Abstract
Purpose
To test a hypothesis that, with 5-fluorocytosine (5-FC) treatment, the co-expression of cytosine deaminase (CD) and uracil phosphoribosyltransferase (UPRT) can lead to greater radiosensitization and bystander effect than CD-expression alone.
Methods and Materials
R3327-AT cell lines stably expressing CD or CDUPRT were generated. The 5-FC and 5-FU cytotoxicity, and the radiosensitivity with/without 5-FC treatment, of these cells were evaluated under both aerobic and hypoxic conditions. The bystander effect was assessed by apoptosis staining and clonogenic survival. The pharmacokinetics of 5-FU and 5-FC metabolism was followed in mice bearing CD- or CDUPRT-expressing tumors using 19F MR spectroscopy (MRS).
Results
CDUPRT-expressing cells were more sensitive to 5-FC and 5-FU than CD-expressing cells. CDUPRT-expression more effectively enhanced the radiosensitizing effect of 5-FC than CD-expression. A 25-fold-lower dose of 5-FC resulted in the same magnitude of radiosensitization in CDUPRT-expressing cells, relative to that in CD-expressing cells. The 5-FC cytotoxicity in co-cultures of parental cells mixed with 10–20% CDUPRT cells was similar as that in 100% CDUPRT cells. 19F MRS measurements showed that expression of CDUPRT leads to enhanced accumulation of fluorine nucleotide (FNuc) in vivo.
Conclusion
Our study indicates that CDUPRT/5-FC strategy may be more effective than CD/5-FC when used as an adjuvant to radiotherapy.
Keywords: cytosine deaminase, uracil phosphoribosyltransferase, 5-fluorocytosine, radiosensitization, bystander effect
Introduction
Gene-directed enzyme prodrug therapy, or as it is commonly called suicide gene therapy, is a promising gene therapy strategy. This approach is based on the delivery of a non-mammalian gene which encodes an enzyme that is non-toxic by itself, but can convert a prodrug into a potent cytotoxin (1). One of the well-characterized suicide gene therapies is the use of cytosine deaminase (CD). CD converts the prodrug 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU). 5-FU is then catalyzed by cellular enzymes to the toxic pyrimidine antimetabolites, such as 5-fluorodeoxyuridine-5’-monophosphate and fluorouridine-5’-triphosphate, which inhibit DNA and RNA synthesis respectively, leading to cell death (2).
The CD/5-FC approach has two advantages in addition to its cytotoxicity: first, 5-FU’s metabolites can enhance radiation response; second, the CD/5-FC approach has a strong bystander effect. The radiosensitization effect is caused by mechanisms such as increased radiation-induced DNA damage, impaired DNA repair, and accumulation of cells in the radiosensitive phases of the cell cycle such as late G1 and early S (3). Transduction of the CD gene followed by 5-FC treatment has been shown to increase radiosensitivity of cancer cells (4, 5). However, there is a paucity of data examining the radiosensitizing effect of the CDUPRT/5-FC strategy. The strong bystander effect of CD/5-FC system is due to the converted 5-FU that can diffuse into neighboring non-CD-expressing cells and cause cytotoxicity (6). The bystander effect may be important in increasing the therapeutic efficacy of gene therapy, because a method that successfully delivers a therapeutic gene to all the tumor cells has not been established.
Although the CD/5-FC strategy holds promise, some challenges still remain. First, certain tumor cells were relatively resistant to 5-FU due to the low efficiency of converting 5-FU into its toxic metabolites (7). This can be circumvented by transduction of the uracil phosphoribosyltransferase (UPRT) gene, which converts 5-FU directly to 5-fluorouridine monophosphate, thus bypassing the rate-limiting reactions catalyzed by other cellular enzymes (8). Simultaneous expression of CD and UPRT genes could act synergistically, resulting in increased sensitivity to 5-FC (8, 9). Second, controversial data are reported regarding whether the tumor microenvironment, such as hypoxia, affects the cytotoxic effect of 5-FU or CD/5-FC approach. Although 5-FU is classified as an oxygen-independent chemotherapeutic agents (10), some tumor cells have been shown to be 5-FU resistant under hypoxic condition (11). Since hypoxic regions are known to exist in a majority of human tumors, and the hypoxic cells are radioresistant, the radiosensitizing ability and bystander effects of the CDUPRT/5-FC approach under hypoxic condition should be of importance to be investigated.
The purpose of this study is to test whether the co-expression of CD and UPRT combined with the 5-FC treatment in R3327-AT rat prostate carcinoma cells can lead to greater radiosensitization and bystander effect than that in cells expressing CD alone, under both aerobic and hypoxic conditions. Our results showed that co-expression of CD and UPRT increased radiosensitization and bystander effects of 5-FC. These data suggest that the CDUPRT/5-FC approach have high potential to improve the therapeutic efficacy of combined enzyme/prodrug gene therapy and radiotherapy.
Materials and Methods
Cell Cultures
R3327-AT cells were obtained from Dr. J. Donald Chapman (CRM Consulting Services, Penticton, BC, Canada) and grown as monolayer in Dulbecco’s Modified Eagle medium (DMEM; Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin (Gemini, West Sacramento, CA). For normoxic culture, cells were incubated in a humidified atmosphere containing 5% CO2 at 37°C; for hypoxic culture, cells were incubated in an In Vivo2 400 Hypoxic workstation (Ruskinn Inc., Cincinnati, OH) with a gas mixture of 0.2% O2, 5.0% CO2 and 94.8% N2 at 37°C.
R3327-AT cell lines stably expressing CD/mDsRed and CD/UPRT/mDsRed fusion genes (designated as CD and CDUPRT cells, respectively) were established as previously described (12). Briefly, the expression plasmids were constructed which contain CD or CDUPRT gene fused with DsRed-Monomer (mDsRed) and a Hygromycin B resistance gene. R3327-AT parental cells were transfected with these plasmids by Lipofectamine2000 (Invitrogen, Carlsbad, CA) for 24 hr according to the manufacturer’s instructions and subsequently cultured in medium containing Hygromycin B (0.2mg/ml; Roche, Mannheim, Germany). Stable transfectants were further selected by fluorescence-activated cell sorting using the cell sorter (Moflo™, Dako, Carpinteria, CA).
