bands (Fig. 6B). ΔGAP3 clearly showed a truncated Mβ in the cell lysate, as well as a truncated MβN in the medium. Very recently, Mβ was also reported to be processed intracellularly at the S2 site located in GAP3 (Fig. 1B). We also used the ΔS2 mutant (whose metalloproteinase-sensitive amino acid sequence is mutated from 583Q to 583L) (29) to test whether S2 is involved in the secretion. The secretion could not be inhibited (Fig. 6C). This suggests that sPmel17 contains GAP3 and that the shedding might occur at the juxtamembrane and/or intramembrane domains.
Figure 6.
ΔKRG or ΔGAP3 does not abrogate the secretion of Pmel17. A) HeLa cells overexpressing mutants of Pmel17, including ΔKRG, ΔGAP3, ΔTM, and ΔCTD, were radiolabeled and chased for 4 h. Cell lysates and medium were immunoprecipitated with NKI/beteb and then analyzed as described previously. B) HeLa cells overexpressing Pmel17-i and ΔGAP3 were radiolabeled and chased for 4 h. Cell lysates and medium were immunoprecipitated with NKI/beteb, then analyzed as described previously. Note that film was developed after a relatively long exposure time to analyze faster-migrating bands in the medium of Pmel17-i and Pmel17-ΔGAP3. C) HeLa cells overexpressing Pmel17-i and ΔS2 were radiolabeled and chased for 4 h; cell lysates and medium were immunoprecipitated with NKI/beteb, then analyzed as described previously.
MβN is fully Golgi modified
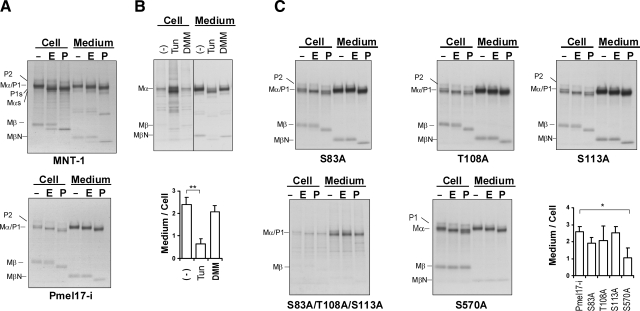
ER-modified (28, 30) and Golgi-modified forms of Pmel17 (14) have been reported to be trafficked to melanosomes. Moreover, only the fully Golgi-modified form of Pmel17 forms the fibrillar matrix detected by HMB45 in stage II melanosomes (10, 11, 15). In earlier reports, Mα and Mβ were shown to be Golgi-modified forms (14, 17). Next, we tried to clarify which form of MβN is secreted in terms of glycosylation. MNT-1 cells and HeLa cells overexpressing Pmel17-i were radiolabeled and then chased. Cell lysates and medium were immunoprecipitated and digested with or without EndoH (which removes ER-modified N-glycans) or PNGaseF (which removes all N-glycans). Mα is largely EndoH resistant and PNGaseF sensitive, while the majority of Mβ is EndoH resistant and PNGaseF sensitive in the cell lysates (Fig. 7A). These results indicate that Mα and Mβ are Golgi-modified forms, as reported previously (14). Mα and Mαs in the medium of MNT-1 cells or in the medium of HeLa cells are largely EndoH resistant and PNGaseF sensitive, as reported previously (14). Interestingly, the majority of MβN is EndoH resistant and PNGaseF sensitive in MNT-1 cells (Fig. 7A). Likewise, MβN is EndoH resistant and PNGaseF sensitive in HeLa cells. These results also suggest that MβN is fully Golgi modified. In other words, sPmel17 (Mα-MβN) is Golgi modified.
Figure 7.
Secreted Pmel17 is fully Golgi modified, and the N-glycan in GAP3 contributes to the secretion. A) MNT-1 cells and HeLa cells overexpressing Pmel17-i were radiolabeled and chased for 1 h (cell lysates of MNT-1 cells) or 4 h (HeLa cells and medium of MNT-1 cells). Cell lysates and medium were immunoprecipitated with NKI/beteb. Immunopurified samples were split into 3 portions; one was digested with EndoH (750 U), another was digested with PNGaseF (750 U), and the third was the untreated control. Digestion reactions were performed for 12 h at 37°C. Digested samples were electrophoresed and visualized by autoradiography. B) MNT-1 cells were radiolabeled and chased in the presence of DMSO, Tun at 2 μg/ml, or DMM at 1 mM for 4 h. Cell lysates and medium were immunoprecipitated with NKI/beteb, separated by electrophoresis, and visualized by autoradiography. Intensities of specific bands, Mα, relative to control are quantified (bottom panel). Graphed data are mean ± sd ratios of Mα intensity in medium/cells from 3 independent experiments. C) HeLa cells overexpressing S83A, T108A, S113A, S83A/T108A/S113A, or S570A were radiolabeled and then analyzed as described above.
N-glycan in GAP3 is important for the secretion of Pmel17
Pmel17 has 5 potential N-glycosylation sites, and 4 of them are thought to be utilized (7, 11, 14, 28). We then examined whether N-glycosylation affects the ectodomain release. We radiolabeled MNT-1 cells and chased them for 4 h with or without Tun (an N-glycosylation inhibitor) or DMM (an N-linked oligosaccharide inhibitor), which prevent the removal of mannose residues on the α1–3 arm of high-mannose structures, and then analyzed the medium (Fig. 7B). sPmel17 was significantly decreased in the presence of Tun; however, the shedding was not totally inhibited. In other words, none of the N-linked glycans are required for the secretion, which was implicit elsewhere (11).
