Abstract
The build-up of species locally within a region by allopatric speciation depends on geographically separated (allopatric) sister populations becoming reproductively incompatible followed by secondary sympatry. Among birds, this has happened frequently in remote archipelagos, spectacular cases including the Darwin's finches (Geospizinae) and Hawaiian honeycreepers (Drepanidinae), but similar examples are lacking in archipelagos nearer to continental landmasses. Of the required steps in the speciation cycle, achievement of secondary sympatry appears to be limiting in near archipelagos and, by extension, in continental regions. Here, I suggest that secondary sympatry might be prevented by apparent competition mediated through pathogens that are locally coevolved with one population of host and are pathogenic in sister populations. The absence of numerous pathogens in remote archipelagos might, therefore, allow sister populations to achieve secondary sympatry more readily and thereby accelerate diversification. By similar reasoning, species should accumulate relatively slowly within continental regions. In this essay, I explore the assumptions and some implications of this model for species diversification.
Keywords: adaptive radiation, allopatry, island birds, parasites, speciation, sympatry
1. Introduction
Speciation is the process through which populations descending from a single ancestor eventually achieve reproductive isolation (de Queiroz 1998). Evolutionary biologists believe that most instances of species formation initially involve geographical isolation of subpopulations (allopatric speciation, Coyne & Orr 2004). Reproductive isolation can arise between such allopatric populations either through random changes in the gene pool or through adaptive divergence (Baker et al. 2005). However, although new evolutionary entities can form in this manner, diversity does not increase locally until reproductively isolated sister populations achieve secondary sympatry. While visiting the Galápagos Islands, Darwin (1839, p. 494) noted that the local mockingbirds (Mimus spp.) differed in appearance from island to island. The divergence of these populations, among which four species currently are recognized (Arbogast et al. 2006), might represent the initial stages of species formation in allopatry, but none of these species co-occurs with any another, in spite of the evidently recent expansion of M. parvulus across several islands (Arbogast et al. 2006).
The Darwin's finches (Geospizinae) in the Galápagos Islands present a distinctly different situation (Grant & Grant 2007). Many of the species of finches have extended their ranges across much of the archipelago and broadly overlap the distributions of other species. Not realizing the common ancestry of the finch species in the Galápagos, Darwin initially missed the significance of these birds to understanding evolution and diversification; their close relationship was later pointed out to him by the ornithologist John Gould (Sulloway 1982). Because the finches are now known to have descended from a single population that colonized the Galápagos, perhaps 2.3 Ma (Sato et al. 2001; Burns et al. 2002), coexistence of finch species following their evolution in allopatry has been the result of subsequent range expansion and establishment of secondary sympatry. Eleven of the 13 recognized species occur on the island of Isabela (Grant 1986), although the process of speciation is not sufficiently complete to prevent frequent hybridization, and some workers have questioned the validity of species limits in the genera Geospiza and Camarhynchus (Zink 2002). A similar build-up of species locally has occurred among the honeycreepers (Drepanidinae) on the Hawaiian Islands (Amadon 1950; James 2004; Pratt 2005). Many island populations, and many entire species, of honeycreepers have become extinct since the arrival of Polynesians, and then Europeans, on the islands. Nevertheless, the combination of extant species and those recognized in the recent fossil record suggests that the drepanid radiation produced at least 59 recent species during the last ca 6 Myr (Fleischer & McIntosh 2001), at least 15 of which were historically present on a single island (Hawaii, Amadon 1950).
(a). The importance of secondary sympatry
In principal, the number of species within a region could grow by allopatric speciation alone. Accordingly, however, geographical ranges would diminish with each new speciation event until the lower potential for geographical isolation and higher rate of extinction of populations with smaller ranges caused diversification to grind to a halt (Rosenzweig 1995). Secondary sympatry of reproductively isolated populations is essential to the build-up of diversity locally as well as within a region. Nowhere is this more obvious than in archipelagos. In contrast to the songbird (Passeriformes) avifaunas of the Hawaiian and Galápagos archipelagos, each of which has supported a spectacular radiation of species, the New Hebrides (Vanuatu) have supported no such radiation (Diamond & Marshall 1976, 1977) and the Lesser Antilles have only a small radiation consisting of four species of thrashers (Hunt et al. 2001). Based on this comparison, Ricklefs & Bermingham (2007a) speculated on the causes of the difference in achieving secondary sympatry among these island groups.
Both the Galápagos and Hawaiian archipelagos are remote and their extant passerine avifaunas are the products, in each case, of six colonization events. The New Hebrides and Lesser Antilles, being closer to continental sources of colonists, have received many more immigrants—24 and 34, respectively—to their contemporary passerine avifaunas. The ecological space in these ‘near’ archipelagos has been filled by colonization while that in the distant archipelagos has been filled primarily by local adaptive radiation. This describes what has happened, but it does not explain why species diversification has been suppressed in the near archipelagos. In fact, islands in the Lesser Antilles and New Hebrides have far fewer species than occur in comparable environments in continental regions (Cox & Ricklefs 1977; Diamond & Marshall 1977). Moreover, new colonists appear to spread readily through both archipelagos (Ricklefs & Bermingham 2007b), suggesting that the ecological space on these islands is relatively porous. Allopatric populations of passerine species are no more divergent morphologically in the Hawaiian and Galápagos archipelagos than they are in the Lesser Antilles (Ricklefs & Bermingham 2007a); diversification of size and shape apparently develops primarily subsequent to secondary sympatry. Thus, ecological incompatibility might not create an important barrier to secondary sympatry in archipelagos like the Lesser Antilles. Higher gene flow between islands in close archipelagos also cannot probably explain infrequent diversification because distances between islands are comparable with distant island groups and differentiation in allopatry is common (Ricklefs & Bermingham 2007a).
Achieving secondary sympatry depends equally on (i) isolation of subpopulations with negligible gene flow between islands, (ii) persistence of isolated populations long enough to develop reproductive incompatibility, most probably through pre-reproductive isolating mechanisms (Price & Bouvier 2002; Price 2008), and (iii) occasional recolonization of islands harbouring sister populations to establish secondary sympatry. Ricklefs & Bermingham (2002) estimated from mitochondrial DNA divergence that populations in the Lesser Antilles can persist on individual islands for several million years; furthermore, many of these populations exhibit episodic phases of secondary expansion within the archipelago (Ricklefs & Bermingham 2001), occasionally reaching back to the continent (Bellemain & Ricklefs 2008). In the Lesser Antilles, however, the commonest result of these phases of expansion is non-overlapping distributions of genetically distinct subpopulations with range boundaries between adjacent islands within the archipelago (Ricklefs & Bermingham 2007a).
