Abstract
Objective
This study compared the methylphenidate (MPH) dose–response profiles of children with the Predominantly Inattentive (PI) and Combined (CB) subtypes of attention-deficit/hyperactivity disorder (ADHD). It is the first such study to enroll a sample comprised exclusively of children, all but one of whom had no prior exposure to ADHD medications.
Method
The design was a double-blind crossover with 1-week exposures to placebo and low, medium, and high, fixed, three times daily (t.i.d.) dosage regimens of immediate-release MPH, administered in random order. Parents and teachers completed weekly behavioral questionnaires (Conners, Swanson, Kotkin, Agler, M-Flynn and Pelham Scale [SKAMP]) and a child psychiatrist provided weekly ratings of symptom severity (ADHD Rating Scale [ADHD-RS]), side effects (Side Effects Rating Scale), and a Clinical Global Impressions–Severity (CGI-S). In addition, laboratory measures of vigilance (Continuous Performance Test [CPT]) and resistance to cognitive interference (Stroop) were administered weekly.
Results
Twenty-five children (15 CB, 10 PI), who met rigorous diagnostic criteria for their ADHD subtype, completed the study. Groups did not differ on demographic variables or severity at baseline. Behavioral questionnaires and clinical ratings indicated significant improvement on MPH for both subtypes but no differences in response profiles of the two groups. Drug effects were predominantly linear for both subtypes. Effects of MPH were significant for the CPT, but not the Stroop, instrument with no differences between ADHD subtypes.
Conclusions
Results support the clinical utility of MPH in the treatment of the PI subtype and provide no evidence of differences in response between the subtypes.
Introduction
The Predominantly Inattentive (PI) subtype of attention-deficit/hyperactivity disorder (ADHD) has been recognized in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) since 1994 (American Psychiatric Association 1994). This subtype shares the inattentiveness of the more commonly diagnosed Combined (CB) subtype, but lacks the accompanying hyperactivity–impulsivity. There may be qualitative differences between the subtypes in attention as well in that children with PI are more likely to display sluggish cognitive tempo (Carlson and Mann 2002; Hartman et al. 2004) and have slower processing speed (Chhabildas et al. 2001; Nigg et al. 2002; Solanto et al. 2007). Other phenotypic differences between the subtypes have been well documented. In particular, children with PI are significantly less likely to display other disruptive behaviors (Eiraldi et al. 1997; Willcutt et al. 1999). In addition, children with PI have distinct patterns of social deficits, characterized by greater passivity, lower aggression (Maedgen and Carlson 2000), and less assertiveness (Solanto et al. 2009). Taken together, these differences suggest that children with the PI subtype do not share a deficit in inhibitory control, which has been hypothesized to be critical to the etiology of the CB subtype (Barkley 1997).
Controversy persists concerning whether PI is truly a separate subtype of ADHD, merely a variant in severity of CB, or an entirely different disorder (Lahey 2001; Milich et al. 2001). Investigations of neurocognitive processes have largely failed to identify endophenotypic differences that might account for the observed phenotypic differences between the subtypes. Two neuropsychological studies found no difference between subtypes in executive function, including inhibitory control, after controlling for differences in intelligence quotient (IQ) or in performance on nonexecutive tasks (Geurts et al. 2004; Solanto et al. 2007). Furthermore, a longitudinal follow-up study showed little stability of the subtypes from preschool through elementary school (Lahey et al. 2005).
A key criterion for differentiation between syndromes is unique response to treatment (Cantwell 1996). Despite the fact that stimulants and atomoxetine are widely used to treat PI as well as CB, there has been little research examining the response of the PI subtype to these medications. Two studies failed to find significant differences between the subtypes in response to methylphenidate (MPH) on the major outcome measures. However, one of these used Diagnostic and Statistical Manual of Mental Disorders, 3rd edition (DSM-III) (American Psychiatric Association, 1980) criteria for subtyping (Barkley et al. 1991), and the other compared only the terminal daily dose (1.0 mg/kg) to placebo (Gorman et al. 2006). The sole dose–response study with children diagnosed according to DSM-IV was a crossover trial with weekly switches to placebo or one of three different doses (18, 36, and 54 mg/day) (Stein et al. 2003). Results indicated that participants with PI were more likely to respond optimally at lower doses than were those with CB. However, both children and adolescents were included in unspecified proportions within each subtype, and it is known that children have different dose–response profiles than do adolescents or adults (Wolraich et al. 2005). Furthermore, diagnosis of ADHD was based on a clinical “best estimate” application of DSM-IV criteria, without use of a structured diagnostic interview or specification of inclusion criteria on parent or teacher questionnaires.
The current study was mounted to compare dose–response profiles of children with PI and CB diagnosed using rigorously applied DSM-IV criteria. Predictions for the comparative response of the subtypes were guided by evidence of divergence in the dose-response and time-action functions for behavioral and cognitive effects of stimulants. A review of the stimulant drug treatment literature indicated that effect sizes (d) have on average been larger for behavioral (0.8–1.0) than for cognitive (0.6–0.8) changes (Spencer et al. 1996). Furthermore, a review of studies that conducted hourly monitoring of activity level and cognitive/attentional function on objective measures (Solanto 2002) revealed longer duration of stimulant effects on the former. Given this literature, and given that the deficits of children with PI are largely in the attentional realm, we predicted preferential response to higher MPH doses in this subtype relative to children with CB.
Methods
The study was approved by the Institutional Review Board at the institution where it was conducted. Participants' parents/guardians and teacher-participants gave written informed consent. Children gave oral and written assent following a description in language understandable to children.
Overview of design and procedures
The design was a double-blind crossover, with week-long exposure to placebo and each of three different dosage regimens of immediate-release MPH in randomized order. Parents and teachers completed weekly behavioral questionnaires, and a child psychiatrist examined the child weekly with respect to symptom levels and side effects. Drug effects were also examined on weekly tests of neurocognitive functions.
