Abstract
Upon antigen stimulation, B lymphocytes differentiate into antibody secreting cells (ASC), most of which undergo apoptosis after a few days of intense Ig production. Differentiation entails expansion of the endoplasmic reticulum (ER) and requires XBP1 but not other elements of the unfolded protein response, like Perk. Moreover, normal and malignant ASC are exquisitely sensitive to proteasome inhibitors, but the underlying mechanisms are poorly understood. Here we analyze the role of CHOP, a transcription factor mediating apoptosis in many cell types that experience high levels of ER stress. CHOP is transiently induced early upon B cell stimulation: covalent IgM aggregates form more readily and IgM secretion is slower in chop-/- cells. Despite these subtle changes, ASC differentiation and lifespan are normal in chop-/- mice. Unlike fibroblasts and other cell types, chop-/- ASC are equally or slightly more sensitive to proteasome inhibitors and ER stressors, implying tissue-specific roles for CHOP in differentiation and stress.
Keywords: UPR, ER stress, plasma cell differentiation, IgM secretion, proteasome inhibitors
1. Introduction
Upon antigen or mitogen stimulation, resting B-lymphocytes undergo a profound architectural and functional metamorphosis differentiating into professional antibody secreting cells (ASC, van Anken et al., 2003; Hendershot and Sitia, 2004). IgM is the main isotype secreted during the primary response. Its biogenesis proceeds stepwise under a stringent quality control schedule [(Anelli et al., 2007) and references therein]: Ig-μ and L chains rapidly assemble into μ2L2 structures, ‘monomers’ in the immunological jargon, before forming secretion-competent hexamers (μ2L2)6 or pentamers [(μ2L2)5+J]. Both species are held together by disulfide bonds through Cys575 in the C-terminal tailpiece (tp) of secretory μ chains (μs). Its inherent complexity and developmental control (Sitia et al., 1990), suggests the existence of dedicated molecular machines assisting and regulating polymerization.
Like most other secretory proteins, Ig are co-translationally translocated into the endoplasmic reticulum (ER) where they attain their proper quaternary structure before proceeding along the exocytic pathway to be secreted. Not surprisingly, therefore, the most dramatic feature of plasma cell differentiation is the expansion of the ER and other secretory organelles (Shohat et al., 1973; van Anken et al., 2003) aimed at accommodating exuberant Ig synthesis. Cells that face an increased secretory demand generally activate the Unfolded Protein Response (UPR), a multidimensional signalling cascade finalized to maintain ER homeostasis. In mammals, this is based on three main branches, initiated by PERK, ATF6 and Ire1, respectively (Ron and Walter, 2007; Schroder and Kaufman, 2006). Ire1 triggers the nonconventional splicing of xbp1 transcripts. As a result, the active form of the XBP1 transcription factor (sXBP1) is produced that overall increases secretory capacity (Shaffer et al., 2004; Sriburi et al., 2007). In the presence of unfolded ER proteins, ATF6 is transported to the Golgi, where the resident proteases SP1 and SP2 release an active transcription factor (Haze et al., 1999; Wu et al., 2007; Yamamoto et al., 2007). By phosphorylating eIF2α, the PKR-like ER kinase PERK attenuates translation of most cap-dependent mRNA (Bertolotti et al., 2000; Harding et al., 2000b), whilst favouring the synthesis of ATF4. In turn, ATF4 induces many protective genes (Lu et al., 2004; Marciniak et al., 2004; Ron and Walter, 2007) and gadd34, which dephosphorylates eIF2α limiting the response (Novoa et al., 2001). Depending on the duration and strength of the stress the UPR can mediate recovery and adaptation or apoptosis (Lin et al., 2007; Rutkowski and Kaufman, 2007). The mechanisms triggering a maladaptive response and apoptosis are not clear (Puthalakath et al., 2007; Szegezdi et al., 2006).
Different cells can selectively exploit the UPR branches (Brewer and Hendershot, 2005; Wu and Kaufman, 2006). For example, the PERK pathway is essential for pancreatic β cells (Harding et al., 2001), but not for plasma cells (Gass et al., 2002; Gass et al., 2008; Zhang et al., 2005). Moreover, there is a considerable degree of cross-talk amongst the three UPR branches (Lee et al., 2002; Ma and Hendershot, 2004; Ron and Walter, 2007; van Huizen et al., 2003; Yamamoto et al., 2007; Yamamoto et al., 2004).
We previously reported that C/EBP homologous protein, (CHOP, also called C/EBPζ or GADD153) (Marciniak et al., 2004; Oyadomari and Mori, 2004), is modestly induced in differentiating I.29μ+ B lymphoma cells (Cenci et al., 2006). Although ER stress is its strongest inducer and CHOP is mostly known as a pro-apoptotic factor (Ma et al., 2002; Oyadomari and Mori, 2004), this transcription factor is also involved in many physiological adaptive processes (Eizirik et al., 1993; Fornace et al., 1988; Luethy and Holbrook, 1992; Oyadomari and Mori, 2004), including mitochondrial (Horibe and Hoogenraad, 2007) and oxidative (Guyton et al., 1996; Tang et al., 2002) stress, amino-acid starvation (Averous et al., 2004; Bruhat et al., 2000), and differentiation of adipocytes (Huang et al., 2005; Li et al., 2006; Tang and Lane, 2000), keratinocytes (Maytin and Habener, 1998) and osteoblasts (Pereira et al., 2006; Shirakawa et al., 2006). Here we investigated the role of CHOP during plasma cell differentiation by comparing wt and chop-/- primary B cells. We found that CHOP is transiently expressed upon mitogen stimulation, but the life span and other parameters of differentiation are similar in wt and chop-/- B splenocytes. However, a subtle phenotype emerged: chop-/- ASC secreted slightly fewer IgM, which tended to accumulate intracellularly as disulfide-linked aggregates, in correlation with lower expression of proteins involved in IgM polymerization. Apoptosis, either spontaneous or induced by proteasome inhibitors (PI) or ER stressors, was similar or slightly increased in chop-/- ASC, implying that in B cells CHOP exerts tissue-specific roles.
2. Materials and Methods
2.1 Cell cultures, spleen B cell isolation and mice handling
Purification of B lymphocytes from mouse spleens was performed by magnetic isolation with anti-CD19 beads (Miltenyi Biotec) following the manufacturer’s instructions. Primary B cells were seeded at an initial cell density of 106/ml in RPMI with 10% FCS endotoxin-free, 100U/ml Pen/Strep, 1mM sodium-pyruvate, 2mM N-glutamine (GIBCO) and 50 μM 2-mercaptoethanol (SIGMA) and induced to differentiate by addition of 20 μg/ml LPS (L2637, SIGMA). Cells were never allowed to reach a density higher than 2-3×106/ml to avoid the risk of starvation. For the in vivo LPS stimulation experiments, 3 mice per point were injected intra-peritoneum with 35 μg/g LPS and B cells were purified as described (Cascio et al., 2008). Primary fibroblasts were obtained by cutting and digesting mice ears in 5μg/mL collagenase-II (Invitrogen) for 2 hrs at 37°C and were grown in DMEM supplemented with 10% FCS, 100U/ml Pen/Strep, 1mM sodium-pyruvate, 2mM N-glutamine. All experiments involving animals were performed following experimental protocols approved by the San Raffaele Scientific Institute Animal Care and Use Committee. Chop-null mice have been previously described (Zinszner et al., 1998). The line was maintained on the FVB/N background (Charles River, Italy).
