Abstract
Low perceived social acceptance is a significant risk factor for emotional difficulties in children. No studies, however, have examined genetic factors that may underlie individual differences in perceived social acceptance. In the present study we examined the relation between polymorphisms on the catechol-O-methyltransferase (COMT) Val158Met and serotonin transporter promoter (5-HTTLPR) genes and perceived social acceptance in 103 adolescent girls. Only the COMT polymorphism was related to perceived social acceptance: Val-allele carriers reported greater perceived social acceptance than did homozygous Met-allele carriers. In a subsample of these participants, homozygous Val-allele carriers reported greater maintenance of positive emotions during stress. This, in turn, predicted social acceptance, suggesting that COMT exerts its effects on social functioning through emotion regulation. These data are the first to show an association between COMT and social functioning in children. Future research might profitably examine emotion regulation as a mediator between COMT and social acceptance.
Introduction
Anyone who remembers middle-school is aware of the significant emotional consequences of perceived popularity among one's peers. Indeed, low perceived social acceptance in youth is associated with increased depressive symptoms and diagnosed depression (Cole et al. 1996; Kistner et al. 1999; Brendgen et al. 2002), as well as with social anxiety (Teachman and Allen 2007). Despite the wealth of evidence linking children's perception of their social status with emotional well-being, we know much less about the role that genes play in the origins of perceived social acceptance. Significant advances have been made in our understanding of the genetic contributions to constructs that are critical to social functioning, such as shyness (Arbelle et al. 2003; Battaglia et al. 2005; Fox et al. 2005) and behavioral inhibition (Fox et al. 2005). No studies, however, have yet investigated the genetic correlates of perceived social acceptance. The current investigation was designed to address this issue by examining the relation between two candidate genes and perceived social acceptance in early adolescents.
The dopaminergic system in particular has been implicated in extraversion (Depue and Collins 1999), which has in turn been related to perceived social acceptance (Jensen-Campbell et al. 2002). Consequently, dopaminergic genes are likely candidates to be associated with perceived social acceptance. One such gene is catechol-O-methyltransferase (COMT), which codes for an enzyme that catabolizes dopamine. A relatively frequent Val158Met polymorphism in the general population, characterized by a methionine (Met) to valine (Val) substitution at codon 158 (Lachman et al. 1996), results in lower levels of COMT enzymatic activity and, consequently, in higher levels of extracellular dopamine, for Met alleles relative to Val alleles (Chen et al. 2004). The Val allele has been found to be associated with decreased corticolimbic reactivity to negative stimuli (Drabant et al. 2006) and increased tolerance of pain via release of endogenous opioids (Zubeita et al. 2007). This ability to regulate emotion conferred by the Val allele may be particularly important for navigating the tumultuous world of adolescent peer relationships (Sroufe 1996).
The second gene we examined for a possible association with perceived social acceptance was the serotonin transporter (5-HTT) gene. A polymorphism on the promoter region of the 5-HTT gene (5-HTTLPR) results in two possible alleles–a short and a long allele. The presence of a short allele has been found to result in reduced serotonin reuptake (Lesch et al. 1996). The short allele has also been associated with increased vulnerability to depression (Caspi et al. 2003), with the presence of anxiety-related personality traits (Lesch et al. 1996), and with increased amygdala responses to negative stimuli (Hariri et al. 2002). Potentially relevant to perceived social acceptance, 5-HTTLPR has also been found to be associated in children with hypothalamic–pituitary–axis (HPA) reactivity to a social stressor (Gotlib et al. 2008), as well as with shyness and behavioral inhibition (Arbelle et al. 2003; Battaglia et al. 2005; Fox et al. 2005), although these latter findings are mixed with respect to whether increased shyness is associated with the short (Battaglia et al. 2005; Fox et al. 2005) or the long (Arbelle et al. 2003) allele.
In Study 1a we examined the association between polymorphisms on the COMT and 5-HTTLPR genes and perceived social acceptance in early adolescent girls. We hypothesized that because of its relation with emotional regulation, the Val allele of the COMT gene would be associated with greater perceived social acceptance than would the Met allele. We further hypothesized that because of its association with shyness and biological reactivity to social stressors, the short allele of the 5-HTTLPR would be associated with lower perceived social acceptance than would the long allele.