The participants were a subset of those who participated in a study of neurocognitive functioning in subtypes of ADHD. Evaluation procedures and eligibility criteria were as described in that paper (Solanto et al. 2007), with the exception that children with co-morbid learning disorders were not eligible for that study, but they were eligible for the current study. Eligibility was assessed in four stages: (1) A phone screen, (2) completion of questionnaires by parents and teachers, (3) face-to-face diagnostic interviews of parent and child, and (4) intellectual and achievement testing. Children who met all inclusion and exclusion criteria were scheduled for a baseline evaluation with a study psychiatrist who obtained medical history and confirmed that there were no contraindications for stimulant treatment.
Recruitment
Children with attention problems were recruited via notices placed on websites of lay self-advocacy groups (e.g., Children and Adults with Attention Deficit/Hyperactivity Disorder [CHADD]), circulated to physicians and schools, placed in local newsletters, and posted on bulletin boards within the hospital.
Phone screen and questionnaires
A phone interview with parents was conducted to ascertain that children were within the appropriate age range, were not currently receiving psychotropic medication, had no chronic medical or neurological conditions, spoke English as a first language, had at least 5 of the 9 DSM-IV inattentive symptoms (to err in the direction of inclusion), and lived near enough to complete the study. Parent and teacher rating scales were sent to families meeting phone screen criteria. Those meeting full criteria on questionnaires, as described below, were invited in for the formal evaluation.
Selection criteria
Inclusion
The following stringent inclusion criteria were used to ensure that children could be unambiguously assigned to one of the two ADHD subgroups: (1) Age between 7 and 12 years. (2) Concordant reports on the Conners' Parent Rating Scale (long form) (CPRS) (Conners et al. 1998) and Conners' Teacher Rating Scale (CTRS) (long form) (Conners et al. 1998), as follows: For the CB group, T-scores ≥65 on both the DSM-IV Inattentive and DSM-IV Hyperactive-Impulsive Scales; for the PI group, T-scores ≥65 on the DSM-IV Inattentive Scale, and <65 on the DSM-IV Hyperactive-Impulsive scale. (3) Diagnosis of ADHD, CB or PI, according to a structured diagnostic interview of the parent (Diagnostic Interview Schedule for Children and Adolescents, DSM-IV version) (Shaffer et al. 1996). (4) Expert clinical diagnosis of ADHD, based on a review of all information collected, including a clinical interview of the parent(s) to obtain the history and a semistructured clinical interview of the child.
Exclusion
Children currently receiving psychotropic medication were excluded. In addition, the following exclusion criteria were applied to both groups. (1) Wechlser Intelligence Scale for Children, 3rd edition (WISC-III) Full Scale IQ <80. (2) Presence of mood disorder, Tourette's disorder, or psychotic disorder, established on the basis of the DISC-IV interview and corroborated by results of the open-ended clinical interview. Co-morbid externalizing disorders were not exclusionary. Co-morbid learning disorders, with the exception of pervasive developmental disorder, were not exclusionary. Reading and arithmetic disorders were diagnosed on the basis of either of the following: Significant difference (p ≤ 0.05) between predicted and actual standard scores for the Reading Composite (or, for arithmetic, Math Reasoning) on the Wechsler Individual Achievement Test (WIAT), or a standard score less than or equal to 85 on either of these WIAT indices. (3) Sensory impairment or chronic medical or neurological condition, including asthma, that required systemic medication. (4) Color-blindness, because the Stroop test has colored stimuli, assessed using the Ishihara plates (Ishihara 1960).
Medication
Dosage regimens were the same as those used successfully in the titration trial of the Multimodal Treatment Study of ADHD (Greenhill et al. 1996). Medication was administered in the morning, at midday and at 3 p.m. The low dosage was 5, 5, and 5 mg; the medium dosage was 10, 10, and 5 mg; and the high dosage was 20, 20, and 10 mg. For children weighing less than 25 kg, the high dosage was 15, 15, and 5 mg. The double-blind trial was preceded by an open-label lead-in week, during which the dose was increased from low to medium or medium to high every 2 days to ascertain any significant side effects. The protocol called for immediate discontinuation of children who experienced serious adverse effects during the lead-in (rating of “severe” or above on any side effect, or any hallucinations). For other children, the protocol allowed for extension of the lead-in to a maximum of 2 weeks to allow for accommodation. Children who did not accommodate (i.e., no side effect rated as more than “moderate”) within 2 weeks of extended lead-in were excluded. No child was excluded from the 4-week trial as a result of side effects experienced during the lead-in period.
Study medications were prepared and coded by the hospital pharmacy using identical gelatin capsules for active medication and placebo.
Weekly assessments and instruments
At baseline and the end of each of the four double-blind study weeks, parents and teachers completed the CPRS or CTRS, and the Swanson, Kotkin, Agler, M-Flynn and Pelham Scale (SKAMP) (Swanson 1992). On the Conners, the T-score on the age- and gender-normed DSM-IV Inattentive subscale was the primary outcome measure. On the SKAMP, the primary outcome was the mean score on the 10-item Attention subscale of functional behaviors in home or school, as rated by parent or teacher respectively, on a 7-point scale ranging from 0 (no impairment) to 6 (maximal impairment).
The study psychiatrist met weekly with the child and parent to measure height, weight, and vital signs and complete the Side Effects Rating Scale (SERS) of 11 side effects on a 4-point scale from 0 (none) to 3 (severe) (Greenhill et al. 1996). The psychiatrist also completed the ADHD Rating Scale (ADHD-RS-IV), consisting of the 9 Inattentive and 9 Hyperactive-Impulsive DSM-IV symptoms of ADHD, each rated on the basis of the parent interview on a 4-point scale ranging from 0 (never or rarely) to 3 (very often) (DuPaul 1998; Faries et al. 2001). The patient was also given a single rating, ranging from 1 (“normal/not ill”) to 7 (“among the most extremely ill patients”) on the National Institute of Mental Health (NIMH) Clinical Global Impressions (CGI) scale of severity (National Institute of Mental Health 1985). Teacher ratings for the just-ended week were made available to the psychiatrist to be included in the determination of the CGI.