Thus, we hypothesized that N-glycosylation is involved in modulating the secretion of Pmel17. If that is correct, which N-linked glycans are involved? To clarify this, we mutated the N-glycosylation sites of Pmel17-i, designated S83A, T108A, S113A, S570A, and S83A/T108A/S113A (a triple mutation). We transfected those constructs into HeLa cells and then radiolabeled them and chased them for 4 h. Medium was analyzed with or without endoglycosidase digestion to determine whether N-glycosylation was successfully affected. Interestingly, for S570A, the secretion was significantly down-regulated, although it was not totally inhibited (Fig. 7C). That did not occur for S83A, T108A, or S113A (Fig. 7C). The MβN fragment contains the N-glycans at N568, and the oligosaccharide enhances but is not required for secretion of sPmel17. Notably, Mα in the medium of S83A was totally EndoH resistant and PNGaseF sensitive, but in the medium of T108A, S113A, or S570A, it was largely EndoH resistant and PNGaseF sensitive. This suggests that the N-glycan at the 81NAS site remains in the high-mannose form even in mature Pmel17. This clearly explains why Mα is largely but not totally EndoH resistant. Mα in the medium of S83A/T108A/S113A-expressing cells was totally EndoH resistant and PNGaseF resistant, which shows that Mα in those mutants is not N-glycosylated. As far as we know, there are no previously reported experimental data that have shown that the N-glycosylation site in the RPT domain is not utilized (7, 11, 14, 28), and this phenomenon has only been presumed. Thus, our data provide important evidence that the N-glycosylation site in the RPT domain of Pmel17 is not utilized. Taken together, the N-glycan in GAP3 can modulate the secretion of Pmel17 but is not required.
sPmel17 is enhanced by PMA or NaN3 but is not inhibited by metal chelators
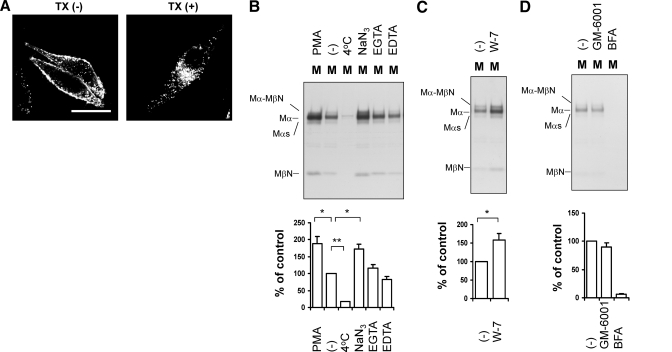
The results described above suggest that sPmel17 is not released by PC cleavage but might be released by a cleavage in the juxtamembrane and/or intramembrane domains. Where does this ectodomain release occur? To determine that, we used HMB50 to immunostain MNT-1 cells with or without a cell-permeabilizing reagent, TX (Fig. 8A). HMB50 reacted mainly with the cell membrane in the absence of TX. Pmel17 is a structural protein that generates the melanosomal matrix fibers (10, 14, 30) but interestingly, it also localized to the cell membrane, which confirms previous reports (11, 16, 23, 31, 40,41,42). MβN was not detected in any of the radiolabeled cell lysates used in this study (Fig. 4A, B). Moreover, Mα-MβN was not detected in radiolabeled HeLa cell lysates overexpressing Pmel17-i-ΔCS or Pmel17-l-ΔCS. Mα-Mβ was reported to be buried in the TX-insoluble fraction (10, 27). Thus, it is possible that Mα-MβΝ is produced intracellularly and is buried in the TX-insoluble fraction making Mα-MβN undetectable in the TX-soluble cell lysates (Fig. 4A, B). However, in this study, we focused on the soluble form of Pmel17, so that even if MβN were buried in the TX-insoluble fraction, this would not contribute to the sPmel17 in the medium. These results suggest that the release of sPmel17 likely occurs in the extracellular milieu but not in intracellular organelles.
Figure 8.
Secretion of Pmel17 is up-regulated by PMA, NaN3, or W-7, but is not inhibited by metal chelators. A) MNT-1 cells were immunocytochemically stained with HMB50 in the presence or absence of TX. Scale bar = 20 μm. B–D) MNT-1 cells were radiolabeled and chased in the presence of 200 nM PMA, DMSO, 20 mM NaN3, 5 mM EGTA, and 5 mM EDTA (B), 50 μM W-7 (C), or 100 nM GM-6001 and 10 μg/ml BFA (D) for 4 h. One sample was chased at 4°C. Medium was analyzed as described previously. Intensities of specific bands, Mα, relative to control are quantified (bottom panel). Graphed data are means ± sd from 3 independent experiments.
The release of extracellular domains has recently been described as ectodomain shedding (43, 44), which raised the question of what regulates the ectodomain shedding of Pmel17. Is it a proteolytic event? In some cases, ectodomain shedding is regulated by matrix metalloproteinases, can be enhanced by PMA or by CaM inhibitors (CaMIs), and can be inhibited by BFA (44,45,46). We radiolabeled MNT-1 cells and chased them for 4 h in the presence of various reagents and then analyzed sPmel17 in the medium (Fig. 8B–D). Note that after radiolabeling, one sample was stored at 4°C for 4 h, while the others were incubated at 37°C. Significant but not complete inhibition of sPmel17 was observed in samples kept at 4°C, which suggests that an enzymatic process is probably involved (47). Oxidative ATP production is blocked by NaN3, and glycolytic ATP production is blocked by removing glucose and adding 2-deoxyglucose (48). In the presence of NaN3, sPmel17 was significantly enhanced. These results suggest that the production of sPmel17 is largely an active energy-independent event. sPmel17 release is significantly enhanced by PMA or by W-7, a CaMI (Fig. 8B, C). The majority of the shedding events are mediated by zinc-dependent metalloproteinases (recently reviewed in ref. 49). Very recently, S2 cleavage in GAP3 was reported to be mediated by metalloproteinases (29), and we tested whether metalloproteinases are involved in Pmel17 shedding. EGTA and EDTA are well-known metal chelators (47), and GM-6001 is a broad-spectrum metalloproteinase inhibitor, but neither of them inhibited sPmel17 (Fig. 8B, D). This implies that metalloproteinases might not be involved with the release of sPmel17. This result is consistent with the fact that ΔGAP3 does not abrogate the secretion of Pmel17 (Fig. 6A, B). However, sPmel17 was totally inhibited by BFA, as reported previously (Fig. 8D). Taken together, the ectodomain shedding of Pmel17 might be a proteolytic event enhanced by PMA, NaN3, or CaMI, and the shedding might occur on the plasma membrane rather than in the cytoplasm.