Study 1a
Methods
Participants
Participants were 103 girls aged 9–14 (M =12.18 years, standard deviation [SD] = 1.53) who, with their mothers, were recruited to participate in a larger project on how people process information (cf. Joormann et al. 2007) through advertisements posted in numerous locations within the local community. To be eligible to participate, the daughters had to have no current or past axis I disorder. Furthermore, the girls' biological mothers had to meet either of two broad criteria: (1) Have no current or past axis I disorder (low-risk daughters, n = 64); or (2) have a history of recurrent episodes of major depressive disorder (MDD) during their daughter's lifetime but no current depression (high-risk daughters, n = 39). Additional details concerning recruitment and assessment of depression and psychopathology are presented elsewhere (Joormann et al. 2007). This study was conducted with the approval of the Stanford University Institutional Review Board.
Demographic information
The girls reported their age, height, weight, ethnicity, and whether they have had their first period. Of the full sample, 75 girls (72.8%) self-identified as Caucasian, 11 (10.7%) as biracial, 10 (9.7%) as Asian-American, 5 (4.9%) as Hispanic (n = 5; 4.9%), and 2 (1.9%) as African American. A body mass index (BMI) was calculated from the girls' height and weight.
Perceived social acceptance
Perceived social acceptance was assessed with the Harter Self-Perception Profile for Children (SPPC) (Harter 1985), a 36-item scale that consists of five six-item domain-specific subscales (Scholastic Competence, Social Acceptance, Athletic Competence, Physical Appearance, and Behavioral Conduct) and one global measure of Self-Worth. In the present study, we focused on the Social Acceptance subscale (reliability α = 0.72), although we also examined the other subscales (α values = 0.78–0.87) to test the specificity of the effects of genes on social acceptance, given the more general association between self-perceptions and adolescent psychopathology (Cole et al. 1997).
Depressive symptomology
The girls completed the 10-item version of the Children's Depression Inventory (CDI-S) (Kovacs 1985), a well-validated self-report measure of children's depressive symptomatology.
Genotyping
To genotype the daughters, saliva was collected using the Oragene Kit (DNA Genotek, Ottawa, Ontario, Canada), an all-in-one system for the collection, preservation, transportation, and purification of DNA from saliva, which provides high-quality DNA and allows for a high success rate of genotyping (Rylander-Rudqvist et al. 2006).
COMT
To genotype the COMT Val158Met polymorphism (Lachman et al. 1996), DNA was amplified with the primers 5′-CTC ATC ACC ATC GAG ATC AA-3′ and 5′-CCA GGT CTG ACA ACG GGT CA-3′. The resulting 109-bp fragment was digested with NlaIII. Digested bands were detected with ethidium bromide staining after gel electrophoresis (4% agarose).
5-HTT
Oligonucleotide primers flanking the 5-HTT–linked polymorphic region (5-HTTLPR) (Heils et al. 1996) and corresponding to the nucleotide positions −1416 to −1397 (stpr5, 5′-GGC GTT GCC GCT CTG AAT GC) and −910 to −888 (stpr3, 5′-GAG GGA CTG AGC TGG ACA ACC AC) of the 5-HTT gene 5′-flanking regulatory region were used to generate 484-bp or 528-bp fragments. The PCR products were electrophoresed through 5% polyacrylamide gel (acrylamide/bis-acrylamide ratio 19:1) at 60 V for 60 minutes.
Results
Demographic characteristics
The allelic frequencies for both COMT and 5-HTTLPR were in Hardy–Weinberg equilibrium, χ2(2,98) = 2.80 and 1.02, respectively, both p values >0.05 1. (The low- and high-risk daughters did not differ in their COMT, χ2(2,103) = 3.12, p > 0.05, or 5-HTTLPR, χ2(2,103) =5.56, p > 0.05, genotypic distributions.) Neither the COMT nor 5-HTTLPR genotype groups differed on any of the demographic or clinical variables (Table 1). The mean CDI score for the sample was 1.97 (SD = 2.3), well below the recommended cut-off of 8 for possible diagnosable depression (Kovacs 1985).
