Abstract
Divergence and speciation can sometimes proceed in the face of, and even be enhanced by, ongoing gene flow. We here study divergence with gene flow in Darwin's finches, focusing on the role of ecological/adaptive differences in maintaining/promoting divergence and reproductive isolation. To this end, we survey allelic variation at 10 microsatellite loci for 989 medium ground finches (Geospiza fortis) on Santa Cruz Island, Galápagos. We find only small genetic differences among G. fortis from different sites. We instead find noteworthy genetic differences associated with beak. Moreover, G. fortis at the site with the greatest divergence in beak size also showed the greatest divergence at neutral markers; i.e. the lowest gene flow. Finally, morphological and genetic differentiation between the G. fortis beak-size morphs was intermediate to that between G. fortis and its smaller (Geospiza fuliginosa) and larger (Geospiza magnirostris) congeners. We conclude that ecological differences associated with beak size (i.e. foraging) influence patterns of gene flow within G. fortis on a single island, providing additional support for ecological speciation in the face of gene flow. Patterns of genetic similarity within and between species also suggest that interspecific hybridization might contribute to the formation of beak-size morphs within G. fortis.
Keywords: sympatric speciation, ecological speciation, disruptive selection, reproductive isolation, gene flow, Darwin's finches
1. Introduction
Strict geographical isolation surely aids speciation in many cases (Mayr 1963; Felsenstein 1981; Coyne & Orr 2004), but there are several contexts where divergence can proceed despite (or even be enhanced by) a lack of geographical isolation. ‘Context 1’ occurs when some initial divergence has occurred in allopatry, and this divergence is then strengthened during a period of secondary contact. Mechanisms that can be important here include ecological character displacement to reduce competition (Schluter 2000b) and reproductive character displacement to reduce maladaptive interbreeding (Brown & Wilson 1956; Grant 1972; Servedio & Noor 2003). ‘Context 2’ is the fission of one initial species into two or more species without strict geographical isolation, i.e. parapatric or purely sympatric speciation. One mechanism likely to be important here is strong disruptive selection (owing to competition or discrete resources) that acts on traits also linked to assortative mating (Fry 2003; Gavrilets 2004; Bolnick & Fitzpatrick 2007). ‘Context 3’ is hybridization between groups following their secondary contact, which can sometimes cause the formation of a new hybrid species (Seehausen 2004; Mallet 2007). Recent theoretical and empirical work on these contexts has spurred a resurgence of interest in the longstanding (Smith 1966; Endler 1973; Felsenstein 1981) hypothesis of ‘divergence with gene flow’ (Rice & Hostert 1993; Dieckmann & Doebeli 1999; Piertney et al. 2001; Bolnick & Fitzpatrick 2007; Doebeli et al. 2007; Berner et al. 2009). Our work focuses on divergence with gene flow in Darwin's finches of the Galápagos Islands, where all three of the above contexts have been invoked.
Context 1: the classic view of speciation in Darwin's finches envisions a three-phase process (Lack 1947; Grant 1999, 2001; Schluter 2000a; Petren et al. 2005; Grant & Grant 2008). In the first phase, a single founding species from the mainland colonizes an island. In the second phase, migrants from that first island colonize additional islands that have different ecological resources, such as different food types. These ecological differences cause divergent selection on foraging traits, particularly beak size and shape, which then undergo adaptive divergence between the islands. In the third phase, a new round of migration between the islands brings partially divergent forms back into secondary contact, where competition further enhances divergence (Lack 1947; Mayr 1963; Grant 1999; Schluter 2000a; Grant & Grant 2006). In this three-phase model, the incipient species continue to diverge following secondary contact because allopatric divergence has led to assortative mating and selection against hybrids that is then manifest in sympatry (Grant & Grant 1993, 1996a,b, 1997a; Grant 1999).
Context 2: two potential cases of purely sympatric speciation have been discussed in Darwin's finches. In one, Grant & Grant (1979) described a population of Geospiza conirostris (large cactus ground finch) on Genovesa Island that was composed of two male types singing different songs and having different beak sizes and foraging habits. These initial distinctions subsequently broke down (Grant & Grant 1989), and no further attention has been directed towards this population. In the other case, Ford et al. (1973) described a population of G. fortis (medium ground finch) at Academy Bay on Santa Cruz Island that was bimodal for beak size. The authors suggested that bimodality was the result of disruptive selection and assortative mating—but this was not tested—and bimodality has since weakened (Hendry et al. 2006). A case has also been made for possible parapatric speciation in Geospiza fuliginosa (small ground finch) between elevation zones on a single island (Kleindorfer et al. 2006).
Context 3: Darwin's finches frequently hybridize (Grant & Grant 1994, 1997a, 1998, 2008; Grant 1999; Sato et al. 1999; Zink 2002; Grant et al. 2005), which might have several consequences for their diversification. First, hybridization between two species on the small island of Daphne Major has led to their morphological and genetic convergence (Grant & Grant 2002; Grant et al. 2004). This convergence was partly the result of changing ecological conditions that increased the fitness of hybrids (Grant & Grant 1996b). Second, hybridization could help to generate new phenotypes that might be able to adapt to new resources (Grant & Grant 1994)—although this has not been confirmed for Darwin's finches.
Common to all of the above contexts is the potential importance of ecological differences that cause divergent or disruptive selection, and thereby promote adaptive divergence. This divergence then becomes coupled to reproductive isolation, a process now called ‘ecological speciation’. Ecological speciation has considerable support from theory and from many natural systems (Schluter 2000a; Rundle & Nosil 2005; Hendry et al. 2007). In Darwin's finches, ecological speciation has been invoked through comparisons of established species (Grant 1999; Grant & Grant 2008), and our work extends these inferences to divergence within species.
(a). Our study
Our work has concentrated on a population of G. fortis at El Garrapatero on Santa Cruz Island that is bimodal for beak size (figure 1; Hendry et al. 2006). The two morphs (i) have beaks adapted for different food types (Herrel et al. 2005; Foster et al. 2008), (ii) produce distinctive vocal mating signals (Podos et al. 2004; Huber & Podos 2006; Herrel et al. 2009) and respond differently to those signals (J. Podos 2007, unpublished data), (iii) show higher survival than birds with intermediate-sized beaks (Hendry et al. 2009), (iv) pair assortatively by beak size (Huber et al. 2007), and (v) show some evidence of genetic divergence (Huber et al. 2007). In short, this population shows potential signs of ecological differentiation in the face of some gene flow.
Figure 1.
Typical small and large beak-size morphs in G. fortis. These two mature males were caught at the same time in the same mist net, and were photographed together. Photo by A. Hendry.
The origin of these beak-size morphs is unknown, with the different possibilities paralleling the general contexts introduced above. For context 1, the two morphs may have originated owing to adaptive divergence on different islands and then came into secondary contact on Santa Cruz (Grant & Grant 2008). As an extension of this context, the two morphs may have originated owing to adaptive divergence between sites on the same island, and then come into secondary contact across the island. For context 2, variation in resources at a given site, or competition for those resources, might have led to a purely sympatric origin of the morphs (Ford et al. 1973). For context 3, hybridization between G. fortis and Geospiza magnirostris (large ground finch) might have originated the large G. fortis morph (Grant & Grant 2008). In the present study, we will not conclusively discriminate among these possibilities. We will instead focus on how ecological/adaptive differences might influence gene flow between the morphs.
Several observations would be particularly informative. First, substantial genetic differences among G. fortis populations at different sites on Santa Cruz would suggest (although not confirm) that spatial isolation on a single island could contribute to speciation. Second, genetic clustering across the island by beak size rather than by location would suggest that gene flow is primarily reduced by ecology/adaptation (diet and beak size) rather than by geography (location). Third, evidence that gene flow is lower between the morphs at the sites where beak-size divergence is greater would further suggest a link between ecology/adaptation and reproductive isolation. Fourth, a continuity in genetic divergence between the G. fortis morphs to divergence between G. fortis and its smaller (G. fuliginosa) and larger (G. magnirostris) congeners would suggest a possible continuity of process from intraspecific divergence to speciation. This last pattern would also be consistent with a possible role for hybridization.
