Abstract
Gonadotropin-releasing hormone acts via cell surface receptors but most human (h) GnRH receptors (GnRHRs) are intracellular. A membrane-permeant nonpeptide antagonist [(2S)-2-[5-[2-(2-axabicyclo[2.2.2]oct-2-yl)-1,1-dimethy-2-oxoethyl]-2-(3,5-dimethylphenyl)-1H-indol-3-yl]-N-(2-pyridin-4-ylethyl)propan-1-amine (IN3)] increases hGnRHR expression at the surface, apparently by facilitating its exit from the endoplasmic reticulum. Here we have quantified GnRHR by automated imaging in HeLa cells transduced with adenovirus expressing hemagglutinin-tagged GnRHR. Consistent with an intracellular site of action, IN3 increases cell surface hGnRHR, and this effect is not blocked or mimicked by membrane-impermeant peptide antagonists [Ac-D2Nal-D4Cpa-D3Pal-Ser-Tyr-d-Cit-Leu-Arg-Pro-d-Ala-NH2 (cetrorelix) and antide]. However, when the C-terminal tail of a Xenopus (X) GnRHR was added (h.XGnRHR) to increase expression, both peptides further increased cell surface GnRHR. Cetrorelix also synergized with IN3 to increase expression of hGnRHR and a G-protein coupling-deficient mutant (A261K-hGnRHR). Cetrorelix also increased cell surface expression of hGnRHR, h.XGnRHR, and mouse GnRHR in gonadotrope-lineage LβT2 cells, and in HeLa cells it slowed h.XGnRHR internalization (measured by receptor-mediated antihemagglutinin uptake). Thus cetrorelix has effects other than GnRHR blockade; it acts as an inverse agonist in internalization assays, supporting the potential importance of ligand-biased efficacy at GnRHR. We also developed an imaging assay for GnRH function based on Ca2+-dependent nuclear translocation of a nuclear factor of activated T cells reporter. Using this in HeLa and LβT2 cells, IN3 and cetrorelix behaved as competitive antagonists when coincubated with GnRH, and long-term pretreatment (16 h) with IN3 reduced its effectiveness as an inhibitor whereas pretreatment with cetrorelix increased its inhibitory effect. This distinction between peptide and nonpeptide antagonists may prove important for therapeutic applications of GnRH antagonists.
Non-peptide and peptide GnRH antagonists can both increase cell surface GnRH receptors but by different mechanisms, increasing trafficking to the surface or slowing internalization, respectively.
GnRH mediates central control of reproduction by stimulating secretion of LH and FSH from gonadotrophs. GnRH-stimulated gonadotropin secretion is blocked by antagonists and mimicked by agonists, but sustained stimulation causes desensitization. Both types of ligand ultimately reduce gonadal steroid levels, which underlie the use of GnRH analogs to treat various forms of steroid-dependent cancers (1,2,3). GnRH acts via Gαq-coupled seven-transmembrane (7TM) receptors to stimulate phospholipase C, causing Ca2+ mobilization and protein kinase C activation (1,2,3,4). In the pituitary, expression of GnRH receptors (GnRHRs) is tightly controlled with levels of GnRHR transcripts and protein being subject to both physiological and pharmacological regulation (1,2,3,4,5).
In addition to GnRH, most vertebrates also express GnRH-II ([His5, Trp7, Tyr8]GnRH), and GnRHRs have evolved in parallel with their ligands. Mammalian type I GnRHRs are unique, in that they lack carboxy-terminal tails (C-tails) (3,4). This is of particular interest in light of the roles for C-tails in other 7TM receptors in which stimulation causes homologous receptor desensitization and internalization by mechanisms involving receptor phosphorylation by G protein receptor kinases. This facilitates binding of arrestins, which prevent G protein activation and also target the receptors for internalization. Because 7TM receptors are typically phosphorylated within the C-tail and these structures are implicated in desensitization and internalization (6,7), their absence is thought to explain why type I mammalian GnRHRs do not show agonist-induced phosphorylation, do not bind arrestins, do not rapidly desensitize, and are internalized very slowly (8,9,10,11,12,13). This fact underlines the importance of other functional parameters (synthesis, degradation, and trafficking) in determining the number of receptors available at the cell surface for activation by the membrane-impermeant cognate ligand.
Cell surface expression of human (h) GnRHR is low (compared with other GnRHRs) in heterologous systems, and this apparently reflects structural features including a primate-specific Lys191 (14,15) and the lack of a second glycosylation site near the N terminus (16) as well as the absence of C-tails (11,12,13,17,18,19). These features all reduce cell surface GnRHR levels, and although the mechanisms are largely unknown, recent work has focused on trafficking to the plasma membrane (PM). It is well established that disease can result from mutations that impair protein trafficking, often causing misfolding and failure to meet quality control for exit from the endoplasmic reticulum (ER) (20). At least 10 diseases are linked to 7TM receptor mutations that cause ER retention and for some of these, pharmacological chaperones have been identified that are thought to increase trafficking to the PM (21,22,23,24). For the hGnRHR, the importance of trafficking is illustrated by point mutants that cause hypogonadotropic hypogonadism. Here, a key observation is that a membrane-permeant nonpeptide GnRHR antagonist (2S)-2-[5-[2-(2-axabicyclo[2.2.2]oct-2-yl)-1,1-dimethy-2-oxoethyl]-2-(3,5-dimethylphenyl)-1H-indol-3-yl]-N-(2-pyridin-4-ylethyl)propan-1-amine (IN3) can “rescue” signaling via most of these mutants (14,15,25). This nonpeptide antagonist is thought to aid protein folding into a conformation needed for ER exit and subsequent trafficking to the PM. Interestingly, IN3 also increased signaling via wild-type GnRHR, suggesting that a large proportion of hGnRHRs do not traffic to the PM (25). We have recently used recombinant adenovirus (Ad) to express epitope-tagged hGnRHR in MCF7 breast cancer cells and found, by confocal microscopy and Western blotting, that the majority of receptors were intracellular (26). Using a method based on fluorescent staining and automated microscopy for GnRHR quantification, we found that a remarkably small proportion of hGnRHRs were at the cell surface in MCF7 cells and that this was dependent upon receptor structure (27). Thus, proportional cell surface expression (PCSE) values were much lower for hGnRHRs than for Xenopus laevis (X) GnRHR, and the hGnRHR PCSE was increased by addition of the XGnRHR tail to the full-length hGnRHR (h.XGnRHR). Receptor localization was also dependent upon cellular context because hGnRHR PCSE was lower in hormone-dependent cancer cell lines (MCF7, T47D, PC3, Du145, Ishikawa) than in gonadotrope-derived (αT4 and LβT2) cells. Importantly, we found that the hGnRHR PCSE was greatly increased by the nonpeptide antagonist and that GnRHRs brought to the PM by IN3 were functional, as illustrated by enhancement of agonist effects on [3H]inositol phosphate (IP) accumulation as well as their antiproliferative and proapoptotic effects in MCF7 cells (26,27,28).
An important observation in support of the idea that membrane-permeant GnRHR antagonists act by facilitating ER exit is that membrane-impermeant peptide antagonists do not mimic pharmacological chaperone effects on trafficking. In accord with this, we found that two peptide antagonists [Ac-D2Nal-D4Cpa-D3Pal-Ser-Tyr-d-Cit-Leu-Arg-Pro-d-Ala-NH2 (cetrorelix) and antide] each failed to increase the PCSE of hGnRHRs in MCF7 cells (27) but both did increase the PCSE of the chimeric h.XGnRHRs. Their maximal effects were considerably lower than those of IN3, and no such effect was seen at the hGnRHR, so it was not clear whether this effect was pertinent to normal receptors. Here, we have explored this further using Ad to express hemagglutinin (HA)-tagged GnRHR in HeLa cells and LβT2 cells. Using automated imaging assays we find that peptide antagonists can increase cell surface expression of hGnRHR either alone or by virtue of synergistic interaction with IN3. Whereas IN3 is thought to increase cell surface GnRHR expression by facilitating transport to the PM, the peptide antagonists apparently do so by slowing internalization from the cell surface. Importantly, the peptide antagonist effects are seen in the absence of GnRH and cannot therefore be attributed to inhibition of GnRH binding. Instead, our data suggest that cetrorelix acts as an inverse agonist (rather than a pure antagonist) in terms of receptor internalization and support the potential importance of ligand-biased efficacy at GnRHR, demonstrating its occurrence with therapeutically relevant ligands, in gonadotrope lineage cells and with nontailed type I mammalian GnRHR.
