Abstract
Melanocortin-3 receptors (Mc3rs) in the central nervous system are involved in expression of anticipatory rhythms and synchronizing clocks maintaining circadian rhythms during restricted feeding (RF) [mice housed under a 12-h light-dark cycle with lights on between zeitgeber time (ZT) 0 to ZT12 fed 60% of normal calories between ZT7 and ZT11]. Because the systems governing circadian rhythms are important for adaptation to RF, we investigated whether Mc3rs are required for metabolic adaption to RF. Mc3r−/− mice subjected to RF exhibited normal weight loss; however, they developed hyperinsulinemia, glucose intolerance, increased expression of lipogenic genes, and increased ketogenesis relative to controls. Rhythmic expression of transcription factors regulating liver clock activity and energy metabolism (Bmal1, Rev-erbα, Pgc1, Foxo1, Hnf4α, and Pck1) was severely compromised in Mc3r−/− mice during RF. Inhibition of neural melanocortin receptors by agouti-related peptide also attenuated rhythmicity in the hepatic expression of these genes during RF. Collectively, these data suggest that neural Mc3rs are important for adapting metabolism and maintaining rhythms of liver metabolism during periods when feeding is restricted to the light cycle.—Sutton, G. M., Begriche, K., Kumar, K. G., Gimble, J. M., Perez-Tilve, D., Nogueiras, R., McMillan, R. P., Hulver, M. W., Tschöp, M. H., Butler, A. A. Central nervous system melanocortin-3 receptors are required for synchronizing metabolism during entrainment to restricted feeding during the light cycle.
Keywords: hypothalamus, circadian biology, liver metabolism, glucose, ketones
The hypothalamus is a critical juncture in the homeostatic circuitry coordinating calorie intake with metabolic activity in peripheral tissues (1). Hypothalamic neurons receive signals of energy status from hormones (e.g., ghrelin, leptin, insulin, and peptide YY3–36) (1) and are regulated by fatty acids and glucose (2, 3). Through projections descending to the pituitary and brainstem, the hypothalamus regulates hepatic glucose production (4,5,6), insulin sensitivity (7, 8), lipogenesis, and fat oxidation (9, 10). Deterioration of the response of hypothalamic neurons to these factors contributes to insulin resistance and dyslipidemia associated with obesity (3, 11, 12).
Melanocortin neurons are one of the hypothalamic circuits involved in energy homeostasis (13). Hypothalamic arcuate (ARC) nucleus neurons expressing proopiomelanocortin (Pomc) secrete melanocyte-stimulating hormones (α-, β- and γ-melanocortin-stimulating hormone), which are agonists for the melanocortin receptors (Mc1r, Mc3r, Mc4r, and Mc5r). A second group of ARC neurons secrete agouti-related peptide (AgRP), an inverse agonist/antagonist at the Mc3rs and Mc4rs (14). Both neurons integrate short- and long-term signals of energy status including glucose, signals from the gut released during meal consumption, and signals from adipocytes indicating energy status (13). Responding to these signals, the melanocortin system regulates satiety, glucose homeostasis, and fatty acid metabolism (13). Outputs from Pomc neurons act to restrain energy balance, preventing weight gain through promotion of satiety and maintenance of energy expenditure. Conversely, AgRP neuronal activity has a positive effect on energy balance, increasing appetite and metabolic efficiency.
Two melanocortin receptors expressed in the central nervous system (CNS) are involved in energy homeostasis (15). The regulation of satiety, autonomic function, energy expenditure, and glucose homeostasis involves Mc4r and is considered to be independent of Mc3r (7, 16). The Mc3r also has a role in energy homeostasis; however, its functions remain unclear. Mc3r mRNA is expressed in areas of the hypothalamus linked to appetite regulation and to autonomic and neuroendocrine circuits affecting peripheral metabolic activity (17). Mc3rs are involved in regulating peripheral metabolism, with Mc3r−/− mice exhibiting a nutrient partitioning phenotype and exacerbated diet-induce obesity (18,19,20,21). Although obesity in Mc3r−/− mice is independent of hyperphagia, we have observed hyperphagia restricted to the lights-on period (13, 21).
Our laboratory has been investigating a potential role for Mc3rs in the expression of food-anticipatory activity. C57BL/6J (B6) mice subjected to a restricted feeding protocol (RF) [60% of normal calories available between zeitgeber time (ZT) 7 and ZT11, corresponding to 1:00–5:00 PM in mice housed in light-dark conditions with lights on between 6:00 AM and 6:00 PM] rapidly develop food anticipatory activity and increased wakefulness before food presentation (22). This behavioral adaptation is severely attenuated in B6 mice lacking Mc3rs (22). The same study showed a link between Mc3rs and neural clock activity. Clocks synchronize rhythms of ingestive behavior and metabolic activity, facilitating anticipation of cyclical changes in the environment (23). The master clock residing in the suprachiasmatic nucleus of the hypothalamus (SCN) synchronizes daily rhythms with photoperiod. However, energy status related to periodic food deprivation-refeeding is the dominant zeitgeber for clocks residing outside the SCN in the brain and in peripheral organs (23). These clocks regulate many enzymatic processes relevant to energy homeostasis, including gluconeogenesis (24). Forced desynchronization of meal time and arousal from the natural circadian rhythm instigates a metabolic syndrome that could increase risk for obesity and diabetes; shift work has also been suggested as a risk factor for metabolic disease (25). Clock mutants become moribund when challenged with phase shifts in food presentation, suggesting a failure to adapt metabolism (26, 27).
Because Mc3rs are involved in energy homeostasis and regulation of peripheral metabolism (13) and the expression of anticipatory rhythms (22), we hypothesized that Mc3rs may also function in metabolic homeostasis during RF. Here we report that metabolic adaptation and liver clock activity are both severely compromised in Mc3r−/− mice during RF. Inhibition of central melanocortin receptors by infusion of the C terminus of AgRP (AgRP82–131) similarly attenuates rhythmicity in liver gene expression during RF but had no effect in mice pair-fed at the onset of the peak period for voluntary food intake. Although weight loss in control and Mc3r−/− mice subjected to RF is comparable, the latter exhibit dissociation between weight loss and improvements in glucose homeostasis. Taken together, these results indicate that hypothalamic melanocortin neurons acting through Mc3rs are essential for metabolic adaptation during RF.
MATERIALS AND METHODS
Animal studies
Tissues used for some of the experiments reported here were from a study that was described previously (22). Male Mc3r−/−, Mc4r−/−, and wild-type (WT) mice were littermates obtained from colonies of heterozygotes backcrossed >10 generations onto the B6 background and maintained at the Pennington Biomedical Research Center (19). AY/a and male B6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All studies were approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee.