Laboratory measures
The Continuous Performance Test (CPT) and Stroop test were administered at each weekly visit within 2 hours of the last administered dose. The Conners CPT measures vigilance (omission errors; reaction time [RT] for hits; standard deviation of hit RT; change in RT as a function of changes in interstimulus interval; and d′, the index of “attentiveness” or ability to discriminate target and nontarget stimuli), as well as impulsivity (commission errors; and beta, indexing bias to respond). The CPT Index score is a weighted average of all performance measures on this task, interpreted as follows: Less than 8 is “good,” 8–11 is “borderline,” greater than 11 is “poor” (Conners 1994).
The paper version of the Stroop was administered and scored using the standard procedures (Golden 1978). The primary outcome measure was the Interference score, which is calculated as the difference between the age-corrected raw Color-Word score and the predicted Color-Word score (Golden 1978) and converted to a T-score such that a higher score indicated better performance.
Data analyses
Data were analyzed using SPSS. Baseline scores for the two groups on demographic and outcome measures were compared by t-test. The effects of MPH were analyzed via repeated-measures multivariate analysis of variance analysis (MANOVA) with one within-subjects factor of Treatment with four levels (Placebo, Low, Medium, and High Dosage) and one between-subjects factor of Group with 2 levels (PI and CB). A MANOVA was conducted for the Clinician ratings on the ADHD-RS and CGI. A separate MANOVA was conducted for Parent and Teacher ratings on the Conners and SKAMP. Secondary analyses, comparing baseline and placebo scores by t-test, was conducted to elucidate placebo effects on these ratings.
MANOVAs were conducted for the eleven individual side effects ratings on the SERS and the 8 outcome measures on the CPT. For the neurocognitive tests, there were four levels of Treatment (placebo, low, medium, and high).
All multivariate tests utilized Wilks' Lambda and all within-subjects tests used the Greenhouse–Geisser F statistic after adjustment of the degrees of freedom based on the Mauchly Test of Sphericity. To maximize the likelihood of finding differences between subtypes (reduce Type II error), planned contrasts were conducted for the subtypes separately even in the absence of a significant interaction between Group and Treatment. Post hoc pairwise tests comparing placebo and drug doses were conducted using the method of least significant differences.
Results
Across the four placebo and drug conditions for the 25 children, data points were missing as follows: Parent Conners, 1%; Teacher Conners, 6%; Parent SKAMP, 1%; Teacher SKAMP, 4%; Side Effects, 0%. Missing scores were replaced by the mean of the scores for that group in that condition.
Study sample
The initial participant group included 30 children. Three children were enrolled but discontinued before receiving any medication. Two children began the double-blind portion of the study but did not complete the trial. One of these children, in the PI group, discontinued due to the development of thrombocytopenic purpura during the first double-blind treatment week (which happened to be placebo). The other child, in the CB group, discontinued due to insomnia and loss of appetite during the low-dose condition, which occurred during the first week of the double-blind treatment protocol.
Twenty-five participants (15 CB and 10 PI) completed the study protocol. One 13 year old, who had been evaluated for the neurocognitive study by age 12, but whose participation in the current study was delayed, was admitted to the PI group There were no significant differences between the CB and PI groups in gender (40% male vs. 50%, respectively); mean age (8.53 ± 1.3 vs. 9.20 ± 1.6); minority representation (53% vs. 20%); full-scale IQ (109 ± 15.80 vs. 113 ± 16.17); oppositional defiant disorder (ODD)% (13% vs. 20%), learning disability (LD)% (40% vs. 20%); or percentage with anxiety (0% vs. 10%). Groups were also equivalent in severity at baseline as ascertained on the Parent DSM-IV Inattentive scale (76.46 ± 9.12 vs. 80.29 ± 5.79); the Teacher DSM-IV Inattentive Scale (69.69 ± 9.51 vs. 69.10 ± 4.95); and CGI (4.73 ± 0.70 vs. 4.20 ± 0.63). As intended by the selection criteria, the subtypes were well-separated with respect to ratings of hyperactivity-impulsivity on the DSM-IV Hyperactive-Impulsive scale completed by the Parent (76.62 ± 10.68 vs. 58.43 ± 12.78, t = 3.40, p = 0.003), and by the Teacher (78.15 ± 9.01 vs. 57.00 ± 10.25, t = 5.0, p ≤ 0.001).
Only 1 child in the sample had a history of treatment with medication for ADHD, but at the time of enrollment this child had been off all medication for more than 1 year.
Subgroups did not differ significantly in mg/kg per day of MPH received in the study, which were as follows for the CB and PI groups, respectively: Low, 0.50 ± 0.12 vs. 0.44 ± 0.13; medium, 0.83 ± 0.20 vs. 0.73 ± 0.21; high, 1.54 ± 0.31 vs. 1.40 ± 0.38.
Paired-sample t-tests revealed that reduction in symptoms from baseline to placebo was significant (two-tailed test) for all parent, teacher, and clinician ratings, with the exception of Conners Parent.
Clinician ratings
ADHD-RS and CGI
For the ADHD-RS, scores were incomplete for four cases in the CB subgroup and two in the PI subgroup. CGI ratings were incomplete for 1 case. These scores were not replaced. MANOVA of ADHD-RS Total Score and CGI ratings yielded a robust effect of Treatment (F = 4.81, p < 0.001, partial eta squared ηp2 = 0.224), as well as a significant effect of Group (F = 3.71, p = 0.047, ηp2 = 0.317), due to worse scores in the CB subgroup. There was no interaction between Group and Treatment. Post hoc univariate tests (Table 1) show a highly significant effect of Treatment on ADHD-RS Total score and significant effect on CGI severity, due in both cases to the linear effect of drug treatment. Pairwise comparisons (Table 2) show significant differences between each dose and placebo, but no significant differences between doses.
Table 1.