Results
Peptide and nonpeptide GnRHR ligands influence cell surface HA-GnRHR expression in HeLa cells
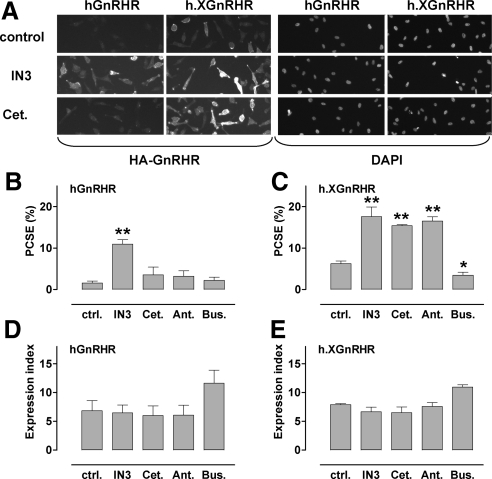
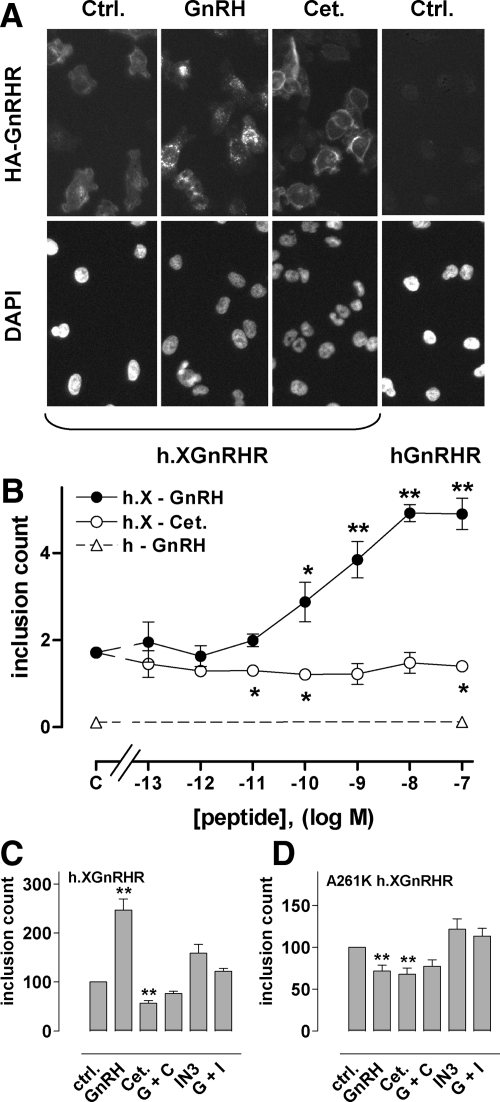
When we compared effects of GnRHR ligands on expression in Ad HA-GnRHR-transduced HeLa cells (Fig. 1A) we found that hGnRHR expression at the cell surface was very low and was increased by a membrane-permeant nonpeptide antagonist (IN3) but not by a peptide antagonist (cetrorelix). Consistent with our earlier work (27,28), quantification revealed that a remarkably small proportion of hGnRHRs were at the cell surface. PCSE was only 1.6%, and this was increased 6-fold (to 11%) by IN3 (Fig. 1B) but was not measurably altered by cetrorelix or by another peptide antagonist (antide) or a peptide agonist [T-BuSer6, Pro9 NH ethylamide-GnRH (buserelin)]. IN3 also increased h.XGnRHR PCSE (from 6.3 to 17.6%), and similar effects were seen with the peptide antagonists (Fig. 1C). None of the compounds had measurable effects on whole-cell expression (Fig. 1, D and E) so PCSE values mirrored cell surface expression (data not shown). In time-course experiments we found that the effects of IN3 on hGnRHR cell surface expression and PCSE were measurable within 2–4 h and maximal after 4–24 h of incubation, and similar time dependence was seen in parallel experiments with h.XGnRHR-expressing cells (data not shown). The peptide antagonists failed to alter the hGnRHR at any time point but in h.XGnRHR-expressing cells the time courses of the peptide antagonist effects were similar to those of IN3 (data not shown).
Figure 1.
Quantification of HA-GnRHR in HeLa cells by automated imaging. HeLa cells transduced with HA-tagged hGnRHR or h.XGnRHR (1 pfu/nl) were incubated 18 h with IN3, cetrorelix (Cet.), antide (Ant.), or buserelin (Bus.) each at (10−6 m), or no addition (control). They were then stained before image acquisition and analysis as described in the Materials and Methods. Panel A contains representative stains from a proportion (∼10%) of fields showing cell-surface HA-GnRHR or DAPI (nuclear) staining in control and IN3- or cetrorelix-treated cells as indicated. Automated algorithms were used to define cell perimeters, EI, and PCSE values. Panels B and C show PCSE values; panels D and E show whole-cell EI values in arbitrary fluorescence units, each pooled from three experiments with triplicate or quadruplicate wells (mean ± sem; n = 3). *, P < 0.05; **, P < 0.01. ctrl., Control.
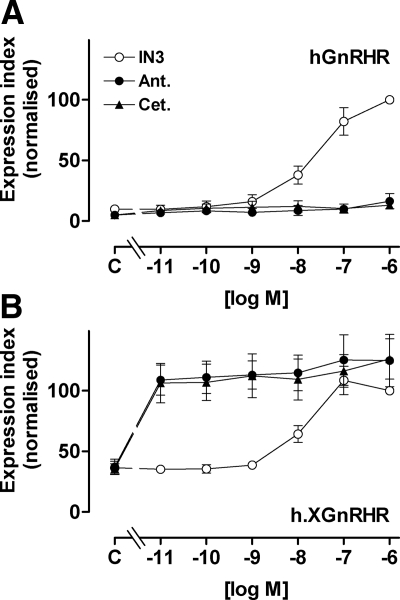
In all experiments in which PCSE values were calculated (Fig. 1 and time course experiments) ligand effects on cell surface expression were paralleled by effects on the proportion of receptors at the cell surface; therefore we determined only cell surface expression for most subsequent experiments. When concentration-response relationships were examined (using 18-h incubations for maximal stimulation) IN3 increased cell surface expression with comparable potencies at hGnRHR and h.XGnRHR (pEC50 values 7.6 ± 0.15 and 7.9 ± 0.17, respectively) (Fig. 2). The two peptide antagonists had no measurable effect on cell surface expression of hGnRHR (Fig. 2A) but caused a pronounced increase in h.XGnRHR expression with pEC50 values of less than 11 and maximal effects comparable to that of IN3 (Fig. 2B).
Figure 2.
Concentration dependence of peptide and nonpeptide antagonists effects on GnRHR expression. HeLa cells were treated as in Fig. 1 except that they were incubated for 18 h with 10−11 to 10−6 m IN3, antide (Ant.), or cetrorelix (Cet.) or without antagonist (C, control) before staining for HA-tagged hGnRHR and h.XGnRHR at the cell surface. The data are cell-surface EI normalized a percent of the value obtained for each receptor in the presence of 10−7 m IN3, and are means ± sems (n = 3–7) from seven experiments, each with triplicate wells. The normalized values were significantly greater than control (P < 0.05) for IN3 at 10−8, 10−7, and 10−6 m (at both receptors) and for all concentrations of peptide antagonists at the h.XGnRHR (panel B), whereas the peptides did not increase expression of the hGnRHR at any concentration (P > 0.05, panel A).