Then where in GAP3 or the TM does the ectodomain shedding occur? ΔGAP3 decreased but could not significantly inhibit ectodomain shedding (Fig. 6A, B), which strongly suggests that the ectodomain shedding occurs in the juxtamembrane and/or intramembrane domains. We designed a series of double alanine mutations of the TM, then transfected them into HeLa cells, radiolabeled the cells, and then analyzed the medium. None of those mutations successfully abrogated the ectodomain shedding of Pmel17 (data not shown). Mβ and Mβl were derived from Pmel17-i and Pmel17-l, respectively (14) (Fig. 5A). This suggests that Mβ and Mβl were produced by CS cleavage only, and the difference was also confirmed (Fig. 5A). Intriguingly, in HeLa cells overexpressing Pmel17-l, faint MβNl as well as strong MβN bands were observed in the medium (Fig. 5A, bottom panel). The difference between Pmel17-i and Pmel17-l is the existence of the additional 7 aa in ΔGAP3 (Fig. 1A). However, ΔGAP3 can still produce sPmel17 (Fig. 6B), which implies the possibility that shedding will also occur at GAP3, as well as at the juxtamembrane and/or the intramembrane domains. The sum of these results suggests that there are ≥2 cleavage points in the juxtamembrane and/or intramembrane domains of Pmel17, which will be characterized in future studies.
DISCUSSION
This study convincingly shows that HMB50 and NKI/beteb require the NTD, PKD, GAP1, and GAP2b domains for reactivity, as shown schematically in Fig. 9. HMB50 and NKI/beteb are monoclonal antibodies raised in mice (3, 4), so the epitopes that they recognize should be a single distinct motif; which implies that Mα (NTD, PKD, GAP1) and Mβ/MβN (GAP2b) are sterically close even after CS cleavage and/or that some of those domains require each other for proper folding of Pmel17, as previously implied (11). Moreover, HMB50 and NKI/beteb do not work well in Western blot analysis, although they function well in immunocytochemistry and in immunoprecipitation (28). This might also suggest that the epitopes of HMB50 and NKI/beteb are conformation dependent. This means that a structural model for Pmel17 could be generated from the epitope mapping in this study. Glycosylated Pmel17 (termed P2) is cleaved to Mα-Mβ within the post-Golgi and/or prelysosomal compartments (27), after which Mα-Mβ is further processed in the RPT domain and in the PKD and/or GAP1 domains to form fibers (10). The Mα-Mβ complex is thought to be separated in stage II melanosomes (10, 11, 50). HMB50 reacts with the lumens of all stages of melanosomes but preferably with stage II melanosomes (14, 16, 23). Combining previously published results with those of this study, independent lines of evidence can now be merged. Mα and Mβ remain cross-linked even in stage II, III, and IV melanosomes. We tentatively conclude that the covalent link between Mα and Mβ exists as long as the HMB50 or NKI/beteb immunoreactivities remain. Shedding mechanisms other than CS cleavage might contribute to the fibrillogenesis (10, 51). One might ask how HMB50 or NKI/beteb reacts with stage II, III, or IV melanosomes. Recently, the RPT domain could form filament structures in the absence of NTD, PKD, GAP1, or GAP2 (22). Moreover, the RPT domain appears to be shed from the Mα-Mβ complex (29). These independent studies prompt us to speculate that part of Mα and part of Mβ are still covalently linked after fibrillogenesis. Another possibility is that the HMB50- or NKI/beteb-reactive epitope is simply present on the NTD, PKD, and GAP1 domains but that depends on the GAP2b domain for proper folding. Again, HMB50 or NKI/beteb do not work well in Western blot analysis (28), and they react with EGFP-N196–254P12b fusion protein, although it does not seem to be properly folded (Fig. 3). From these considerations, although we can not completely rule out the latter possibility at this time, we conclude that the NTD, PKD, GAP1, and GAP2b domains are required for the immunoreactivity of HMB50 or NKI/beteb. Yet the precise epitope remains unknown.
Figure 9.
Schematic of HMB50 and NKI/beteb epitopes and the secreted form of Pmel17 (sPmel17). HMB50 and NKI/beteb react with parts of NTD, PKD, GAP1, and GAP2b (indicated by horizontal line). Some of the Mα-Mβ complex is further processed in premelanosomes or in melanosomes (Fig. 1), and some is further cleaved into the secreted form, Mα-MβN, probably by multiple ectodomain shedding at spanning or juxtamembrane domains. However, the exact cleavage points are currently unknown (indicated by question mark). Remainder of Mβ is annotated as MβC. Mα and MβN remain linked in the medium. Checkmark indicates a fully matured N-glycan; ν indicates a high-mannose-type or hybrid-type N-glycan.
