Abstract
The filamentous bacteriophage are highly immunogenic particles that can be used as carrier proteins for peptides and presumably other haptens and antigens. Our previous work demonstrated that the antibody response was better focused against a synthetic peptide if it was conjugated to phage as compared to the classical carrier, ovalbumin. We speculated that this was due, in part, to the relatively low surface complexity of the phage. Here, we further investigate the phage as an immunogenic carrier, and the effect reducing its surface complexity has on the antibody response against peptides that are either displayed as recombinant fusions to the phage coat or are chemically conjugated to it. Immunodominant regions of the minor coat protein, pIII, were removed from the phage surface by excising its N1 and N2 domains (Δ3 phage variant), whereas immunodominant epitopes of the major coat protein, pVIII, were altered by reducing the charge of its surface-exposed N-terminal residues (Δ 8 phage variant). Immunization of mice revealed that the Δ3 variant was less immunogenic than wild-type (WT) phage, whereas the Δ8 variant was more immunogenic. The immunogenicity of two different peptides was tested in the context of the WT and Δ3 phage in two different forms: (i) as recombinant peptides fused to pVIII, and (ii) as synthetic peptides conjugated to the phage surface. One peptide (MD10) in its recombinant form produced a stronger anti-peptide antibody response fused to the WT carrier compared to the Δ3 phage carrier, and did not elicit a detectable anti-peptide response in its synthetic form conjugated to either phage carrier. This trend was reversed for a different peptide (4E10L), which did not produce a detectable anti-peptide antibody response as a recombinant fusion; yet, as a chemical conjugate to Δ3 phage, but not WT phage, it elicited a highly focused anti-peptide antibody response that exceeded the anti-carrier response by ~ 65-fold. The results suggest that focusing of the antibody response against synthetic peptides can be improved by decreasing the antigenic complexity of the phage surface.
Keywords: filamentous phage, vaccine, epitope targeting, peptide
1. Introduction
There is a growing need for vaccines that focus antibody (Ab) responses against conserved, neutralizing epitopes on highly variable pathogens, such as HIV-1, hepatitis C virus, and influenza virus. However, such epitopes may be weakly immunogenic, with other epitopes dominating the Ab response [1–3]. Conjugate vaccines enhance immune responses through chemical conjugation of a targeted antigen (e.g., a microbial protein, peptide or polysaccharide) to an immunogenic carrier protein. The targeted antigens are typically poorly immunogenic by themselves, and thus are attached to carriers, which provide T-cell epitopes (TCEs) and other stimulatory factors that enhance Ab responses and the development of immunological memory. However, carriers themselves are typically large and complex proteins that may generate unwanted Ab and T-cell cross-reactivities upon immunization [4]. Furthermore, it is not clear whether Ab responses elicited by the carrier affect or skew the Ab response against the targeted antigen. Several studies have shown that altering an immunodominant epitope (e.g., by point mutation or glycosylation) can redirect Ab responses to other epitopes on a peptide or protein [1, 5–8] and can affect neutralizing activity [9]. However, to our knowledge no studies have addressed the possibility of altering a carrier protein’s epitopes to improve the Ab response to an associated hapten, peptide or carbohydrate antigen.
Previously, we reported that the filamentous phage carrier, f1.K, better focused the Ab response against a synthetic peptide than the traditional carrier, ovalbumin (OVA), and hypothesized that this was in part due to the reduced surface complexity (i.e., restricted number of B-cell epitopes on the phage surface) of the phage particle compared to OVA [10], as most of the phage surface comprises the 12 N-terminal residues of the major coat protein, pVIII. Based on these results, we proposed that the phage surface could be engineered to reduce the number of surface-exposed immunodominant B-cell epitopes, and that such modifications would both enhance and focus the Ab response against a peptide (synthetic or recombinant) or other antigen, as compared to peptides presented in the context of wild-type (WT) phage carrier. We use these terms to mean that the anti-peptide Ab titers and the ratio of anti-peptide to anti-carrier Ab titers, respectively, are higher than those elicited by the WT phage carrier. Thus, our goal was to further engineer filamentous phage to serve as a vaccine carrier that would both enhance and focus the Ab response against weakly immunogenic regions on pathogens, including non-protein and polysaccharide antigens.
The filamentous phage has a number of characteristics that make it an excellent model system for studying Ab responses. It is highly immunogenic, eliciting high-titer serum Abs even at low doses and without adjuvant [10, 11]. While phage elicit T cell responses [12], its immunogenicity is also enhanced by virion-associated lipopolysaccharide (LPS) [13, 14] and the phage’s particulate nature. Unlike classical antigens (like OVA), small amounts of phage will produce a detectable "burst" of Ab-secreting cells in the blood by the third day after subcutaneous immunization without adjuvant (our unpublished results), making it well suited for studies of early stages in the cellular response to immunization. Despite its intensity, the Ab response against the phage is restricted to the twelve N-terminal residues of the major coat protein, pVIII [15], and to the N1 and N2 domains of the minor coat protein, pIII. Moreover, the phage is relatively easy to engineer and to use for chemical conjugation [10]. It has been used as an immunogenic carrier for producing Abs against many different peptides fused to the N-terminus of pVIII ([16, 17] and thoroughly reviewed in [11]), or conjugated to the phage coat [10], and we previously showed that it can focus the Ab response against a conjugated peptide, by increasing the ratio of the anti-peptide to anti-phage serum titer [10].
To reduce the immunogenicity of pIII, a single-stranded DNA (ssDNA) mutagenesis procedure was used to delete a segment encoding the N1 and N2 domains of pIII from the phage genome (Δ3 phage). To reduce the immunogenicity of pVIII, three residues (E2A, D5S and K8Q [15]) that are required for Ab binding to pVIII (i.e., critical binding residues; CBRs [18, 19]) were modified in both WT phage (Δ8 phage) and Δ3 phage (Δ8Δ3 phage). Thus, we tested as carriers: i) WT phage, ii) Δ3 phage bearing WT pVIII and truncated pIII, iii) Δ8 phage bearing modified pVIII and WT pIII, and iv) Δ8Δ3 phage, a double mutant bearing both modified pVIII and truncated pIII. Ab responses produced by mice immunized with the different phage variants revealed that Δ3 phage were significantly less immunogenic than WT phage. Unexpectedly, phage bearing the Δ8 modification produced stronger Ab responses than WT phage. Thus, the Δ3 phage was chosen for testing as a carrier for enhancing and/or focusing the Ab response against recombinantly displayed or synthetically conjugated peptides.
We examined the impact of removing the outer domains of pIII on the Ab response against two weakly immunogenic peptides (MD10 and 4E10LA/B; 4E10LA and 4E10LB are virtually identical peptides differing by a single amino acid residue) that were recombinantly displayed on the phage coat or chemically conjugated to it. The anti-peptide Ab response elicited by the recombinant MD10 peptide-pVIII fusion was stronger when displayed on WT carrier as compared to display on the Δ3 carrier, whereas anti-peptide responses were undetectable for either carrier bearing chemically-conjugated peptide. In contrast, the anti-peptide Ab response elicited by synthetic 4E10LB peptide was far stronger against peptide conjugated to Δ3 carrier, as compared to WT carrier; remarkably, the Ab response against this peptide dwarfed the response against the Δ3 carrier. No anti-peptide Ab response was observed for WT or Δ3 phage displaying 4E10LA peptide as a recombinant fusion. Taken together, our results show that removal of immunodominant domains of pIII decreased phage immunogenicity, and enhanced and focused the Ab response against one of the two chemically-conjugated synthetic peptides tested, but neither of the recombinantly displayed ones. We surmise this effect depends on a given peptide's copy number, its inherent immunogenicity, and the manner in which it is presented on the phage surface.
2. Materials and methods
2.1 Materials and animals
Five-week-old BALB/c mice were purchased from Charles River Laboratories Inc. (Saint-Constant, QC). The f8-5 phage (WT phage) is described in [20]. The gel-purified oligonucleotide primer for making the Δ3 phage (primer 1: 5’p TGCCATTTTTTCATAATCAAAATCACCGGAACCTTCAACAGTTTCAGCGGAGTGAGAATA GAAAGG 3’) was synthesized by the NAPS Unit at the University of British Columbia. Complementary oligonucleotides for producing Δ8 phage were produced by Invitrogen Corp. (Carlsbad, CA) with the following sequences: Δ8#1: 5’ pGCGGGTGACTCTCCCGCACAGGCGGCCTTTGACTCCCTGCAAG, and Δ8#2: 5’ CTAGCTTGCAGGGAGTCAAAGGCCGCCTGTGCGGGAGAGTCACCCGCTGCA; both were gel-purified by urea-PAGE [21]. The primers used to sequence gene3 were pIIIFwd: 5’ CAAGCTGTTTAAGAAATTCACCTCG and pIIIRev: 5’ GCCCTTTTTAAGGAAAGTAAGCAGA, and those used to sequence gene8 were pVIIIFwd: 5’ GGTTGGTGCCTTCGTAGTGGC and pVIIIRev: 5’ GCGAATAATAATTTTTTCACG. The MD10 peptide [22] was selected from a phage-displayed peptide library by monoclonal (M)Ab SYA/J6, which was elicited against an oligosaccharide from the LPS of Shigella flexneri [23]. The synthetic MD10 peptide that was used in ELISAs is biotinylated and bears the sequence NH3+-MDWNMHAAGG-ornithine(biotin)-K-CONH2 (MW: 1556.1, 95% pure, synthesized at Alberta Peptide Institute, University of Alberta, Edmonton); the synthetic MD10 peptide that was conjugated to phage bears the sequence NH3+-MDWNMHAAGGC-CONH2 (MW: 1190.2, 95% pure, synthesized by EZBiolab Inc., Westfield, IN). The 4E10LB synthetic peptide was selected from a phage-displayed random peptide library using MAb 4E10, a broadly neutralizing Ab against HIV-1; its affinity for the MAb was further optimized through selection from a sublibrary whose sequences were biased toward the consensus sequence obtained in the primary selection [24]. The 95% pure 4E10LB sequence, NH3+-AEPAENNWFMLTYFLAAEGC-CONH2 (MW: 2255.87) was synthesized by EZBiolab Inc. The crosslinking agent, N-succinimidyl-3-(2-pyridyldithio)propionate (SPDP), which contains a 6.8 Å cleavable spacer arm, was from Pierce Chemical Co. (Rockford, IL); the 2-pyridyldithio group reacts optimally with sulfhydryls (at the C-terminus of the peptide) and the N-hydroxysuccinimide (NHS) ester reacts with primary amines on the N-terminus of the phage coat and Lys residues. Recombinant streptavidin from Streptomyces avidinii was produced in Escherichia coli by Roche Diagnostics (Indianapolis, IN).