Interaction between peptide and nonpeptide antagonists in regulation of GnRHR expression
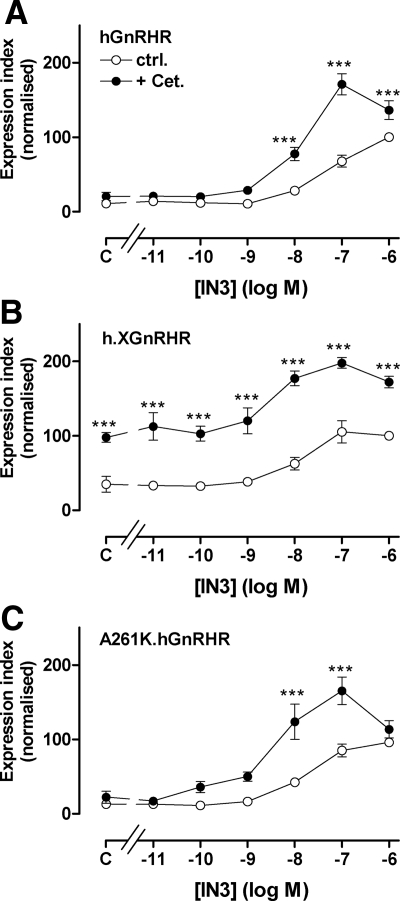
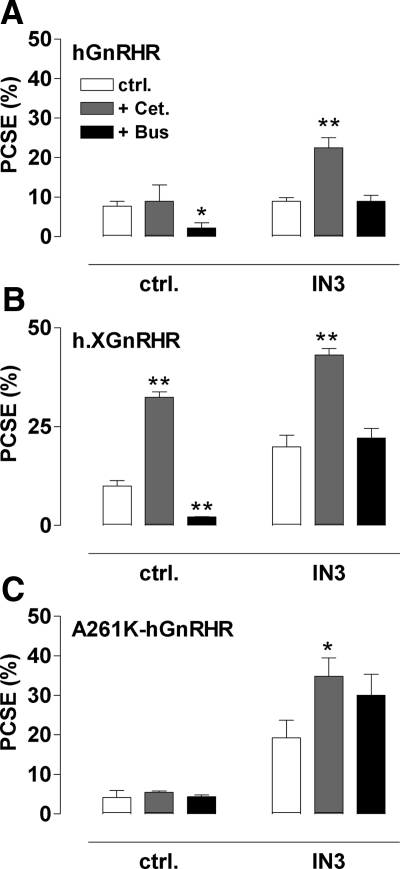
When h.XGnRHR-expressing cells were incubated for 18 h with varied concentrations of IN3 with or without 10−7 m cetrorelix, IN3 caused the expected concentration-dependent increase in cell surface expression of both receptors, and cetrorelix also increased expression of h.XGnRHR but not the hGnRHR (Fig. 3). However, cetrorelix also increased the response to IN3 mediated by both receptors. This effect was most obvious for the hGnRHR (Fig. 3A) in which there was clear synergy between the two antagonists. Thus, the effect of 10−8m IN3 on hGnRHR expression was increased approximately 4-fold by cetrorelix, despite the fact that the peptide had no measurable effect alone. We also assessed whether this synergy was dependent upon signaling using hGnRHR with an A261K mutation that prevents G protein activation (29). As shown (Fig. 3C), IN3 caused a concentration-dependent increase in A261K.hGnRHR expression, and cetrorelix had no effect alone but synergized with IN3 to increase expression at 10−8 and 10−7m IN3. In separate experiments we determined the effects of cetrorelix and buserelin on PCSE (Fig. 4). Consistent with the expression index data, 10−7 m cetrorelix was only able to increase hGnRHR or A261K-hGnRHR PCSE in the presence of IN3 (10−8 m) although it increased h.XGnRHR PCSE in the presence and absence of IN3. Buserelin also caused the expected reduction in h.XGnRHR PCSE but did not alter A261K-hGnRHR PCSE, in accord with earlier work showing that agonist-induced down-regulation of cell surface GnRHR is dependent upon signaling in this model (28).
Figure 3.
Interaction between peptide and nonpeptide antagonist effects on GnRHR expression index. HeLa cells were treated as for Fig. 1 except that they were transduced with HA-tagged hGnRHR (panel A), h.XGnRHR (panel B), or A261K.hGnRHR (panel C) and then incubated 18 h in medium with 10−11 to 10−6 m IN3 or without IN3 (C, control) with 0 (ctrl.) or 10−7 m cetrorelix (Cet.) before staining. The data shown are the cell-surface EI normalized as a percent of the value for each receptor in the presence of 10−6 m IN3 and are means ± sem (n = 3–6) from six experiments, each with triplicate wells. Two-way ANOVAs revealed that IN3, cetrorelix, and the interaction between them were all significant variables with all three receptors (P < 0.001), and post hoc tests revealed significant differences between effects with and without cetrorelix (***, P < 0.001). Two-way ANOVA of data with 0 or 10−7 m cetrorelix data subtracted (i.e. analysis of the IN3-induced increment alone) also revealed that IN3, cetrorelix, and the interaction between them as significant variables (P < 0.001). Synergism between the antagonists is further evidenced by the fact that the increase in hGnRHR expression caused by 10−8 and 10−7m IN3 was greater in the presence of cetrorelix than in its absence for hGnRHR and A261K.hGnRHR (P < 0.05). ctrl., Control.
Figure 4.
Interaction between peptide and nonpeptide antagonist effects on GnRHR PCSE. Cells transduced with hGnRHR, h.XGnRHR, or A261K-hGnRHR (panels A–C, respectively) were treated as in Fig. 3 except that incubations were with 0 (ctrl.) or 10−8 m IN3, either alone (ctrl.) or with 10−7 m cetrorelix (Cet.) or buserelin (Bus.). Whole-cell and cell-surface EI values were determined, enabling calculation of the PCSE values (as above). The data shown are means ± sem (n = 3) from three separate experiments each with triplicate wells. *, P < 0.05; **, P < 0.01 compared with the appropriate receptor and IN3-matched control. ctrl., Control.
HA-GnRHR Internalization
Because we suspected that cetrorelix might increase cell surface GnRHR by slowing internalization, we tested this using a recently described imaging assay (28) in which cell surface HA-GnRHRs are loaded with anti-HA antibody at 21 C and then washed and incubated for 60 min at 37 C to permit internalization. When cells were incubated at 37 C in control medium, h.XGnRHR staining was relatively evenly distributed over the cell surface (Fig. 5A) but when 10−7 m GnRH was present during the 37 C incubation the h.XGnRHR had a more punctuate distribution with puncta often concentrated around the nucleus (Fig. 5A; see also Ref. 28). This redistribution reflects receptor-mediated and agonist-induced internalization into the endosomal pathway because very little staining is seen in cells expressing GnRHR without HA tags (data not shown) and because transferrin colocalizes with GnRHR in many of these puncta (28). To quantify this effect we used an automated imaging algorithm to define the perimeters of the nuclei, the cell body, and also these small intensely stained inclusions. The number of inclusions within the image area defined by the nuclear stain and collar (inclusion count) was used as a measure of GnRHR internalization (28). As expected, we found that the inclusion count in hGnRHR expressing cells was too low for meaningful assessment of ligand effects (because there are too few HA-hGnRHR at the cell surface for anti-HA labeling at 21 C; Fig. 5, A and B), whereas GnRH caused a dose-dependent increase in inclusion count for h.XGnRHR-expressing cells (Fig. 5B; see also Ref. 28). Importantly, we found that cetrorelix also caused significant reductions in inclusion count at concentrations of 10−11, 10−10, and 10−7 m (Fig. 5B). In a separate series of experiments we tested for functional interactions between GnRHR ligands. As expected, h.XGnRHR internalization was increased by 10−7 m GnRH and decreased by 10−7 m cetrorelix (Fig. 5C). Interestingly, IN3 did not reduce the HA-h.XGnRHR inclusion count, and GnRH failed to stimulate internalization in the presence of either antagonist (Fig. 5C). We also tested for dependence on signaling and found that GnRH failed to increase the HA-A261K-h.XGnRHR inclusion count. Indeed, both GnRH and cetrorelix caused modest (but statistically significant) reductions in inclusion count with this construct (Fig. 5D).
Figure 5.
Effects of agonists and antagonists on HA-GnRHR internalization. Panel A, HeLa cells transduced with HA-tagged hGnRHR or h.XGnRHR were loaded with anti-HA at 21 C, and then washed and incubated for 60 min at 37 C in medium with the 0 or 10−7 m GnRH or cetrorelix (Cet.) as indicated (only control cells are shown for HA-hGnRHR). These cells were then fixed, permeabilized, and stained before imaging as described in Materials and Methods. The images shown are from small proportions (∼10%) of representative fields, demonstrating the more punctate distribution of HA-hXGnRHR in GnRH-stimulated cells. This effect was quantified using imaging algorithms to determine the inclusion count (numbers of brightly stained puncta over and around the nucleus) as described in Materials and Methods. Panel B, Cells were transduced, loaded with anti-HA, and then incubated for 60 min at 37 C in medium with the 0 or 10−13 to 10−7 m GnRH or cetrorelix (Cet.) or with no addition (C, control) before image acquisition and analysis as above. The data show means ± sem (n = 3) from three separate experiments each with triplicate wells. HA-hGnRHR inclusion counts were too low for assessment of ligand effects (open triangles, HA-hGnRHR inclusion counts with 0 or 10−7 m GnRH), whereas inclusion counts for the HA-hXGnRHR were increased by GnRH and decreased by cetrorelix. *, P < 0.05; **, P < 0.01 compared with receptor-matched control. Panels C and D, Cells transduced with HA-h.XGnRHR or HA-A261K-h.XGnRHR were loaded with anti-HA and then washed and incubated 60 min at 37 C in medium with the no addition (ctrl.), with 10−7 m GnRH (GnRH or G), 10−7 cetrorelix (Cet. or C), 10−6 m IN3 (I) or with combinations of agonist and antagonist as indicated. They were then imaged and analyzed as above. The data shown are means ± sem (n = 3) from three separate experiments each with triplicate wells. **, P < 0.01 compared with receptor-matched control. Ctrl., Control.