Intracerebroventricular infusion of AgRP
Liver tissue was obtained from a study in which male B6 mice were infused intracerebroventricularly with AgRP82–131 (0.05 nmol/d for 14 d) or artificial cerebrospinal fluid (acsf), and then subjected to pair-feeding (4.5 g/d at ZT12/6:00 PM) or RF, as described previously (22).
Gene expression
For measurement of genes normalized to cyclophilin B by quantitative RT-PCR with the ABI 7900 system (Applied Biosystems Inc., Foster City, CA, USA), primer-probe combinations were used as described previously (19, 22).
Blood chemistry
Plasma lipids were measured on a Beckman Synchron CX7 (Beckman Coulter, Fullerton, CA, USA). For glucose tolerance tests (GTTs), mice were deprived of food overnight, and blood samples were taken at time 0 (baseline) or 15–60 min after an i.p. injection of glucose (1 mg/kg). For the pyruvate tolerance test, ad libitum fed WT and Mc3r−/− mice were deprived of food for 12 h, with food taken away at lights on (6:00 AM). Mice were given a bolus of 1 g/kg pyruvate dissolved in saline at just before lights out, and blood glucose was measured every 15 min for 1 h.
Measurement of nuclear and cytosolic Foxo1 protein
Forty milligrams of fresh liver tissue was homogenized in 400 μl of ice-cold CER-I solution (Pierce Biotechnology, Rockford, IL) supplemented with 40 μl of protease inhibitor cocktail (Pierce Biotechnology). Nuclear fractions were separated from cytoplasm by differential centrifugation, and the protein concentration was determined by using the BCA Protein Assay Kit (Pierce Biotechnology). To assess hepatic expression of Foxo1, aliquots (25 μg) of nuclear or cytoplasm proteins were resolved on 4–20% SDS-polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA, USA), transferred onto polyvinyl pyrrolidone membranes (Bio-Rad Laboratories), and probed with a polyclonal anti-rabbit Foxo1 antibody kindly provided by Dr. H. Dong (University of Pittsburgh School of Medicine, Pittsburgh, PA, USA) (28). To normalize cytoplasm and nuclear loadings, blots were stripped and incubated, respectively, with a monoclonal mouse antibody against β-actin (Sigma-Aldrich Corp., St. Louis, MO, USA) or a polyclonal rabbit antibody against fibrillarin (Abcam, Cambridge, MA, USA).
Statistics
All data are presented as means ± se. Effects of genotype within feeding paradigm ZT were analyzed post hoc using Student’s t tests. In experiments in which mice were euthanized at ZT6 and ZT18, a 2-way ANOVA was used to test for significance of genotype and ZT. For analysis of citrate synthase data, a 3-way ANOVA (genotype, feeding paradigm, and diet) was performed using SAS 9.1.3 (SAS Institute, Cary, NC, USA). The Bonferroni correction was applied when Student’s t test was used for post hoc analysis of multiple groups.
RESULTS
Mc3rs are required for normal rhythms of clock activity in liver
Mc3r−/− and WT mice subjected to RF exhibit a reduction in energy expenditure commensurate with reduced caloric consumption; however, a difference in postprandial whole-body substrate preference in Mc3r−/− mice suggested increased use of fatty acids (22). Because the liver clock regulates both glucose production (24) and lipid metabolism (29), we measured clock activity in liver samples collected from our previous study (22). A schematic diagram describing the RF protocol is provided in Fig. 1A. In brief, 2-mo-old male B6 Mc3r−/− mice and WT controls housed in 12-h light-dark conditions (lights on between 6:00 AM and 6:00 PM) were allowed to feed ad libitum or were subjected to RF for 7 d. On d 8 of the study, food was not presented, and mice were euthanized for tissue collection at 6:00 AM, 12:00 PM, 6:00 PM, and 12:00 AM (corresponding to ZT 0, 6, 12, and 18).
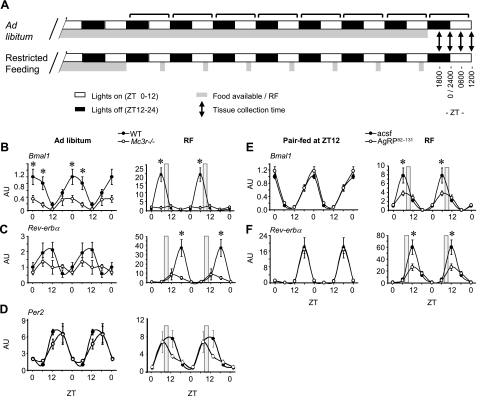
Figure 1.
Mc3r signaling is required for normal liver clock activity during RF. A) Schematic diagram of the RF protocol used for these experiments. Note that food was not available on the day of tissue and serum collection. B–D) Double-plotted expression pattern of Bmal1 (B), Rev-erbα (C), and Per2 (D) in liver of WT and Mc3r−/− mice fed ad libitum or subjected to RF (gray bar). E, F) Double-plotted expression pattern of Bmal1 (E) and Rev-erbα (F) in liver of mice treated i.c.v. with AgRP82–131 or acsf pair-fed a nonlimiting amount of food (4.5 g) at ZT12 (6:00 PM, onset of lights off; left panels) or subjected to RF (right panels). *P < 0.05 vs. corresponding WT. AU, arbitrary units.
Rhythms in the expression of Bmal1 and Rev-erbα in WT and Mc3r−/− mice fed ad libitum or after 7 d of RF are shown in Fig. 1. During ad libitum feeding, WT mice exhibited the predicted rhythm in Bmal1 and Rev-erbα expression, with a 12-h phase difference in peak expression (Fig. 1B, C, left panels). Bmal1 expression was significantly lower in Mc3r−/− mice at ZT0 and ZT6, indicating reduced amplitude (Fig. 1B, left panel). Rhythmicity in expression of Rev-erbα mRNA was also reduced (Fig. 1C, left panel). As reported previously (30, 31), RF was associated with a phase shift in rhythmic expression in WT mice, with peak expression at ZT6 and ZT18, respectively (Fig. 1B, C, right panels). This pattern of expression of Bmal1 or Rev-erbα was severely disrupted in Mc3r−/− mice (Fig. 1B, C, right panels).