2.2 Engineering Δ3 phage: removing the pIII N1 and N2 domains from f8-5
To remove regions of the f8-5 genome encoding the N1 and N2 domains of pIII, ssDNA mutagenesis was performed using a single primer that anneals at two sites, encoding both the pIII signal peptide and the CT domain, and thus “loops out” a 744-base pair (bp) segment of DNA. This loop is bypassed during synthesis of the second DNA strand, resulting in deletion of its DNA (Figure 1 A and B). To prepare ssDNA for cloning, f8-5 virions were propagated at a large scale using standard methods [25], and ssDNA was purified from polyethylene glycol (PEG)/NaCl-precipitated virions by phenol-chloroform extraction followed by ethanol precipitation [25]. DNA quality and size were confirmed by 0.8% agarose gel electrophoresis of viral DNA from SDS-lysed phage in 4× GBB buffer [26]. To anneal Primer 1 to the ssDNA template, 13 pmol Primer 1 and 700 fmol f8-5 ssDNA were mixed in 100 µl annealing buffer (10 mM Tris-HCl pH 7.5, 500 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA)), so that the final ratio of primer to template was 18:1. The mixture was incubated at 72°C for 10 minutes, then slowly cooled to 30°C and placed on ice. The primed template (100 µl) was added to an extension reaction containing dNTPs (26 µM each), 50 units (u) T4 ligase (Invitrogen) and 16.25 u Sequenase Version 2.0 DNA polymerase (USB Corp., Cleveland, OH) in a total volume of 150 µl T4 DNA ligase buffer (Invitrogen). The extension reaction was incubated on ice for 5 minutes, at 25°C for 5 minutes, then at 37°C for one hour. The reaction was then brought to a volume of 2.2 ml with water and concentrated using an Ultra-15 10K NMWCO Amicon® centrifugal filter device (Millipore Corp., Bedford, MA), followed by one wash with 4 ml 0.1× Tris-EDTA buffer (10 mM Tris-HCl pH 7.5, 1 mM EDTA), and one wash with 4 ml water. The retentate was dried in a Speed Vac, dissolved in water, then split between two aliquots of electrocompetent E. coli MC1061 cells and electroporated in 0.1 cm cuvettes using a Bio-Rad gene pulser [27].
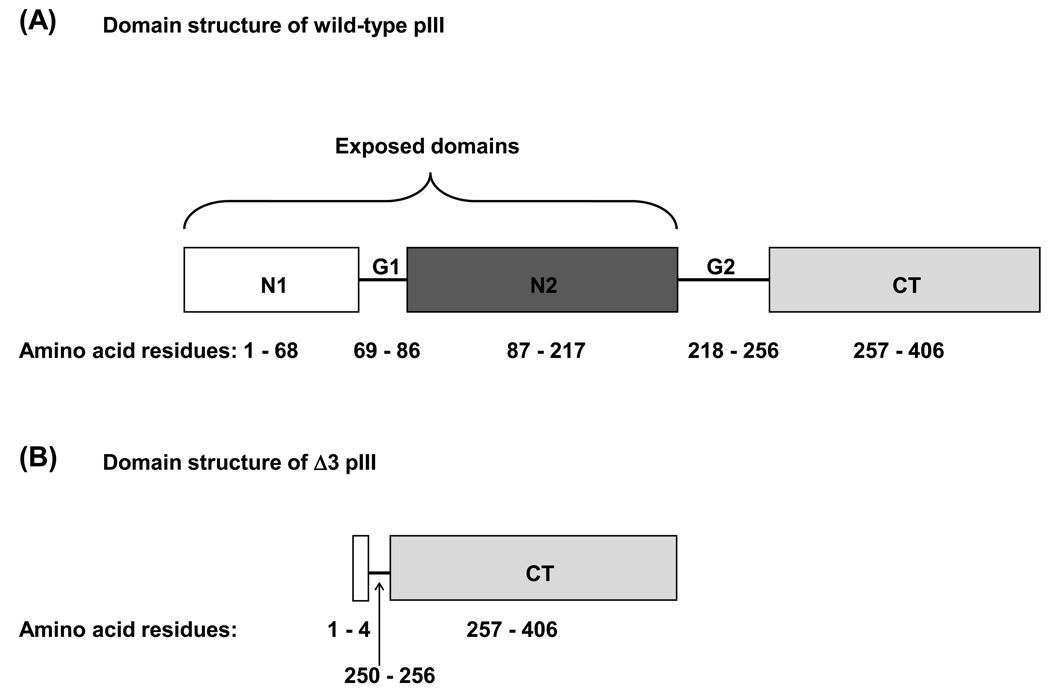
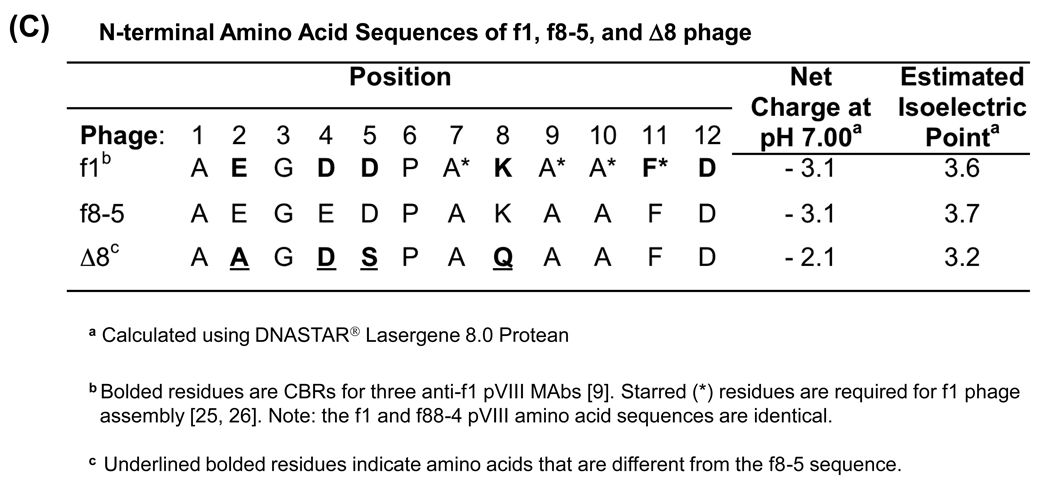
Figure 1.
Pictorial depiction of wild-type and engineered filamentous phage coat proteins. (A) Domain structure of WT pIII. The bracket indicates the surface-exposed N1 and N2 domains of pIII that were removed, leaving (B) the Δ3 variant comprising four N-terminal residues, part of the second glycine-rich linker (G2) and the C-terminal (CT) domain to allow phage assembly into particles containing a single ssDNA genome. (C) N-terminal amino acid sequence of mature pVIII from f1 phage, f8-5 phage, and the modified Δ8 phage variant showing alterations to CBRs.
After induction of the tetracycline (tet) resistance gene in NZY/tet (0.2 µg/ml) for 1 hour at 37°C, E. coli MC1061 cells transformed with the mutagenesis reaction were grown in a 400-ml overnight (O/N) NZY/tet (10 µg/ml) culture at 37°C, and replicative form (RF) DNA was purified from bacterial pellets using a Qiagen® plasmid midi kit using the manufacturer’s protocol for low copy-number plasmids. To select for the desired RF, 10 µg of total RF DNA was digested with Bme1580I and BseRI (New England Biolabs, Ipswich, MA), whose restriction sites are present in gene3 of WT phage but not in the Δ3 variant). The digested RF DNA was purified by phenol-chloroform extraction followed by ethanol precipitation, and 1.6 µg was used to transform electrocompetent E. coli MC1061 cells. The transformed cells were propagated as above and RF DNA was prepared for a second round of negative selection. After selection, an undigested RF DNA band, corresponding to the expected genome size of the desired clone, was gel-purified and used to transform electrocompetent E. coli MC1061 cells. Transformed cells were induced and plated on NZY agar containing 40 µg/ml tet, and 100 single colonies were picked, grown O/N at 37°C in 2 ml NZY/tet (10 µg/ml) starter cultures. Culture supernatants were screened for phage production by agarose gel electrophoresis of viral DNA as described. Of the 11 clones that produced phage, 5 bore the desired sequence (Figure 1 B).
2.3 Altering immunodominant residues on pVIII on f8-5 and Δ3 phage
To alter CBRs on pVIII that are known to be essential for binding by MAbs raised against whole phage (Δ8 variant, Figure 1 C), f8-5 and Δ3 RF were digested with PstI and NheI (New England Biolabs) to excise the DNA encoding the N-terminal region of pVIII, followed by gel purification to remove the digested fragment. Oligonucleotides Δ3#1, and Δ3#2, which encode complementary sites, were annealed in equimolar amounts as described above. The resulting fragment, bearing overhangs for PstI and NheI restriction sites, was ligated to digested f8-5 or Δ3 RF using T4 DNA ligase (New England Biolabs). The ligation mixtures were used to transform Subcloning Efficiency™ DH5α™ Competent Cells (Invitrogen) according to the manufacturer’s instructions. After induction in NZY/tet (0.2 µg/ml), transformed cells were selected on NZY agar containing 40 µg/ml tet and single clones were screened by digestion with BamHI (New England Biolabs); this site is absent in clones bearing the changes to gene8. Clones were confirmed by DNA sequencing.
2.4 Large-scale preparation of f8-5, Δ3, Δ8, and Δ8Δ3 phage
Large-scale phage stocks were prepared using standard methods [25], with the exception that heat-shock transformation of phage RF DNA was used in place of infection; Δ3 phage are rendered non-infectious by the removal of the N1 and N2 domains of pIII. Fifty-µl aliquots of CaCl2-competent E. coli MC1061 cells were transformed by heat shock [28] with 500 ng phage RF. Tet-resistant transformed cells were induced and plated on NZY/tet agar plates and single colonies were grown in 2-ml starter cultures as described. Large-scale cultures (500 ml - 1 L NZY/tet (10 µg/ml)) were inoculated with 500 µl - 1 ml of starter culture and grown at 37 °C with shaking (250 rpm) for ~20 hours, and virions were purified from the culture medium by PEG/NaCl precipitation [25], followed by CsCl density gradient centrifugation [29]. Phage concentrations were determined by absorbance measurement as described in Smith and Scott [29] and confirmed by agarose gel electrophoresis of viral DNA as described.