2.2 Flow cytometry
2-5×105 B cells were washed in PBS/0.5% BSA and stained with each of the following reagents: 0.5 μg/ml propidium iodide, 1:200 anti-CD138 PE-conjugated (BD Pharmingen), 1:200 anti-CD38 FITC-conjugated (BD Pharmingen), 1:10.000 anti-IgM-μ chain (Cappel) FITC-conjugated, 1:200 anti-TCR-α chain FITC conjugated (BD Pharmingen) or with the Annexin V-propidium Iodide staining kit (BD Pharmingen) following manufacturer’s instructions.
Samples data were acquired with either Cytomics FC500 flow cytometer (Beckman Coulter) or LSR-II flow cytometer (BD Bioscience) and data were analyzed with FCS Express (De Novo Software) analysis software. Normalization and dose-response non-linear regression analysis was performed with Prism version 5.0 (GraphPad Software).
2.3 Relative real time PCR
Total RNA was isolated from cells by Trizol (Invitrogen) following the manufacturer’s instructions and quality of purified RNA was checked by OD 260-280 readings and by electrophoresis separation. 0.5-1 μg of total RNA was reverse-transcribed by the Super-Script II kit (Invitrogen) in a final volume of 25 μl. Real time PCR was performed with Sybr Green Master Mix in a final volume of 25 μl by the ABI7900 HT thermal cycler (Applied Biosystems). Data were analyzed by the SDS 2.0 software (Applied Biosystems). The increase of each transcript at a certain time point of differentiation relatively to day 0 was calculated by the ΔΔCt method using histone 3 and/or β-actin as reference genes. The following primers were used: CHOP - FW: GCG ACA GAG CCA GAA TAA CA RW: ACC AGG TTC TGC TTT CAG GT; BiP - FW: TAT TGG AGG TGG GCA AAC CAA G RW: CGC TGG GCA TCA TTG AAG TAA G; Ero1α - FW: CCG AAA AAC TGA TCG CAA AT RW: CCG TCC TCC TCA GTG AAC AT; XBP1 spliced forms - FW: GGA GTG GAG TAA GGC TGG TG RW: CCA GAA TGC CCA AAA GGA TA; XBP1 total - FW: TTT GGG CAT TCT GGA CAA GT RW: AAA GGG AGG CTG GTA AGG AA; EDEM - FW: ACT GAT TCC AAA CAG CCC TT RW: GGA TCC CTG TCT TGG TGT TT; ERdj4 - FW: GCC ATG AAG TAC CAC CCT GA RW: CTT TCC GAC TAT TGG CAT CC; β-actin - FW TGC TAT GTT GCT CTA GAC TTC GAG RW TGC CAC AGG ATT CCA TAC CCA; H3 - FW: GTG AAG AAA CCT CAT CGT TAC AGG CCT GGT RW: CTG CAA AGC ACC AAT AGC TGC ACT CTG GAA.
2.4 Protein gradient and western blotting
Total cell lysates (to detect IgM assembly intermediates) were prepared by lysing cells in a buffer containing 25 mM Tris, 100 mM NaCl, 3 mM EDTA, 2% SDS plus freshly added protease inhibitors and 10 mM NEM, followed by sonication to remove DNA and boiling for 10 mins. Post nuclear supernatants (to detect ERdj3, HERP, BiP, ERp44, ERGICp53, Ero1α) were prepared by lysis in 10 mM Tris HCl pH 7.4, 150 mM NaCl, 1% NP40 plus freshly added protease inhibitors and 10 mM NEM. Nuclear extracts (to detect CHOP and sXBP1) were prepared by lysing the cells in harvest buffer (10 mM HEPES, 50 mM NaCl, 0.5 M sucrose, 0.1 mM EDTA, 0.5% Triton X-100 plus freshly added 1 mM DTT and protease inhibitors), and lysing the nuclear pellet in nuclei buffer (10 mM HEPES, 500 mM NaCl, 0.1 mM EDTA, 0.1 mM EGTA, 0.1%NP40 plus freshly added 1 mM DTT and protease inhibitors).
To detect IgM assembly intermediates, total cell lysates were separated on gradient polyacrylamide running gels (2-14%) with 2.5% stacking gels. To detect other proteins conventional SDS-PAGE was performed. Gels were transferred to nitrocellulose sheets as previously described (Cenci et al., 2006). Wt and chop-/- LPS stimulated cells were lysed in SDS, sonicated and centrifuged on continuous sucrose gradients (5-25%) at 40,000 rpm for 400 min at 4°C on a SW41Ti (Beckman Coulter). 39 fractions were collected and aliquots analyzed by dot-blot assays with anti-μ antibodies.
Anti-ERp44 and anti-Ero1α monoclonal antibodies were produced by hybridoma cell cultures. The anti-ERGICp53 rabbit serum was kindly provided by Dr. HP Hauri (Basel, Switzerland). XBP1 and CHOP antibodies were from Santa Cruz Biotechnology. Anti-μ rabbit antibody was from Zymed, anti- κ antibody was from Southern Biotechnology and anti-J was a polyclonal serum produced by PRIMM (Milano, Italy). Anti-actin was from SIGMA. WB images were acquired with the Chemidoc-it Imaging System (UVP) and processed with Adobe Photoshop 7.0 (Adobe Systems Inc.). Densitometric analysis was performed by the Image Quant 5.2 software (Molecular Dynamics).
2.5 Secretion assays
Differentiating B cells were washed twice in PBS and cultured in Hybridoma medium (GIBCO) in the absence of FCS for 4 hrs at a cell density of 106/ml. Protease inhibitors and 10 mM NEM were added to the media at the end of the incubation period and the cells were discarded by sequential centrifugation at 300g first and at 17000g afterwards. 10 μl of media were analyzed by ELISA. The ELISA plate was pre-coated with 1:200 anti-μ rabbit purified IgG (Zymed), blocked with 3% milk in PBS/0.05% Tween 20 and media diluted to 100 μl in PBS were incubated O/N at 4°C. After washing (PBS/0.05% Tween 20) a secondary anti-μ-HRP 1:1000 (Zymed) was incubated for 1hr at RT. The assay was developed with SigmaFast OPD (SIGMA) fast and read at 450nm. Alternatively IgM secretion in the presence of 2-ME was measured by dot blotting 10μl of secretion media (as described above) on nitrocellulose membrane, decorated with HRP conjugated anti μ Ab (Zymed) . ELISA on mice IgM titers was performed as above utilizing 100μl of 1:15000 diluted sera collected by tail vein bleeding.