(b). Pathogens and secondary sympatry
To explain the low rate of secondary sympatry in birds of the Lesser Antilles, Ricklefs & Bermingham (2007a) suggested that incompatibility among sister populations might result from parasites that coevolve low virulence with host populations locally, but are pathogenic in sister populations lacking recent exposure to the allopatric parasite. The mechanism is thus ‘apparent competition’ (Holt 1977; Holt & Lawton 1994), in which potentially shared parasites cause incompatibility between two host populations in sympatry. Such parasites could either prevent the invasion of an island or result in the exclusion of the local population by a new colonist, effectively preventing secondary sympatry of host populations in both cases. Although considerable attention has been paid to the effects of both generalized and specialized predators on range limits in prey populations (Holt & Barfield 2009), little theory has been developed for the effects of specialized pathogens. The outcomes of pathogen–host interactions undoubtedly depend on the particulars of their coevolutionary interactions. However, as summarized below, sufficient empirical evidence has accumulated on the impacts of introduced pathogens that prevention of secondary sympatry by pathogen incompatibility is plausible.
Apparent competition might be less of a factor in remote archipelagos, such as the Hawaiian and Galápagos Islands, if fewer parasite lineages colonized or persisted in such locations. Although the original host populations undoubtedly carried their own parasites, these might have been lost over time. In addition, fewer colonizing species in remote archipelagos would have led to smaller parasite species pools and fewer parasites available to switch hosts. By implication, continental biotas could harbour a correspondingly broader array of parasite species, and apparent competition consequently might inhibit secondary sympatry even more than in near archipelagos. Accordingly, the rate of species diversification would be highest in remote archipelagos and lowest in diverse continental assemblages. Similarly, we would expect sympatry among related lineages to be high in remote archipelagos and low within the major continents.
2. Evidence in support of the pathogen hypothesis
Several observations are consistent with the hypothesis that coevolved pathogens interfere with secondary sympatry and, potentially, can inhibit diversification.
(a). Many populations are adversely affected by introduced pathogens
The history of the spread of humans around the globe is replete with colonists bringing devastating diseases that become epidemic in susceptible native populations (Diamond & Marshall 1977; McNeill 1998). Examples in animal populations include West Nile Virus (Lanciotti et al. 1999; Kramer & Bernard 2001), Mycoplasma gallisepticum (Dhondt et al. 1998), the malaria parasite Plasmodium relictum in the Hawaiian avifauna (van Riper et al. 1986), myxomatosis virus in the introduced European rabbit populations of Australia (Fenner & Ratcliffe 1965), rinderpest (Plowright 1982) and canine distemper virus (McCarthy et al. 2007). The recently discovered white-nose fungus in Myotis and Perimyotis bats in the northeastern USA has caused severe population declines of several species but apparently does not affect the co-distributed Eptesicus fuscus (Blehert et al. 2009). Many of these pathogens are viruses, and they may be spread by vectors or by direct contact. In general, ‘emerging diseases’ commonly represent the movement of an endemic parasite into a new host, often in a new geographical region (Daszak et al. 2000).
(b). Pathogens have been implicated in the competitive displacement of one species by another
The case of the introduced North American gray (or grey) squirrel (Sciurus carolinensis), which has replaced the native red squirrel (S. vulgaris) over much of the UK, is a widely cited example. The interaction between these species possibly has involved the emergence of a novel poxvirus that is deadly to red squirrels but seemingly harmless to grey squirrels, which are nonetheless carriers of the pathogen and a source of infection (Sainsbury et al. 2000; Tompkins et al. 2003; McInnes et al. 2006). The key to this interaction is the ability of the grey squirrel to support an endemic viral parasite that is highly pathogenic in a closely related population. A caecal nematode appears to be responsible for the exclusion of grey partridge by ring-necked pheasant (Tompkins et al. 2000a,b, 2001). A similar case concerns the decimation of native noble crayfish (Astacus astacus) populations by the crayfish plague, the freshwater fungal pathogen Aphanomyces astaci, which was introduced into European lakes with the western North American signal crayfish Pacifastacus leniusculus, a species that is resistant to the fungus but an effective carrier (Alderman 1996; Josefsson & Andersson 2001; Oidtmann et al. 2002).
(c). Local coevolution of host and parasite populations
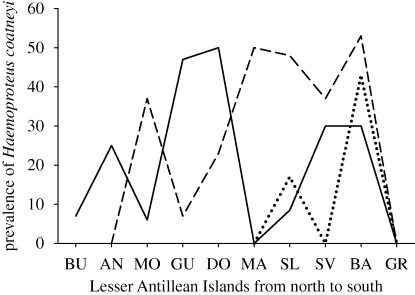
Local coevolution is widely observed in nature (Thompson 2005) and has been demonstrated in the case of haemosporidian parasites of birds, for example, by a strong species × island interaction in the prevalence of the parasites in passerine birds of the Lesser Antilles (Apanius et al. 2000; Fallon et al. 2005; figure 1). In this example, a single lineage of parasite (Haemoproteus coatneyi), characterized in our analyses by its mitochondrial cytochrome b sequence, exhibits different prevalence levels in the same host species (e.g. the bananaquit Coereba flaveola) on different islands; bananaquits apparently escape infection completely on the island of Martinique, even though the parasite is present there in other species. Although prevalence might reflect pathogen or vector abundance rather than host susceptibility, the Lesser Antillean bullfinch (Loxigilla noctis) exhibits an inverse trend in the prevalence of the same parasite throughout the Lesser Antilles, with higher infection rates where the parasite is uncommon in bananaquits. To the extent that parasite prevalence represents the relative susceptibility of a host population to a particular parasite organism, these patterns suggest local coevolution. In particular, where H. coatneyi is prevalent in one host species, it is sparse in another. Host sharing tends to involve closely related host species (e.g. Bensch et al. 2000; Ricklefs & Fallon 2002; Ricklefs et al. 2004; Davies & Pedersen 2008), providing opportunities for the evolution of resistance in one sister population to depress a susceptible sister population. Even in the case of novel pathogens introduced into a region, potential host species vary in their innate resistance to infection and the production of pathogenic effects (e.g. Wheeler et al. 2009).
Figure 1.
Prevalence (%) of the haemosporidian parasite Haemoproteus (Parahaemoproteus) coatneyi in the bananaquit Coereba flaveola, Lesser Antillean bullfinch L. noctis and black-faced grassquit Tiaris bicolor in the Lesser Antilles. Islands, from north to south are BU, Barbuda; AN, Antigua; MO, Montserrat; GU, Guadeloupe; DO, Dominica; MA, Martinique; SL, St Lucia; SV, St Vincent; BA, Barbados; GR, Grenada. Haemoproteus coatneyi is the only haemosporidian present on Barbados. All three host species are common on all the islands. Solid line, bananaquit; dashed line, bullfinch; dotted line, grassquit.
