Abstract
The study of ingestive behaviour has an extensive history, starting as early as 1918 when Wallace Craig, an animal behaviourist, coined the terms ‘appetitive’ and ‘consummatory’ for the two-part sequence of eating, drinking and sexual behaviours. Since then, most ingestive behaviour research has focused on the neuroendocrine control of food ingestion (consummatory behaviour). The quantity of food eaten, however, is also influenced by the drive both to acquire and to store food (appetitive behaviour). For example, hamster species have a natural proclivity to hoard food and preferentially alter appetitive ingestive behaviours in response to environmental changes and/or metabolic hormones and neuropeptides, whereas other species would instead primarily increase their food intake. Therefore, with the strong appetitive component to their ingestive behaviour that is relatively separate from their consummatory behaviour, they seem an ideal model for elucidating the neuroendocrine mechanisms underlying the control of food hoarding and foraging. This review focuses on the appetitive side of ingestive behaviour, in particular food hoarding, attempting to integrate what is known about the neuroendocrine mechanisms regulating this relatively poorly studied behaviour. An hypothesis is formed stating that the direction of ‘energy flux’ is a unifying factor for the control of food hoarding.
Keywords: ingestive behaviour, energy balance, hoarding, appetitive behaviour, food intake, foraging
1. Introduction
Ingestive behaviour has a long history of study and currently has a place in the fields of animal behaviour, learning and memory, psychology, physiology and neuroscience. In 1918, Wallace Craig, an animal behaviourist, coined the terms ‘appetitive’ and ‘consummatory’ for the two-part sequence of behaviours required for eating, drinking and reproduction (Craig 1918). More specifically, he defined appetitive behaviours as motivated, species-specific behaviours involved in seeking a goal object (e.g. food, water, a mate), ultimately bringing the animal into physical contact with the goal object (Craig 1918). By contrast, the consummatory behaviours (from consummate not consume) are reflexive, stereotyped and are the final act once the goal object has been obtained (Craig 1918), in the case of ingestive consummatory behaviour—eating.
The primary purpose of this review is to focus on the appetitive ingestive behaviour of food hoarding, and to a lesser extent, food foraging, and attempt to integrate what is known about the neuroendocrine mechanisms controlling these behaviours. We will focus on studies done in small mammals where external, as well as peripheral and central, factors affecting these behaviours have been studied in relation to energy balance. It is beyond the scope of this review to cover the interesting work in the field on hoarding and memory in various bird and squirrel species. More specifically, we will focus our review on the offspring of wild trapped animals that are considered ‘natural food hoarders’ because they are documented hoarders in nature, but have been studied in the laboratory, such as Syrian hamsters (Mesocricetus auratus; Murphy 1985) and Siberian hamsters (Phodopus sungorus). Occasionally, we will supplement this information with studies of laboratory rats, which are not natural hoarders (Pisano & Storer 1948; Calhoun 1962; Lore & Flannelly 1978; Takahashi & Lore 1980; Whishaw & Whishaw 1996), but instead carry food from the source to a safe place to eat. A paper by Wolfe in 1939 (Wolfe 1939) opened the door to the laboratory study of food hoarding by demonstrating that it was quantifiable. Several years later, Morgan et al. (1943) generated a ‘deficit hypothesis’ that continues to guide many hoarding studies today. The deficit hypothesis proposes that animals hoard owing to a growing energetic deficit that eventually reaches a threshold that triggers food hoarding (Morgan Stellar & Johnson 1943). This type of deficit was initially not thought to be equivalent to the short-term deficit induced by ‘hunger’; instead, the signal to hoard was thought to be generated across a longer time period of days or weeks (Bindra 1948). This deficit hypothesis for food hoarding is similar to the notion embodied in the ‘lipostatic theory’ of Kennedy (1953) where food intake is stimulated when there are deficits in fat stores. We review the hoarding literature below in light of the deficit model and show that although some data appear to support this hypothesis, it is difficult to reconcile many other data with it. We present, as we have suggested in the past (Bartness & Day 2003), a different hypothesis that borrows liberally from the metabolic control of food intake (Friedman 1991; Friedman & Rawson 1994) and fertility (Wade & Schneider 1992; Wade et al. 1996). Specifically, we propose that ‘energy flux’ is important for changes in food-hoarding behaviour. That is, it may be that the signals that trigger foraging/hoarding are generated from central neuropeptides, endocrine or other circulating factors associated with the movement of available metabolic fuels from one compartment (e.g. glycogen from liver or muscle or lipid from adipose tissue stores) to tissues where they can be oxidized. This hypothesis accounts for more of the data involving external and internal influences on food hoarding than is accounted for by the deficit hypothesis, as the reader will discover below.
Before beginning our discussions of external and internal factors affecting food hoarding, we ask what seems to be a question with a simple answer, but is not. Why do animals hoard food? Studies of birds indicate that food hoarding is an adaptive strategy for animals living in an environment with an unpredictable food supply (Pravosudov & Clayton 2002), and this may be true for all species to some degree (for a review, see Vander Wall 1990). For birds that fly, food hoarding appears to make sense physically because storage of calories for future use as lipid energy in white adipose tissue (WAT) can hamper takeoff for flight (Brodin 2000). Why do non-bird species hoard food where flight considerations do not exist? There are no firm answers to this question, but under the conditions found in nature, animals have evolved to become energy efficient. Therefore, food hoarding is likely to be more energy efficient than fat storage because food hoarding does not require food to be eaten, catabolized and converted into a storable form of energy internally until mobilized when it then can be oxidized. Increases in body mass as fat also can increase predation risk as ambulation becomes more cumbersome and/or slow. Given that food hoarding appears more energy efficient than conversion of food to lipid and carbohydrate stores, why is food hoarding not the primary form of energy storage for all animals? There are several mitigating factors against food hoarding, which include food spoilage and theft of food by others as primary reasons (for a review, see Vander Wall 1990).
2. External factors
(a). Food shortage (food deprivation/food restriction)
Food availability in nature is often suboptimal and unpredictable, unlike the Utopian conditions in the laboratory; therefore, significant allocations of time and effort are required to find and secure food to meet energy demands. Food shortages in the wild are emulated in the laboratory by withdrawing food completely (food deprivation) or by decreasing food allotments (food restriction). The first laboratory studies of food hoarding were done using rats, and these non-natural hoarders will exhibit hoarding (food carrying) if sufficiently food deprived (Morgan et al. 1943; Herberg & Blundell 1967; Fantino & Cabanac 1980). In addition to increasing hoarding/carrying after food deprivation, rats also overeat (Baker 1955), as do most animals across many taxa including humans (for a review, see Bartness & Demas 2004). An energetic regulatory puzzle exists for all hamster species tested to date because they fail to exhibit the prolonged and pronounced overeating after food deprivation (Syrian (M. auratus) Silverman & Zucker 1976; Turkish (Mesocricetus brandti) Rowland 1982; Siberian (P. sungorus) Bartness & Clein 1994; Chinese (Cricetulus griseus) Billington et al. 1984). Siberian and Syrian hamsters, for example, are physically capable of overeating (e.g. calorically diluted diets (Rowland 1982; Wood & Bartness 1996a); hypothalamic lesions in paraventricular hypothalamus (PVH) and ventromedial hypothalamus (Bartness et al. 1985; Rowland et al. 1986; Bittman et al. 1991)). Silverman & Zucker (1976) speculated that selection pressures for building food caches to counteract shortfalls in food availability led to the elimination of post-fast-induced hyperphagia in hamsters. We repeatedly tested the notion that Siberian hamsters ‘over-hoard food’, rather than ‘overeat’, in response to food deprivation. In these studies, we found that, similar to birds (Smulders 1998) and laboratory rats (Whishaw et al. 1990), Siberian hamsters will consume food when it is presented after a period of deprivation (Bartness & Clein 1994; Wood & Bartness 1996b; Bartness 1997; Day & Bartness 2003; Keen-Rhinehart & Bartness 2007b, 2008), but unlike rats and most other species, food hoarding, but not food intake, is markedly increased after initial small increases in food intake post-fast in Siberian hamsters (Bartness & Clein 1994; Wood & Bartness 1996b; Bartness 1997; Day & Bartness 2003; Keen-Rhinehart & Bartness 2007b, 2008). Similar to Siberian hamsters, Syrian hamsters also increase food hoarding rather than food intake post-fast, albeit at somewhat lower levels (Lea & Tarpy 1986; Schneider & Buckley 2001; cf. Wong & Jones 1985). Thus, it appears that the control of food intake and food hoarding are separable in hamsters, making it possible, in principle, to determine the underlying neuroendocrine mechanisms responsible for controlling each behaviour.
(b). Food availability (foraging effort)
The relation between food hoarding and food availability is infrequently studied in the laboratory. From an energetic standpoint, one might expect an inverted ‘U’ function relating food hoard size to foraging effort. That is, with an over-abundant food supply food hoarding should be minimal, and at low foraging efforts food hoarding should increase. According to this function, food hoarding eventually should decline with increasing foraging effort as it imposes an increasing energy expenditure, requiring food to be eaten immediately rather than saved for later. Some of the initial studies on this topic were conducted in laboratory rats and Syrian hamsters. Early studies in laboratory rats indicate that they behave in an energy-efficient manner relative to foraging effort. For example, they decrease food-hoard size as the tube leading from their home cage to a food source is lengthened (Cabanac & Swiergiel 1989). Analogous findings of decreases in food hoarding have been reported when Syrian hamsters are required to work for food by pressing a bar an increasing number of times to obtain food pellets (Lea & Tarpy 1986). In these examples, however, the effort required to obtain the food is not very demanding compared with the requirements of foraging in nature. Therefore, we investigated the relation between food-hoard size and foraging effort in a model that requires more substantial energy expenditure to obtain food.