2.6 Pulse chase assays
In pulse-chase experiments cells were incubated 5 min in methionine/cysteine-free medium (Gibco) supplemented with 1% dialized FBS and labelled for 10 min with 0,4 mCi/ml labelling mix (Easy tag Expres35s, PerkinElmer). Labelled cells were washed twice in HBSS (Sigma) 4°C and incubated for various time periods. At each time point cells were lysed in 1% NP40, protein inhibitors cocktail (Roche). Pellet were lysed in 2%SDS. Dried gels were exposed with Storage Phosphor screen (GE) and analyzed wit Typhoon (GE).
3. Results
3.1 Normal development and terminal differentiation of B cells in chop-/- mice
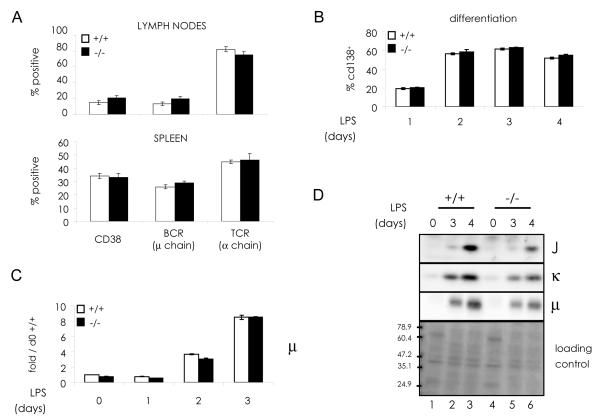
Wild type (wt) and chop-/- mice displayed similar proportions of B and T lymphocytes in their spleens and lymph nodes (Fig.1 A). Peripheral lymphoid organs showed the same gross morphology, and comparable numbers of CD19+ cells could be purified from spleens of either genotype (data not shown). Moreover, the frequency of CD138+/khigh plasma cells in the BM was similar in chop-/- and wt mice (mean ± SEM: 0.20 ± 0.03 and 0.17 ± 0.02, respectively). These observations indicate that CHOP is not essential for B cell development. To study the role of CHOP during terminal B cell differentiation, primary CD19+ splenocytes from wt and chop-/- mice (Oyadomari and Mori, 2004; Zinszner et al., 1998) were stimulated in vitro with LPS (Iwakoshi et al., 2003; Shaffer et al., 2004). Under these conditions, splenic B cells readily differentiate into ASC, as determined by their immunofluorescent staining for surface CD138 (Fig.1 B) and intense and diffuse intracellular Ig (data not shown), reaching about 60% of the cultures at day 3. LPS-induced differentiation proceeded normally in chop-/- mice, as indicated by similar kinetics of CD138+ surface expression (Fig.1 B) and intracellular accumulation of IgM subunits (Fig.1 C and D).
Fig. 1. CHOP deletion does not affect B cell differentiation.
A) Cells harvested from lymph nodes and spleens were stained with fluorescent antibodies against B (CD38 and BCR) and T (TCRα) cell markers and analyzed by flow cytometry (average of 3 experiments ± SEM).
B-D) B splenocytes from wt and chop-/- mice were incubated in the presence of LPS and differentiation towards antibody secreting plasma cells analyzed by various parameters. B) Surface expression of CD138/syndecan-1 was measured by flow cytometry (average of 3 experiments ± SEM). C) Ig-μ transcript levels were measured by relative real time PCR. The fold increase at each time point with respect to wt day 0 was calculated by the ΔΔCt method, using histone 3 as reference gene. Error bars represent the SEM for the average of PCR triplicates of the same samples. D) Ig-J, κ and μ chains protein expression was assessed by sequentially probing the same filter with specific antibodies. Ponceau staining of the nitrocellulose filter is presented as loading control.
3.2 CHOP is transiently induced during primary antibody secreting cell differentiation
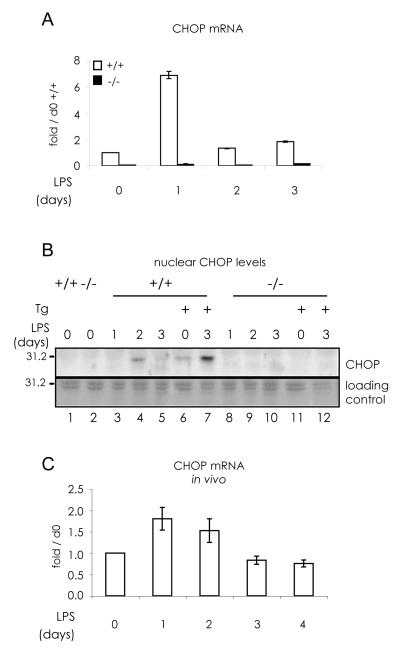
CHOP was transiently upregulated (days 1-2) in LPS-stimulated wild type B cells (Fig.2 A and B). As expected, no signal was obtained by real time PCR (panel A) or Western blots (B) when chop-/- splenocytes were utilized. Chop transcripts increased at day 1 of LPS stimulation and became barely detectable thereafter as differentiation towards antibody secreting cells (ASC) proceeded. In other experiments, transcription and synthesis peaked at day 2, but we never observed CHOP in the nucleus at day 3 or after (Fig.2 B). However, the ER stressor thapsigargin (Th) caused nuclear accumulation in LPS-stimulated B splenocytes, indicating that CHOP silencing is not irreversible.
Fig. 2. Transient expression of CHOP during B lymphocyte differentiation.
A) CD19+ B splenocytes from wt and chop-/- mice were cultured in vitro with 20 μg/ml LPS for the indicated time points. chop mRNA expression pattern during differentiation was followed by relative real time PCR as in Fig.1 C.
B) Nuclear accumulation of CHOP protein in B splenocytes, differentiated as in panel A, was followed by Western blot analyses. When indicated, cells were treated with 2 μg/ml thapsigargin (Th) for 3 hrs. Ponceau staining of a region of the nitrocellulose filter is shown as loading control.
C) CHOP is transiently induced also in in vivo differentiating B cells. c57/BL6 mice were injected intra-peritoneally with LPS. After LPS injection, 3 mice were sacrificed at each time point and differentiating B cells were purified and mixed to extract total RNA. CHOP transcript was detected as in panel A.
Since CHOP is induced by various types of stress, and isolation and in vitro culture expose cells to higher pO2 than that found in physiological conditions, we determined if similar CHOP expression patterns could be observed in in vivo differentiation assays. To this aim, CD38+ cells were isolated at the indicated time points from the spleen of wild type mice challenged with an intra-peritoneal injection of LPS (Fig. 2 C). With this in vivo stimulation protocol, about one third of splenocytes become CD138+ ASC at day 3 (Cascio et al., 2008). Real time PCR analyses confirmed that chop mRNA is up-regulated at early time points also during in vivo differentiation.