Western Blot
Equal amount (20 µg) of protein samples from CD and CDUPRT cells incubated under aerobic or 0.2% O2 for 48 hr were analyzed by Western blotting using the sheep anti-yCD polyclonal antibody (Biotrend, Cologne, Germany) and the horseradish peroxidase-labeled bovine anti-sheep IgG (Santa Cruz, Santa Cruz, CA). The protein expression was visualized using the Supersignal chemiluminescent substrate (Pierce, Rockford, IL).
Fluorescence Microscopy and Flow Cytometric Analysis
For fluorescence microscopy, CD, CDUPRT and parental cells were plated into 4-chamber slides and incubated for 48 hr under aerobic or hypoxic condition. The fluorescent images were acquired with a fluorescence microscope (Axiovert 200M, Zeiss). For flow cytometric analysis, the cells were plated into 6-well plates and incubated under aerobic or hypoxic condition for 48 hr. The fluorescence of mDsRed was measured at the cell sorter, and data analyzed with the Flowjo program (Tree Star Inc, Ashland, OR)
Drug Cytotoxicity and Colony Formation Assay
R3327-AT, CD, or CDUPRT cells were incubated under aerobic or hypoxic condition for 24 hr, and then treated with 5-FC or 5-FU at various concentrations for an additional 24 hr under the same condition. After drug treatment, cells were trypsinized, counted, serially diluted, plated in 60 mm dishes and cultured for 10–14 days. The colonies were then stained and counted. The plating efficiency (PE), the number of colonies divided by the number of cells plated, was calculated. The surviving fraction is the PE of treated cells normalized to the PE of non-treated cells. Cell survival curves were constructed by plotting the surviving fractions as a function of prodrug concentration.
Bystander Effect
CD or CDUPRT cells were mixed with parental cells at different ratios (2:98, 5:95, 10:90, 20:80 and 50:50) and plated into 60 mm dishes. Twenty-four hours after plating, one half of the dishes were placed in the hypoxic workstation (0.2% O2) for 24 hr. Subsequently, 5-FC was added to a final concentration of 50 µg/ml to CD-expressing group, or 5 µg/ml to CDUPRT-expressing groups. After a 24 hrs’ 5-FC treatment cell survival was determined by the colony formation assay.
Annexin V-FITC Apoptosis Staining
CD and CDUPRT cells, and mixed cultures of CD or CDUPRT cells and parental cells (20:80) were treated with 5-FC (50 µg/ml to CD cells and 5 µg/ml to CDUPRT cells) for 48 hr. Immediately after treatment these cells were stained with the Annexin-V-FITC (Biovision, Mountain View, CA) according to the manufacturer’s instructions. Flow cytometric and flurescence microscopic analysis were performed subsequently.
Radiation Survival Assay
The effect of 5-FC treatment on the radiosensitivity of CD and CDUPRT cells was studied: CD and CDUPRT cells were first treated with 50 µg/ml and 2 µg/ml of 5-FC, respectively, for 24 hr under aerobic or hypoxic condition. At the end of treatment these cells were irradiated with graded doses using a Cs-137 unit (Mark 1 model 68, Shephard and Associate, San Fernando, CA) at the dose rate of ~2.0 Gy/min, and cell survival determined by colony formation assay. Both aerobic and hypoxic conditions were maintained until the end of irradiation. In parallel, the radiosensitivity of aerobic or hypoxic R3327-AT parental, CD and CDUPRT cells without 5-FC treatment was also determined and used as controls.
To study the effect of radiation combined with 5-FC treatment on the bystander cells, mixture of 80% R3327-AT parental cells and 20% CD or CDUPRT cells were cultured. These mixed cells were treated with 100 µg/ml (for CD cells) or 5 µg/ml 5-FC (for CDUPRT cells), respectively, under aerobic or hypoxic condition for 24 hr. At the end of treatment, they were irradiated and cell survivals determined by colony formation assay. The R3327-AT parental cells were used as the controls.
Surviving fractions were normalized to the PE of non-irradiated cells treated with or without 5-FC treatment, and plotted as a function of radiation dose to generate radiation survival curves. These curves were fitted to the linear-quadratic equation and the doses to reduce surviving fraction to 0.1, 0.01, and 0.001 were calculated for each survival curve. The sensitization enhancement ratio (SER) was calculated as a ratio between the doses required to reduce the surviving fraction in the control group (radiation only) and treatment group (5-FC plus radiation) to the same level.
Magnetic resonance spectroscopy
All experiments were approved by the Institutional Animal Care and Use Committee of Memorial Sloan-Kettering Cancer Center. CD or CDUPRT cells (5×106 in 0.1ml PBS) were implanted subcutaneously into the hind limb of male athymic nu/nu mice (NCI, Frederick, MD) as previously described [12]. When tumors had grown to a size of 200–400 mm3 mice were used for in vivo 19F MRS measurements.
In vivo 19F MRS was performed as previously described [12] using a Bruker 7T spectrometer with a 31 cm wide horizontal bore magnet. A home built two-turn radiofrequency solenoid coil with a diameter of 12 mm tuned to 282 MHz was used to collect 19F MR data. Briefly, mice were anesthetized with isoflurane through a nose cone and positioned in a specially designed animal holder as previously described [12] with a jig that immobilized the tumor bearing limb in an extended fashion away from the body so that the tumor was directly inside the coil. 19F MR spectra were acquired and averaged over 10 min before and after the i.v. administration of 150mg/kg 5-FU or 150mg/kg 5-FC via a tail vein catheter, for a maximum of 2-h using a single pulse with a TR of 1 sec and sweep width of 15 000 Hz. A glass sphere (18µl) filled with 75 mmol/L sodium fluoride (NaF) was used as an external reference for 19F MRS quantitation purposes. The MR time domain was analyzed using XsOs NMR (Columbia University). The spectral area of the acquired data was obtained from a Lorentzian fit to the spectra after line broadening (30Hz). Metabolite ratios were calculated by normalizing their resonance-peak areas to that of NaF. Graphic representation of the spectroscopic data (5-FC/NaF, 5-FU/NaF and FNuc/NaF vs time) shows the average ± SD of the given ratio at each time point.
Statistical analysis
Averages are presented as the mean ± SE. Difference in cytotoxicity and radiosensitivity was determined using the Student’s t test. A p value of <0.01 was considered statistically significant.
