Abstract
Objective
The aim of this study was to determine the effect on family quality of life (QOL) of clonidine (CLON) and methylphenidate (MPH), used alone and in combination, in treating attention-deficit/hyperactivity disorder (ADHD).
Methods
Two proxy QOL measures were used in a multicenter, double-blind, placebo-controlled 16-week trial of 122 children, ages 7–12 years, with ADHD. Children were randomized to one of four groups in which they received MPH, CLON, a combination of drugs, or placebo. QOL was measured with the Daily Hassles Scale and the Impact on Family Scale at baseline and at 16 weeks.
Results
In a general linear model repeated measures analysis, treatment groups improved over a 16-week period compared to placebo for Daily Hassles and Impact on Family, as well as in symptoms measured by the ADHD Rating Scale. QOL measures correlated moderately with efficacy and symptom measures.
Conclusion
This study provides evidence that measures of QOL for the family are sensitive to pharmacological treatment of ADHD. The correlation pattern of the QOL measures with symptom and efficacy variables supported family QOL as a related but separate construct. Clonidine for Attention-Deficit/Hyperactivity Disorder Treatment Study (CAT) Trial Registry Name: Clinicaltrials.gov; ID Number, NCT00031395; URL, http://clinicaltrials.gov/ct/show/NCT00031395?order=8/.
Introduction
Health-care professionals regard the improvement of a patient's quality of life (QOL) as an important treatment goal, in addition to and beyond that of symptom relief. Measuring QOL in the pediatric population involves the assessment of a wide range of factors concerning the child's physical, emotional, and social functioning. Although comprehensive pediatric measures of QOL (Varni et al. 2001; Landgraf et al. 2002) are becoming well established, there is a continued effort among researchers to develop age- and disease-specific QOL measures (Landgraf et al. 2002; Sawyer et al. 2002). Recent attention given to psychiatric illnesses shows that their effect on QOL is significant and in some cases is comparable to or worse than that of some chronic physical illnesses (Varni et al. 2001; Sawyer et al. 2002; Bastiaansen et al. 2004).
Attention-deficit/hyperactivity disorder (ADHD) is the most prevalent psychiatric disorder in children, affecting approximately 5–10% of school children in the United States (Polanczyk et al. 2007). Several studies have documented the association of symptoms of ADHD with lower QOL (Varni et al. 2001; Klassen et al. 2004; Matza et al. 2004; Escobar et al. 2005; Rentz et al. 2005). Moreover, there is ample evidence showing increased family and parental stress and parental marital discord in the families of children with ADHD (Fischer 1990; Anastopoulos et al. 1992; Johnston and Mash 2001; Podolski and Nigg 2001; Harrison and 2002). Parental and family stress is most likely an important factor leading parents to seek out treatment for their child. Reducing family and parent stress should be an important treatment goal, considering the central role of the parent and family in the child's upbringing.
Definitions of QOL vary, although all are multidimensional and usually include physical, emotional, and social components. Two of the most prevalent measures of QOL in the pediatric population are the Child Health Questionnaire (CHQ) (Landgraf et al. 1996) and the Pediatric Quality of Life Inventory (PedsQL) (Varni et al. 2001), both of which have physical and psychosocial factors that can be further broken down into specific subscales. The addition of “family relationships” as a QOL dimension has been proposed previously in comprehensive models (Cummins 1997; Hagerty et al. 2001) and is represented by several subscales in the CHQ. Measures of personal strain in the parents, financial burden, and social disruption for the family have been used for many years, although they are not previously referred to as measures of QOL (Stein and Reissman 1980; Greenberg and Crnic 1990).
A challenge facing developers of QOL measures is to construct scales that are sensitive to the effects of treatment. The results from studies examining the effects of treatment on QOL in ADHD have been mixed. For example, treatment may affect symptoms, but not necessarily QOL (Brown et al. 2006). However, a few studies report a pharmacological treatment effect on QOL in adults (Adler et al. 2007). Other studies have reported treatment effects in children as defined by the PedsQL (Sallee et al. 2003) and also by the psychosocial subscale of the CHQ (Perwien et al. 2004). These studies demonstrate the validity of using QOL measures to assess treatment response.