2.5 Western blot analysis
The coat proteins from 2×1010 (f8-5) or 2.3×1010 (Δ3) CsCl-purified phage particles (comprising 640 ng pVIII) were separated by SDS-PAGE [30], then transferred onto PVDF Immobilon-PSQ transfer membranes (Millipore) using a Trans-Blot SD Semi-Dry Transfer Cell (Bio-Rad Laboratories, Richmond, CA) [31]. Membranes were blocked O/N at 4°C with 5% (w/v) skim milk (Bio-Rad Laboratories, Hercules, CA) in Tris-buffered saline (TBS; 50 mM Tris-HCl, pH 7.5, 150 mM NaCl), then incubated with pooled mouse-anti-phage immune sera in TBS containing 2.5% (w/v) dried skim milk and 0.05% (v/v) Tween 20 (Sigma, St. Louis, MO) for 2 hours at room temperature (RT). Serum dilutions were normalized according to their average anti-phage IgG titers: 1/3,000 for anti-f8-5, 1/1,000 for anti-Δ3, 1/8,000 for anti-Δ8, and 1/14,000 for anti-Δ8Δ3. Membranes were washed with TBS containing 5% dried skim milk for 20 minutes, then four times in TBS containing 0.1% Tween 20. Mouse Abs were detected with goat-anti-mouse IgG (Fc)-horseradish peroxidase (HRP) conjugate (Pierce) at a dilution of 1/1500. After washing with TBS containing 0.1% Tween 20, the bound HRP conjugate was detected with an ECL western blot detection reagent kit (Amersham International PLC, Buckinghamshire, United Kingdom), and exposed to Safelight Medical X-ray film (Fuji Photo Film Co. Ltd., Tokyo, Japan) for visualization.
2.6 Preparation of hybrid phage bearing recombinant MD10 or 4E10LA peptide
Hybrid phage bearing recombinant MD10 or 4E10LA peptide were prepared by co-transforming E. coli MC1061 cells with phage RF DNA and an ampicillin (amp)-resistant plasmid (pBRXN/MD10/pVIII or pBRXN/4E10LA/pVIII) [32] bearing the recombinant gene8 cassette from f88-4 expressing either the MD10 peptide-pVIII or the 4E10LA peptide-pVIII fusion protein. CaCl2-competent E. coli MC1061 cells were transformed by heat shock [28] with one of the above plasmids, and selected on NZY agar plates containing 100 µg/ml amp O/N at 37°C. A single amp-resistant colony was grown O/N at 37°C in 2 ml NZY/amp (100 µg/ml) and made CaCl2-competent [28]. These cells were then transformed by heat shock with WT, Δ3, or Δ8Δ3 phage RF DNA, and selected on NZY agar containing 40 µg/ml tet and 100 µg/ml amp, allowing selection of clones bearing both phage RF DNA and either of pBRXN/MD10/pVIII or pBRXN/4E10LA/pVIII. Single colonies were selected and used to prepare large-scale cultures of virions using standard methods [25], with the exception that 10 µg/ml tet and 100 µg/ml amp were used in culture, along with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) to induce the expression of the MD10/pVIII or 4E10LA/pVIII fusion proteins. The resulting virions were purified from the culture medium by PEG/NaCl precipitation followed by CsCl density-gradient centrifugation. The presence of the MD10 or 4E10LA peptides on hybrid virions was confirmed by ELISA with MAbs SYA/J6 or 4E10 (see below); similar amounts of peptide were observed on the f8-5 and Δ3 phage (f8-5/MD10rec vs. Δ3/MD10rec and f8-5/4E10LArec vs. Δ3/4E10LArec, respectively).
2.7 Conjugation of MD10 or 4E10LB peptide to f8-5 and Δ3 phage
The synthetic MD10 and 4E10LB peptides were conjugated to f8-5 and Δ3 phage using the crosslinking agent SPDP. Phage particles were activated with the crosslinking agent in a reaction containing 250 µg phage protein (7.8 × 1012 particles for f8-5, and 8.8 × 1012 particles for Δ3) and 1 mM final concentration of SPDP, in a total volume of 1.4 ml phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 12 mM Na2HPO4, 1.2 mM KH2PO4, pH 7.4) (166 µg/ml phage protein). The reaction was incubated for 1.5 hours with rotation at RT, and quenched by the addition of 166 µl 3M Tris-HCl, pH 8.3 (2 mM final concentration) with incubation for 15 minutes at RT. To remove excess crosslinker from the activated phage, the volume was brought up to 5 ml with PBS, and virions were purified by PEG/NaCl precipitation. Phage were resuspended in 1.4 ml PBS containing 1 mM EDTA, and 160 µg MD10 or 4E10LB peptide was added, bringing the final concentrations of phage and peptide to 166 µg/ml of phage protein, and 106 µg/ ml of peptide (0.03 mM and 0.5 mM, respectively). These amounts produced a ratio of ~16 peptide molecules for each pVIII molecule. After incubation with the activated virions for two days with rotation at 4°C, unbound peptide was removed by three rounds of PEG/NaCl precipitation. Phage treated with SPDP crosslinker and blocked with cysteine (SPDP-Cys) were also prepared as described above except that 80 µl 100 mM L-cysteine (final concentration 5 mM) (Sigma) was added instead of peptide. Conjugates were stored at 4°C in PBS.
2.8 Immunization schedule
As summarized in Supplemental Table S1, groups of 6–8 week old BALB/c mice (n=5) were immunized five times via subcutaneous (SC) injection at two sites between the shoulder blades with 100 µl PBS containing 10 µg phage protein (3.1 × 1011 virions for f8-5, Δ8, f8-5/MD10rec, f8-5/MD10conj, f8-5/4E10LArec, f8-5/4E10LBconj and f8-5/SPDP-Cys or 3.4 × 1011 virions for Δ3, Δ8Δ3, Δ3/MD10rec, Δ3/MD10conj, Δ3/4E10LArec, Δ3/4E10LBconj and Δ3/SPDP-Cys). Calculations of phage protein are based on the amount of pVIII per virion, which is determined by the genome size (1 pVIII molecule for every ~2.3 – 2.4 nucleotides in the phage genome [33, 34]; mature pVIII is 50 residues long). Immunizations were performed at weeks 0, 2, 4, 6, and 8, and blood was collected via the saphenous vein at weeks 0, 2, 4, 6, and 8; final bleeds and euthanasia were performed on week 10 by cardiac puncture under CO2 anaesthesia. All protocols involving mice were approved by the SFU Animal Care Committee in compliance with the Canadian Council on Animal Care. Blood was allowed to clot at RT for 24 hours, and sera were collected by centrifugation and stored at −20°C. Identical conditions were used in immunizations with the MD10, 4E10LB and SPDP-Cys phage conjugates with the exception that 2 µg phage protein was used. Lower amounts of phage were used to equalize the amount of peptide on the different immunogens, since phage conjugates typically carry at least five times more peptide than phage bearing recombinantly displayed peptide [10].
2.9 ELISA and determination of serum titers
ELISAs were performed using titrated sera as follows: 1 µg streptavidin in 35 µl or 200 ng 4E10LB peptide in 35 µl TBS was adsorbed to wells of Costar High-binding Easy Wash microtiter/96-well plates (Corning Inc., Corning, NY) O/N at 4°C. The following day, wells were aspirated and plates were washed once with 200 µl/well TBS, then 1010 biotinylated f8-5, Δ3, Δ8, or Δ8Δ3 phage particles, or 200 ng biotinylated-MD10 peptide were added in 35 µl TBS to wells containing streptavidin and allowed to bind for ≥30 minutes. Each well was blocked with 200 µl TBS containing 2% (w/v) bovine serum albumin (BSA; Sigma) for 1 hour at 37°C, and then washed three times with TBS containing 0.1% Tween 20. Sera were diluted in TBS containing 0.1% Tween 20 and 1% BSA, starting at a 1/100 dilution, and titrated in 1/3 dilutions. The titrated sera, in a volume of 35 µl, were added to each well and incubated for 2 hours at RT. The wells were then washed six times with TBS containing 0.1% Tween 20 and bound Ab was detected by a 45-minute incubation at RT with goat anti-mouse IgG (Fc):HRP conjugate (Pierce) diluted 1/1500 in TBS containing 0.1% Tween 20 and 1% BSA. HRP was detected with 35 µl 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS; Sigma) solution containing H2O2 [25]. The OD405–490 of each well was measured using a SpectraMax ELISA plate reader (Molecular Devices, Sunnyvale, CA). The data from each titration, after subtraction of background signals from sera plated on BSA-coated wells, were analyzed to give the dilution factor (the inverse of the titer) corresponding to the half-maximal OD405–490 reading (approximately 0.55 OD405–490 for the majority of serum samples). Serum samples that showed weak binding, but did reach the half-maximal OD405–490, were assigned a titer of 50. The arithmetic mean and standard error of the mean are reported for each treatment group.
2.10 Statistical analysis
Where appropriate, data were analyzed by ANOVA [35] using MS Excel to determine the variance within and between groups. Differences between groups were considered significant if the ANOVA p-value was below 0.05. Standard error of the mean was calculated using MS Excel.
3. Results
3.1 Removal of immunodominant epitopes from phage coat proteins, pIII and pVIII
The minor coat protein pIII comprises three structural domains: the exposed N1 and N2 domains (shown in Figure 1 A), which elicit a significant proportion of the Ab response to filamentous phage, and the C-terminal (CT) domain, which is required for phage assembly. To remove the N1 and N2 domains of pIII, an oligonucleotide was synthesized that is complementary to two sites in the f8-5 genome. This oligonucleotide anneals to viral DNA at a 5’ site (encoding the signal peptide and N-terminus of mature pIII) and a 3’ site (encoding glycine linker #2 adjacent to the N-terminal portion of the CT domain) of gene3; as it does not anneal to the intervening sequence, it essentially “loops out” the DNA encoding the N1 and N2 domains. Thus, after synthesis of the second strand of viral DNA, a small fraction of the resulting RF contains the deletion. WT RF encoding the N1 and N2 regions was cleaved by restriction enzymes Bme1580I and BseRI (whose sites are present in the “looped out” DNA sequence). Thus, after the initial synthesis and ligation, the RF was amplified in E. coli MC1061 cells, the WT fraction encoding N1 and N2 was linearized and the resulting product was used to transform E. coli cells. After two rounds of this negative selection, 5 out of 100 single phage clones produced by the RF bore the desired mutation. As shown in Figure 1 B, the Δ3 clone expresses the CT domain of pIII fused to the four N-terminal residues of the N1 domain, which were preserved in order to maintain the signal peptide cleavage site.