For a long time, sPmel17 was thought to be Mα, not Mα-MβN (3, 14, 17). Those reports were based on immunoprecipitation studies in which melanoma cells were radiolabeled and immunoprecipitated using Pmel17-specific antibodies, after which they were denatured, electrophoresed, and visualized by radiography. From our results, the Mα-MβN complex is split into Mα and MβN by such denaturation. Some earlier studies noted the existence of MβN, but none of them characterized it further. Vogel and Esclamado (3) detected Mα and a faster-migrating band in the medium, and they thought it to be a breakdown product of Mα. Berson et al. (14) detected Mα and a faster-migrating band in the medium; however, that other band was generated by postlysis degradation. The existence of the disulfide bond and the PC cleavage at the CS might have previously hidden the existence of MβN. PCs surely cleave P2 to Mα-Mβ (27), which was confirmed in this study. However, PC inhibitors did not reduce the secretion of sPmel17 but a higher-molecular-weight product, uncleaved Mα-MβN, was detected. We convincingly show that the cleavage of Pmel17 by PCs and its secretion are independent events.
The Mα-Mβ complex is mainly found in the TX-insoluble fraction of cell lysates (10, 27); however, the Mα-MβN complex is secreted into the medium. Interestingly, uncleaved Mα-MβN is also secreted. The difference between Mβ and MβN seems to be closely associated with the solubility. Recombinant Mα reconstituted from Escherichia coli inclusion bodies were shown to have amyloid characteristics (22, 51). Combining that result with ours, the MβN fragment might play some role in its solubility. The β-amyloid (βA) peptide is the main component of the proteinaceous filaments that form the amyloid plaques found in Alzheimer’s disease (AD) (52). Ectodomain shedding of β-amyloid precursor protein (β-APP) produced βA. α-, β-, and γ-secretases are involved in the shedding. The βA42 (42-residue) fragment shed by β- and γ-secretases tends to form fibrils (53) and is the major component in patients with AD (54). Similarly, Pmel17 generates a secreted form, Mα-MβN, probably by ectodomain shedding; however, Mα-Mβ produces insoluble amyloid-like fibrils, i.e., the fibrillar matrix of stage II melanosomes. Very recently, metalloproteinases were reported to be involved in the shedding of the GAP3 domain in intracellular Pmel17 (29). However, in our study, ΔGAP3, as well as ΔS2, still produced sPmel17, and metalloproteinase inhibitors could not inhibit its shedding. These results suggest that the mechanism of intracellular processing and that of ectodomain shedding are apparently distinct phenomena. Intriguingly, secreted Pmel17-l seems to contain MβN and MβNl (Fig. 5A, bottom panel). These data suggest that the shedding has multiple mechanisms, as is the case for β-APP.
We and others have reported that the lumenal domains of Pmel17 are crucial for its intracellular trafficking (10, 15, 40). NTD, PKD, GAP1, and GAP2 (specifically GAP2b; unpublished results) are required but RPT, KRG, GAP3, and CTD are not important for the intracellular trafficking of Pmel17 (10). Similarly, ΔKRG and ΔGAP3 were secreted but CTD is required for the secretion of Pmel17. The trafficking of Pmel17 to melanosomes remains widely discussed. Some Pmel17 is directly sorted to the plasma membrane and then is endocytosed, while other Pmel17 molecules are sorted to LAMP-2 positive organelles (endosomes, lysosomes, or melanosomes) (15, 16, 40). Pmel17 has a putative dileucine-based signal (ENSPLL) in its CTD. Equivalent dileucine-based signals are found in tyrosinase-related protein-1, tyrosinase, and QNR-71 (the quail homologue of NMB) and mutations of those sorting signals cause misrouting of those proteins (55,56,57,58). Our preliminary experiments suggest that mutation of the dileucine-based sorting signal of Pmel17 does not affect its secretion (unpublished results). There have been several previous reports that the dileucine-based sorting signal of Pmel17 does not affect its localization to the plasma membrane (refs. 40,41,42 and unpublished results) but is important for the internalization process (41, 42). Combining those results with ours in this study, the CTD seems to be crucial for shedding but is not required for the localization to the plasma membrane or for the intracellular trafficking. Recently, the ENSPLL sequence has been shown to be an internalization signal required for efficient endocytosis and for accumulation in melanosomes in melanocytes (41). However, in HeLa cells, this signal does not appear to be essential for accumulation in endosomes, probably due to robust clathrin-independent endocytosis (41). Thus, there still remains the possibility that intracellular trafficking of Pmel17 depends on the cell type.
How Pmel17 is trafficked to the plasma membrane is also in dispute (11, 16, 23, 31). One proposed pathway is that immature Pmel17, termed P1, escapes from the Golgi stack and then is trafficked to the plasma membrane (23, 31). This mechanism is supported by the fact that HMB45 has no reactivity, and a strong P1 band is detected in the membrane fraction of MNT-1 cells, and that the ER-modified form of Pmel17 can be detected on the cell surface by immunoelectron microscopy (23, 31). Another proposed pathway is that Pmel17 is trafficked to the plasma membrane through the trans-Golgi network (TGN) (16). This mechanism is also supported by an ultrastructural study using bovine serum albumin-gold (16) and by flow cytometry using a polyclonal antibody that reacts with the ER-modified form of Pmel17 (11). Previous studies reported that the secreted Mα is largely Golgi modified (14, 17). In this study, we characterized sPmel17 more accurately, and we convincingly show that sPmel17 (Mα-MβN) is largely Golgi-modified and is derived from P2. This means that it is a late Golgi-modified form and that P1 might not be secreted, even if it is trafficked to the plasma membrane (23, 31).
Moreover, we found that the N-glycan at the N81 site is a high-mannose type or hybrid type and that the N-glycans at N106, N111, and N568 are the complex type. As expected from previous reports (7, 14, 17), we also proved that N321 is not utilized. O-Glycosylation has been reported to affect the intracellular trafficking of Pmel17 (23), while other studies have suggested that N- and O-glycosylation are not required for the trafficking of Pmel17 to endocytic compartments (11). Our study shows that S570A decreases the secretion of Pmel17, which implies two possibilities: One is that sheddases might not work efficiently in the absence of an N-glycan at N568, while another is that Pmel17 might not localize efficiently to the plasma membrane without an N-glycan at N568. However, we cannot distinguish between these two mechanisms at this time and must wait for the identification of the sheddases involved in Pmel17 secretion.