In our studies, we adapted the foraging effort model of Perrigo & Bronson (1985) to our food-hoarding paradigm (Bartness & Clein 1994) to incorporate two characteristics of hoarding and foraging in nature—effort and distance. We tested the effects of increased foraging effort on foraging (pellets earned), food intake (pellets eaten) and hoarding (pellets hoarded) by housing Siberian hamsters in a foraging/hoarding system. Because we will continue to report the effects of various manipulations on food hoarding/foraging and intake by Siberian hamsters housed in this foraging/hoarding system throughout this review, we briefly describe it here. Two cages are positioned one above the other and are connected by approximately 1.52 m tubing that has corners and straightways for both horizontal and vertical climbs. The bottom cage represents an underground burrow, is dark and contains bedding and nesting material. The top cage represents an above-ground foraging area, is lit and contains a running wheel and a water bottle. Food pellets (75 mg) are presented contingent upon the completion of a programmed number of wheel revolutions in the upper cage. In our first experiments, foraging efforts ranged from 10 revolutions per pellet (5.24 m per pellet) to 200 revolutions per pellet (104.8 m per pellet). We also included a group of hamsters where food is available non-contingently, but the running wheel is available for locomotor activity without any contingency (free wheel/free food) to control for the effects of exercise per se. This enables us to determine whether a treatment non-specifically increases or decreases wheel-running, thereby stopping us from attributing such responses to alterations in the motivation to forage (earn) food. Finally, we included a sedentary control group where food also is available non-contingently, but the wheel is blocked from turning (blocked wheel/free food) to control for the effects of exercise on their behaviour or physiology.
In these studies, food intake remained mostly constant, with the exception of a slight increase at 10 revolutions per pellet (Day & Bartness 2001). Food-hoard size exhibited an inverse ‘U’ function with increases in foraging effort, as expected. That is, animals with an active running wheel in their cage (free wheel/free food group) hoarded four times as much food as the sedentary controls (blocked wheel/free food group). At the lower foraging efforts (10 and 50 revolutions), food hoarding also was greater (threefold increase) than that of the sedentary controls (blocked wheel/free food), but it was equal to or below sedentary control levels for animals forced to forage at the highest efforts (100 and 200 revolutions, respectively; Day & Bartness 2001). These studies, along with those from laboratory rats and birds, appear to confirm that food hoarding is an adaptive strategy to maintain energy homeostasis when food availability is either limited or unpredictable.
3. Peripheral physiological factors
There are a variety of peripheral factors that influence ingestive behaviour including gonadal steroids, metabolic hormones and adiposity, some of which have been reviewed previously (Bartness & Day 2003). In terms of the response to short-term energetic challenges that simulate decreased fuel utilization (e.g. chemical-induced glucoprivation, lipoprivation), there is little evidence that hamsters increase their food hoarding (Bartness et al. 1995; Demas & Bartness 1999). There is also a general consensus that food hoarding is inversely related to circulating oestradiol concentration (Estep et al. 1978; Coling & Herberg 1982), indicating that food hoarding appears to be inhibited when females would be seeking a mate or mating, which makes sense from an adaptive standpoint. Here, we have chosen to focus on some of the more recent studies on the control of food hoarding by peripheral factors that examine the connection between food hoarding and adiposity as well as the metabolic hormones, leptin and ghrelin.
(a). Inverse relation between adiposity and food hoarding
Some data suggest that body fat storage is inversely related to food-hoarding behaviour, which appears to support the deficit hypothesis. Specifically, it is a widely held hypothesis that decreases in body fat tend to increase food hoarding, perhaps to generate an external energy source to compensate for reduced internal energy sources (Herberg & Blundell 1970; Fantino & Cabanac 1980; Bartness & Clein 1994). In hoarding studies with Syrian hamsters (Schneider & Buckley 2001), it appears that the hamsters naturally separate out into two groups of high and low hoarders. The body mass of each group, however, is the same, and for this species, body mass and body fat correlate well (Bartness & Wade 1984; Bartness et al. 1984, 1987; Wade & Bartness 1984). Thus, for Syrian hamsters, the inverse relation between body mass/fat and food-hoard size is not supported (Schneider & Buckley 2001). We do not see this dichotomous separation of high and low hoarders in Siberian hamsters, however, but although not explicitly tested or rigorously investigated, we repeatedly find no apparent relation between the natural variations in body fat and variations in food hoarding in this species.
In a study examining the effects of foraging effort on food hoarding as well as on body fat levels (Day & Bartness 2001), we noticed that the epididymal white adipose tissue (EWAT) pad that surrounds the testes was decreased across all the foraging efforts even though total body fat only decreased at the highest foraging effort (Day & Bartness 2001). Therefore, we methodically and explicitly manipulated lipid levels by excising varying amounts of WAT by removing both EWAT pads (EWAT lipectomy; EWATx), both inguinal WAT pads (IWATx; each IWAT pad is approximately twice the size of an EWAT pad) or both EWAT and IWAT pads (EWATx + IWATx; Dailey & Bartness 2008b). We predicted that the greater the surgically induced lipid deficit, the greater the magnitude of the hoarding response; however, this was not found. Specifically, EWATx caused the greatest increase in food hoarding even though IWATx produced approximately twice and EWATx + IWATx three times the lipid deficit of EWATx in Siberian hamsters required to forage for their food (Dailey & Bartness 2008b). Food intake was not affected by lipectomy in this experiment (Dailey & Bartness 2008b) or in all lipectomy experiments across a wide range of animal species (for a review, see Mauer et al. 2001). Collectively, it appears that the magnitude of a lipid deficit does not affect appetitive or consummatory behaviours; rather the loss of specific fat pads (EWAT) can preferentially stimulate increases in food hoarding. Why would this occur? The EWAT pad may be particularly critical because it is important for the energy supply, and perhaps, gamete growth factors, to the reproductive organs. That is, EWATx inhibits spermatogenesis completely in laboratory rats (Srinivasan et al. 1986). Therefore, the deficit hypothesis was not supported by the lipectomy studies reviewed above because the site of the lipid deficit, rather than the total lipid deficit, seems to be more important.
Outside of these lipectomy studies on food hoarding by Siberian hamsters (Wood & Bartness 1997; Dailey & Bartness 2008a), there are several studies in support of a potential relation between body mass/fat and food hoarding. Food deprivation and restriction studies of normal laboratory rats, as well as dietary obese (‘supermarket diet’-fed; Winn & Herberg 1985) and genetically obese (Zucker fa/fa) rats (Herberg & Winn 1982), demonstrate increases in food hoarding/carrying with increases in food deprivation/restriction. Seemingly strong evidence for an inverse relation between body mass/fat and food hoarding is that obese animals hoard food after the same per cent loss of body mass (fat) as chow-fed (Winn & Herberg 1985) and lean (Fa/Fa) controls (Herberg & Winn 1982). In addition to the inverse relation between body fat and food hoarding/carrying in laboratory rats that sometimes is found, support for this notion also comes from other species. For example, unlike some species of rat that fatten during pregnancy (e.g. eastern wood rats, Nemotoma floridana; McClure 1987) and human females, Siberian hamsters lose 40–50% of their body fat during pregnancy and lactation (Schneider & Wade 1987) and instead increase food hoarding during pregnancy and lactation (Bartness 1997). Some evidence also supports the notion that increased internal energy stores (body fat levels) decrease food hoarding. For example, laboratory mice fed lipid-rich supplements are heavier than non-supplemented mice and hoard/carry less food (Ross & Smith 1953), as do high-fat diet-fed Siberian hamsters that also have increases in body mass (Wood & Bartness 1996a) and body fat (A. D. Wood & T. J. Bartness 1996, unpublished observations) compared with their thinner, chow-fed controls. Overall, despite the lack of a positive relation between the level of decreased body fat by lipectomy and food hoarding, these data appear to suggest a potential relation between decreases in body fat and increases in food hoarding (but see below for a different interpretation). Whether it is decreases in total body fat or, as we will contend below, increases in the energy flux away from WAT and into highly metabolically active tissues such as liver, muscle and brain, how do such changes in body fat or energy flux ultimately trigger increases in food-hoarding behaviour? Moreover, in the case of fat-pad-specific effects of lipectomy on food hoarding, how would the brain ‘know’ which fat pads were removed or lipid depleted?
One possible conduit from WAT to the central nervous system (CNS) is via the sensory innervation of WAT. That is, WAT has sensory innervation (Fishman & Dark 1987) and we have recently traced the central sensory circuits from WAT to the brain using a transneuronal viral tract tracer, the H129 strain of herpes simplex I virus (Song et al. 2009). We have also tested the possibility that WAT sensory innervation informs the CNS of peripheral lipid stores in Siberian hamsters (Shi & Bartness 2005). Specifically, bilateral EWAT sensory denervation was accomplished by intra-WAT pad microinjections of capsaicin, a sensory neurotoxin, resulting in a local and selective sensory denervation (Bartness & Day 2003; Shi & Bartness 2005). EWAT sensory denervation triggers increases in the mass of intact WAT pads analogous to the increases in the mass of non-excised WAT pads after real EWATx (Mauer & Bartness 1994, 1996, 1997; Bartness & Day 2003; Shi & Bartness 2005) even though there is no actual lipid deficit. Thus, although not tested explicitly as of this writing, sensory denervation of EWAT would be predicted to increase food hoarding in a manner similar to real lipectomy (Wood & Bartness 1997; Dailey & Bartness 2008a). Therefore, the intriguing possibility exists that the brain is informed of body fat stores through sensory nerve projections that could control appetitive, as well as consummatory, ingestive behaviours.
There are additional data that do not support an inverse relation between body mass/fat and food hoarding, in addition to the effects of graded lipectomy (Dailey & Bartness 2008a). For example, decreases in body fat mass are not necessary for increases in food hoarding (Fantino & Cabanac 1984; Wood & Bartness 1996a), nor are increases in body fat always associated with decreases in food hoarding (Herberg & Blundell 1970; Gosselin & Cabanac 1996; Wood & Bartness 1997). One possible resolution to some of these discrepancies is that the measures of body mass and body fat (whole animal carcass composition) are not sensitive enough to detect shifts in oxidizable metabolic fuel availability resulting from lipid accumulation or mobilization. Therefore, an alternative view, as noted above, is the notion that energy flux may be a key controlling factor affecting food hoarding. This view supports most of the existing data on food hoarding, not only the increased food-hoarding-associated decreases in body fat (pregnancy and lactation (Bartness 1997); food restriction (Mauer & Bartness 1994; Wood & Bartness 1996b; Day et al. 1999) and lipectomy (Wood & Bartness 1997; Dailey & Bartness 2008a)), but also the increases in food hoarding not associated with reductions in total body fat such as seen with small increases in foraging effort (Day & Bartness 2001), the nearly immediate effects of changes in the caloric density of the diet, either dilution or enhancement (Wood & Bartness 1996a), and the acute peripheral or central administration of appetite-stimulating peptides as discussed below. Therefore, it may be that there are common underlying processes for the metabolic control of food hoarding that are shared with those controlling food intake (Friedman & Stricker 1976) and fertility (Wade & Schneider 1992) as we have postulated previously (Day & Bartness 2001).
