Abstract
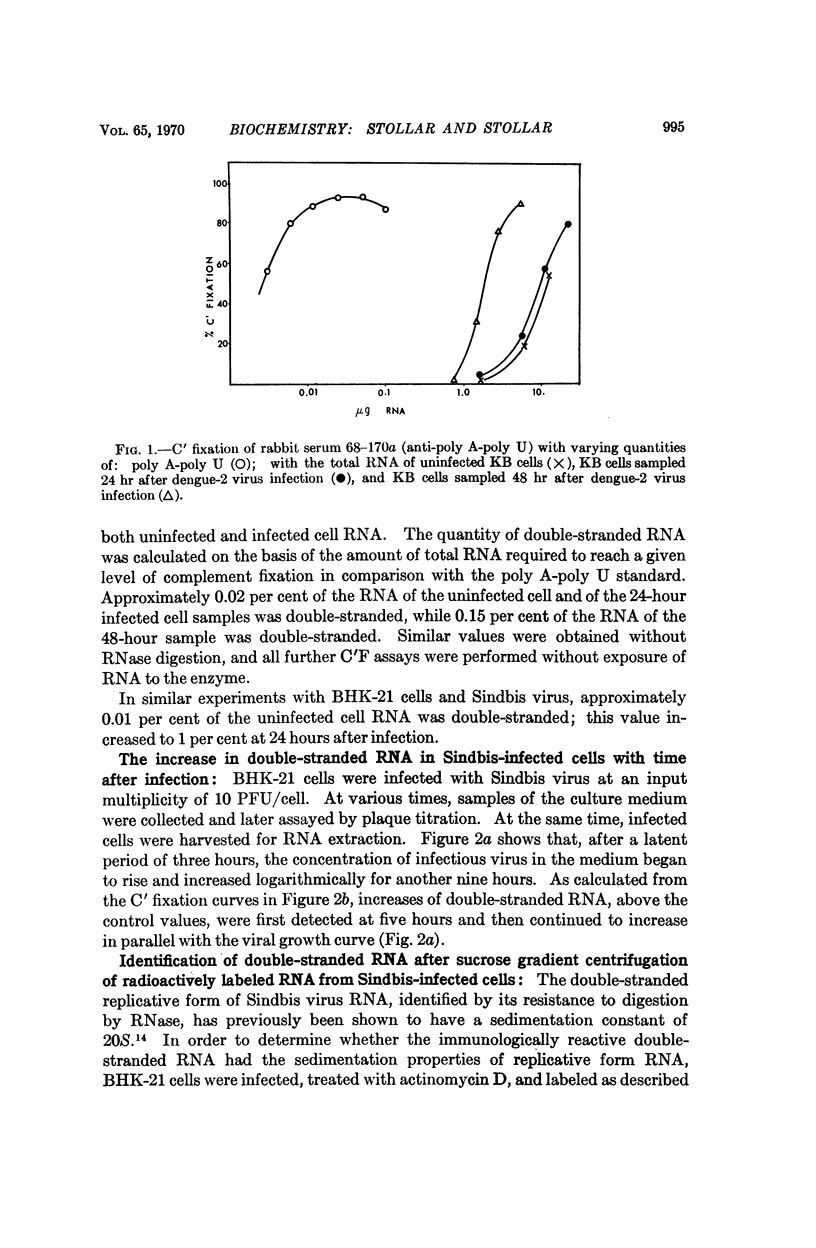
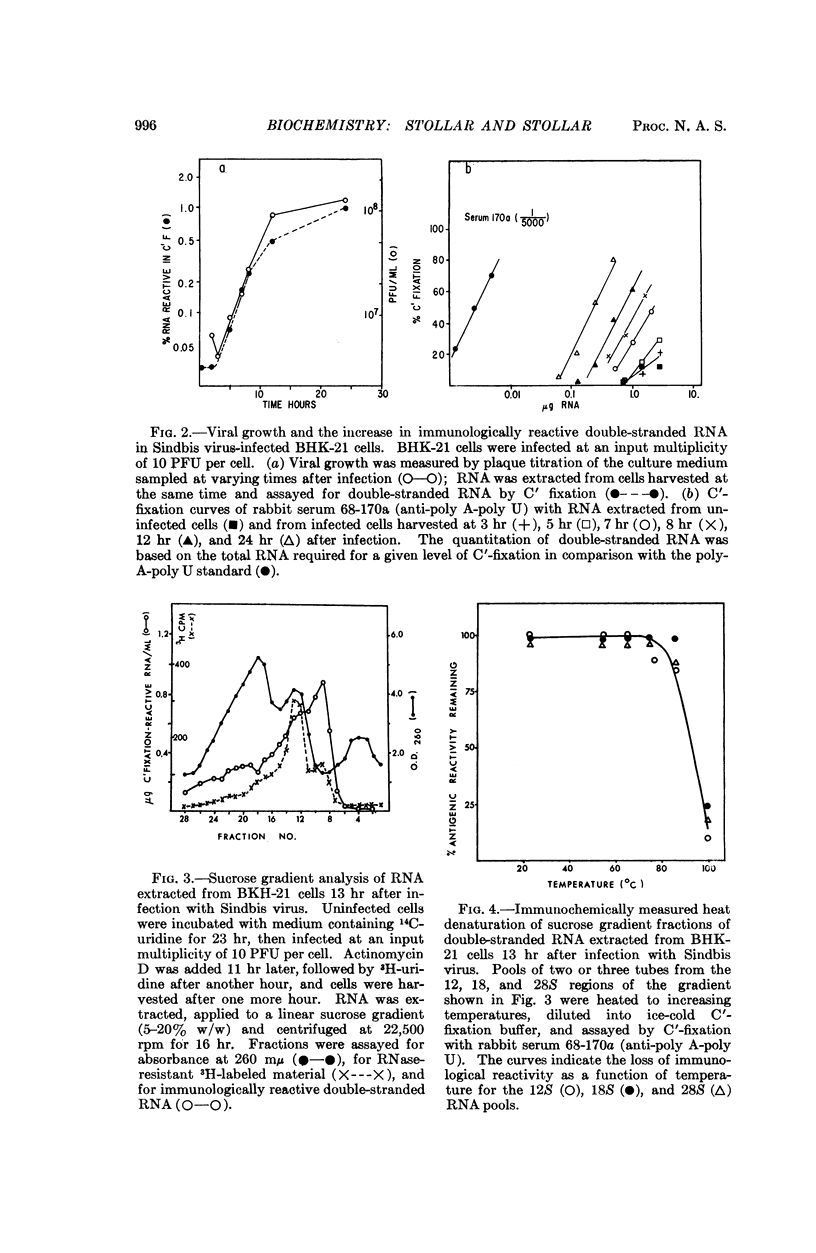
A quantitative complement fixation assay which specifically measures double-stranded RNA has been used to study this RNA extracted from uninfected and arbovirus-infected cells. The double-straned RNA of the uninfected BHK-21 cells sedimented in the 12S region in sucrose gradients. The double-stranded RNA of Sinbis virus-infected cells, as measured immunochemically, included a predominant peak at 12S, a smaller 18S peak, and polydisperse material extending into the 26-30S region. All classes of this RNA detected immunochemically showed a sharp thermal denaturation curve, and increased in amount progressively during infection, with the 12S peak predominant at all times.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colby C., Duesberg P. H. Double-stranded RNA in vaccinia virus infected cells. Nature. 1969 Jun 7;222(5197):940–944. doi: 10.1038/222940a0. [DOI] [PubMed] [Google Scholar]
- Doty P., Boedtker H., Fresco J. R., Haselkorn R., Litt M. SECONDARY STRUCTURE IN RIBONUCLEIC ACIDS. Proc Natl Acad Sci U S A. 1959 Apr;45(4):482–499. doi: 10.1073/pnas.45.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Nutrition needs of mammalian cells in tissue culture. Science. 1955 Sep 16;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Franklin R. M. Symposium on replication of viral nucleic acids. I. Formation and properties of a replicative intermediate in the biosynthesis of viral ribonucleic acid. Bacteriol Rev. 1966 Jun;30(2):267–278. doi: 10.1128/br.30.2.267-278.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B., TSUGITA A. Purification of viral RNA by means of bentonite. Virology. 1961 May;14:54–58. doi: 10.1016/0042-6822(61)90131-3. [DOI] [PubMed] [Google Scholar]