In addition to removing the exposed domains of pIII, immunodominant CBRs near the N-terminus of pVIII were modified to reduce Ab binding. Knessel et al. (1991) defined the epitopes for three murine MAbs against the pVIII of f1 phage (Δ8 in Figure 1 C) to CBRs Glu2, Asp4, Asp5, Pro6, Lys8, Phe11 and Asp12 [15]. One of these residues, Phe11, is also critical for phage assembly [36, 37], and thus was not altered. Four pVIII variants were designed in order of increasing complexity. The first contained three amino acid changes (AAGDSPAQAAFD, Figure 1 C) whereas the second contained changes to five residues (AAGSSPAQAAFN) [15]. Two additional pVIII variants bearing more drastic changes were also designed: one composed mostly of Gly and Ala residues (AAGGGPAAAAFN), and one (ADPAQAFN) in which four residues were deleted (E2, G3, D4 and A9). All changes were successfully introduced into both the f8-5 and Δ3 genomes, but only the first variant (AAGDSPAQAAFD) produced virions at practical titers. Since we wanted to examine the combined influence of pVIII and pIII changes on immunogenicity, the variant with three amino-acid replacements (AAGDSPAQAAFD) was chosen for future study, and named Δ8.
3.2 Phage bearing the Δ3 and Δ8 modifications have altered immunogenicity and expose a unique sets of epitopes
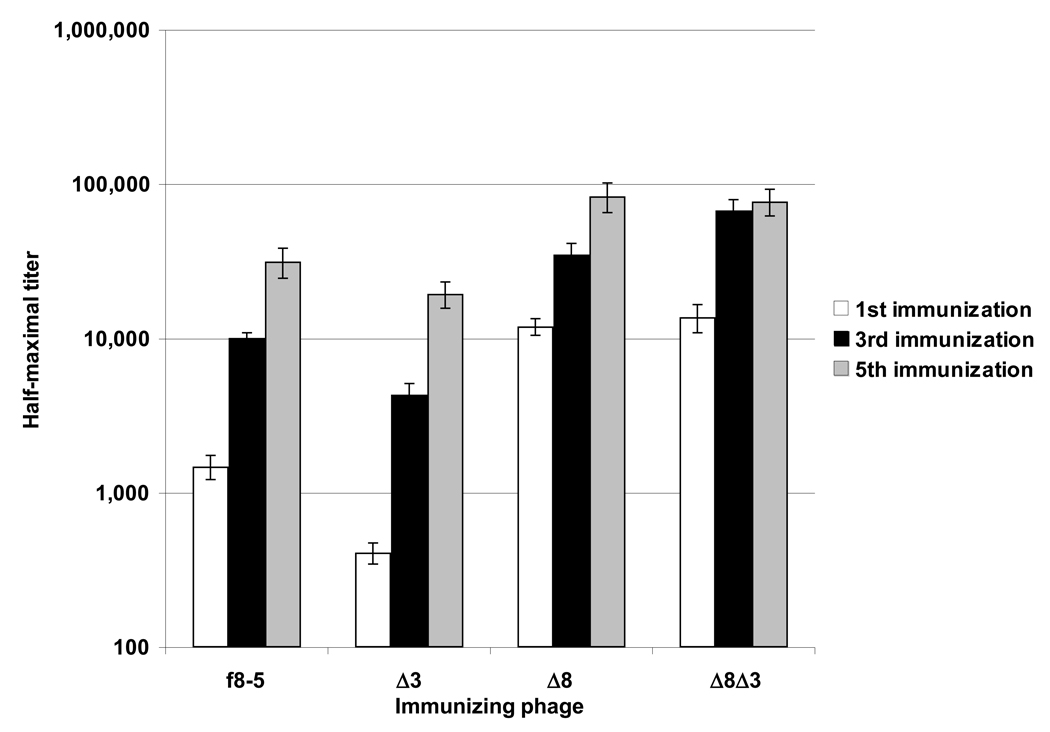
The relative immunogenicities of the f8-5, Δ3, Δ8, and Δ8Δ3 phage were tested in BALB/c mice; average half-maximal Ab titers are shown in Figure 2. Mice were immunized five times with 10 µg phage protein, and half-maximal Ab titers were determined against the immunizing phage by ELISA after the first, third, and fifth immunizations. The anti-phage titers elicited by the Δ3 phage were significantly lower than those elicted by WT phage, starting from the first immunization in which average anti-Δ3 titers were 3.5-fold lower than anti-f8-5 ones. This trend was not as pronounced after the third and fifth immunizations, after which anti-f8-5 titers exceeded anti-Δ3 ones by 2.3-fold and 1.6-fold, respectively. Thus, it appears that removal of immunodominant domains of pIII reduced the immunogenicity of the virion. Unexpectedly, the Δ8 modification (which was designed to minimize immunogenic residues) significantly enhanced the immunogenicity of the phage, and this enhancement was not dependent on the presence or absence of the pIII N1 and N2 domains (Figure 2). After the first immunization, anti-Δ8 and Δ8Δ3 Ab responses exceeded those of WT phage by ~9 fold, although after the fifth immunization this difference was reduced to ~2.6-fold, indicating that phage carrying the Δ8 modification are significantly more immunogenic than WT phage.
Figure 2.
Average half-maximal anti-f8-5, Δ3, Δ8, or Δ8Δ3 Ab titers after one, three, and five immunizations. Error bars show standard error of the arithmetic mean. ANOVA was used to compare the significance between immunization groups at the first, third and fifth immunizations (p-values for 1st, 2nd, 3rd immunizations: < 1×10−5). Note that the Y-axis is log scaled.
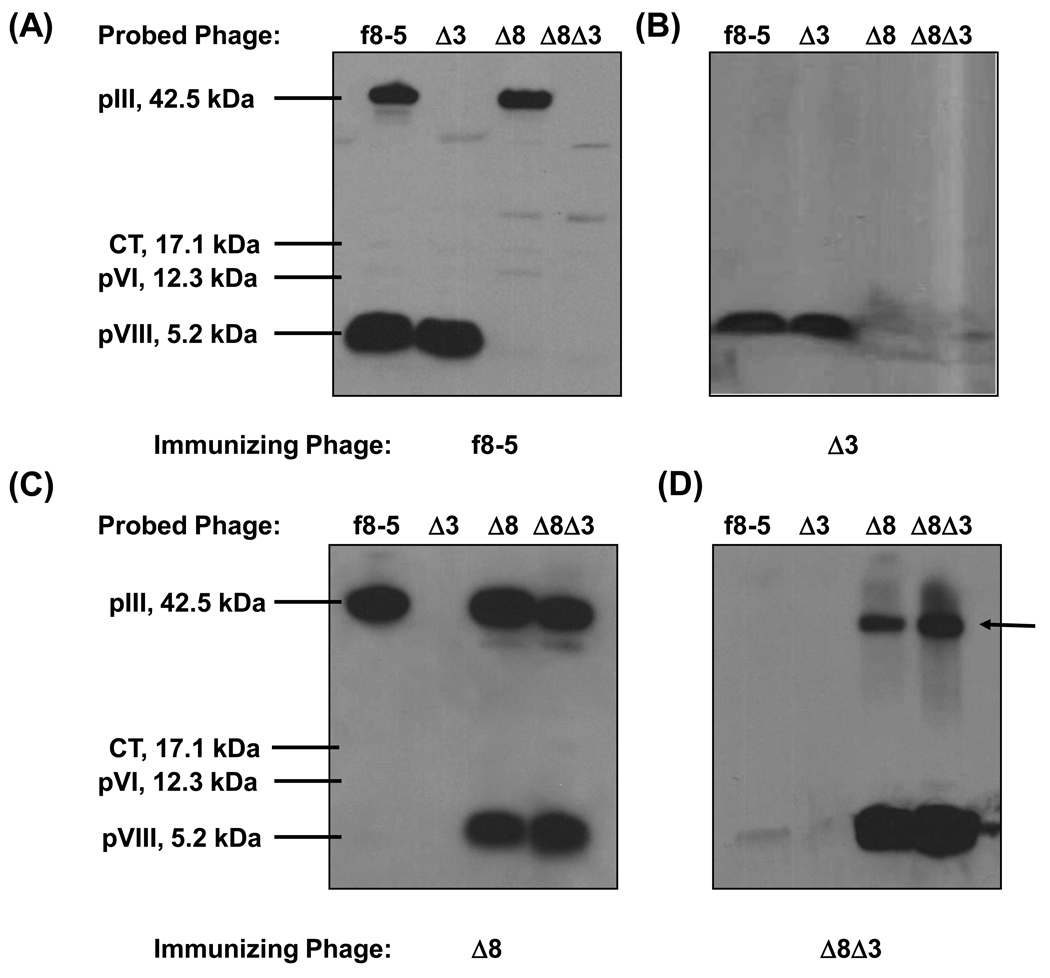
To further characterize the Ab response against the modified virions, the distribution of Ab reactivities to both WT and engineered phage coat proteins was explored by western blot using as probe antisera produced against f8-5, Δ3, Δ8, and Δ8Δ3 phage (Figure 3). Sera were diluted based on their average IgG titers against WT phage to ensure comparability. As shown in Figure 3 A, antisera against f8-5 reacted with WT pVIII and pIII but not significantly with Δ8 pVIII; this trend was also observed in the western blot probed with anti-Δ3 sera (compare Figure 3 A and B). As expected, there was no detectable binding to pIII by the anti-Δ3 sera, confirming that the CT domain of Δ3 pIII is not exposed on the assembled virion. As shown in Figure 3 C and D, immunization with Δ8 and Δ8Δ3 phage produced Abs that bound strongly to Δ8 pVIII, but not to WT pVIII. Taken together, these results indicate that altering CBRs on pVIII produced phage that were more immunogenic than WT phage, whereas deletion of the N1 and N2 domains of pIII reduced the immunogenicity of the phage. In addition, WT pVIII and Δ8 pVIII bear qualitatively distinct epitopes, since in western blots Abs produced against one do not cross-react significantly with the other. This effect was less pronounced for phage binding in ELISAs, in which a greater degree of Ab cross-reactivity was observed between phage variants (Supplemental Figure S1); however, it may be that these Abs were directed against LPS associated with the phage coat, which would have been present on all intact phage variants, but would not have been detected in western blots (see below and Supplemental Figure S2).