(b). Metabolic hormones: leptin and ghrelin
Food deprivation engenders a host of central and peripheral changes in physiology that include alterations in metabolic hormones. For example, food deprivation decreases circulating leptin and increases circulating ghrelin concentrations, the latter an acylated stomach-secreted peptide (Ariyasu et al. 2001; Asakawa et al. 2001) in hamsters (Tups et al. 2004), laboratory rats (Tschop et al. 2000), mice (Toshinai et al. 2001; Moesgaard et al. 2004) and humans (Kojima et al. 1999; Date et al. 2000). Considerable effort has focused on the effects of these ‘metabolic hormones’ on consummatory ingestive behaviour, but only relatively recently have these hormones been investigated for their role in the control of appetitive ingestive behaviours such as food hoarding.
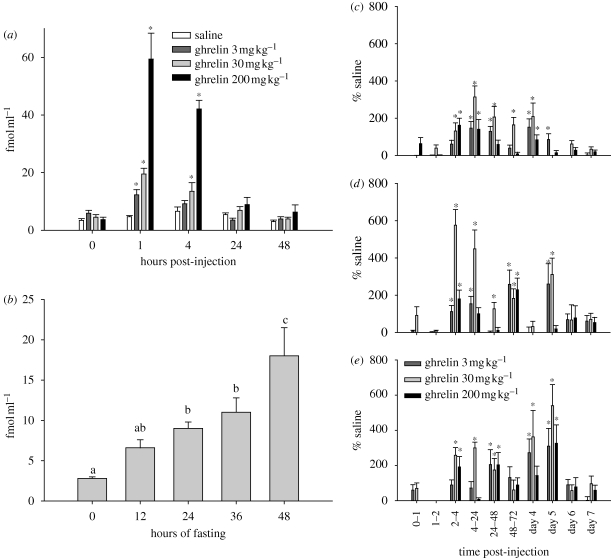
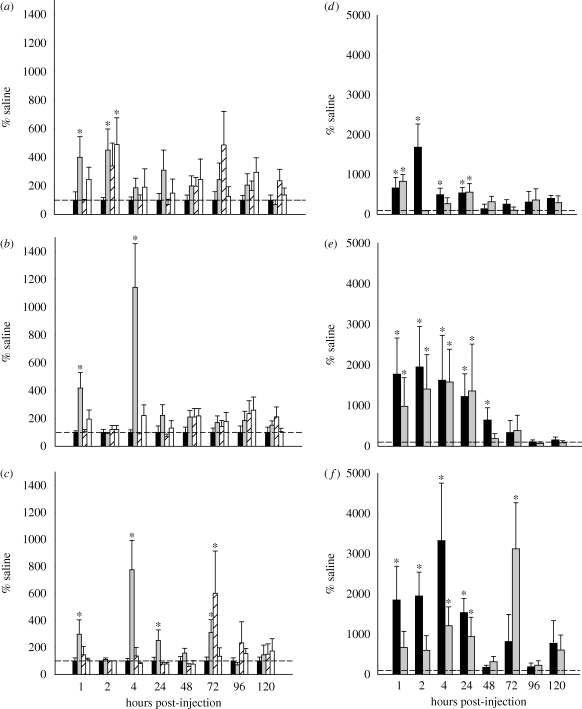
Circulating ghrelin appears to stimulate appetite via actions in the hypothalamus (Tschop et al. 2000; Asakawa et al. 2001; Bagnasco et al. 2003; Olszewski et al. 2003) and brainstem (Faulconbridge et al. 2003). Therefore, we tested the involvement of ghrelin in the control of food hoarding in Siberian hamsters using our foraging/hoarding system described above. In these studies, we administered ghrelin in doses that result in circulating concentrations similar to those generated naturally by 24–48 h of food deprivation (figure 1a,b; Keen-Rhinehart & Bartness 2005). This resulted in significant increases in food hoarding (figure 1c–e; Keen-Rhinehart & Bartness 2005). Interestingly, these increases in food hoarding after a single peripheral ghrelin injection lasted up to 5 days (figure 1e) even though there was no evidence of increased circulating ghrelin 24 h post-injection (Keen-Rhinehart & Bartness 2005). Food deprivation of Siberian hamsters also triggers prolonged increases in food hoarding (e.g. Bartness & Clein 1994; Bartness 1997). Therefore, peripheral ghrelin treatment may have increased appetitive behaviour by mimicking the effects of food deprivation.
Figure 1.
(a,b) Effects of (a) ghrelin treatment on plasma active ghrelin concentrations at time points from 0 to 48 h post-injection and (b) 0–48 h of food deprivation on plasma active ghrelin concentrations at 12-h time intervals, p < 0.05 for different letters. (c–e) Mean + s.e.m. food hoarding (pellets hoarded) as a percentage of the saline-injected controls for the effects of peripheral ghrelin treatment. (c) Hamsters with no foraging requirement and a blocked running wheel (blocked wheel), (d) hamsters with no foraging requirement but a freely moving wheel (free wheel group), and (e) hamsters with a foraging requirement of 10 revolutions per food pellet (10 revolutions per pellet group), *p < 0.05 compared with saline injection. Adapted from Keen-Rhinehart & Bartness (2005). Copyright © American Physiological Society.
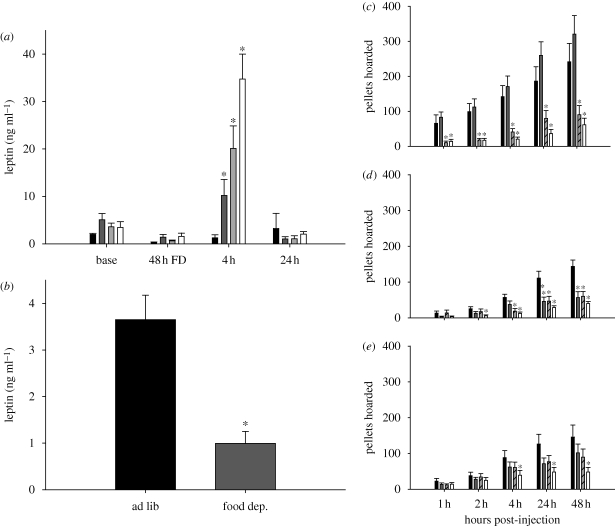
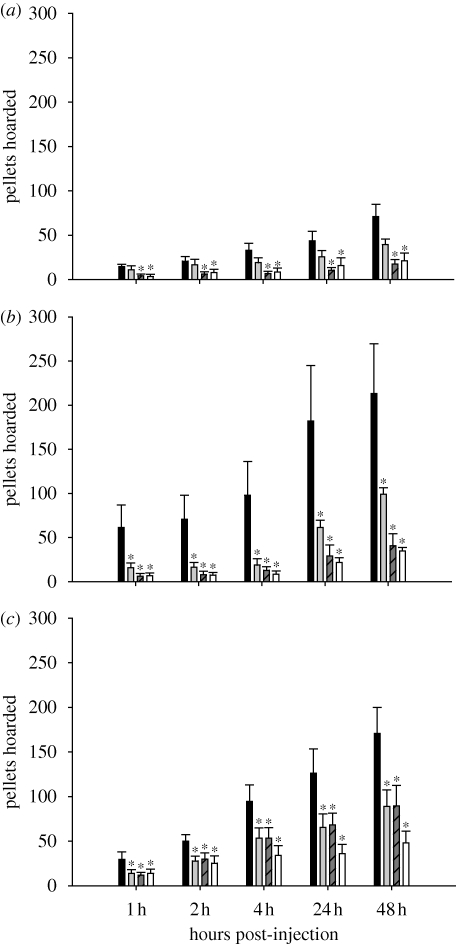
Leptin, another metabolic hormone, acts in opposition of ghrelin to inhibit food intake (e.g. Asakawa et al. 2001). Circulating leptin concentrations decrease with food deprivation and increase with feeding (Ahima et al. 1996; Boden et al. 1996) and, moreover, leptin treatment decreases food intake (Atcha et al. 2000; Klingenspor et al. 2000; Dhillon et al. 2001). Therefore, leptin could serve as a signal to decrease food hoarding via action in some key areas of the brain known to affect food intake, such as the arcuate nucleus of the hypothalamus (Arc; Palkovits 2003) or in the brainstem (Grill et al. 2002). It seems unlikely, however, that the decreases in circulating leptin after lipectomy (Harris et al. 2002; Dailey & Bartness 2008a) could account for the fat pad-specific effects of lipectomy on food hoarding, discussed above, because leptin is secreted by all fat pads into the blood and could not convey the specific site of the lipid loss. Exogenously administered leptin can affect food hoarding in hamsters. Specifically, in food-deprived, non-foraging Syrian hamsters, systemic leptin injection blocked decreased food-deprivation-induced increases in hoarding (Schneider & Buckley 2001). More recently, our laboratory conducted experiments in Siberian hamsters using the previously described foraging/hoarding system to determine whether central or peripheral leptin treatment could block the effects of food deprivation on food hoarding. In these studies, peripherally administered leptin prevented food-deprivation-induced decreases in circulating leptin (figure 2a,b; Keen-Rhinehart & Bartness 2008) and attenuated food-deprivation-induced increases in food hoarding, being most effective in hamsters with the lowest foraging effort requirement (figure 1c–e; Keen-Rhinehart & Bartness 2008). It is generally accepted that leptin penetrates into the brain to act centrally to affect ingestive behaviour (Elmquist 2001), although evidence of leptin receptors on vagal sensory nerves (Buyse et al. 2001) suggests an additional means by which peripheral leptin could affect food intake (but ultimately acting centrally as well). Indeed, in our studies, leptin more effectively blocked food-deprivation-induced increases in food hoarding when given centrally, inhibiting food-deprivation-induced increases in food hoarding up to 48 h post-injection regardless of foraging requirement (figure 3a–c; Keen-Rhinehart & Bartness 2008). Therefore, leptin can affect food hoarding, most probably via action in the brain, and its effects can be modulated by foraging effort requirements. Collectively, leptin and ghrelin appear to provide opposing forces to help maintain energy homeostasis by directly or ultimately acting centrally to alter appetitive ingestive behaviours, especially food hoarding, with environmental factors such as foraging effort modulating their effects. Recent studies from our laboratory have attempted to determine the neuropeptidergic factors that might be downstream of these metabolic hormones.
Figure 2.