More evidence is needed to determine the effects of treatment on the varied factors that comprise QOL and which measures are sensitive to pharmacological treatment. Therefore, we used two proxy measures of QOL for families in a multicenter, randomized, double-blind, placebo-controlled study of clonidine (CLON), methylphenidate (MPH), and drug combination in children with primary ADHD as part of the Clonidine for Attention-Deficit/Hyperactivity Disorder Treatment Study (CAT study.) Efficacy and safety data for the CAT study have been reported in separate publications (Daviss et al. 2008; Palumbo et al. 2008).
The CAT study group used several secondary outcome measures, including the two proxy QOL measures used in this study—the Impact on Family questionnaire (Greenberg and Crnic 1990) and the Parenting Daily Hassles Scale (Crnic and Greenberg 1990.) These two scales are referred to here as “proxy” measures, because they have not been previously identified as measures of QOL. Furthermore, they have not been validated as QOL measures, insofar as they have not been correlated with known measures of QOL. However, the items in both measures tapped into day-to-day activities that would seem to fall under the construct of family QOL. The conceptualization of this study was thus done after the data collection had been completed.
The purpose of the current paper was to examine the effect of the two aforementioned commonly used pharmacologic agents on the QOL of families with children who have ADHD. The hypothesis was that families whose children engaged in active treatment would experience greater improvement in QOL versus families whose children are treated with placebo.
Methods
Subjects
Methods for the CAT study are detailed in two previous papers, and summarized briefly here (Daviss et al. 2008; Palumbo et al. 2008). The CAT study was supported by a National Institute of Neurological Disorders and Stroke (NINDS) grant. The study enrolled 122 children of any race and ethnic background, ages 7–12, in a multicenter, double-blind, placebo-controlled 16-week trial. The informed consent process was approved by the internal review board at each research site. After a thorough explanation of the procedures, risks, and benefits, parents of the children signed a consent form and the child participants signed an assent form. Each child met the criteria for ADHD set forth in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR) (American Psychiatric Association 2000), as determined by the Diagnostic Interview Schedule for Children (DISC) (Fisher et al. 1993). Additionally, a teacher in regular contact with the child had to supply two separate qualifying ratings as follows: First, the teacher had to indicate a sufficient number of ADHD symptoms, rated as “pretty much” or “very much” on the Disruptive Behaviors Disorders Rating Scale (Pelham et al. 1992). Second, the teacher had to rate the severity of the ADHD symptoms above specified cutoff scores (boys, grades 2–3 = 10; grade 4 and above = 9; girls, grades 2–3 = 7, grade 4 and above = 6) on the Iowa Conners' Teacher Rating Scale (Pelham et al. 1989). Also, a parent in daily contact with the child had to indicate the presence of sufficient symptoms at home as measured by the Iowa Conners' Parent Rating Scale. Finally, the investigator had to rate global functioning less than or equal to 70 on the Child Global Assessment Scale (C-GAS) (Shaffer et al. 1983), with difficulty evident in at least two areas, such as school and home. Of the measures mentioned in this paragraph, only the Conners' Teacher Rating Scale was used in the analyses to provide evidence for construct validity of the two proxy QOL measures.
Children were excluded for: Evidence of a tic disorder, major depression, pervasive developmental disorder, autism, psychosis, mental retardation, anorexia nervosa, bulimia, a serious cardiovascular (e.g., significant hypotension, congenital heart disease) or other medical disorder that would preclude the safe use of MPH or CLON, impaired renal function (a routine urinalysis was performed), or pregnancy (a urine pregnancy test was performed for all adolescent girls). Additionally, family history of long QT syndrome, cardiomyopathy, or premature (age ≤ 45 years) sudden death were also exclusions.
To enroll, subjects could not receive any other medications for the treatment of ADHD or other associated behavioral symptoms. Any such treatment had to be discontinued at least 6 weeks (2 weeks for MPH) before enrollment. All children and parents received protocol-based behavioral interventions and were not allowed to receive any other treatments during the course of the 16-week study. Prior use of MPH or CLON, whether judged to be beneficial or not, was permitted.
Children at four sites (University of Cincinnati, University of Rochester, State University of New York at Buffalo, and University of Pittsburgh) were randomly assigned to one of four groups in which they received placebo, MPH only, CLON only, or a combination of both drugs (COMB). All investigators, study coordinators, teachers, parents, and children were blinded to the treatment assignments.