(d). Distribution anomalies
The distributions of many species of bird in the West Indies cannot be attributed readily to dispersal limitation or competition for resources (Lack 1976) and are thus candidates for exclusion by pathogens. Among the best known of these anomalies is the absence of the bananaquit C. flaveola from Cuba. The bananaquit is the most abundant bird in the West Indies and it inhabits every island in the archipelago, regardless of size, with the exception of Cuba (Bond 1956; Raffaele et al. 1998). Dispersal evidently is not a problem because bananaquits are known from the cays off the north coast of Cuba, and may breed there (Garrido & Kirkconnell 2000). In addition, a lineage of the bananaquit occurs in Quintana Roo, Mexico, separated by Cuba from its closest relatives in the Bahamas Islands (Bellemain et al. 2008). Lack (1976) and MacArthur & Wilson (1967) suggested that competitors, including wintering North American warblers and the native red-legged honeycreeper, might exclude the bananaquit from Cuba. However, the bananaquit is distributed throughout South and Central America and is abundant on the islands of Hispaniola and Puerto Rico, which also host dense populations of wintering migrants (Raffaele et al. 1998; Latta et al. 2003).
A similar distribution anomaly involves the absence of the pearly eyed thrasher (Margarops fuscatus) from the mainland of Hispaniola, although it is a common breeder on Soana and Beata Islands close to the south and east coasts of Hispaniola (apparently now on the mainland in the far eastern end of the island, Latta et al. 2006) and on the southern Bahamas Islands to the north. These and other examples of gaps in the distribution of otherwise widespread species have many potential explanations, among which is that a local population of another host species harbours an endemic parasite that is pathogenic in the missing species. Both Cuba and Hispaniola have many endemic species of birds that might serve as reservoirs for locally coevolved disease organisms, which could prevent the invasion of susceptible species.
(e). Parapatric (abutting) distributions of closely related species are common in regions of high species richness
Non-overlapping distributions of closely related populations are often attributed to competition between the species or, in cases in which the populations interbreed, to the reduced fitness of hybrids (Cadena 2007), although this is not observed in some hybrid zones (e.g. Flockhart & Wiebe 2009). Regardless of their causes, parapatric distributions sometimes give way to sympatry with time as barriers to population overlap are dismantled. Classic cases of parapatric distributions involve replacements of related species, primarily members of the same genus, along altitudinal gradients. For example, Terborgh (1971) attributed 36 per cent of lower limits and 28 per cent of upper limits of birds along an altitudinal transect in the eastern Andes of Peru to competition from closely related species (his table 5); he further suggested that these numbers were underestimates in some cases owing to difficulties regarding the taxonomic identification of congeners. Terborgh's fig. 9 shows the parapatric ranges of species in several genera, including many with gaps between the distributions of related species. These gaps would not be expected if exploitative competition were the cause (unless perhaps, a third competitor was distributed between them), but they might reflect interactions through pathogens if vectors carried the parasites beyond the altitudinal ranges of the hosts.
Diamond (1972) also commented extensively on altitudinal parapatry in the ranges of congeneric species in New Guinea. Diamond (1972) suggested that through the initial stages of species formation, incipient species were so similar ecologically that distributional overlap was precluded by competitive exclusion. For example, ‘… one finds sequences of two, three, or even four closely related species replacing each other abruptly with altitude’ (Diamond 1972, p. 764; 1973). Further, ‘… spatial segregation is the dominant mode of ecological segregation through at least stage six of speciation. It is the sole mode of segregation in 88 of the 119 cases … among submodes of spatial segregation, the most prevalent is altitude, acting along in 63 cases and acting in combination with other modes and spatial submodes in 48 cases. Often, but not always, the altitudinal border between the two species is sharp: the highest individual of the low-altitude species abuts and is interspecifically territorial with the lowest individual of the high-altitude species’ (Diamond 1986, pp. 105–106). In the case of altitudinal replacement among two species of Crateroscelis warblers (Diamond 1973, fig. 6), both species reached their highest densities close to their shared boundary.
(f). Host populations on remote islands have relatively few parasites
The evidence on this point is sparse owing to a dearth of comparative studies of disease prevalence in continental and island settings. Beadell et al. (2007) found reduced prevalence of haemosporidian parasites in populations of several species of bird on remote islands of the western Pacific Ocean. Birds of the Hawaiian Islands apparently lacked such parasites before the introduction of P. relictum (Warner 1968; van Riper et al. 1986). Surveys of a variety of common diseases of domestic poultry in the Galápagos Islands failed to find evidence of related infections in native species (Soos et al. 2008). We have not found haemosporidian parasites in samples of Darwin's finches (R. E. Ricklefs and P. G. Parker, unpublished data). The isolated island of Barbados to the east of the Lesser Antilles has only a single common lineage of haemosporidian parasite, which exhibits expanded host breadth compared with that of the same parasite lineage in the Lesser Antilles, where it coexists with many others (Fallon et al. 2005) (figure 1).
(g). Island populations should exhibit low resistance to introduced pathogens
The prediction of reduced resistance follows from the hypothesis that remote archipelagos have reduced parasite pressure for maintaining defences against infection. In this case, we would expect introduced parasites, to which island populations have had no previous exposure, to encounter little resistance to infection. A difficulty with this prediction is that populations should lack explicit resistance to novel specialized parasites, regardless of the local parasite environment. Thus, the prediction depends on the maintained level of generalized resistance selected by the local parasite community.
Few comparative studies have addressed this prediction and the evidence on this point is mixed (Matson 2006). Low genetic variation has been reported in many island populations, and this is generally attributed to founder effects and loss of genetic diversity through drift (bottleneck effects) over long periods (Beadell et al. 2007; Siddle et al. 2007). In the Galápagos hawk (Buteo galapagoensis), genetic (minisatellite) diversity in populations decreases with smaller island size, as does the concentration of circulating natural antibodies, while the abundance of biting (amblyceran) feather lice increases (Whiteman et al. 2006). In contrast, natural antibodies and other measures of immune system function appeared no different in matched mainland and island populations of birds (Matson 2006).