3.3 Induction of UPR elements in the absence of CHOP
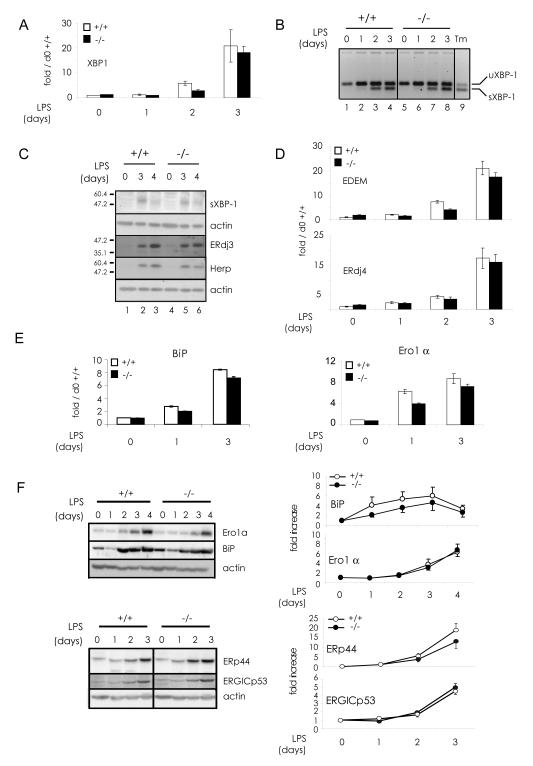
Confirming our previous results (Cenci et al., 2006; van Anken et al., 2003), numerous components of the UPR increased during ASC differentiation (Fig. 3). Altogether, the absence of CHOP had minor effects on the process. The transcription and splicing of xbp1 mRNA and the appearance of sXBP1 protein showed the same dynamics in wild type and chop-/- cells (Fig.3 A, B and C). Consistently, the mRNA levels of its targets edem, ERdj4 (Kanemoto et al., 2005; Lee et al., 2003; Yoshida et al., 2003) and ERdj3 (Shen and Hendershot, 2007) were similar (Fig.3 C and D). At closer inspection, however, the induction of some UPR elements was slightly less marked in chop-/- ASC. This was the case for BiP, both at the mRNA and protein level (Fig.3 E and F), and for ERp44 (Fig.3 F). Together with CHOP, HERP is a UPR target dually regulated by the ATF6-XBP1 and PERK/ATF4 pathways, via ERSEs and a C/EBP-ATF composite element, respectively (Ma and Hendershot, 2004). HERP is an ER localized protein involved in ER associated degradation (ERAD) and protects against ER stress-induced apoptosis (Liang et al., 2006; Okuda-Shimizu and Hendershot, 2007). We found HERP induction in wild type as well as in chop-/- ASC at the latest days of differentiation (Fig.3 C). Since previous studies showed that ero1α expression is regulated by CHOP in mouse embryonic fibroblasts (Marciniak et al., 2004), we analyzed the expression patterns of this key oxidase (Fig.3 E and F). Ero1α transcripts were slightly less abundant at days 1 and 3 of LPS stimulation in chop-/- cells, whilst Ero1α protein levels were similar.
Fig. 3. UPR signaling in chop-/- antibody secreting cells.
B splenocytes from wt and chop-/- mice were incubated in the presence of LPS as described in Fig.2 A before RNA and protein extracts were obtained to study the expression pattern of various UPR components.
A) Total XBP1 transcripts were quantified by relative real-time PCR as in Fig.1 C.
B) XBP1 activation was assessed by gel electrophoresis of RT-PCR products obtained with primers flanking the XBP1 26b intron that allow to discriminate the unspliced (uXBP1) and the spliced (sXBP1) forms of XBP1 mRNA. In lane 9, I.29μ+ cells were treated with tunicamycin (Tm) as XBP1 activation positive control.
C) Protein levels of sXBP1, ERdj3 and HERP were analyzed by western blotting with the corresponding antibodies. Actin was used as loading control.
D) EDEM and ERdj4 transcripts were quantified as in panel A.
E) BiP and Ero1α transcripts were quantified as in panels A and D.
F) Ero1α, BiP, ERp44 and ERGICp53 protein expression was analyzed as in panel C. Densitometric analyses are shown in the right panels. Data are reported as fold increase relative to the protein amounts measured at day 0, normalized to actin (average of 4 experiments ± SEM).
3.4 CHOP expression is important for efficient IgM polymerization and secretion
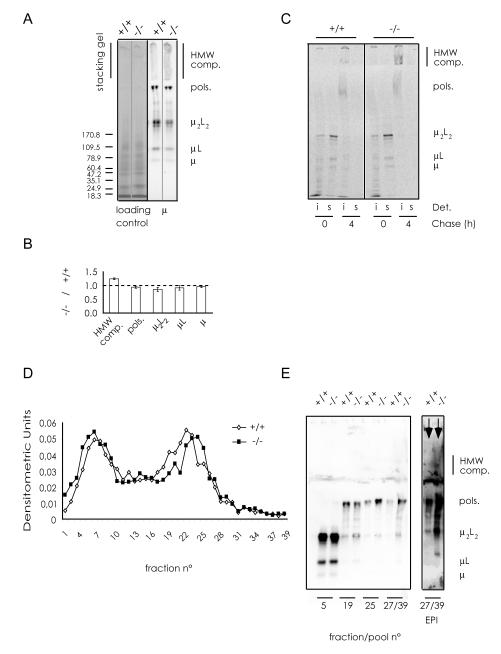
The most important feature of plasma cells is their ability to efficiently produce, assemble and secrete high amounts of Ig. As shown above, the increase in the synthesis of IgM subunits was similar in wild type and chop-/- ASC (Fig.1 C and D). However, a subtle phenotype became evident when we compared IgM assembly by non-reducing western blotting. As shown in Fig.4, chop-/- ASC accumulated more high molecular weight (HMW) complexes than wild type cells upon LPS stimulation (panel A), at the expenses of μ2L2 and μL assemblies (panel B). These HMW species contained J, L and μ chains and consisted of disulphide-bonded aggregates, as they were no longer detectable after heating samples in DTT (data not shown). Pulse chase assays (Fig.4 C, S1 and S2) were performed to confirm the presence of HMW complexes and analyze the kinetics of formation. Clearly, HMW complexes were more abundant in the detergent insoluble fraction of chop-/- ASC than in controls (Fig.4 C and S1B). At day 3 after LPS stimulation, they peaked after 1 hour of pulse and slowly decreased thereafter (Fig. S1B) despite similar amounts of μ2L2 were synthesized during the pulse (Fig. S1A). At day 4, intracellular polymers (hexamers and pentamers) were easily detectable in both the detergent soluble and insoluble fractions (Fig. S2). Detergent insoluble HMW complexes were slightly more abundant in chop-/- cells (lanes 9-10); polymers and HMW complexes persisted for longer times (Fig. S2, compare lanes 5 and 10), suggesting less efficient intracellular transport and secretion. To further characterize this phenotype, lysates from wt and chop-/- ASC were fractionated on continuous sucrose gradients and the distribution of IgM in individual fractions investigated by dot blot assays (Fig.4 D). Relevant fractions were then analyzed electrophoretically under non-reducing conditions (Fig.4 E). In chop-/- ASC, IgM migrated deeper in the gradient. Also polymers and lower assemblies were more abundant in fractions 27 and higher, suggesting the presence of non-covalent complexes.