Figure 3.
Immunization with modified phage variants redirects the Ab response to different sets of epitopes. Pooled sera from groups of 5 mice, immunized three times with WT f8-5 phage (A), or phage variants Δ3 (B), Δ8 (C), or Δ8Δ3 (D) were used to probe equivalent amounts of all four phage variants in western blots. The phage variants being probed are named along sample lanes at the top of each blot, and the immunizing phage are named on the bottom. The arrow indicates an unusual band produced by immunization with Δ8 and Δ8Δ3 phage.
Interestingly, the Δ8 modification appeared to elicit Abs against novel epitopes or proteins on virions bearing Δ8 pVIII. As shown in Figure 3 D (see arrow), anti-Δ8 and anti-Δ8Δ3 sera bound a protein with the same apparent mobility as WT pIII. This band was not pIII because: (i) the anti-Δ8Δ3 sera were elicited against phage lacking the N1 and N2 domains of pIII, (ii) both anti-Δ8 and anti-Δ8Δ3 sera produced this band in the Δ8Δ3 phage lanes, and (iii) both anti-Δ8 and anti-Δ8Δ3 sera do not bind to WT pIII on f8-5 phage (Figure 3 C and D). Thus, Ab reactivity against a ~42 kDa protein, or protein aggregate, is elicited by immunization with the Δ8 phage,
3.3 The Δ3 phage does not elicit focused Ab responses against recombinantly displayed or chemically conjugated MD10 peptide
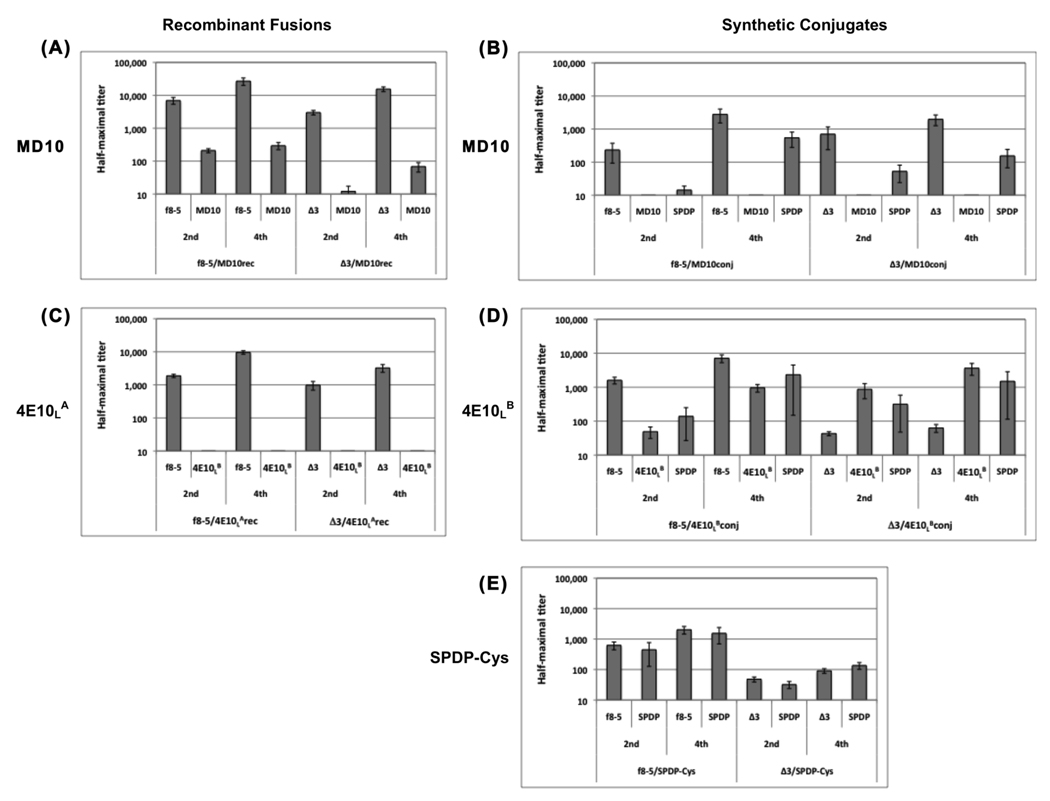
To determine the Ab-enhancing and/or -focusing ability (e.g., any observed increase in the ratio of anti-peptide to anti-carrier Ab responses) of the modified phage, we displayed the weakly immunogenic peptide, MD10, on the surface of f8-5, Δ3 and Δ8Δ3 phage by expressing it as an N-terminal fusion to pVIII or by chemically conjugating it to the phage surface (a complete list of phage immunogens is found in Supplemental Table S1). The MD10 peptide was selected from a phage-displayed random peptide library by SYA/J6, a MAb specific for the LPS of Shigella flexneri [22]. The relative copy number of the MD10 peptide on the three phage carriers was assessed by ELISA, in which SYA/J6 was titrated on immobilized phage bearing recombinant or conjugated peptide. SYA/J6 showed relatively similar binding to recombinant f8-5 and Δ3 phage bearing the MD10 peptide (f8-5/MD10rec and Δ3/MD10rec, respectively; Supplemental Figure S3 A), indicating similar copy numbers. However, the recombinant MD10 peptide on the Δ8Δ3 carrier showed weaker binding indicating lower peptide copy number (data not shown); thus, it was not further studied as a carrier. In addition, SYA/J6 showed relatively similar titration profiles on MD10 peptide chemically conjugated to f8-5 and to Δ3 phage (f8-5/MD10conj and Δ3/MD10conj, respectively; Supplemental Figure S3 B). ELISAs for the recombinant and conjugate phage were not directly comparable as they were not performed side-by-side, but the similarity of their titration profiles (as compared to the MD10 peptide-streptavidin complex positive control) suggests that their peptide copy numbers were roughly the same. A further indication of peptide incorporation into the phage conjugates was revealed by SDS-PAGE analysis comparing the MD10-phage conjugates to phage conjugates containing no peptide (i.e., SPDP-Cys); this showed a unique band that ran just behind the pVIII band for the peptide-containing conjugates but not those containing SPDP-Cys (Supplemental Figure S4 A). Mice were immunized with 10 µg recombinant phage protein or 2 µg conjugate phage protein at two-week intervals, and an ELISA was used to measure half-maximal Ab titers against synthetic MD10 peptide, f8-5 phage, Δ3 phage, and the SPDP crosslinker conjugated to BSA and blocked with cysteine; the average titer for each group of 5 mice is shown in Figure 4 A and B.
Figure 4.
Average half-maximal Ab titers against phage (f8-5 or Δ3), peptide and SPDP crosslinker produced by mice immunized two and four times with phage bearing recombinant or chemically-conjugated MD10 or 4E10L peptides. Immune sera from: (A) phage displaying recombinant MD10 peptide; (B) MD10 synthetic peptide conjugated to phage via the SPDP crosslinker; (C) phage displaying recombinant 4E10LA peptide; (D) 4E10LB synthetic peptide conjugated to phage via the SPDP crosslinker; (E) SPDP crosslinker conjugated to phage and blocked with cysteine. Error bars show standard error of the arithmetic mean for each immunization group. Note that the Y-axis is log scaled.
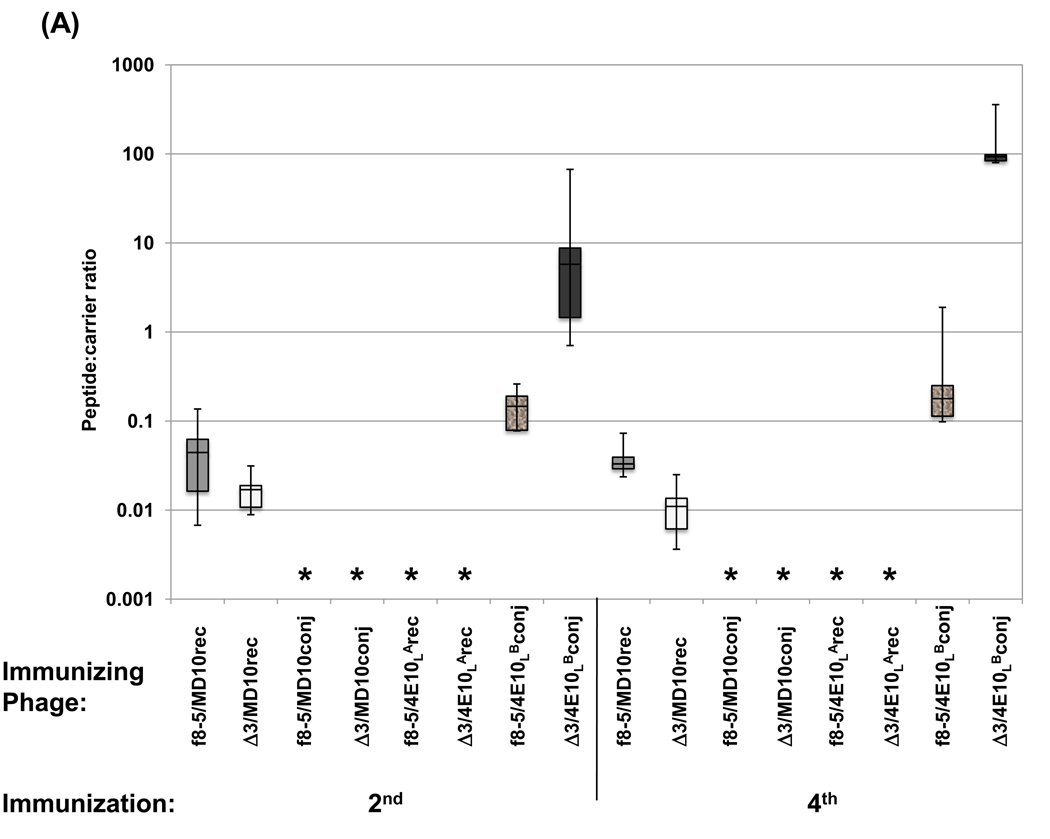
Two and four immunizations with Δ3 phage consistently elicited anti-phage Ab titers that were significantly lower than those elicited by WT phage, though they were within the same order of magnitude (Figure 4 A). However, the Δ3 modification did not enhance anti-MD10 peptide Ab responses. After four immunizations with Δ3/MD10rec phage, anti-MD10 Ab titers were barely detectable, reaching only 70, whereas f8-5/MD10rec phage produced significant anti-peptide Ab titers that reached 300. The ratios of peptide:carrier Ab responses from individual mice are shown in Figure 5 A, and reveal that the Δ3 modification did not enhance or focus the Ab response against the MD10 peptide compared to WT phage. The anti-peptide Ab response produced with the Δ3 phage carrier was approximately 100-fold lower than the anti-phage Ab response, whereas the anti-peptide Ab response produced by the WT carrier was only 10-fold lower than the anti-phage response. Taken together, these data show that the Δ3 modification reduced the anti-phage Ab response, but did not focus the Ab response against this weakly immunogenic recombinant peptide.