Results
CDUPRT expression enhances the 5-FU and 5-FC sensitivity of R3327-AT cells more than CD Expression
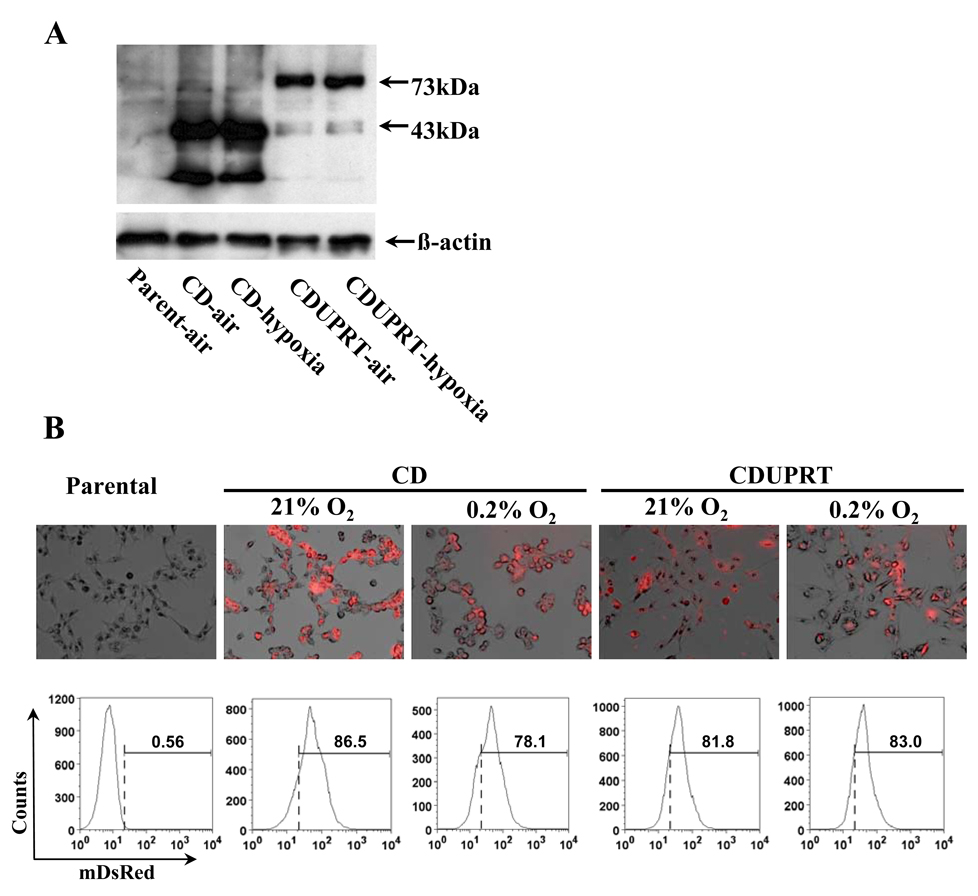
R3327-AT cell lines stably expressing CD/mDsRed or CDUPRT/mDsRed were successfully established. The expression of the respective fusion proteins was confirmed by western blot, flow cytometry and fluorescent microscopy (Fig. 1) and their functions were tested using 5-FU and 5-FC cytotoxicity assay (Fig. 2). Western blot analysis (Fig. 1A) with anti-CD antibody revealed a 43-kDa band in CD samples and a 73-kDa band in CDUPRT samples, which corresponded to the predicted sizes of CD/mDsRed and CD/UPRT/mDsRed proteins respectively. The expression of CD/mDsRed and CD/UPRT/mDsRed fusion proteins are clearly visible under fluorescence microscopy (Fig.1B, upper panel), with the mDsRed signals in CD cells stronger than those in CDUPRT cells. Flow cytometric analysis (Fig.1B, lower panel) confirmed the microscopic findings. In all experiments and analyses described above there was no significant difference in the expression of the fusion proteins between aerobic and hypoxic cells.
Figure 1.
Protein expression detected by western blot (A), fluorescent microscopy (B, upper panel, ×200 magnification) and analyzed by flow cytometry (B, lower panel). Western blotting was performed with anti-yeast CD antibody. Fluorescence images were overlaid with the phase-contrasted images. In flow cytometric analysis, the gate (dashed line) was set to 0.56% mDsRed positive in parental cells. The mDsRed positive ratios in CD, CDUPRT cells according to the gate were showed above the bars.
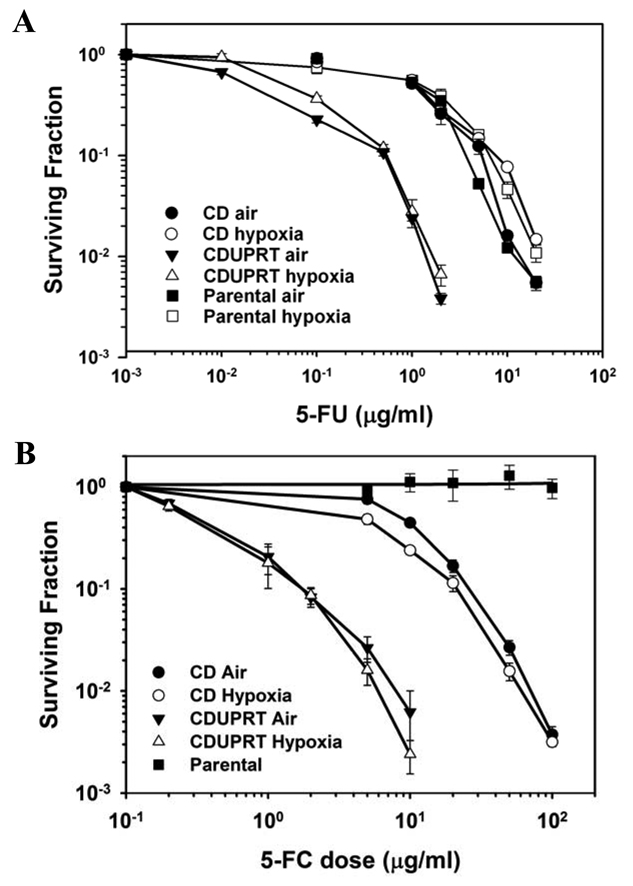
Figure 2.