Fig. 4. CHOP promotes optimal assembly of IgM.
A) IgM polymerization was analyzed by non-reducing western blots. Total protein extracts from wt and chop-/- B cells after 4 days of LPS stimulation were subjected to SDS-PAGE in non-reducing conditions and blotted. The nitrocellulose filter was incubated with anti-μ antibodies and the main IgM assembly intermediates were identified. Immuno-decoration with anti-κ or anti-J antibodies (not shown) confirmed the identification of the assembly intermediates.
B) Quantification of the relative amount of each IgM species in chop-/- ASC with respect to the wt. The percentage of IgM species (HMW complexes, polymers, μ2L2, μL, μ) relative to the sum of all the species was calculated after densitometric analyses of non-reducing western blots as the one shown in A. The histogram reports the ratios between each IgM species in chop-/- and wt samples as determined in four independent experiments (n=4; average ± SEM).
C) Wt and chop-/- B splenocytes, stimulated in vitro with LPS (day 3), were pulsed for 10 min with 35S-labeled aminoacids and chased for 0 or 4 hours. NP40 soluble (s) and insoluble (i) fractions were immunoprecipitated with anti-μ and resolved by SDS-PAGE under non-reducing conditions. More HMW complexes are found in the insoluble fraction of chop-/- cells (compare lanes 7 and 3).
D) Lysates from wt and chop-/- B splenocytes, stimulated in vitro with LPS for 4 days, were centrifuged on continuous sucrose gradients. 39 fractions were collected and aliquots analyzed by dot-blot assays with anti-μ antibodies.
E) Individual or pooled fractions of the gradient shown in D, were resolved by SDS-PAGE under non-reducing conditions and blots immuno-decorated with anti-μ to highlight the main assembly intermediates. An enhancement of the two lanes corresponding to fractions 27-39 is shown on the right (Enhanced Pixel Intensity, EPI) to better appreciate the presence of HMW complex. Note that in chop-/- cells HMW complexes (and also some polymers and μ2L2) are more abundant in fractions 27-39 than in control splenocytes.
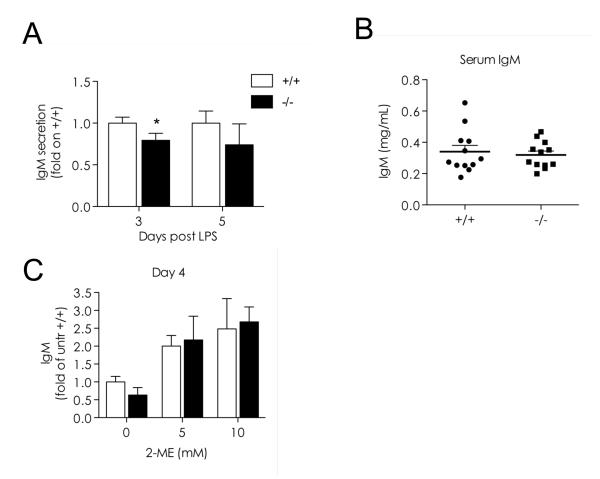
To determine whether the accumulation of HMW complexes correlated with impaired secretion, we quantified extracellular IgM in wild type and chop-/- LPS cultures. At all time points analyzed (day 3, 4 or 5 after stimulation), chop-/- cells secreted fewer IgM (Fig.5 A and C). Serum IgM levels were also slightly lower in chop-/- animals (Fig. 5 B), even though these mice displayed no overt immunological phenotype.
Fig. 5. CHOP is important for efficient IgM secretion.
A) IgM released into the medium by wt and CHOP cells stimulated with LPS for the indicated times was quantified by ELISA (106 cells/ml for 4 hrs). The data indicate the concentration found in the spent medium of chop-/- cells relative to the corresponding wt cells. The data for day 3 and 5 represent the average of two independent experiments analyzed in duplicate (n=4; average ± SEM, * indicates a p value < 0.05 (Student’s T test)).
B) Serum IgM levels were determined by ELISA. The results from 12 mice per group are shown in the scatter plot together with mean ± SEM. C) Effects of the reducing agent 2-mercaptoethanol (2-ME) on IgM secretion 4 days after LPS stimulation in the presence or absence of the indicated concentrations (mM) of 2-ME. IgM concentration in the media (106 cells/ml for 4 hrs) was measured by dot-blot assay, and expressed as relative changes assuming the secretion of untreated wt cells as one (average of 2 independent experiments ± SEM).
Next, we reasoned that if aberrant disulfide bonds were responsible for intracellular accumulation, secretion could be induced by adding the reducing agent 2-ME (Alberini et al., 1990). Clearly, IgM secretion was induced to a higher level in chop-/- than in wt ASC: the differences in secretion efficiency observed in the absence of the reducing agent were drastically attenuated upon 2ME treatment (Fig.5 C), a finding that confirms the post-translational nature of the phenotype observed.
3.5 CHOP is dispensable for spontaneous and stress-induced ASC apoptosis
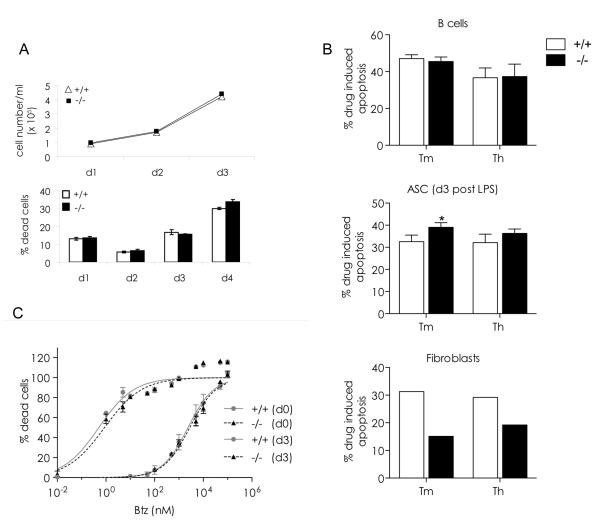
The patterns of proliferation and death were similar in wild type and chop-/- LPS cultures (Fig.6 A and additional results not shown) indicating that CHOP plays no essential role in ASC apoptosis (Cenci et al., 2006). Although it is well accepted that during the UPR CHOP mainly plays a pro-apoptotic role (Marciniak et al., 2004; Oyadomari et al., 2002; Oyadomari and Mori, 2004), CHOP was previously suggested to have an anti-apoptotic role in oligodendrocytes (Southwood et al., 2002) and murine myeloma cells (Cudna and Dickson, 2006). We thus wondered whether CHOP deletion affected B cell sensitivity to ER stress- or PI-induced death. When challenged with tunicamycin (Tm) or thapsigargin (Th), chop-/- ASC were slightly more sensitive to these agents than wild type cells. As expected, the reverse was true for fibroblasts (panel B), suggesting that CHOP may exert a tissue-specific protective role in differentiating B cells.
Fig. 6. Chop is dispensable for ASC apoptosis.