The shedding of extracellular domains of transmembrane proteins by this kind of specific proteolytic process in the juxtamembrane is a well-recognized phenomenon. Shedding of membrane proteins is apparently a universal characteristic of eukaryotic cells (44). However, shedding is, nevertheless, restricted to a limited subset of membrane proteins, which are usually type I, type II, or glycosylphosphatidylinositol-anchored membrane proteins (44), which have the following features. One characteristic of the activity of membrane secretases is their highly regulated nature. The activation of intracellular second messenger systems, such as phorbol esters, which are well-characterized activators of protein kinase C (PKC), or intracellular Ca2+ pathways, induces shedding (44, 59). Shed proteins might also contain as yet undefined recognition motifs in their extracellular domains that signal stalk cleavage (60). Candidate shedding proteases are unusual disintegrin Zn-metalloproteinases in many cases (49). In this study, we conclude that Pmel17 undergoes ectodomain shedding by which sPmel17 is released. Moreover, the shedding might occur mainly at the plasma membrane and is less likely within the cells. Pmel17 ectodomain shedding is enhanced by PMA, as occurs for many membrane-localized molecules (61). An important intracellular mediator in the actions of Ca2+ is CaM (45), which has been shown to be involved in the regulation of shedding of several membrane proteins, including β-APP (46). The relationship between pigmented melanocytes and CaM has been poorly investigated (62). We show in this study that a CaMI stimulates the shedding of Pmel17. The ectodomain shedding of CD44 has been reported to be stimulated by a CaMI, and the CaMI-induced shedding is mediated by a disintegrin and metalloproteinase (ADAM)-10, whereas shedding of CD44 induced by PMA is mediated by ADAM-17 (63). Thus, stimulation of the secretion by PMA and CaMI might be via different pathways. Interestingly, metal chelators or a metalloproteinase inhibitor did not inhibit the shedding of Pmel17. This implies that metalloproteinases might not be closely associated with sPmel17. Similar to the β-APP shedding by α-secretase or to the shedding of CD100, sheddases independent of metals might be involved in Pmel17 shedding (47, 61). Taken together, Pmel17 shedding is a regulated proteolytic process induced by PMA or CaMI, whereas it is largely ATP and metalloproteinase independent. The precise mechanism responsible for the shedding of Pmel17 is as yet unclear. Although further studies are required, Pmel17 is released via a specific proteolytic pathway, and the mechanism involved in that shedding is probably different from those previously described (44, 49). Ectodomain shedding was initially believed to be a cell-surface event; however, there is increasing evidence that ectodomain cleavage can also take place in intracellular compartments, such as exosomes (64). Tumor-derived exosomes could be a source of tumor antigens (65). Pmel17 secretion might have some association with exosomes; however, this remains an unsolved problem at this time.
Pmel17 was originally identified as a melanoma-specific antigen and then was shown to contribute to the amyloid-like fibrillogenesis involved in melanosome structure (10, 14, 51). In this study, we show that sPmel17 is composed of fully Golgi-modified Mα and MβN fragments and is released by regulated ectodomain shedding. There are several melanoma serum markers currently used in the clinic (66, 67), and sPmel17 might be an important candidate for a novel melanoma-specific serum marker. We are now further exploring this possibility.
Acknowledgments
We gratefully thank Dr. Michael S. Marks (University of Pennsylvania, Philadelphia, PA, USA) for kindly providing the Pmel17-i, Pmel17-l, and Pmel17-s vectors; Dr. Richard N. Sifers (Baylor College of Medicine, Houston, TX, USA) for the kind gift of hATM-pcDNA3.1; Dr. Donald F. Steiner (University of Chicago, Chicago, IL, USA) for the kind gift of pCMV-furin; Dr. Susan H. Garfield and Dr. Stephen M. Wincovitch for expert advice on confocal microscopy; and Dr. Motozo Yamashita for useful suggestions. This research was supported, in part, by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
References
- Dlugosz A, Merlino G, Yuspa S H. Progress in cutaneous cancer research. J Investig Dermatol Symp Proc. 2002;7:17–26. doi: 10.1046/j.1523-1747.2002.19631.x. [DOI] [PubMed] [Google Scholar]
- Gown A M, Vogel A M, Hoak D, Gough F, McNutt M A. Monoclonal antibodies specific for melanocytic tumors distinguish subpopulations of melanocytes. Am J Pathol. 1986;123:195–203. [PMC free article] [PubMed] [Google Scholar]
- Vogel A M, Esclamodo R M. Identification of a secreted Mr 95,000 glycoprotein in human melanocytes and melanomas by a melanocyte specific monoclonal antibody. Cancer Res. 1988;48:1286–1294. [PubMed] [Google Scholar]
- Vennegoor C, Hageman P, Van Nouhuijs H, Ruiter D J, Calafat J, Ringens P J, Rumke P. A monoclonal antibody specific for cells of the melanocyte lineage. Am J Pathol. 1988;130:179–192. [PMC free article] [PubMed] [Google Scholar]
- Adema G J, de Boer A J, Vogel A M, Loenen W A M, Figdor C G. Molecular characterization of the melanocyte lineage-specific antigen gp100. J Biol Chem. 1994;269:20126–20133. [PubMed] [Google Scholar]