Table 1.
Demographic Variables by Genotype
| Genotype (n) | Age in years (SD) | Caucasian, % (n) | BMI (SD) | CDI (SD) | Postpubertal, % (n)a |
|---|---|---|---|---|---|
| COMT | |||||
| Val/val (21) | 12.14 (1.62) | 62% (13) | 20.84 (3.51) | 2.05 (2.19) | 50% (10) |
| Val/met (60) | 12.12 (1.55) | 77% (46) | 19.98 (2.97) | 1.84 (2.35) | 51% (21) |
| Met/met (22) | 12.41 (1.40) | 73% (16) | 19.40 (3.20) | 2.23 (2.37) | 53% (9) |
| 5-HTTLPR | |||||
| l/l (23) | 12.39 (1.27) | 87% (20) | 20.47 (2.62) | 2.27 (2.66) | 55% (11) |
| l/s (55) | 12.06 (1.63) | 75% (41) | 19.64 (3.30) | 1.85 (2.42) | 45% (18) |
| s/s (25) | 12.44 (1.45) | 56% (14) | 20.47 (3.18) | 1.96 (1.65) | 61% (11) |
Only 78 of the 103 participants reported their menarche status.
There were no significant genotype differences on any of the demographic variables.
BMI = body mass index; CDI = child depression inventory; COMT = catechol-O-methyltransferase; 5-HTTLPR = serotonin transporter promoter region; SD = standard deviation; n = number.
Genes and social acceptance
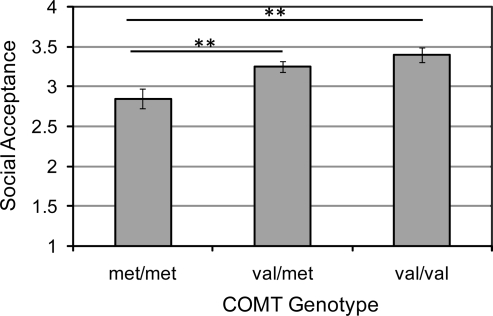
We tested our hypotheses that COMT and 5-HTTLPR would predict differences in perceived social acceptance by conducting one-way analyses of variance (ANOVA) separately for COMT and 5-HTTLPR. As predicted, there was an effect of COMT genotype on perceived social acceptance, F(2,100) = 7.18, p = 0.001, R2 = 0.13 (Fig. 1), which survived Bonferroni correction. (When we included risk status as a factor, COMT still predicted social acceptance, F(2, 97) = 6.94, p = 0.002. The main effect of risk status and the interaction of risk status and COMT on social acceptance both were nonsignificant, F values = 1.4 and 0.36, respectively. Because population stratification is always a concern in gene–behavior studies, we assessed whether Caucasian and non-Caucasian participants differed in their genotypic distributions or scores on perceived social acceptance. Failing to support a population stratification explanation of the data, there were no differences between Caucasian and non-Caucasian participants in their COMT, χ2(2,103) =1.71, p > 0.05, or 5-HTTLPR, χ2(2,103) = 5.98, p > 0.05, genotypic distributions, which were in Hardy–Weinberg equilibrium in both groups, or in perceived social acceptance, t(101) = 0.82, p > 0.05.) Planned two-tailed contrasts revealed that girls who were homozygous for the Met allele reported significantly lower perceived social acceptance than did both heterozygous girls, t(80) = 3.08, p = 0.003, and girls homozygous for the Val allele, t(41) = 3.59, p = 0.001, who did not differ significantly from each other, t(75) = 1.17, not significant (N.S.). To assess the specificity of this effect of the COMT genotype on social functioning, we also examined the effect of the COMT genotype on the other five subscales of the SPPC. The COMT genotype did not predict scores on any of the remaining four self-perception scales or on the self-worth scale, F values = 0.34–1.8, all p values > 0.05.
FIG. 1.
The association between COMT Val158Met polymorphism and perceived social acceptance. Bars are standard errors of the mean. **p < 0.01. COMT = Catechol-O-methyltransferase.