The double-blind treatment period consisted of an 8-week dosage titration period and an 8-week maintenance dosage period. The maximal allowed daily dosage of clonidine was 0.6 mg/day (divided no more than four times daily, with first doses given as early as first thing in the morning, and last doses generally given within an hour of bedtime). The maximal allowed daily dosage of MPH (immediate release) was 60 mg/day (divided no more than three times daily, given in the morning and at noon, with the same or a lower third dose prescribed at 4 p.m.). Researchers used a flexible-dose titration procedure to reproduce standard clinical practice and to enable the determination of an optimal (or highest tolerated) dose for each child. “Optimal” dosage was defined as the one that allowed the subject to reach a “good” level of school functioning, with no further room for improvement, and with an acceptable level of side effects. Children who did not reach a “good” level of functioning were limited to a dose with tolerable side effects or the maximum dose.
Measures
After successful screening, a baseline (week 0) evaluation visit occurred within 2 weeks. At this time, the following ratings were performed: Impact on Family questionnaire (Greenberg and Crnic 1990), Parenting Daily Hassles Scale (Crnic and Greenberg 1990), and the ADHD Rating Scale (DuPaul et al.1998). The ratings were repeated at week 16 (the final week of the trial) and include those below.
Impact on Family Questionnaire
Ten items were used from a 22-item scale (plus two optional items) that measures parent reports of financial burden, social disruption of the family, and personal strain related to their child's problems. Examples include: “It is hard to give much attention to my spouse/friend or my other children because of the needs of my child,” and “Special family activities are often spoiled because of my child's behavior.” The questionnaire instructed parents to rate their level of agreement with each item on a 5-point Likert scale (0 = strongly disagree, 1 = disagree, 2 = not sure, 3 = agree, 4 = strongly agree). Twelve of the 22 items in the Impact on Family scale exhibited significant floor effects for which greater than 60% of responses were “disagree” or “strongly disagree. These items were omitted, and the remaining 10 items (see Appendix A) were combined for an adapted version used in subsequent statistical tests. Scores were computed by taking the mean of the 10 items. The Cronbach alpha was 0.86 at baseline and 0.91 at week 16 for these 10 items (potential range 0–4). All adjustments to the scale were made prior to examining the data for treatment effects; however, it was known to the authors that including the omitted items would likely attenuate statistical differences between treatment groups.
Parenting Daily Hassles Questionnaire
This is a 20-item scale measuring routine events that can make life difficult for families with young children (see Appendix B). Examples include: “The kids won't listen or do what they are asked without being nagged,” and “The kids are hard to manage in public (grocery store, shopping center, restaurant).” Parents were instructed to rate how much of a hassle each event was in the past month on a 5-point Likert scale (1 = none, 2 = mild, 3 = moderate, 4 = significant, 5 = big). A mean of the 20 items was calculated for each subject (potential range 1–5). Cronbach alpha was 0.90 at baseline and 0.92 at week 16.
ADHD Rating Scale
A child psychiatrist completed this 18-item symptom measure, which is linked directly to DSM-IV diagnostic criteria for ADHD. The scale can be divided into two subscales—hyperactivity/impulsivity and inattention. All items were Likert-type, using the following 5-point scale: 0 = Not at all, 1 = Just a little, 2 = Pretty much, 3 = Very much, U = unknown. Means for each child of the total score and both subscales were computed (potential ranges 0–3).
Statistical methods
Outcome variables were analyzed only for children who completed at least 8 weeks of the study (the completion point of the titration phase.) If a child withdrew prematurely (after 8 weeks, but before 16 weeks), the investigating site attempted to collect data for all measures intended for the final visit. For all analyses, if there was missing data or early withdrawal, the last available observation for each child was carried forward and imputed for that visit.
Statistical tests compared the groups using a general linear model (GLM) repeated measures procedure. Because comparisons of all four separate groups (GLM repeated measures) were not significant, the tested hypotheses were based on the comparison of subjects on any of the three treatments relative to those on placebo, as a post hoc analysis.
Results
Demographic and clinical characteristics
Unless otherwise noted, results are presented as means ± standard deviations. Of 201 eligible patients, 122 were enrolled and randomized between October, 2000, and April, 2004. The majority of participants were male; 98 boys and 24 girls enrolled with a mean age of 9.5 ± 1.6 years. There were 95 Caucasian, 13 black, 8 Hispanic, and 6 of other racial background participants. Detailed demographic and clinical characteristics of the children are presented in Palumbo et al. (2008). There were no significant differences in demographic or clinical data at baseline comparing subjects on placebo relative to those receiving an active treatment.