- Adema G J, Bakker A B H, de Boer A J, Hohenstein P, Figdor C G. pMel17 is recognized by monoclonal antibodies NKI-beteb, HMB-45, and HMB-50 and by anti-melanoma CTL. Brit J Cancer. 1996;73:1044–1048. doi: 10.1038/bjc.1996.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos A C, Truschel S T, Raposo G, Marks M S. The Silver locus product Pmel17/gp100/Silv/ME20: controversial in name and in function. Pigment Cell Res. 2005;18:322–336. doi: 10.1111/j.1600-0749.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries T J, Smeets M, de Graaf R, Hou-Jensen K, Brocker E B, Renard N, Eggermont A M, van Muijen G N P, Ruiter D J. Expression of gp100, MART-1, tyrosinase, and S100 in paraffin-embedded primary melanomas and locoregional, lymph node and visceral metastases: implications for diagnosis and immunotherapy. A study conducted by the EORTC Melanoma Cooperative Group. J Pathol. 2001;193:13–20. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH729>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Thomson W, Mackie R M. Comparison of five antimelanoma antibodies for identification of melanocytic cells on tissue sections in routine dermatopathology. J Am Acad Dermatol. 1989;21:1280–1284. doi: 10.1016/s0190-9622(89)70344-3. [DOI] [PubMed] [Google Scholar]
- Hoashi T, Muller J, Vieira W D, Rouzaud F, Kikuchi K, Tamaki K, Hearing V J. The repeat domain of the melanosomal matrix protein PMEL17/GP100 is required for the formation of organellar fibers. J Biol Chem. 2006;281:21198–21208. doi: 10.1074/jbc.M601643200. [DOI] [PubMed] [Google Scholar]
- Harper D C, Theos A C, Herman K E, Tenza D, Raposo G, Marks M S. Premelanosome amyloid-like fibrils are composed of only Golgi-processed forms of Pmel17 that have been proteolytically processed in endosomes. J Biol Chem. 2008;283:2307–2322. doi: 10.1074/jbc.M708007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing V J. Biogenesis of pigment granules: A sensitive way to regulate melanocyte function. J Dermatol Sci. 2005;37:3–14. doi: 10.1016/j.jdermsci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Seiji M, Fitzpatrick T B, Simpson R T, Birbeck M S C. Chemical composition and terminology of specialized organelles (melanosomes and melanin granules) in mammalian melanocytes. Nature. 1963;197:1082–1084. doi: 10.1038/1971082a0. [DOI] [PubMed] [Google Scholar]
- Berson J F, Harper D C, Tenza D, Raposo G, Marks M S. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol Biol Cell. 2001;12:3451–3464. doi: 10.1091/mbc.12.11.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos A C, Truschel S T, Tenza D, Hurbain I, Harper D C, Berson J F, Thomas P C, Raposo G, Marks M S. A lumenal domain-dependent pathway for sorting to intraluminal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell. 2006;10:343–354. doi: 10.1016/j.devcel.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Tenza D, Murphy D M, Berson J F, Marks M S. Distinct protein sorting and localization to premelanosomes, melanosomes and lysosomes in pigmented melanocytic cells. J Cell Biol. 2001;152:809–823. doi: 10.1083/jcb.152.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresh G A, Wang W-C, Beam K S, Malacko A R, Hellstrom I, Hellstrom K E, Marquardt H. Differential processing and secretion of the melanoma-associated ME20 antigen. Arch Biochem Biophys. 1994;311:95–102. doi: 10.1006/abbi.1994.1213. [DOI] [PubMed] [Google Scholar]
- Bycroft M, Bateman A, Clarke J, Hamill S J, Sandford R, Thomas R L, Chothia C. The structure of a PKD domain from polycystin-1: implications for polycystic kidney disease. EMBO J. 1999;18:297–305. doi: 10.1093/emboj/18.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols S E, Harper D C, Berson J F, Marks M S. A novel splice variant of Pmel17 expressed by human melanocytes and melanoma cells lacking some of the internal repeats. J Invest Dermatol. 2003;121:821–830. doi: 10.1046/j.1523-1747.2003.12474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano F, Martinez-Esparza M, Jiménez-Cervantes C, Hill S P, Lozano J A, García-Borrón J C. New insights on the structure of the mouse silver locus and on the function of the silver protein. Pigment Cell Res. 2000;13:118–124. doi: 10.1111/j.0893-5785.2000.130821.x. [DOI] [PubMed] [Google Scholar]
- Kwon B S, Chintamaneni C D, Kozak C A, Copeland N G, Gilbert D J, Jenkins N A, Barton D E, Francke U, Kobayashi Y, Kim K K. A melanocyte-specific gene, Pmel 17, maps near the silver coat color locus on mouse chromosome 10 and is a syntenic region on human chromosome 12. Proc Natl Acad Sci U S A. 1991;88:9228–9232. doi: 10.1073/pnas.88.20.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey R P, Shewmaker F, McPhie P, Monterroso B, Thurber K, Wickner R B. The repeat domain of the melanosome fibril protein Pmel17 forms the amyloid core promoting melanin synthesis. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0906509106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia J C, Rouzaud F, Julien S, Chen K G, Passeron T, Yamaguchi Y, Abu-Asab M, Tsokos M, Costin G E, Yamaguchi H, Jenkins L M, Nagashima K, Appella E, Hearing V J. Sialylated core 1 O-glycans influence the sorting of Pmel17/gp100 and determine its capacity to form fibrils. J Biol Chem. 2007;282:11266–11280. doi: 10.1074/jbc.M608449200. [DOI] [PubMed] [Google Scholar]