Expression of CD or CDUPRT sensitizes R3327-AT cells to 5-FU (A) and 5-FC (B) under aerobic (closed symbols) or hypoxic condition (open symbols). Surviving fractions of parental (□, ■), CD (○, ●) and CDUPRT (Δ, ▲) cells after a 24 hr exposure to 5-FU or 5-FC are shown as a function of drug dose. Data are presented as the mean ± SE from three repeated experiments.
The sensitivity of CD and CDUPRT cells to 5-FU or 5-FC is shown in Fig. 2. Both wild-type and CD-expressing cells was sensitive to 5-FU treatment. Presumably due to the more efficient conversion of 5-FU to 5-FUMP by UPRT, CDUPRT-expressing cells were much more sensitive to 5-FU compared to CD-expressing cells (Fig 2A). As clearly demonstrated in Fig. 2B, the survival of CD and CDUPRT cells treated with 5-FC decreased as a function of drug dose, while 5-FC was not toxic to the parental cells even at the highest concentration tested (100 µg/ml). Comparison of survival curves for CD and CDUPRT cells indicated that the CDUPRT cells were much more sensitive to 5-FC than CD cells. For each cell line, the sensitivity to 5-FC or 5-FU exposure under aerobic and hypoxic conditions was indistinguishable.
The survival curves for CD and CDUPRT cells were parallel, specifically below SF of 0.1, and the 5-FC doses required to result in the same level of cytotoxicity were approximately 10 times higher for CD cells than for CDUPRT cells. Based on these results, different doses of 5-FC were used for CD and CDUPRT cells to result in same level of cytotoxicity in subsequent experiments.
Expression of CDUPRT with 5-FC treatment increases the bystander effects under aerobic and hypoxic conditions
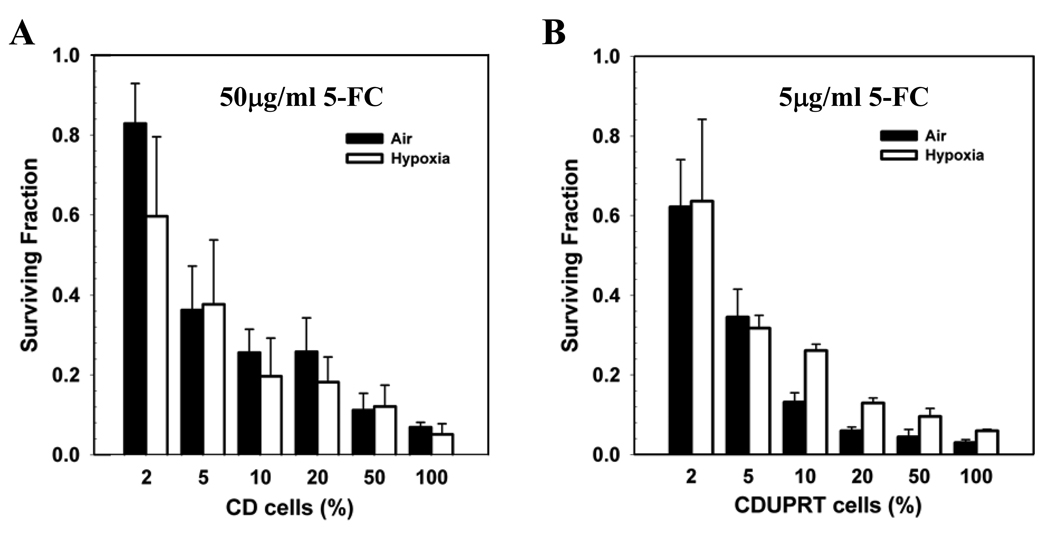
To examine the bystander effects, CD or CDUPRT cells were mixed with parental R3327-AT cells in various ratios and exposed to 5-FC, and cell survivals were determined. Figure 3A (CD cells) and 3B (CDUPRT cells) clearly showed the significant bystander effect. For example, the presence of as little as 5% CDUPRT or CD cells decreased the surviving fraction to ~0.4. Surviving fractions decreased further with an increase in the percentage of CD or CDUPRT cells. Notably, the 5-FC dose given to CDUPRT cells (5 µg/ml) was 10-fold lower than that to CD cells (50 µg/ml). The oxygen status of the cells did not result in observable difference in the bystander effect in CD cells (P>0.01, Fig. 3A). In the CDUPRT mixture, there was a difference between the air and hypoxia groups with air being more effective at high percentage (10%–50%) of CDUPRT cells (P<0.01, Fig. 3B). This is due to the slower growth rate of CDUPRT cells compared with parental cells under prolonged hypoxic condition. For example, the percentage of CDUPRT cells in the 20% cell mixture decreased to less than 10% after exposure to 0.2% O2 for 48 hr (data not shown).
Figure 3.
The bystander effects in CD (A) and CDUPRT (B) cells after a 24 hr 5-FC treatment. CD or CDUPRT cells were mixed with parental R3327-AT cells in various ratios and exposed to 5-FC (50 µg/ml to CD cells and 5 µg/ml to CDUPRT cells) under aerobic (solid bars) or hypoxic (open bars) condition, and cell survivals were determined by colony formation assay. The mean ± SE of three repeated experiments are shown.
