Abstract
Objective
To determine the association of changes on nailfold capillaroscopy with clinical findings and genotype in children with juvenile dermatomyositis (DM), in order to identify potential differences in disease course over 36 months.
Methods
At diagnosis of juvenile DM in 61 children prior to the initiation of treatment, tumor necrosis factor α (TNFα) −308 allele and DQA1*0501 status was determined, juvenile DM Disease Activity Scores (DAS) were obtained, and nailfold capillaroscopy was performed. The disease course was monitored for 36 months. Variations within and between patients were assessed by regression analysis.
Results
At diagnosis, shorter duration of untreated disease (P = 0.05) and a lower juvenile DM skin DAS (P = 0.035) were associated with a unicyclic disease course. Over 36 months, end-row loop (ERL) regeneration was associated with lower skin DAS (P < 0.001) but not muscle DAS (P = 0.98); ERL regeneration and decreased bushy loops were associated with a shorter duration of untreated disease (P = 0.04 for both). At 36 months, increased ERL regeneration (P = 0.007) and improvement of skin DAS (P < 0.001) and muscle DAS (P = 0.025) were associated with a unicyclic disease course.
Conclusion
Early treatment of juvenile DM may lead to a unicyclic disease course. The non-unicyclic disease course usually involves continuing skin manifestations with persistent nailfold capillaroscopy changes. The correlation of nailfold capillaroscopy results with cutaneous but not with musculoskeletal signs of juvenile DM over a 36-month period suggests that the cutaneous and muscle vasculopathies have different pathophysiologic mechanisms. These findings indicate that efforts to identify the optimal treatment of cutaneous features in juvenile DM require greater attention.
Juvenile dermatomyositis (DM) is a systemic inflammatory microvasculopathy that primarily affects skin and muscle. The pathogenesis of juvenile DM involves an immune-mediated process triggered by environmental factors in a genetically susceptible host. Although evidence of an antecedent illness in the 3–4 months before onset of juvenile DM and strong expression of interferon-α/β–induced genes in muscle of untreated children with juvenile DM (1) have suggested a possible microbial trigger (2), polymerase chain reaction studies of affected muscle failed to identify bacterial or viral genomic material (3). HLA–DQA1*0501 and other HLA markers (DQA1*301 and DRB*0301) appear to be major risk factors for the development of juvenile DM, but do not predict disease chronicity (4). In patients with untreated juvenile DM, increased synthesis of tumor necrosis factor α (TNFα), a proinflammatory cytokine, is common, and TNFα −308A polymorphism has been associated with a prolonged disease course, pathologic calcifications (5), increased thrombospondin 1 production, and small vessel occlusion (6).
In juvenile DM, systemic immunosuppressive therapy is guided by clinical findings, especially those reflecting muscle disease, as well as laboratory results. Recent analysis showed that serum levels of creatine-phosphokinase, aldolase, lactate dehydrogenase, and serum glutamic oxaloacetic transaminase sometimes fall within the normal range in untreated children who have had juvenile DM symptoms for >4 months, despite the fact that they had clinical evidence of active cutaneous and muscle inflammation as well as persistent immunologic abnormalities (7). More sensitive indicators of disease activity are needed to help predict disease course and guide therapy.
Periungual telangiectasia is a characteristic feature of juvenile DM. Nailfold capillaroscopy is a noninvasive technique that provides quantitative information about capillary end-row loop (ERL) loss, areas of avascularity, and formation of bushy loops (BLs) (capillary dilatations and branching arboreal capillary loops) (8). In children with untreated juvenile DM, these features reflect ongoing juvenile DM disease activity in the skin, but not muscle, suggesting distinct pathophysiologic mechanisms in skin versus muscle (9). To extend this observation, we conducted a retrospective observational study over 36 months, of children with newly diagnosed, initially untreated juvenile DM. The main goal of this study was to determine the association of nailfold capillaroscopy changes with demographic features (sex, race, and age at disease onset), duration of untreated disease; disease activity in skin and muscle, TNFα −308 and HLA–DQA1*0501 alleles, and disease course. Additionally, these variables were used to assess possible predictors of the disease course at the time of diagnosis and to determine differences in disease course over 36 months.