(a,b) Effects of (a) leptin treatment on plasma leptin concentrations at time points from 0 to 24 h post-injection and (b) 48 h of food deprivation on plasma leptin concentrations. Black bars, leptin (0); dark grey bars, leptin (10 µg); light grey bars, leptin (40 µg); white bars, leptin (80 µg), *p < 0.05 compared with ad libitum baseline concentrations. (c–e) Effects of peripheral leptin treatment on food hoarding in (c) hamsters with no foraging requirement and a blocked running wheel (blocked wheel), (d) hamsters with no foraging requirement but a freely moving wheel (free wheel group), and (e) hamsters with a foraging requirement of 10 revolutions per food pellet (10 revolutions per pellet group), *p < 0.05 compared with saline injection. Black bars, leptin (0); grey bars, leptin (10 µg); striped bars, leptin (40 µg); white bars, leptin (80 µg). 48 h FD, 48 h of food deprivation. Adapted from Keen-Rhinehart & Bartness (2008). Copyright © American Physiological Society.
Figure 3.
Ability of central leptin treatment to counteract the effects of food deprivation on food hoarding in (a) hamsters with no foraging requirement and a blocked running wheel (blocked wheel), (b) hamsters with no foraging requirement but a freely moving wheel (free wheel group), and (c) hamsters with a foraging requirement of 10 revolutions per food pellet (10 revolutions per pellet group), *p < 0.05 compared with saline injection. Black bar, leptin (0); grey bar, leptin (1.25 µg); striped bar, leptin (2.5 µg); white bar, leptin (5 µg). Adapted from Keen-Rhinehart & Bartness (2008). Copyright © American Physiological Society.
4. Central factors
Although neural substrates underlying the consummatory ingestive behaviour of feeding abound, even in Siberian hamsters (Boss-Williams & Bartness 1996; Wood & Bartness 1997; Schuhler et al. 2003, 2004; Paul et al. 2005; Pelz & Dark 2007), the neural substrates underlying the appetitive ingestive behaviour of food hoarding have been virtually unknown until recently. Several studies published within the past 4 years have attempted to remedy this lack of information (Day & Bartness 2004; Day et al. 2005; Keen-Rhinehart & Bartness 2007a,b; Dailey & Bartness 2009). These studies provide a centrepiece for understanding the central factors that control food hoarding. Prior to these studies, Woods et al. (1998) proposed that the neuropeptides shown to stimulate food intake may instead bring animals into contact with food, the appetitive phase of the ingestive sequence (Craig 1918). That is, neuropeptides might trigger food-seeking behaviour and, if the food is readily present requiring little or no effort, as in home-cage testing, then the consummatory phase would proceed automatically (Woods et al. 1998). These findings led us to formulate the hypothesis that neuropeptide regulators of food intake (e.g. neuropeptide Y (NPY), α-melanocyte-stimulating hormone (α-MSH; Kim et al. 2000)) also, or instead, may control appetitive responses such as food hoarding and/or foraging. Therefore, we recently began a series of studies testing neuropeptide candidates likely to control food hoarding, some of the results of which are shown and/or discussed here. We began these experiments by examining neuropeptides whose gene expression and/or content changed with food deprivation, a condition promoting marked increases in food hoarding, but not food intake in this species as discussed above (Bartness & Clein 1994; Day & Bartness 2003; Keen-Rhinehart & Bartness 2007a,b, 2008). For example, food deprivation increases the synthesis of mRNA for appetite-stimulating (orexigenic) neuropeptides, NPY and agouti-related protein (AgRP), and decreases proopiomelanocortin (POMC), a precursor of the appetite inhibiting (anorexigenic) peptide, α-MSH, in the arcuate nucleus in the hypothalamus of Siberian hamsters (Reddy et al. 1999; Mercer et al. 2000). These responses to food deprivation by Siberian hamsters are consistent with those for these peptides in the arcuate nucleus of laboratory rats or mice (Schwartz et al. 1995; Ebihara et al. 1999). Although changes in gene expression do not necessarily reflect changes in neuropeptide release in the terminal fields, they often are consistent with such changes (e.g. NPY release in the PVH with food deprivation; Sahu et al. 1995), suggesting that food deprivation ultimately increases the release of the orexigenic peptides NPY and AgRP and inhibits the release of the anorexigenic peptide α-MSH at the terminal fields of these arcuate nucleus neurons (e.g. PVH and the perifornical area (PFA) of the hypothalamus).
NPY is one of the most potent factors promoting food intake studied to date (Clark et al. 1984; Stanley & Leibowitz 1984). Therefore, we investigated the involvement of NPY and its receptors in the control of food hoarding, knowing that NPY administered into the third ventricle stimulates food intake in Siberian hamsters in standard home-cage tests (Boss-Williams & Bartness 1996). Using the foraging/hoarding system, NPY also increases food intake substantially (approx. 200–300%), but these increases are not as impressive as the increases in food hoarding (approx. 300–1100% increases; Day et al. 2002).
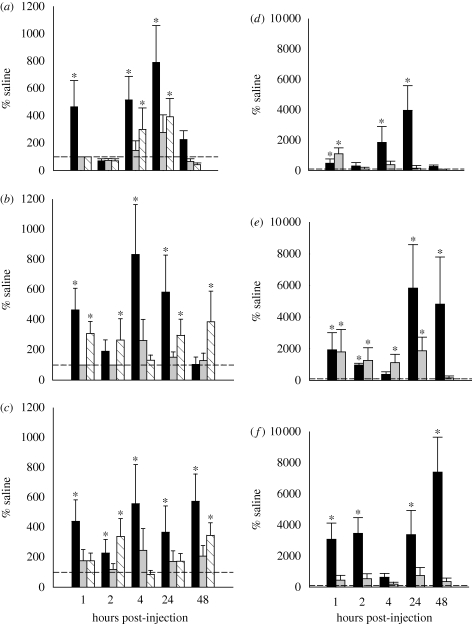
NPY has five receptor subtypes (Y1–Y5), and of these receptors, the Y1 and Y5 receptor subtypes have been especially implicated in the control of ingestive behaviour (Duhault et al. 2000). We tested which of these two Y receptor subtypes underlie the stimulation of food hoarding by Siberian hamsters. Third ventricular acute injection of the NPY Y1 ([Pro34]NPY) or Y5 ([D-Trp34]NPY) receptor (R) agonists in Siberian hamsters revealed that the Y1R agonist increases food hoarding more than food intake, whereas the Y5R agonist produces larger increases in food intake compared with food hoarding (Day et al. 2005). Therefore, the increases in food intake observed after intracerebroventricular NPY treatment in Siberian hamsters (Boss-Williams & Bartness 1996; Day et al. 2005) are likely to be mediated by the Y5R, whereas increases in appetitive behaviours appear to occur through NPY action at the Y1R subtype (Day et al. 2005). To make a compelling case for the physiological function of a peptide, however, or in this case one of the receptors, it is essential that a receptor ‘antagonist’ produces the opposite effect of exogenous administration of the peptide or blocks the physiological response thought to involve the peptide (Smith 1999). Therefore, in order to confirm the role of the NPY Y1R in the appetitive ingestive behavioural response to food deprivation/ghrelin, we conducted studies to determine whether an NPY Y1R antagonist (1229U91) could block food deprivation- or ghrelin-induced increases in food hoarding. In these studies, third ventricular injection of 1229U91 mildly attenuated the ability of ghrelin to stimulate food hoarding (figure 4a–c; Keen-Rhinehart & Bartness 2007b), indicating that some other mediator must be at least partially responsible for ghrelin-induced increases in food hoarding. 1229U91, the NPY Y1R antagonist, significantly attenuated the effects of food deprivation on food hoarding (figure 4d–f; Keen-Rhinehart & Bartness 2007b), suggesting the role of this receptor subtype in food-deprivation-induced increases in food hoarding. The ability to counteract the effects of food deprivation and ghrelin treatment on food hoarding was highly dependent on foraging effort, with 1229U91 completely blocking food-deprivation-induced increases in food hoarding by animals required to run 10 wheel revolutions for each food pellet (figure 4f; Keen-Rhinehart & Bartness 2007b). Although third ventricular injection of a Y1R agonist ([Pro34]NPY) to Siberian hamsters tested in the same foraging/hoarding apparatus increases food hoarding more markedly than does a Y5R agonist ([D-Trp34]NPY), as noted above, the relative inability of this Y1R to inhibit food hoarding illustrates the likely multiplicity of NPY receptors involved in food hoarding, similar to that for food intake by laboratory rats and mice (Iyengar et al. 1999).
Figure 4.
Ability of an NPY Y1R antagonist (1229U91) to counteract the effects of (a–c) ghrelin treatment (Black bar, saline + ghrelin; grey bar, saline + 1229U91; striped bar, ghrelin + 1229U91)) and (d–f) food deprivation (FD) on food hoarding (black bar, FD; grey bar, FD + 1229U91). Effects of central 1229U91 treatment on food hoarding after peripheral ghrelin treatment in (a) hamsters with no foraging requirement and a blocked running wheel (blocked wheel), (b) hamsters with no foraging requirement but a freely moving wheel (free wheel group), and (c) hamsters with a foraging requirement of 10 revolutions per food pellet (10 revolutions per pellet group). Effects of central 1229U91 treatment on food hoarding after 48 h of food deprivation in (d) hamsters with no foraging requirement and a blocked running wheel (blocked wheel), (e) hamsters with no foraging requirement but a freely moving wheel (free wheel group), and (f) hamsters with a foraging requirement of 10 revolutions per food pellet (10 revolutions per pellet). *p < 0.05 compared with saline injection. Adapted from Keen-Rhinehart & Bartness (2007b). Copyright © American Physiological Society.
In the studies reviewed above, NPY or its receptor agonists and antagonists were injected into the third ventricle, yielding little information beyond some involvement of central sites close to the ventricles involved in controlling food hoarding. In laboratory rats, microinjection of NPY or a Y1R agonist into the PVH (Stanley & Leibowitz 1984) or the PFA (Stanley & Thomas 1993) elicits a robust increase in food intake. Both sites express NPY receptors (Parker & Herzog 2000) and contain numerous NPY immunoreactive fibres (de Quidt & Emson 1986). By contrast, prior or coinjection of an NPY Y1R antagonist into the PVH blocks the ability of PVH NPY injections to increase food intake (Wieland et al. 1998; Yokosuka et al. 1999), suggesting that the NPY-induced increase in food intake after PVH injection is due to Y1R involvement. Therefore, we injected the NPY Y1R antagonist, 1229U91, into the PVH and PFA to determine whether it would block the effects of food deprivation on food hoarding. In these studies, 1229U91 injected into the PFA, but not the PVH, almost completely blocked food-deprivation-induced increases in food hoarding, regardless of foraging effort (Dailey & Bartness 2009). Collectively, the results from these studies support the view that the role of NPY in ingestive behaviour is not restricted to consummatory ingestive behaviour (eating), but also may be important in increasing appetitive ingestive behaviours. These results also further dissociate the effects of NPY injected into the PFA and PVH on appetitive and consummatory ingestive behaviours, respectively, and demonstrate that Y1Rs appear to mediate changes in food hoarding more so than food intake.