We test for the above signatures of ecological speciation by using microsatellite loci to analyse population structure on Santa Cruz Island, specifically in relation to (i) the small and large G. fortis beak-size morphs (figure 1), (ii) the different sampling sites (figure 2), and (iii) the different granivorous ground finch species (G. fuliginosa, G. fortis and G. magnirostris). At one of the collection sites (El Garrapatero), both small and large beak-size morphs are present and the beak-size distribution is bimodal (Hendry et al. 2006; Huber et al. 2007; figure 3). At a second site (Academy Bay), both small and large morphs are present, but the historically strong bimodality in beak size demonstrated by Ford et al. (1973) has since weakened (Hendry et al. 2006; figure 3). At a third site (Borrero Bay), the large morph is rare (Grant et al. 1976; Hendry et al. 2006; figure 3).
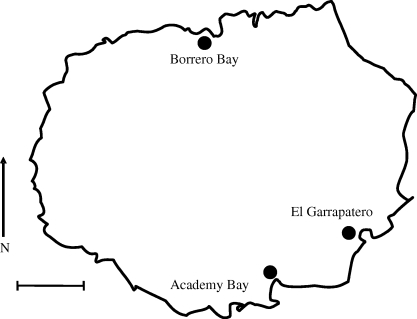
Figure 2.
Collection sites on Santa Cruz Island, Galapágos. Scale bar, 10 km.
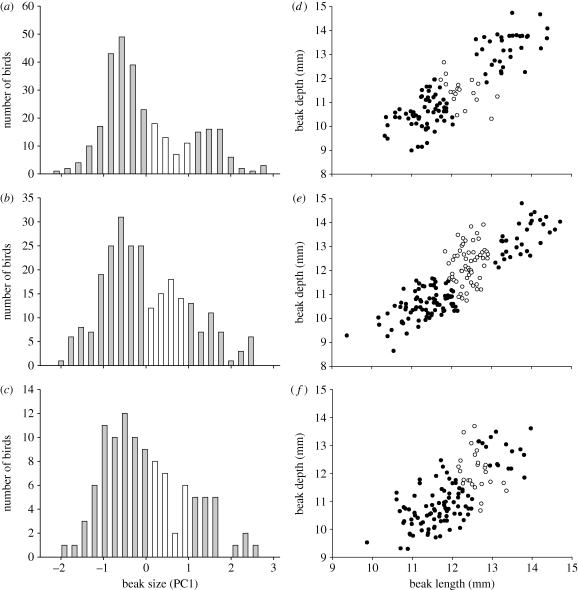
Figure 3.
Distribution of beak sizes in G. fortis at three different sites on Santa Cruz Island in 2004. At El Garrapatero, (d) small and large morphs are both common and (a,d) intermediates are relatively few. At Academy Bay, (e) small and large morphs are both common and (b,e) intermediates are less rare. (c,f) At Borrero Bay, the large morph is rare. These data are from Hendry et al. (2006). (a–c) The white sections on the histograms and (d–f) the open circles on the graphs represent the ‘intermediate beak-size class’ omitted from the genetic analyses (§2).
2. Material and methods
(a). Sampling
Sampling took place in January through March of 2003–2005. Finches were captured in mist nets and then measured (following Grant 1999) for beak length (anterior edge of nares to tip of upper mandible), beak depth (at the nares) and beak width (base of lower mandible). The ulnar vein was pricked with a needle, and the blood was blotted onto filter paper treated with ethylenediamine tetraacetic acid (EDTA). Captured birds were banded to avoid repeated sampling, and were then released at their site of capture. Sample sizes for G. fortis were 518 birds at Academy Bay, 419 birds at El Garrapatero and 113 birds at Borrero Bay. All of these sites are low-elevation arid zone habitats (Wiggins & Porter 1971), where G. fortis is most abundant. Logistic and monetary constraints prevented sampling on additional islands.
We also took blood samples from the other granivorous ground finches found on Santa Cruz: 10 G. fuliginosa (small ground finch) from El Garrapatero, six G. magnirostris (large ground finch) from El Garrapatero, and 14 G. magnirostris from Academy Bay. Larger sample sizes were not possible owing to limitations on our permits. We did not analyse the closely related Geospiza scandens (cactus finch) because divergence for this species is on a different divergent ecological (cactus seeds, pollen and nectar) and morphological (beak length relative to depth) axis than for the other three Geospiza (Lack 1947; Bowman 1961; Grant 1999; Foster et al. 2008).
Individual G. fortis with beak-size data was assigned either to the small or to the large beak-size categories. Specifically, we used PC1 scores (based on beak length, depth and width) in a two-step cluster analysis with all G. fortis together to identify the beak size dividing point that best separated the two clusters (PC1 = 0.312). Birds close to the dividing point could not be confidently assigned to either category, and so we defined three beak-size ‘classes’. The large beak-size class had 150 birds with beaks more than 0.5 standard deviations larger than the dividing point defined by the cluster analysis. The small beak-size class had 342 birds with beaks more than 0.5 standard deviations smaller than the dividing point. The intermediate beak-size class had 174 birds with beak sizes within 0.5 standard deviations of the dividing point. We focus our presentation on comparisons of the small and large beak-size classes—because we can be certain that these birds represent the small and large beak-size morphs. Regardless, our conclusions do not change in analyses that (i) consider the intermediate-size class as an additional group (electronic supplementary material, appendix S1) or (ii) divide the birds into small and large beak-size categories based strictly on the above dividing point—i.e. without excluding any G. fortis (electronic supplementary material, appendix S2).
(b). Genetic data
Total DNA was extracted from blood samples with a modified standard proteinase K phenol–chloroform protocol (Sambrook et al. 1989). DNA was amplified by PCR and screened for allelic variation at 11 di-nucleotide microsatellite loci: Gf03, Gf04, Gf05, Gf07, Gf08, Gf09, Gf10, Gf11, Gf12, Gf13 and Gf16 (Petren 1998). Multiplex PCR reactions in a final volume of 10 µl were carried out under the thermocycling conditions specified by Petren (1998). Four fluorescent-labelled primers (FAM, HEX, PET and TET) were used to label the 5′ tail of the different loci. A multicapillary ABI 3100-base station was used to score the microsatellite fragments.
Raw genotypes were imported into Genalex v. 6 (Peakall & Smouse 2006), where they were converted into formats suitable for various software packages. We then used Genepop (Raymond & Rousset 1995) v. 3.4 and Fstat (Goudet 1995) v. 2.9.3.2 to calculate basic population genetic parameters: allelic diversity, observed (Ho) and expected (He) heterozygosities, and fixation indices (FIS). We also used Genepop to test for Hardy–Weinberg deficits and for linkage disequilibrium.
(c). Population structure
Analyses of G. fortis population structure began with statistical comparisons between a priori defined groups based on various combinations of beak-size class (large or small) and collection site (El Garrapatero, Academy Bay or Borrero Bay). We first partitioned the total genetic variation with analysis of molecular variance (AMOVA) based on 10 000 permutations in Arlequin v. 3.1 (Excoffier et al. 2005). We then used hierarchical F-statistics in Hierfstat (Goudet 2005). Both analyses were performed for (i) beak-size classes nested within sites and (ii) sites nested within beak-size classes.
We next tested for genetic differences between (i) all combinations of beak-size class and site, (ii) beak-size classes only (i.e. sites pooled within a beak-size class), and (iii) sites only (i.e. beak-size classes pooled within a site). These analyses involved (i) Fisher's exact probability tests for genetic differentiation in Genepop (Raymond & Rousset 1995), (ii) Wright's F-statistics (FST) according to Weir & Cockerham (1984) and with confidence intervals from Fstat (Goudet 1995), and (iii) Slatkin's (1995) R-statistics (RST).