Post Hoc Univariate Main Effects, Interactions, and Contrasts for Effects of Group and Treatment
| |
Effects for combined sample and for subtypes analyzed separately (F-values) |
Treatment effect contrasts (F-Values) |
|||||
|---|---|---|---|---|---|---|---|
| Measure | Group | Treatment | Group × treatment | Partial eta squared (ηp2) for treatment effect | Linear | Quadratic | Cubic |
| ADHD-RS Total | 7.62b | 9.94d | 0.22 | 0.369 | 20.60d | 2.29 | 3.44a |
| Combined | 5.88c | 0.370 | 15.96c | 1.36 | 0.67 | ||
| Inattentive | 4.51b | 0.392 | 6.63b | 1.46 | 2.58 | ||
| CGI Severity | 5.66b | 3.35b | 1.05 | 0.165 | 7.73b | 0.53 | 1.08 |
| Combined | 2.08 | 0.172 | 7.31b | 0.74 | 0.24 | ||
| Inattentive | 2.12 | 0.232 | 2.26 | 0.05 | 3.72a | ||
| Conners' Parent IN | 4.30b | 4.60c | 0.54 | 0.167 | 10.03c | 0.11 | 1.79 |
| Combined | 3.03a | 0.178 | 5.86b | 0.36 | 0.17 | ||
| Inattentive | 2.05 | 4.51a | 0.51 | 1.38 | |||
| Conners' Teacher IN | 0.47 | 1.14 | 0.03 | 0.047 | 5.56b | 0.12 | 0.00 |
| Combined | 0.74 | 0.050 | 3.37a | 0.17 | 0.00 | ||
| Inattentive | 0.46 | 2.27 | 0.10 | 0.00 | |||
| SKAMP Parent IN | 4.36b | 4.53b | 1.63 | 0.164 | 7.48b | 1.84 | 0.60 |
| Combined | 1.40 | 0.091 | 0.78 | 2.76 | 1.99 | ||
| Inattentive | 4.36b | 9.38b | 0.24 | 0.03 | |||
| SKAMP Teacher IN | 0.47 | 4.66c | 0.09 | 0.168 | 9.89c | 0.49 | 0.44 |
| Combined | 2.75a | 0.164 | 5.43b | 0.51 | 0.74 | ||
| Inattentive | 2.35 | 5.12b | 0.10 | 0.02 | |||
| Side Effects (Total Score) | 1.29 | 6.21c | 0.51 | 0.213 | 6.96b | 12.03c | 0.35 |
| Combined | 3.98b | 0.221 | 4.84b | 5.12b | 1.77 | ||
| Inattentive | 3.77b | 0.295 | 4.80a | 9.58b | 0.46 | ||
| Stroop–Interference Score | 1.77 | 0.61 | 0.40 | 0.031 | 1.25 | 0.24 | 0.59 |
| Combined | 0.74 | 0.058 | 0.18 | 1.42 | 0.27 | ||
| Inattentive | 0.34 | 0.046 | 1.23 | 0.03 | 0.26 | ||
| CPT–Index Score | 0.27 | 4.23c | 0.40 | 0.174 | 8.17b | 0.01 | 1.19 |
| Combined | 1.95 | 0.140 | 3.26a | 0.76 | 0.24 | ||
| Inattentive | 2.84a | 0.262 | 5.99b | 0.38 | 1.00 | ||
| CPT–Hit RT | 0.11 | 3.05a | 0.32 | 0.132 | 5.31b | 0.63 | 1.74 |
| Combined | 2.38 | 0.165 | 4.07a | 0.05 | 1.92 | ||
| Inattentive | 1.26 | 0.136 | 2.25 | 1.11 | 0.34 | ||
| CPT–Hit RT SE | 0.02 | 9.44c | 1.18 | 0.321 | 16.16c | 0.62 | 0.76 |
| Combined | 10.12c | 0.457 | 15.55c | 0.61 | 3.58a | ||
| Inattentive | 1.97 | 0.198 | 3.76a | 0.15 | 0.19 | ||
| CPT–Omission | 0.40 | 9.21d | 1.32a | 0.315 | 24.98d | 0.07 | 0.00 |
| Combined | 7.12c | 0.372 | 17.33c | 1.89 | 0.03 | ||
| Inattentive | 4.26b | 0.347 | 10.32b | 1.08 | 0.02 | ||
| CPT–Commission | 0.09 | 2.33a | 0.68 | 0.105 | 5.79b | 0.05 | 0.21 |
| Combined | 1.84 | 0.133 | 3.62a | 0.63 | 0.04 | ||
| Inattentive | 1.35 | 0.144 | 2.98 | 0.71 | 0.73 | ||
| CP–Attentiveness (d') | 0.13 | 9.09d | 0.53 | 0.312 | 22.07d | 1.31 | 0.84 |
| Combined | 5.25c | 0.304 | 10.18c | 0.06 | 0.00 | ||
| Inattentive | 4.39b | 0.355 | 17.63c | 1.12 | 2.62 | ||
| CPT–Beta | 0.73 | 11.30d | 0.08 | 0.361 | 21.76d | 0.94 | 1.11 |
| Combined | 7.10c | 0.372 | 12.41c | 1.08 | 0.31 | ||
| Inattentive | 4.80b | 0.375 | 10.77b | 0.16 | 0.72 | ||
| CPT–Hit RT ISI Change | 0.04 | 3.40b | 1.11 | 0.145 | 4.39b | 3.95a | 0.36 |
| Combined | 3.87b | 0.244 | 3.86a | 7.67b | 1.19 | ||
| Inattentive | 0.68 | 0.078 | 1.53 | 0.12 | 0.16 | ||
Results in boldface are for the total sample. Subgroups were also tested separately in planned contrasts. Within-subjects tests used the Greenhouse–Geisser F-statistic.
p < 0.10.
p < 0.05.
p < 0.01.
p < 0.001.
Abbreviations: ADHD-RS = ADHD Rating Scale; CGI = Clinical Global Impressions; IN = Inattention subscale; SKAMP = Swanson, Kotkin, Agler, M-Flynn and Pelham Scale; CPT = Continuous Performance Test; RT = reaction time; SE = standard error; ISI = interstimulus interval.
Table 2.