Oscillators in the liver are hypothesized to be regulated by local and systemic inputs. The oscillator involving Bmal1 and Rev-erbα is considered to be cell autonomous, whereas a second system regulating a smaller subset of genes responds to systemic signals (32). Although Bmal1 and Rev-erbα exhibited a marked suppression of rhythm in Mc3r−/− mice, rhythms in the expression of Per2 mRNA were only modestly affected by genotype (Fig. 1D). Together, these data suggest that inputs affecting the Bmal1/Rev-erbα oscillator are affected by loss of Mc3rs and are probably external in origin because Mc3rs are not expressed in liver (17). The other component of the clock system thought to respond to systemic signals retains a form of rhythmicity.
We next determined whether acute blockade of CNS melanocortin signaling interferes with regulation of Bmal1 and Rev-erbα expression. Male B6 mice were administered AgRP82–131 (0.05 nmol/d), which is an antagonist/inverse agonist at the Mc3r and Mc4r (33, 34), or acsf for 14 d as described previously (22). Mice receiving AgRP82–131 or acsf were either pair-fed a defined amount of food (4.5 g) at ZT12 (corresponding to onset of the dark phase at 6:00 PM) or subjected to RF using the protocol shown in Fig. 1A. Body weight and food intake data for this experiment were reported previously (22).
In pair-fed groups, central infusion of AgRP82–131 had no effect on rhythmicity of Bmal1 or Rev-erbα expression in liver (Fig. 1E, F, left panels). RF again resulted in a phase shift in the rhythm in Bmal1 and Rev-erbα expression in control mice (Fig. 1E, F, right panels). As observed in Mc3r−/− mice, a marked reduction in peak expression of both genes was observed in mice treated with AgRP82–131 during RF (Fig. 1E, F, right panels).
Mc3r−/− mice subjected to RF develop hyperinsulinemia and glucose intolerance
Previous observations suggested that loss of Mc3r function does not severely compromise glucose homeostasis in mice fed standard chow ad libitum (18,19,20). To investigate whether impaired entrainment of Mc3r−/− mice to RF during the light cycle affects glucose homeostasis, we measured serum insulin and glucose (Fig. 2A, B). In ad libitum fed animals, serum insulin and glucose levels of Mc3r−/− mice were within the normal range at ZT0, 6, and 12. At ZT18, there was a tendency (P=0.07) for hyperinsulinemia in Mc3r−/− mice; however, the difference was modest.
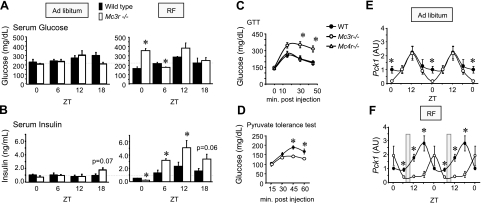
Figure 2.
Abnormal glucose homeostasis in Mc3r−/− mice subjected to RF. A, B) Serum glucose (A, left panel) and insulin (B, left panel) values in Mc3r−/− mice fed ad libitum at ZT0, 6, 12, and 18 were unremarkable. Mc3r−/− mice subjected to RF exhibited marked abnormalities in serum glucose (A, right panel) and insulin (B, right panel). At ZT0, Mc3r−/− mice subjected to RF were hyperglycemic and hypoinsulinemic (P<0.05). Hyperglycemia may have been resolved at other time points through compensatory increases in serum insulin. *P < 0.05 vs. corresponding WT. C) Mc3r−/− mice subjected to RF for 2 wk are glucose-intolerant compared with Mc4r−/− and WT mice subjected to RF. *P < 0.05 vs. WT and Mc4r−/−. *P < 0.05 vs. WT. D) Reduced conversion of pyruvate to glucose in ad libitum fed Mc3r−/− mice relative to WT mice. *P < 0.05 vs. WT. E, F) Abnormal rhythmic expression of liver Pck1 mRNA in Mc3r−/− mice is amplified during RF. In ad libitum fed animals (E), Mc3r−/− mice exhibited a lower nadir of Pck1 mRNA expression at ZT0. With RF (F), Mc3r−/− mice exhibited 3- to 4-fold lower levels of expression at ZT12 and ZT18 relative to controls. *P < 0.05 vs. corresponding WT. AU, arbitrary units.
The RF protocol used for these studies stimulates food anticipatory behavior by using a negative energy balance that results in an ∼10% loss of body weight (22). This negative energy balance was associated with lower serum glucose levels at some but not all time points in WT mice (Fig. 2A). However, the response of Mc3r−/− mice to RF was different. Remarkably, Mc3r−/− mice subjected to RF exhibited an increase in serum glucose at ZT0 (serum glucose at ZT0 was 212±8 and 352±24 mg/dl in Mc3r−/− mice fed ad libitum or subjected to RF, respectively, P=0.002). A likely explanation for this hyperglycemia is insufficient insulin production, as serum insulin levels were significantly lower in Mc3r−/− mice relative to controls at this time point (Fig. 2B). However, at the other time points Mc3r−/− mice were hyperinsulinemic, which was statistically significant at ZT6 and ZT12 (both P<0.05), whereas there was a strong tendency at ZT18 (P=0.06) (Fig. 2B).
We next performed studies to investigate how RF affects glucose homeostasis using GTT. We compared Mc3r−/− mice with two models of obesity due to impaired Mc4r function. In the first study, we examined glucose tolerance of male Mc3r−/− (n=8), Mc4r−/− (n=7), and WT (n=8) mice subjected to RF for 2 wk. The tests were performed in the morning (i.e., after the overnight period when food was not available). Mc4r−/− mice were significantly heavier than WT and Mc3r−/− mice before RF (body weight for WT mice was 28.8±0.8 g, for Mc3r−/− mice was 25.1±0.9 g, and for Mc4r−/− mice was 32.4±1.8 g; P<0.05, Mc4r−/− vs. WT and Mc3r−/− mice). Weight loss with RF was not affected by genotype (weight loss for WT mice, 3.3±0.7 g; for Mc3r−/− mice, 3.2±0.6 f; and for Mc4r−/− mice, 2.7±0.6 g). However, after 2 wk of RF Mc3r−/− mice were glucose-intolerant relative to both WT and Mc4r−/− mice (Fig. 2C). We also examined the consequence of RF on glucose tolerance in lethal yellow (Ay/a) mice, a mouse model of late-onset obesity and type 2 diabetes (35). The Ay/a mice used for this study were obese and markedly glucose-intolerant; as predicted, weight loss associated with RF dramatically improved glucose tolerance (data not shown).