Finally, we used multilocus genotypes to infer population structure. Two of these analyses were conducted without reference to a priori defined groups. First, we used the Bayesian clustering method in Structure (Pritchard et al. 2000) v. 2.1 to infer the likelihood of K = 1–5 clusters that minimize Hardy–Weinberg and linkage disequilibrium. Given the modest genetic differences (§3), these analyses were run under an admixture model with correlated allele frequencies using a 50 000 burn-in period and 500 000 Monte Carlo Markov chain iterations. Second, we performed similar analyses in Baps (Corander et al. 2004) v. 4.14, here using an admixture model and runs consisting of 10 000 iterations. Third, we used factorial correspondence analysis in Genetix (Belkhir et al. 2004) v. 4.0 to visualize variation between the a priori defined (as above) small and large beak-size classes at each site.
Variation among the three Geospiza species was examined by repeating the above analyses (as appropriate) using the three species as separate groups. For this analysis, all G. fortis individuals, including the intermediate-size class, were pooled.
(d). Gene flow
We also estimated contemporary gene flow to check for congruence with the above analyses of historical population structure. For this analysis, we used the partial Bayesian assignment method (Cornuet et al. 1999; Paetkau et al. 2004) implemented in Geneclass (Piry et al. 2004) with 10 000 simulations. This method (i) estimates contemporary gene flow as the number of first generation ‘migrants’ (and is therefore conceptually quite different from the historical inferences of population structure reported above) and (ii) is still useful when some potential source populations (here, other sites on Santa Cruz or other islands) have not been sampled (Cornuet et al. 1999). We felt that Geneclass was more appropriate than Structure for assignment tests because the latter assigns proportions of genomes to candidate populations and assumes all the candidate populations have been included in the analysis. Previous studies have shown that Geneclass performs well in detecting contemporary migrants (Cornuet et al. 1999; Eldridge et al. 2001; Berry et al. 2004; Paetkau et al. 2004). We did not use Migrate because simulation studies have found that it performs poorly when estimating gene flow (Abdo et al. 2004; Slatkin 2005; Chapuis et al. 2009).
3. Results
(a). Variation within G. fortis
All of the loci showed moderate to high levels of variation (table 1). One locus (Gf10) turns out to be Z-linked (Petren et al. 2005) and was therefore omitted from subsequent analyses. Of the 10 remaining loci, six (Gf03, Gf04, Gf09, Gf11, Gf12 and Gf16) showed significant heterozygote deficits when all G. fortis were pooled together (four after sequential Bonferroni correction; table 1). Five pairings of loci in this pooled sample showed significant linkage disequilibrium (two pairs after sequential Bonferroni correction, results not shown). These deviations from Hardy–Weinberg and linkage equilibria probably reflect population structure within G. fortis—because these loci apparently lack null alleles (Petren 1998; Keller et al. 2001; Petren et al. 2005) and are not physically linked to each other (Petren 1998). In the following two paragraphs, we first describe differences between a priori defined beak-size classes (small versus large) and collection sites (Academy Bay, El Garrapatero and Borrero Bay) based on standard population genetic tests. We then examine the groupings revealed by analysing multilocus genotypes, whether defined a priori or not.
Table 1.
Allelic diversity in the combined sample of all G. fortis from Santa Cruz Island, Galapágos. Columns indicate the total number of individuals genotyped (n), the number of alleles identified (Na), observed heterozygosity (Ho), expected heterozygosity (He), FIS estimates following Weir & Cockerham (1984) and the significance of Hardy–Weinberg deficits. Bold entries are those that remain significant after sequential Bonferroni correction.
| locus | n | Na | Ho | He | FIS | HW |
|---|---|---|---|---|---|---|
| Gf03 | 986 | 17 | 0.827 | 0.841 | 0.017 | <0.001 |
| Gf04 | 989 | 8 | 0.479 | 0.469 | −0.020 | 0.012 |
| Gf05 | 983 | 13 | 0.663 | 0.664 | 0.002 | 0.276 |
| Gf07 | 861 | 20 | 0.849 | 0.873 | 0.028 | 0.137 |
| Gf08 | 902 | 28 | 0.906 | 0.927 | 0.023 | 0.056 |
| Gf09 | 903 | 21 | 0.630 | 0.636 | 0.011 | <0.001 |
| Gf10 | 988 | 14 | 0.242 | 0.473 | 0.489 | <0.001 |
| Gf11 | 860 | 35 | 0.890 | 0.936 | 0.049 | <0.001 |
| Gf12 | 781 | 23 | 0.874 | 0.900 | 0.029 | <0.001 |
| Gf13 | 847 | 16 | 0.850 | 0.862 | 0.014 | 0.401 |
| Gf16 | 857 | 13 | 0.799 | 0.797 | −0.002 | 0.014 |
| average | 897 | 18 | 0.728 | 0.762 | 0.058 | <0.001 |
Some subtle genetic differences were evident among the sites (tables 2–4), but we here concentrate on the much greater differences between the beak-size classes. First, the percentage of molecular variation attributable to beak-size classes was greater than that attributable to differences between collection sites, and the former was highly significant when size classes were nested within collection sites (table 2). Second, hierarchical F-statistics revealed that differentiation between beak-size classes (classes nested within total: Fclasses/total = 0.011, p = 0.001, and classes nested within sites: Fclasses/sites = 0.0203, p = 0.035) was greater than that between collection sites (sites nested within total: Fsites/total = 0.021, p = 0.737 and sites nested within classes: Fsites/classes = 0.0122, p = 0.001). Third, the two beak-size classes were quite distinct when the birds were pooled across all sites: exact test p < 0.0001, FST = 0.011 (CI = 0.006–0.034), and RST = 0.017. Fourth, when birds were not pooled across sites, all pairwise comparisons between the small and large beak-size classes (within or between sites) were significant—except for those involving the rare large Borrero Bay birds (table 3). Amplifying this last point, differentiation between beak-size classes at a given site was greatest for El Garrapatero, lower for Academy Bay and absent for Borrero Bay (table 3).
Table 2.
Analysis of molecular variance (AMOVA) for Santa Cruz G. fortis. Sampled birds represent two beak-size classes (small and large) from the three collection sites (Academy Bay, Borrero Bay and El Garrapatero). These analyses were based on 10 polymorphic microsatellite loci (i.e. excluding Gf10). Levels of significance were extracted after 10 000 permutations, as implemented by Arlequin v. 3.1.
| source of variation | sum of squares | variance components | percentage of variation | p-value |
|---|---|---|---|---|
| sites nested within beak-size classes | ||||
| between size classes | 23.249 | 0.04373 | 1.11 | 0.096 |
| among sites within size classes | 19.734 | 0.00706 | 0.18 | <0.001 |
| within sites | 3786.277 | 3.87145 | 98.70 | <0.001 |
| beak-size classes nested within sites | ||||
| among sites | 12.238 | −0.01902 | −0.49 | 0.793 |
| between size classes within sites | 30.745 | 0.04602 | 1.18 | <0.001 |
| within size classes | 10244.091 | 3.89103 | 99.31 | <0.001 |
Table 3.
Genetic differentiation in G. fortis between beak-size classes (S, small; L, large) from different collection sites (AB, Academy Bay; BB, Borrero Bay; and EG, El Garrapatero). n represents sample sizes, p-values for genic differentiation are above the diagonal. Bold entries are those that remained significant after sequential Bonferroni correction. FST values are below the upper diagonal, with asterisks (*) indicating 95% confidence intervals that do not overlap with zero. RST values are below the lower diagonal.
| n | ABS | ABL | BBS | BBL | EGS | EGL | |
|---|---|---|---|---|---|---|---|
| Academy Bay S (ABS) | 118 | <0.001 | 0.029 | 0.004 | 0.001 | <0.001 | |
| Academy Bay L (ABL) | 60 | 0.012* | <0.001 | 0.023 | <0.001 | 0.101 | |
| Borrero Bay S (BBS) | 53 | 0.001 | 0.015* | 0.093 | 0.002 | <0.001 | |
| Borrero Bay L (BBL) | 24 | 0.004* | 0.007* | 0.001 | <0.001 | 0.044 | |
| El Garrapatero S (EGS) | 171 | 0.002* | 0.019* | <0.001 | 0.005* | <0.001 | |
| El Garrapatero L (EGL) | 66 | 0.012* | 0.0001 | 0.013* | 0.005 | 0.016* | |
| Academy Bay S (ABS) | 118 | ||||||
| Academy Bay L (ABL) | 60 | 0.017 | |||||
| Borrero Bay S (BBS) | 53 | 0.001 | 0.021 | ||||
| Borrero Bay L (BBL) | 24 | 0.009 | 0.008 | 0.007 | |||
| El Garrapatero S (EGS) | 171 | 0.003 | 0.023 | <0.001 | 0.017 | ||
| El Garrapatero L (EGL) | 66 | 0.025 | <0.001 | 0.029 | <0.001 | 0.030 |
Table 4.