Means and Pairwise Comparisons for Behavioral Measures and Side Effects
| |
Mean (SD) |
||||
|---|---|---|---|---|---|
| Measure | Baseline | Placebo | Low | Medium | High |
| ADHD-RS Total Score | 33.21 (9.69) | 26.58 (11.39)a | 18.63 (10.45)b | 18.21 (10.34)b | 15.21 (9.21)b |
| Combined | 37.55 (6.38) | 30.64 (10.10) | 23.18 (9.65) | 21.36 (10.25) | 19.55 (8.95) |
| Inattentive | 27.25 (10.65) | 21.00 (11.24) | 12.38 (8.38) | 13.88 (9.36) | 9.25 (5.78) |
| CGI Severity | 4.52 (0.71) | 4.00 (1.12)a | 3.44 (1.16)b | 3.28 (1.10)a,b | 3.08 (0.81)b |
| Combined | 4.73 (.70) | 4.2 (1.15) | 3.87 (.92) | 3.4 (.99) | 3.4 (.74) |
| Inattentive | 4.20 (.63) | 3.7 (1.06) | 2.80 (1.23) | 3.10 (1.29) | 2.60 (.70) |
| Conners' Parent IN | 77.99 (8.05) | 65.80 (13.98)a | 61.64 (10.39)a | 61.56 (11.84)a | 56.96 (12.41)b |
| Combined | 76.46 (9.12) | 69.20 (13.44) | 64.93 (10.89) | 63.27 (12.39) | 61.07 (13.44) |
| Inattentive | 80.29 (5.79) | 60.70 (13.84) | 56.70 (7.62) | 59.00 (11.10) | 50.80 (7.70) |
| Conners' Teacher IN | 69.46 (7.87) | 63.28 (10.55)a | 61.52 (7.98)a,b | 60.48 (6.55)a,b | 60.04 (10.57)b |
| Combined | 69.69 (9.51) | 64.13 (10.74) | 62.13 (7.17) | 61.20 (6.04) | 61.07 (10.60) |
| Inattentive | 69.10 (4.95) | 62.00 (10.71) | 60.60 (9.38) | 59.40 (7.44) | 58.50 (10.89) |
| SKAMP Parent IN | 2.78 (1.10) | 1.97 (1.34)a | 1.55 (1.18)a,b | 1.53 (1.32)b | 1.39 (1.42)b |
| Combined | 2.77 (1.14) | 2.26 (1.34) | 1.80 (1.25) | 2.00 (1.47) | 1.90 (1.58) |
| Inattentive | 2.79 (1.11) | 1.54 (1.30) | 1.17 (0.98) | 0.81 (0.62) | 0.61 (0.58) |
| SKAMP Teacher IN | 2.60 (1.04) | 2.49 (1.55)a | 2.22 (1.34)a,b | 1.79 (1.02)b | 1.74 (1.24)b |
| Combined | 2.69 (1.24) | 2.61 (1.60) | 2.37 (1.43) | 1.86 (0.87) | 1.89 (1.01) |
| Inattentive | 2.47 (0.67) | 2.30 (1.53) | 1.99 (1.22) | 1.69 (1.25) | 1.50 (1.56) |
| Side Effects–Total Score | 2.64 (2.27) | 1.60 (2.00)a | 1.80 (2.02)a,b | 3.28 (3.41)b | 5.08 (4.47)c |
| Combined | 2.80 (2.62) | 1.47 (1.25) | 2.40 (2.23) | 3.87 (3.64) | 5.53 (5.21) |
| Inattentive | 2.40 (1.71) | 1.80 (2.86) | 0.90 (1.29) | 2.40 (2.99) | 4.40 (3.20) |
Results in boldface are for the total sample. Figures in parentheses are standard deviations. Pairwise comparisons did not include baseline. Means with nonoverlapping letters are significantly different (p < 0.05).
Abbreviations: SD = Standard deviation; ADHD-RS = ADHD Rating Scale; CGI = Clinical Global Impressions; IN = Inattention subscale; SKAMP = Swanson, Kotkin, Agler, M-Flynn and Pelham Scale.
Parent and teacher ratings
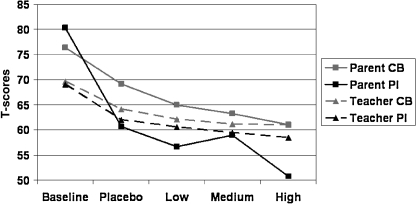
MANOVA of parent and teacher ratings yielded a significant effect of Treatment (F = 2.33, p = 0.009, ηp2 = 0.123) but no effect of Group nor Group × Treatment interaction. Post hoc univariate tests (Table 1) show that the effect of Treatment was highly significant for the Parent Conners (Fig. 1) and for the Parent and Teacher SKAMP ratings, attributable to a linear reduction in symptoms for both subgroups with the introduction of drug and with increasing drug dose. Quadratic and cubic components of the treatment effect were not significant for the subgroups considered together or separately.
FIG. 1.
Means and Pairwise Comparisons for Neurocognitive Measures
Pairwise comparisons (Table 2) yielded significant symptom reductions for the medium and high dosages, compared to placebo, for both the Parent and Teacher SKAMP. For the Parent Conners ratings, however, only the high dose was significantly different from placebo, suggesting that ratings of impairment on the SKAMP are more sensitive to drug effects than the symptom ratings on the Conners.
Side effects
The Total SERS score was significant for the effect of Treatment but not for the interaction between Group and Treatment (Table 1). Both the linear and quadratic components of the Treatment effect were significant. Means (Table 2) revealed that Total SERS exceeded baseline and placebo only at the highest dose.
Scores for individual side effects were entered into a MANOVA that yielded a significant main effect of Treatment (F = 1.950, p = 0.001) but no interaction with Group. Univariate analyses showed that Treatment caused an increase in side effects for: Appetite (F = 7.996, p ≤ 0.001), sleep (F = 5.32, p = 0.001), and stomachaches (F = 3.348, p = 0.032), with marginal Treatment effects on headaches (F = 2.822, p = 0.054) and picks at skin or fingers (F = 1.059, p = 0.053). Examination of means for individual items revealed that the apparent decrease in side effects relative to baseline and placebo for the PI subgroup at the lowest dose was due to a reduction in ratings for side effects involving sleep, stomachaches, worrying, and tearfulness. Ratings for these side effects increased at higher doses for both subgroups.