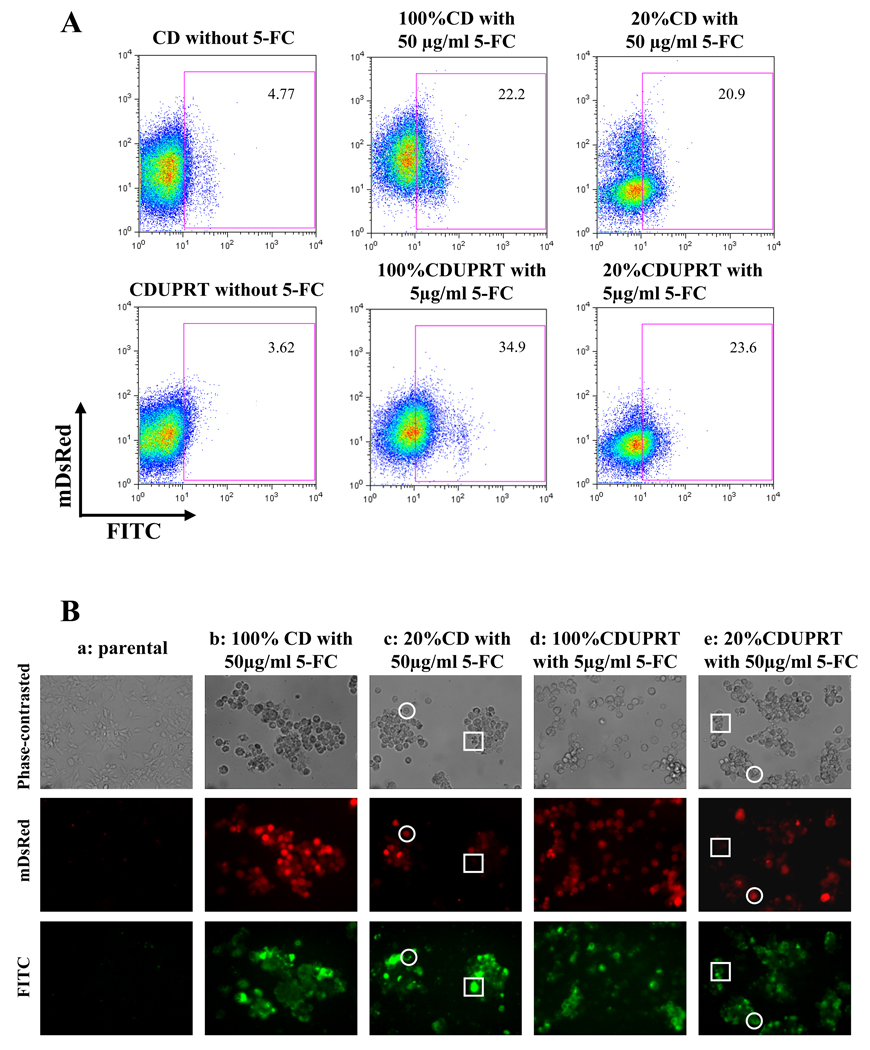
The bystander effect was also evaluated by another endpoint, cellular apoptosis. In Fig. 4A, flow cytometric analysis for untreated CD and CDUPRT cells showed low background staining with annexin-V (4.8% and 3.6, respectively). Treatments with 5-FC for 48 hr (50 µg/ml for CD cells or 5 µg/ml for CDUPRT cells) induced a significant amount of apoptosis in 100% CDUPRT (or 100% CD) cells or in a cell mixture of 20% CDUPRT (or 20% CD) cells and 80% parental cells. The apoptosis indexes in the 100% CD and CDUPRT cells were 22.2%, and 34.9% respectively, and those in the cell mixture containing 20% CD or 20% CDUPRT cells were 20.9% or 23.6%, respectively. This bystander effect can also be visualized by fluorescent microscopy. As shown in Fig. 4B, phase-contrasted images (upper panel), mDsRed (middle panel) and FITC (lower panel) fluorescence images were acquired for the parental cells (a), 100% CD cells (b), a mixture of 20% CD cells and 80% parental cells (c), 100% CDUPRT cells (d) and a mixture of 20% CDUPRT and 80% parental cells (e). For parental cells, 5-FC treatment (50 µg/ml) resulted in insignificant amount of apoptosis. In contrast, in 100% CD and 100% CDUPRT cells (red cells in the middle panel), 5-FC treatment (50 µg/ml to CD cells and 5 µg/ml to CDUPRT cells) induced many apoptotic cells (green cells in the lower panel). Consistent with the flow cytometric findings, 5-FC treatment induced similar amount of apoptosis in 20% CD or CDUPRT cells mixed with 80% parental cells as those in 100% CD or CDUPRT cells. Fig 4B (c) and (e) clearly showed that when the CD or CDUPRT cells were mixed with the parental cells, 5-FC treatments induced apoptosis not only in the CD or CDUPRT cells (cells in open circles, showing both red and FITC fluorescence) but also in the bystander parental cells (cells in open squares, showing no red signal but clearly detectable FITC signal).
Figure 4.
Bystander effect in CD and CDUPRT cells after a 48 hr 5-FC treatment. Apoptosis is used as an endpoint and assessed by anti-AnnexinV-FITC staining. In flow cytometric analysis (4A), a rectangle gate was set with unstained parental cells. The numbers in the rectangle show the Annexin-V-FITC positive ratios. Under fluorescence microscopy (4B), phase-contrasted (upper row), mDsRed (middle row) and FITC (lower row) images from same field were acquired. See details in text.
Expression of CDUPRT enhances the radiosensitizing effect of 5-FC under aerobic and hypoxic condition
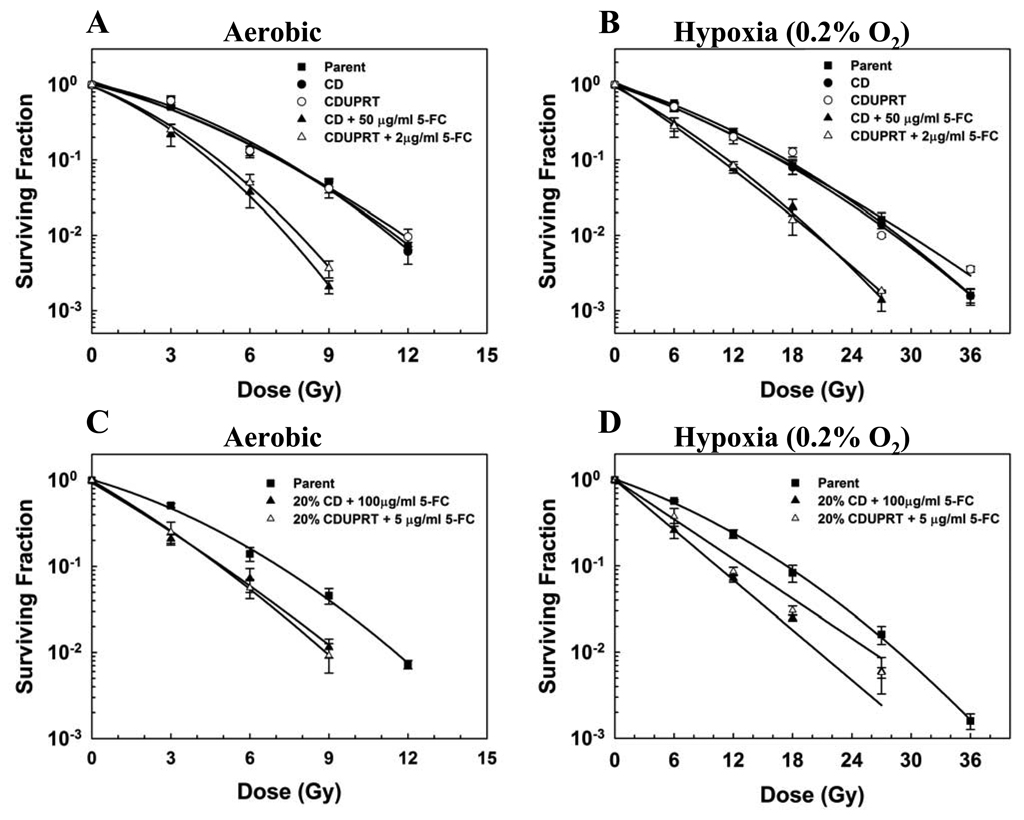
To study the effect of 5-FC on the radiation response of cells expressing either CD or CDUPRT, cells were exposed to 5-FC for 24 hr, at the end of this treatment, cells were irradiated and the surviving fractions were determined. Results in Fig. 5 showed that, without 5-FC treatment, the radiosensitivity of CD (solid circles) and CDUPRT cells (open circles) was similar to that of the parental cells (open square) under aerobic (Fig. 5A) and hypoxic conditions (Fig. 5B). 5-FC treatments significantly enhanced the radiation response of both CD and CDUPRT cells. This radiosensitization effect was greater for CDUPRT cells (open triangle) than for CD cells (solid triangle). This is evidenced by that a ~ 25 fold lower concentration of 5-FC was required to radiosensitize CDUPRT cells to a similar extent as CD cells. The SERs were 1.54 ± 0.05 and 1.54 ± 0.03 for aerobic CD cells treated with 50 µg/ml 5-FC and CDUPRT cells treated with 2 µg/ml 5-FC, respectively. The SER for hypoxic CD and CDUPRT cells were 1.37 ± 0.002 and 1.39 ± 0.02, respectively.