Influence of peptide and nonpeptide antagonists on GnRHR function
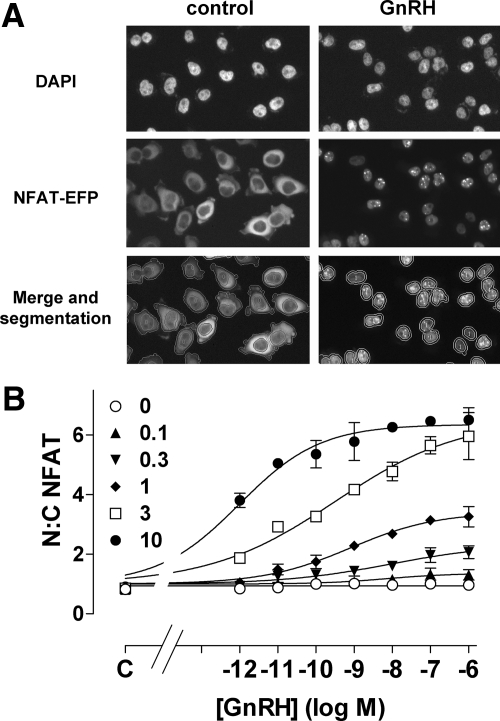
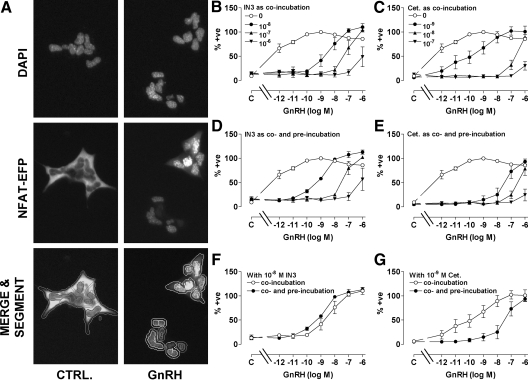
We next explored functional correlates of the antagonist effects on GnRHR expression. To do so we developed an imaging-based readout for GnRHR activation. Nuclear factors of activated T cells (NFATs) are transcription factors that play crucial roles in the immune system (30). They are cytoplasmic in unstimulated cells but, when dephosphorylated by calcineurin, translocate to the nucleus where they regulate transcription. Because calcineurin is activated by calmodulin, the Ca2+/calmodulin/calcineurin-dependent nuclear translocation of NFAT can provide a readout for activation of phospholipase C-coupled 7TM receptors. When HeLa cells were transduced with Ad hGnRHR as well as Ad expressing an NFAT-emerald fluorescent protein (EFP) reporter, the EFP was distributed throughout the cytoplasm. GnRH caused a pronounced increase in the proportion of NFAT-EFP within the nucleus (Fig. 6A) and this effect was maximal within 30–60 min (data not shown). Automated image analysis was used to calculate the nuclear-cytoplasmic (N:C) NFAT-EFP ratio, and this revealed that GnRH caused a dose-dependent increase in N:C NFAT-EFP ratio and that the potency and efficacy of this effect were both dependent upon Ad hGnRHR titer (Fig. 6B). Thus GnRH had greater potency and efficacy in cells transduced with Ad hGnRHR at 10 plaque-forming units/nl (pfu/nl) (EC50 ∼ 10−12 m; maximum increase from 1 to >6) than in cells transduced at 1 pfu/nl (EC50 ∼ 10−9 m; maximum increase from 1–3) and had no measurable effect in control cells receiving no Ad hGnRHR (Fig. 6B). For subsequent experiments we used Ad HA-hGnRHR at 1 pfu/nl and also calculated the proportion of cells in which the N:C NFAT-EFP ratio was more than 1.5. Very few control HeLa cells (0.4 ± 0.3%) had N:C NFAT-EFP ratios of more than 1.5, and this increased to 52.7 ± 2.6% in cells stimulated with 10−7 m GnRH, providing a simple and robust readout for GnRHR activation.
Figure 6.
Influence of GnRH on NFAT-EFP localization. A, HeLa cells were transduced with N-terminal HA-tagged hGnRHR (1 pfu/nl) and with Ad expressing the NFAT-EFP reporter and then cultured 18 h before stimulation for 45 min with 0 or 10−7 m GnRH. The cells were fixed, nuclei were stained (DAPI), and digital images were acquired as described in Materials and Methods. Representative images are shown for control and GnRH-stimulated wells (each <5% of the cells imaged in each field). The lower images illustrate segmentation and the application of a filter to define the proportion of cells in which the N:C NFAT-EFP ratio was less than 1.5 (0) or more than 1.5 (1). B, Cells were treated as above except that GnRH concentration was 10−12 to 10−6 m or 0 (C, control), and Ad HA-hGnRHR titer was 0 or 0.1–10 pfu/nl, as indicated. Each point shows the N:C NFAT-EFP ratio from three separate wells (mean ± sem) from a representative experiment.
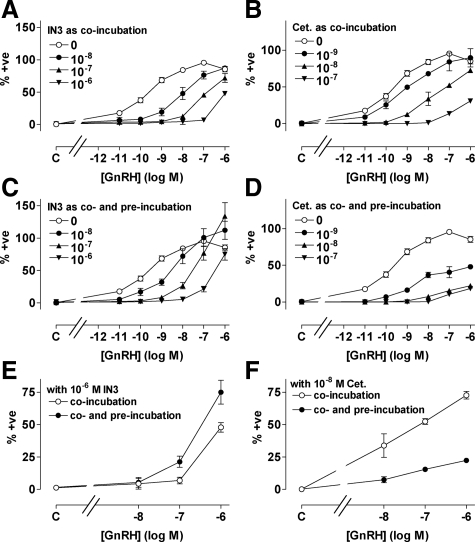
GnRH caused a dose-dependent nuclear translocation of NFAT-EFP with a pEC50 value of 9.8 ± 0.1, and this effect was inhibited by coincubation with IN3 or cetrorelix, both of which shifted GnRH dose-response curves rightward (Fig. 7, A and B). We also constructed GnRH dose-response curves in cells pretreated (18 h) with varied concentrations of IN3 or cetrorelix before stimulation with GnRH still in the presence of the same antagonist (i.e. in cells co- and preincubated with IN3 or cetrorelix). Again, IN3 inhibited GnRH effects, but there was a tendency for IN3 (10−8 and 10−7 m) to increase maximal GnRH effects, and the rightward shift in the GnRH dose-response curves was less pronounced. Consequently, effects of GnRH on NFAT-EFP translocation were lower in cells coincubated with GnRH and IN3 than in cells preincubated with IN3 and then coincubated with agonist and antagonist (compare panels A and C of Fig. 7). These responses are measured under conditions of equal proportional receptor occupancy so the difference is due to the IN3 pretreatment. We also determined the effect of cetrorelix pretreatment and found that its inhibitory effect was more pronounced when provided during the pretreatment and coincubation period, than during the coincubation alone (compare panels B and D of Fig. 7). This distinction between the antagonists is most evident when curves are compared for a single antagonist concentration with or without antagonist pretreatment. Thus, for example, pretreatment with 10−7 m IN3 caused 2- to 4-fold increases in responses to 10−8 to 10−6 m GnRH, demonstrating increased expression of functional GnRHR at the cell surface (Fig. 7E), but the opposite effect is seen with 10−8 m cetrorelix, where pretreatment actually increased the inhibitory effect (Fig. 7F).
Figure 7.
Influence of IN3 and cetrorelix (Cet.) on GnRH-stimulated NFAT-EFP translocation. HeLa cells transduced with HA-tagged hGnRHR (1 pfu/nl) and the NFAT-EFP reporter were incubated 45 min with 10−11 to 10−6 m GnRH or without agonist (C, control) before imaging. Effects of IN3 (left panels) and cetrorelix (right panels) were also determined by coincubation of agonists and antagonists as indicated (panels A and B). GnRH dose-response curves were also generated in cells preincubated (18 h) with the indicated concentrations of IN3 or cetrorelix and then coincubated (45 min) with GnRH and the same antagonist concentration as used in the pretreatment (panels C and D). The effect of the preincubation is illustrated for a single antagonist concentration by comparison of curves with antagonist coincubation alone, and those with co- and preincubation with IN3 (10−7 m; panel E−data from panels A and C) or cetrorelix (10−8 m, panel F−data from panels B and D). The figures show the proportion of cells in which the N:C NFAT-EFP ratio was more than 1.5, normalized (as a percent of the internal control maximal response). Values shown are means ± sems (n = 3–4) from four separate experiments, each with triplicate wells. GnRH responses with antagonist coincubation differed significantly (P < 0.05) from those with antagonist co- and preincubation at 10−8 to 10−6 m GnRH (for both antagonists, panels E and F). +ve, Positive.