Genetic differentiation between G. fortis (all three beak-size classes pooled) from the three collection sites on Santa Cruz Island. p-values for genic differentiation are above the diagonal. Bold entries are those that remained significant after sequential Bonferroni correction. FST values are below the upper diagonal, with asterisks (*) indicating 95% confidence intervals that do not overlap with zero. RST values are below the lower diagonal.
| Academy Bay | Borrero Bay | El Garrapatero | |
|---|---|---|---|
| Academy Bay | 0.010 | <0.001 | |
| Borrero Bay | 0.002* | <0.001 | |
| El Garrapatero | 0.003* | 0.002* | |
| Academy Bay | |||
| Borrero Bay | 0.001 | ||
| El Garrapatero | 0.003 | 0.003 |
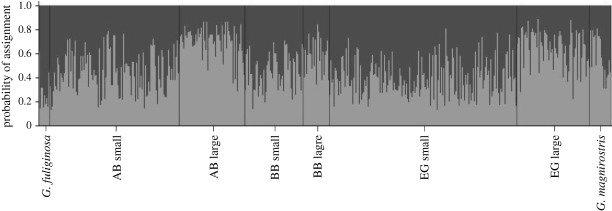
The above findings were mirrored in analyses of multilocus genotypes. Bayesian analyses in Structure recovered two clusters (figure 4) that roughly corresponded to the small and large beak-size classes. For example, 63 per cent of birds in the large beak-size class were assigned with the highest probability to one cluster, whereas 60 per cent of birds in the small beak-size class were assigned with the highest probability to the other cluster. When each collection site was considered separately, Structure found only a single cluster at each site, which is not surprising given the very limited power of this method when genetic differentiation is modest (Pritchard et al. 2000; Evanno et al. 2005; Waples & Gaggiotti 2006). Similar to Structure, Baps largely recovered the two beak-size classes when using the entire dataset, but not when each site was analysed separately (results not shown). Finally, factorial correspondence analysis revealed that the differences between morphs were greatest at El Garrapatero, lower at Academy Bay and absent at Borrero Bay (figure 5).
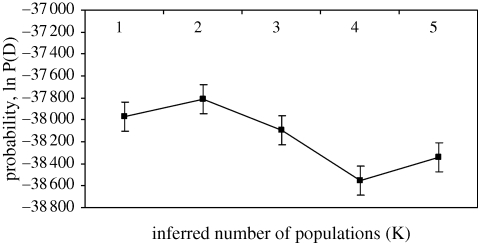
Figure 4.
Two clusters are most likely when combining all G. fortis in Structure v. 2.1. This conclusion holds when using the ad hoc criterion of Evanno et al. (2005). The consensus of five simulations following the parameters described in §2 is shown. Error bars show the variation in the probability of assignment.
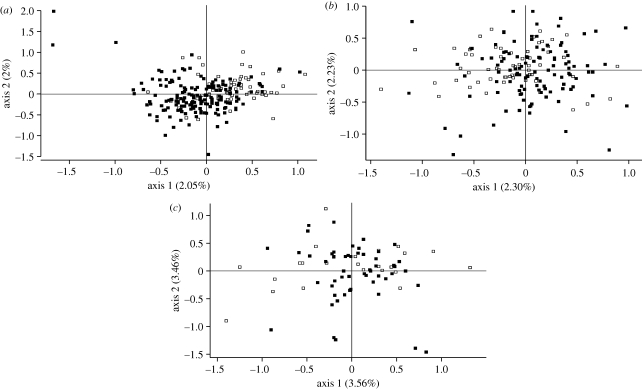
Figure 5.
Population structure in G. fortis as visualized through factorial correspondence analysis of multilocus genotypes (Genetix v. 3.1). The black and white squares are small and large beak-size classes, respectively. (a) At El Garrapatero, the two morphs occupy different, although partly overlapping distributions. (b) At Academy Bay and (c) Borrero Bay, the two morphs occupy similar distributions, although some slight separation is evident, particularly for Academy Bay.
(b). Variation among species
Genetic differentiation between the G. fortis beak-size classes represents a small-scale version of the differences between G. fortis and its smaller (G. fuliginosa) and larger (G. magnirostris) granivorous congeners. First, differentiation (FST) between the small and large beak-size classes (pooled across sites) was roughly one-half to one-third of that between G. fortis (pooled across morphs and sites) and either G. fuliginosa or G. magnirostris (table 5). Second, small G. fortis were genetically more similar to G. fuliginosa than to G. magnirostris, whereas large G. fortis were genetically more similar to G. magnirostris than to G. fuliginosa (table 5). These results were supported in analyses of multilocus genotypes. When Structure (figure 6) and Baps (results not shown) were asked to assign birds to only two clusters, these programs found (i) a strong separation between G. fuliginosa and G. magnirostris and (ii) that small G. fortis tended to be placed in the G. fuliginosa cluster and large G. fortis tended to be placed in the G. magnirostris cluster.
Table 5.
Genetic differentiation within and between the three granivorous ground finch species. Geospiza fortis is represented by the small and large beak-size classes and by all size classes (including intermediates) pooled. p-values for genetic differentiation are above the diagonal. Bold entries are those that remained significant after sequential Bonferroni correction. FST values are below the upper diagonal, with asterisks (*) indicating 95% confidence intervals that do not overlap with zero. RST values are below the lower diagonal.
| G. fortis (small) | G. fortis (large) | G. fuliginosa | G. magnitrostris | G. fortis (all) | |
|---|---|---|---|---|---|
| G. fortis (small) | <0.001 | 0. 01 | <0.001 | — | |
| G. fortis (large) | 0.011* | <0.001 | <0.001 | — | |
| G. fuliginosa | 0.022* | 0.041* | <0.001 | <0.001 | |
| G. magnitrostris | 0.031* | 0.021* | 0.082* | <0.001 | |
| G. fortis (all) | — | — | 0.029* | 0.026* | |
| G. fortis (small) | |||||
| G. fortis (large) | 0.017 | ||||
| G. fuliginosa | 0.001 | 0.036 | |||
| G. magnitrostris | 0.014 | 0.001 | 0.054 | ||
| G. fortis (all) | — | — | 0.011 | 0.001 |
Figure 6.
Bayesian clustering analysis of population structure performed in Structure v. 2.1. Black bars represent the separation between different groups: G. fuliginosa, G. fortis (AB small, AB large, BB small, BB large, EG small and EG large) and G. magnirostris, AB, Academy Bay; BB, Borerro Bay; EG, El Garrapatero.
(c). Gene flow
Estimates of contemporary gene flow from Geneclass were consistent with key results from the above analyses of population structure: (i) gene flow was lower between beak-size morphs than within them, both within and between sites and (ii) gene flow was lower between the beak-size classes at El Garrapatero than at other sites (tables 6 and 7). Similar results were obtained both when including the intermediate-size class and when using a single dividing point between the large and small size class (electronic supplementary material, appendices S3 and S4).
Table 6.