Another well-studied central appetite-stimulating factor is AgRP, an endogenous melanocortin (MC) receptor inverse agonist (i.e. it blocks the receptor from its naturally occurring agonist, α-MSH, or exogenously applied agonists such as melanotan II (MTII), but it triggers post-receptor effects as well; Ollmann et al. 1997). AgRP is almost exclusively cosynthesized by arcuate nucleus NPY neurons in laboratory rats and Siberian hamsters (Hahn et al. 1998; Mercer et al. 2000). AgRP gene expression increases with food deprivation in the arcuate nucleus of laboratory rats and of Siberian hamsters (Hahn et al. 1998; Mercer et al. 2000) as noted above. In addition, third ventricular, PVH or dorsomedial hypothalamic nucleus injections of AgRP in rats profoundly stimulates food intake, doing so for several days (Hagan et al. 2000; Kim et al. 2000; Wirth & Giraudo 2000). Previous studies also indicate that the ghrelin-induced increases in food intake in rats are mediated by the melanocortin 3 and 4 receptors (MC-3/4Rs; Kamegai et al. 2000, 2001; Nakazato et al. 2001). Therefore, we injected the MC-3/4R inverse agonist, AgRP, into the third ventricle of Siberian hamsters acclimated to our foraging/hoarding apparatus and found significant increases in foraging (approx. 75–400%), food intake (approx. 100–150%) and especially food hoarding (approx. 200–1200%; Day & Bartness 2004), perhaps suggesting a more important role of the MC receptors in food hoarding than food intake in this species. To determine if AgRP is important for the endogenous stimulation of food hoarding in response to food deprivation and ghrelin, we blocked the central action of AgRP at the MC-3/4Rs using the MC-3/4R agonist, MTII. Recall that MTII is a synthetic version of α-MSH, the natural agonist ligand for MC-3/4Rs, which is a by-product of cleavage of a large precursor peptide, POMC (Chronwall 1985) and an inhibitor of food intake in rats (Poggioli et al. 1986). Unlike the NPY Y1R antagonist 1229U91 (Keen-Rhinehart & Bartness 2007b), MTII almost completely blocked the ability of ghrelin to increase food hoarding regardless of foraging effort (figure 5a–c; Keen-Rhinehart & Bartness 2007a). MTII was much less effective in inhibiting food-deprivation-stimulated food hoarding (figure 5d–f; Keen-Rhinehart & Bartness 2007a). These data support the notion that food-deprivation-induced increases in circulating ghrelin concentrations increase AgRP antagonism of the MC-3/4Rs thereby primarily stimulating food hoarding by Siberian hamsters. Because MTII was not able to effectively block food-deprivation-induced increases in food hoarding, however, it is likely that other factors are important for the stimulation of food hoarding. As discussed above, NPY is one of the most likely candidates for this additional factor responsible for the stimulation of food hoarding in response to food deprivation.
Figure 5.
Ability of a melanocortin-3/4R agonist (MTII) to counteract the effects of (a–c) peripheral ghrelin treatment (black bar, saline; grey bar, saline + ghrelin; striped bar, MTII + saline; white bar, MTII + ghrelin) and (d–f) food deprivation (FD) on food hoarding (black bar, FD; grey bar, FD + MTII). Effects of central MTII treatment on food hoarding after peripheral ghrelin treatment in (a) hamsters with no foraging requirement and a blocked running wheel (blocked wheel), (b) hamsters with no foraging requirement but a freely moving wheel (free wheel group), and (c) hamsters with a foraging requirement of 10 revolutions per food pellet (10 revolutions per pellet group). Effects of central MTII treatment on food hoarding after 48 h of food deprivation in (d) hamsters with no foraging requirement and a blocked running wheel (blocked wheel), (e) hamsters with no foraging requirement but a freely moving wheel (free wheel group), and (f) hamsters with a foraging requirement of 10 revolutions per food pellet (10 revolutions per pellet group). *p < 0.05 compared with saline injection. Adapted from Keen-Rhinehart & Bartness (2007a). Copyright © Elsevier.
5. Conclusions
We have reviewed much of the neuroendocrine and endocrine mechanisms underlying food hoarding in several rodent species studied in the laboratory in an attempt to synthesize what is known about the environmental and physiological controllers of the appetitive ingestive behaviours of food hoarding, and to a lesser degree food foraging. The apparent inverse relation between body fat levels and food hoarding (Herberg & Blundell 1970; Fantino & Cabanac 1980; Bartness & Clein 1994) appears to have many exceptions. One possible way to reconcile the discordant data is to view the events that trigger food hoarding as changes in energy flux rather than measurable changes in body fat per se. Clearly, more intensive investigations are needed to propel this notion further, such as has been done for the study of the metabolic controls of food intake (Friedman 1995; Scheurink & Nolan 1996) and of reproduction (for a review, see Wade & Schneider 1992; Wade et al. 1996) where many of the key aspects of energy metabolism involved in these responses have been elucidated.
Collectively, the studies using Siberian hamsters in a foraging/hoarding system discussed in this review illustrate the relations among food deprivation, ghrelin, leptin, NPY/AgRP arcuate nucleus neurons and food hoarding, suggesting the following, albeit simplified scenario. First, food deprivation increases plasma ghrelin, among other responses, as evidenced by a positive relation between the length of food deprivation and circulating ghrelin concentrations (Ariyasu et al. 2001; Keen-Rhinehart & Bartness 2005). Presumably, ghrelin then stimulates arcuate nucleus NPY/AgRP neurons that express the ghrelin receptor, growth hormone secretagogue receptor 1a (Kamegai et al. 2000; Mondal et al. 2005), among other sites, to increase the expression and release of these two peptides into the PVH and other projection sites, such as the PFA, to increase these appetitive ingestive behaviours. In support of this notion is the ability of central NPY or AgRP to trigger impressive food-deprivation-like increases in food hoarding in ad libitum-fed hamsters, with NPY receptor antagonists or MC-3/4 receptor agonists each partially inhibiting the effects of food deprivation and ghrelin on food hoarding. In addition, we have new data that indicate that third ventricular coinjection of NPY and AgRP, each at doses that do not stimulate foraging, hoarding or food intake (i.e. subthreshold doses of each) potently increase all three behaviours, especially food hoarding, as well as activating neurons in a number of brain sites, as indicated by production of the early-immediate gene product c-fos, providing evidence for the synergistic action of these two neuropeptides in the stimulation of food hoarding (Keen-Rhinehart et al. in preparation).
Understanding the underlying the basis for fundamental appetitive behaviours like food hoarding that are so pervasive across animal taxa, including humans (e.g. Dodd et al. 1977), may have import implications for understanding the development of obesity. Therefore, we hope that this review will stimulate thinking and greater emphasis on the control of each phase of the ingestive behaviour sequence—appetitive and consummatory phases, resulting in new models and experiments to yield a broader understanding of the search for, storage of and consumption of food.
Acknowledgements
This work was supported in part by NIDDK grant R37-DK-078358 to T.J.B.
Footnotes
One contribution of 10 to a Theme Issue ‘Integrating ecology, psychology and neurobiology within a food-hoarding paradigm’.