Mc3r regulate hepatic glucose production
To determine whether reduced clock activity in the liver of Mc3r−/− mice affects glucose production, we performed a pyruvate tolerance test. The conversion of exogenously administered pyruvate to glucose was markedly impaired in ad libitum fed male Mc3r−/− mice compared with controls (Fig. 2D). We next measured the expression of cytosolic phosphoenolpyruvate carboxykinase (Pck1), a rate-limiting enzyme involved in gluconeogenesis. Under ad libitum feeding conditions, hepatic Pck1 expression exhibited a rhythmic pattern (Fig. 2E). In Mc3r−/− mice, expression relative to that in WT mice was reduced at the nadir observed at ZT0; however, the peak level of expression was normal (Fig. 2E). As observed with the liver clock, the effect of genotype on Pck1 expression was far more severe during RF, with Mc3r−/− mice subjected to RF exhibiting a marked reduction in the amplitude of Pck1 expression at ZT6, ZT12, and ZT18 relative to that in controls (Fig. 2F).
Central melanocortin signaling synchronizes rhythmic expression of transcription factors involved in energy metabolism
The liver clock regulates rhythmic expression of a significant portion of the transcriptome including pathways involved in lipid and glucose metabolism (23). To further investigate the impact of impaired clock regulation on hepatic energy metabolism, we examined the expression of four key genes encoding transcription factors that are responsible for regulating expression of genes involved in glucose and fatty acid metabolism (Pgc1α, Pgc1β, Foxo1, and Hnf4α). In the ad libitum groups, rhythms in the expression of all four genes were grossly normal (Fig. 3A, left panels). However, with RF the loss of Mc3r signaling was associated with marked disruptions of the normal pattern of expression. As observed for Bmal1 and Rev-erbα, RF amplified the rhythm of gene expression, with Pgc1α, Pgc1β, Foxo1, and Hnf4α exhibiting maxima at ZT18 (Fig. 3A, right panels). This coincides with the peak observed in Rev-erbα expression (Fig. 1A, B, right panels).
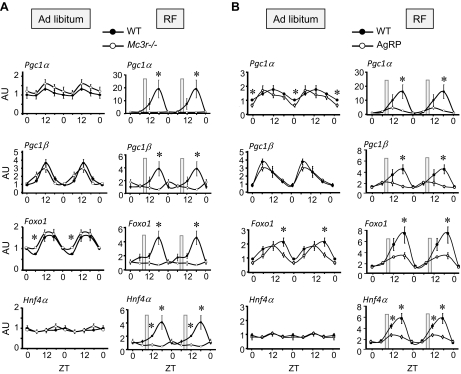
Figure 3.
Rhythmicity of expression of key transcriptional regulators of metabolism is dependent on Mc3r during RF. A) Rhythmicity of expression of Pcg1a, Pgc1β, Foxo1, and Hnf4α is phase-shifted and amplified during RF (compare left panels with right panels) in WT mice. In contrast, Mc3r−/− mice subjected to RF exhibit a marked loss of rhythmicity of expression during peak activity levels in WT controls. B) Amplification of rhythms of expression of Pcg1a, Pgc1β, Foxo1, and Hnf4α by RF is attenuated by the central infusion of AgRP82–131 (0.05 nmol/d). Mice receiving AgRP82–131 or acsf exhibited comparable rhythmicity of gene expression when food was presented at the onset of dark period. However, the phase shift in expression associated with RF observed in controls was attenuated by centrally administered AgRP82–131. *P < 0.05 vs. corresponding acsf.
We then determined whether acute pharmacological inhibition of central melanocortin signaling would similarly affect rhythms in the expression of these genes. Again, RF was associated with a phase shift and increased amplitude in expression of Pgc1α, Pgc1β, Foxo1, and Hnf4α relative to that in the control group (Fig. 3B, compare left and right panels). Although central infusion of AgRP82–131 was associated with minimal or at most modest changes in the rhythm of expression of Pgc1α, Pgc1β, Foxo1, and Hnf4α in the pair-fed group (Fig. 3B, left panels), the infusion of AgRP82–131 during RF was associated with a marked reduction in the amplitude of expression in all four genes (Fig. 3B, right panels).
To determine whether reduced expression of Foxo1 mRNA in liver of Mc3r−/− mice affects Foxo1 protein levels, we used livers from a second study. Cytoplasmic and nuclear protein lysates were prepared from fresh tissue harvested at ZT6 and ZT18 from WT and Mc3r−/− mice subjected to 7 d of RF. Nuclear levels of Foxo1 protein were lower in Mc3r−/− mice at both times (Fig. 4A). However, Foxo1 protein levels in the cytoplasm were not affected by genotype. These results suggest reduced nuclear localization of Foxo1 in liver of Mc3r−/− mice during RF. Because no increase in cytosolic protein Foxo1 levels was observed, total Foxo1 protein levels are probably also reduced.
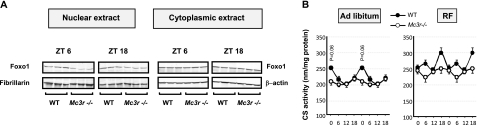
Figure 4.
Reduced nuclear content of Foxo1 protein at ZT6 and ZT18 in Mc3r−/− mice subjected to RF relative to controls (A) and reduced rhythmicity of CS activity in Mc3r−/− mice (B). A) Levels of Foxo1 protein were determined by Western blot in cytoplasmic and nuclear extracts of WT and Mc3r−/− mice subjected to 7d of RF. Nuclear levels of Foxo1 were lower in Mc3r−/− mice; however, there was no difference in cytosolic Foxo1 protein levels. B) Liver CS activity levels exhibit a rhythmic pattern in WT mice fed ad libitum (left panel) that is attenuated in Mc3r−/− mice. RF is associated with increased CS activity, irrespective of genotype (P<0.01; right panel). However, CS activity remains lower in Mc3r−/− mice.
Pgc1α and Pgc1β are transcriptional coactivators involved in regulating the expression of genes involved in mitochondrial oxidative phosphorylation (36), whereas Pgc1α also regulates expression of clock genes in liver (37). To determine whether the reduced expression of Pgc1α and Pgc1β affects mitochondrial function, we measured the activity of the mitochondrial enzyme citrate synthase (CS) in liver samples from WT and Mc3r−/− mice used for assessment of gene expression in Figs. 1 and 3. CS is a soluble enzyme of the tricarboxylic acid cycle residing in the mitochondrial matrix and provides an indication of mitochondrial activity. Overall, analysis of CS data indicated a highly significant effect of genotype (P=0.0014) and feeding time (P<0.0001) on CS activity (Fig. 4B). In ad libitum fed mice, a rhythm in CS activity was evident in WT mice (Fig. 3B, left panel) with a peak in activity at ZT0 corresponding to the middle of the dark phase and peak ingestive behavior. The amplitude of the rhythm was reduced in Mc3r−/− mice, with a tendency for low CS activity at ZT0 (P=0.06). Overall, RF was associated with increased CS activity (P<0.01, independent of genotype). Again, CS activity was lower in Mc3r−/− mice (Fig. 4B, right panel, P<0.01 for RF in WT vs. RF in Mc3r−/− mice).