- Angles-Cano E, Rojas G. Apolipoprotein(a): structure-function relationship at the lysine-binding site and plasminogen activator cleavage site. Biol Chem. 2002;383:93–99. doi: 10.1515/BC.2002.009. [DOI] [PubMed] [Google Scholar]
- Scanu A M, Edelstein C. Kringle-dependent structural and functional polymorphism of apolipoprotein (a) Biochim Biophys Acta. 1995;1256:1–12. doi: 10.1016/0005-2760(95)00012-2. [DOI] [PubMed] [Google Scholar]
- Kuliawat R, Santambrogio L. A mutation within the transmembrane domain of melanosomal protein Silver (Pmel17) changes lumenal fragment interactions. Eur J Cell Biol. 2009;88:653–667. doi: 10.1016/j.ejcb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson J F, Theos A C, Harper D C, Tenza D, Raposo G, Marks M S. Proprotein convertase cleavage liberates a fibrillogenic fragment of a resident glycoprotein to initiate melanosome biogenesis. J Cell Biol. 2003;161:521–533. doi: 10.1083/jcb.200302072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto K, Watabe H, Valencia J C, Kushimoto T, Kobayashi T, Appella E, Hearing V J. Epitope mapping of the melanosomal matrix protein gp100 (PMEL17): Rapid processing in the endoplasmic reticulum and glycosylation in the early Golgi compartment. J Biol Chem. 2004;279:28330–28338. doi: 10.1074/jbc.M401269200. [DOI] [PubMed] [Google Scholar]
- Kummer M P, Maruyama H, Huelsmann C, Baches S, Weggen S, Koo E H. Formation of Pmel17 amyloid is regulated by juxtamembrane metalloproteinase cleavage, and the resulting C-terminal fragment is a substrate for gamma-secretase. J Biol Chem. 2009;284:2296–2306. doi: 10.1074/jbc.M808904200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushimoto T, Basrur V, Valencia J C, Matsunaga J, Vieira W D, Muller J, Appella E, Hearing V J. A new model for melanosome biogenesis based on the purification and mapping of early melanosomes. Proc Natl Acad Sci U S A. 2001;98:10698–10703. doi: 10.1073/pnas.191184798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia J C, Watabe H, Chi A, Rouzaud F, Chen K G, Vieira W D, Takahashi K, Yamaguchi Y, Berens W, Nagashima K, Shabanowitz J, Hunt D F, Appella E, Hearing V J. Sorting of Pmel17 to melanosomes through the plasma membrane by AP1 and AP2: evidence for the polarized nature of melanocytes. J Cell Sci. 2006;119:1080–1091. doi: 10.1242/jcs.02804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur R P, Bigler S A, Skelly M, Gown A M. Anti-melanoma monoclonal antibody HMB45 identifies an oncofetal glycoconjugate associated with immature melanosomes. J Histochem Cytochem. 1992;40:207–212. doi: 10.1177/40.2.1552165. [DOI] [PubMed] [Google Scholar]
- Chiamenti A M, Vella F, Bonetti F, Pea M, Ferrari S, Martignoni G, Benedetti A, Suzuki H. Anti-melanoma monoclonal antibody HMB-45 on enhanced chemiluminescence-Western blotting recognizes a 30–35 kDa melanosome-associated sialated glycoprotein. Melanoma Res. 1996;6:291–298. doi: 10.1097/00008390-199608000-00003. [DOI] [PubMed] [Google Scholar]
- Hoashi T, Watabe H, Muller J, Yamaguchi Y, Vieira W D, Hearing V J. MART-1 is required for the function of the melanosomal matrix protein Pmel17/gp100 and the maturation of melanosomes. J Biol Chem. 2005;280:14006–14016. doi: 10.1074/jbc.M413692200. [DOI] [PubMed] [Google Scholar]
- Wu Y, Swulius M T, Moremen K W, Sifers R N. Elucidation of the molecular logic by which misfolded alpha 1-antitrypsin is preferentially selected for degradation. Proc Natl Acad Sci U S A. 2003;100:8229–8234. doi: 10.1073/pnas.1430537100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean F, Stella K, Thomas L, Liu G, Xiang Y, Reason A J, Thomas G. alpha1-antitrypsin Portland, a bioengineered serpin highly selective for furin: application as an antipathogenic agent. Proc Natl Acad Sci U S A. 1998;95:7293–7298. doi: 10.1073/pnas.95.13.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguay S J, Milewski W M, Young B D, Nakayama K, Steiner D F. Processing of wild-type and mutant proinsulin-like growth factor-IA by subtilisin-related proprotein convertases. J Biol Chem. 1997;272:6663–6670. doi: 10.1074/jbc.272.10.6663. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Nakagawa T, Kasai K, Banno T, Duguay S J, van de Ven W J M, Murakami K, Nakayama K. A second mutant allele of furin in the processing-incompetent cell line, LoVo. Evidence for involvement of the homo B domain in autocatalytic activation. J Biol Chem. 1995;270:26565–26569. doi: 10.1074/jbc.270.44.26565. [DOI] [PubMed] [Google Scholar]
- Miranda L, Wolf J, Pichuantes S, Duke R, Franzusoff A. Isolation of the human PC6 gene encoding the putative host protease for HIV-1 gp160 processing in CD4+ T lymphocytes. Proc Natl Acad Sci U S A. 1996;93:7695–7700. doi: 10.1073/pnas.93.15.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage S, Lapointe R. Melanosomal targeting sequences from gp100 are essential for MHC Class II-restricted endogenous epitope presentation and mobilization to endosomal compartments. Cancer Res. 2006;66:2423–2432. doi: 10.1158/0008-5472.CAN-05-2516. [DOI] [PubMed] [Google Scholar]