References
- Ahima R. S., Prabakaran D., Mantzoros C. S., Qu D., Lowell B. B., Maratos-Flier E., Flier J. S.1996Role of leptin in the neuroendocrine response to fasting. Nature 382, 250–252 (doi:10.1038/382250a0) [DOI] [PubMed] [Google Scholar]
- Ariyasu H., et al. 2001Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J. Clin. Endocrinol. Metab. 86, 4753–4758 (doi:10.1210/jc.86.10.4753) [DOI] [PubMed] [Google Scholar]
- Asakawa A., et al. 2001Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 120, 337–345 (doi:10.1053/gast.2001.22158) [DOI] [PubMed] [Google Scholar]
- Atcha Z., Cagampang F. R., Stirland J. A., Morris I. D., Brooks A. N., Ebling F. J., Klingenspor M., Loudon A. S.2000Leptin acts on metabolism in a photoperiod-dependent manner, but has no effect on reproductive function in the seasonally breeding Siberian hamster (Phodopus sungorus). Endocrinology 141, 4128–4135 (doi:10.1210/en.141.11.4128) [DOI] [PubMed] [Google Scholar]
- Bagnasco M., Tulipano G., Melis M. R., Argiolas A., Cocchi D., Muller E. E.2003Endogenous ghrelin is an orexigenic peptide acting in the arcuate nucleus in response to fasting. Regul. Pept. 111, 161–167 (doi:10.1016/S0167-0115(02)00283-5) [DOI] [PubMed] [Google Scholar]
- Baker R. A.1955The effects of repeated deprivation experiences on feeding behavior. J. Comp. Physiol. Psychol. 48, 37–42 (doi:10.1037/h0048051) [DOI] [PubMed] [Google Scholar]
- Bartness T. J.1997Food hoarding is increased by pregnancy, lactation and food deprivation in Siberian hamsters. Am. J. Physiol. 272, R118–R125 [DOI] [PubMed] [Google Scholar]
- Bartness T. J., Clein M. R.1994Effects of food deprivation and restriction, and metabolic blockers on food hoarding in Siberian hamsters. Am. J. Physiol. 266, R1111–R1117 [DOI] [PubMed] [Google Scholar]
- Bartness T. J., Day D. E.2003Food hoarding: a quintessential anticipatory appetitive behavior. Prog. Psychobiol. Physiol. Psychol. 18, 69–100 (doi:10.1016/S0363-0951(03)80007-5) [Google Scholar]
- Bartness T. J., Demas G. E.2004Comparative studies of food intake: lessons from non-traditionally studied species. In Food and fluid intake, vol. 14 (eds Stricker E. M., Woods S. C.), pp. 423–467 New York, NY: Plenum [Google Scholar]
- Bartness T. J., Wade G. N.1984Photoperiodic control of body weight and energy metabolism in Syrian hamsters (Mesocricetus auratus): role of pineal gland, melatonin, gonads, and diet. Endocrinology 114, 492–498 (doi:10.1210/endo-114-2-492) [DOI] [PubMed] [Google Scholar]
- Bartness T. J., Ruby N. F., Wade G. N.1984Dietary obesity in exercising or cold-exposed Syrian hamsters. Physiol. Behav. 32, 85–90 (doi:10.1016/0031-9384(84)90075-1) [DOI] [PubMed] [Google Scholar]
- Bartness T. J., Bittman E. L., Wade G. N.1985Paraventricular nucleus lesions exaggerate dietary obesity but block photoperiod-induced weight gains and suspension of estrous cyclicity in Syrian hamsters. Brain Res. Bull. 14, 427–430 (doi:10.1016/0361-9230(85)90020-6) [DOI] [PubMed] [Google Scholar]
- Bartness T. J., Wade G. N., Goldman B. D.1987Are the short photoperiod decreases in serum prolactin responsible for the seasonal changes in energy balance in Syrian and Siberian hamsters? J. Exp. Zool. 244, 437–454 (doi:10.1002/jez.1402440310) [DOI] [PubMed] [Google Scholar]
- Bartness T. J., Morley J. E., Levine A. S.1995Effects of food deprivation and metabolic fuel utilization on the photoperiodic control of food intake in Siberian hamsters. Physiol. Behav. 57, 61–68 (doi:10.1016/0031-9384(94)00203-H) [DOI] [PubMed] [Google Scholar]
- Billington C. J., Morley J. E., Levine A. S., Gerritsen G. C.1984Feeding systems in Chinese hamsters. Am. J. Physiol. 2447, R405–R411 [DOI] [PubMed] [Google Scholar]
- Bindra D.1948The nature of motivation for hoarding food. J. Comp. Physiol. Psychol. 41, 211–218 (doi:10.1037/h0055698) [DOI] [PubMed] [Google Scholar]
- Bittman E. L., Bartness T. J., Goldman B. D., DeVries G. J.1991Suprachiasmatic and paraventricular control of photoperiodism in Siberian hamsters. Am. J. Physiol. 260, R90–R101 [DOI] [PubMed] [Google Scholar]
- Boden G., Chen X., Mozzoli M., Ryan I.1996Effect of fasting on serum leptin in normal human subjects. J. Clin. Endocrinol. Metab. 81, 3419–3423 (doi:10.1210/jc.81.9.3419) [DOI] [PubMed] [Google Scholar]
- Boss-Williams K. A., Bartness T. J.1996NPY stimulation of food intake in Siberian hamsters is not photoperiod dependent. Physiol. Behav. 59, 157–164 [DOI] [PubMed] [Google Scholar]
- Brodin A.2000Why do hoarding birds gain fat in winter in the wrong way? Suggestions from a dynamic model. Behav. Ecol. 11, 27–31 (doi:10.1093/beheco/11.1.27) [Google Scholar]
- Buyse M., et al. 2001Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the vagus nerve. Eur. J. Neurosci. 14, 64–72 (doi:10.1046/j.0953-816x.2001.01628.x) [DOI] [PubMed] [Google Scholar]
- Cabanac M., Swiergiel A. H.1989Rats eating and hoarding as a function of body weight and cost of foraging. Am. J. Physiol 26, R952–R957 [DOI] [PubMed] [Google Scholar]
- Calhoun J. B.1962The ecology and sociology of the Norway rat Washington, DC: US Government Printing Office [Google Scholar]
- Chronwall B. M.1985Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides 6, 1–11 (doi:10.1016/0196-9781(85)90128-7) [DOI] [PubMed] [Google Scholar]
- Clark J. T., Kalra P. S., Crowley W. R., Kalra S. P.1984Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115, 427–429 (doi:10.1210/endo-115-1-427) [DOI] [PubMed] [Google Scholar]
- Coling J. G., Herberg L. J.1982Effect of ovarian and exogenous hormones on defended body weight, actual body weight, and the paradoxical hoarding of food by female rats. Physiol. Behav. 29, 687–691 (doi:10.1016/0031-9384(82)90239-6) [DOI] [PubMed] [Google Scholar]
- Craig W.1918Appetites and aversions as constituents of instincts. Biol. Bull. 34, 91–107 (doi:10.2307/1536346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey M. E., Bartness T. J.2008aFat pad-specific effects of lipectomy on foraging, food hoarding and food intake. Am. J. Physiol. 94, R321–R328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey M. E., Bartness T. J.2008bFat pad-specific effects of lipectomy on foraging, food hoarding, and food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R321–R328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey M. J., Bartness T. J.2009Appetitive and consummatory ingestive behaviors stimulated by PVH and perifornical area NPY injections. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R877–R892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y., Kojima M., Hosoda H., Sawaguchi A., Mondal M. S., Suganuma T., Matsukura S., Kangawa K., Nakazato M.2000Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141, 4255–4261 (doi:10.1210/en.141.11.4255) [DOI] [PubMed] [Google Scholar]
- Day D. E., Bartness T. J.2001Effects of foraging effort on body fat and food hoarding by Siberian hamsters. J. Exp. Zool. 289, 162–171 (doi:10.1002/1097-010X(20010215)289:3<162::AID-JEZ2>3.0.CO;2-N) [PubMed] [Google Scholar]
- Day D. E., Bartness T. J.2003Fasting-induced increases in hoarding are dependent on the foraging effort level. Physiol. Behav. 78, 655–668 (doi:10.1016/S0031-9384(03)00052-0) [DOI] [PubMed] [Google Scholar]
- Day D. E., Bartness T. J.2004Agouti-related protein increases food hoarding, but not food intake by Siberian hamsters. Am. J. Physiol. 286, R38–R45 [DOI] [PubMed] [Google Scholar]
- Day D. E., Mintz E. M., Bartness T. J.1999Diet self-selection and food hoarding after food deprivation by Siberian hamsters. Physiol. Behav. 68, 187–194 (doi:10.1016/S0031-9384(99)00167-5) [DOI] [PubMed] [Google Scholar]
- Day D. E., Mintz E. M., Bartness T. J.2002Diet choice exaggerates food hoarding, intake and pup survival across reproduction. Physiol. Behav. 75, 143–157 (doi:10.1016/S0031-9384(01)00655-2) [DOI] [PubMed] [Google Scholar]
- Day D. E., Keen-Rhinehart E., Bartness T. J.2005Role of NPY and its receptor subtypes in foraging, food hoarding, and food intake by Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R29–R36 [DOI] [PubMed] [Google Scholar]
- Demas G. E., Bartness T. J.1999Effects of food deprivation and metabolic fuel utilization on food hoarding by jirds (Meriones shawi). Physiol. Behav. 67, 243–248 (doi:10.1016/S0031-9384(99)00066-9) [DOI] [PubMed] [Google Scholar]
- de Quidt M. E., Emson P. C.1986Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system–II. Immunohistochemical analysis. Neuroscience 18, 545–618 [DOI] [PubMed] [Google Scholar]
- Dhillon H., Kalra S. P., Prima V., Zolotukhin S., Scarpace P. J., Moldawer L. L., Muzyczka N., Kalra P. S.2001Central leptin gene therapy suppresses body weight gain, adiposity and serum insulin without affecting food consumption in normal rats: a long-term study. Regul. Pept. 99, 69–77 (doi:10.1016/S0167-0115(01)00237-3) [DOI] [PubMed] [Google Scholar]
- Dodd D. K., Stalling R. B., Bedell J.1977Grocery purchases as a function of obesity and assumed food deprivation. Int. J. Obes. 1, 43–47 [PubMed] [Google Scholar]
- Duhault J., et al. 2000Food intake regulation in rodents: Y5 or Y1 NPY receptors or both? Can. J. Physiol. Pharmacol. 78, 173–185 (doi:10.1139/cjpp-78-2-173) [PubMed] [Google Scholar]
- Ebihara K., et al. 1999Involvement of agouti-related protein, an endogenous antagonist of hypothalamic melanocortin receptor, in leptin action. Diabetes 48, 2028–2033 (doi:10.2337/diabetes.48.10.2028) [DOI] [PubMed] [Google Scholar]
- Elmquist J. K.2001Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Int. J. Obes. Relat. Metab. Disord. 25, S78–S82 (doi:10.1038/sj.ijo.0801918) [DOI] [PubMed] [Google Scholar]
- Estep D. Q., Lanier D. L., Dewsbury D. A.1978Variation of food hoarding with the estrous cycle of Syrian golden hamsters (Mesocricetus auratus). Horm. Behav. 11, 259–263 (doi:10.1016/0018-506X(78)90029-6) [DOI] [PubMed] [Google Scholar]