The effects of introduced pathogens documented in some island populations suggest reduced resistance of island taxa to infection, although similar epidemics have also been reported from continental species. A survey of introduced diseases in island and continental settings might favour heightened susceptibility in island populations, but I am not aware of a quantitative analysis. Certainly, some spectacular cases of invasive diseases have been reported from remote islands. Among the most famous are introduced avian poxvirus, avian malaria (P. relictum), and Toxoplasma gondii in the Hawaiian avifauna (van Riper et al. 1986; Work et al. 2000). Many endemic species are susceptible to malaria parasites, which cause a high level of morbidity and mortality (Atkinson et al. 1995, 2000, 2001), although the underlying genetic basis for susceptibility has not been established (Jarvi et al. 2004). Some studies point to a role for major histocompatibility complex (MHC) alleles in resistance against certain parasites (Westerdahl et al. 2005; Bonneaud et al. 2006), and MHC diversity is thought to be reduced in island populations, but with little supporting evidence (Vincek et al. 1997).
Susceptibility to malaria varies among species in the native Hawaiian avifauna. For example, Hawaiian thrushes (Myadestes) and Hawaiian crows (Massey et al. 1996) become infected but apparently can control the disease at benign levels (Atkinson et al. 2001). Many native and introduced species are not seriously affected by avian malaria, and there is evidence that some populations are beginning to evolve resistance to the parasite (Woodworth et al. 2005). In the Galápagos Islands, the Galápagos dove is apparently extremely susceptible to infection by Haemoproteus spp. parasites, with prevalence approaching 100 per cent and high infection intensities (Santiago-Alarcon et al. 2008). In this case, the origin of the parasite, particularly the timing of its arrival in the archipelago, is unclear, although several parasite lineages present in the Galápagos populations of doves are closely related, if not genetically identical, to continental parasites (Santiago-Alarcon et al. in press). Thus, it is likely that Haemoproteus is a recent arrival in Galápagos doves.
Patterns of infection among Hawaiian birds are mirrored to some degree by disease in the human populations of Hawaii. Toxoplasma gondii is widespread among Pacific Islanders, reaching prevalences of 85–100% on many islands (Wallace 1976). However, on Oahu and Hawaii, prevalence depended on ethnic group, with the Japanese population, derived recently from a highly diverse region, showing the lowest prevalence. On the island of Taiwan, close to the Asian continent, few individuals tested positive for Toxoplasma antibody. As one would expect, susceptibility to parasites in island populations extends beyond micro-organisms, as in the case of nestlings of Darwin's finches (Geospiza) on the Galápagos Islands, which are heavily infested by blood-sucking larvae of the introduced fly Philornis spp. (Fessl et al. 2006).
3. Conclusions
(a). Isolation, disease and rate of diversification
The hypothesis that coevolved (i.e. specialized) disease organisms can limit secondary sympatry and thus rate of diversification, combined with the assumption that such disease organisms are less frequent on remote islands, implies that rate of diversification should be relatively high on remote islands and relatively low in diverse continental settings. Comparisons among archipelagos are consistent with this in that adaptive radiations of some island taxa have involved high rates of species formation associated with the build-up of sympatric populations (table 1). It is certainly true, however, that many island colonists fail to diversify and that rapid radiations also occur in continental settings. The examples in table 1 are highly selected, do not separate species formation and extinction and ignore time heterogeneity in rates. They are meant primarily to illustrate the need for a comparative approach to documenting rates of species formation in different geographical settings.
Table 1.
Estimated average rates (Myr−1) of diversification (speciation–extinction), calculated as ln(species)/time, for several groups of island and continental taxa.
| taxon | location | species | age (Myr) | diversification rate (Myr−1) | reference |
|---|---|---|---|---|---|
| Darwin's finches | Galápagos Islands | 13 | 2.3 | 1.12 | Sato et al. (2001) |
| honeycreepers | Hawaiian Islands | >57 | 4.5 | 0.90 | Fleischer et al. (1998) |
| thrashers | Lesser Antilles | 4 | 2.5 | 0.55 | Hunt et al. (2001) |
| Dendroica warblers | Nearctic | 27 | 4.5 | 0.73 | Lovette & Bermingham (1999) |
| obligate ant-following Thamnophilidae | Neotropics | 16 | 5.1 | 0.54 | Brumfield et al. (2007) |
| silverswords (Compositae) | Hawaiian Islands | 28 | 5.2 | 0.56 | Baldwin & Sanderson (1998) |
A core prediction of the hypothesis that pathogens depress secondary sympatry and species diversification is that sympatry among related species decreases with increased species richness. One of the hallmarks of the honeycreeper radiation in the Hawaiian Islands and the finch radiation in the Galápagos Islands is the high degree of sympatry among closely related species (Grant 1986; Pratt 2005). The wood warblers (Parulidae) of North America represent one of the most rapid continental radiations of passerine birds and as many as 22 of 42 common species can occur locally (Lovette & Hochachka 2006), suggesting few barriers to sympatry in this group. In contrast, the greater number of geographical subspecies and allopatric phylogenetic lineages per species in the tropics suggests that higher diversity makes sympatry more difficult (Martin & Tewksbury 2008). In each case, of course, this might be due to direct competitive effects as well (Holt & Barfield 2009).
Another prediction of the pathogen hypothesis of species diversification is that the mechanism would be most applicable where host taxa have evolved specific defences against specific pathogens, as in the case of an antibody-based immune response. Thus, insects and plants, for example, which depend on more generalized defences (Burdon & Thrall 1999; Kraaijeveld & Godfray 1999; Hultmark 2003; Vijendravarma et al. 2009), should form secondary sympatry more readily and thus diversify more rapidly. Certainly, these groups exhibit very high diversity. Relatively few studies of plants and insects have reported apparent competition, particularly based on specialized pathogens (e.g. Grosholz 1992; Morris et al. 2004, 2005; Collinge & Ray 2006; Carvalheiro et al. 2008; Orrock et al. 2008; van Veen et al. 2008).
(b). Testing the pathogen hypothesis
The most definitive test of the hypothesis that pathogens can prevent secondary sympatry of sister host populations would be reciprocal translocation. Introductions of alien populations are ethically unacceptable and logistically difficult, although historical introductions, both accidental and intentional, have provided uncontrolled ‘experimental’ results consistent with the hypothesis (§2b). Under some circumstances, it would be possible to maintain alien individuals in captivity yet exposed to ambient pathogens, for example, by caging birds in natural habitats, and this approach would be worth pursuing. Where pathogens can be isolated and cultured, it would be possible to conduct cross-infections in a laboratory setting. One roadblock for experimental tests will be determining the identity of the pathogen or pathogens that prevent secondary sympatry, if they exist at all. Accordingly, genetic differentiation of particular pathogens between closely related host species, or of host genes for resistance to particular pathogens (e.g. Bonneaud et al. 2006), would be relatively uninformative with respect to identifying causative agents or testing the pathogen hypothesis for parapatry.