Contrary to predictions, there was no effect of 5-HTTLPR3 on perceived social acceptance, F(2,100) = 0.11, p > 0.05, or any of the other self-perception subscales, F values between 0.13 and 0.88, all p values > 0.05. (A subset of participants was also genotyped for the A/G SNP within the 5-HTTLPR insertion that recent research has shown modifies the L and S alleles [rendering the L alleles with the G SNP to act similarly to the S alleles; Wendland et al. 2006]. Even using this alternative genotyping classification, there was no significant association between 5-HTTLPR and social acceptance.)
Study 1b
In Study 1a, we found a link between the COMT Val158Met polymorphism and perceived social acceptance. Those participants with a Val allele reported greater social acceptance than did participants homozygous for the Met allele. In Study 1b, conducted with a subset of these girls, we further examined this association between COMT and social acceptance by assessing participants' affective responses to a stressor. One prominent predictor of high perceived social acceptance in children is the ability to regulate emotion (Maughan et al. 2007). The COMT Val allele seems to confer an advantage in emotional regulation, as evidenced by decreased reactivity to negative stimuli (Drabant et al. 2006) and increased analgesia to pain (Zubeita et al. 2003). Therefore, we hypothesized that girls with a Val allele who report high social acceptance would exhibit an enhanced regulatory response to a stressor, reflected by decreased negative and/or increased positive emotional responses.
Methods
Participants
Participants were a subsample of the girls from Study 1a (age M = 11.92, standard error [SE] = 0.26) who completed the stressful task and provided emotion ratings. The genotype characteristics of COMT Val158Met of this subsample were met/met = 6, val/met = 21, val/val = 11.
Emotion ratings
Before, during, and after the stressor, participants reported how they felt (from 1 = not at all to 7 = very) using four emotion terms: Happy, stressed, excited, and upset. Happy and excited were averaged to form a positive emotion index, and stressed and upset were averaged to form a negative emotion index.
Procedure
Participants first completed baseline emotion ratings. Next, participants underwent a 15-minute laboratory stress induction, which consisted of two tasks. In the first task, participants completed a serial subtraction task for 3 minutes in which they began at 400 and counted backwards by 7's as quickly and accurately as possible. If they made a mistake, they were told by the experimenter to start over. If participants completed this subtraction task before the 3 minutes were over, they started over at 4000 and counted backwards by 17's. Following this task, participants were administered the 12-minute Ewart Social Competence Interview (SCI) (Ewart et al. 2002), in which participants discussed details of stressful life situations with the experimenter. Immediately following this stress session, participants completed another set of emotion ratings. Following the stressor, participants watched a neutral videotape about Denali National Park for 27 minutes and then completed their final set of emotion ratings.
Results
To test our hypothesis that girls with at least one copy of the COMT Val allele would exhibit decreased negative emotion and/or increased positive emotion during stress, we submitted both the negative and positive emotion ratings to 3 (COMT genotypes val/val, val/met, met/met) × 3 (rating period = prestress, stress, poststress) mixed ANOVAs. The analysis conducted on the negative emotion ratings yielded only a significant main effect of rating period, F(2,70) = 13.92, p < 0.001. Participants reported increased negative emotions from prestress (M = 1.93, SE = 0.13) to stress (M = 2.43, SE = 0.21), t(37) = 2.61, p = 0.013, and decreased negative emotions from stress to post-stress (M = 1.53, SE = 0.12), t(37) = 5.02, p < 0.001. Neither the main effect of COMT nor the interaction of COMT and rating period was significant (F values = 0.12, 0.92, respectively).