- Theos A C, Berson J F, Theos S C, Herman K E, Harper D C, Tenza D, Sviderskaya E V, Lamoreux M L, Bennett D C, Raposo G, Marks M S. Dual loss of ER export and endocytic signals with altered melanosome morphology in the silver mutation of Pmel17. Mol Biol Cell. 2006;17:3598–3612. doi: 10.1091/mbc.E06-01-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robila V, Ostankovitch M, Altrich-Vanlith M L, Theos A C, Drover S, Marks M S, Restifo N, Engelhard V H. MHC Class II presentation of gp100 epitopes in melanoma cells requires the function of conventional endosomes and is influenced by melanosomes. J Immunol. 2008;181:7843–7852. doi: 10.4049/jimmunol.181.11.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinakaran G, Koo E H. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N M, Karran E H, Turner A J. Membrane protein secretases. Biochem J. 1997;321:265–279. doi: 10.1042/bj3210265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Vorherr T, Carafoli E. Calmodulin-binding domains: just two faced or multi-faceted? Trends Biochem Sci. 1995;20:38–42. doi: 10.1016/s0968-0004(00)88949-5. [DOI] [PubMed] [Google Scholar]
- Diaz-Rodriguez E, Esparis-Ogando A, Montero J C, Yuste L, Pandiella A. Stimulation of cleavage of membrane proteins by calmodulin inhibitors. Biochem J. 2000;346:359–367. [PMC free article] [PubMed] [Google Scholar]
- Elhabazi A, Delaire S, Bensussan A, Boumsell L, Bismuth G. Biological activity of soluble CD100. I. The extracellular region of CD100 is released from the surface of T lymphocytes by regulated proteolysis. J Immunol. 2001;166:4341–4347. doi: 10.4049/jimmunol.166.7.4341. [DOI] [PubMed] [Google Scholar]
- Swanson R A, Benington J H. Astrocyte glucose metabolism under normal and pathological conditions in vitro. Dev Neurosci. 1996;18:515–521. doi: 10.1159/000111448. [DOI] [PubMed] [Google Scholar]
- Qi H, Rand M D, Wu X, Sestan N, Wang W, Rakic P, Xu T, Artavanis-Tsakonas S. Processing of the notch ligand delta by the metalloprotease Kuzbanian. Science. 1999;283:91–94. doi: 10.1126/science.283.5398.91. [DOI] [PubMed] [Google Scholar]
- Raposo G, Marks M S. Melanosomes: dark organelles enlighten endosomal membrane transport. Nat Rev Mol Cell Biol. 2007;8:786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler D M, Koulov A V, Alory-Jost C, Marks M S, Balch W E, Kelly J W. Functional amyloid formation within mammalian tissue [Online] PLoS Biol. 2005;4:e6. doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R E, Bertram L. New frontiers in Alzheimer’s disease genetics. Neuron. 2001;32:181–184. doi: 10.1016/s0896-6273(01)00476-7. [DOI] [PubMed] [Google Scholar]
- Jarrett J T, Berger E P, Lansbury P T., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Vijayasaradhi S, Xu Y, Bouchard B, Houghton A N. Intracellular sorting and targeting of melanosomal membrane proteins: identification of signals for sorting of the human brown locus protein, gp75. J Cell Biol. 1995;130:807–820. doi: 10.1083/jcb.130.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo P A, Frank D W, Bieler B M, Berson J F, Marks M S. A cytoplasmic sequence in human tyrosinase defines a second class of di-leucine-based sorting signals for late endosomal and lysosomal delivery. J Biol Chem. 1999;274:12780–12789. doi: 10.1074/jbc.274.18.12780. [DOI] [PubMed] [Google Scholar]
- Honing S, Sandoval I V, von Figura K. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R, Planque N, Martin P, Dewitte F, Saule S, Hoflack B. The AP-3-dependent targeting of the melanosomal glycoprotein QNR-71 requires a di-leucine-based sorting signal. J Cell Sci. 2001;114:2831–2841. doi: 10.1242/jcs.114.15.2831. [DOI] [PubMed] [Google Scholar]
- Lambert D W, Clarke N E, Hooper N M, Turner A J. Calmodulin interacts with angiotensin-converting enzyme-2 (ACE2) and inhibits shedding of its ectodomain. FEBS Lett. 2008;582:385–390. doi: 10.1016/j.febslet.2007.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadhukhan R, Sen G C, Ramchandran R, Sen I. The distal ectodomain of angiotensin-converting enzyme regulates its cleavage-secretion from the cell surface. Proc Natl Acad Sci U S A. 1998;95:138–143. doi: 10.1073/pnas.95.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribas J, Borroto A. Protein ectodomain shedding. Chem Rev. 2002;102:4627–4638. doi: 10.1021/cr010202t. [DOI] [PubMed] [Google Scholar]
- Dowdy J C, Anthony F A, Costlow M E. Topical W-7 inhibits ultraviolet radiation-induced melanogenesis in Skh:HR2 pigmented hairless mice. Photodermatol Photoimmunol Photomed. 1995;11:143–148. doi: 10.1111/j.1600-0781.1995.tb00156.x. [DOI] [PubMed] [Google Scholar]
- Nagano O, Murakami D, Hartmann D, De S B, Saftig P, Iwatsubo T, Nakajima M, Shinohara M, Saya H. Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca2+ influx and PKC activation. J Cell Biol. 2004;165:893–902. doi: 10.1083/jcb.200310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovronsky D M, Moore D B, Milla M E, Doms R W, Lee V M. Protein kinase C-dependent alpha-secretase competes with beta-secretase for cleavage of amyloid-beta precursor protein in the trans-Golgi network. J Biol Chem. 2000;275:2568–2575. doi: 10.1074/jbc.275.4.2568. [DOI] [PubMed] [Google Scholar]
- Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- Bosserhoff A K, Kaufmann M, Kaluza B, Bartke I, Zirngibl H, Hein R, Stolz W, Buettner R. Melanoma-inhibiting activity, a novel serum marker for progression of malignant melanoma. Cancer Res. 1997;57:3149–3153. [PubMed] [Google Scholar]
- Ito S, Kato T, Maruta K, Fujita K, Kurahashi T. Determination of DOPA, dopamine, and 5-S-cysteinyl-DOPA in plasma, urine, and tissue samples by high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1984;311:154–159. doi: 10.1016/s0378-4347(00)84702-7. [DOI] [PubMed] [Google Scholar]