Bidirectional rates of contemporary gene flow in G. fortis between beak-size classes (S, small; L, large) from different collection sites (AB, Academy Bay; BB, Borrero Bay; EG, El Garrapatero). n and p represent sample sizes and statistical significance of the probability of assignment, respectively. These results were obtained using the Paetkau et al. (2004) assignment method as implemented in the software Geneclass 2.0.
| n | p | ABS | ABL | BBS | BBL | EGS | EGL | |
|---|---|---|---|---|---|---|---|---|
| Academy Bay S (ABS) | 118 | 0.014 | 7 | 8 | 1 | 12 | 6 | |
| Academy Bay L (ABL) | 60 | 0.015 | 4 | 1 | 2 | 4 | 14 | |
| Borrero Bay S (BBS) | 53 | 0.006 | 7 | 1 | 6 | 8 | 3 | |
| Borrero Bay L (BBL) | 24 | 0.011 | 6 | 3 | 4 | 2 | 4 | |
| El Garrapatero S (EGS) | 171 | 0.021 | 12 | 3 | 9 | 1 | 5 | |
| El Garrapatero L (EGL) | 66 | 0.021 | 3 | 12 | 2 | 3 | 2 |
Table 7.
Bidirectional rates of contemporary gene flow in G. fortis (all three beak-size classes pooled) from the three collection sites on Santa Cruz Island. p represents statistical significance of the probability of assignment. These results were obtained using the Paetkau et al. (2004) assignment method as implemented in the software Geneclass 2.0.
| p | Academy Bay | Borrero Bay | El Garrapatero | |
|---|---|---|---|---|
| Academy Bay | 0.001 | 21 | 26 | |
| Borrero Bay | 0.001 | 14 | 16 | |
| El Garrapatero | 0.001 | 33 | 23 |
4. Discussion
All of our data and analyses were congruent in revealing significant population structure and limitations to gene flow within G. fortis on Santa Cruz Island. This result shows the potential for partial reproductive isolation to be maintained (or perhaps to even originate) on a single island. This conclusion seems to run counter to persistent skepticism about bird diversification on single islands (Coyne & Price 2000; Grant 2001; Phillimore et al. 2008). Perhaps divergence can be more easily maintained here owing to the reasonable size (986 km2), elevation (869 m.a.s.l.) and ecological diversity of Santa Cruz Island (Wiggins & Porter 1971; Grant 1999; Parent & Crespi 2006). That is, larger and higher islands often have a greater diversity of niches into which adaptive radiation may proceed (Ricklefs & Lovette 1999; Losos & Schluter 2000; Ryan et al. 2007). In addition, larger islands allow for greater isolation-by-distance, which may facilitate divergence in response to spatially structured selection (Doebeli & Dieckmann 2003; Gavrilets & Vose 2005; Gavrilets et al. 2007). We now detail how the population structure of G. fortis on Santa Cruz is associated with space (collection sites) and with ecological traits (beak size). We then turn to a further consideration of the origins/maintenance of this variation.
(a). Patterns of differentiation and gene flow
A significant fraction of the observed population structure and limitations to gene flow in G. fortis on Santa Cruz Island could be attributed to differences among collection sites (table 3). This result seems to support sporadic suggestions that spatial separation, particularly when coupled with ecological differences, might contribute to the divergence of birds on a single island (see also Blondel et al. 1999; Postma & van Noordwijk 2005; Kleindorfer et al. 2006; Ryan et al. 2007; Christensen & Kleindorfer 2009). In our study, however, the population structure attributable to space was very small—although it might have been greater if we had examined G. fortis at more distant and ecologically divergent sites, such as different altitudinal zones (e.g. for G. fuliginosa, see Kleindorfer et al. 2006). At present, however, we must conclude that although spatial separation can certainly aid diversification in parapatry (Doebeli & Dieckmann 2003; Gavrilets & Vose 2005; Gavrilets et al. 2007), we have no evidence that this process has been particularly important in Darwin's finches.
The most striking pattern in our data was that most of the population structure and gene flow restriction was associated with beak size. That is, birds in the small and large beak-size classes showed moderate, and highly significant, genetic differentiation both within and between sites (table 3). Indeed, birds with similar beak sizes were more genetically similar across sites than were birds of different beak sizes within sites (table 3). These patterns probably reflect limited overall gene flow between the morphs, rather than linkage to genes for beak size (e.g. BMP4, Abzhanov et al. 2004), because similar patterns were evident at multiple unlinked neutral loci. The observed clustering by beak size, rather than by site, could have two basic explanations. One is that the beak-size morphs originated at one or a few sites and then spread out to occupy more sites with limited gene flow between the morphs at each site. Another is that the two morphs split independently at multiple sites and then interbred across sites within each morph. Either way, patterns of gene flow within G. fortis on Santa Cruz Island are mainly associated with ecology (different foraging adaptations) rather than geography.
Evidence for the importance of ecology is strengthened when divergence and gene flow between the small and large G. fortis morphs is compared across the three collection sites. Genetic differentiation is greatest, and gene flow lowest, at El Garrapatero (table 3 and figure 5), where the population is currently bimodal for beak size (figure 3; Hendry et al. 2006). Genetic differentiation is weaker at Academy Bay (table 3 and figure 5), where bimodality was strong in the past (Ford et al. 1973) but has since weakened (Hendry et al. 2006). Genetic differentiation is largely absent at Borrero Bay (table 3 and figure 5), where the large morph is rare (Hendry et al. 2006). This spatial coupling of genetic and phenotypic differentiation might reflect either of two opposite, but complementary, causal effects (Räsänen & Hendry 2008). On the one hand, increasing gene flow between the morphs might constrain their ability to differentiate, with this effect being greatest at Borrero Bay. On the other hand, increasing adaptive divergence between the morphs might reduce gene flow (i.e. ecological speciation), with this effect being greatest at El Garrapatero.
Additional insights are made possible by comparing divergence and gene flow within G. fortis to that between G. fortis and its smaller (G. fuliginosa) and larger (G. magnirostris) congeners. First, divergence between the G. fortis beak-size morphs was approximately half of that between G. fortis and each of the two other species. Second, the small G. fortis morph was genetically most similar to G. fuliginosa and the large G. fortis morph was genetically most similar to G. magnirostris. Here, again, greater ecological/adaptive differences (between relative to within species) is associated with lower gene flow. In addition, the fact that divergence within G. fortis is a small-scale version of divergence among the three species suggests that processes maintaining reproductive isolation between the two morphs, such as disruptive selection (Hendry et al. 2009) and assortative mating (Huber et al. 2007), might reflect those that contribute to speciation in the group. Our analysis of within-species variation thus supports previous arguments based on between-species variation (Lack 1947; Grant 1999; Grant & Grant 2008) about the importance of ecological speciation in Darwin's finches.
(b). Potential scenarios for diversification
As noted in the introduction, several scenarios have been proposed for the origin of G. fortis beak-size morphs, each matching a different expected context for divergence with gene flow. We cannot here determine which scenario is correct, but we can provide some further insight into the various possibilities.
The first context was initial divergence in different locations followed by further divergence after secondary contact. For Darwin's finches, this initial divergence is typically postulated to have occurred among islands (Lack 1947; Grant 1999, 2001; Schluter 2000a; Petren et al. 2005; Grant & Grant 2008), whereas our data are relevant to the possibility of initial divergence between sites on the same island. We found relatively little support for this possibility given that genetic divergence was very small between sites on the same island (table 4). This result is not definitive, however, because substantial divergence in selected traits/genes might occur even when divergence in neutral markers is absent (Nosil et al. 2009). Moreover, we did not examine all possible divergent environments on Santa Cruz, with higher elevations being a possible site of greater divergence (Kleindorfer et al. 2006).
The second context was fully sympatric speciation. We have no data to directly address this possibility but it seems worthwhile to at least entertain further. The reason is that the ‘magic trait’ conditions thought to favour fully sympatric speciation (Fry 2003; Gavrilets 2004; Bolnick & Fitzpatrick 2007) are present in Geospiza. First, disruptive selection on beak size occurs between sympatric species (Schluter & Grant 1984; Schluter et al. 1985; Grant & Grant 2006) and between the sympatric G. fortis morphs (Hendry et al. 2009). Second, differences in beak size, and the resulting differences in song (Podos 2001; Huber & Podos 2006; Herrel et al. 2009), generate assortative mating between sympatric species (Ratcliffe & Grant 1983; Grant & Grant 1996a, 1997b) and between the sympatric G. fortis morphs (Huber et al. 2007). Third, beak size is highly heritable (Keller et al. 2001), males learn their songs from their fathers (Grant & Grant 1989), and females prefer to mate with males that sing songs similar to their father (Grant & Grant 1998). In short, beak size in Darwin's finches seems a particularly likely candidate for a magic trait.