Two less direct studies would be relevant. First, comparative surveys of disease prevalence and diversity between islands and continents, among biomes, and among taxa are virtually non-existent and should be a high priority (e.g. Soos et al. 2008). Of course, most pathogens of wild species have not been described, and screening would be limited to agents for which diagnostic tests have been developed for commercially important species (e.g. many viruses) or that can be observed directly (e.g. haemosporidians and trypanosomes). Second, where pathogen pressure can be assessed, time to achieve secondary sympatry (Barraclough & Vogler 2000; Lovette & Hochachka 2006) would be expected to increase in direct relation to the diversity and prevalence of pathogens. I believe we currently lack the empirical foundation to apply these tests. Clearly, comparative surveys of pathogens in natural populations should become a high priority on a broad scale.
Acknowledgements
I thank Bob Holt, Bob Zink and an anonymous reviewer for discussion and comments, and the National Science Foundation of the United States (DEB-0089226, DEB-0542390) for support of research on West Indian birds and their haemosporidian parasites.
Footnotes
One contribution of 13 to a Theme Issue ‘Darwin's Galápagos finches in modern evolutionary biology’.
References
- Alderman D. J.1996Geographical spread of bacterial and fungal diseases of crustaceans. Rev. Sci. Tech. 15, 603–632 [DOI] [PubMed] [Google Scholar]
- Amadon D.1950The Hawaiian honeycreepers (Aves, Drepaniidae). Bull. Am. Mus. Nat. Hist. 95, 151–262 [Google Scholar]
- Apanius V., Yorinks N., Bermingham E., Ricklefs R. E.2000Island and taxon effects in parasitism and resistance of Lesser Antillean birds. Ecology 81, 1959–1969 [Google Scholar]
- Arbogast B. S., Drovetski S. V., Curry R. L., Boag P. T., Seutin G., Grant P. R., Grant B. R., Anderson D. J.2006The origin and diversification of Galápagos mockingbirds. Evolution 60, 370–382 [PubMed] [Google Scholar]
- Atkinson C. T., Woods K. L., Dusek R. J., Sileo L. S., Iko W. M.1995Wildlife disease and conservation in Hawaii: pathogenicity of avian malaria (Plasmodium relictum) in experimentally infected iiwi (Vestiaria coccinea). Parasitology 111(Suppl.), S59–S69 (doi:10.1017/S003118200007582X) [DOI] [PubMed] [Google Scholar]
- Atkinson C. T., Dusek R. J., Woods K. L., Iko W. M.2000Pathogenicity of avian malaria in experimentally-infected Hawaii Amakihi. J. Wildlife Dis. 36, 197–204 [DOI] [PubMed] [Google Scholar]
- Atkinson C. T., Lease J. K., Drake B. M., Shema N. P.2001Pathogenicity, serological responses, and diagnosis of experimental and natural malarial infections in native Hawaiian thrushes. Condor 103, 209–218 (doi:10.1650/0010-5422(2001)103[0209:PSRADO]2.0.CO;2) [Google Scholar]
- Baker A. J., Huynen L. J., Haddrath O., Millar C. D., Lambert D. M.2005Reconstructing the tempo and mode of evolution in an extinct clade of birds with ancient DNA: The giant moas of New Zealand. Proc. Natl Acad. Sci. USA 102, 8257–8262 (doi:10.1073/pnas.0409435102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin B. G., Sanderson M. J.1998Age and rate of diversification of the Hawaiian silversword alliance (Compositae). Proc. Natl Acad. Sci. USA 95, 9402–9406 (doi:10.1073/pnas.95.16.9402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough T. G., Vogler A. P.2000Detecting the geographical pattern of speciation from species-level phylogenies. Am. Nat. 155, 419–434 (doi:10.1086/303332) [DOI] [PubMed] [Google Scholar]
- Beadell J. S., Atkins C., Cashion E., Jonker M., Fleischer R. C.2007Immunological change in a parasite-impoverished environment: divergent signals from four island taxa. PLoS ONE 2, e896 (doi:10.1371/journal.pone.0000896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemain E., Ricklefs R. E.2008Are islands the end of the colonization road? Trends Ecol. Evol. 23, 461–468 (doi:10.1016/j.tree.2008.05.001) [DOI] [PubMed] [Google Scholar]
- Bellemain E., Bermingham E., Ricklefs R. E.2008The dynamic evolutionary history of the bananaquit (Coereba flaveola) in the Caribbean revealed by a multigene analysis. BMC Evol. Biol. 8, 240 (doi:10.1186/1471-2148-8-240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch S., Stjernman M., Hasselquist D., Ostman O., Hansson B., Westerdahl H., Pinheiro R. T.2000Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc. R. Soc. Lond. B 267, 1583–1589 (doi:10.1098/rspb.2000.1181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blehert D. S., et al. 2009Bat white-nose syndrome: an emerging fungal pathogen? Science 323, 227 (doi:10.1126/science.1163874) [DOI] [PubMed] [Google Scholar]
- Bond J.1956Checklist of birds of the West Indies. Philadelphia, PA: Academy of Natural Sciences [Google Scholar]
- Bonneaud C., Perez-Tris J., Federici P., Chastel O., Sorci G.2006Major histocompatibility alleles associated with local resistance to malaria in a passerine. Evolution 60, 383–389 [PubMed] [Google Scholar]
- Brumfield R. T., Tello J. G., Cheviron Z. A., Carling M. D., Crochet N.2007Phylogenetic conservatism and antiquity of a tropical specialization: army-ant-following in the typical antbirds (Thamnophilidae). Mol. Phylogenet. Evol. 45, 1–13 (doi:10.1016/j.ympev.2007.07.019) [DOI] [PubMed] [Google Scholar]
- Burdon J. J., Thrall P. H.1999Spatial and temporal patterns in coevolving plant and pathogen associations. Am. Nat. 153, S15–S33 (doi:10.1086/303209) [DOI] [PubMed] [Google Scholar]
- Burns K. J., Hackett S. J., Klein N. K.2002Phylogenetic relationships and morphological diversity in Darwin's finches and their relatives. Evolution 56, 1240–1252 [DOI] [PubMed] [Google Scholar]
- Cadena C. D.2007Testing the role of interspecific competition in the evolutionary origin of elevational zonation: an example with Buarremon brush-finches (Aves, Emberizidae) in the Neotropical mountains. Evolution 61, 1120–1136 (doi:10.1111/j.1558-5646.2007.00095.x) [DOI] [PubMed] [Google Scholar]