Increased dependency of Mc3r−/− mice on fatty acids for energy production during RF
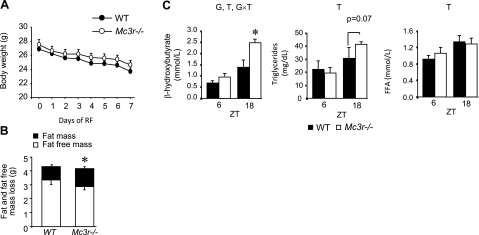
The data obtained thus far strongly suggest that CNS Mc3r are required for maintaining normal glucose homeostasis during RF. When glucose levels are insufficient due either to food deprivation or impaired insulin activity, fatty acids become the predominant substrate for ATP production. Increased preference for fat oxidation was in fact indicated in our earlier analysis of whole-body substrate preference by indirect calorimetry in Mc3r−/− and control mice subjected to RF (22). To determine whether the abnormal adaptation of substrate preference would affect weight loss in Mc3r−/− mice during RF, we performed a study in which we measured weight loss and body composition by nuclear magnetic resonance. At the start of the study, Mc3r−/− mice exhibited modest obesity typical of this model, with increased fat mass (FM) (FM for WT was 2.3±0.4 g, n=8, and for Mc3r−/− was 4.5±0.4 g, n=10; P<0.01), but no significant difference in weight (26.9±0.4 g for WT and 27.5±0.7 g for Mc3r−/− mice, P=0.51). As observed previously, absolute weight loss associated with RF was not affected by genotype (Fig. 5A). However, the proportion of weight loss attributable to FM compared with fat-free mass (FFM) was greater in Mc3r −/− mice relative to that in controls (Fig. 5B). The reduction in FM + FFM was similar in WT and Mc3r−/− mice (4.3±0.3 vs. 4.1±0.3 g, P=0.74). The loss of FM as a percentage of FM + FFM was significantly greater in Mc3r−/− mice (31±2 vs. 23±2%, P<0.05).
Figure 5.
A, B) Weight loss (A) broken down by FM or FFM (B) and serum chemistry analyses in WT and Mc3r−/− mice subjected to RF. Absolute weight loss was not significantly affected by genotype (A); however, the proportion of weight loss attributable to fat mass was proportionately greater in Mc3r−/− mice (B). *P < 0.05 vs. FM in WT. C) Left panel: serum ketones in WT and Mc3r−/− mice subjected to RF. Serum ketones were elevated at ZT18 compared with ZT6, and this difference was greater in Mc3r−/− mice. Serum triglycerides (middle panel) and free fatty acids (right panel) were also higher at ZT18, and this was not significantly affected by genotype. There was, however, a tendency (P=0.07) for higher triglycerides in Mc3r−/− mice at ZT18. Letters above graphs indicate significance (P<0.05) of an effect of genotype (G), zeitgeber time (T), or an interaction indicating that the effect of time is dependent on genotype (G×T).*P < 0.05 vs. corresponding WT.
We next measured serum lipid metabolites at ZT6 and ZT18 in WT and Mc3r−/− mice after 7 d of RF. Two-way ANOVA indicated significant effects of genotype and time on serum ketones, with a marked and statistically significant increase in serum β-hydroxybutyrate observed at ZT18 and a tendency for a modest difference at ZT6 (Fig. 5C). Serum triglycerides and FFA levels were affected by time but not by genotype, although Mc3r−/− mice exhibited a tendency (P=0.07) for increased triglycerides relative to WT mice at ZT18. (Fig. 5C).
Ketone bodies result from incomplete β-oxidation in liver mitochondria and are elevated during fasting and uncontrolled diabetes. We subsequently analyzed the expression of key lipid regulatory enzymes and transcription factors involved in fatty acid oxidation. The expression of carnitine palmitoyltranserase 1a, the enzyme catalyzing the transfer of long-chain fatty acids to carnitine for transport across the mitochondrial inner membrane, was significantly increased in Mc3r−/− mice compared with that in control mice at ZT6 (1.7±0.09 vs. 1.0±0.08, P=0.002) but not at ZT18 (2.2±0.08 vs. 1.8±0.12, P=0.10). However, the expression of peroxisome proliferator-activated receptor-α (PPARα), acyl-coenzyme A dehydrogenase, and acyl-CoA oxidase, which are involved in mitochondrial and peroxisomal fatty acid oxidation, was not significantly affected by genotype (data not shown).
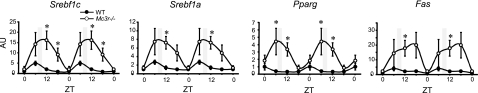
Although Mc3r−/− mice lost more FM compared with controls, the difference is quite small (∼0.3 g or 12 kJ) (Fig. 5B), making the adipose triglyceride pool an unlikely source of fatty acids. Moreover, these studies used a low-fat, high-carbohydrate diet (10% kJ from fat, 20% kJ from protein, and 70% kJ from carbohydrate, mostly in the form of cornstarch and sucrose) as the energy source (22). Hyperinsulinemia can increase the conversion of carbohydrate to fatty acid through stimulation of sterol regulatory element binding transcription factor 1 (Srebf1) (38, 39). The nuclear receptor PPARγ may also regulate hepatic lipogenesis in some conditions (40). Mc3r−/− mice exhibited a markedly increased expression of genes encoding or nuclear receptors regulating lipogenesis (Srebf1a and 1c and Pparg), as well as a key regulatory enzyme (fatty acid synthase) (Fig. 6). All of these genes exhibited a more pronounced rhythmic pattern of expression in Mc3r−/− mice correlating with the degree of hyperinsulinemia (Fig. 3B).
Figure 6.
Increased expression of lipogenic genes in liver of Mc3r−/− mice relative to controls during RF. Expression profile of transcription factors that regulate expression of genes involved in lipogenesis (Srebf1a, Srebf1c, and Pparg) was increased at ZT6, 12, and 18 but not at ZT0, which coincides with a period of hyperinsulinemia (compare with Fig. 2 B, left panel). Expression of Fas, a lipogenic enzyme, was also higher at these time points. *P < 0.05 vs. corresponding WT.