Neurocognitive tests
There were no significant differences between subtypes on placebo for any of the cognitive measures.
Continuous Performance Test
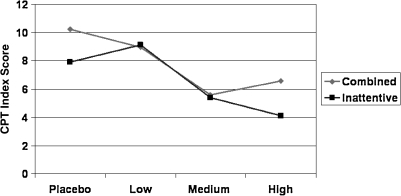
Complete CPT data were available for analysis for 13 CB and 9 PI participants. (Missing data points for the neurocognitive test results were not replaced by means.) MANOVA for the combined sample yielded a significant main effect of Treatment (F = 2.53, p < 0.001, ηp2 = 0.275). Univariate analyses (Table 1) yielded significant effects of Treatment on all measures with the exceptions of Hit RT and Commission Errors, for which the effect was a trend. For all significant drug effects, only the linear component was significant. There were no significant effects or interactions involving Group. The means of one or more drug doses differed significantly from placebo for all CPT measures (Table 3). Mean scores were reduced from the clinical range on placebo to the normal range on one or more drug doses for: Beta, Omission errors, and CPT-RT interstimulus intervals (ISI). Also significantly improved by drug was the CPT Index (Fig. 2). CPT scores most sensitive to drug effects were Beta, Hit RT SE, Omission errors, and Attentiveness.
Table 3.
Means and Pairwise Comparisons for Neurocognitive Measures
| |
Mean (SD) |
|||
|---|---|---|---|---|
| Measure | Placebo | Low | Medium | High |
| Stroop–Interference1 | 55.24 (4.81)a | 54.24 (4.44)a | 55.52 (4.75)a | 56.05 (4.02)a |
| Combined | 55.23 (5.25) | 53.54 (3.33) | 54.38 (3.25) | 55.62 (4.86) |
| Inattentive | 55.25 (4.33) | 55.38 (5.90) | 57.38 (6.32) | 56.75 (2.19) |
| CPT–Index Score2 | 9.41 (4.74)a | 8.39 (6.03)a | 5.64 (6.21)b | 5.17 (6.33)b |
| Combined | 10.24 (5.61) | 8.95 (6.86) | 5.60 (5.99) | 6.56 (6.75) |
| Inattentive | 8.81 (2.54) | 9.13 (5.11) | 5.50 (6.65) | 4.11 (5.76) |
| CPT–Hit RT | 51.15 (17.33)a | 55.70 (12.75)a | 55.51 (10.87)a | 57.85 (12.88)b |
| Combined | 51.44 (19.94) | 56.15 (14.27) | 55.94 (12.17) | 59.66 (13.84) |
| Inattentive | 50.74 (13.81) | 55.05 (10.97) | 54.90 (9.35) | 55.24 (11.64) |
| CPT–Hit RT SE | 59.96 (14.15)a | 52.24 (13.40)b | 49.33 (14.13)b | 44.64 (13.40)c |
| Combined | 61.84 (15.40) | 51.32 (14.39) | 49.43 (16.85) | 42.35 (14.71) |
| Inattentive | 57.23 (12.48) | 53.57 (12.56) | 49.19 (9.89) | 47.93 (11.23) |
| CPT–Omission3 | 81.56 (17.85)a | 71.86 (29.48)a,b | 64.46 (32.91)b,c | 58.60 (25.28)c |
| Combined | 82.74 (13.29) | 67.01 (32.71) | 59.26 (33.47) | 55.67 (28.82) |
| Inattentive | 79.85 (23.80) | 78.87 (24.13) | 71.97 (32.50) | 61.39 (20.42) |
| CPT–Commission | 55.28 (10.69)a | 53.95 (11.03)a,b | 51.80 (10.28)b | 50.49 (12.92)b |
| Combined | 55.52 (9.88) | 54.79 (11.08) | 53.41 (6.82) | 49.99 (11.18) |
| Inattentive | 54.93 (12.38) | 52.74 (11.51) | 49.48 (14.05) | 51.36 (15.78) |
| CPT–Attentiveness (d') | 61.72 (10.96)a | 57.84 (9.20)b | 54.15 (8.77)c | 53.33 (11.59)b,c |
| Combined | 60.69 (8.43) | 57.40 (8.92) | 54.60 (4.95) | 52.05 (8.02) |
| Inattentive | 63.21 (14.31) | 58.48 (10.10) | 53.49 (12.83) | 55.18 (15.80) |
| CPT–Beta | 77.59 (22.42)a | 69.32 (21.71)a | 60.05 (17.86)b | 57.59 (16.60)b,c |
| Combined | 75.78 (21.64) | 66.03 (21.67) | 57.41 (17.98) | 55.10 (15.04) |
| Inattentive | 80.20 (24.59) | 74.07 (22.14) | 63.87 (18.03) | 61.18 (18.96) |
| CPT–RT ISI Change | 68.32 (15.36)a | 58.29 (14.96)b | 57.11 (13.33)b | 57.12 (10.63)b |
| Combined | 71.99 (17.70) | 56.64 (14.38) | 56.81 (14.82) | 56.67 (12.45) |
| Inattentive | 63.02 (9.81) | 60.69 (16.34) | 57.54 (11.68) | 57.76 (7.94) |
Results in boldface are for the total sample. Standard deviations are indicated in parentheses. Means with nonoverlapping letters are significantly different (p < 0.05). With the exceptions noted below, all scores are T-scores. For all CPT measures, a higher score indicates worse performance.
For the Stroop, a higher T-score indicates better performance.
The CPT–Index score is an absolute score.
CPT-Omission errors are expressed as percentiles in the normative population.
Abbreviations: SD = Standard deviation; CPT = Continuous Performance Test; RT = reaction time; SE = standard error; ISI = interstimulus interval.
FIG. 2.