Primary splenic B cells from wt and chop-/- mice were stimulated in vitro with LPS and assayed for apoptotic sensitivity to proteasome inhibitors (PI) and ER stress.
A) Similar proliferation and apoptosis of LPS-induced wild-type and chop-/- B cells. B cells were stimulated with LPS for the indicated days, and cell numbers counted (top panel). The percentage of dead cells was assessed by flow cytometry upon staining of the cells with propidium iodide (n=3; average ± SEM) (bottom panel).
B) Hypersensitivity to ER stressors in chop-/- plasma cells. Resting (top) and LPS-stimulated B cells (middle panel) were treated for 16 hrs with tunicamycin (Tm, 0.5 μg/ml) or thapsigargin (Th, 1 μg/ml) and apoptosis assessed by flow cytometry. Graphs express the percentage of propidium iodide positive cells after subtracting the basal levels of cell death in untreated cells (average±SEM: 43.3±3.8 and 43.5±3.6 for wt and chop -/- unstimulated B cells and 25.4±2.0 and 28.4±2.0 for wt and chop -/- LPS stimulated B cells, respectively) (Bars represent the average of 2 independent experiments ± SEM, at least 2 mice per experiment; * indicates a p value < 0.05 (Student’s T test)). Bottom panel: Primary fibroblasts were treated for 48 hrs with Tm (2 μg/ml) or Th (2.5 μg/ml) and apoptosis assessed as above. One representative experiment is shown.
C) Apoptotic sensitivity to PI in resting and LPS-stimulated wt and chop-/- B cells. Primary B cells, unstimulated and stimulated 3 days with LPS, were treated for 16 hrs with the indicated doses of bortezomib (Btz) and apoptosis assessed as described in B. Data were normalized and fitted by non-linear regression with a normalized dose-response curve (R2>0.9) and the curves are shown. 95% confidence interval of EC50 are: d0 +/+ : 1.7-3.2 μM, d0 −/− : 2.1-3.6 μM; d3 +/+ : 0.2-1.2 nM, d3 −/− : 0.4 to 1.9 nM (average of 2 or more independent experiments ± SEM at least 2 mice per experiment).
Normal and malignant ASC are exquisitely sensitive to PI (Bianchi et al., 2009; Cascio et al., 2008; Cenci et al., 2006; Neubert et al., 2008), but the mechanisms that cause their death remain unclear. The observation that chop levels increase upon PI administration suggested an involvement of CHOP in PI-induced apoptosis (Meister et al., 2007; Neubert et al., 2008). Confirming our previous results (Bianchi et al., 2009; Cenci et al., 2006; Cascio et al., 2008), LPS-activated ASC were more sensitive to Bortezomib (Btz) than resting B cells. However, no differences were detected between wild type and chop-/- mice, indicating that CHOP is dispensable for spontaneous or Btz-induced cell death.
4. Discussion
Originally described as a tripartite response to ER stress, the UPR is emerging as a collection of integrated pathways, which are differentially required in various tissues. For instance, Ire1 and XBP1 (Iwakoshi et al., 2003; Reimold et al., 2001), but not PERK (Gass et al., 2008; Zhang et al., 2005), are required for terminal B cell differentiation. Our data demonstrate that despite its transient induction following LPS stimulation of B cells, CHOP is dispensable for differentiation and life span control. Nonetheless, a subtle phenotype became evident when IgM polymerization and secretion were analyzed, chop-/- cells being slightly less efficient than their normal counterparts. Therefore, despite the fact that its expression is transient and limited to the first days of differentiation, CHOP seems to contribute to the full development of IgM folding and quality control machineries.
How is the transient appearance of CHOP controlled? Let us first consider its activation: CHOP induction in ER stress is severely attenuated in perk-/- and in eIF2αS51A knock-in MEFs (Harding et al., 2000a; Scheuner et al., 2001; Wu et al., 2007). ATF4 over-expression cannot rescue its expression in perk-/- cells, implying that signal(s) other than ATF4 are required downstream eIF2α phosphorylation (Harding et al., 2000a). Consistent with its PERK-independent expression, ASC lacking this sensor do not show significant impairment in IgM secretion (Gass et al., 2008). Furthermore, the kinetics of CHOP induction upon LPS stimulation differ from those of other UPR target genes exemplified by HERP, an element thought to be similarly regulated (Ma and Hendershot, 2004). LUMAN/CREB3 (Liang et al., 2006) and/or other unidentified factor(s), could mediate the differential regulation of CHOP and HERP during ASC differentiation.
Several kinase(s) can phosphorylate eIF2α (e.g. GCN2 or PKR) independently from PERK, perhaps as a result of the profound metabolic and structural rearrangements that ensue in B cells following antigen or mitogen activation (van Anken et al., 2003). The stress imposed by preparing to massive Ig synthesis include peroxide generation ((Reth, 2002), Bertolotti M. et al. submitted), increased energy production, protein synthesis and amino-acid import (Cenci and Sitia, 2007), and virtually all these events can induce CHOP. Whatever its activation mechanism, the expression of CHOP is dramatically attenuated after a few days of differentiation in both primary and lymphoma B cells (Cenci et al., 2006). Its disappearance is not due to a dominant inhibitory effect: pharmacologic ER stressors efficiently induce its expression, implying the existence of subtle regulatory mechanisms.
In most cell types analyzed so far, and particularly in pancreatic β cells, CHOP expression correlates with the transition from an adaptive to a maladaptive UPR. Indeed, its absence retards the onset of overt diabetes (Oyadomari et al., 2002) and protects MEFs from excessive ER stress (Marciniak et al., 2004; Zinszner et al., 1998). It was hence surprising to observe that ASC lacking CHOP were equally or slightly more sensitive to Tm or Th. This effect was tissue specific, because chop-/- fibroblasts obtained from the same animals died less when exposed to Tm or Th, and chop-/- oligodendrocytes were more resistant to proteotoxic P0 species (Pennuto et al., 2008). A recent study supports an anti-apoptotic role of CHOP in the B cell lineage, murine myeloma cells with undetectable levels of CHOP expression exhibiting increased sensitivity to Tm (Cudna and Dickson, 2006). Tissue specific CHOP effects were reported also during the differentiation of adipocytes, osteoblasts and keratinocytes (Li et al., 2006; Maytin and Habener, 1998; Pereira et al., 2006; Shirakawa et al., 2006; Tchkonia et al., 2007). Different expression of proteins known to form heterodimers with CHOP (e.g. C/EBP and ATF family members (Chen et al., 1996; Oyadomari and Mori, 2004)) and the interplay amongst these transcription factors could determine the final output of the response on different tissues or cell types.
Our data indicate that neither spontaneous ASC apoptosis nor their hypersensitivity to PI require CHOP. This observation is of interest in view of the fact that PI are used as a first-line treatment against myeloma. In principle therefore, CHOP inhibitors could be used to avoid adverse effects of the therapy without impacting on its efficacy against myeloma cells.