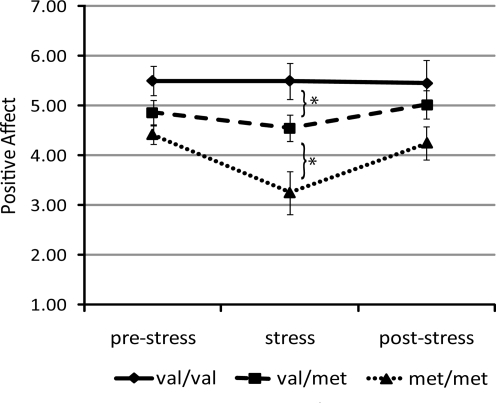
The analysis of the positive emotion ratings also yielded a significant main effect of rating period, F(1.9,70) = 6.90, p = 0.002. In contrast to negative emotion, however, the main effect of COMT was significant in this analysis, F(2,35) = 3.81, p = 0.032; moreover, both these main effects were qualified by a significant interaction of COMT and rating period, F(4,70) = 2.62, p = 0.042. Consistent with our hypothesis, this interaction was characterized by a significant quadratic trend, F(2,35) = 4.17, p = 0.024, but no linear trend, F(2,35) = 0.58, p > 0.05, suggesting that the groups differed significantly in the middle (stress) period (Fig. 2). Indeed, separate univariate ANOVAs (by COMT genotype) of positive emotion ratings at each emotion period revealed that the groups differed in their positive emotional responses during only the stress period, F(2,35) = 6.72, p = 0.003. Participants homozygous for the Val allele reported greater positive emotions during the stress period than did heterozygous Val/Met participants, t(30) =2.07, p = 0.047, who, in turn, reported greater positive emotions than did participants homozygous for the Met allele, t(25) = 2.29, p = 0.03. The three genotype groups did not differ significantly at either the prestress (F[2,35] = 2.51) or posttest (F[2,35] = 1.63) periods, both p values > 0.05.
FIG. 2.
The association between COMT Val158Met polymorphism and positive emotions before, during, and after a laboratory stressor. Bars are standard errors of the mean. *p < 0.05. COMT = Catechol-O-methyltransferase.
Inspection of the data suggests that the difference among the groups during the stress period was due to Val/Val participants maintaining positive emotion throughout the stressor and participants with a Met allele decreasing positive emotion from prestress to stress. The results of within-group ANOVAs for each COMT group conducted on positive emotion ratings across the three rating periods were consistent with this formulation. For Val/Val participants, there was no effect of rating period on emotion ratings, F(2, 35) = 0.02, p > 0.05. In contrast, for participants with a Met allele there were significant effects of rating period on emotion ratings (Val/Met, F(2,35) = 3.41, p = 0.046; Met/Met, F(2,35) = 10.75, p = 0.013). For both of these groups, positive emotions during the stress period were significantly lower than they were during the nonstress periods (pre- and poststress), t values =2.28 and 4.24, both p values < 0.05 for Val/Met and Met/Met, respectively. (Consistent with the null findings from Study 1a, there were no main effects of 5-HTTLPR on either positive, F[2,35] = 1.1, p > 0.05, or negative emotion ratings, F[2,35] = 0.17, p > 0.05. There was also no interaction between 5-HTTLPR genotype and rating period for either positive, F[4,70] = 0.95, p > 0.05 or negative emotion ratings, F[4,70] =0.33, p > 0.05.)
Finally, also supporting our hypothesis, positive emotion during the stress period was positively correlated with perceived social acceptance, r(38) = 0.37, p = 0.02. There was no relation between negative emotion during the stress period and perceived social acceptance, r(38) = −0.20, p > 0.05.
Discussion
The present findings add to the literature documenting associations between genes and social functioning by demonstrating a link between the COMT Val158Met polymorphism and perceived social acceptance in adolescents. A large body of research has established that low perceived social acceptance is a risk factor for emotional difficulties in adolescents (Cole et al. 1996; Kistner et al. 1999; Brendgen et al. 2002; Teachman and Allen 2007). The current study is the first, however, to demonstrate a genetic association with this risk factor. Specifically, adolescent girls who had at least one copy of the COMT Val allele reported greater perceived social acceptance than did girls who were homozygous for the Met allele.