Patients and Methods
Patients
Untreated children with definite juvenile DM (10), seen in the immunology/rheumatology clinic at Children's Memorial Medical Center between 1994 and 2004, were enrolled in the study after informed consent was obtained from their legal guardians. Patients were excluded if they had <2 nailfold capillaroscopy studies or juvenile DM Disease Activity Score (DAS) assessments (11) in 36 months, prior treatment, or a diagnosis of overlap syndrome. Study participants were classified according to sex, race (white versus nonwhite), age at disease onset, duration of untreated disease, disease course, and TNFα −308 and HLA–DQA1*0501 allele status. Data on age at the time the first symptom of juvenile DM (rash or muscle weakness) was recognized, and on the time between onset of juvenile DM and the first administration of systemic immunosuppressive therapy (7), were recorded.
Nailfold capillaroscopy and DAS assessments
Nail-fold capillaroscopy, skin DAS, and muscle DAS assessments were administered at the first diagnostic outpatient visit (baseline) and every 6 (±3) months during a maximum of 36 (±6) months of followup. Nailfold capillaroscopy results and DAS findings were each graded by the same individuals for each patient at each time point, without knowledge of the results of the other assessments. Nailfold capillaroscopy analyses were performed as previously described (9). The nailfold capillaroscopy images were analyzed for the number of BLs and the total number of loops (ERLs) per 3-mm section over 8 digits. The severity of juvenile DM involvement was assessed using the validated juvenile DM DAS (11). The total DAS (see Appendix A, available on the Arthritis & Rheumatism Web site at http://www.mrw.interscience.wiley.com/suppmat/0004-3591/suppmat/) is a 20-point scale with 2 subscales, one for musculoskeletal findings (assessing muscle function and extent of weakness, with a maximum possible score of 11) and the other for dermatologic findings (assessing the extent and severity of rash, presence of Gottron's papules, and/or telangiectasia [defined as tiny, superficial dilated blood vessels with no particular pattern] of nailfolds, palate, and/or eyelids, with a maximum possible score of 9). A higher DAS represents greater disease activity. Nailfold capillaroscopy was also performed on 33 healthy children as controls, after informed consent was obtained from legal guardians.
Genotyping
TNFα −308 polymorphisms were identified as TNFα −308A (AA/AG) or TNFα −308G (GG). HLA–DQA1*0501 status was determined as previously described (1).
Classification of disease course
Disease course was classified as unicyclic or non-unicyclic. Unicyclic course was defined as stable clinical status, with a total DAS remaining at ≤3 at all assessments during the 36-month observation period. Our clinical experience suggests that a total DAS of ≤3 is associated with clinically inactive, stable disease. Non-unicyclic course was defined as a disease course showing evidence of continued clinical activity, with a DAS of >3. In order to verify the consistency of the definition of unicyclic and non-unicyclic disease course, a subset of patients was classified based on 5-year followup data. The disease course classification using 3-year and 5-year data was then compared. In the unicyclic disease course group, most of the children had discontinued all immunosuppressive therapy before 36 months and had no recurrence in the 2–3 years after discontinuation. Those in the non-unicyclic disease course group were observed to have disease activity necessitating either continuous or repeated immunosuppressive therapy for 36 months. This report does not focus on the type of therapy received by the children (dose, route, or frequency) since the goal of the study was to identify the association of the type of disease course with periungual telangiectasia, regardless of the treatment utilized.
Statistical analysis
Values obtained in children with untreated juvenile DM were compared with those obtained after up to 36 months of therapy. Continuous changes were analyzed using the values collected every 6 months (±3 months) for up to 36 months. Repeated-measures linear regression analysis was used to determine the association between the primary predictor (DAS) and nailfold capillaroscopy outcomes. Time in the longitudinal model was treated as a continuous variable. A mixed-effects approach was used to model intra- and interpatient variation. The primary focus of the analyses was interpatient comparisons; intrapatient variability was included to account for the correlations between the longitudinal observations in individual patients. Other covariates considered were sex, race, age at disease onset, duration of untreated disease, disease course, and TNFα −308 and HLA–DQA1*0501 status. Association of baseline nailfold capillaroscopy and DAS results with disease course was explored one predictor at time, using linear regression models and controlling for the duration of untreated disease. Data are presented as the mean ± SD. Data were analyzed using SAS version 9.1. P values less than 0.05 were considered significant.