DISCUSSION
Analysis of the phenotype of melanocortin receptor-knockout mice back-crossed onto the C57BL/6J strain indicates that the functions of the Mc3rs pertaining to glucose homeostasis are modest under normal conditions of ad libitum feeding compared with those of the Mc4rs (7, 19). However, the results from this study indicate that Mc3rs are indeed important for maintaining glucose homeostasis, a function that is especially important during RF. The RF protocol used involves a food deprivation phase lasting ∼20 h, which is interrupted by a 4-h period of food consumption proportional to 60% of normal calorie intake (22). In rodents, this pattern of scheduled feeding coincides with a rapid response of hypothalamic melanocortin neurons (41, 42). AgRP neuronal activity dominates during food deprivation, acting to increase metabolic efficiency and promoting appetite. After meal ingestion, dominance rapidly shifts to Pomc neurons. Increased Mc4r activity during this phase promotes satiety and maintains thyroid function, energy expenditure, and glucose utilization (13). Mc3rs are abundant in the mediobasal hypothalamus (43) and are regulated in parallel by neuropeptide secreted from primary melanocortin neurons. However, the function of these neurons has remained unclear. The results of the current study and those from our previous analysis of behavior (22) suggest that Mc3r-coupled outputs are central to synchronizing wakefulness, activity, and metabolism during times when food availability is restricted to the light cycle.
CNS Mc3rs are required for maintaining rhythmicity of expression of genes encoding core components of transcription factor complexes that regulate genes involved in diverse hepatic energy metabolism functions. Foxo1 regulates gluconeogenesis and lipid metabolism (44). Hnf4α regulates glucose metabolism, lipid transport, and xenobiotic metabolism (45). The two members of the Pgc1 family also regulate transcription of genes involved in glucose production and mitochondrial activity, with Pgc1α acting as a coactivator of Hnf4α and Foxo1 (36, 44).
The interaction between Mc3r genotype and RF to affect fasting insulin and glucose was dependent on ZT. Mc3r−/− mice subjected to RF exhibited hypoinsulinemia and hyperglycemia at ZT0, suggesting insufficient insulin production. In contrast, insulin and glucose data at other ZTs suggested that Mc3r−/− mice were producing sufficient insulin to compensate for insulin resistance. Mc3r−/− mice were also shown to be glucose intolerant relative to WT mice during RF. β-Cell function in Mc3r−/− mice subjected to RF may be compromised during the transition between lights-off and lights-on (ZT0), with hyperglycemia resulting from insufficient insulin production and insulin resistance. At other times, insulin secretion is sufficient to maintain normal serum glucose levels, although the mice are glucose intolerant and are probably insulin resistant. This result suggests a circadian component in the regulation of insulin production by the pancreas that is also affected by loss of Mc3r activity. Evidence for the circadian regulation of insulin secretion has been reported, including regulation by melatonin acting through the melanotonin receptor 1B expressed in the pancreas (46).
With severe and chronic negative energy balance, amino acids released by proteolysis from muscle can provide an additional source of substrate for gluconeogenesis. In the current studies it was noteworthy that a substantial proportion of weight loss may have been FFM. Mc3r−/− mice subjected to RF appear unable to efficiently produce and utilize glucose and may actually expend energy converting carbohydrate to fatty acids in the liver. Ketones resulting from incomplete oxidation of fatty acids may also constitute a significant energy source in Mc3r−/− mice, compensating for impaired production and utilization of glucose. The correlation between serum insulin and the expression of lipogenic genes in liver indicates that hyperinsulinemia may contribute to this process by increasing the activity of Srebf1, a master regulator of the expression of genes encoding lipogenic enzymes (38, 39). This result and the observation of reduced expression of gluconeogenic genes in liver suggests that the liver of Mc3r−/− mice may be responding normally to hyperinsulinemia during RF. The phenotype of Mc3r−/− mice subjected to RF therefore probably involves mixed or tissue-specific insulin resistance. The glucose intolerance that develops in Mc3r−/− mice subjected to RF could indicate a selective insulin resistance in other insulin target tissues, such as skeletal muscle and adipose tissue. The resulting hyperinsulinemia then regulates the expression of insulin-responsive genes in liver, such as Pck1, Srebf1, and Fas. Clearly, further studies are required to determine the sites of insulin resistance, and the mechanisms explaining how this occurs despite a state of negative energy balance.
Clock mechanisms synchronizing metabolism with the circadian day developed early during evolution and have been described in life forms ranging from prokaryotes to complex metazoans (23). For surface-dwelling vertebrates, light is the principal zeitgeber synchronizing the “master” clock, which resides in the SCN. However, restricting nutrient availability to defined periods at intervals of 24 h induces rhythms of locomotory activity and increased wakefulness that anticipate food presentation (47). RF is also associated with a phase shift in the rhythmic pattern of clock gene expression outside the SCN (30, 31), suggesting an involvement of non-SCN clocks in entrainment to feeding time. Although the entrainment of behavior exhibits some characteristics suggesting the involvement of a clock mechanism, elucidating the site and mechanisms governing this process has been difficult (48). The involvement of known clock genes in the expression of food anticipatory rhythms has recently been questioned (26, 49). There is, however, agreement that the dominant zeitgeber for clocks residing outside the SCN is related to energy metabolism (23, 50) and that clock outputs regulate many aspects of energy metabolism essential for energy homeostasis, including glucose production and lipid processing (24, 51). Reduced expression of anticipatory activity previously reported in clock mutants may reflect impaired metabolic adaptation, leading to moribund behavior and increased mortality (26, 49). We have not observed increased mortality in Mc3r−/− mice subjected to RF. However, the current results are consistent with the development of a metabolic condition associated with reduced capacity for glucose production and utilization and an increased dependency on fatty acids for energy production.
The inhibition of liver clock activity by centrally administered AgRP was only observed in mice subjected to RF, with no effect observed in mice fed ad libitum. Inhibition of the liver clock by AgRP is therefore not due to a pharmacological impairment of melanocortin tone in the CNS per se. Rather, the effect of centrally administered AgRP on liver clock activity appears to be dependent on an interaction with mechanisms required for adaptation to feeding outside the normal circadian rhythm established by photoperiod and the circadian clock. This interpretation is consistent with the induction of what could be a second oscillator system that integrates temporal cues of nutrient consumption with energy status. During RF, this system dominates the regulation of signals emanating from the CNS that are required for synchronizing metabolism and liver clock activity.