The third context was interspecific hybridization leading to the origin of one of the morphs. The most probable scenario here would be that the large morph was originally formed through interbreeding between G. magnirostris and small-beaked G. fortis. This possibility is worth considering given that (i) the small G. fortis morph is widespread, whereas the large G. fortis morph is not (Grant et al. 1976; Hendry et al. 2006), (ii) the large G. fortis morph is morphologically and genetically intermediate between the small G. fortis morph and G. magnirostris, and (iii) we recorded one instance of a large morph G. fortis female mating with a G. magnirostris male (Huber et al. 2007). Our study system might therefore represent a chance to further consider the possibility of ecologically based hybrid speciation.
5. Summary
The beak-size morphs of G. fortis on Santa Cruz Island represent a case of divergence with gene flow being maintained (or driven) by ecological differences. The two morphs have different foraging morphology that is clearly adapted for different food types, and this divergence has led to reproductive barriers that include disruptive selection and assortative mating. These associations are largely maintained across Santa Cruz Island as a whole, overwhelming minor spatial restrictions on gene flow. Space does interact with beak size, however, in that sites where birds show greater morphological divergence are also sites where they show greater genetic divergence (lower gene flow). Moreover, patterns of genetic variation are associated with beak size not only within species, but also between species. All of these observations point to ecological/adaptive differences as the main driver of the reproductive isolation in this system.
Acknowledgements
Logistical support and permits were provided by the Galápagos National Park Service and the Charles Darwin Research Station. Field assistance was provided by Ana Gabela, Sarah Huber and David Delaney. Laboratory assistance was provided by Maribel González, Jesús Mavárez and Oris Sanjur. K. Petren provided primer aliquots for the DNA analyses. L. Keller, K. Petren and J. S. Moore also provided helpful comments on an earlier draft of this manuscript. J. Raeymaekers and X. Thibert-Plante provided useful suggestion for data analysis. Anonymous reviewers gave helpful comments on the last version of the manuscript. This research was funded by the Secretaría Nacional de Ciencia, Tecnología e Innovación and El Instituto para la Formación y Aprovechamiento de los Recursos Humanos, Panama (L.F.d.L.); the US National Science Foundation (J.P.); the Natural Sciences and Engineering Research Council of Canada (A.P.H.); and the Smithsonian Tropical Research Institute (E.B.).
Footnotes
One contribution of 13 to a Theme Issue ‘Darwin's Galápagos finches in modern evolutionary biology’.
References
- Abdo Z., Crandall K. A., Joyce P.2004Evaluating the performance of likelihood methods for detecting population structure and migration. Mol. Ecol. 13, 837–851 (doi:10.1111/j.1365-294X.2004.02132.x) [DOI] [PubMed] [Google Scholar]
- Abzhanov A., Protas M., Grant B. R., Grant P. R., Tabin C. J.2004Bmp4 and morphological variation of beaks in Darwin's finches. Science 305, 1462–1465 (doi:10.1126/science.1098095) [DOI] [PubMed] [Google Scholar]
- Belkhir K., Borsa P., Chikhi L., Raufaste N., Bonhomme F.2004Genetix 4.05, logiciel sous Windows TM pour la génétique des populations. In Laboratoire génome, populations, interactions, CNRS UMR 5171 Montpellier, France: Université de Montpellier II [Google Scholar]
- Berner D., Grandchamp A., Hendry A. P., Feder J.2009Variable progress toward ecological speciation in parapatry: stickleback across eight lake–stream transitions. Evolution 63, 1740–1753 (doi:10.1111/j.1558-5646.2009.00665.x) [DOI] [PubMed] [Google Scholar]
- Berry O., Mandy D. T., Stephen D. S.2004Can assignment tests measure dispersal? Mol. Ecol. 13, 551–561 (doi:10.1046/j.1365-294X.2004.2081.x) [DOI] [PubMed] [Google Scholar]
- Blondel J., Dias P. C., Perret P., Maistre M., Lambrechts M. M.1999Selection-based biodiversity at a small spatial scale in a low-dispersing insular bird. Science 285, 1399–1402 (doi:10.1126/science.285.5432.1399) [DOI] [PubMed] [Google Scholar]
- Bolnick D. I., Fitzpatrick B.2007Sympatric speciation: theory and empirical data. Annu. Rev. Ecol. Syst. 38, 459–487 (doi:10.1146/annurev.ecolsys.38.091206.095804) [Google Scholar]
- Bowman R. I.1961Morphological differentiation and adaptation in the Galápagos finches. Univ. Calif. Publ. Zool. 48, 1–326 [Google Scholar]
- Brown W. L., Jr, Wilson E. O.1956Character displacement. Syst. Zool. 5, 49–64 (doi:10.2307/2411924) [Google Scholar]
- Chapuis M. P., Lecoq M., Michalakis Y., Loiseau A., Sword G. A., Piry S., Estoup A.2009Do outbreaks affect genetic population structure? A worldwide survey in Locusta migratoria, a pest plagued by microsatellite null alleles. Mol. Ecol. 17, 3640–3653 (doi:10.1111/j.1365-294X.2008.03869.x) [DOI] [PubMed] [Google Scholar]
- Christensen R., Kleindorfer S.2009Jack-of-all-trades or master of one? Variation in foraging specialisation across years in Darwin's Tree finches (Camarhynchus spp.). J. Ornithol. 150, 383–391 (doi:10.1007/s10336-008-0358-y) [Google Scholar]
- Corander J., Waldmann P., Marttinen P., Sillanpää M. J.2004Baps 2: enhanced possibilities for the analysis of genetic population structure. Bioinformatics 20, 2363–2369 (doi:10.1093/bioinformatics/bth250) [DOI] [PubMed] [Google Scholar]
- Cornuet J. M., Piry S., Luikart G., Estoup A., Solignac M.1999New methods employing multilocus genotypes to select or exclude populations as origins of individuals. Genetics 153, 1989–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J. A., Orr H. A.2004Speciation Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- Coyne J. A., Price T. D.2000Little evidence for sympatric speciation in island birds. Evolution 54, 2166–2171 [DOI] [PubMed] [Google Scholar]
- Dieckmann U., Doebeli M.1999On the origin of species by sympatric speciation. Nature 400, 354–357 (doi:10.1038/22521) [DOI] [PubMed] [Google Scholar]
- Doebeli M., Dieckmann U.2003Speciation along environmental gradients. Nature 421, 259–264 (doi:10.1038/nature01274) [DOI] [PubMed] [Google Scholar]
- Doebeli M., Blok H. J., Leimar O., Dieckmann U.2007Multimodal pattern formation in phenotype distributions of sexual populations. Proc. R. Soc. B. 274, 347–357 (doi:10.1098/rspb.2006.3725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge M. D. B., Kinnear J. E., Onus M. L.2001Source population of dispersing rock-wallabies (Petrogale lateralis) identified by assignment tests on multilocus genotypic data. Mol. Ecol. 1310, 2867–2876 [DOI] [PubMed] [Google Scholar]
- Endler J. A.1973Gene flow and population differentiation: studies of clines suggest that differentiation along environmental gradients may be independent of gene flow. Science 179, 243–250 (doi:10.1126/science.179.4070.243) [DOI] [PubMed] [Google Scholar]
- Evanno G., Regnaut S., Goudet J.2005Detecting the number of clusters of individuals using the software Structure: a simulation study. Mol. Ecol. 14, 2611–2620 (doi:10.1111/j.1365-294X.2005.02553.x) [DOI] [PubMed] [Google Scholar]