Dosing
Mean end-of-study dosages in groups on active treatment were as follows: CLON, 0.24 ± 0.11 mg/day; MPH, 30.2 ±18.9 mg/day; COMB, CLON 0.23 ± 0.13 mg/day and MPH 25.4 + 18.2 mg/day. At the end of the study, the average MPH daily dosage was 0.76 ± 0.54 mg/kg for subjects taking MPH (MPH or COMB). For additional dosing data, see Daviss et al. (2008).
Subject disposition
A total of 46 subjects withdrew from the study prematurely. Twenty out of 30 (67%) were from the placebo group, 8 of which were due to lack of efficacy. Eleven (38%) withdrew from the MPH group, five (16%) from the CLON group, and eight (25%) from the COMB group.
Correlations of Impact on Family and Parenting Daily Hassles with other measures
Table 1 shows the intermeasure correlations within the same time point between the family QOL measures and ADHD symptoms. Subject numbers for these pairwise correlations varied because of missing data. All correlations shown were significant. Correlations of baseline measures are shown above the diagonal and correlations of week 16 measures are shown below the diagonal. The Parenting Daily Hassles and Impact on Family scales were strongly correlated with one another at both time points, and moderately correlated with ADHD symptoms (week 16), and teacher-rated efficacy (Abbreviated Symptoms Questionnaire, ASQ).
Table 1.
Summary Statistics for Outcome Variables
| 1. | 2. | 3. | 4. | 5. | 6. | |
|---|---|---|---|---|---|---|
| 1. Impact on Family | — | 0.66a(102) | 0.24b(101) | 0.19(105) | 0.15(105) | 0.17(105) |
| 2. Parenting Daily Hassles | 0.65a(92) | — | 0.30a(98) | 0.15(102) | 0.02(102) | 0.21b(102) |
| 3. ASQ-T | 0.43a(91) | 0.31a(91) | — | 0.65a(118) | 0.37a(118) | 0.66a(118) |
| 4. ADHD-RS Overall | 0.46a(90) | 0.35a(91) | 0.59a(98) | — | 0.78b(122) | 0.85a(122) |
| 5. ADHD-RS Inattentive | 0.37a(90) | –0.27a(91) | 0.42a(98) | 0.89a(99) | — | 0.34a(122) |
| 6. ADHD-RS Hyperactive-Impulsive | 0.45a(90) | 0.35a(91) | 0.64a(98) | 0.92a(99) | 0.66a(99) | — |
Correlations of baseline measures are shown above, and of week 16 measures are shown below the diagonal.
p < 0.01 for two-tailed test.
p < 0.05 for two-tailed test.
Abbreviations: ADHD = Attention-deficit/hyperactivity disorder; ASQ-T = Abbreviated Symptoms Questionnaire–Teacher; ADHD-RS = Attention-Deficit/Hyperactivity Disorder Rating Scale.
Outcome measures
Overall, the treatment groups combined improved more than the placebo group on most of the outcome measures presented in Table 2. These results are consistent with primary efficacy results presented in Palumbo et al. (2008), although that study found differences among the three treatment groups. As mentioned above, comparisons of the separate four treatment groups in this study were not statistically significant for Parenting Daily Hassles or Impact on Family. Results of only the treatment groups combined versus the placebo group are presented below. The number of subjects in each group (Table 2) varies slightly depending on missing data. Data from the Parenting Daily Hassles and the Impact on Family questionnaires were not available from some of the families in the study.
Table 2.
Summary Statistics for Outcome Variables
| Variable | Group (n) | Baseline | Week 16 | Difference (CI) | Effect sizea |
|---|---|---|---|---|---|
| Parenting Daily Hassles | Treatment (68) | 2.38 (0.66) | 1.96 (.67) | 0.42b (0.29, 0.55) | 0.069 |
| Placebo (16) | 2.38 (0.72) | 2.32 (.71) | 0.07b (–0.17, 0.31) | ||
| Impact on Family | Treatment (69) | 1.99 (0.92) | 1.55 (1.00) | 0.45b (0.26, 0.63) | 0.056 |
| Placebo (16) | 1.80 (0.86) | 1.82 (1.02) | –0.03b (–0.36, 0.31) | ||
| ADHD-RS total | Treatment (81) | 1.81 (0.60) | 1.17 (0.75) | 0.64b (0.46, 0.81) | 0.058 |
| Placebo (18) | 1.80 (0.67) | 1.63 (0.75) | 0.17b (–0.05, 0.39) |
Partial eta squared.