We reported previously that the CNS Mc3rs are important for the expression of food anticipatory behavior responding to RF and for maintaining clock activity in cortical areas of the brain (22). Here we elected to investigate the role of Mc3rs in regulating the liver clock. The rationale for this study was based on previous data linking the hypothalamic melanocortin system to the regulation of hepatic glucose metabolism (6, 8, 52) and on observations that the liver clock responds more rapidly to phase shifts in meal presentation relative to other metabolic tissues (31). The current results demonstrate that the outputs from a well-defined neuronal system are involved in synchronizing the activity of the liver clock with feeding cues, as indicated by dramatic reductions in the expression of Bmal1 and Rev-erbα mRNA. This could be due to direct regulation of a system for transmitting signals of circadian timing to the liver. Alternatively, the deterioration of liver clock activity could be a secondary effect associated with the development of metabolic perturbations observed in Mc3r−/− mice during RF, such as selective insulin resistance in nonhepatic tissues having a feedback effect on the liver.
The results of the current study, along with our previously published data (22), suggest that melanocortin neurons expressing Mc3r may be a conduit linking signals of energy status with the systems involved in entraining behavioral rhythms and synchronizing metabolism with phase shifts of food intake. The neuroanatomy of the melanocortin system may provide an explanation for variability in studies examining the effects of hypothalamic and extrahypothalamic lesions on food entrainment. Mc3r mRNA is expressed in ∼30 sites distributed throughout the hypothalamus and limbic structures, including several hypothalamic nuclei, septum, hippocampus, olfactory cortex, thalamus, amygdala, and ventral tegmental area (17). Information from endocrine and neural signals of food intake may thus be transmitted from the arcuate nucleus to multiple regions of the CNS involved in regulating complex behaviors and to signals emanating from the CNS affecting the liver clock. The signals from the CNS that transmit information of photoperiod to peripheral clocks are unknown but may involve circulating factors (53).
Mc3r−/− mice are a unique mouse model of obesity that exhibits a dissociation of weight loss from improvements in glucose metabolism. Mice with impaired Mc4r activity exhibited improvements in glucose tolerance with RF, as would be predicted for obese mice losing weight because of reduced calorie intake. Indeed, after 2 wk of RF Mc3r−/− mice exhibit glucose intolerance relative to WT and Mc4r−/− mice. Most published data suggest that regulation of glucose homeostasis by melanocortins involves neurons expressing Mc4r and that Mc3r has little function in maintaining insulin sensitivity (7, 16). The current results indicate that the role of Mc3r as a significant factor in the neural circuitry linking nutrient consumption with glucose homeostasis needs to be reassessed.
To summarize, the results of this study demonstrate that CNS Mc3rs are required for metabolic adaptation to RF. In the absence of Mc3rs, RF is associated with the development of a metabolic syndrome characterized by reduced glucose utilization, abnormal substrate preference, and desynchronization of the liver clock. Mice, being nocturnal, are most active during the lights-off period, which is also the time when they consume most of their calories. The central nervous melanocortin system acting through Mc3rs seems to have a critical role in regulating systems that are involved in maintaining glucose homeostasis during periods when food intake is restricted to the light cycle.
Acknowledgments
We thank Dr. Jennifer Rood, Dr. Robert Koza, Dr. Jose Galgani, Brian Goh, Emily Meyer, Armand Centanni, and Xiying Wu for technical assistance. We thank Dr. H. Henry Dong (Rangos Research Center, Children’s Hospital of Pittsburgh, Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh, PA) for providing the Foxo1 antibody. Funding for this work was provided by the National Institutes of Health (DK073189 to A.A.B.). A.A.B. and J.M.G. acknowledge the support of the Pennington Biomedical Research Foundation and Clinical Nutrition Research Unit Center grant, “Nutritional Programming: Environmental and Molecular Interactions” (1P30 DK072476), sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases. The authors declare that they have no competing interests.
References
- Obici S. Molecular targets for obesity therapy in the brain. Endocrinology. 2009;150:2512–2517. doi: 10.1210/en.2009-0409. [DOI] [PubMed] [Google Scholar]
- Levin B E. Neuronal glucose sensing: still a physiological orphan? Cell Metab. 2007;6:252–254. doi: 10.1016/j.cmet.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Caspi L, Wang P Y, Lam T K. A balance of lipid-sensing mechanisms in the brain and liver. Cell Metab. 2007;6:99–104. doi: 10.1016/j.cmet.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Pocai A, Obici S, Schwartz G J, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005;1:53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Pocai A, Lam T K, Gutierrez-Juarez R, Obici S, Schwartz G J, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic KATP channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Juarez R, Obici S, Rossetti L. Melanocortin-independent effects of leptin on hepatic glucose fluxes. J Biol Chem. 2004;279:49704–49715. doi: 10.1074/jbc.M408665200. [DOI] [PubMed] [Google Scholar]
- Zhou L, Sutton G M, Rochford J J, Semple R K, Lam D D, Oksanen L J, Thornton-Jones Z D, Clifton P G, Yueh C Y, Evans M L, McCrimmon R J, Elmquist J K, Butler A A, Heisler L K. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6:398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest. 2001;108:1079–1085. doi: 10.1172/JCI12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T K, Gutierrez-Juarez R, Pocai A, Bhanot S, Tso P, Schwartz G J, Rossetti L. Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nat Med. 2007;13:171–180. doi: 10.1038/nm1540. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh J M, Sutton G M, Pfluger P T, Castaneda T R, Neschen S, Hofmann S M, Howles P N, Morgan D A, Benoit S C, Szanto I, Schrott B, Schurmann A, Joost H G, Hammond C, Hui D Y, Woods S C, Rahmouni K, Butler A A, Farooqi I S, O'Rahilly S, Rohner-Jeanrenaud F, Tschop M H. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007;117:3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M G, Cowley M A, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- Parton L E, Ye C P, Coppari R, Enriori P J, Choi B, Zhang C Y, Xu C, Vianna C R, Balthasar N, Lee C E, Elmquist J K, Cowley M A, Lowell B B. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- Garfield A S, Lam D D, Marston O J, Przydzial M J, Heisler L K. Role of central melanocortin pathways in energy homeostasis. Trends Endocrinol Metab. 2009;20:203–215. doi: 10.1016/j.tem.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Small C J, Kim M S, Stanley S A, Mitchell J R, Murphy K, Morgan D G, Ghatei M A, Bloom S R. Effects of chronic central nervous system administration of agouti-related protein in pair-fed animals. Diabetes. 2001;50:248–254. doi: 10.2337/diabetes.50.2.248. [DOI] [PubMed] [Google Scholar]
