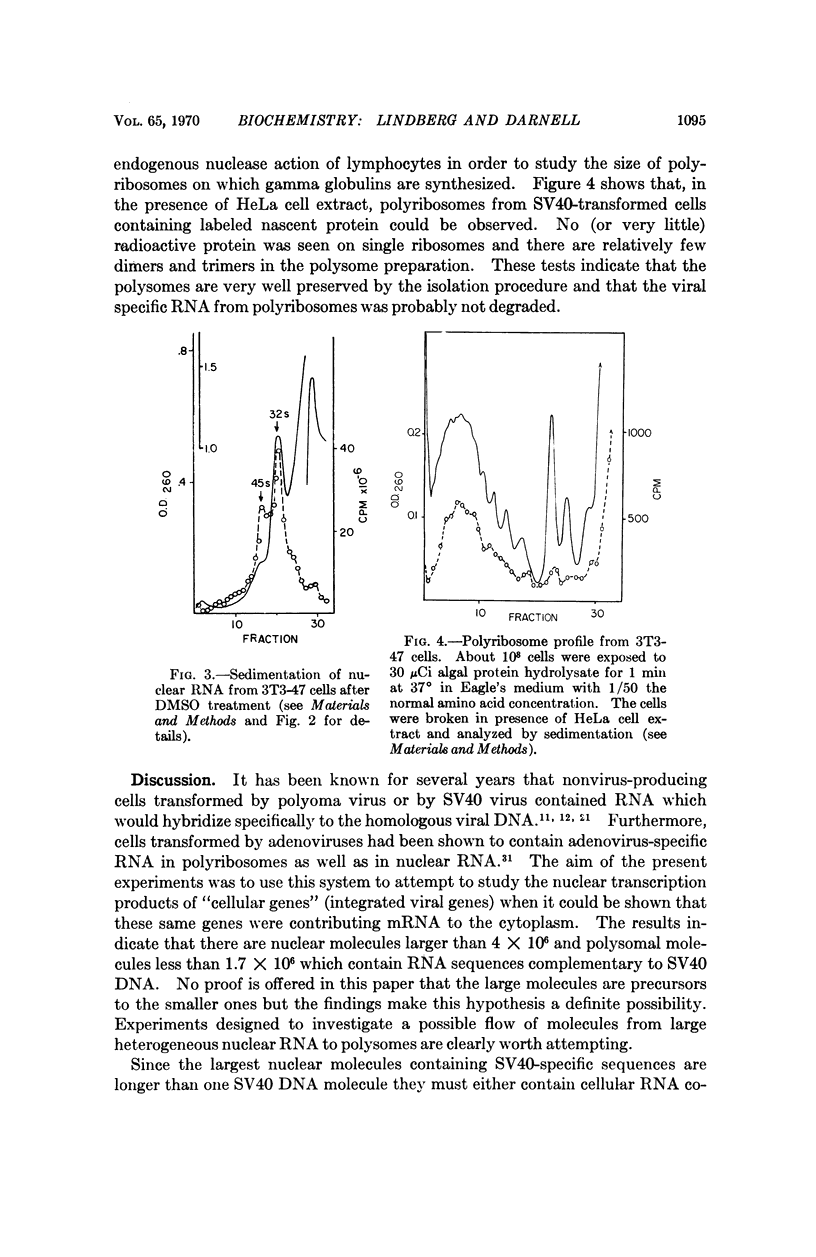
Abstract
Cells transformed by the oncogenic virus SV40 are known to contain viral DNA integrated into cellular DNA and to produce virus-specific RNA. It has been shown that nuclear molecules containing virus-specific sequences are considerably longer than presumed virus-specific mRNA molecules from cytoplasmic polyribosomes. This finding suggests the possibility that cytoplasmic mRNA is derived by the specific cleavage of larger nuclear RNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Winocour E., Sachs L. Characterization of the simian virus 40-specific RNA in virus-yielding and transformed cells. J Mol Biol. 1968 Feb 14;31(3):415–429. doi: 10.1016/0022-2836(68)90418-x. [DOI] [PubMed] [Google Scholar]
- Attardi G., Parnas H., Hwang M. I., Attardi B. Giant-size rapidly labeled nuclear ribonucleic acid and cytoplasmic messenger ribonucleic acid in immature duck erythrocytes. J Mol Biol. 1966 Sep;20(1):145–182. doi: 10.1016/0022-2836(66)90123-9. [DOI] [PubMed] [Google Scholar]
- BALTIMORE D. IN VITRO SYNTHESIS OF VIRAL RNA BY THE POLIOVIRUS RNA POLYMERASE. Proc Natl Acad Sci U S A. 1964 Mar;51:450–456. doi: 10.1073/pnas.51.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L. Virus-specific RNA in cells productively infected or transformed by polyoma virus. J Mol Biol. 1966 Apr;16(2):359–373. doi: 10.1016/s0022-2836(66)80179-1. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V., BLACK P. H. THE NUCLEIC ACID OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:388–392. doi: 10.1016/0042-6822(64)90176-x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Ribonucleic acids from animal cells. Bacteriol Rev. 1968 Sep;32(3):262–290. doi: 10.1128/br.32.3.262-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Fujinaga K., Green M. Mechanism of viral carcinogenesis by deoxyribonucleic acid mammalian viruses. IV. Related virus-specific ribonucleic acids in tumor cells induced by "highly" oncogenic adenovirus types 12, 18, and 31. J Virol. 1967 Jun;1(3):576–582. doi: 10.1128/jvi.1.3.576-582.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Kasai T., Bautz E. K. Regulation of gene-specific RNA synthesis in bacteriophage T4. J Mol Biol. 1969 May 14;41(3):401–417. doi: 10.1016/0022-2836(69)90284-8. [DOI] [PubMed] [Google Scholar]
- McConkey E. H., Hopkins J. W. Molecular weights of some HeLa ribosomal RNA's. J Mol Biol. 1969 Feb 14;39(3):545–550. doi: 10.1016/0022-2836(69)90144-2. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Smith I., Holtzman E. Ribosomal RNA synthesis and processing in a particulate site in the HeLa cell nucleus. Science. 1966 Nov 11;154(3750):786–789. doi: 10.1126/science.154.3750.786. [DOI] [PubMed] [Google Scholar]