This pattern of results is consistent with the proposed “warrior–worrier” model of the function of the COMT Val158Met polymorphism (Stein et al. 2006). This model proposes that whereas Met-allele carriers exhibit better memory/attention but also increased anxiety (worrier), Val-allele carriers exhibit decreased anxiety and better regulatory responses to negative stimuli (warrior). Extending findings of previous studies that support this model (Zubeita et al. 2007; Drabant et al. 2006), the current study provides evidence that the Val allele is associated with continued positive emotions during stress, whereas the Met allele is associated with diminished positive emotions during stress. This maintenance of positive emotions during stress was also correlated with perceived social acceptance, suggesting that this is a possible pathway through which COMT exerts its effects. Interestingly, COMT did not influence negative emotional responses during stress, suggesting that the difference in positive emotions among participants with the Val and Met alleles was not due to how stressful they perceived the stressor to be, but rather, the degree to which they recruited positive emotions to cope with the stressor. This coactivation of positive and negative emotions during stress for participants with a Val allele is a highly effective coping response (Folkman and Moskowitz 2000) that they may use more broadly to navigate the tumultuous world of adolescent social relationships. More explicit investigation of this and other possible mediating factors will help to elucidate precisely how COMT influences perceived social acceptance.
Although perceived competence is often treated as an aggregate measure of multiple domains of competence, including social, scholarly, athletic, physical, and behavioral domains, there is clearly discriminant validity among measures of competence in these multiple areas (Harter 1982). In fact, social acceptance in particular seems to be related consistently to childhood psychopathology (Kistner et al. 1999; Zimmer-Gembeck et al. 2007). Importantly, in the current study, COMT predicted differences only in perceived social acceptance and not in competence more broadly, thereby highlighting the specificity of the role of COMT in social functioning and documenting the importance of distinguishing perceived social acceptance from other domains of competence. Moreover, it is also important to note that COMT was not related to level of depressive symptomatology, further underscoring the specificity of the association of COMT and social acceptance.
This relation between COMT and social functioning has implications for pharmacological interventions that target the COMT gene. For example, the phasic-tonic dopamine hypothesis predicts that val-allele carriers, who normally have lower performance on tasks requiring sustained cognitive activation, would improve on these tasks with increased extrasynaptic dopamine (Bilder et al. 2004). Indeed, amphetamine has been shown to increase cortical efficiency (i.e., a reduction in brain activation required for the same level of performance) for Val-allele homozygotes on a working memory task (Mattay et al. 2003). Our finding, however, suggests that Val-allele carriers, with their presumably lower levels of extrasynaptic dopamine, exhibit better social functioning than do Met-allele homozygotes. Thus, although pharmacological interventions that increase dopamine levels in Val-allele carriers may have positive effects on cognitive functioning, they may also have detrimental effects on social functioning. Although speculative, the possible adverse effects of such pharmacological treatments on social functioning highlight the importance of assessing this construct in investigations of dopaminergic interventions.
Contrary to our second hypothesis, we found no association between 5-HTTLPR and perceived social acceptance. We had hypothesized that 5-HTTLPR would be associated with social acceptance because of the previously found association between 5-HTTLPR and shyness and behavioral inhibition (Arbelle et al. 2003; Battaglia et al. 2005; Fox et al. 2005). It is important to note, however, that these previous findings are equivocal with respect to whether it was the long or short allele that was associated with shyness. Further, evidence suggests that shyness may be better predicted by an interaction between 5-HTTLPR and maternal social support (Fox et al. 2005), which we did not assess. This null finding could also reflect the inconsistency in the literature about the relation between shyness and social acceptance (Fordham and Stevenson-Hinde 1999). Shyness is only one of many possible factors underlying social acceptance (Hay et al. 2004), and its association with social acceptance is often contingent on other variables, such as age and loneliness (Fordham and Stevenson-Hinde 1999).
The participants in this study were part of a larger project in which adolescent girls are selected based on their vulnerability to psychopathology (i.e., on the presence or absence of recurrent episodes of maternal depression during the daughters' lifetime), with the added criterion that the girls have no current or past diagnosable psychopathology. One advantage of using these selection criteria is that we are able to examine the relation between genes and perceived social acceptance prior to a first onset of diagnosable psychopathology, and without the confound of current symptomatology. Indeed, the majority of participants in our sample reported CDI scores well below the cut off for diagnosable depression, and none had experienced a diagnosable axis I disorder. Moreover, by including high-risk girls in our sample, we have greater power to examine in the future whether these genes interact with risk level to predict the occurrence of psychopathology. At the same time, however, a limitation of this sample is that we excluded adolescent girls with current or past psychopathology, thus limiting our ability to generalize the obtained results to the broader population of adolescent girls. We also did not collect ancestry information from the girls. Although there were no differences between Caucasians and non-Caucasians in their genotypic distributions or their perceived social acceptance, without controlling for possible confounding effects of ancestry we cannot with certainty rule out population stratification as a possible explanation for our findings. Finally, despite the obtained significant relation between COMT and social acceptance, this prescreened sample was relatively small for a gene–behavior association study. Future investigations should replicate these findings in a larger, more heterogeneous sample.