- Cone R D. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cone R D. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- Roselli-Rehfuss L, Mountjoy K G, Robbins L S, Mortrud M T, Low M J, Tatro J B, Entwistle M L, Simerly R B, Cone R D. Identification of a receptor for γ melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci U S A. 1993;90:8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A S, Marsh D J, Trumbauer M E, Frazier E G, Guan X M, Yu H, Rosenblum C I, Vongs A, Feng Y, Cao L, Metzger J M, Strack A M, Camacho R E, Mellin T N, Nunes C N, Min W, Fisher J, Gopal-Truter S, MacIntyre D E, Chen H Y, Van der Ploeg L H. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Sutton G M, Trevaskis J L, Hulver M W, McMillan R P, Markward N J, Babin M J, Meyer E A, Butler A A. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology. 2006;147:2183–2196. doi: 10.1210/en.2005-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A A, Kesterson R A, Khong K, Cullen M J, Pelleymounter M A, Dekoning J, Baetscher M, Cone R D. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- Butler A A. The melanocortin system and energy balance. Peptides. 2006;27:281–290. doi: 10.1016/j.peptides.2005.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton G M, Perez-Tilve D, Nogueiras R, Fang J, Kim J K, Cone R D, Gimble J M, Tschop M H, Butler A A. The melanocortin-3 receptor is required for entrainment to meal intake. J Neurosci. 2008;28:12946–12955. doi: 10.1523/JNEUROSCI.3615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C B, Takahashi J S, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia K A, Storch K F, Weitz C J. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey K M, Bass J. Obeying the clock yields benefits for metabolism. Proc Natl Acad Sci U S A. 2009;106:4069–4070. doi: 10.1073/pnas.0901304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch K F, Weitz C J. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci U S A. 2009;106:6808–6813. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger R E, Buijs R M, Challet E, Escobar C, Landry G J, Kalsbeek A, Pevet P, Shibata S. Standards of evidence in chronobiology: critical review of a report that restoration of Bmal1 expression in the dorsomedial hypothalamus is sufficient to restore circadian food anticipatory rhythms in Bmal1−/− mice. J Circadian Rhythms. 2009;7:3. doi: 10.1186/1740-3391-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu S, Altomonte J, Perdomo G, He J, Fan Y, Kamagate A, Meseck M, Dong H H. Aberrant Forkhead box O1 function is associated with impaired hepatic metabolism. Endocrinology. 2006;147:5641–5652. doi: 10.1210/en.2006-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staels B. When the clock stops ticking, metabolic syndrome explodes. Nat Med. 2006;12:54–55. doi: 10.1038/nm0106-54. [DOI] [PubMed] [Google Scholar]
- Dudley C A, Erbel-Sieler C, Estill S J, Reick M, Franken P, Pitts S, McKnight S L. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B, Schaad O, Reinke H, Saini C, Schibler U. Regulation of circadian gene expression in liver by systemic signals and hepatocyte oscillators. Cold Spring Harb Symp Quant Biol. 2007;72:319–330. doi: 10.1101/sqb.2007.72.041. [DOI] [PubMed] [Google Scholar]
- Ollmann M M, Wilson B D, Yang Y K, Kerns J A, Chen Y, Gantz I, Barsh G S. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Rossi M, Kim M S, Morgan D G, Small C J, Edwards C M, Sunter D, Abusnana S, Goldstone A P, Russell S H, Stanley S A, Smith D M, Yagaloff K, Ghatei M A, Bloom S R. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of α-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- Robinson S W, Dinulescu D M, Cone R D. Genetic models of obesity and energy balance in the mouse. Annu Rev Genet. 2000;34:687–745. doi: 10.1146/annurev.genet.34.1.687. [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman B M. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Liu C, Li S, Liu T, Borjigin J, Lin J D. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- Perlemuter G, Bigorgne A, Cassard-Doulcier A M, Naveau S. Nonalcoholic fatty liver disease: from pathogenesis to patient care. Nat Clin Pract Endocrinol Metab. 2007;3:458–469. doi: 10.1038/ncpendmet0505. [DOI] [PubMed] [Google Scholar]
- Petersen K F, Dufour S, Savage D B, Bilz S, Solomon G, Yonemitsu S, Cline G W, Befroy D, Zemany L, Kahn B B, Papademetris X, Rothman D L, Shulman G I. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova O, Haluzik M, Matsusue K, Cutson J J, Johnson L, Dietz K R, Nicol C J, Vinson C, Gonzalez F J, Reitman M L. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- Johnstone L E, Fong T M, Leng G. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab. 2006;4:313–321. doi: 10.1016/j.cmet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Singru P S, Sanchez E, Fekete C, Lechan R M. Importance of melanocortin signaling in refeeding-induced neuronal activation and satiety. Endocrinology. 2007;148:638–646. doi: 10.1210/en.2006-1233. [DOI] [PubMed] [Google Scholar]
- MacNeil D J, Howard A D, Guan X, Fong T M, Nargund R P, Bednarek M A, Goulet M T, Weinberg D H, Strack A M, Marsh D J, Chen H Y, Shen C P, Chen A S, Rosenblum C I, MacNeil T, Tota M, MacIntyre E D, Van der Ploeg L H. The role of melanocortins in body weight regulation: opportunities for the treatment of obesity. Eur J Pharmacol. 2002;450:93–109. doi: 10.1016/s0014-2999(02)01989-1. [DOI] [PubMed] [Google Scholar]
- Gross D N, van den Heuvel A P, Birnbaum M J. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- Gupta R K, Kaestner K H. HNF-4α: from MODY to late-onset type 2 diabetes. Trends Mol Med. 2004;10:521–524. doi: 10.1016/j.molmed.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Peschke E. Melatonin, endocrine pancreas and diabetes. J Pineal Res. 2008;44:26–40. doi: 10.1111/j.1600-079X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- Stephan F K. The “other” circadian system: food as a zeitgeber. J Biol Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Davidson A J. Search for the feeding-entrainable circadian oscillator: a complex proposition. Am J Physiol. 2006;290:R1524–R1526. doi: 10.1152/ajpregu.00073.2006. [DOI] [PubMed] [Google Scholar]
- Pendergast J S, Nakamura W, Friday R C, Hatanaka F, Takumi T, Yamazaki S. Robust food anticipatory activity in BMAL1-deficient mice. PLoS ONE. 2009;4:e4860. doi: 10.1371/journal.pone.0004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnen H. Circadian rhythms: a circadian loop asSIRTs itself. Science. 2009;324:598–599. doi: 10.1126/science.1174132. [DOI] [PubMed] [Google Scholar]
- Duez H, Staels B. Rev-erbα gives a time cue to metabolism. FEBS Lett. 2008;582:19–25. doi: 10.1016/j.febslet.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Konner A C, Janoschek R, Plum L, Jordan S D, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh G S, Kahn C R, Cowley M A, Ashcroft F M, Bruning J C. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Guo H, Brewer J M, Champhekar A, Harris R B, Bittman E L. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci U S A. 2005;102:3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]