Abstract
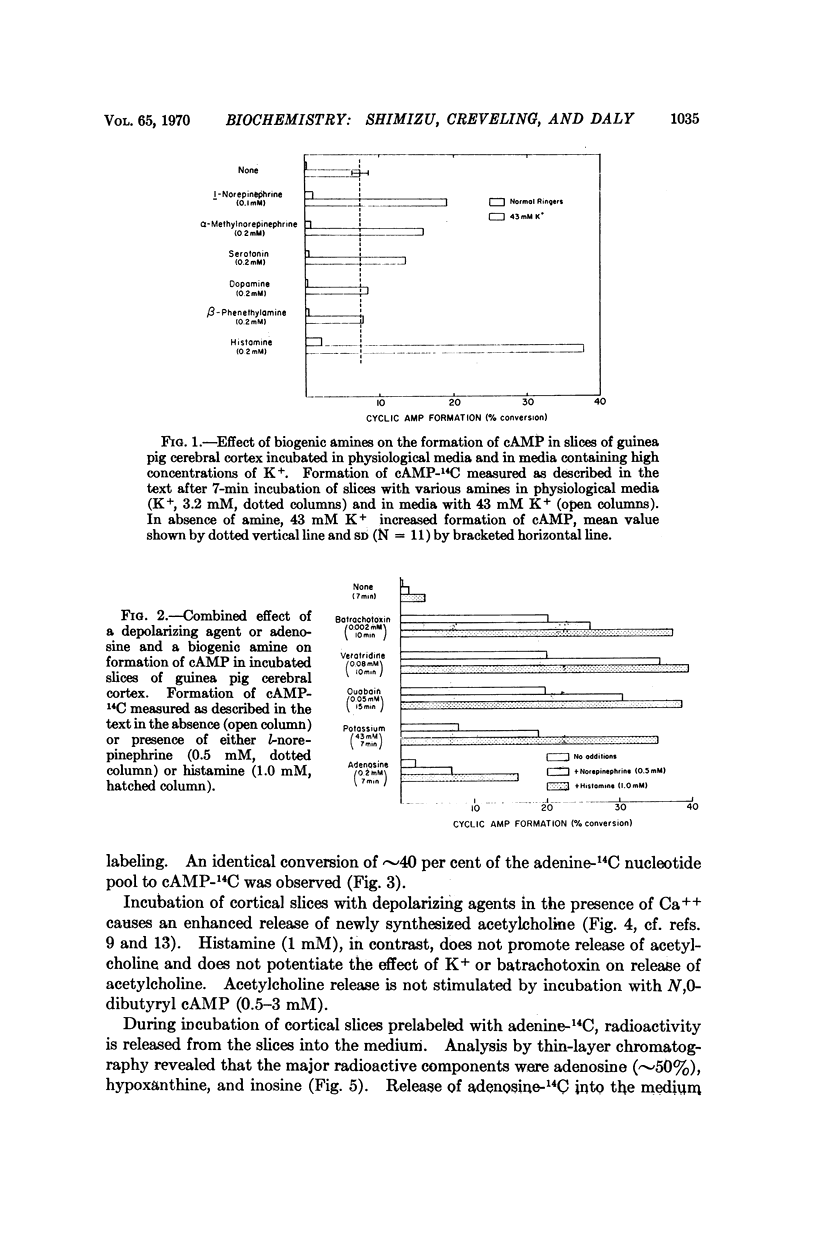
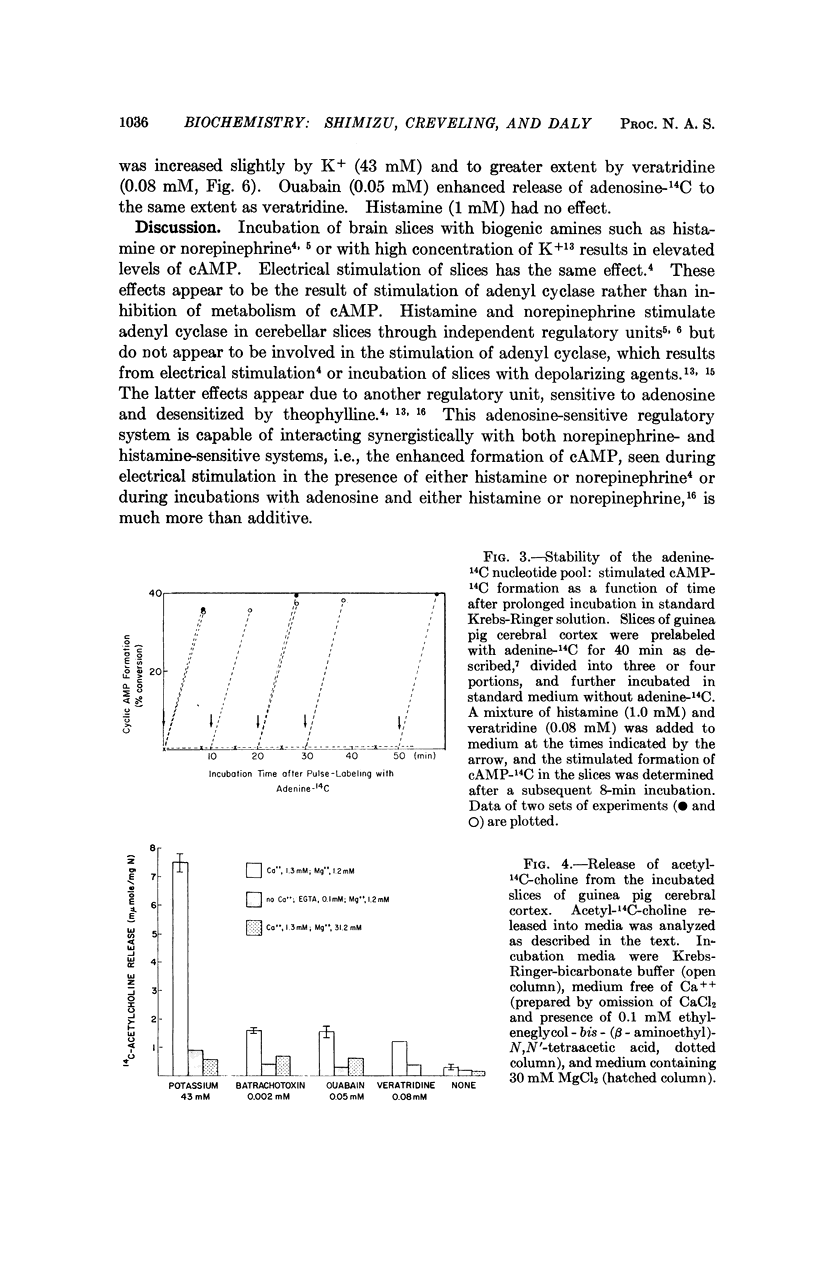
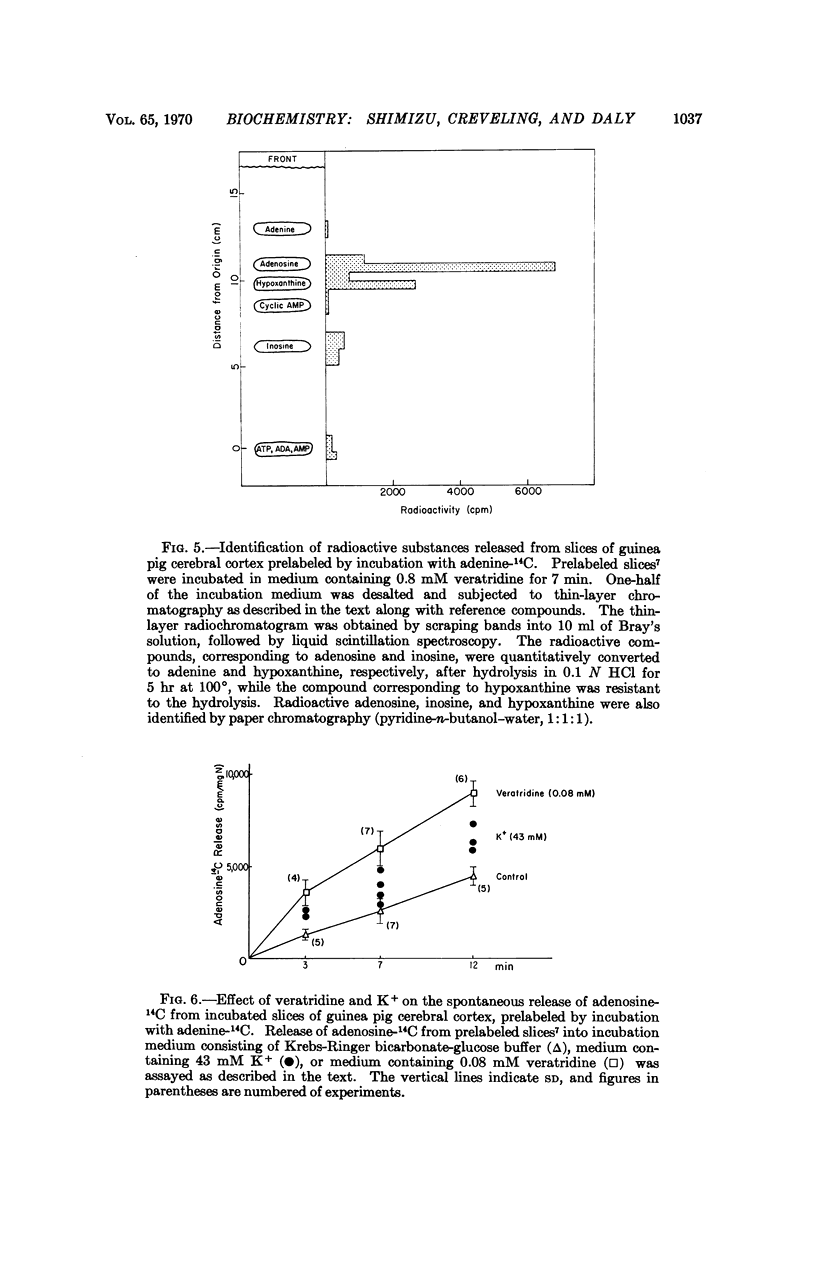
When cortical slices are incubated with adenine-14C, adenine nucleotides are labeled in a small and relatively stable pool. The ATP-14C of this pool is readily converted to cAMP-14C during incubations with depolarizing agents, such as K+, ouabain, veratridine, or batrachotoxin. During incubations with these agents, release of acetylcholine and of adenosine into the medium is enhanced. The increase in release of adenosine parallels the enhanced formation of cAMP-14C elicited by depolarizing agents, providing further evidence that adenosine may serve to couple electrical activity in the central nervous system with formation of cAMP. When adenosine or a depolarizing agent are incubated, together with a biogenic amine, such as histamine, serotonin, or norepinephrine, the combined effect on cAMP-14C formation in cortical slices is much more than additive. Extracellular levels of biogenic amines could in this manner modulate cAMP formation and biochemical responses in nervous tissue during electrical activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BDOLAH A., SCHRAMM M. THE FUNCTION OF 3'5' CYCLIC AMP IN ENZYME SECRETION. Biochem Biophys Res Commun. 1965 Feb 3;18:452–454. doi: 10.1016/0006-291x(65)90730-8. [DOI] [PubMed] [Google Scholar]