Means and Pairwise Comparisons for Neurocognitive Measures
The MANOVA tests conducted separately for the subtypes yielded a significant main effect of Treatment on the CPT for the CB subgroup [F = 2.48, p = 0.001, ηp2 = 0.402] and a trend for an effect of Treatment for the PI subgroup [F = 1.55, p = 0.097, ηp2 = 0.416].
Stroop Color-Word Test
Complete Stroop data were available for 13 CB and 8 PI participants. Analysis of variance (ANOVA) yielded no significant main effects or interactions (Table 1). Mean age-adjusted T-scores were in the average range (Table 3) for both subgroups in all placebo and drug conditions.
Discussion
Results revealed significant effects of MPH for both the CB and PI subtypes, but little difference in the response of these two subgroups. For both subtypes, there was a linear decrease in symptoms with increasing drug dose, whether rated by clinicians, parents, or teachers.
Performance on the CPT also improved as a linear function of dosage for both subgroups. The CPT appeared somewhat more sensitive to drug effects than did the behavioral measures, evidencing improvement at the lowest dose, as well as differences between doses for some measures. Scores indexing vigilance (e.g., Attentiveness) as well as impulsivity (e.g., Beta) yielded significant treatment effects. These results are consistent with many reports of the sensitivity of the CPT to stimulant effects in children with ADHD (Losier et al. 1996), but provided no indication of a differential response by subtype.
Effects of MPH on the Stroop have been examined in three other studies, with one study of a small sample (n = 18) reporting significant improvement at the subjects' usual clinical dose (Langleben et al. 2006), and two others, each employing larger samples and multiple doses finding no effect (Bedard et al. 2002; Scheres et al. 2003). Thus, our study contributes to the balance of negative results on this test.
A limitation of the study is the relatively small sample size. However, it must be emphasized that all of these children met rigorous criteria not only for ADHD, but also for subtype. In addition, all but one were stimulant-naïve. These features enhance the homogeneity of the samples. The repeated-measures design provides additional power. Examination of means provided no suggestion of a difference between subgroups in dose–response curves. Consistent with this interpretation, a post hoc power analysis indicated that a total sample size of 152 would be necessary for 80% power to detect a significant difference (p ≤ 0.05) for the Treatment × Group interaction for the ADHD-RS-Total score, given the small magnitude of the corresponding F statistic (0.22) and ηp2 (0.013).
In summary, the results of this study confirm that MPH is an effective treatment for children with the Predominantly Inattentive form of ADHD. In addition, the findings of similar dose–response profiles for the subtypes across different informants (parent, teacher, and clinician) and on a widely used psychometric test of attention (CPT) provide no support for the hypothesis of biological differences between the subtypes.
Disclosures
Dr. Solanto serves on the Medical Advisory Board of Shire Pharmaceuticals. Dr. Newcorn is a recipient of grants for research support from Eli Lilly and McNeil Pediatrics. He is a consultant and/or advisor for Eli Lilly, Novartis, McNeil, Shire, Lupin, Abbott, Psychogenics, BioBehavioral Diagnostics Company, as well as a speaker for McNeil and Novartis. Dr. Ivanov has consulted for Shire and received research support from Shire and Eli Lily. Drs. Vail, Gilbert, and Lara have no conflicts of interest or financial ties to disclose.
Footnotes
This research was funded by NIMH Grant R21 MH62945 to the first author.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3th. Washington (DC): American Psychiatric Association; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington (DC): American Psychiatric Association; 1994. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA. DuPaul GJ. McMurray MB. Attention deficit disorder with and without hyperactivity: Clinical response to three dose levels of methylphenidate. Pediatrics. 1991;87:519–531. [PubMed] [Google Scholar]
- Bedard AC. Ickowicz A. Tannock R. Methylphenidate improves Stroop naming speed, but not response interference, in children with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2002;12:301–309. doi: 10.1089/104454602762599844. [DOI] [PubMed] [Google Scholar]
- Cantwell DP. Classification of child and adolescent psychopathology. J Child Psychol Psychiatry. 1996;37:3–12. doi: 10.1111/j.1469-7610.1996.tb01377.x. [DOI] [PubMed] [Google Scholar]
- Carlson CL. Mann M. Sluggish cognitive tempo predicts a different pattern of impairment in the attention deficit hyperactivity disorder, predominantly inattentive type. J Clin Child Adolesc Psychol. 2002;31:123–129. doi: 10.1207/S15374424JCCP3101_14. [DOI] [PubMed] [Google Scholar]
- Chhabildas N. Pennington B. Willcutt EG. A comparison of the neuropsychological profiles of the DSM-IV subtypes of ADHD. J Abnorm Child Psychol. 2001;29:529–540. doi: 10.1023/a:1012281226028. [DOI] [PubMed] [Google Scholar]
- Conners CK. The Conners' Continuous Performance Test. Toronto: Multi-Health Systems; 1994. [Google Scholar]
- Conners CK. Sitarenios G. Parker J. Epstein JN. Revision and restandardization of the Conners' Teacher Rating Scale (CTRS-R): Factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- Conners CK. Sitarenios G. Parker JDA. Epstein JN. The revised Conners' Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;25:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ. Power TJ. Anastopoulos AD. Reid R. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretations. New York: Guilford Press; 1998. [Google Scholar]
- Eiraldi RB. Power TJ. Nezu CM. Patterns of comorbidity associated with subtypes of attention-deficit/hyperactivity disorder among 6- to 12- year-old children. J Am Acad Child Adolesc Psychiatry. 1997;36:503–514. doi: 10.1097/00004583-199704000-00013. [DOI] [PubMed] [Google Scholar]