- Carvalheiro L. G., Buckley Y. M., Ventim R., Fowler S. V., Memmott J.2008Apparent competition can compromise the safety of highly specific biocontrol agents. Ecol. Lett. 11, 690–700 (doi:10.1111/j.1461-0248.2008.01184.x) [DOI] [PubMed] [Google Scholar]
- Collinge S. K., Ray C. (eds) 2006Disease ecology: community structure and pathogen dynamics Oxford: Oxford University Press [Google Scholar]
- Cox G. W., Ricklefs R. E.1977Species diversity, ecological release, and community structuring in Caribbean land bird faunas. Oikos 29, 60–66 [Google Scholar]
- Coyne J. A., Orr H. A.2004Speciation Sunderland, MA: Sinauer Associates [Google Scholar]
- Darwin C. R.1839Narrative of the surveying voyages of His Majesty's Ships Adventure and Beagle between the years 1826 and 1836, describing their examination of the southern shores of South America, and the Beagle's circumnavigation of the globe. London, UK: Henry Colburn; (Journal and remarks, 1832–1836) [Google Scholar]
- Daszak P., Cunningham A. A., Hyatt A. D.2000Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287, 443–449 (doi:10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- Davies T. J., Pedersen A. B.2008Phylogeny and geography predict pathogen community similarity in wild primates and humans. Proc. R. Soc. B 275, 1695–1701 (doi:10.1098/rspb.2008.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Queiroz K.1998The general lineage concept of species, species criteria, and the process of speciation: a conceptual unification and terminological recommendations. In Endless forms: species and speciation (eds Howard D. J., Berlocher S. H.), pp. 57–75 Oxford: Oxford University Press [Google Scholar]
- Dhondt A. A., Tessaglia D. L., Slothower R. L.1998Epidemic mycoplasmal conjunctivitis in house finches from eastern North America. J. Wildlife Dis. 34, 265–280 [DOI] [PubMed] [Google Scholar]
- Diamond J. M.1972Avifauna of the Eastern Highlands of New Guinea Cambridge, MA: Nuttall Ornithological Club [Google Scholar]
- Diamond J. M.1973Distributional ecology of New Guinea birds. Science 179, 759–769 (doi:10.1126/science.179.4075.759) [DOI] [PubMed] [Google Scholar]
- Diamond J. M.1986Evolution of ecological segregation in the New Guinea montane avifauna. In Community ecology (eds Diamond J., Case T. J.), pp. 98–125 New York, NY: Harper & Row [Google Scholar]
- Diamond J. M., Marshall A. G.1976Origin of the New Hebridean avifauna. Emu 76, 187–200 [Google Scholar]
- Diamond J. M., Marshall A. G.1977Distributional ecology of New Hebridean birds: a species kaleidoscope. J. Anim. Ecol. 46, 703–727 (doi:10.2307/3636) [Google Scholar]
- Fallon S. M., Bermingham E., Ricklefs R. E.2005Host specialization and geographic localization of avian malaria parasites: a regional analysis in the Lesser Antilles. Am. Nat. 165, 466–480 (doi:10.1086/428430) [DOI] [PubMed] [Google Scholar]
- Fenner F., Ratcliffe F. N.1965Myxomatosis London, UK: Cambridge University Press [Google Scholar]
- Fessl B., Kelindorfer S., Tebbich S.2006An experimental study on the effects of an introduced parasite in Darwin's finches. Biol. Conserv. 127, 55–61 (doi:10.1016/j.biocon.2005.07.013) [Google Scholar]
- Fleischer R. C., McIntosh C. E.2001Molecular systematics and biogeography of the Hawaiian avifauna. Stud. Avian Biol. 22, 51–60 [Google Scholar]
- Fleischer R. C., McIntosh C. E., Tarr C. L.1998Evolution on a volcanic conveyor belt: using phylogeographic reconstructions and K-Ar-based ages of the Hawaiian Islands to estimate molecular evolutionary rates. Mol. Ecol. 7, 533–545 (doi:10.1046/j.1365-294x.1998.00364.x) [DOI] [PubMed] [Google Scholar]
- Flockhart D. T. T., Wiebe K. L.2009Absence of reproductive consequences of hybridization in the Northern Flicker (Colaptes auratus) hybrid zone. Auk 126, 351–358 (doi:10.1525/auk.2009.08086) [Google Scholar]
- Garrido O. H., Kirkconnell A.2000Field guide to the birds of Cuba Ithaca, New York: Comstock Publishing Associates of Cornell University Press [Google Scholar]
- Grant P. R.1986Ecology and evolution of Darwin's finches Princeton, NJ: Princeton University Press [Google Scholar]
- Grant P. R., Grant B. R.2007How and why species multiply: the radiation of Darwin's finches Princeton, New Jersey: Princeton University Press [Google Scholar]
- Grosholz E. D.1992Interactions of intraspecific, interspecific, and apparent competition with host–pathogen population dynamics. Ecology 73, 507–514 (doi:10.2307/1940756) [Google Scholar]
- Holt R. D.1977Predation, apparent competition, and the structure of prey communities. Theor. Popul. Biol. 12, 197–229 (doi:10.1016/0040-5809(77)90042-9) [DOI] [PubMed] [Google Scholar]
- Holt R. D., Barfield M.2009Trophic interactions and range limits: the diverse roles of predation. Proc. R. Soc. B 276, 1435–1442 (doi:10.1098/rspb.2008.1536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R. D., Lawton J. H.1994The ecological consequences of shared natural enemies. Ann. Rev. Ecol. Syst. 25, 495–520 (doi:10.1146/annurev.es.25.110194.002431) [Google Scholar]
- Hultmark D.2003Drosophila immunity: paths and patterns. Curr. Opin. Immunol. 15, 12–19 (doi:10.1016/S0952-7915(02)00005-5) [DOI] [PubMed] [Google Scholar]
- Hunt J. S., Bermingham E., Ricklefs R. E.2001Molecular systematics and biogeography of Antillean thrashers, tremblers, and mockingbirds (Aves: Mimidae). Auk 118, 35–55 (doi:10.1642/0004-8038(2001)118[0035:MSABOA]2.0.CO;2) [Google Scholar]
- James H. F.2004The osteology and phylogeny of the Hawaiian finch radiation (Fringillidae: Drepanidini), including extinct taxa. Zool. J. Linn. Soc. 141, 207–255 (doi:10.1111/j.1096-3642.2004.00117.x) [Google Scholar]