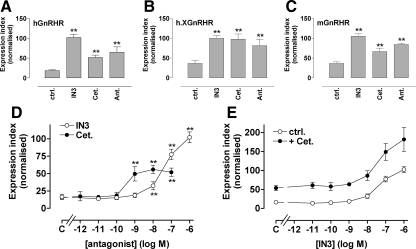
In the final experiments, we assessed whether the peptide and nonpeptide antagonist effects seen in HeLa cells also occurred in LβT2 cells. We have previously shown that approximately 5% of hGnRHR is expressed at the cell surface in Ad hGnRHR-transduced LβT2 cells and that this can be increased to 15% by IN3 (27). Extending this, we found that IN3, cetrorelix, and antide all increased cell surface expression of hGnRHR and h.XGnRHR (Fig. 8, A and B). Because these are murine gonadotrope-derived cells, we also transduced them with Ad expressing HA-tagged mouse (m)GnRHR and again, cell surface expression was found to be increased not only by IN3, but also by cetrorelix and antide (Fig. 8C). In dose-response experiments monitoring effects on hGnRHR expression in LβT2 cells, IN3 had greater efficacy but lower potency than cetrorelix (Fig. 8D). However, the synergistic effect of IN3 and cetrorelix on cell surface hGnRHR expression in HeLa cells (Fig. 3) was not seen in LβT2 cells, in which the antagonist effects were additive (Fig. 8E). We also used NFAT-EFP translocation to monitor GnRHR signaling via the endogenous mGnRHR of LβT2 cells. As shown (Fig. 9), GnRH caused a dose-dependent nuclear translocation of NFAT-EFP, and this effect was inhibited by IN3 and cetrorelix. When coincubated with GnRH, both antagonists shifted GnRH dose-response curves rightward (Fig. 9, B and C). When cells were preincubated with IN3 before incubation with GnRH (in the continued presence of IN3), the dose-response curves were similar to those obtained with GnRH/IN3 coincubation (compare panels B and D of Fig. 9), but when cells were preincubated with cetrorelix before incubation with GnRH (in the continued presence of cetrorelix), the inhibitory effect of cetrorelix was considerably increased (compare panels C and E of Fig. 9). This distinction between the antagonists is most evident when GnRH dose-response curves are compared for single-antagonist concentrations with or without antagonist pretreatment. Thus, for example, pretreatment with 10−8 m IN3 increased the potency of GnRH approximately 4-fold (increasing the pEC50 value from 8.3 ± 0.2 to 8.9 ± 0.1; P < 0.05) whereas cetrorelix pretreatment reduced GnRH potency more than 100-fold (reducing the pEC50 value from 9.7 ± 0.6 to 7.4 ± 0.2, P < 0.01) (Fig. 9, F and G).
Figure 8.
Effects of peptide and nonpeptide antagonists on GnRHR expression in gonadotrope lineage cells. Panels A–C, LβT2 cells transduced with Ad HA-hGnRHR, HA-h.XGnRHR, or HA-mGnRHR were incubated 18 h with 10−7 m IN3, cetrorelix (Cet.), or antide (Ant.) before cell-surface hGnRHR quantification as above. Panels D and E, Cells were treated as above except that they were transduced with Ad HA-hGnRHR and the 18-h incubation was with the indicated concentrations of IN3 or cetrorelix (panel D) or with varied concentrations of IN3 with or without 10−7 m cetrorelix (panel E). Data shown are the cell-surface GnRHR EI and are means ± sems (n = 3–8) pooled from repeated experiments after normalization to the maximal internal control value with IN3. ANOVA with post hoc Dunnett's tests revealed that the effects of all three antagonists were significant at all three receptors (panels A–D; *, P < 0.05; **, P < 0.01). Two-way ANOVA of the data in panel E revealed IN3 and cetrorelix as significant variables (P < 0.01), but the IN3-cetrorelix interaction term was not significant (P > 0.05). ctrl., Control.
Figure 9.
Influence of IN3 and cetrorelix (Cet.) on GnRH-stimulated NFAT-EFP translocation in gonadotroph-lineage cells. LβT2 cells were transduced with the NFAT-EFP reporter (10 pfu/nl) and were stimulated 45 min with the indicated concentrations of GnRH as above (Fig. 7) except that they were not transduced with Ad GnRHR. Panel A shows representative images for control and 10−7 m GnRH-stimulated wells, and the lower images illustrate image segmentation and the application of a filter to define the proportion of cells in which the N:C NFAT-EFP ratio is more than 1.25. Effects of IN3 and cetrorelix were also determined by coincubation of the indicated doses of agonists and antagonists (panels B and C). GnRH concentration-response curves were also generated in cells preincubated (18 h) with the indicated concentrations of IN3 or cetrorelix and then coincubated (45 min) with the indicated concentration of GnRH and the same antagonist concentration as used in the pretreatment (panels D and E). The effect of the preincubation is illustrated for single antagonist concentrations by comparison of curves with antagonist coincubation alone and those with co- and preincubation with 10−8 m IN3 (panel F; data from panels B and D) or 10−9 m cetrorelix (panel G; data from panels C and E). The figures show the proportion of cells in which the N:C NFAT-EFP ratio was more than 1.25, normalized (as a percent of the internal control maximal response). Values shown are means ± sems (n = 3–4) from four separate experiments, each with triplicate wells.
Discussion
Using automated imaging assays to monitor GnRHR localization and function, we have found that only a small proportion of HA-hGnRHRs are located at the cell surface after Ad-mediated transduction in HeLa cells and that this proportion depends upon receptor structure (i.e. is increased by XGnRHR tail addition) and can be increased by IN3 (Figs. 1–3; see also Ref. 28). This nonpeptide antagonist also increased cell surface hGnRHR expression in hormone-dependent cancer cell lines (27) and in gonadotrope lineage LβT2 cells (Ref. 27 and Fig. 8). These data support recent work suggesting that most hGnRHRs are actually intracellular and that IN3 acts as a pharmacological chaperone increasing their number and proportion at the cell surface by increasing their ER exit (14,15,21,22,23,24,25,26,27,28). A key line of evidence for intracellular IN3 action is that its effects are not mimicked by peptide antagonists, and our data with hGnRHRs in MCF7 (27) and HeLa cells (Figs. 1 and 2) are consistent with this. However, we have found that two peptide antagonists increase cell surface h.XGnRHR expression. Because this effect was only seen with the chimera (Ref. 27 and Fig. 1), we initially assumed it was not relevant to wild-type GnRHRs, but we now show that the two peptide antagonists also increase cell surface expression of the hGnRHRs in two cell lines. In HeLa cells this effect was only seen in conjunction with IN3 [i.e. IN3 and cetrorelix acted synergistically (Fig. 3)] but in LβT2 cells, both antide and cetrorelix alone increased cell surface expression of hGnRHR (and mGnRHR). Indeed, cetrorelix was more potent than IN3 at increasing h.XGnRHR expression in HeLa cells and hGnRHR in the murine gonadotrope line (Fig. 8).