Results
Patient characteristics
Over a 10-year period, 96 children were diagnosed as having untreated juvenile DM by one of the authors (LMP). Of these, 19 had received immunosuppressive therapy before their first nailfold capillaroscopy, 7 had no TNFα allele analysis, 2 had overlap syndrome, and 7 had only 1 followup visit. The remaining 61 patients were included in the study. The mean ± SD followup time was 29.6 ± 10.2 months (range 3.2–38.6). There were a total of 375 concurrent nailfold capillaroscopy and DAS observations during the 36-month observation period. Forty-nine patients (80%) had >5 observations. Followup data were available on 40 patients at 36 months and on 31 patients at 60 months. Sex, race, disease course, and TNFα −308 and HLA–DQA1*0501 allele status are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of the 61 children with juvenile dermatomyositis*
| Sex | |
| Male | 12 (20) |
| Female | 49 (80) |
| Race | |
| White | 49 (80) |
| Nonwhite | 12 (20) |
| TNFα −308 promoter gene | |
| AA/GA | 20 (33) |
| GG | 41 (67) |
| DQA1*0501 allele† | |
| Negative | 30 (50) |
| Positive | 30 (50) |
| Disease course over 36 months | |
| Non-unicyclic | 36 (59) |
| Unicyclic | 25 (41) |
Values are the number (%).
TNFα = tumor necrosis factor α.
Not tested in 1 patient.
Disease activity and nailfold capillaroscopy results
At baseline, the 61 patients had a mean ± SD of 5.63 ± 1.70 ERLs/mm (range 3.13–10.29) and 0.13 ± 0.15 BLs/mm (range 0–0.71), a skin DAS of 5.59 ± 1.45 (range 0–8), and a muscle DAS of 5.20 ± 2.92 (range 0–10). At 36 months (n = 40), the number of ERLs/mm was 6.32 ± 1.35 (range 3.13–9.33), the number of BLs/mm was 0.15 ± 0.16 (range 0–71), the skin DAS was 2.63 ± 2.35 (range 0–7), and the muscle DAS was 0.56 ± 0.88 (range 0–3). The 25th quartile and 75th quartile values of the number of ERLs/mm in the 33 healthy controls were 7.61/mm and 8.94/mm, respectively.
Results of genotype analysis for TNFα −308 and HLA–DQA1*0501
TNFα −308 genotype analysis revealed that 20 (32.8%) of the patients with juvenile DM had the AA/GA allele and 41 (67.2%) had the GG allele. Thirty patients (50%) were positive for the HLA–DQA1*0501 allele and 30 (50%) were negative for the allele; DQA1*0501 analysis was not performed on 1 patient.
Disease course
The disease course was non-unicyclic in 36 patients (59%) and unicyclic in 25 (41%). Among the 31 children on whom 5-year followup data were available (mean ± SD 78 ± 6 months), a majority (81%) of those with a unicyclic disease course at 3 years continued to be classified as having a unicyclic course at 5 years.
Potential baseline predictors of disease course
Shorter duration of untreated disease (P = 0.05) and lower skin DAS at baseline (P = 0.035) were significantly associated with a unicyclic disease course (Table 2). Type of disease course was not significantly associated with sex (P = 0.21), age at disease onset (P = 0.44), number of ERLs/mm (P = 0.14), number of BLs/mm (P = 0.78), muscle DAS (P = 0.65), TNFα −308 polymorphism (P = 0.11), or HLA–DQA1*0501 allele (P = 0.19).
Table 2.
Differences in disease features between juvenile DM patients with a unicyclic disease course and those with a non-unicyclic disease course*
| Unicyclic disease course (n = 25 at baseline; n = 17 after 36 months of treatment) | Non-unicyclic disease course (n = 36 at baseline; n = 23 after 36 months of treatment) | P | |
|---|---|---|---|
| Duration of untreated disease, months | 6.92 ± 6.11 (0.26–20.80) | 15.61 ± 24.64 (0.69–111.01) | 0.05 |
| Age at disease onset, years | 5.53 ± 2.73 (1.51–11.61) | 6.21 ± 4.04 (1.09–15.25) | 0.44 |
| Nailfold capillaroscopy and DAS findings at baseline | |||
| ERLs, per mm | 5.96 ± 1.74 (3.5–9.17) | 5.32 ± 1.62 (3.13–10.29) | 0.14 |
| BLs, per mm | 0.11 ± 0.13 (0–0.46) | 0.14 ± 0.17 (0–0.71) | 0.78 |
| Skin DAS | 5.12 ± 1.48 (0–7) | 5.92 ± 1.36 (3–8) | 0.035 |
| Muscle DAS | 5.0 ± 2.94 (0–10) | 5.35 ± 2.93 (0–10) | 0.65 |
| Nailfold capillaroscopy and DAS findings after 36 months of immunosuppressive treatment | |||
| ERLs, per mm | 7.22 ± 0.95 (5.81–9.33) | 5.65 ± 1.22 (3.13–9.04) | 0.007 |
| BLs, per mm | 0.12 ± 0.12 (0–0.33) | 0.18 ± 0.17 (0–0.71) | 0.53 |
| Skin DAS | 0.65 ± 0.79 (0–2) | 4.09 ± 2.02 (0–7) | <0.001 |
| Muscle DAS | 0.24 ± 0.44 (0–1) | 0.80 ± 1.04 (0–3) | 0.025 |
Values are the mean ± SD (range).