- Reich P. R., Black P. H., Weissman S. M. Nucleic acid homology studies of SV 40 virus-transformed and normal hamster cells. Proc Natl Acad Sci U S A. 1966 Jul;56(1):78–85. doi: 10.1073/pnas.56.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHARFF M. D., UHR J. W. FUNCTIONAL RIBOSOMAL UNIT OF GAMMA-GLOBULIN SYNTHESIS. Science. 1965 Apr 30;148(3670):646–648. doi: 10.1126/science.148.3670.646. [DOI] [PubMed] [Google Scholar]
- STRAUSS J. H., Jr, SINSHEIMER R. L. Purification and properties of bacteriophage MS2 and of its ribonucleic acid. J Mol Biol. 1963 Jul;7:43–54. doi: 10.1016/s0022-2836(63)80017-0. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer K., Marcaud L. Remarques sur les ARN messagers polycistroniques dans les cellules animales. Bull Soc Chim Biol (Paris) 1965;47(8):1697–1713. [PubMed] [Google Scholar]
- Soeiro R., Birnboim H. C., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. II. Base composition and cellular localization of a heterogeneous RNA fraction. J Mol Biol. 1966 Aug;19(2):362–372. doi: 10.1016/s0022-2836(66)80010-4. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Darnell J. E. Competition hybridization by "pre-saturation" of HeLa cell DNA. J Mol Biol. 1969 Sep 28;44(3):551–562. doi: 10.1016/0022-2836(69)90379-9. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Vaughan M. H., Warner J. R., Darnell J. E., Jr The turnover of nuclear DNA-like RNA in HeLa cells. J Cell Biol. 1968 Oct;39(1):112–118. doi: 10.1083/jcb.39.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Kelly R. B., Sinsheimer R. L. Denaturation of RNA with dimethyl sulfoxide. Biopolymers. 1968 Jun;6(6):793–807. doi: 10.1002/bip.1968.360060604. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. SUCCESSIVE TRANSFORMATIONS OF AN ESTABLISHED CELL LINE BY POLYOMA VIRUS AND SV40. Science. 1965 Jan 29;147(3657):513–514. doi: 10.1126/science.147.3657.513. [DOI] [PubMed] [Google Scholar]
- Westphal H., Dulbecco R. Viral DNA in polyoma- and SV40-transformed cell lines. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1158–1165. doi: 10.1073/pnas.59.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa-Fukada M., Fukada T., Kawade Y. Characterization of rapidly labeled ribonucleic acid of animal cells in culture. Biochim Biophys Acta. 1965 Jul 15;103(3):383–398. doi: 10.1016/0005-2787(65)90132-2. [DOI] [PubMed] [Google Scholar]