Conclusion
Perceived social acceptance is well documented as both a cause and a consequence of emotional well being. The present results are the first to elucidate aspects of one possible pathway between genes and emotional well being, that involving COMT Val158Met and perceived social acceptance in early adolescence. It will be important in future research to examine other aspects of this pathway, including possible mediating factors between COMT and perceived social acceptance, and the ability of this pathway to predict changes in emotional well being.
Footnotes
This research was supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Affective Disorders (NARSAD) to Jutta Joormann and by a Distinguished Scientist Award from NARSAD and Grant MH074849 from the National Institute of Mental Health to Ian H. Gotlib.
Disclosures
Drs. Waugh, Dearing, Joormann, and Gotlib declare that, except for income received from their primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Acknowledgments
The authors thank Kirsten Gilbert, Yamanda Wright, Joachim Hallmayer, and Lin Xioyang for their help with this study.
References
- Arbelle S. Benjamin J. Golin M. Kremer I. Belmaker RH. Ebstein RP. Relation of shyness in grade school children to the genotype for the long form of the serotonin transporter promoter region polymorphism. Am J Psychiatry. 2003;160:671–676. doi: 10.1176/appi.ajp.160.4.671. [DOI] [PubMed] [Google Scholar]
- Battaglia M. Ogliari A. Zanoni A. Citterio A. Pozzoli U. Giorda R. Maffei C. Marino C. Influence of the serotonin transporter promoter gene and shyness on children's cerebral responses to facial expressions. Arch Gen Psychiatry. 2005;62:85–94. doi: 10.1001/archpsyc.62.1.85. [DOI] [PubMed] [Google Scholar]
- Bilder RM. Volavka J. Lachman HM. Grace AA. The catechol-O-methyltransferase polymorphism: Relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Brendgen M. Vitaro F. Turgeon L. Poulin F. Assessing aggressive and depressed children's social relations with classmates and friends: A matter of perspective. J Abnorm Child Psychol. 2002;30:609–624. doi: 10.1023/a:1020863730902. [DOI] [PubMed] [Google Scholar]
- Caspi A. Sugden K. Moffitt TE. Taylor A. Craig IW. Harrington H. McClay J. Mill J. Martin J. Braithwaite A. Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chen J. Lipska BK. Halim N. Ma QD. Matsumoto M. Melhem S. Kolachana BS T.M. H. Herman MM. Apud J. Egan MF. Kleinman JE. Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DA. Martin JM. Powers B. Truglio R. Modeling causal relations between academic, social competence, depression: A multitrait-multimethod longitudinal study of children. J Abnorm Psychol. 1996;105:258–270. doi: 10.1037//0021-843x.105.2.258. [DOI] [PubMed] [Google Scholar]
- Cole DA. Martin JM. Powers B. A competency-based model of child depression: A longitudinal study of peer, parent, teacher, and self-evaluations. J Child Psychol Psychiatry. 1997;38:505–514. doi: 10.1111/j.1469-7610.1997.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Depue RA. Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Drabant EM. Hariri AR. Meyer-Lindenberg A. Munoz KE. Mattay VS. Kolachana BS. Egan MF. Weinberger DR. Catechol-O-methyltransferase ValÂ1-sup-5-sup-8Met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 2006;63:1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- Ewart CK. Jorgensen RS. Suchday S. Chen E. Matthews KA. Measuring stress resilience, coping in vulnerable youth: The social competence interview. Psycholog Assess. 2002;14:339–352. doi: 10.1037//1040-3590.14.3.339. [DOI] [PubMed] [Google Scholar]
- Folkman S. Moskowitz JT. Positive affect and the other side of coping. Am Psychol. 2000;55:647–654. doi: 10.1037//0003-066x.55.6.647. [DOI] [PubMed] [Google Scholar]
- Fordham K. Stevenson-Hinde J. Shyness, friendship quality, and adjustment during middle childhood. J Child Psychol Psychiatry. 1999;40:757–768. [PubMed] [Google Scholar]