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Browning E. T., Schulman M. P. (14C) acetylcholine synthesis by cortex slices of rat brain. J Neurochem. 1968 Dec;15(12):1391–1405. doi: 10.1111/j.1471-4159.1968.tb05921.x. [DOI] [PubMed] [Google Scholar]
- Dudley H. W. Observations on acetylcholine. Biochem J. 1929;23(5):1064–1074. doi: 10.1042/bj0231064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin V. G. Enhancement of brain glutamate dehydrogenase activity and glutamate oxidation by adenine nucleotides. Mol Pharmacol. 1969 Nov;5(6):615–624. [PubMed] [Google Scholar]
- Fleischer N., Donald R. A., Butcher R. W. Involvement of adenosine 3',5'-monophosphate in release of ACTH. Am J Physiol. 1969 Nov;217(5):1287–1291. doi: 10.1152/ajplegacy.1969.217.5.1287. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Singer J. J. Evidence for a role of cyclic AMP in neuromuscular transmission. Proc Natl Acad Sci U S A. 1969 Sep;64(1):134–141. doi: 10.1073/pnas.64.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P., Rudolph S. A., Sturtevant J. M. Enthalpy of hydrolysis of the 3' bond of adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate. J Biol Chem. 1969 Sep 10;244(17):4798–4800. [PubMed] [Google Scholar]
- Kakiuchi S., Rall T. W., McIlwain H. The effect of electrical stimulation upon the accumulation of adenosine 3',5'-phosphate in isolated cerebral tissue. J Neurochem. 1969 Apr;16(4):485–491. doi: 10.1111/j.1471-4159.1969.tb06847.x. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Rall T. W. The influence of chemical agents on the accumulation of adenosine 3',5'-Phosphate in slices of rabbit cerebellum. Mol Pharmacol. 