Figure 5.
Radiosensitizing effect of 5-FC on CD and CDUPRT cells under aerobic (A) and hypoxic (B) conditions, and radiosensitization of bystander cells co-cultured with CD or CDUPRT cells under aerobic (C) and hypoxic (D) conditions. See details in text. The average values of surviving fractions of three independent experiments (± SE) are shown.
We then examined the radiosensitizing effect of 5-FC on bystander cells. Co-cultures of 20% CD or CDUPRT cells and 80% parental cells were first treated with 5-FC for 24 hr and then irradiated immediately afterwards. Similar to 100% CD and CDUPRT cells, 5-FC also sensitized the radiation response of these mixed populations under aerobic (Fig. 5C) and hypoxic conditions (Fig. 5D). The radiosensitizing effect of 5-FC was also greater for CDUPRT mixtures (open triangle) than for CD mixtures (solid triangle). This is evidenced by that the 5-FC dose to induce the same magnitude of radiosensitization for the CDUPRT mixture was approximately 20 fold lower than the dose for CD mixtures. The SERs in co-cultured CD and CDUPRT cells under aerobic conditions were 1.22 ± 0.12 and 1.33 ± 0.07, and were 1.68 ± 0.07 and 1.25 ± 0.08 under hypoxic conditions, respectively.
Expression of CDUPRT leads to enhanced accumulation of FNuc in vivo determined by 19F MR spectroscopy
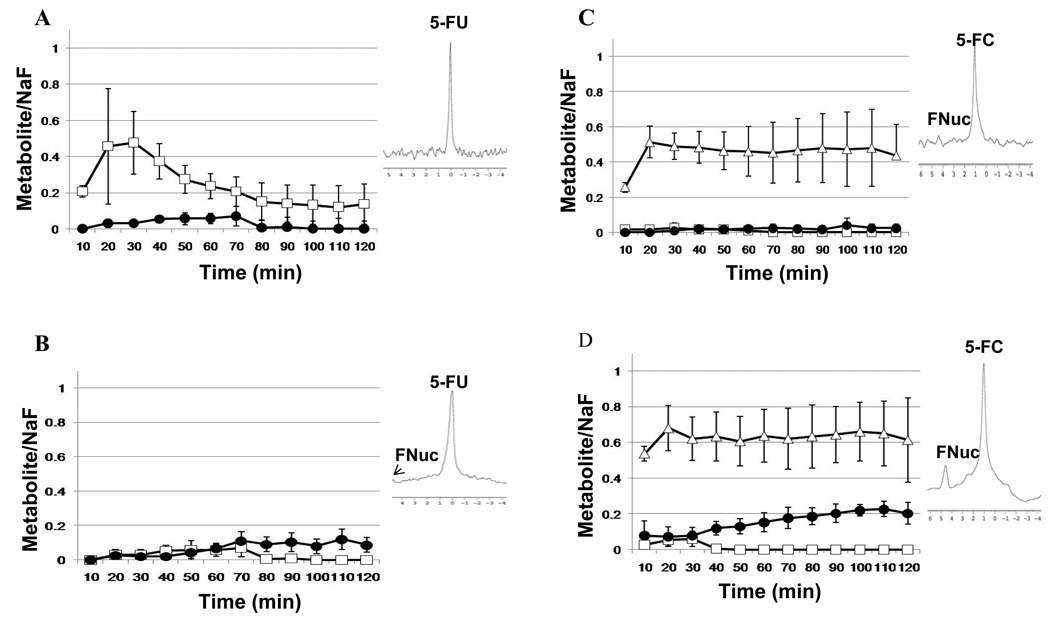
The pharmacokinetics of 5-FU and 5-FC metabolism was followed in mice bearing CD- or CDUPRT-expressing tumors using 19F MR spectroscopy (Fig.6). Following administration of 150mg/kg (i.v) 5-FU or 150mg/kg (i.v) 5-FC, serial spectra were acquired sequentially every 10 min over a 2-h time course. Tumors bearing CD (n=3) and tumors bearing CDUPRT (n=4) treated with 5-FU showed there was an overall increase in initial uptake of 5-FU, followed by a steady decrease over time eventually reaching a plateau after 80 min in both CD and CDUPRT tumors (Fig. 6, A and B). Conversion of 5-FU to FNuc (4.8–5ppm), could be detected within 20 min following 5-FU administration, which increased steadily throughout the time course of data acquisition (Fig.6, A and B). In addition to the anabolite (FNuc), 5-FU catabolites fluoro-β-ureido-propanoic acid (FUPA: −17.2ppm) and α-fluoro-β-alanine (FBAL: −19.0ppm) were also visible (data not shown). Following administration of 150mg/kg 5-FC (i.v), rapid conversion of 5-FC to 5-FU occurred within 10 min following injection, resulting in the detection of FNuc in one out of the three CD tumors, where levels remained stable for 2 hr (Fig. 6C). In agreement with the in vitro results in the current study, CDUPRT expressing tumors were more efficient in the conversion of 5-FC to 5-FU leading to the production of measureable levels of FNucs which increased over time (Fig. 6D). No detection of the catabolites FUPA or FBAL could be observed in CDUPRT or CD tumors.