- Fantino M., Cabanac M.1980Body weight regulation with a proportional hoarding response in the rat. Physiol. Behav. 24, 939–942 (doi:10.1016/0031-9384(80)90153-5) [DOI] [PubMed] [Google Scholar]
- Fantino M., Cabanac M.1984Effect of a cold ambient temperature on the rat's food hoarding behavior. Physiol. Behav. 32, 183–190 (doi:10.1016/0031-9384(84)90127-6) [DOI] [PubMed] [Google Scholar]
- Faulconbridge L. F., Cummings D. E., Kaplan J. M., Grill H. J.2003Hyperphagic effects of brainstem ghrelin administration. Diabetes 52, 2260–2265 (doi:10.2337/diabetes.52.9.2260) [DOI] [PubMed] [Google Scholar]
- Fishman R. B., Dark J.1987Sensory innervation of white adipose tissue. Am. J. Physiol. 253, R942–R944 [DOI] [PubMed] [Google Scholar]
- Friedman M. I.1991Metabolic control of calorie intake. In Appetite and nutrition (eds Friedman R., Tordoff M. G., Kare M. R.), pp. 19–38 New York, NY: Marcel Dekker [Google Scholar]
- Friedman M. I.1995Control of energy intake by energy metabolism. Am. J. Clin. Nutr. 62, 1096S–1100S [DOI] [PubMed] [Google Scholar]
- Friedman M. I., Rawson N. E.1994Fuel metabolism and appetite control. In Appetite and body weight regulation: sugar, fat and macronutrient substitutes (eds Fernstrom J. D., Miller G. D.), pp. 63–76 Boca Raton, FL: CRC Press [Google Scholar]
- Friedman M. I., Stricker E. M.1976The physiological psychology of hunger: a physiological perspective. Psychol. Rev. 83, 409–431 (doi:10.1037/0033-295X.83.6.409) [PubMed] [Google Scholar]
- Gosselin C., Cabanac M.1996Ever higher: constant rise of body weight set-point in growing Zucker rats. Physiol. Behav. 60, 817–821 [DOI] [PubMed] [Google Scholar]
- Grill H. J., Schwartz M. W., Kaplan J. M., Foxhall J. S., Breininger J., Baskin D. G.2002Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143, 239–246 (doi:10.1210/en.143.1.239) [DOI] [PubMed] [Google Scholar]
- Hagan M. M., Rushing P. A., Pritchard L. M., Schwartz M. W., Strack A. M., Van Der Ploeg L. H., Woods S. C., Seeley R. J.2000Long-term orexigenic effects of AgRP-(83–132) involve mechanisms other than melanocortin receptor blockade. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R47–R52 [DOI] [PubMed] [Google Scholar]
- Hahn T. M., Breininger J. F., Baskin D. G., Schwartz M. W.1998Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1, 271–272 [DOI] [PubMed] [Google Scholar]
- Harris R. B., Hausman D. B., Bartness T. J.2002Compensation for partial lipectomy in mice with genetic alterations of leptin and its receptor subtypes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R1094–R1103 [DOI] [PubMed] [Google Scholar]
- Herberg L. J., Blundell J. E.1967Lateral hypothalamus: hoarding behavior elicited by electrical stimulation. Science 155, 349–350 (doi:10.1126/science.155.3760.349) [DOI] [PubMed] [Google Scholar]
- Herberg L. J., Blundell J. E.1970Non-interaction of ventromedial and lateral hypothalamic mechanisms in the regulation of feeding and hoarding behaviour in the rat. Q. J. Exp. Psychol. 22, 133–141 [DOI] [PubMed] [Google Scholar]
- Herberg L. J., Winn P.1982Body-weight regulatory mechanisms and food hoarding in hereditarily obese (fa/fa) and lean (Fa/Fa) Zucker rats. Physiol. Behav. 29, 631–635 (doi:10.1016/0031-9384(82)90231-1) [DOI] [PubMed] [Google Scholar]
- Iyengar S., Li D. L., Simmons R. M.1999Characterization of neuropeptide Y-induced feeding in mice: do Y1-Y6 receptor subtypes mediate feeding? J. Pharmacol. Exp. Ther. 289, 1031–1040 [PubMed] [Google Scholar]
- Kamegai J., Tamura H., Shimizu T., Ishii S., Sugihara H., Wakabayashi I.2000Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology 141, 4797–4800 (doi:10.1210/en.141.12.4797) [DOI] [PubMed] [Google Scholar]
- Kamegai J., Tamura H., Shimizu T., Ishii S., Sugihara H., Wakabayashi I.2001Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and agouti-related protein mRNA levels and body weight in rats. Diabetes 50, 2438–2443 (doi:10.2337/diabetes.50.11.2438) [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E., Bartness T. J.2005Peripheral ghrelin injections stimulate food intake, foraging and food hoarding in Siberian hamsters. Am. J. Physiol. 288, R716–R722 [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E., Bartness T. J.2007aMTII attenuates ghrelin- and food deprivation-induced increases in food hoarding and food intake. Horm. Behav. 52, 612–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen-Rhinehart E., Bartness T. J.2007bNPY Y1 receptor is involved in ghrelin- and fasting-induced increases in foraging, food hoarding, and food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1728–R1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen-Rhinehart E., Bartness T. J.2008Leptin inhibits food-deprivation-induced increases in food intake and food hoarding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1737–R1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen-Rhinehart E., Teubner B., Bartness T. J.In preparation Third ventricular co-injection of sub-threshold doses of NPY and AgRP stimulate food hoarding, foraging and intake. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy G. C.1953The role of depot fat in the hypothalamic control of food intake in the rat. Proc. R. Soc. Lond. B 140, 578–592 (doi:10.1098/rspb.1953.0009) [DOI] [PubMed] [Google Scholar]
- Kim M. S., et al. 2000Hypothalamic localization of the feeding effect of agouti-related peptide and alpha-melanocyte-stimulating hormone. Diabetes 49, 177–182 (doi:10.2337/diabetes.49.2.177) [DOI] [PubMed] [Google Scholar]
- Klingenspor M., Niggemann H., Heldmaier G.2000Modulation of leptin sensitivity by short photoperiod acclimation in the Djungarian hamster, Phodopus sungorus. J. Comp. Physiol. 170, 37–43 [DOI] [PubMed] [Google Scholar]
- Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K.1999Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 (doi:10.1038/45230) [DOI] [PubMed] [Google Scholar]
- Lea S. E. G., Tarpy R. M.1986Hamsters' demand for food to eat and hoard as a function of deprivation and cost. Anim. Behav. 34, 1759–1768 (doi:10.1016/S0003-3472(86)80262-7) [Google Scholar]
- Lore R. K., Flannelly D. J.1978Comparative studies of wild and domestic rats: some difficulties in isolating the effects of genotype and environment. Aggress. Behav. 7, 253–257 (doi:10.1002/1098-2337(1981)7:3<253::AID-AB2480070308>3.0.CO;2-F) [Google Scholar]
- Mauer M. M., Bartness T. J.1994Body fat regulation following partial lipectomy in Siberian hamsters is photoperiod-dependent and fat pad-specific. Am. J. Physiol. 266, R870–R878 [DOI] [PubMed] [Google Scholar]
- Mauer M. M., Bartness T. J.1996Photoperiod-dependent fat pad mass and cellularity changes following partial lipectomy in Siberian hamsters. Am. J. Physiol. 270, R383–R392 [DOI] [PubMed] [Google Scholar]
- Mauer M. M., Bartness T. J.1997Fat pad-specific compensatory mass increases after varying degrees of partial lipectomy in Siberian hamsters. Am. J. Physiol. 273, 2117–2123 [DOI] [PubMed] [Google Scholar]
- Mauer M. M., Harris R. B. S., Bartness T. J.2001The regulation of total body fat: lessons learned from lipectomy studies. Neurosci. Biobehav. Rev. 25, 15–28 (doi:10.1016/S0149-7634(00)00047-6) [DOI] [PubMed] [Google Scholar]
- McClure P. A.1987The energetics of reproduction and life histories of cricetine rodents (Neotoma floridana and Sigmodon hispidus). Symp. Zoolog. Soc. Lond. 57, 241–258 [Google Scholar]
- Mercer J. G., Moar K. M., Ross A. W., Morgan P. J.2000Regulation of leptin receptor, POMC and AGRP gene expression by photoperiod and food deprivation in the hypothalamic arcuate nucleus of the male Siberian hamster (Phodopus sungorus). Appetite 34, 109–111 (doi:10.1006/appe.1999.0301) [DOI] [PubMed] [Google Scholar]
- Moesgaard S. G., Ahren B., Carr R. D., Gram D. X., Brand C. L., Sundler F.2004Effects of high-fat feeding and fasting on ghrelin expression in the mouse stomach. Regul. Pept. 120, 261–267 (doi:10.1016/j.regpep.2004.03.018) [DOI] [PubMed] [Google Scholar]
- Mondal M. S., Date Y., Yamaguchi H., Toshinai K., Tsuruta T., Kangawa K., Nakazato M.2005Identification of ghrelin and its receptor in neurons of the rat arcuate nucleus. Regul. Pept. 126, 55–59 (doi:10.1016/j.regpep.2004.08.038) [DOI] [PubMed] [Google Scholar]
- Morgan C. T., Stellar E., Johnson O.1943Food-deprivation and hoarding in rats. J. Comp. Physiol. 35, 275–295 [Google Scholar]
- Murphy M. R.1985History of the capture and domestication of the Syrian golden hamster. In The hamster: reproduction and behavior (ed. Seigel H. I.), pp. 3–22 New York, NY: Plenum Press [Google Scholar]
- Nakazato M., Murakami N., Date Y., Kojima M., Matsuo H., Kangawa K., Matsukura S.2001A role for ghrelin in the central regulation of feeding. Nature 409, 194–198 (doi:10.1038/35051587) [DOI] [PubMed] [Google Scholar]
- Ollmann M. M., Wilson B. D., Yang Y. K., Kerns J. A., Chen Y., Gantz I., Barsh G. S.1997Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278, 135–138 (doi:10.1126/science.278.5335.135) [DOI] [PubMed] [Google Scholar]
- Olszewski P. K., Grace M. K., Billington C. J., Levine A. S.2003Hypothalamic paraventricular injections of ghrelin: effect on feeding and c-Fos immunoreactivity. Peptides 24, 919–923 (doi:10.1016/S0196-9781(03)00159-1) [DOI] [PubMed] [Google Scholar]
- Palkovits M.2003Hypothalamic regulation of the food intake. Ideggyogy. Sz 56, 288–302 [PubMed] [Google Scholar]
- Parker R., Herzog H.2000Localization of Y-receptor subtype mRNAs in rat brain by digoxigenin labeled in situ hybridization. Methods Mol. Biol. 153, 165–183 [DOI] [PubMed] [Google Scholar]
- Paul M. J., Freeman D. A., Park J. H., Dark J.2005Neuropeptide Y induces torpor-like hypothermia in Siberian hamsters. Brain Res. 1055, 83–92 (doi:10.1016/j.brainres.2005.06.090) [DOI] [PubMed] [Google Scholar]