DM = dermatomyositis; DAS = juvenile DM Disease Activity Score; ERLs = end-row loops; BLs = bushy loops.
Nailfold capillaroscopy changes over 36 months and their associations
Longitudinal data analysis with time as a continuous measure (using all nailfold capillaroscopy studies from baseline up to 36 months of systemic therapy) showed that increased ERL regeneration and decreased BL numbers were associated with shorter duration of untreated disease (both P = 0.04). ERL regeneration was associated with lower skin DAS (P < 0.001) but was not associated with muscle DAS (P = 0.98). There were no statistically significant associations of either ERL or BL changes with sex (P = 0.33 and P = 0.12, respectively), race (P = 0.19 and P = 0.14, respectively), age at disease onset (P = 0.46 and P = 0.44, respectively), TNFα −308 polymorphism (P = 0.32 and P = 0.78, respectively), or presence of HLA–DQA1*0501 (P = 0.15 and P = 0.93, respectively).
Differences between unicyclic and non-unicyclic disease course over 36 months
Increased ERL regeneration (P = 0.007) and improvement in the skin DAS (P < 0.001) and muscle DAS (P = 0.025) were each associated with a unicyclic disease course in children with juvenile DM after the start of therapy (Figures 1A–C and Table 2). Figure 1D shows a composite view of ERL, skin DAS, and muscle DAS data at 36 months (n = 40) and 60 months (n = 31), demonstrating a lack of significant change between the 2 time points. There was no association between BL changes and disease course (P = 0.53) (Table 2).
Figure 1.
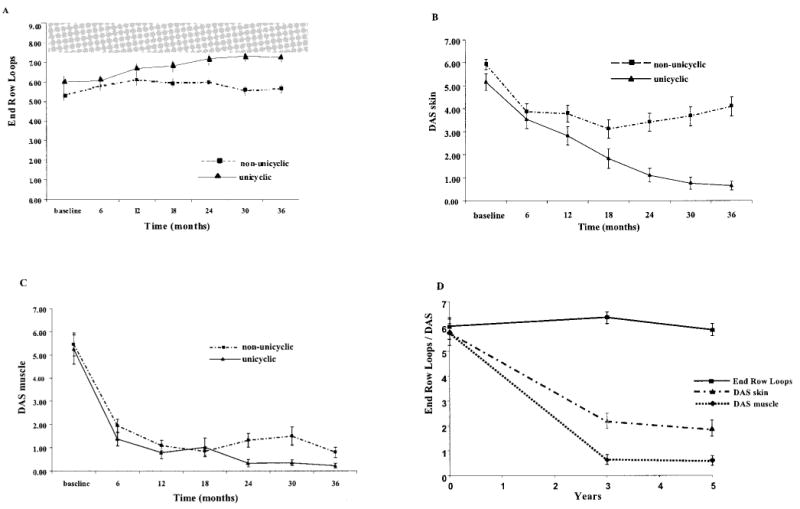
Differences in study parameters between juvenile dermatomyositis (DM) patients with a unicyclic and those with a non-unicyclic disease course over 36 months of treatment. Patients were untreated at study initiation. A, Regeneration of end-row loops. Shaded area shows the 25th to 75th quartile values in 33 healthy control children. B, Juvenile DM skin Disease Activity Score (DAS). C, Juvenile DM muscle DAS. D, End-row loop regeneration, skin DAS, and muscle DAS findings at 60-month followup compared with those at 36-month followup, showing relatively little change between the 2 time points. Values are the mean ± SD.