Our data reveal that a clinically used peptide antagonist increases cell surface expression of normal (rather than chimeric) human GnRHR, raising the question of mechanism. It presumably acted at the cell surface because GnRH and its peptide analogs do not pass freely through the PM and because cetrorelix and antide did not mimic or block IN3 effects on hGnRHR expression in MCF7 cells or HeLa cells (i.e. the peptides do not access the intracellular site of IN3 action). The additive or synergistic effects of IN3 and cetrorelix on hGnRHR expression also indicate distinct sites of action. For other 7TM receptors (e.g. δ-opioid receptors), agonists accelerate trafficking to the PM (31), and cetrorelix can apparently mimic agonist effects in other systems (32). However, this is an unlikely mechanism for the cetrorelix effect for three reasons. First, cetrorelix is a pure antagonist in many assays (Refs. 33 and 34; see also Figs. 7 and 9). Second, GnRH and the agonist buserelin did not increase cell surface hGnRHRs or h.XGnRHRs whereas cetrorelix and antide did (Figs. 1 and 3, Ref. 28, and data not shown). Third, the A261K mutation that prevents signaling did not prevent the synergistic effects of IN3 and cetrorelix on hGnRHR expression (Fig. 3). An alternative possibility is that the peptide antagonists act at the surface to slow internalization. Using high-throughput imaging (28), we found that agonists stimulate the trafficking, down-regulation, and/or internalization of hGnRHRs and h.XGnRHRs, and now show that cetrorelix actually slows h.XGnRHR internalization. We could not quantify hGnRHR internalization (because expression was too low), but the fact that cetrorelix slows h.XGnRHR internalization and synergizes with IN3 to increase PM expression of both hGnRHR and h.XGnRHR, is consistent with action at the cell surface to slow internalization of hGnRHR as well as h.XGnRHR. In this scenario, peptide antagonists effectively trap GnRHR at the PM so that they are ineffective when cell surface expression is low. This could explain the fact that the peptide antagonists alone have no effect on cell surface HA-hGnRHR expression in MCF7 cells (28) and HeLa cells (Fig. 1) but did increase surface expression in LβT2 cells (Fig. 8) because the number and proportion of HA-hGnRHRs at the cell surface is higher in ligand naïve LβT2 cells than in MCF7 and HeLa cells. Similarly the low cell-surface expression of HA-hGnRHR compared with HA-h.XGnRHR could explain why the peptide antagonists increased expression of the chimeric receptor but not that of the HA-hGnRHR in MCF7 and HeLa cells. It could also contribute to the marked potency of the peptides at increasing PM expression of h.XGnRHRs (Fig. 1) because receptor trapping at the cell surface could have a cumulative effect throughout the 18-h incubation period. However, extrapolation of these possibilities to the in vivo situation is hampered by the fact that the number and proportion of hGnRHR at the cell surface of human gonadotropes is unknown.
An obvious question with our data is whether the HA tag influences receptor function. The lack of validated antibodies prevents comparison of hGnRHR localization with and without tags, but we have found that N-terminal HA tags do not influence affinity, specificity, cell surface expression levels, or potency in radioligand binding and [3H]IP accumulation assays with hGnRHR and h.XGnRHR (Refs. 26,27,28 and data not shown). Moreover, the fact that the A261K mutation prevented agonist-induced HA-GnRHR internalization (Fig. 5) and down-regulation (28) is consistent with the known dependence of GnRHR internalization on signaling (29) and, most importantly, effects of IN3 on HA-GnRHR expression at the cell surface are paralleled by increased functional responses. Here, we have explored function using NFAT-EFP translocation. We know of no previous work showing that GnRH causes NFAT translocation, but the effects were not unexpected because GnRHRs mediate Ca2+ mobilization and calmodulin activation (1,2,3). This would be expected to activate calcineurin, causing NFAT translocation to the nucleus (30). Indeed, an NFAT-dependent transcription reporter has recently been used to characterize GnRHR ligands (35). Here we show that GnRH causes dose-dependent nuclear translocation of NFAT-EFP, and using this as a readout for GnRHR activation, found that pretreatment with 10−7 m IN3 increased hGnRHR-mediated responses to GnRH (Fig. 7E). Because receptor number dictates response amplitude in this assay (Fig. 6), these data clearly show that the IN3 pretreatment increased functional GnRHRs at the cell surface. Similar conclusions were reached in an earlier study in which [3H]IP accumulation was used as a readout for GnRHR activation (27), but the NFAT-EFP assay is much less labor intensive. We were unable to make similar comparisons of hGnRHR expression and function in LβT2 cells because we could not have distinguished between signaling of the transduced hGnRHR and the endogenous mGnRHR. However, GnRH caused NFAT-EFP translocation in these cells and this effect was inhibited by antide and cetrorelix. Moreover, IN3 pretreatment clearly increased endogenous mGnRHR-mediated GnRH NFAT-EFP translocation. This most likely reflects an IN3-induced increase in functional endogenous mGnRHR at the cell surface paralleling its ability to increase hGnRHR and mGnRHR expression in these gonadotrope lineage cells.
When testing whether cetrorelix increases functional GnRHR at the cell surface, we were surprised to find that overnight pretreatment with cetrorelix actually increased its inhibitory effects in both cell types (Figs. 7 and 9). Indeed, when HeLa cells were coincubated with cetrorelix and GnRH, it appeared to behave as a competitive antagonist, shifting the dose-response relationship rightward (Fig. 7B), but with overnight incubation it reduced the maximum response as expected for an irreversible antagonist (Fig. 7D). The reason for this is not clear, but it is unlikely to simply reflect time taken for cetrorelix association with the receptors because very similar data were obtained when cetrorelix effects were compared using 5- and 15-min preincubations (data not shown). An intriguing possibility is that with long-term (i.e. 18 h) cetrorelix incubation, insurmountable antagonism develops (for at least a proportion of hGnRHRs). Irrespective of mechanism, our data reveal a fundamental difference between antagonists; IN3 pretreatment reduced its effectiveness as an inhibitor of GnRH signaling (Figs. 7E and 9F) because it increased cell surface GnRHR expression, whereas the cetrorelix pretreatment increased its inhibitory effect (Figs. 7F and 9G) despite the fact that it can increase cell surface GnRHR expression. This distinction underlines the potential importance of insurmountable antagonism as nonpeptide antagonists are further developed for clinical applications exploiting GnRHR blockade (36,37).
The effects of cetrorelix reported here have broad implications for understanding of GnRHR function. Conventional receptor theory assumes that there are single inactive and active receptor conformations and that antagonists occupy the former, whereas agonists induce or stabilize the latter. It is increasingly recognized, however, that there are multiple active conformations for 7TM receptors, including GnRHR (38,39,40). Indeed, these distinct conformations may be preferentially induced or stabilized by different ligands and may couple differentially to distinct effectors, providing the basis for ligand-directed trafficking of receptor signaling (also known as “ligand-biased efficacy”). Using readouts for type I mammalian GnRHR-mediated Gq/11 activation ([3H]IP accumulation and NFAT-EFP translocation), GnRH and buserelin are full agonists and cetrorelix is thought to be a full antagonist (33,34), influencing receptor function solely by inhibiting agonist effects. In contrast, in the HA-h. XGnRHR internalization assay, GnRH and buserelin are agonists but cetrorelix acts as an inverse agonist, reducing internalization in the absence of GnRH. Most importantly, the peptide antagonist effects reveal the existence of an antagonist-occupied GnRHR conformation at the cell surface that differs from that of the unoccupied receptor. Similar conclusions were reached for h.XGnRHR in MCF7 cells (27), but it was not clear whether this effect was pertinent to normal type I mammalian GnRHR. The data herein show that this functional distinction between unoccupied and cetrorelix-occupied GnRHRs holds true for hGnRHR in HeLa cells (Fig. 3) and for hGnRHRs and mGnRHRs in LβT2 cells (Fig. 8). Thus, we have found evidence for ligand-biased efficacy at GnRHR with therapeutically relevant ligands (cetrorelix and buserelin), normal (i.e. nontailed) receptors, and in gonadotrope lineage cells.
In summary, we show that peptide and nonpeptide antagonists both increase cell surface GnRHR expression in HeLa and LβT2 cells. The fact that IN3 increased GnRHR signaling reinforces the notion that there is a large potentially functional intracellular reserve of GnRHRs. We also show that peptide antagonists increase cell-surface expression of hGnRHR, h.XGnRHR, and mGnRHR (either alone or in synergy with IN3) and that cetrorelix slows h.XGnRHR internalization. Although we could not measure hGnRHR internalization directly, we suggest that a similar mechanism (i.e. slowing internalization) underlies the ability of cetrorelix to increase cell surface hGnRHR expression. These peptide antagonist effects are seen in the absence of GnRH and therefore reveal actions other than inhibition of GnRH binding. Our data reveal that cetrorelix can be an inverse agonist (rather than a pure antagonist) of GnRHR internalization and support the likely importance of ligand-biased efficacy, demonstrating its occurrence with therapeutically relevant ligands, in gonadotrope lineage cells and with nontailed type I mammalian GnRHR. Finally, we observed a clear distinction between the effects of the nonpeptide antagonist in which pretreatment opposes its inhibitory effect (on GnRHR-mediated NFAT-EFP translocation), and a peptide antagonist in which pretreatment enhanced antagonist potency. This distinction may prove to be important for therapeutic applications of GnRH antagonists.