- Excoffier L., Laval G., Schneider S.2005Arlequin version 3.0: an integrated software package for population genetics data analysis. Evol. Bioinfo. Online 1, 47–50 [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J.1981Skepticism towards Santa Rosalia, or why are there so few kinds of animals? Evolution 35, 124–138 (doi:10.2307/2407946) [DOI] [PubMed] [Google Scholar]
- Ford H. A., Parkin D. T., Ewing A. W.1973Divergence and evolution in Darwin's finches. Biol. J. Linn. Soc. 5, 289–295 (doi:10.1111/j.1095-8312.1973.tb00707.x) [Google Scholar]
- Foster D. J., Podos J., Hendry A. P.2008A geometric morphometric appraisal of beaks shape in Darwin's finches. J. Evol. Biol. 21, 263–275 [DOI] [PubMed] [Google Scholar]
- Fry J. D.2003Multilocus models of sympatric speciation: Bush versus Rice versus Felsenstein. Evolution 57, 1735–1746 [DOI] [PubMed] [Google Scholar]
- Gavrilets S.2004Fitness landscapes and the origin of species Princeton, NJ: Princeton University Press [Google Scholar]
- Gavrilets S., Vose A.2005Dynamic patterns of adaptive radiation. Proc. Natl Acad. Sci. USA 102, 18 040–18 045 (doi:10.1073/pnas.0506330102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilets S., Vose A., Barluenga M., Salzburger W., Meyer A.2007Case studies and mathematical models of ecological speciation. 1. Cichlids in a crater lake. Mol. Ecol. 16, 2893–2909 (doi:10.1111/j.1365-294X.2007.03305.x) [DOI] [PubMed] [Google Scholar]
- Goudet J.1995Fstat (Version 1.2): a computer program to calculate F-statistics. Heredity 86, 485–486 [Google Scholar]
- Goudet J.2005Hierfstat, a package for R to compute and test hierarchical F-statistics. Mol. Ecol. Notes 5, 184–186 (doi:10.1111/j.1471-8286.2004.00828.x) [Google Scholar]
- Grant P. R.1972Convergent and divergent character displacement. Biol. J. Linn. Soc. 4, 39–68 (doi:10.1111/j.1095-8312.1972.tb00690.x) [Google Scholar]
- Grant P. R.1999Ecology and evolution of Darwin's finches Princeton, NJ: Princeton University Press [Google Scholar]
- Grant P. R.2001Reconstructing the evolution of birds on islands: 100 years of research. Oikos 92, 385–403 (doi:10.1034/j.1600-0706.2001.920301.x) [Google Scholar]
- Grant B. R., Grant P. R.1979Darwin's finches: population variation and sympatric speciation. Proc. Natl Acad. Sci. USA 76, 2359–2363 (doi:10.1073/pnas.76.5.2359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P. R., Grant B. R.1989Sympatric speciation in Darwin's finches. In Speciation and its consequences (eds Otte D., Endler J. A.). Sunderland, MA: Sinauer Associates [Google Scholar]
- Grant B. R., Grant P. R.1993Evolution of Darwin's finches caused by a rare climatic event. Proc. R. Soc. Lond. B. 251, 111–117 (doi:10.1098/rspb.1993.0016) [Google Scholar]
- Grant P. R., Grant B. R.1994Phenotypic and genetic effects of hybridization in Darwin's finches. Evolution 48, 297–316 (doi:10.2307/2410094) [DOI] [PubMed] [Google Scholar]
- Grant B. R., Grant P. R.1996aCultural inheritance of song and its role in the evolution of Darwin's finches. Evolution 50, 2471–2487 (doi:10.2307/2410714) [DOI] [PubMed] [Google Scholar]
- Grant B. R., Grant P. R.1996bHigh survival of Darwin's finch hybrids: effects of beak morphology and diets. Ecology 77, 500–509 (doi:10.2307/2265625) [Google Scholar]
- Grant P. R., Grant B. R.1997aHybridization, sexual imprinting, and mate choice. Am. Nat. 149, 1–28 (doi:10.1086/285976) [Google Scholar]
- Grant P. R., Grant B. R.1997bMating patterns of Darwin's finch hybrids determined by song and morphology. Biol. J. Linn. Soc. 60, 317–343 (doi:10.1111/j.1095-8312.1997.tb01499.x) [Google Scholar]
- Grant B. R., Grant P. R.1998Hybridization and speciation in Darwin's finches: the role of sexual imprinting on a culturally transmitted trait. In Endless forms: species and speciation (eds Howard D. J., Berlocher S. H.). Oxford, UK: Oxford University Press [Google Scholar]
- Grant P. R., Grant B. R.2002Unpredictable evolution in a 30-year study of Darwin's finches. Science 296, 707–711 (doi:10.1126/science.1070315) [DOI] [PubMed] [Google Scholar]
- Grant P. R., Grant B. R.2006Evolution of character displacement in Darwin's finches. Science 313, 224–226 (doi:10.1126/science.1128374) [DOI] [PubMed] [Google Scholar]
- Grant P. R., Grant B. R.2008How and why species multiply: the radiation of Darwin's finches Princeton, NJ: Princeton University Press [Google Scholar]
- Grant P. R., Grant B. R., Smith J. N. M., Abbott I. J., Abbott L. K.1976Darwin's finches: population variation and natural selection. Proc. Natl Acad. Sci. USA 73, 257–261 (doi:10.1073/pnas.73.1.257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P. R., Grant B. R., Markert J. A., Keller L. F., Petren K.2004Convergent evolution of Darwin's finches caused by introgressive hybridization and selection. Evolution 58, 1588–1599 [DOI] [PubMed] [Google Scholar]
- Grant P. R., Grant B. R., Petren K.2005Hybridization in the recent past. Am. Nat. 166, 56–67 (doi:10.1086/430331) [DOI] [PubMed] [Google Scholar]
- Hendry A. P., Grant P. R., Grant B. R., Ford H. A., Brewer M. J., Podos J.2006Possible human impacts on adaptive radiation: beak size bimodality in Darwin's finches. Proc. R. Soc. B 273, 1887–1894 (doi:10.1098/rspb.2006.3534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry A. P., Nosil P., Rieseberg L. H.2007The speed of ecological speciation. Func. Ecol. 21, 455–464 (doi:10.1111/j.1365-2435.2007.01240.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry A. P., Huber S. K., de León L. F., Herrel A., Podos J.2009Disruptive selection in a bimodal population of Darwin's finches. Proc. R. Soc. B 276, 753–759 (doi:10.1098/rspb.2008.1321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrel A., Podos J., Huber S. K., Hendry A. P.2005Bite performance and morphology in a population of Darwin's finches: implications for the evolution of beak shape. Func. Ecol. 19, 43–48 (doi:10.1111/j.0269-8463.2005.00923.x) [Google Scholar]
- Herrel A., Podos J., Vanhooydonck B., Hendry A. P.2009Force–velocity trade-off in Darwin's finch jaw function: a biomechanical basis for ecological speciation? Func. Ecol. 23, 119–125 (doi:10.1111/j.1365-2435.2008.01494.x) [Google Scholar]
- Huber S. K., Podos J.2006Beak morphology and song features covary in a population of Darwin's finches (Geospiza fortis). Biol. J. Linn. Soc. 88, 489–498 (doi:10.1111/j.1095-8312.2006.00638.x) [Google Scholar]
- Huber S. K., de León L. F., Hendry A. P., Berminghan E., Podos J.2007Reproductive isolation of sympatric morphs in a population of Darwin's finches. Proc. R. Soc. B. 274, 1709–1714 (doi:10.1098/rspb.2007.0224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L. F., Grant P. R., Grant B. R., Petren K.2001Heritability of morphological traits in Darwin's finches: misidentified paternity and maternal effects. Heredity 87, 325–336 (doi:10.1046/j.1365-2540.2001.00900.x) [DOI] [PubMed] [Google Scholar]
- Kleindorfer S., Chapman T. W., Winkler H., Sulloway F. J.2006Adaptive divergence in contiguous populations of Darwin's small ground finch (Geospiza fuliginosa). Evol. Ecol. Res. 8, 357–372 [Google Scholar]