Difference of baseline and week 16 mean.
Abbreviations: CI = 95% Confidence interval; ADHD-RS = Attention-Deficit/Hyperactivity Disorder Rating Scale.
Efficacy measures
Primary efficacy (and safety) results are reported in two separate articles (Daviss et al. 2008; Palumbo et al. 2008). Briefly, the primary efficacy outcome was the change score on the Conners' Abbreviated Symptoms Questionnaire for Teachers (ASQ-T), and secondary outcomes included the ASQ-Parent (ASQ-P) and C-GAS.
Quality of Life and Symptom measures
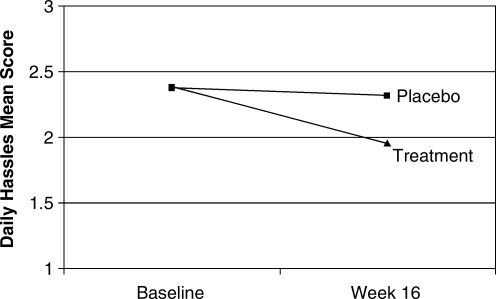
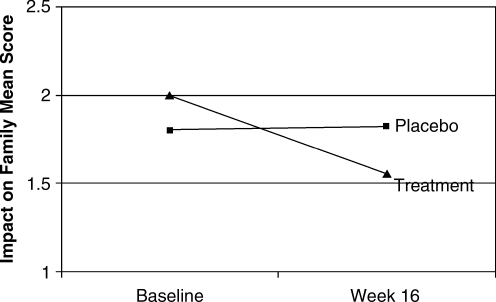
For the Parenting Daily Hassles Scale, the combined treatment groups improved by a mean of 0.42 (17.7%) compared to a mean change of 0.07 (2.9%) for the placebo group (F[1,82] = 6.07, p = 0.016), as shown in Fig. 1. Similarly, the combined treatment groups improved on the Impact on Family scale by a mean of 0.45 (22.3%) compared to the placebo group, which was slightly worse by a mean of 0.03 (−1.4%) (F[1,83] = 4.95, p = 0.029), as shown in Fig. 2. Finally, the Attention-Deficit/Hyperactivity Disorder Rating Scale (ADHD-RS) total score improved for the treatment group by a mean of 0.64 (35.3%) compared to a mean of 0.17 (9.5%) for placebo (F[1,97] = 5.94, p = 0.017).
FIG. 1.
Mean change from baseline on Daily Hassles Scale.
FIG. 2.
Mean change from baseline on the Impact on Family Scale.
Discussion
This study provided some evidence for a measurable effect of active treatments with MPH and/or CLON on QOL measures for families with children who have ADHD. The results were consistent with the previously published efficacy article from this study and support the conclusion that treatment was efficacious across several domains. One explanation for the improvement of family QOL is that the medications improve symptoms that make daily activities difficult for the parents. For example, an inattentive or hyperactive child would be difficult to manage during an outing to a store, and medications that reduce these symptoms simply make the shopping easier for the parent to accomplish. The use of two proxy measures for family QOL in this study requires some discussion of the validity of the measurement model below.
In addition to traditional measures of efficacy and safety, there is a growing recognition of the importance of assessing disease impact on QOL. An increasing number of “quality of life” measures are being constructed and validated, although few have been used in an experimental design that would allow an assessment of their sensitivity to drug treatment. Several general QOL measures for children have emerged as standards, but there is still no consensus on specific QOL measures for ADHD. General measures may be seen as superior because of their comprehensive coverage of QOL domains. However, they may fail to demonstrate sensitivity reliably for select QOL domains that are more salient for specific diseases. The most promising measures in terms of sensitivity for ADHD seem to be those that assess the psychosocial domain.