- Pelz K. M., Dark J.2007ICV NPY Y1 receptor agonist but not Y5 agonist induces torpor-like hypothermia in cold-acclimated Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R2299–R2311 [DOI] [PubMed] [Google Scholar]
- Perrigo G., Bronson F. H.1985Behavioral and physiological responses of female house mice to foraging variation. Physiol. Behav. 34, 437–440 (doi:10.1016/0031-9384(85)90208-2) [DOI] [PubMed] [Google Scholar]
- Pisano R. G., Storer T. I.1948Burrows and feeding of the Norway rat. J. Mammalog. 29, 374–383 (doi:10.2307/1375126) [Google Scholar]
- Poggioli R., Vergoni A. V., Bertolini A.1986ACTH-(1-24) and alpha-MSH antagonize feeding behavior stimulated by kappa opiate agonists. Peptides 7, 843–848 (doi:10.1016/0196-9781(86)90104-X) [DOI] [PubMed] [Google Scholar]
- Pravosudov V. V., Clayton N. S.2002A test of the adaptive specialization hypothesis: population differences in caching, memory, and the hippocampus in black-capped chickadees (Poecile atricapilla). Behav. Neurosci. 116, 515–522 (doi:10.1037/0735-7044.116.4.515) [PubMed] [Google Scholar]
- Reddy A. B., Cronin A. S., Ford H., Ebling F. J.1999Seasonal regulation of food intake and body weight in the male Siberian hamster: studies of hypothalamic orexin (hypocretin), neuropeptide Y (NPY) and pro-opiomelanocortin (POMC). Eur. J. Neurosci. 11, 3255–3264 (doi:10.1046/j.1460-9568.1999.00746.x) [DOI] [PubMed] [Google Scholar]
- Ross I., Smith W. I.1953The hoarding behavior of the mouse II. The role of deprivation, satiation and stress. J. Genet. Psychol. 82, 279–297 [DOI] [PubMed] [Google Scholar]
- Rowland N. E.1982Failure by deprived hamsters to increase food intake: some behavioral and physiological determinants. J. Comp. Physiol. Psychol. 96, 591–603 (doi:10.1037/h0077905) [DOI] [PubMed] [Google Scholar]
- Rowland N. E., Miceli M. O., Malsbury C. W., Baile C. A., Della-Fera M. A., Gingerich R. L., Caputo F. A.1986Medial hypothalamic lesions in Syrian hamsters: characterization of hyperphagia and weight gain. Physiol. Behav. 36, 513–521 (doi:10.1016/0031-9384(86)90324-0) [DOI] [PubMed] [Google Scholar]
- Sahu A., Dube M. G., Phelps C. P., Sninsky C. A., Kalra P. S., Kalra S. P.1995Insulin and insulin-like growth factor II suppress neuropeptide Y release from the nerve terminals in the paraventricular nucleus: a putative hypothalamic site for energy homeostasis. Endocrinology 136, 5718–5724 (doi:10.1210/en.136.12.5718) [DOI] [PubMed] [Google Scholar]
- Scheurink A. J. W., Nolan L. J.1996Food intake, fuel homeostasis, and the autonomic nervous system. Appetite 26, 304 (doi:10.1006/appe.1996.0026) [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Buckley C. A.2001Food hoarding is increased by food deprivation and decreased by leptin treatment in Syrian hamsters. Am. J. Physiol. 285, R1021–R1029 [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Wade G. N.1987Body composition, food intake, and brown fat thermogenesis in pregnant Djungarian hamsters. Am. J. Physiol. 253, R314–R320 [DOI] [PubMed] [Google Scholar]
- Schuhler S., Horan T. L., Hastings M. H., Mercer J. G., Morgan P. J., Ebling F. J.2003Decrease of food intake by MC4-R agonist MTII in Siberian hamsters in long and short photoperiods. Am. J. Physiol. 284, R227–R232 [DOI] [PubMed] [Google Scholar]
- Schuhler S., Horan T. L., Hastings M. H., Mercer J. G., Morgan P. J., Ebling F. J.2004Feeding and behavioural effects of central administration of the melanocortin 3/4-R antagonist SHU9119 in obese and lean Siberian hamsters. Behav. Brain Res. 152, 177–185 (doi:10.1016/S0166-4328(03)00260-2) [DOI] [PubMed] [Google Scholar]
- Schwartz M. W., Sipols A. J., Grubin C. E., Baskin D. G.1995Differential effect of fasting on hypothalamic expression of gene encoding neuropeptide Y, galanin, and glutamic acid decarboxylase. Brain Res. Bull. 31, 361–367 (doi:10.1016/0361-9230(93)90228-4) [DOI] [PubMed] [Google Scholar]
- Shi H., Bartness T. J.2005White adipose tissue sensory nerve denervation mimics lipectomy-induced compensatory increases in adiposity. Am. J. Physiol. 289, R514–R520 [DOI] [PubMed] [Google Scholar]
- Silverman H. J., Zucker I.1976Absence of post-fast food compensation in the golden hamster (Mesocricetus auratus). Physiol. Behav. 17, 271–285 (doi:10.1016/0031-9384(76)90076-7) [DOI] [PubMed] [Google Scholar]
- Smith G. P.1999Introduction to the reviews on peptides and the control of food intake and body weight. Neuropeptides 33, 323–328 (doi:10.1054/npep.1999.0056) [DOI] [PubMed] [Google Scholar]
- Smulders T. V.1998A game theoretical model of the evolution of food hoarding: applications to the Paridae. Am. Nat. 151, 356–366 (doi:10.1086/286124) [DOI] [PubMed] [Google Scholar]
- Song C. K., Schwartz G. J., Bartness T. J.2009Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R501–R511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan V., Thombre D. P., Lakshmanan S., Chakrabarty A. S.1986Effect of removal of epididymal fat on spermatogenesis in albino rats. Indian J. Exp. Biol. 24, 487–488 [PubMed] [Google Scholar]
- Stanley B. G., Leibowitz S. F.1984Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 35, 2635–2642 (doi:10.1016/0024-3205(84)90032-8) [DOI] [PubMed] [Google Scholar]
- Stanley B. G., Thomas W. J.1993Feeding responses to perifornical hypothalamic injection of neuropeptide Y in relation to circadian rhythms of eating behavior. Peptides 14, 475–481 (doi:10.1016/0196-9781(93)90135-4) [DOI] [PubMed] [Google Scholar]
- Takahashi L. K., Lore R. K.1980Foraging and food hoarding of wild Rattus norvegicus in an urban environment. Behav. Neural Biol. 29, 527–531 (doi:10.1016/S0163-1047(80)92863-0) [DOI] [PubMed] [Google Scholar]
- Toshinai K., Mondal M. S., Nakazato M., Date Y., Murakami N., Kojima M., Kangawa K., Matsukura S.2001Upregulation of ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem. Biophys. Res. Commun. 281, 1220–1225 (doi:10.1006/bbrc.2001.4518) [DOI] [PubMed] [Google Scholar]
- Tschop M., Smiley D. L., Heiman M. L.2000Ghrelin induces adiposity in rodents. Nature 407, 908–913 (doi:10.1038/35038090) [DOI] [PubMed] [Google Scholar]
- Tups A., Helwig M., Khorooshi R. M., Archer Z. A., Klingenspor M., Mercer J. G.2004Circulating ghrelin levels and central ghrelin receptor expression are elevated in response to food deprivation in a seasonal mammal (Phodopus sungorus). J. Neuroendocrinol. 16, 922–928 (doi:10.1111/j.1365-2826.2004.01251.x) [DOI] [PubMed] [Google Scholar]
- Vander Wall S. B.1990Food hoarding in animals Chicago, IL: University of Chicago Press [Google Scholar]
- Wade G. N., Bartness T. J.1984Seasonal obesity in Syrian hamsters: effects of age, diet, photoperiod, and melatonin. Am. J. Physiol. 247, R328–R334 [DOI] [PubMed] [Google Scholar]
- Wade G. N., Schneider J. E.1992Metabolic fuels and reproduction in female mammals. Neurosci. Biobehav. Rev. 16, 235–272 (doi:10.1016/S0149-7634(05)80183-6) [DOI] [PubMed] [Google Scholar]
- Wade G. N., Schneider J. E., Li H. Y.1996Control of fertility by metabolic cues. Am. J. Physiol. 270, E1–E19 [DOI] [PubMed] [Google Scholar]
- Whishaw I. Q., Whishaw G. E.1996Conspecific aggression influences food carrying: studies on a wild population of Rattus norvegicus. Aggress. Behav. 22, 47–66 (doi:10.1002/(SICI)1098-2337(1996)22:1<47::AID-AB5>3.0.CO;2-R) [Google Scholar]
- Whishaw I. Q., Oddie S. D., McNamara R. K., Harris T. L., Perry B. S.1990Psychophysical methods for study of sensory-motor behavior using a food-carrying (hoarding) task in rodents. J. Neurosci. Meth. 32, 123–133 (doi:10.1016/0165-0270(90)90168-F) [DOI] [PubMed] [Google Scholar]
- Wieland H. A., Engel W., Eberlein W., Rudolf K., Doods H. N.1998Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br. J. Pharmacol. 125, 549–555 (doi:10.1038/sj.bjp.0702084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn P., Herberg L. J.1985Changes in actual versus defended body weight elicited by a varied, palatable (‘supermarket’) diet in rats. Physiol. Behav. 35, 683–687 (doi:10.1016/0031-9384(85)90397-X) [DOI] [PubMed] [Google Scholar]
- Wirth M. M., Giraudo S. Q.2000Agouti-related protein in the hypothalamic paraventricular nucleus: effect on feeding. Peptides 21, 1369–1375 (doi:10.1016/S0196-9781(00)00280-1) [DOI] [PubMed] [Google Scholar]
- Wolfe J. B.1939An exploratory study of food-storing in rats. J. Comp. Psychol. 28, 97–108 (doi:10.1037/h0060894) [Google Scholar]
- Wong R., Jones C. H.1985A comparative analysis of feeding and hoarding in hamsters and gerbils. Behav. Process. 11, 301–308 (doi:10.1016/0376-6357(85)90024-5) [DOI] [PubMed] [Google Scholar]
- Wood A. D., Bartness T. J.1996aCaloric density affects food hoarding and intake by Siberian hamsters. Physiol. Behav. 59, 897–903 (doi:10.1016/0031-9384(95)02167-1) [DOI] [PubMed] [Google Scholar]
- Wood A. D., Bartness T. J.1996bFood deprivation-induced increases in hoarding by Siberian hamsters are not photoperiod-dependent. Physiol. Behav. 60, 1137–1145 (doi:10.1016/0031-9384(96)00173-4) [DOI] [PubMed] [Google Scholar]
- Wood A. D., Bartness T. J.1997Partial lipectomy, but not PVN lesions, increases food hoarding by Siberian hamsters. Am. J. Physiol. 272, R783–R792 [DOI] [PubMed] [Google Scholar]
- Woods S. C., Figlewicz D. P., Madden L., Porte D., Jr, Sipols A. J., Seeley R. J.1998NPY and food intake: discrepancies in the model. Regul. Pept. 75–76, 403–408 (doi:10.1016/S0167-0115(98)00095-0) [DOI] [PubMed] [Google Scholar]
- Yokosuka M., Kalra P. S., Kalra S. P.1999Inhibition of neuropeptide Y (NPY)-induced feeding and c-Fos response in magnocellular paraventricular nucleus by a NPY receptor antagonist: a site of NPY action. Endocrinology 140, 4494–4500 (doi:10.1210/en.140.10.4494) [DOI] [PubMed] [Google Scholar]