Discussion
The analysis of 375 observations of nailfold capillaroscopy, muscle DAS, and skin DAS obtained from 61 initially untreated children with juvenile DM during a 36-month treatment period showed that ERL loss was associated with the cutaneous but not the musculoskeletal signs of juvenile DM, suggesting that damage to skin and damage to muscle may have distinct pathophysiologic mechanisms. A longer duration of untreated disease correlated with worsening of the nailfold capillaroscopy changes. These results are consistent with our previous finding of correlations between ERL changes and severity of the rash and duration of disease in newly diagnosed, untreated children with juvenile DM (9). Persistent rash is not merely a cosmetic challenge; it can be associated with complications such as cutaneous calcinosis and may represent persistent vasculopathy which could lead to long-term complications if untreated. A decreased nailfold capillaroscopy score was recently shown to correlate with gastrointestinal vasculopathy and decreased absorption of orally administered corticosteroids (12). Our finding of a strong correlation between nailfold capillaroscopy changes and chronic cutaneous manifestations in both treated and untreated patients with juvenile DM suggests that early and more aggressive management should be considered, even in the absence of muscular weakness.
The disease course in children with juvenile DM has traditionally been classified as unicyclic, polycyclic, or chronic continuous (13). Previous data (5) suggest, and these new longitudinal nailfold capillaroscopy data confirm, that a simpler classification of unicyclic and non-unicyclic disease course may be more useful. Patients with a polycyclic and chronic continuous course overlap in their clinical features, prognoses, and recommended medical interventions; therefore, in the present study they were combined into 1 group, called non-unicyclic disease. The definition of a unicyclic disease course as a total DAS of ≤3 at all assessments (≥2 assessments in all cases) during a 36-month period is both novel and helpful. This definition is new and empirical and differs from previous uses of the term, in which patients were classified as having unicyclic disease if they had 1 episode of juvenile DM symptoms with permanent remission 2 years after diagnosis and therapy. Further studies are needed to validate this new definition.
A longer duration of untreated disease was found to be associated not only with persistent nailfold capillaroscopy abnormalities, but also with a non-unicyclic disease course. Consistent with previous findings (14), early, effective therapy was associated with a higher likelihood of a unicyclic pattern. The inflammatory disease process itself evolves, and duration of untreated disease is a critical element in the pathophysiology of the illness (7). Nailfold capillaroscopy data at diagnosis cannot be used to predict the course of disease, but if the vascular abnormalities persist, they provide another sensitive parameter of chronic disease activity and damage and should prompt the clinician to consider continued and aggressive therapy. Since nailfold capillaroscopy findings correlated with skin disease activity, the observation of nailfold capillaroscopy abnormalities in the setting of subtle skin changes is often very useful, providing insight into the disease process. Our results indicate that neither TNFα −308 nor DQA1*0501 is a useful marker for predicting the chronicity of the disease in treated juvenile DM patients, and neither was associated with nailfold capillary changes. Investigators at our institution previously reported that the TNFα −308A polymorphism was associated with disease chronicity (5). The present seemingly contradictory results likely reflect the shift toward more aggressive early therapy at our center, leading to a milder disease course with significant reduction in the development of pathologic calcifications.
In children with treated juvenile DM, a unicyclic disease course was associated with increased ERL regeneration and improvement in both skin DAS and muscle DAS at 36 months, which was maintained at 60 months in 81% of patients. The improvement of skin signs was observed to be a very gradual process, in parallel with the regeneration of capillary ERLs. The muscle inflammation, in contrast, responded much more quickly to systemic immunosuppressive treatment, with a rapid decrease in the muscle DAS. We conclude that the non-unicyclic disease course is, in general, a reflection of continuing skin involvement with persistent nail-fold capillaroscopy changes, given that current medical intervention results in rapidly reversing muscle disease but not in rapid change in skin disease. Specific therapy for the cutaneous inflammation in juvenile DM would improve the outcome of this often chronic illness.
Acknowledgments
Dr. Pachman's work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH (grant 1-R0-1-AR-48289), the National Arthritis Foundation, and Cure JM.
Footnotes
Author Contributions: Dr. Pachman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Christen-Zaech, Paller, Pachman.
Acquisition of data. Sundberg, Pachman.
Analysis and interpretation of data. Christen-Zaech, Seshadri, Sund-berg, Paller, Pachman.