Materials and Methods
Materials and cell culture
Peptides were from Sigma (Poole, UK) except for buserelin and cetrorelix, which were kindly provided by Professor J. Sandow (Aventis Pharma GmbH, Frankfurt am Main, Germany) and Dr. T. Reissmann (ASTA Medica AG, Frankfurt, Germany), respectively. The membrane-permeant indole GnRH antagonist IN3 was from Dr. Ashton Wallace (Merck and Co., Inc., Rahway, NJ). Culture media were from GIBCO (Paisley, Scotland, UK), and plastic ware was from Corning (supplied by Appleton Woods, Birmingham, UK) or Nunc (supplied by Fisher, Loughborough, UK). Sera were from First Link (Brierly Hill, UK), and antibodies were from Invitrogen (Paisley, Scotland, UK) or Cambridge Biosciences (Cambridge, UK). HeLa cells were cultured in DMEM with 10% fetal calf serum, 2 mm l-glutamine, 50 IU/ml penicillin, and 50 μg/ml streptomycin (culture medium). LβT2 cells were maintained in culture medium on Matrigel-treated plates. Matrigel (from BD Bioscience, Lutterworth, UK) was used according to the manufacturer's instructions. For imaging, cells were plated in black-sided and clear-bottomed 96-well plates from Corning (supplied by Appleton Woods). GnRHRs with N-terminal (exofacial) HA tags were expressed in these cells using recombinant, E1-deleted Ad-expressing HA-hGnRHR, HA-mGnRHR, HA-XGnRHR, or HA-h.XGnRHR, prepared as described elsewhere (13,26,27,28,29,30). These were routinely used at 1–3 pfu/nl, although in one experiment Ad HA-hGnRHR titer was varied between 0.1 and 10 pfu/nl. For some experiments cells were also transduced with Ad-expressing NFAT-EFP (2–10 pfu/nl). These were purchased from GE Healthcare, Ltd. (Little Chalfont, UK). In all cases, cells were transduced by infection with Ad (6-h incubation) the day after plating, and assays were then performed within 24 h of transduction.
Quantification of receptor expression
Cell surface and whole-cell HA-GnRHRs were measured by fluorescence microscopy using an automated system for image acquisition (IN Cell Analyzer 1000; GE Healthcare UK Ltd.), and validated algorithms for image analysis (IN Cell Analyzer version 1.0) as described elsewhere (27,28). Briefly, cells were grown in 96-well plates (2500–5000 cells per well), infected with Ad HA-GnRHR (as above), and left 18 h before staining. For cell-surface receptor staining, they were incubated 1 h at 4 C with primary antibody (mouse monoclonal anti-HA-11, clone 16B12, stock at 5–7 mg/ml diluted 1:200 in DMEM with 1% BSA) and then washed with ice-cold PBS, fixed (30 min in 2% paraformaldehyde in PBS), and then permeabilized (10 min in PBS with 0.1% Triton X-100). The cells were then washed (three times), blocked (1 h in PBS with 0.1% Triton X-100 and 1% BSA), and incubated 1 h with the secondary antibody (Alexa Fluor 488 conjugated goat antimouse IgG at 1:500 in PBS with 0.1%Triton X-100 and 1% BSA). They were then washed with PBS, incubated with 0.3 μm 4′,6-diamidino-2-phenylindole (DAPI, 15 min), and washed again before imaging. For whole-cell staining, cells were washed with PBS, fixed, permeabilized, and blocked as above before being exposed to the anti-HA primary antibody. They were then washed with PBS (three times) and incubated with Alexa Fluor 488-conjugated antimouse IgG and DAPI. Digital images were acquired, collecting one to four fields per well with a 10× objective (Plan Apochromat, numerical aperture 0.45) to obtain images of 100-1000 cells (per well) in a total imaged area of 0.6–2.4 mm2. The images were segmented and quantified using the IN Cell 1000 Analyzer software (Dual Area Analysis Algorithm version 1.0). This analysis provided fluorescence intensity in arbitrary fluorescence units per cell and per well. We also defined the proportion of imaged cells expressing measurable HA-GnRHR (cells in which fluorescence was >10% above background) and compounded these values as an expression index (EI). In some cases the EI obtained without permeabilization (cell surface EI) was expressed as a % of the EI with permeabilization (whole-cell EI) to calculate PCSE. Nonspecific labeling was negligible, with these protocols as revealed by the low fluorescence in control cells receiving no Ad or by omission of primary or secondary antibody (data not shown).
Quantification of receptor internalization
Receptor internalization was measured using a recently described imaging assay (28). Briefly, intact HA-GnRHR-transduced HeLa cells were incubated 60 min at 21 C with mouse anti-HA (1:200) in DMEM with 2% fetal calf serum, washed (to remove unbound antibody), and then incubated 60 min at 37 C in DMEM with 2% BSA with the test compounds. They were then washed in ice-cold PBS, after which they were fixed, permeabilized, stained with secondary antibody and DAPI, and then used for image capture and quantification of whole-cell HA-GnRHR expression as above. With this protocol GnRH causes a time-, temperature-, and agonist-dependent increase in HA-GnRHR staining within small punctuate regions that are often concentrated around the nucleus (28). To quantify this redistribution, we used automated software (Dual Area Analysis Algorithm version 1.0; GE Healthcare) to define nuclear perimeters, and then to add a collar of 2 μm around the nucleus and identify the small intensely stained regions as inclusions. For each cell we determined the number of inclusions in the area defined by the nucleus and collar and the mean value for each well (28).
Quantification of receptor signaling
Translocation of NFAT-EFP from the cytoplasm to the nucleus was used as a readout for GnRHR activation. To do so, cells were infected with Ad NFAT-EFP (and Ad HA-GnRHR) as above. After pretreatments, cells were stimulated with GnRH for 45 min and washed, fixed, and permeabilized. Cells were then incubated with 0.3 μm DAPI (15 min) and washed with PBS before imaging. Digital images were acquired as above and analyzed using IN Cell 1000 software (Dual Area Analysis Algorithm) to quantify fluorescence in the nucleus and cytoplasm (background subtracted). The N:C NFAT-EFP ratio was calculated for each cell, and the mean N:C ratio was calculated for each field of view. In several experiments the proportion of cells responding to treatment was also calculated, using a N:C ratio threshold as described in the figure legends.
Statistical analysis and data presentation
The figures show the data (mean ± sem) of three or four wells in single experiments that are representative of at least two experiments, or show data pooled from at least three independent experiments. Where data are normalized for pooling, normalization details are given in the legends. Statistical analysis was by one- or two-way ANOVA and post hoc tests (detailed in legends) accepting P < 0.05 as statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. A. Wallace (Merck and Co. Inc., Rahway, NJ), Professor J. Sandow (Aventis Pharma GmbH, Frankfurt am Main, Germany), and Dr. T. Reissmann (ASTA Medica AG, Frankfurt am Main, Germany) for providing IN3, buserelin, and cetrorelix, respectively, and to Professor P. Mellon (University of California, San Diego, CA) for providing the LβT2 cells.
Footnotes
This work was supported by the Wellcome Trust (Project Grant Awards 062918 and 076557 and Equipment Grant 078407, to C.A.M.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 15, 2009
Abbreviations: Ad, Adenovirus; C-tail, carboxyl-terminal tail; DAPI, 4′6-amidino-2-phenylindole; EFP, emerald fluorescent protein; EI, expression index; ER, endoplasmic reticulum; GnRH, mammalian GnRH; GnRHR, GnRH receptor (prefixed h for human, m for mouse, X for Xenopus laevis type I, and h.X for human GnRHR with added C-tail from Xenopus GnRHR); IN3, (2S)-2-[5-[2-(2-axabicyclo[2.2.2]oct-2-yl)-1,1-dimethy-2-oxoethyl]-2-(3,5-dimethylphenyl)-1H-indol-3-yl]-N-(2-pyridin-4-ylethyl)propan-1-amine; N:C, nuclear-cytoplasmic; NFAT, nuclear factor of activated T-cells; NFAT-EFP, NFAT1c fused with EFP; PCSE, proportional cell surface expression; pfu, plaque-forming units; PM, plasma membrane; 7TM, seven transmembrane.