Both the Parenting Daily Hassles and Impact on Family scales had adequate internal consistency, demonstrating an important aspect of measurement reliability. Both measures were moderately correlated with symptoms and efficacy outcomes, supporting the argument that they represent related but different variables, and thus providing evidence for construct validity in this application. As mentioned earlier, both scales were conceptualized as proxy measures of QOL based primarily on face validity, although neither had data-based evidence for validity for measuring QOL. However the results in this study lend some support for the content validity of both scales as measures of family QOL. The authors recognize that the degree of content validity for both measures depends on validation in future studies.
The current study has several potential limitations related to the measurement model. First, the results presented here are based on measures that focus primarily on the effects of ADHD on the family, which is only one domain in the broadly defined QOL construct. The CAT study planners included several secondary outcomes, but not established measures of QOL. Second, the scales in this paper have not been previously validated as QOL measures, and no previous study uses an adjusted version of the Impact on Family scale. Furthermore, the measures are based on subjective ratings by the parent and may or may not tap the child's sense of subjective well being, or their assessment of family functioning. There is evidence that parents and children differ in their ratings of QOL (Waters et al. 2003).
Whereas the design of this study potentially enabled us to test interaction between CLON and MPH on QOL outcomes, the statistical tests were not significant for the two proxy QOL measures. Consequently, the conclusions are limited to an “active treatment” versus “placebo” comparison, rather than a comparison of different treatment groups. Additionally, with the increased number of statistical tests comes an increased chance of false positive results, although there is some reassurance that the treatment group was different from the placebo group for both QOL measures. Considering the post hoc nature of the analyses, results in this study should be considered exploratory.
This study provides exploratory evidence that QOL measures of the impact on the parent and family can be useful in gauging the treatment response. Health-care professionals should expect to see a continuing burgeoning of QOL measures for a variety of psychiatric illnesses. More research will help to establish the validity and the utility of QOL measures to assess response to treatment.
Appendix A. Items of the Revised Impact on Family Questionnaire
I spend a great deal of time with teachers and other professionals concerning my child's behavior.
It is hard to give much attention to my spouse/friend or my other children because of the needs of my child.
Fatigue is a problem for me because of my child's behavior.
Nobody understands the burden I carry because of my child.
I worry about what will happen to my child in the future (when he/she grows up).
There is much fighting within our family (e.g., between myself and my spouse, between our children) because of my child's behavior.
Special family activities are often spoiled because of my child's behavior.
There are few quiet, calm moments in our home because of my child's behavior.
It is hard for me to get my housework done when my child is at home.
I am sometimes embarrassed by my child's behavior in front of the neighbors.
Appendix B. Items of the Parenting Daily Hassles Questionnaire
Continually cleaning up messes of toys or food.
Being nagged, whined at, complained to.
Meal-time difficulties with picky eaters, complaining, etc.
The kids won't listen or do what they are asked without being nagged.
Baby-sitters are hard to find.
The kids schedules (like pre-school or other activities) interfere with meeting your own household needs.
Sibling arguments or fights require a ‘referee.’
The kids demand that you entertain them or play with them.
The kids resist or struggle with you over bed-time.
The kids are constantly underfoot, interfering with other chores.
The need to keep a constant eye on where the kids are and what they are doing.
The kids interrupt adult conversations or interactions.
Having to change your plans because of unprecedented child needs.
The kids get dirty several times a day requiring changes of clothing.
Difficulties in getting privacy (e.g., in the bathroom.)
The kids are hard to manage in public (grocery store, shopping center, restaurant).
Difficulties in getting kids ready for outings and leaving on time.
Difficulties in leaving kids for a night out or at school or day care.
The kids have difficulties with friends (eg. fighting, trouble, getting along, or no friends available).
Having to run extra errands to meet the kids needs.
Footnotes
Additional contributions to this study were made by Michael P. McDermott, Ph.D.
This work is supported by the National Institute of Neurological Disorders and Stroke (NINDS) Grant 5R01 NS 039087 (PI, Dr. Sallee).
Disclosures
Dr. Palumbo is employed by Pfizer pharmaceuticals and is an adjunct faculty member at The University of Rochester. Dr. Sallee is a consultant to Shire and Otsuka. Dr. Daviss is an independent reviewer of psychiatric evaluations for a study sponsored by Lexicor Inc. and is also a paid speaker for Quintiles Medical Education. Dr. Buckstein is a consultant for Quintiles CME, and is involved with an edited book for Routledge Publishing.