- Fox NA. Nichols KE. Henderson HA. Rubin K. Schmidt L. Hamer D. Ernst M. Pine DS. Evidence for a gene-environment interaction in predicting behavioral inhibition in middle childhood. Psychol Sci. 2005;16:921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Gotlib IH. Joormann J. Minor KL. Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63 doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. Mattay VS. Tessitore A. Kolachana B. Fera F. Goldman D. Egan MF. Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Harter S. The perceived competence scale for children. Child Dev. 1982;53:87–97. [PubMed] [Google Scholar]
- Harter S. Manual for the Self-Perception Profile for Children. Denver (Colorado): University of Denver; 1985. [Google Scholar]
- Hay DF. Payne A. Chadwick A. Peer relations in childhood. J Child Psychol Psychiatry. 2004;45:84–108. doi: 10.1046/j.0021-9630.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- Heils A. Teufel A. Petri S. Stober G. Riederer P. Bengel D. Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Jensen-Campbell LA. Adams R. Perry DG. Workman KA. Furdella JQ. Egan SK. Agreeableness, extraversion, and peer relations in early adolescence: Winning friends and deflecting aggression. J Res Personality. 2002;36:224–251. [Google Scholar]
- Joormann J. Talbot L. Gotlib IH. Biased processing of emotional information in girls at risk for depression. J Abnorm Psychol. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kistner J. Balthazor M. Risi S. Burton C. Predicting dysphoria in adolescence from actual and perceived peer acceptance in childhood. J Clin Child Psychol. 1999;28:94–104. doi: 10.1207/s15374424jccp2801_8. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The children's depression inventory. Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- Lachman HM. Papolos DF. Saito T. Yu YM. Szumlanski CL. Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lesch KP. Bengel D. Heils A. Sabol SZ. Greenberg DB. Petri S. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Mattay VS. Goldberg TE. Fera F. Hariri AR. Tessitore A. Egan M. Kolachana B. Callicott JH. Weinberger DR. Catechol-O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan A. Cicchetti D. Toth SL. Rogosch FA. Early-occurring maternal depression and maternal negativity in predicting young children's emotion regulation and socioemotional difficulties. J Abnorm Child Psychol. 2007;35:685–703. doi: 10.1007/s10802-007-9129-0. [DOI] [PubMed] [Google Scholar]
- Rylander-Rudqvist T. Hakansson N. Tybring G. Wolk A. Quality and quantity of saliva DNA obtained from the self-administered Oragene method: A pilot study on the cohort of Swedish men. Cancer Epidemiol Biomarkers Prev. 2006;15:1742–1745. doi: 10.1158/1055-9965.EPI-05-0706. [DOI] [PubMed] [Google Scholar]
- Sroufe LA. Cambridge, UK: Cambridge University Press; 1996. Emotional development: The organization of emotional life in the early years. [Google Scholar]
- Stein DJ. Newman TK. Savitz J. Ramesar R. Warriors versus worriers: The role of COMT gene variants. CNS Spectrums. 2006;11:745–748. doi: 10.1017/s1092852900014863. [DOI] [PubMed] [Google Scholar]
- Teachman B. Allen J. Development of social anxiety: Social interaction predictors of implicit and explicit fear of negative evaluation. J Abnorm Child Psychol. 2007;35:63–78. doi: 10.1007/s10802-006-9084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland JR. Martin BJ. Kruse MR. Lesch KP. Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11 doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Zimmer-Gembeck MJ. Hunter TA. Pronk R. A model of behaviors, peer relations and depression: Perceived social acceptance as a mediator and the divergence of perceptions. J Social Clin Psychol. 2007;26:273–302. [Google Scholar]
- Zubeita J-K. Heitzig MM. Smith YR. Bueller JA. Xu K. Xu Y. Koeppe RA. Stohler CS. Goldman D. COMT val158met genotype affects mu-opiod neurotransmitter responses to a pain stressor. Science. 2007;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]