- Jarvi S. I., Tarr C. L., McIntosh C. E., Atkinson C. T., Fleischer R. C.2004Natural selection of the major histocompatibility complex (Mhc) in Hawaiian honeycreepers (Drepanidinae). Mol. Ecol. 13, 2157–2168 (doi:10.1111/j.1365-294X.2004.02228.x) [DOI] [PubMed] [Google Scholar]
- Josefsson M., Andersson B.2001The environmental consequences of alien species in the Swedish lakes Malaren, Hjalmaren, Vanern and Vattern. Ambio 30, 514–521 [DOI] [PubMed] [Google Scholar]
- Kraaijeveld A. R., Godfray H. C. J.1999Geographic patterns in the evolution of resistance and virulence in Drosophila and its parasitoids. Am. Nat. 153, S61–S74 (doi:10.1086/303212) [DOI] [PubMed] [Google Scholar]
- Kramer L. D., Bernard K. A.2001West Nile virus in the Western Hemisphere. Curr. Opin. Infect. Dis. 14, 519–525 [DOI] [PubMed] [Google Scholar]
- Lack D.1976Island biology illustrated by the land birds of Jamaica. Berkeley, CA: University of California Press [Google Scholar]
- Lanciotti R. S., et al. 1999Origin of the West Nile Virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286, 2333–2337 (doi:10.1126/science.286.5448.2333) [DOI] [PubMed] [Google Scholar]
- Latta S. C., Rimmer C. C., McFarland K. P.2003Winter bird communities in four habitats along an elevational gradient on Hispaniola. Condor 105, 179–197 (doi:10.1650/0010-5422(2003)105[0179:WBCIFH]2.0.CO;2) [Google Scholar]
- Latta S. C., Rimmer C. C., Keith A., Wiley J., Raffaele H., McFarland K. P., Fernandez E.2006Birds of the Dominican Republic and Haiti Princeton, NJ: Princeton University Press [Google Scholar]
- Lovette I. J., Bermingham E.1999Explosive speciation in the New World Dendroica warblers. Proc. R. Soc. Lond. B 266, 1629–1636 (doi:10.1098/rspb.1999.0825) [Google Scholar]
- Lovette I. J., Hochachka W. M.2006Simultaneous effects of phylogenetic niche conservatism and competition on avian community structure. Ecology 87, S14–S28 (doi:10.1890/0012-9658(2006)87[14:SEOPNC]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- MacArthur R. H., Wilson E. O.1967The theory of island biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- Martin P. R., Tewksbury J. J.2008Latitudinal variation in subspecific diversification of birds. Evolution 62, 2775–2778 (doi:10.1111/j.1558-5646.2008.00489.x) [DOI] [PubMed] [Google Scholar]
- Massey J. G., Graczyk T. K., Cranfield M. R.1996Characteristics of naturally acquired Plasmodium relictum capistranoae infections in naive Hawaiian crows (Corvus hawaiiensis) in Hawaii. J. Parasitol. 82, 182–185 (doi:10.2307/3284139) [PubMed] [Google Scholar]
- Matson K.2006Are there differences in immune function between continental and insular birds? Proc. R. Soc. B 273, 2267–2274 (doi:10.1098/rspb.2006.3590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy A. J., Shaw M. A., Goodman S. J.2007Pathogen evolution and disease emergence in carnivores. Proc. R. Soc. B 274, 3165–3174 (doi:10.1098/rspb.2007.0884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes C. J., Wood A. R., Thomas K., Sainsbury A. W., Gurnell J., Dein F. J., Nettleton P. F.2006Genomic characterization of a novel poxvirus contributing to the decline of the red squirrel (Sciurus vulgaris) in the UK. J. Gen. Virol. 87, 2115–2125 (doi:10.1099/vir.0.81966-0) [DOI] [PubMed] [Google Scholar]
- McNeill W. H.1998Plagues and peoples New York: Anchor Books/Doubleday [Google Scholar]
- Morris R. J., Lewis O. T., Godfray H. C. J.2004Experimental evidence for apparent competition in a tropical forest food web. Nature 428, 310–313 (doi:10.1038/nature02394) [DOI] [PubMed] [Google Scholar]
- Morris R. J., Lewis O. T., Godfray H. C. J.2005Apparent competition and insect community structure: towards a spatial perspective. Annales Zoologici Fennici 42, 449–462 [Google Scholar]
- Oidtmann B., Heitz E., Rogers D., Hoffmann R. W.2002Transmission of crayfish plague. Dis. Aquat. Organ. 52, 159–167 (doi:10.3354/dao052159) [DOI] [PubMed] [Google Scholar]
- Orrock J. L., Witter M. S., Reichman O. J.2008Apparent competition with an exotic plant reduces native plant establishment. Ecology 89, 1168–1174 (doi:10.1890/07-0223.1) [DOI] [PubMed] [Google Scholar]
- Plowright W.1982The effects of rinderpest and rinderpest control on wildlife in Africa. Symp. Zool. Soc. Lond. 50, 1–28 [Google Scholar]
- Pratt H. D.2005The Hawaiian honeycreepers. Oxford, UK: Oxford University Press [Google Scholar]
- Price T.2008Speciation in birds Greenwood Village, Colorado: Roberts and Company [Google Scholar]
- Price T. D., Bouvier M. M.2002The evolution of F-1 postzygotic incompatibilities in birds. Evolution 56, 2083–2089 [PubMed] [Google Scholar]
- Raffaele H., Wiley J., Garrido O., Keith A., Raffaele J.1998A guide to the birds of the West Indies Princeton, NJ: Princeton University Press [Google Scholar]
- Ricklefs R. E., Bermingham E.2001Nonequilibrium diversity dynamics of the Lesser Antillean avifauna. Science 294, 1522–1524 (doi:10.1126/science.1065005) [DOI] [PubMed] [Google Scholar]
- Ricklefs R. E., Bermingham E.2002The concept of the taxon cycle in biogeography. Global Ecol. Biogeogr. 11, 353–361 (doi:10.1046/j.1466-822x.2002.00300.x) [Google Scholar]
- Ricklefs R. E., Bermingham E.2007aThe causes of evolutionary radiations in archipelagoes: passerine birds in the Lesser Antilles. Am. Nat. 169, 285–297 (doi:10.1086/510730) [DOI] [PubMed] [Google Scholar]
- Ricklefs R. E., Bermingham E.2007bThe West Indies as a laboratory of ecology and evolution. Phil. Trans. R. Soc. B 363, 2393–2413 (doi:10.1098/rstb.2007.2068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs R. E., Fallon S. M.2002Diversification and host switching in avian malaria parasites. Proc. R. Soc. Lond. B 269, 885–892 (doi:10.1098/rspb.2001.1940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs R. E., Fallon S. M., Bermingham E.2004Evolutionary relationships, cospeciation, and host switching in avian malaria parasites. Syst. Biol. 52, 111–119 (doi:10.1080/10635150490264987) [DOI] [PubMed] [Google Scholar]