- LANGRIDGE R., GOMATOS P. J. The structure of RNA. Reovirus RNA and transfer RNA have similar three-dimensional structures, which differ from DNA. Science. 1963 Aug 23;141(3582):694–698. doi: 10.1126/science.141.3582.694. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Burge B. W., Coady H. M. Intracellular conversion of the RNA of sindbis virus to a double-stranded form. Virology. 1967 Oct;33(2):239–249. doi: 10.1016/0042-6822(67)90143-2. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Schwartz E. F., Stollar B. D. Antibodies to polyadenylate-polyuridylate copolymers as reagents for double strand RNA and DNA-RNA hybrid complexes. Biochem Biophys Res Commun. 1969 Apr 10;35(1):115–120. doi: 10.1016/0006-291x(69)90490-2. [DOI] [PubMed] [Google Scholar]
- Stevens T. M., Schlesinger R. W. Studies on the nature of dengue viruses. I. Correlation of particle density, infectivity, and RNA content of type 2 virus. Virology. 1965 Sep;27(1):103–112. doi: 10.1016/0042-6822(65)90147-9. [DOI] [PubMed] [Google Scholar]
- Stollar V., Stevens T. M., Schlesinger R. W. Studies on the nature of dengue viruses. II. Characterization of viral RNA and effects of inhibitors of RNA synthesis. Virology. 1966 Oct;30(2):303–312. doi: 10.1016/0042-6822(66)90105-x. [DOI] [PubMed] [Google Scholar]
- Stollar V. Studies on the nature of dengue viruses. IV. The structural proteins of type 2 dengue virus. Virology. 1969 Nov;39(3):426–438. doi: 10.1016/0042-6822(69)90091-9. [DOI] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- Weissmann C., Ochoa S. Replication of phage RNA. Prog Nucleic Acid Res Mol Biol. 1967;6:353–399. doi: 10.1016/s0079-6603(08)60530-9. [DOI] [PubMed] [Google Scholar]