References
- Conn PM, Huckle WR, Andrews WV, McArdle CA 1987 The molecular mechanism of action of gonadotropin releasing hormone (GnRH) in the pituitary. Recent Prog Horm Res 43:29–68 [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Catt KJ 1995 Expression and signal transduction pathways of gonadotropin-releasing hormone receptors. Recent Prog Horm Res 50:161–205 [DOI] [PubMed] [Google Scholar]
- Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR 2004 Gonadotropin-releasing hormone receptors. Endocr Rev 25:235–275 [DOI] [PubMed] [Google Scholar]
- Cheng CK, Leung PC 2005 Molecular biology of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and their receptors in humans. Endocr Rev 26:283–306 [DOI] [PubMed] [Google Scholar]
- Rispoli LA, Nett TM 2005 Pituitary gonadotropin-releasing hormone receptor: structure, distribution and regulation of expression. Anim Reprod Sci 88:57–74 [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ 2002 Seven transmembrane receptors. Nat Rev Mol Cell Biol 3:639–650 [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ 2002 The role of β-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci 115:455–465 [DOI] [PubMed] [Google Scholar]
- Davidson JS, Wakefield IK, Millar RP 1994 Absence of rapid desensitization of the mouse gonadotropin-releasing hormone receptor. Biochem J 300:299–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle CA, Forrest-Owen W, Willars G, Davidson J, Poch A, Kratzmeier M 1995 Desensitization of gonadotropin-releasing hormone action in the gonadotrope-derived α T3-1 cell line. Endocrinology 136:4864–4871 [DOI] [PubMed] [Google Scholar]
- McArdle CA, Willars GB, Fowkes RC, Nahorski SR, Davidson JS, Forrest-Owen W 1996 Desensitization of gonadotropin-releasing hormone action in αT3-1 cells due to uncoupling of inositol 1,4,5-trisphosphate generation and Ca2+ mobilization. J Biol Chem 271:23711–23717 [DOI] [PubMed] [Google Scholar]
- Heding A, Vrecl M, Bogerd J, McGregor A, Sellar R, Taylor PL, Eidne KA 1998 Gonadotropin-releasing hormone receptors with intracellular carboxyl-terminal tails undergo acute desensitization of total inositol phosphate production and exhibit accelerated internalization kinetics. J Biol Chem 273:11472–11477 [DOI] [PubMed] [Google Scholar]
- McArdle CA, Franklin J, Green L, Hislop JN 2002 Signaling, cycling and desensitization of gonadotrophin-releasing hormone receptors. J Endocrinol 173:1–11 [DOI] [PubMed] [Google Scholar]
- Hislop JN, Everest HM, Flynn A, Harding T, Uney JB, Troskie BE, Millar RP, McArdle CA 2001 Differential internalization of mammalian and non-mammalian gonadotropin-releasing hormone receptors. Uncoupling of dynamin-dependent internalization from mitogen-activated protein kinase signaling. J Biol Chem 276:39685–39694 [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM 2004 Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic 5:821–837 [DOI] [PubMed] [Google Scholar]
- Conn PM, Ulloa-Aguirre A, Ito J, Janovick JA 2007 G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev 59:225–250 [DOI] [PubMed] [Google Scholar]
- Davidson JS, Flanagan CA, Zhou W, Becker II, Elario R, Emeran W, Sealfon SC, Millar RP 1995 Identification of N-glycosylation sites in the gonadotropin-releasing hormone receptor: role in receptor expression but not ligand binding. Mol Cell Endocrinol 107:241–245 [DOI] [PubMed] [Google Scholar]
- Lin X, Janovick JA, Brothers S, Blömenrohr M, Bogerd J, Conn PM 1998 Addition of catfish gonadotropin-releasing hormone (GnRH) receptor intracellular carboxyl-terminal tail to rat GnRH receptor alters receptor expression and regulation. Mol Endocrinol 12:161–171 [DOI] [PubMed] [Google Scholar]
- Blomenröhr M, Heding A, Sellar R, Leurs R, Bogerd J, Eidne KA, Willars GB 1999 Pivotal role for the cytoplasmic carboxyl-terminal tail of a nonmammalian gonadotropin-releasing hormone receptor in cell surface expression, ligand binding, and receptor phosphorylation and internalization. Mol Pharmacol 56:1229–1237 [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Katz A, Sun YM, Lopes J, Illing N, Millar RP, Davidson JS 1998 Contrasting internalization kinetics of human and chicken gonadotropin-releasing hormone receptors mediated by C-terminal tail. J Endocrinol 156:R9–R12 [DOI] [PubMed] [Google Scholar]
- Kopito RR, Ron D 2000 Conformational disease. Nat Cell Biol 2:E207–E209 [DOI] [PubMed] [Google Scholar]
- Bernier V, Lagacé M, Bichet DG, Bouvier M 2004 Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab 15:222–228 [DOI] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier M 2000 Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human δ opioid receptor. J Biol Chem 275:13727–13736 [DOI] [PubMed] [Google Scholar]
- Petäjä-Repo UE, Hogue M, Bhalla S, Laperrière A, Morello JP, Bouvier M 2002 Ligands act as pharmacological chaperones and increase the efficiency of δ opioid receptor maturation. EMBO J 21:1628–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CM, Brady AE, Nickols HH, Wang Q, Limbird LE 2004 Membrane trafficking of G-protein coupled receptors. Annu Rev Pharmacol Toxicol 44:559–609 [DOI] [PubMed] [Google Scholar]
- Conn PM, Knollman PE, Brothers SP, Janovick JA 2006 Protein folding as posttranslational regulation: evolution of a mechanism for controlled plasma membrane expression of a g protein-coupled receptor. Mol Endocrinol 20:3035–3041 [DOI] [PubMed] [Google Scholar]
- Sedgley KR, Finch AR, Caunt CJ, McArdle CA 2006 Intracellular gonadotropin-releasing hormone receptors in breast cancer and gonadotrope lineage cells. J Endocrinol 191:625–636 [DOI] [PubMed] [Google Scholar]
- Finch AR, Sedgley KR, Caunt CJ, McArdle CA 2008 Plasma membrane expression of GnRH receptors: regulation by antagonists in breast, prostate, and gonadotrope cell lines. J Endocrinol 196:353–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch AR, Caunt CJ, Armstrong SP, McArdle CA 2009 Agonist-induced internalization and down-regulation of gonadotropin releasing hormone receptors. Am J Physiol Cell Physiol 291:C591–C600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myburgh DB, Millar RP, Hapgood JP 1998 Alanine-261 in intracellular loop III of the human gonadotropin-releasing hormone receptor is crucial for G-protein coupling and receptor internalization. Biochem J 331:893–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F 2005 NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol 84:488–499 [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Guan JS 2006 Role of delivery and trafficking of δ-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol Sci 27:324–329 [DOI] [PubMed] [Google Scholar]
- Emons G, Müller V, Ortmann O, Schulz KD 1998 Effects of LHRH analogues on mitogenic signal transduction in cancer cells. J Steroid Biochem Mol Biol 65:199–206 [DOI] [PubMed] [Google Scholar]
- Schally AV, Halmos G, Rekasi Z, Arencibia-Jiminez JM 2001 The actions of luteinizing hormone-releasing hormone agonists, antagonists and cytotoxic analogues on the luteinizing hormone-releasing hormone receptors on the pituitary and tumors. Infertil Reprod Med Clin North Am 12:17–44 [Google Scholar]
- Blithe DL 2001 Applications for GnRH antagonists. Trends Endocrinol Metab 12:238–240 [DOI] [PubMed] [Google Scholar]
- Heitman LH, Ye K, Oosterom J, Ijzerman AP 2008 Amiloride derivatives and a nonpeptide antagonist bind at two distinct allosteric sites in the human gonadotropin-releasing hormone receptor. Mol Pharmacol 73:1808–1815 [DOI] [PubMed] [Google Scholar]
- Betz SF, Zhu YF, Chen C, Struthers RS 2008 Non-peptide gonadotropin-releasing hormone receptor antagonists. J Med Chem 26:3331–3348 [DOI] [PubMed] [Google Scholar]
- Kohout TA, Xie Q, Reijmers S, Finn KJ, Guo Z, Zhu YF, Struthers RS 2007 Trapping of a nonpeptide ligand by the extracellular domains of the gonadotropin-releasing hormone receptor results in insurmountable antagonism. Mol Pharmacol 72:238–247 [DOI] [PubMed] [Google Scholar]
- Caunt CJ, Hislop JN, Kelly E, Matharu AL, Green LD, Sedgley KR, Finch AR, McArdle CA 2004 Regulation of gonadotropin-releasing hormone receptors by protein kinase C: inside out signaling and evidence for multiple active conformations. Endocrinology 145:3594–3602 [DOI] [PubMed] [Google Scholar]
- Maudsley S, Davidson L, Pawson AJ, Chan R, López de Maturana R, Millar RP 2004 Gonadotropin-releasing hormone (GnRH) antagonists promote proapoptotic signaling in peripheral reproductive tumor cells by activating a Gαi-coupling state of the type I GnRH receptor. Cancer Res 64:7533–7544 [DOI] [PubMed] [Google Scholar]
- López de Maturana R, Pawson AJ, Lu ZL, Davidson L, Maudsley S, Morgan K, Langdon SP, Millar RP 2008 Gonadotropin-releasing hormone analog structural determinants of selectivity for inhibition of cell growth: support for the concept of ligand-induced selective signaling. Mol Endocrinol 22:1711–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.