- Rosenzweig M. L.1995Species diversity in space and time Cambridge, UK: Cambridge University Press [Google Scholar]
- Sainsbury A. W., Nettleton P., Gilray J., Gurnell J.2000Grey squirrels have high seroprevalence to a parapoxvirus associated with deaths in red squirrels. Anim. Conserv. 3, 229–233 (doi:10.1111/j.1469-1795.2000.tb00107.x) [Google Scholar]
- Santiago-Alarcon D., Whiteman N. K., Parker P. G., Ricklefs R. E., Valkiunas G.2008Patterns of parasite abundance and distribution in island populations of Galápagos endemic birds. J. Parasitol. 94, 584–590 [DOI] [PubMed] [Google Scholar]
- Santiago-Alarcon D., Outlaw D. C., Ricklefs R. E., Parker P. G.In press Haplotype diversity of haemosporidian parasites in New World doves (Columbiformes), with emphasis on the endemic Galápagos dove. Int. J. Parasitol [DOI] [PubMed] [Google Scholar]
- Sato A., Tichy H., O'Huigin C., Grant P. R., Grant B. R., Klein J.2001On the origin of Darwin's finches. Mol. Biol. Evol. 18, 299–311 [DOI] [PubMed] [Google Scholar]
- Siddle H. V., Kreiss A., Eldridge M. D., Noonan E., Clarke C. J., Pyecroft S., Woods G. M., Belov K.2007Transmission of a fatal clonal tumor by biting occurs due to depleted MHC diversity in a threatened carnivorous marsupial. Proc. Natl Acad. Sci. USA 104, 16 221–16 226 (doi:10.1073/pnas.0704580104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soos C., Padilla L. R., Iglesias A., Gottdenker N., Bédon M. C., Rios A., Parker P. G.2008Comparison of pathogens in a broiler and backyard chickens on the Galápagos Islands: implications for transmission to wildlife. Auk 125, 445–455 (doi:10.1525/auk.2008.06235) [Google Scholar]
- Sulloway F. J.1982The Beagle collections of Darwin's finches (Geospizinae). Bull. Brit. Mus. (Nat. Hist.) Histor. Ser. 43, 49–94 [Google Scholar]
- Terborgh J.1971Distribution on environmental gradients: theory and a preliminary interpretation of distributional patterns in the avifauna of the Cordillera Vilcabamba, Peru. Ecology 52, 23–40 (doi:10.2307/1934735) [Google Scholar]
- Thompson J. N.2005The geographic mosaic of coevolution Chicago: University of Chicago Press [Google Scholar]
- Tompkins D. M., Draycott R. A. H., Hudson P. J.2000aField evidence for apparent competition mediated via the shared parasites of two gamebird species. Ecol. Lett. 3, 10–14 (doi:10.1046/j.1461-0248.2000.00117.x) [Google Scholar]
- Tompkins D. M., Greenman J. V., Robertson P. A., Hudson P. J.2000bThe role of shared parasites in the exclusion of wildlife hosts: Heterakis gallinarum in the ring-necked pheasant and the grey partridge. J. Anim. Ecol. 69, 829–840 (doi:10.1046/j.1365-2656.2000.00439.x) [DOI] [PubMed] [Google Scholar]
- Tompkins D. M., Greenman J. V., Hudson P. J.2001Differential impact of a shared nematode parasite on two gamebird hosts: implications for apparent competition. Parasitology 122, 187–193 (doi:10.1017/S0031182001007247) [DOI] [PubMed] [Google Scholar]
- Tompkins D. M., White A. R., Boots M.2003Ecological replacement of native red squirrels by invasive greys driven by disease. Ecol. Lett. 6, 189–196 (doi:10.1046/j.1461-0248.2003.00417.x) [Google Scholar]
- van Riper C., III, van Riper S. G., Goff M. L., Laird M.1986The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol. Monogr. 56, 327–344 (doi:10.2307/1942550) [Google Scholar]
- van Veen F. J. F., Muller C. B., Pell J. K., Godfray H. C. J.2008Food web structure of three guilds of natural enemies: predators, parasitoids and pathogens of aphid. J. Anim. Ecol. 77, 191–200 (doi:10.1111/j.1365-2656.2007.01325.x) [DOI] [PubMed] [Google Scholar]
- Vijendravarma R. K., Kraaijeveld A. R., Godfray H. C. J.2009Experimental evolution shows Drosophila melanogaster resistance to a microsporidian pathogen has fitness costs. Evolution 63, 104–114 (doi:10.1111/j.1558-5646.2008.00516.x) [DOI] [PubMed] [Google Scholar]
- Vincek V., Ohuigin C., Satta Y., Takahata N., Boag P. T., Grant P. R., Grant B. R., Klein J.1997How large was the founding population of Darwins finches. Proc. R. Soc. Lond. B 264, 111–118 (doi:10.1098/rspb.1997.0017) [Google Scholar]
- Wallace G. D.1976The prevalence of toxoplasmosis on Pacific Islands, and the influence of ethnic group. Am. J. Trop. Med. Hyg. 25, 48–53 [DOI] [PubMed] [Google Scholar]
- Warner R. E.1968The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. Condor 70, 101–120 (doi:10.2307/1365954) [Google Scholar]
- Westerdahl H., Waldenstrom J., Hansson B., Hasselquist D., von Schantz T., Bensch S.2005Associations between malaria and MHC genes in a migratory songbird. Proc. R. Soc. B 272, 1511–1518 (doi:10.1098/rspb.2005.3113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler S. S., Barker C. M., Fang Y., Armijos M. V., Carroll B. D., Husted S., Johnson W. O., Reisen W. K.2009Differential impact of West Nile virus on California birds Condor 111, 1–20 (doi:10.1525/cond.2009.080013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman N. K., Matson K. D., Bollmer J. L., Parker P. G.2006Disease ecology in the Galápagos Hawk (Buteo galapagoensis): host genetic diversity, parasite load and natural antibodies. Proc. R. Soc. B 273, 797–804 (doi:10.1098/rspb.2005.3396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth B. L., et al. 2005Host population persistence in the face of introduced vector-borne diseases: Hawaii amakihi and avian malaria. Proc. Natl Acad. Sci. USA 102, 1531–1536 (doi:10.1073/pnas.0409454102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work T. M., Massey J. G., Rideout B. A., Gardiner C. H., Ledig D. B., Kwok O. C., Dubey J. P.2000Fatal toxoplasmosis in free-ranging endangered ‘Alala from Hawaii. J. Wildlife Dis. 36, 205–212 [DOI] [PubMed] [Google Scholar]
- Zink R. M.2002A new perspective on the evolutionary history of Darwin's finches. Auk 119, 864–871 (doi:10.1642/0004-8038(2002)119[0864:ANPOTE]2.0.CO;2) [Google Scholar]