- Faries DE. Yalcin I. Harder D. Heiligenstein JH. Validation of the ADHD Rating Scale as a clinician-administered and scored instrument. J Attent Disord. 2001;5:107–115. [Google Scholar]
- Geurts HM. Verte S. Oosterlaan J. Roeyers H. Sergeant JA. ADHD subtypes: Do they differ in their executive functioning profile? Arch Clin Neuropsychol. 2004;20:457–477. doi: 10.1016/j.acn.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Golden CJ. The Stroop Color and Word Test: A Manual for Clinical Uses. Chicago (Illinois): Stoelting Co.; 1978. [Google Scholar]
- Gorman EB. Klorman R. Thatcher JE. Borgstedt AD. Effects of methylphenidate on subtypes of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45:808–816. doi: 10.1097/01.chi.0000214191.57993.dd. [DOI] [PubMed] [Google Scholar]
- Greenhill LL. Abikoff HB. Arnold LE. Cantwell DP. Conners CK. Elliott G. Hechtman L. Hinshaw SP. Hoza B. Jensen PS. March JS. Newcorn J. Pelham WE. Severe JB. Swanson JM. Vitiello B. Wells K. Medication treatment strategies in the MTA study: Relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry. 1996;35:1304–1313. doi: 10.1097/00004583-199610000-00017. [DOI] [PubMed] [Google Scholar]
- Hartman CA. Willcutt EG. Rhee SH. Pennington BF. The relation between sluggish cognitive tempo and DSM-IV ADHD. J Abnorm Child Psychol. 2004;32:491–503. doi: 10.1023/b:jacp.0000037779.85211.29. [DOI] [PubMed] [Google Scholar]
- Ishihara S. Ishihara Test for Color Blindness. 1960. http://www.toledo-bend.com/colorblind/links.asp http://www.toledo-bend.com/colorblind/links.asp
- Lahey BB. Should the combined and predominantly inattentive types of ADHD be considered distinct and unrelated disorders? Not now, at least. Clinical Psychol: Sci Pract. 2001;8:494–497. [Google Scholar]
- Lahey BB. Pelham WE. Loney J. Lee SS. Willcutt E. Instability of the DSM-IV Subtypes of ADHD from preschool through elementary school. Arch Gen Psychiatry. 2005;62:896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- Langleben DD. Monterosso J. Elman I. Ash B. Krikorian G. Austin G. Effect of methylphenidate on Stroop Color-Word task performance in children with attention deficit hyperactivity disorder. Psychiatry Res. 2006;141:315–320. doi: 10.1016/j.psychres.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Losier BJ. McGrath PJ. Klein RM. Error patterns on the continuous performance test in non-medicated and medicated samples of children with and without ADHD: A meta-analytic review. J Child Psychol Psychiatry. 1996;37:971–987. doi: 10.1111/j.1469-7610.1996.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Maedgen JW. Carlson CL. Social functioning and emotional regulation in the attention deficit hyperactivity disorder subtypes. J Clin Child Adolesc Psychol. 2000;29:30–42. doi: 10.1207/S15374424jccp2901_4. [DOI] [PubMed] [Google Scholar]
- Milich R. Balentine A. Lynam D. ADHD Combined Type and ADHD Predominantly Inattentive Type are distinct and unrelated disorders. Clinical Psychol: Scie Pract. 2001;8:463–488. [Google Scholar]
- Nigg JT. Blaskey LG. Huang-Pollock CL. Rappley MD. Neuropsychological executive functions and DSM-IV subtypes. J Am Acad Child Adolesc Psychiatry. 2002;41:59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. CGI: Clinical Global Impressions Scale—NIMH. Psychopharmacol Bull. 1985;21:839–844. [Google Scholar]
- Scheres A. Oosterlaan J. Swanson J. Morein-Zamir S. Meiran N. Schut H. Vlasveld L. Sergeant JA. The effect of methylphenidate on three forms of response inhibition in boys with AD/HD. J Abnorm Child Psychol. 2003;31:105–120. doi: 10.1023/a:1021729501230. [DOI] [PubMed] [Google Scholar]
- Shaffer D. Fisher P. Dulcan M. Davies M. Piacentini J. Schwab-Stone M. Lahey BB. Bourdon K. Jensen PS. Bird HR. Canino G. Regier D. The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3): Description, acceptability, prevalence rates, and performance in the MECA study. J Am Acad Child Adolesc Psychiatry. 1996;35:865–877. doi: 10.1097/00004583-199607000-00012. [DOI] [PubMed] [Google Scholar]
- Solanto MV. Dopamine dysfunction in AD/HD: Integrating clinical and basic neuroscience research. Behav Brain Res. 2002;130:65–71. doi: 10.1016/s0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- Solanto MV. Gilbert SN. Raj A. Zhu J. Pope-Boyd S. Stepak B. Vail L. Newcorn JH. Neurocognitive functioning in AD/HD, Predominantly Inattentive Subtype. J Abnorm Child Psychol. 2007;35:729–744. doi: 10.1007/s10802-007-9123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV. Pope-Boyd S. Tryon W. Stepak B. Social functioning in predominantly inattentive and combined subtypes of children with ADHD. J Attent Disord. 2009;13:107–116. doi: 10.1177/1087054708320403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T. Biederman J. Wilens TE. Harding M. O'Donnell D. Griffin S. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35:409–432. doi: 10.1097/00004583-199604000-00008. [DOI] [PubMed] [Google Scholar]
- Stein M. Sarampote CS. Waldman ID. Robb AS. Conlon C. Pearl PL. Black DO. Seymour KE. Newcorn JH. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003;112:e404. doi: 10.1542/peds.112.5.e404. [DOI] [PubMed] [Google Scholar]
- Swanson J. School-Based Assessments and Interventions for ADD Students. Irvine (California): K.C. Publications; 1992. [Google Scholar]
- Willcutt EG. Pennington BF. Chhabildas NA. Friedman MC. Alexander J. Psychiatric comorbidity associated with DSM-IV ADHD in a nonreferred sample of twins. J Am Acad Child Adolesc Psychiatry. 1999;38:1355–1362. doi: 10.1097/00004583-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Wolraich ML. Wibbelsman CJ. Brown TE. Evans SW. Gotlieb EM. Knight JR. Ross EC. Shubiner HH. Wender EH. Wilens TE. Attention-deficit/hyperactivity disorder among adolescents: A review of the diagnosis, treatment, and clinical implications. Pediatrics. 2005;115:1734–1746. doi: 10.1542/peds.2004-1959. [DOI] [PubMed] [Google Scholar]