5 Conclusions
Our observations regarding a cell type-specific role for the transcription factor CHOP support the idea that there is no universal constrain specifying CHOP’s role as a death gene; the latter is more likely due to the cellular context in which other C/EBP proteins (which CHOP heterodimerizes with) are expressed. In other words, CHOP is simply one of the worker bees of the UPR regulating various downstream targets to promote fitness under selection pressure. The existent literature has emphasized the failure of this homeostatic principle under conditions of severe ER stress, whereas our study on B cells has uncovered a plausible example of the positive selection that promoted CHOP’s evolution and retention as a UPR target gene.
Supplementary Material
Acknowledgements
We thank Tiziana Anelli, Stefania Ceppi, Luca Rampoldi, Cristina Scielzo, Larry Wrabetz and all the members of our laboratories for providing helpful suggestions, discussions and essential reagents, Elena Pasqualetto and Luisa Massardi for technical help, Ana Fella and Raffaella Brambati for secretarial assistance. The financial support of Associazione Italiana per la Ricerca sul Cancro (AIRC), Fondazione Cariplo (NOBEL project), Ministero Istruzione Universitá e Ricerca (MIUR-PRIN) and Telethon – Italy (Grant no. GGP06155) is gratefully acknowledged.
Abbreviations
- ASC
antibody secreting cells
- ATF
activating transcription factor
- BCR
B cell receptor
- C/EBP
CCAAT-enhancer binding protein
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- ERSE
ER stress element
- Ig
immunoglobulin
- PC
plasma cell
- PI
proteasome inhibitor
- UPR
unfolded protein response
- Th
thapsigargin
- Tm
tunicamycin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberini CM, Bet P, Milstein C, Sitia R. Secretion of immunoglobulin M assembly intermediates in the presence of reducing agents. Nature. 1990;347:485–7. doi: 10.1038/347485a0. [DOI] [PubMed] [Google Scholar]
- Anelli T, Ceppi S, Bergamelli L, Cortini M, Masciarelli S, Valetti C, Sitia R. Sequential steps and checkpoints in the early exocytic compartment during secretory IgM biogenesis. Embo J. 2007;26:4177–88. doi: 10.1038/sj.emboj.7601844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averous J, Bruhat A, Jousse C, Carraro V, Thiel G, Fafournoux P. Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J Biol Chem. 2004;279:5288–97. doi: 10.1074/jbc.M311862200. [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Bianchi G, Oliva L, Cascio P, Pengo N, Fontana F, Cerruti F, Orsi A, Pasqualetto E, Mezghrani A, Calbi V, Palladini G, Giuliani N, Anderson KC, Sitia R, Cenci S. The proteasome load versus capacity balance determines apoptotic sensitivity of multiple myeloma cells to proteasome inhibition. Blood. 2009;113(13):3040–9. doi: 10.1182/blood-2008-08-172734. [DOI] [PubMed] [Google Scholar]
- Brewer JW, Hendershot LM. Building an antibody factory: a job for the unfolded protein response. Nat Immunol. 2005;6:23–9. doi: 10.1038/ni1149. [DOI] [PubMed] [Google Scholar]
- Bruhat A, Jousse C, Carraro V, Reimold AM, Ferrara M, Fafournoux P. Amino acids control mammalian gene transcription: activating transcription factor 2 is essential for the amino acid responsiveness of the CHOP promoter. Mol Cell Biol. 2000;20:7192–204. doi: 10.1128/mcb.20.19.7192-7204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio P, Oliva L, Cerruti F, Mariani E, Pasqualetto E, Cenci S, Sitia R. Dampening Ab responses using proteasome inhibitors following in vivo B cell activation. Eur J Immunol. 2008;38:658–67. doi: 10.1002/eji.200737743. [DOI] [PubMed] [Google Scholar]
- Cenci S, Mezghrani A, Cascio P, Bianchi G, Cerruti F, Fra A, Lelouard H, Masciarelli S, Mattioli L, Oliva L, Orsi A, Pasqualetto E, Pierre P, Ruffato E, Tagliavacca L, Sitia R. Progressively impaired proteasomal capacity during terminal plasma cell differentiation. Embo J. 2006;25:1104–13. doi: 10.1038/sj.emboj.7601009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci S, Sitia R. Managing and exploiting stress in the antibody factory. FEBS Lett. 2007;581:3652–7. doi: 10.1016/j.febslet.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Chen BP, Wolfgang CD, Hai T. Analysis of ATF3, a transcription factor induced by physiological stresses and modulated by gadd153/Chop10. Mol Cell Biol. 1996;16:1157–68. doi: 10.1128/mcb.16.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudna RE, Dickson AJ. Engineering responsiveness to cell culture stresses: growth arrest and DNA damage gene 153 (GADD153) and the unfolded protein response (UPR) in NS0 myeloma cells. Biotechnol Bioeng. 2006;94:514–21. doi: 10.1002/bit.20861. [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Bjorklund A, Cagliero E. Genotoxic agents increase expression of growth arrest and DNA damage--inducible genes gadd 153 and gadd 45 in rat pancreatic islets. Diabetes. 1993;42:738–45. doi: 10.2337/diab.42.5.738. [DOI] [PubMed] [Google Scholar]
- Fornace AJ, Jr., Alamo I, Jr., Hollander MC. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci U S A. 1988;85:8800–4. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JN, Gifford NM, Brewer JW. Activation of an unfolded protein response during differentiation of antibody-secreting B cells. J Biol Chem. 2002;277:49047–54. doi: 10.1074/jbc.M205011200. [DOI] [PubMed] [Google Scholar]
- Gass JN, Jiang HY, Wek RC, Brewer JW. The unfolded protein response of B-lymphocytes: PERK-independent development of antibody-secreting cells. Mol Immunol. 2008;45:1035–43. doi: 10.1016/j.molimm.2007.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton KZ, Xu Q, Holbrook NJ. Induction of the mammalian stress response gene GADD153 by oxidative stress: role of AP-1 element. Biochem J. 1996;314(Pt 2):547–54. doi: 10.1042/bj3140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000a;6:1099–108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–63. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000b;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–99. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot LM, Sitia R. Immunoglobulin assembly and secretion. In: Honjo T, Alt FW, Neuberger MS, editors. Molecular Biology of B Cells. Elsevier; London: 2004. pp. 261–273. [Google Scholar]
- Horibe T, Hoogenraad NJ. The chop gene contains an element for the positive regulation of the mitochondrial unfolded protein response. PLoS ONE. 2007;2:e835. doi: 10.1371/journal.pone.0000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Lane MD, Tang QQ. Effect of serum on the down-regulation of CHOP-10 during differentiation of 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 2005;338:1185–8. doi: 10.1016/j.bbrc.2005.10.057. [DOI] [PubMed] [Google Scholar]
- Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003;4:321–9. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- Kanemoto S, Kondo S, Ogata M, Murakami T, Urano F, Imaizumi K. XBP1 activates the transcription of its target genes via an ACGT core sequence under ER stress. Biochem Biophys Res Commun. 2005;331:1146–53. doi: 10.1016/j.bbrc.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–59. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002;16:452–66. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Huang HY, Chen JG, Jiang L, Liu HL, Liu DG, Song TJ, He Q, Ma CG, Ma D, Song HY, Tang QQ. Lactacystin inhibits 3T3-L1 adipocyte differentiation through induction of CHOP-10 expression. Biochem Biophys Res Commun. 2006;350:1–6. doi: 10.1016/j.bbrc.2006.08.188. [DOI] [PubMed] [Google Scholar]