Manuscript preparation. Christen-Zaech, Seshadri, Paller, Pachman.
Statistical analysis. Seshadri.
References
- 1.Tezak Z, Hoffman EP, Lutz JL, Fedczyna TO, Stephan D, Bremer EG, et al. Gene expression profiling in DQA1*0501+ children with untreated dermatomyositis: a novel model of pathogenesis. J Immunol. 2002;168:4154–63. doi: 10.4049/jimmunol.168.8.4154. [DOI] [PubMed] [Google Scholar]
- 2.Pachman LM, Lipton R, Ramsey-Goldman R, Shamiyeh E, Abbott K, Mendez EP, et al. History of infection before the onset of juvenile dermatomyositis: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Research Registry. Arthritis Rheum. 2005;53:166–72. doi: 10.1002/art.21068. [DOI] [PubMed] [Google Scholar]
- 3.Pachman LM, Litt DL, Rowley AH, Hayford JR, Caliendo J, Heller S, et al. Lack of detection of enteroviral RNA or bacterial DNA in magnetic resonance imaging–directed muscle biopsies from twenty children with active untreated juvenile dermatomyositis. Arthritis Rheum. 1995;38:1513–8. doi: 10.1002/art.1780381019. [DOI] [PubMed] [Google Scholar]
- 4.Mamyrova G, O'Hanlon TP, Monroe JB, Carrick DM, Malley JD, Adams S, et al. Immunogenetic risk and protective factors for juvenile dermatomyositis in Caucasians. Arthritis Rheum. 2006;54:3979–87. doi: 10.1002/art.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pachman LM, Liotta-Davis MR, Hong DK, Kinsella TR, Mendez EP, Kinder JM, et al. TNFα-308A allele in juvenile dermatomyositis: association with increased production of tumor necrosis factor α, disease duration, and pathologic calcifications. Arthritis Rheum. 2000;43:2368–77. doi: 10.1002/1529-0131(200010)43:10<2368::AID-ANR26>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Lutz J, Huwiler KG, Fedczyna T, Lechman TS, Crawford S, Kinsella TR, et al. Increased plasma thrombospondin-1 (TSP-1) levels are associated with the TNFα-308A allele in children with juvenile dermatomyositis. Clin Immunol. 2003;103:250–63. doi: 10.1006/clim.2001.5212. [DOI] [PubMed] [Google Scholar]
- 7.Pachman LM, Abbott K, Sinacore JM, Amoruso L, Dyer A, Lipton R, et al. Duration of illness is an important variable for untreated children with juvenile dermatomyositis. J Pediatr. 2006;148:247–53. doi: 10.1016/j.jpeds.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Dolezalova P, Young SP, Bacon PA, Southwood TR. Nailfold capillary microscopy in healthy children and in childhood rheumatic diseases: a prospective single blind observational study. Ann Rheum Dis. 2003;62:444–9. doi: 10.1136/ard.62.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith RL, Sundberg J, Shamiyah E, Dyer A, Pachman LM. Skin involvement in juvenile dermatomyositis is associated with loss of end row nailfold capillary loops. J Rheumatol. 2004;31:1644–9. [PubMed] [Google Scholar]
- 10.Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 11.Bode RK, Klein-Gitelman MS, Miller ML, Lechman TS, Pachman LM. Disease activity score for children with juvenile dermatomyositis: reliability and validity evidence. Arthritis Rheum. 2003;49:7–15. doi: 10.1002/art.10924. [DOI] [PubMed] [Google Scholar]
- 12.Rouster-Stevens KA, Gursahaney A, Ngai KL, Daru JA, Pachman LM. Pharmacokinetic study of oral prednisolone compared with intravenous methylprednisolone in patients with juvenile dermatomyositis. Arthritis Rheum. 2008;59:222–6. doi: 10.1002/art.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spencer CH, Hanson V, Singsen BH, Bernstein BH, Kornreich HK, King KK. Course of treated juvenile dermatomyositis. J Pediatr. 1984;105:399–408. doi: 10.1016/s0022-3476(84)80012-8. [DOI] [PubMed] [Google Scholar]
- 14.Fisler RE, Liang MG, Fuhlbrigge RC, Yalcindag A, Sundel RP. Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis. J Am Acad Dermatol. 2002;47:505–11. doi: 10.1067/mjd.2002.122196. [DOI] [PubMed] [Google Scholar]