Figure 5.
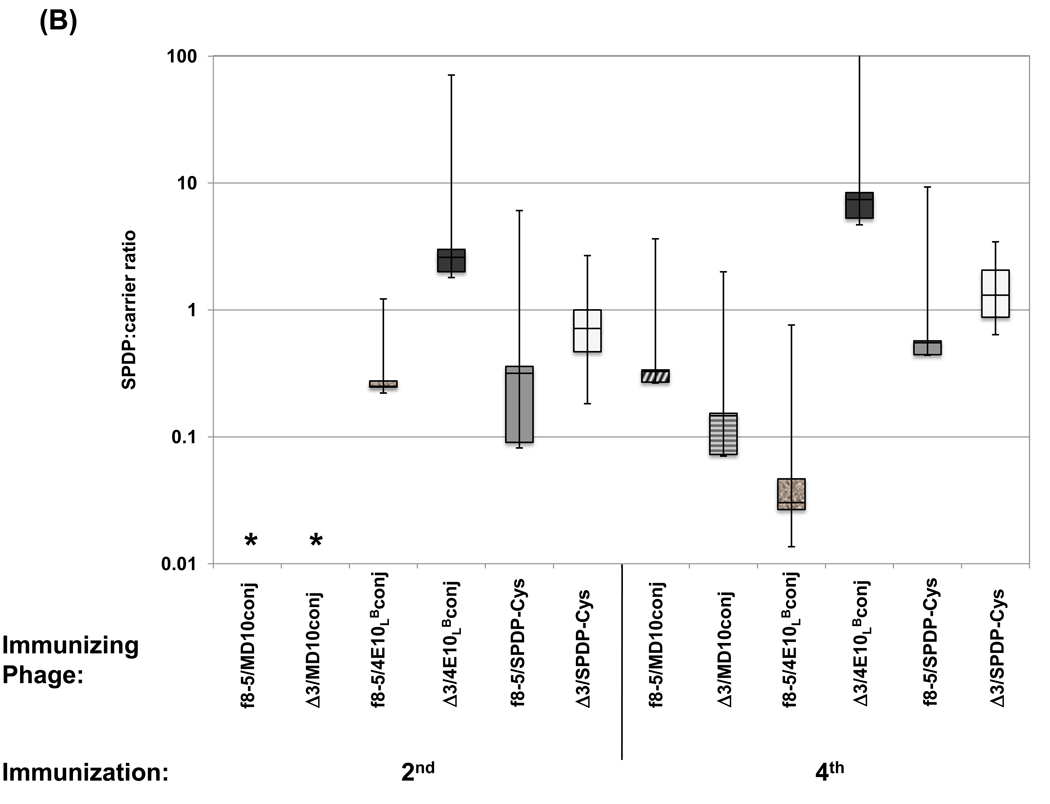
Boxplot showing (A) ratios of anti-peptide to anti-phage half-maximal Ab titers calculated for individual mice or (B) ratios of anti-SPDP crosslinker to anti-phage half-maximal Ab titers calculated for individual mice immunized two and four times with phage bearing recombinant or chemically-conjugated MD10 or 4E10L peptides. Asterisks denote instances where titer ratios could not be determined due to undetectable anti-peptide, anti-SPDP or anti-phage Ab responses. Note that the Y-axis is log scaled.
We also examined the effect of the Δ3 modification on the Ab response to synthetic MD10 peptide chemically conjugated to phage, as peptide-phage conjugates previously produced focused Ab responses against a synthetic peptide [10]. The anti-f8-5 and anti-Δ3 Ab titers were roughly similar after the second and fourth immunizations; both were significantly lower than the anti-phage Ab titers observed using phage carriers displaying a recombinant peptide (compare Figure 4 A and B), probably due to coating of the phage particles with the SPDP crosslinker. After four immunizations, no significant anti-MD10 peptide titers were observed for either the f8-5 or Δ3 phage carrier. Thus, the Ab response against the recombinant MD10 peptide was significantly greater than that against its synthetic counterpart, and once again, the Δ3 carrier did not focus the Ab response against the MD10 peptide as compared to WT carrier.
3.4 Δ3 phage focus the Ab response against the chemically conjugated synthetic 4E10LB peptide, but not against recombinant 4E10LA peptide
The extent to which minimization of carrier epitopes focuses Ab responses against a peptide may depend upon the relative immunogenicity of the peptide being tested. Thus, we repeated the above experiments using another peptide, 4E10LA, which was selected using the broadly neutralizing anti-HIV-1 MAb, 4E10. The 4E10LA peptide (sequence: NH3+-AEPAEANWFMLTYFLAAEGC-CONH2; residue 6, underlined, is Ala) proved impossible to synthesize, and thus the less hydrophobic 4E10LB peptide (sequence: NH3+-AEPAENNWFMLTYFLAAEGC-CONH2; residue 6 is Asn), which binds MAb 4E10 as well or better than 4E10LA, was used for conjugation and in ELISA. Our unpublished immunization studies in rabbits indicated that conjugates containing synthetic 4E10LB peptide were reliably immunogenic, but that 4E10LA peptide displayed as a recombinant fusion was only weakly immunogenic. As with the case for the MD10 peptide, the 4E10 peptides were either displayed on the surface of f8-5 and Δ3 phage (f8-5/4E10LArec and Δ3/4E10LArec, respectively), or attached to the phage surface by chemical conjugation (f8-5/4E10LBconj and Δ3/4E10LBconj, respectively). MAb 4E10 showed relatively similar binding in ELISA to recombinant 4E10LA on both f8-5 and Δ3 phage carriers (Supplemental Figure S3 C). Comparison of f8-5 and Δ3 phage carriers bearing chemically conjugated synthetic 4E10LB peptide showed that the two carriers bore similar copy numbers of conjugated peptide (Supplemental Figure S3 D). Once again, SDS-PAGE analysis of phage conjugates revealed the aforementioned unique band behind pVIII only with the peptide conjugates but not the SPDP-Cys conjugate (Supplemental Figure S4 B).
Recombinant Δ3 phage displaying 4E10LA produced anti-phage Ab titers that were lower than WT phage but within the same order of magnitude, similar to the results with Δ3 and WT phage displaying the MD10 peptide (compare Figure 4 A and C). Once again, the Δ3 modification did not enhance anti-peptide Ab responses. After four immunizations, the anti-4E10LA Ab responses remained undetectable, regardless of the phage carrier type.
Phage conjugates comprising the 4E10LB synthetic peptide behaved quite differently from the MD10 peptide conjugates. As shown in Figure 4 D, anti-phage Ab titers produced with Δ3/4E10LBconj were lower than those produced with f8-5/4E10LBconj, and were not significantly boosted after the fourth immunization, whereas the anti-phage titers were significantly boosted by f8-5/4E10LBconj, by both 4E10LA recombinant phage (Figure 4 C) and by all of the MD10 recombinant phage (Figure 4 A) and conjugates (Figure 4 B). In contrast to the anti-phage responses, the anti-peptide Ab responses produced by both 4E10LB conjugates were boosted between the second and fourth immunizations. Moreover, the Δ3/4E10LB conjugate compared to the f8-5/4E10LBconj, both enhanced the Ab response against the 4E10LB peptide (Figure 4 D), and focused it, as reflected by the peptide:carrier ratios from individual mice (Figure 5 A). Taken together, the results suggest that removal of immunodominant domains on pIII allowed the Ab response to be boosted against the synthetic 4E10LB peptide, but not the phage carrier, and in so doing, focused the Ab response against the peptide. This was not the case for the nearly identical 4E10LA peptide displayed as a recombinant fusion, or for the MD10 peptide in any form.
3.5 The SPDP crosslinker elicits an anti-SPDP Ab response
In addition to measuring relative anti-peptide and anti-phage Ab responses, we also measured the Ab response to the SPDP crosslinking agent blocked with cysteine. SPDP is reported to be weakly immunogenic [38], and immunization studies conducted with similar conjugates in rabbits did not elicit a significant anti-SPDP Ab response (data not shown). Here, we observed significant reactivity against SPDP-treated BSA by sera from mice immunized with phage-peptide conjugates (Figure 4 B and D) or with phage conjugated to Cys (f8-5/SPDP-Cys and Δ3/SPDP-Cys, respectively; Figure 4E); no binding was detected against untreated BSA (not shown). The anti-SPDP titers tended to follow the anti-phage titers, and the ratio of reactivity to SPDP and to carrier was below 2.0; this was true for all of the conjugates (Figure 5 B), with the notable exception of the Δ3/4E10LB conjugate, whose anti-SPDP titer appeared to follow the anti-peptide titer, producing average SPDP-Cys:carrier ratios of 15 and 23 after the second and fourth immunizations, respectively (Figure 5 B). Thus, it appears that the Δ3 phage enhanced and focused the Ab response against both the 4E10LB peptide and the SPDP crosslinker.
4. Discussion
We report here for the first time the engineering of a vaccine carrier based on the hypothesis that removing or altering immunodominant epitopes on an immunogen can redirect the Ab response against other epitopes, including haptens, on its surface. Novel filamentous phage carriers were created by removing the N1 and N2 domains of pIII (Δ3 modification), and by altering charged CBRs on pVIII so as to reduce Ab binding (Δ8 modification). Phage bearing individual modifications to pIII or pVIII (Δ3 or Δ8), or phage bearing both modifications (Δ8Δ3) were tested for immunogenicity and for their ability to enhance Ab titers against recombinant or chemically-conjugated peptides, and to focus them in relation to the Ab titers against the phage carrier.