1968 Jul;4(4):367–378. [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- Miyamoto E., Kuo J. F., Greengard P. Adenosine 3',5'-monophosphate-dependent protein kinase from brain. Science. 1968 Jul 4;165(3888):63–65. [PubMed] [Google Scholar]
- Rasmussen H., Tenenhouse A. Cyclic adenosine monophosphate, CA++, and membranes. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1364–1370. doi: 10.1073/pnas.59.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Shimizu H., Daly J. W., Creveling C. R. A radioisotopic method for measuring the formation of adenosine 3',5'-cyclic monophosphate in incubated slices of brain. J Neurochem. 1969 Dec;16(12):1609–1619. doi: 10.1111/j.1471-4159.1969.tb10360.x. [DOI] [PubMed] [Google Scholar]
- Siggins G. R., Hoffer B. J., Bloom F. E. Cyclic adenosine monophosphate: possible mediator for norepinephrine effects on cerebellar Purkinje cells. Science. 1969 Sep 5;165(3897):1018–1020. doi: 10.1126/science.165.3897.1018. [DOI] [PubMed] [Google Scholar]
- Simpson L. L. The role of calcium in neurohumoral and neurohormonal extrusion processes. J Pharm Pharmacol. 1968 Dec;20(12):889–910. doi: 10.1111/j.2042-7158.1968.tb09672.x. [DOI] [PubMed] [Google Scholar]
- Turtle J. R., Littleton G. K., Kipnis D. M. Stimulation of insulin secretion by theophylline. Nature. 1967 Feb 18;213(5077):727–728. doi: 10.1038/213727a0. [DOI] [PubMed] [Google Scholar]