- Liang G, Audas TE, Li Y, Cockram GP, Dean JD, Martyn AC, Kokame K, Lu R. Luman/CREB3 induces transcription of the endoplasmic reticulum (ER) stress response protein Herp through an ER stress response element. Mol Cell Biol. 2006;26:7999–8010. doi: 10.1128/MCB.01046-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–9. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luethy JD, Holbrook NJ. Activation of the gadd153 promoter by genotoxic agents: a rapid and specific response to DNA damage. Cancer Res. 1992;52:5–10. [PubMed] [Google Scholar]
- Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–65. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. Herp is dually regulated by both the endoplasmic reticulum stress-specific branch of the unfolded protein response and a branch that is shared with other cellular stress pathways. J Biol Chem. 2004;279:13792–9. doi: 10.1074/jbc.M313724200. [DOI] [PubMed] [Google Scholar]
- Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–77. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maytin EV, Habener JF. Transcription factors C/EBP alpha, C/EBP beta, and CHOP (Gadd153) expressed during the differentiation program of keratinocytes in vitro and in vivo. J Invest Dermatol. 1998;110:238–46. doi: 10.1046/j.1523-1747.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- Meister S, Schubert U, Neubert K, Herrmann K, Burger R, Gramatzki M, Hahn S, Schreiber S, Wilhelm S, Herrmann M, Jack HM, Voll RE. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67:1783–92. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K, Wiethe C, Winkler TH, Kalden JR, Manz RA, Voll RE. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14:748–55. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–22. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda-Shimizu Y, Hendershot LM. Characterization of an ERAD pathway for nonglycosylated BiP substrates, which require Herp. Mol Cell. 2007;28:544–54. doi: 10.1016/j.molcel.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–32. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–9. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Pennuto M, Tinelli E, Malaguti M, Del Carro U, D’Antonio M, Ron D, Quattrini A, Feltri ML, Wrabetz L. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron. 2008;57:393–405. doi: 10.1016/j.neuron.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira RC, Stadmeyer L, Marciniak SJ, Ron D, Canalis E. C/EBP homologous protein is necessary for normal osteoblastic function. J Cell Biochem. 2006;97:633–40. doi: 10.1002/jcb.20660. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–49. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, Grusby MJ, Alt F, Glimcher LH. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–7. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3:1129–34. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007;32:469–76. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–76. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. Divergent roles of IRE1alpha and PERK in the unfolded protein response. Curr Mol Med. 2006;6:5–36. doi: 10.2174/156652406775574569. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H, Yu X, Yang L, Tan BK, Rosenwald A, Hurt EM, Petroulakis E, Sonenberg N, Yewdell JW, Calame K, Glimcher LH, Staudt LM. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 2004;21:81–93. doi: 10.1016/j.immuni.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Shen Y, Hendershot LM. Identification of ERdj3 and OBF-1/BOB-1/OCA-B as direct targets of XBP-1 during plasma cell differentiation. J Immunol. 2007;179:2969–78. doi: 10.4049/jimmunol.179.5.2969. [DOI] [PubMed] [Google Scholar]
- Shirakawa K, Maeda S, Gotoh T, Hayashi M, Shinomiya K, Ehata S, Nishimura R, Mori M, Onozaki K, Hayashi H, Uematsu S, Akira S, Ogata E, Miyazono K, Imamura T. CCAAT/enhancer-binding protein homologous protein (CHOP) regulates osteoblast differentiation. Mol Cell Biol. 2006;26:6105–16. doi: 10.1128/MCB.02429-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohat M, Janossy G, Dourmashkin RR. Development of rough endoplasmic reticulum in mouse splenic lymphocytes stimulated by mitogens. Eur J Immunol. 1973;3:680–7. doi: 10.1002/eji.1830031106. [DOI] [PubMed] [Google Scholar]
- Sitia R, Neuberger M, Alberini C, Bet P, Fra A, Valetti C, Williams G, Milstein C. Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell. 1990;60:781–90. doi: 10.1016/0092-8674(90)90092-s. [DOI] [PubMed] [Google Scholar]
- Southwood CM, Garbern J, Jiang W, Gow A. The unfolded protein response modulates disease severity in Pelizaeus-Merzbacher disease. Neuron. 2002;36:585–96. doi: 10.1016/s0896-6273(02)01045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriburi R, Bommiasamy H, Buldak GL, Robbins GR, Frank M, Jackowski S, Brewer JW. Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis. J Biol Chem. 2007;282:7024–34. doi: 10.1074/jbc.M609490200. [DOI] [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–5. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JR, Nakamura M, Okura T, Takata Y, Watanabe S, Yang ZH, Liu J, Kitami Y, Hiwada K. Mechanism of oxidative stress-induced GADD153 gene expression in vascular smooth muscle cells. Biochem Biophys Res Commun. 2002;290:1255–9. doi: 10.1006/bbrc.2002.6336. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Lane MD. Role of C/EBP homologous protein (CHOP-10) in the programmed activation of CCAAT/enhancer-binding protein-beta during adipogenesis. Proc Natl Acad Sci U S A. 2000;97:12446–50. doi: 10.1073/pnas.220425597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Pirtskhalava T, Thomou T, Cartwright MJ, Wise B, Karagiannides I, Shpilman A, Lash TL, Becherer JD, Kirkland JL. Increased TNFalpha and CCAAT/enhancer-binding protein homologous protein with aging predispose preadipocytes to resist adipogenesis. Am J Physiol Endocrinol Metab. 2007;293:E1810–9. doi: 10.1152/ajpendo.00295.2007. [DOI] [PubMed] [Google Scholar]
- van Anken E, Romijn EP, Maggioni C, Mezghrani A, Sitia R, Braakman I, Heck AJR. Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity. 2003;18:243–253. doi: 10.1016/s1074-7613(03)00024-4. [DOI] [PubMed] [Google Scholar]
- van Huizen R, Martindale JL, Gorospe M, Holbrook NJ. P58IPK, a novel endoplasmic reticulum stress-inducible protein and potential negative regulator of eIF2alpha signaling. J Biol Chem. 2003;278:15558–64. doi: 10.1074/jbc.M212074200. [DOI] [PubMed] [Google Scholar]
- Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell Death Differ. 2006;13:374–84. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–64. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–76. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K. Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem. 2004;136:343–50. doi: 10.1093/jb/mvh122. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K. A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell. 2003;4:265–71. doi: 10.1016/s1534-5807(03)00022-4. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wong HN, Song B, Miller CN, Scheuner D, Kaufman RJ. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest. 2005;115:268–81. doi: 10.1172/JCI21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.