Figure 6.
Pharmacokinetics of 150 mg/kg 5-FU and 150mg/kg 5-FC given i.v. 10–120 min after administration in (A) CD expressing tumors treated with 5-FU (n=3), (B) CDUPRT expressing tumors with 5-FU treatment (n=4) (C) CD tumors treated with 5-FC (n=3), and (D) CDUPRT tumors treated with 5-FC (n=3). Results show the mean ± SD of the ratio of 5-FU/NaF (□), 5-FC/NaF (□) and FNuc/NaF (●). Representative in vivo 19F MR spectra of CD and CDUPRT tumors at 60 min after injection with 5-FU or 5-FC are shown alongside the pharmacokinetic plots, respectively. The chemical shift of 5-FU is normalized to 0 ppm, 5-FC at 1 ppm and FNuc between 4.8–5 ppm.
Discussion
5-FU is one of the anticancer drugs most commonly used in combination with radiotherapy, and has been shown in prospective clinical trials to enhance radiation response and to improve local control in some human malignancies (13, 14). However, the lack of differential effects between tumors and normal tissues limit the potential therapeutic efficacy of combining high-dose 5-FU with radiotherapy. Approaches to transduce CD gene into tumor cells followed by 5-FC administration can produce 5-FU electively at the tumor site, while reducing normal tissue toxicities (4–6).
A previous study demonstrated that adenovirus-mediated expression of the E. coli CD gene in conjunction with the 5-FC treatment resulted in radiosensitization (4). However, the application of bacterial CD is limited by its inefficiency in converting of 5-FC to 5-FU. By comparison, yeast CD has been shown to convert 5-FC more effectively and provide greater radiosensitization effect in animal tumor models (5, 15). Recently, a phase I clinical trial for newly-diagnosed prostate cancer has used the yeast CD in double suicide gene therapy in combination with intensity-modulated radiation therapy (16). Consistent with these findings, our results showed that yeast CD expression dramatically improved the sensitivity of R3327-AT cells to 5-FC (Fig. 2), more than that observed in bacterial T cells (17). Our data also demonstrated that 5-FC treatment was able to sensitize CD-expressing cells to radiation (Fig. 6), consistent with findings by others (5, 18).
n important feature of CD/5-FC strategy is its strong bystander effect. Huber et al. (19) observed significant tumor regression after 5-FC treatment in tumors that contained only 2% CD-expressing cells. In contrast, there was little bystander effect in bacterial CD-transduced R3327-AT cells, probably a result of the low extracellular 5-FU level (20). In the present study, we observed strong bystander effects in cells expressing yeast CD. This is likely due to the higher enzyme activity produced by yeast CD compared to that produced by bacterial CD (5). More importantly, our study demonstrated that CDUPRT/5-FC system can effectively produce stronger bystander effect even at a 5-FC concentration 10-fold lower than that used for CD cells. This was evidenced by the fact that similar clonegenic survival and apoptosis index were fould in 100% CDUPRT cell population and cell mixture consisted of only 20% CDUPRT cells (Fig. 3 and 4). The annexin-V FITC stained were erfomed after 24 hr or 48 hr 5-FC treatment. Howover, only results of 48 hr treatment were shown because of clearer evidence.
Given the fact that 5-FU must be metabolized to the cytotoxic fluorinated nucleotides, it is not surprising that co-transduction of UPRT gene with CD gene could significantly improve the 5-FC cytotoxicity. It is reasonable to hypothesize that the CDUPRT/5-FC system can enhance the radiosensitizing effect of 5-FC, because this system can increase the production of 5-FU and its cytotoxic metabolites, leading to radiosensitization. However, there is a paucity of data examining the radiosensitizing effect of the CDUPRT/5-FC strategy (21). Our results clearly showed that co-expression of CD and UPRT not only significantly increased the sensitivity to 5-FC (Fig. 2), but also improved the radiosensitizing effect of 5-FC relative to that induced by CD expression (Fig. 6). This is evidenced by the fact that a 25-fold lower concentration of 5-FC was required to radiosensitize CDUPRT cells to a similar extent as that required to radiosensitize CD cells. More importantly, approximately a 20-fold lower concentration of 5-FC resulted in a similar radiosensitizing effect to the bystander cells co-cultured with CDUPRT cells compared to those with CD cells. This stronger radiosensitizing effect of CDUPRT/5-FC compared to CD/5-FC was also demonstrated under hypoxic condition. These findings suggest that CDUPRT/5-FC approach may have a great potential as an adjuvant to radiotherapy.
A major difficulty in suicide gene therapy for the clinical application is a lack of methods that can accurately measure the transduction efficiency of the therapeutic genes and monitor the biological function of the transgene. An advantage of CD/5-FC and CDUPRT/5-FC approaches is that the conversion of 5-FC to 5-FU and other fluorinated nucleotides can be monitored by the 19F MRS, as our results (Fig. 6) and other reports shown [22,23]. At present, we are generating adenovirus carrying CDUPRT for gene therapy and will utilize in vivo MRS imaging to non-invasively evaluate the efficacy of the transgene expression/activity by monitoring drug metabolism.
Acknowledgments
This work was supported in part by grants from National Institutes of Health (PO1 CA115675, RO1 CA56909, and R33 CA109772).