When phage bearing the Δ3, Δ8 and Δ8Δ3 modifications were compared to WT phage as immunogens, it was observed that removal of the N1 and N2 domains of pIII produced phage that were significantly less immunogenic than WT phage (Figure 2), perhaps due to loss of TCEs. In contrast, the Δ8 modification enhanced immunogenicity, in that both the anti-Δ8 and anti-Δ8Δ3 titers were significantly higher than the titer against WT phage (Figure 2). Interestingly, the Δ8 modification may have exposed new epitopes on the phage (see Figure 3). Immunization with Δ8 or Δ8Δ3 phage elicited Abs that reacted weakly with WT pVIII (Figure 3), as well as Abs against an unidentified protein with approximately the same electrophoretic mobility as full-length pIII. Most likely, this band represents aggregated Δ8 pVIII molecules [39, 40] or a bacterial protein or protein aggregate (e.g., ompA; personal communication, J. Rakonjac, Massey University, NZ) that adheres to the virion via nonspecific interactions with modified pVIII (Figure 1C). Bacterial proteins or other constituents (e.g., LPS) that non-specifically associate with phage in general may also explain the discrepancy we observed between the western blot analyses (Figure 3) and phage ELISAs (Supplemental Figure S1); both WT and Δ3 phage elicited Ab reactivity with S. flexneri LPS (Supplemental Figure S2) and E. coli LPS (our unpublished data).
The Δ3 phage was compared to WT phage as a carrier for two weakly immunogenic recombinant peptides, MD10 [23] and 4E10LA [24], which were previously selected by MAbs against the LPS of S. flexneri and the HIV envelope protein, gp41, respectively, from phage libraries displaying peptides as fusions to the N-terminus of pVIII. WT phage displaying recombinant MD10 peptide produced a much stronger anti-peptide Ab response than the corresponding Δ3 phage (Figure 4 A), and neither phage carrier elicited a detectable anti-peptide Ab response against recombinant 4E10LA peptide, even after four immunizations (Figure 4 C). While we cannot rule out the possibility that the lack of cross-reactivity of sera against the phage-borne 4E10LA peptide with 4E10LB synthetic peptide was due to the single amino acid difference (N6A) between the two, it is more likely that removal of the immunodominant domains of pIII failed to focus the Ab responses against the MD10 and 4E10LA recombinant peptides.
We previously had shown that phage bearing a chemically-conjugated synthetic peptide produced a stronger and more focused anti-peptide Ab response than phage displaying the same peptide in recombinant form [10], and reasoned that the Δ3 modification might better focus the Ab response against chemically-conjugated synthetic peptides. The SPDP hetero-bifunctional crosslinker used in this study produces stable conjugates that are more immunogenic than their non-crosslinked counterparts [41, 42]. While mice immunized with WT and Δ3 phage displaying recombinant MD10 peptide produced significant peptide reactivity, those immunized with either phage carrier bearing chemically-conjugated MD10 peptide did not produce any detectable anti-peptide response (compare Figure 4 A and B). In contrast, mice immunized with phage bearing chemically-conjugated 4E10LB peptide elicited high anti-peptide titers (Figure 4 D), with the Δ3 carrier exhibiting significantly higher peptide:carrier ratios than the WT carrier (Figure 5 A). Thus, the anti-peptide Ab response was enhanced and focused by removal of immunodominant pIII domains from the phage carrier only for the phage/4E10LB conjugate, but not for the phage/MD10 conjugate or for either peptide displayed recombinantly.
There may be several reasons why only the Δ3/4E10LB conjugate produced a focused anti-peptide Ab response. The 4E10LB peptide may be inherently more immunogenic than the MD10 peptide and/or may contain its own TCEs. The MD10 peptide is known to be only weakly immunogenic, as others have shown that multiple immunizations with this peptide in high copy number, in multiple doses containing adjuvant, were required to produce detectable Ab responses [43]. It is also possible that the higher copy number of chemically conjugated synthetic peptides relative to recombinant peptides [10] could result in stronger anti-peptide Ab responses in the former, perhaps by enhanced crosslinking of B-cell receptors. This is supported by studies using B cells expressing anti-hen egg lysozyme (HEL) B-cell receptors, which suggest that antigen valency can contribute to enhanced antigen processing and presentation of class II major histocompatibility complex (MHC II) epitopes to T cells [44]. Another study demonstrated that increasing antigen valency results in increased calcium signalling and enhanced B-cell receptor clustering [45].
Surprisingly, conjugation did not enhance the Ab response against the MD10 peptide compared to recombinant display; this could be due to the lower dose of conjugate vs. recombinant phage (Supplemental Table S1) used for immunization. Furthermore, peptides may not have similar conformations displayed as recombinant fusions vs. as synthetic conjugates, which may affect their immunogenicity. For example, the 4E10LB peptide is extremely hydrophobic and adheres to phage particles even in the absence of crosslinker (our unpublished observation. Extensive washing and PEG precipitation effectively removes non-specifically bound peptide, but not SPDP-conjugated peptide; thus, recombinant 4E10LA peptide fused to pVIII may embed itself in recesses on the phage coat, rendering it less accessible for Ab binding. In contrast, chemical conjugation of the synthetic 4E10LB peptide may better expose it on the phage surface via attachment to the bulky SPDP crosslinker. In addition, it has been noted that the affinities of MAbs are typically greater for the recombinant peptides they select from phage libraries than for their synthetic peptide counterparts [46–48], suggesting that fusion to pVIII itself can contribute to peptide affinity, either through flanking sequences, or through conformational stabilization via the peptide bond (as opposed to a flexible linker). These effects, too, may be peptide-dependent.
The observation that the focused Ab responses produced by Δ3 phage were weaker in overall magnitude than those produced by WT phage was not entirely surprising, since the removal of the N1 and N2 domains of pIII very likely reduced MHC II TCEs on the phage. Poor T cell help is undesirable in a vaccine carrier, as protective immunity against pathogens (e.g., smallpox) often requires vigorous B- and T-cell responses against the same microbial antigen [49]. A lack of T cell help could be overcome by incorporating strong, foreign MHC II TCEs into the phage particle; previous studies have done so using class I MHC TCEs from HIV-1, OVA, and Hepatitis B virus [50–52], and an MHC II peptide from the staphylococcal superantigen, TSST-1 [53]. However, in these examples, TCEs were fused to the N-terminus of pIII or pVIII, exposing them for binding by B-cell receptors [54], which may result in an anti-TCE Ab responses and/or interfere with TCE presentation on MHC II molecules [55, 56]. Anti-TCE Ab binding could be avoided by inserting a TCE into regions of pVIII that are not surface-exposed, such as its amphipathic or hydrophobic domains, which can tolerate drastic changes if the modified pVIII is in low copy number compared to WT pVIII [36]. A second strategy to enhance the Ab response to haptens carried by Δ3 phage would be to engineer molecular adjuvants into the phage coat (e.g., onto the CT domain of pIII) such as C3d, a complement cleavage product that crosslinks CD21 on B cells [57, 58], bacterial flagellin, which functions by activating dendritic cells through Toll-like receptor 5 signalling [59, 60]. Alternatively, adjuvants such as CpG ODN could be chemically conjugated to the phage surface; the adjuvant, LPS, is already associated with phage virions [12, 13].
Conjugate vaccines are likely to become increasingly important because they create a covalent linkage between a targeted antigen and a strongly immunogenic protein carrier. Studies by Batista and Neuberger (2000) and others have shown that B-lymphocytes can extract antigen that is non-covalently bound to a surface, in a process now called trogocytosis [61]. This can result in the separation of antigenic constituents from the surface of a virion or immune complex, and limiting B- and T-cell responses by confining them to individual antigens [49]. Covalent linkage of two antigens from the same organism has been shown to produce lasting Ab responses; a low-dose conjugate vaccine comprising the polysaccharide of Pneumococcus crosslinked to one of its membrane proteins produced stronger secondary IgG responses than 108 live or whole, killed cells [62]. Therefore, covalent conjugation to a carrier has the potential advantage of dependably eliciting an immune response against a desired protein or non-protein antigen, region or domain that is part of a more complex pathogen.
Filamentous phage can be used as highly immunogenic carrier proteins [10]. In this study, we investigated the effect immunodominant epitopes on the phage might have in the Ab response against weakly immunogenic peptides linked to the phage surface via recombinant fusion or covalent crosslinks. It was shown that alteration or removal of immunodominant epitopes on the phage redistributed the Ab response to other epitopes on the phage coat. In addition, a modified phage carrier focused the Ab response against a chemically conjugated synthetic peptide, but not against the same peptide displayed as a recombinant fusion, or against a different peptide, regardless of its method of display. It is possible that targeting of Ab responses towards displayed peptides, and away from irrelevant carrier epitopes, could be further improved by associating TCEs and molecular adjuvants with the phage coat.