References
- Adler LA. Spencer TJ. Levine LR. Ramsey JL. Tamura R. Kelsey D. Ball SG. Allen AJ. Biederman J. Functional outcomes in the treatment of adults with ADHD. J Attention Disorders. 2007;20:1–8. doi: 10.1177/1087054707308490. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders., 4th edition, Text Revision (DSM-IV-TR) Washington (DC): American Psychiatric Association; 2000. [Google Scholar]
- Anastopoulos AD. Guevremont DC. Shelton TL. DuPaul GJ. Parenting stress among families of children with attention-deficit hyperactivity disorder. J Abnormal Child Psych. 1992;20:503–520. doi: 10.1007/BF00916812. [DOI] [PubMed] [Google Scholar]
- Bastiaansen D. Koot HM. Ferdinand RF. Verhulst FC. Quality of life in children with psychiatric disorders: Self-, parent, and clinician report. J Am Acad Child Adolesc Psychiatry. 2004;43:221–230. doi: 10.1097/00004583-200402000-00019. [DOI] [PubMed] [Google Scholar]
- Brown RT. Perwien A. Faries DE. Kratochvil CJ. Vaughan BS. Atomoxetine in the management of children with ADHD: Effects on quality of life and school functioning. Clin Pediatrics. 2006;45:819–827. doi: 10.1177/0009922806294219. [DOI] [PubMed] [Google Scholar]
- Crnic KA. Greenberg MT. Minor parenting stresses with young children. Child Dev. 1990;61:1628–1637. doi: 10.1111/j.1467-8624.1990.tb02889.x. [DOI] [PubMed] [Google Scholar]
- Cummins RA. Assessing Quality of Life in Brown. In: Roy , editor. Quality of Life for People with Disabilities. Models, Research and Practice. Cheltenham: Stanley Thornes (Publishers) Ltd.; 1997. [Google Scholar]
- Daviss W. Patel N. Robb A. McDermott M. Bukstein O. Pelham W. Palumbo D. Harris P. Sallee F. Clonidine for Attention-Deficit/Hyperactivity Disorder: II. ECG changes and adverse events analysis. J Am Acad Child Adolesc Psychiatry. 2008;47:189–198. doi: 10.1097/chi.0b013e31815d9ae4. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ. Power TJ. Anastopoulos AD. Reid R. ADHD Rating Scale-IV: Checklist, Norms, Clinical Interpretation. New York: Guilford; 1998. [Google Scholar]
- Escobar R. Soutullo CA. Hervas A. Gastaminza X. Polavieja P. Gilaberte I. Worse quality of life for children with newly diagnosed attention-deficit/hyperactivity disorder, compared with asthmatic and healthy children. Pediatrics. 2005;116:364–369. doi: 10.1542/peds.2005-0386. [DOI] [PubMed] [Google Scholar]
- Fischer M. Parenting stress and the child with attention deficit hyperactivity disorder. J Clin Child Psychol. 1990;19:337–346. [Google Scholar]
- Fisher PW. Shaffer D. Piacentini JC. Sensitivity of the Diagnostic Interview Schedule for Children, 2nd edition (DISC-2.1) for specific diagnoses of children, adolescents. J Am Acad Child Adolesc Psychiatry. 1993;32:666–667. doi: 10.1097/00004583-199305000-00026. [DOI] [PubMed] [Google Scholar]
- Greenberg MT. Crnic KA. Minor parenting stresses with young children. Child Dev. 1990;61:1628–1637. doi: 10.1111/j.1467-8624.1990.tb02889.x. [DOI] [PubMed] [Google Scholar]
- Hagerty MR. Cummins RA. Ferriss AL. Land K. Michalos AC. Peterson M, et al. Quality of life indexes for national policy. Review and Agenda for Research, Social Indicators Research. 2001;55:1–96. [Google Scholar]
- Harrison C. Sofronoff K. ADHD and parental psychological distress: role of demographics, child behavioral characteristics, and parental cognitions. J Am Acad Child Adolesc Psychiatry. 2002;41:703–711. doi: 10.1097/00004583-200206000-00010. [DOI] [PubMed] [Google Scholar]
- Klassen AF. Miller A. Fine S. Health-related quality of life in children and adolescents who have a diagnosis of attention-deficit/hyperactivity disorder. Pediatrics. 2004;114:541–547. doi: 10.1542/peds.2004-0844. [DOI] [PubMed] [Google Scholar]