References
- 1.Greco O, Dachs GU. Gene directed enzyme/prodrug therapy of cancer: historical appraisal and future prospectives. J Cell Physiol. 2001;187:22–36. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1060>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 2.Daher GC, Harris BE, Diasio RB. Metabolism of pyrimidine analogues and their nucleosides. Pharmacol Ther. 1990;48:189–222. doi: 10.1016/0163-7258(90)90080-l. [DOI] [PubMed] [Google Scholar]
- 3.Hwang HS, Davis TW, Houghton JA, et al. Radiosensitivity of thymidylate synthase-deficient human tumor cells is affected by progression through the G1 restriction point into S-phase: implications for fluoropyrimidine radiosensitization. Cancer Res. 2000;60:92–100. [PubMed] [Google Scholar]
- 4.Anello R, Cohen S, Atkinson G, et al. Adenovirus mediated cytosine deaminase gene transduction and 5-fluorocytosine therapy sensitizes mouse prostate cancer cells to irradiation. J Urol. 2000;164:2173–2177. [PubMed] [Google Scholar]
- 5.Kievit E, Nyati MK, Ng E, et al. Yeast cytosine deaminase improves radiosensitization and bystander effect by 5-fluorocytosine of human colorectal cancer xenografts. Cancer Res. 2000;60:6649–6655. [PubMed] [Google Scholar]
- 6.Trinh QT, Austin EA, Murray DM, et al. Enzyme/prodrug gene therapy: comparison of cytosine deaminase/5-fluorocytosine versus thymidine kinase/ganciclovir enzyme/prodrug systems in a human colorectal carcinoma cell line. Cancer Res. 1995;55:4808–4812. [PubMed] [Google Scholar]
- 7.Kanai F, Kawakami T, Hamada H, et al. Adenovirus-mediated transduction of Escherichia coli uracil phosphoribosyltransferase gene sensitizes cancer cells to low concentrations of 5-fluorouracil. Cancer Res. 1998;58:1946–1951. [PubMed] [Google Scholar]
- 8.Tiraby M, Cazaux C, Baron M, et al. Concomitant expression of E. coli cytosine deaminase and uracil phosphoribosyltransferase improves the cytotoxicity of 5-fluorocytosine. FEMS Microbiol Lett. 1998;167:41–49. doi: 10.1111/j.1574-6968.1998.tb13205.x. [DOI] [PubMed] [Google Scholar]
- 9.Erbs P, Regulier E, Kintz J, et al. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene. Cancer Res. 2000;60:3813–3822. [PubMed] [Google Scholar]
- 10.Teicher BA, Lazo JS, Sartorelli AC. Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 1981;41:73–81. [PubMed] [Google Scholar]
- 11.Sasabe E, Zhou X, Li D, et al. The involvement of hypoxia-inducible factor-1alpha in the susceptibility to gamma-rays and chemotherapeutic drugs of oral squamous cell carcinoma cells. Int J Cancer. 2007;120:268–277. doi: 10.1002/ijc.22294. [DOI] [PubMed] [Google Scholar]
- 12.Xing L, Deng X, Kotedia K, et al. Non-invasive molecular and functional imaging of cytosine deaminase and uracil phosphoribosyltransferase fused with red fluorescence protein. Acta Oncol. 2008:1–10. doi: 10.1080/02841860802256475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adelstein DJ, Saxton JP, Lavertu P, et al. A phase III randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy alone in resectable stage III and IV squamous cell head and neck cancer: preliminary results. Head Neck. 1997;19:567–575. doi: 10.1002/(sici)1097-0347(199710)19:7<567::aid-hed2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Smith TJ, Ryan LM, Douglass HO, Jr, et al. Combined chemoradiotherapy vs. radiotherapy alone for early stage squamous cell carcinoma of the esophagus: a study of the Eastern Cooperative Oncology Group. Int J Radiat Oncol Biol Phys. 1998;42:269–276. doi: 10.1016/s0360-3016(98)00232-6. [DOI] [PubMed] [Google Scholar]
- 15.Kievit E, Bershad E, Ng E, et al. Superiority of yeast over bacterial cytosine deaminase for enzyme/prodrug gene therapy in colon cancer xenografts. Cancer Res. 1999;59:1417–1421. [PubMed] [Google Scholar]
- 16.Freytag SO, Movsas B, Aref I, et al. Phase I trial of replication-competent adenovirus-mediated suicide gene therapy combined with IMRT for prostate cancer. Mol Ther. 2007;15:1016–1023. doi: 10.1038/mt.sj.6300120. [DOI] [PubMed] [Google Scholar]
- 17.Corban-Wilhelm H, Ehemann V, Becker G, et al. Comparison of different methods to assess the cytotoxic effects of cytosine deaminase and thymidine kinase gene therapy. Cancer Gene Ther. 2004;11:208–214. doi: 10.1038/sj.cgt.7700667. [DOI] [PubMed] [Google Scholar]
- 18.Khil MS, Kim JH, Mullen CA, et al. Radiosensitization by 5-fluorocytosine of human colorectal carcinoma cells in culture transduced with cytosine deaminase gene. Clin Cancer Res. 1996;2:53–57. [PubMed] [Google Scholar]
- 19.Huber BE, Austin EA, Richards CA, et al. Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci U S A. 1994;91:8302–8306. doi: 10.1073/pnas.91.17.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corban-Wilhelm H, Hull WE, Becker G, et al. Cytosine deaminase and thymidine kinase gene therapy in a Dunning rat prostate tumour model: absence of bystander effects and characterisation of 5-fluorocytosine metabolism with 19F-NMR spectroscopy. Gene Ther. 2002;9:1564–1575. doi: 10.1038/sj.gt.3301834. [DOI] [PubMed] [Google Scholar]
- 21.Kambara H, Tamiya T, Ono Y, et al. Combined radiation and gene therapy for brain tumors with adenovirus-mediated transfer of cytosine deaminase and uracil phosphoribosyltransferase genes. Cancer Gene Ther. 2002;9:840–845. doi: 10.1038/sj.cgt.7700506. [DOI] [PubMed] [Google Scholar]
- 22.Gade TP, Koutcher JA, Spees WM, et al. Imaging transgene activity in vivo. Cancer Res. 2008;68:2878–2884. doi: 10.1158/0008-5472.CAN-07-6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamstra DA, Lee KC, Tychewicz JM, et al. The use of 19F spectroscopy and diffusion-weighted MRI to evaluate differences in gene-dependent enzyme prodrug therapies. Mol Ther. 2004;10:916–928. doi: 10.1016/j.ymthe.2004.07.022. [DOI] [PubMed] [Google Scholar]