- Lack D.1947Darwin's finches Cambridge, UK: Cambridge University Press [Google Scholar]
- Losos J. B., Schluter D.2000Analysis of an evolutionary species-area relationship. Nature 408, 847–850 (doi:10.1038/35048558) [DOI] [PubMed] [Google Scholar]
- Mallet J.2007Hybrid speciation. Nature 446, 279–283 (doi:10.1038/nature05706) [DOI] [PubMed] [Google Scholar]
- Mayr E.1963Animal species and evolution Cambridge, UK: Belknap Press of Harvard University Press [Google Scholar]
- Nosil P., Funk D. J., Ortiz-Barrientos D.2009Divergent selection and heterogeneous genomic divergence. Mol. Ecol. 18, 375–402 (doi:10.1111/j.1365-294X.2008.03946.x) [DOI] [PubMed] [Google Scholar]
- Paetkau D., Slade R., Burden M., Estoup A.2004Genetic assignment methods for the direct, real-time estimation of migration rate: a simulation-based exploration of accuracy and power. Mol. Ecol. 13, 55–65 (doi:10.1046/j.1365-294X.2004.02008.x) [DOI] [PubMed] [Google Scholar]
- Parent C. E., Crespi B. J.2006Sequential colonization and diversification of Galápagos endemic land snail genus Bulimulus (Gastropoda, Stylommatophora). Evolution 60, 2311–2328 [PubMed] [Google Scholar]
- Peakall R., Smouse P. E.2006Genalex 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 6, 288–295 (doi:10.1111/j.1471-8286.2005.01155.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petren K.1998Microsatellites primers from Geospiza fortis and cross species amplification in Darwin's finches. Mol. Ecol. 12, 1782–1784 [DOI] [PubMed] [Google Scholar]
- Petren K., Grant P. R., Grant B. R., Keller L. F.2005Comparative landscape genetics and the adaptive radiation of Darwin's finches: the role of peripheral isolation. Mol. Ecol. 14, 2943–2957 (doi:10.1111/j.1365-294X.2005.02632.x) [DOI] [PubMed] [Google Scholar]
- Phillimore A. B., Orme C. D. L., Thomas G. H., Blackburn T. M., Bennett P. M., Gaston K. J., Owens I. P. F.2008Sympatric speciation in birds is rare: insights from range data and simulations. Am. Nat. 171, 646–657 (doi:10.1086/587074) [DOI] [PubMed] [Google Scholar]
- Piertney S. B., Summers R., Marquiss M.2001Microsatellite and mitochondrial DNA homogeneity among phenotypically diverse crossbill taxa in the UK. Proc. R. Soc. Lond. B. 268, 1511–1517 (doi:10.1098/rspb.2001.1015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piry S., Alapetite A., Cornuet J. M., Paetkau D., Baudouin L., Estoup A.2004Geneclass2: a software for genetic assignment and first-generation migrant detection. J. Heredity 95, 536–539 (doi:10.1093/jhered/esh074) [DOI] [PubMed] [Google Scholar]
- Podos J.2001Correlated evolution of morphology and vocal signal structure in Darwin's finches. Nature 409, 185–188 (doi:10.1038/35051570) [DOI] [PubMed] [Google Scholar]
- Podos J., Southall J. A., Rossi-Santos M. R.2004Vocal mechanics in Darwin's finches: correlation of beak gape and song frequency. J. Exp. Biol. 207, 607–619 (doi:10.1242/jeb.00770) [DOI] [PubMed] [Google Scholar]
- Postma E., van Noordwijk A. J.2005Gene flow maintains a large genetic difference in clutch size at a small spatial scale. Nature 433, 65–68 (doi:10.1038/nature03083) [DOI] [PubMed] [Google Scholar]
- Pritchard J. K., Stephens M., Donnelly P.2000Inference of population structure using multilocus genotype data. Genetics 155, 45–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räsänen K., Hendry A. P.2008Disentangling interactions between adaptive divergence and gene flow when ecology drives diversification. Ecol. Lett. 11, 624–636 (doi:10.1111/j.1461-0248.2008.01176.x) [DOI] [PubMed] [Google Scholar]
- Ratcliffe L. M., Grant P. R.1983Species recognition in Darwin's finches (Geospiza, Gould). I: discrimination by morphological cues. Anim. Behav. 31, 1139–1153 (doi:10.1016/S0003-3472(83)80021-9) [Google Scholar]
- Raymond M., Rousset F.1995Genepop (version 1.2) population genetics software for exact test and ecumenicism. Heredity 86, 248–249 [Google Scholar]
- Rice W. R., Hostert E. E.1993Perspective: laboratory experiments on speciation: what have we learned in forty years? Evolution 47, 1637–1653 (doi:10.2307/2410209) [DOI] [PubMed] [Google Scholar]
- Ricklefs R. E., Lovette I. J.1999The roles of island area per se and habitat diversity in the species-area relationships of four Lesser Antillean faunal groups. J. Anim. Ecol. 68, 1142–1160 (doi:10.1046/j.1365-2656.1999.00358.x) [Google Scholar]
- Rundle H. D., Nosil P.2005Ecological speciation. Ecol. Lett. 8, 336–352 (doi:10.1111/j.1461-0248.2004.00715.x) [Google Scholar]
- Ryan P. G., Bloomer P., Moloney C. L., Grant T. J., Delport W.2007Ecological speciation in South Atlantic Island finches. Science 315, 1420–1423 (doi:10.1126/science.1138829) [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E. F., Maniatis T.1989Molecular cloning: a laboratory manual, 2nd edn.Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Sato A., O'hUigin C., Figueroa F., Grant P. R., Grant B. R., Tichy H., Klein J.1999Phylogeny of Darwin's finches as revealed by mtDNA sequences. Proc. Natl Acad. Sci. USA 96, 5101–5106 (doi:10.1073/pnas.96.9.5101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D.2000aThe ecology of adaptive radiation Oxford, UK: Oxford University Press [Google Scholar]
- Schluter D.2000bEcological character displacement in adaptive radiation. Am. Nat. 156, S4–S16 (doi:10.1086/303412) [Google Scholar]
- Schluter D., Grant P. R.1984Determinants of morphological patterns in communities of Darwin's finches. Am. Nat. 123, 175–196 (doi:10.1086/284196) [Google Scholar]
- Schluter D., Price T. D., Grant P. R.1985Ecological character displacement in Darwin's finches. Science 227, 1056–1059 (doi:10.1126/science.227.4690.1056) [DOI] [PubMed] [Google Scholar]
- Seehausen O.2004Hybridization and adaptive radiation. Trends Ecol. Evol. 19, 198–207 (doi:10.1016/j.tree.2004.01.003) [DOI] [PubMed] [Google Scholar]
- Servedio M. R., Noor M. A. F.2003The role of reinforcement in speciation: theory and data meet. Annu. Rev. Ecol. Syst. 34, 339–364 (doi:10.1146/annurev.ecolsys.34.011802.132412) [Google Scholar]
- Slatkin M.1995A measure of population subdivision based on microsatellite allele frequencies. Genetics 139, 457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatkin M.2005Seeing ghosts: the effect of unsampled populations on migration rates estimated for sampled populations. Mol. Ecol. 14, 67–73 (doi:10.1111/j.1365-294X.2004.02393.x) [DOI] [PubMed] [Google Scholar]
- Smith J. M.1966Sympatric speciation. Am. Nat. 100, 637–650 (doi:10.1086/282457) [Google Scholar]
- Waples R., Gaggiotti O.2006What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity. Mol. Ecol. 15, 1419–1439 (doi:10.1111/j.1365-294X.2006.02890.x) [DOI] [PubMed] [Google Scholar]
- Weir B. S., Cockerham C. C.1984Estimating F-statistics for the analysis of population structure. Evolution 38, 1358–1370 (doi:10.2307/2408641) [DOI] [PubMed] [Google Scholar]
- Wiggins I. L., Porter D. M.1971Flora of the Galápagos Islands Stanford, CA: Stanford University Press [Google Scholar]
- Zink R.2002A new perspective on the evolutionary history of Darwin's finches. The Auk 119, 864–871 (doi:10.1642/0004-8038(2002)119[0864:ANPOTE]2.0.CO;2) [Google Scholar]