- Johnston C. Mash EJ. Families of children with attention-deficit/hyperactivity disorder: Review and recommendations for future research. Clin Child Family Psychol Rev. 2001;4:183–207. doi: 10.1023/a:1017592030434. [DOI] [PubMed] [Google Scholar]
- Landgraf JM. Abetz L. Ware JE. The CHQ: A User's Manual (2nd printing). Boston, MA: HealthAct, 1st printing. Boston (Massachusetts): The Health Institute; 1996. [Google Scholar]
- Landgraf JM. Rich M. Rappaport L. Measuring quality of life in children with attention-deficit/hyperactivity disorder and their families: Development and evaluation of a new tool. Arch Pediatr Adolesc Med. 2002;156:384–391. doi: 10.1001/archpedi.156.4.384. [DOI] [PubMed] [Google Scholar]
- Matza LS. Rentz AM. Secnik K. Swensen AR. Revicki DA. Michelson D, et al. The link between health-related quality of life and clinical symptoms among children with attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2004;25:166–174. doi: 10.1097/00004703-200406000-00005. [DOI] [PubMed] [Google Scholar]
- Palumbo D. Sallee F. Pelham W. Buckstein O. Daviss W. McDermott M. Clonidine for attention-deficit/hyperactivity disorder: I. Efficacy and tolerability outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47:180–188. doi: 10.1097/chi.0b013e31815d9af7. [DOI] [PubMed] [Google Scholar]
- Pelham WE. Milich R. Murphy DA. Normative data on the Iowa Conners Teacher Rating Scale. J Clin Child Psychol. 1989;18:259–262. [Google Scholar]
- Pelham WE. Gnagy EM. Greenslade KE. Milich R. Teacher ratings of DSM-III-R symptoms of the disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Perwien AR. Faries DE. Kratochvil CJ. Sumner CR. Kelsey DK. Allen AJ. Improvement in health-related quality of life in children with ADHD: An analysis of placebo controlled studies of atomoxetine. J Dev Behav Pediatrics. 2004;25:264–271. doi: 10.1097/00004703-200408000-00006. [DOI] [PubMed] [Google Scholar]
- Podolski CL. Nigg JT. Parent stress and coping in relation to child ADHD severity and associated child disruptive behavior problems. J Clin Child Psychol. 2001;30:503–513. doi: 10.1207/S15374424JCCP3004_07. [DOI] [PubMed] [Google Scholar]
- Polanczyk G. Silva de Lima M. Horta BL. Biederman J. Rohde LA. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Rentz A. Matza L. Secnik K. Swensen A. Revicki D. Psychometric validation of the child health questionnaire (CHQ) in a sample of children and adolescents with attention-deficit/hyperactivity disorder. Quality Life Res. 2005;14:719–734. doi: 10.1007/s11136-004-0832-9. [DOI] [PubMed] [Google Scholar]
- Sallee FR. Ambrosini PJ. Lopez FA. Shi L. Michaels MA. Health-related quality of life and treatment satisfaction and preference in a community assessment study of extended-release mixed amphetamine salts for children with attention-deficit/hyperactivity disorder. J Outcomes Res. 2003;7:21–33. [Google Scholar]
- Sawyer MG. Whaites L. Rey JM. Hazell PL. Graetz BW. Baghurst P. Health-related quality of life of children and adolescents with mental disorders. J Am Acad Child Adolesc Psychiatry. 2002;41:530–537. doi: 10.1097/00004583-200205000-00010. [DOI] [PubMed] [Google Scholar]
- Shaffer D. Gould MS. Brasic J. A children's global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Stein REK. Reissman CK. The development of an Impact-on-Family Scale: Preliminary findings. Medical Care. 1980;18:456–472. doi: 10.1097/00005650-198004000-00010. [DOI] [PubMed] [Google Scholar]
- Varni JW. Seid M. Kurtin PS. PedsQL4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Waters E. Stewart-Brown S. Fitzpatrick R. Agreement between adolescent self-report and parent reports of health and well-being: results of an epidemiological study. Child Care Health Dev. 2003;29:501–509. doi: 10.1046/j.1365-2214.2003.00370.x. [DOI] [PubMed] [Google Scholar]