Supplementary Material
Acknowledgments
We thank animal care technicians L. van der Wal, M. Dearden, and K. Buettner at the Simon Fraser University Animal Resource Center for their care and assistance with the mice used in this study, and B.M. Pinto (Simon Fraser University) and D.R. Bundle (University of Alberta, Edmonton) for providing S. flexneri LPS and MAb SYA/J6. This work was supported by the National Institutes of Health grants AI44395 and AI4911 to J.K.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cleveland SM, Buratti E, Jones TD, North P, Baralle F, McLain L, et al. Immunogenic and antigenic dominance of a nonneutralizing epitope over a highly conserved neutralizing epitope in the gp41 envelope glycoprotein of human immunodeficiency virus type 1: its deletion leads to a strong neutralizing response. Virology. 2000;266(1):66–78. doi: 10.1006/viro.1999.0041. [DOI] [PubMed] [Google Scholar]
- 2.Zwick MB. The membrane-proximal external region of HIV-1 gp41: a vaccine target worth exploring. AIDS. 2005;19(16):1725–1737. doi: 10.1097/01.aids.0000189850.83322.41. [DOI] [PubMed] [Google Scholar]
- 3.Schotsaert M, De Filette M, Fiers W, Saelens X. Universal M2 ectodomain-based influenza A vaccines: preclinical and clinical developments. Expert Rev Vaccines. 2009;8(4):499–508. doi: 10.1586/erv.09.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inic-Kanada A, Stojanovic M, Zivkovic I, Kosec D, Micic M, Petrusic V, et al. Murine monoclonal antibody 26 raised against tetanus toxoid cross-reacts with beta2-glycoprotein I: its characteristics and role in molecular mimicry. Am J Reprod Immunol. 2009;61(1):39–51. doi: 10.1111/j.1600-0897.2008.00660.x. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A, Sarkar S, Nazabal C, Balasundaram G, Rao KV. B cell responses to a peptide epitope. I. The cellular basis for restricted recognition. J Immunol. 1996;157(7):2779–2788. [PubMed] [Google Scholar]
- 6.Chiesa MD, Martensen PM, Simmons C, Porakishvili N, Justesen J, Dougan G, et al. Refocusing of B-cell responses following a single amino acid substitution in an antigen. Immunology. 2001;103(2):172–178. doi: 10.1046/j.1365-2567.2001.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pantophlet R, Wilson IA, Burton DR. Hyperglycosylated mutants of human immunodeficiency virus (HIV) type 1 monomeric gp120 as novel antigens for HIV vaccine design. J Virol. 2003;77(10):5889–5901. doi: 10.1128/JVI.77.10.5889-5901.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheerlinck JP, DeLeys R, Saman E, Brys L, Geldhof A, De Baetselier P. Redistribution of a murine humoral immune response following removal of an immunodominant B cell epitope from a recombinant fusion protein. Mol Immunol. 1993;30(8):733–739. doi: 10.1016/0161-5890(93)90144-z. [DOI] [PubMed] [Google Scholar]
- 9.Garrity RR, Rimmelzwaan G, Minassian A, Tsai WP, Lin G, de Jong JJ, et al. Refocusing neutralizing antibody response by targeted dampening of an immunodominant epitope. J Immunol. 1997;159(1):279–289. [PubMed] [Google Scholar]
- 10.van Houten NE, Zwick MB, Menendez A, Scott JK. Filamentous phage as an immunogenic carrier to elicit focused antibody responses against a synthetic peptide. Vaccine. 2006;24(19):4188–4200. doi: 10.1016/j.vaccine.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Houten NE, Scott JK. Phage libraries for developing antibody targeted diagnostics and vaccines. In: Sidhu SS, editor. Phage display in biotechnology and drug discovery. Boca Raton: CRC Press/Taylor & Francis; 2005. pp. 165–254. [Google Scholar]
- 12.Willis AE, Perham RN, Wraith D. Immunological properties of foreign peptides in multiple display on a filamentous bacteriophage. Gene. 1993;128(1):79–83. doi: 10.1016/0378-1119(93)90156-w. [DOI] [PubMed] [Google Scholar]
- 13.Grabowska AM, Jennings R, Laing P, Darsley M, Jameson CL, Swift L, et al. Immunisation with phage displaying peptides representing single epitopes of the glycoprotein G can give rise to partial protective immunity to HSV-2. Virology. 2000;269(1):47–53. doi: 10.1006/viro.2000.0185. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson F, Tsagozis P, Lundberg K, Parsa R, Mangsbo SM, Persson MA, et al. Tumor-specific bacteriophages induce tumor destruction through activation of tumor-associated macrophages. J Immunol. 2009;182(5):3105–3111. doi: 10.4049/jimmunol.0800224. [DOI] [PubMed] [Google Scholar]
- 15.Kneissel S, Queitsch I, Petersen G, Behrsing O, Micheel B, Dubel S. Epitope structures recognised by antibodies against the major coat protein (g8p) of filamentous bacteriophage fd (Inoviridae) J Mol Biol. 1999;288(1):21–28. doi: 10.1006/jmbi.1999.2676. [DOI] [PubMed] [Google Scholar]
- 16.Parmley SF, Smith GP. Filamentous fusion phage cloning vectors for the study of epitopes and design of vaccines. Adv Exp Med Biol. 1989;251:215–218. doi: 10.1007/978-1-4757-2046-4_21. [DOI] [PubMed] [Google Scholar]
- 17.Irving MB, Pan O, Scott JK. Random-peptide libraries and antigen-fragment libraries for epitope mapping and the development of vaccines and diagnostics. Curr Opin Chem Biol. 2001;5(3):314–324. doi: 10.1016/S1367-5931(00)00208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geysen HM, Tainer JA, Rodda SJ, Mason TJ, Alexander H, Getzoff ED, et al. Chemistry of antibody binding to a protein. Science. 1987;235(4793):1184–1190. doi: 10.1126/science.3823878. [DOI] [PubMed] [Google Scholar]
- 19.Davies DR, Cohen GH. Interactions of protein antigens with antibodies. Proc Natl Acad Sci U S A. 1996;93(1):7–12. doi: 10.1073/pnas.93.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrenko VA, Smith GP, Mazooji MM, Quinn T. Alpha-helically constrained phage display library. Protein Eng. 2002;15(11):943–950. doi: 10.1093/protein/15.11.943. [DOI] [PubMed] [Google Scholar]
- 21.Ellington A, Pollard JD., Jr Chapter 2. Purification of oligonucleotides using denaturing polyacrylamide gel electrophoresis. Curr Protoc Mol Biol. 2001 doi: 10.1002/0471142727.mb0212s42. Unit2 12. [DOI] [PubMed] [Google Scholar]
- 22.Harris SL, Craig L, Mehroke JS, Rashed M, Zwick MB, Kenar K, et al. Exploring the basis of peptide-carbohydrate crossreactivity: evidence for discrimination by peptides between closely related anti- carbohydrate antibodies. Proc Natl Acad Sci U S A. 1997;94(6):2454–2459. doi: 10.1073/pnas.94.6.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vyas NK, Vyas MN, Chervenak MC, Johnson MA, Pinto BM, Bundle DR, et al. Molecular recognition of oligosaccharide epitopes by a monoclonal Fab specific for Shigella flexneri Y lipopolysaccharide: X-ray structures and thermodynamics. Biochemistry. 2002;41(46):13575–13586. doi: 10.1021/bi0261387. [DOI] [PubMed] [Google Scholar]
- 24.Bahr SL. Peptide markers for the HIV-1 neutralizing antibody 4E10 [dissertation] Burnaby: Simon Fraser University; 2004. [Google Scholar]
- 25.Bonnycastle LLC, Mehroke JS, Rashed M, Gong X, Scott JK. Probing the basis of antibody reactivity with a panel of constrained peptide libraries displayed by filamentous phage. J Mol Biol. 1996;258(5):747–762. doi: 10.1006/jmbi.1996.0284. [DOI] [PubMed] [Google Scholar]
- 26.Bonnycastle LLC, Shen J, Menendez A, Scott JK. General phage methods. In: Barbas C III, Burton DR, Scott JK, Silverman GJ, editors. Phage Display: A laboratory manual. Vol. 15. Plainview, New York: Cold Spring Harbor Laboratory Press; 2001. pp. 1–30. [Google Scholar]
- 27.Dower WJ, Miller JF, Ragsdale CW. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Smith GP, Scott JK. Libraries of peptides and proteins displayed on filamentous phage. Methods Enzymol. 1993;217:228–257. doi: 10.1016/0076-6879(93)17065-d. [DOI] [PubMed] [Google Scholar]
- 30.Zwick MB, Shen J, Scott JK. Homodimeric peptides displayed by the major coat protein of filamentous phage. J Mol Biol. 2000;300(2):307–320. doi: 10.1006/jmbi.2000.3850. [DOI] [PubMed] [Google Scholar]
- 31.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonnycastle LLC, Brown KL, Tang J, Scott JK. Assaying phage-borne peptides by phage capture on fibrinogen or streptavidin. Biol Chem. 1997;378(6):509–515. doi: 10.1515/bchm.1997.378.6.509. [DOI] [PubMed] [Google Scholar]
- 33.Marvin DA, Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marvin DA, Welsh LC, Symmons MF, Scott WR, Straus SK. Molecular structure of fd (f1, M13) filamentous bacteriophage refined with respect to X-ray fibre diffraction and solid-state NMR data supports specific models of phage assembly at the bacterial membrane. J Mol Biol. 2006;355(2):294–309. doi: 10.1016/j.jmb.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 35.Ashcroft SJH. Practical statistics for the biological sciences : simple pathways to statistical analyses. Basingstoke: Palgrave; 2002. [Google Scholar]
- 36.Roth TA, Weiss GA, Eigenbrot C, Sidhu SS. A minimized M13 coat protein defines the requirements for assembly into the bacteriophage particle. J Mol Biol. 2002;322(2):357–367. doi: 10.1016/s0022-2836(02)00769-6. [DOI] [PubMed] [Google Scholar]
- 37.Stopar D, Spruijt RB, Hemminga MA. Anchoring mechanisms of membrane-associated M13 major coat protein. Chem Phys Lipids. 2006;141(1–2):83–93. doi: 10.1016/j.chemphyslip.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Peeters JM, Hazendonk TG, Beuvery EC, Tesser GI. Comparison of four bifunctional reagents for coupling peptides to proteins and the effect of the three moieties on the immunogenicity of the conjugates. J Immunol Methods. 1989;120(1):133–143. doi: 10.1016/0022-1759(89)90298-6. [DOI] [PubMed] [Google Scholar]
- 39.Spruijt RB, Wolfs CJ, Hemminga MA. Aggregation-related conformational change of the membrane-associated coat protein of bacteriophage M13. Biochemistry. 1989;28(23):9158–9165. doi: 10.1021/bi00449a030. [DOI] [PubMed] [Google Scholar]
- 40.Stopar D, Spruijt RB, Wolfs CJ, Hemminga MA. In situ aggregational state of M13 bacteriophage major coat protein in sodium cholate and lipid bilayers. Biochemistry. 1997;36(40):12268–12275. doi: 10.1021/bi970747a. [DOI] [PubMed] [Google Scholar]
- 41.Leibl H, Tomasits R, Eibl MM, Mannhalter JW. Adjuvant/carrier activity of inactivated tick-borne encephalitis virus. Vaccine. 1998;16(4):340–345. doi: 10.1016/s0264-410x(97)80911-5. [DOI] [PubMed] [Google Scholar]
- 42.Szu SC, Stone AL, Robbins JD, Schneerson R, Robbins JB. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J Exp Med. 1987;166(5):1510–1524. doi: 10.1084/jem.166.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borrelli S, Hossany RB, Pinto BM. Immunological evidence for functional rather than structural mimicry by a Shigella flexneri Y polysaccharide-mimetic peptide. Clin Vaccine Immunol. 2008;15(7):1106–1114. doi: 10.1128/CVI.00050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim YM, Pan JY, Korbel GA, Peperzak V, Boes M, Ploegh HL. Monovalent ligation of the B cell receptor induces receptor activation but fails to promote antigen presentation. Proc Natl Acad Sci U S A. 2006;103(9):3327–3332. doi: 10.1073/pnas.0511315103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puffer EB, Pontrello JK, Hollenbeck JJ, Kink JA, Kiessling LL. Activating B cell signaling with defined multivalent ligands. ACS Chem Biol. 2007;2(4):252–262. doi: 10.1021/cb600489g. [DOI] [PubMed] [Google Scholar]
- 46.Zwick MB, Bonnycastle LL, Menendez A, Irving MB, Barbas CF, 3rd, Parren PW, et al. Identification and characterization of a peptide that specifically binds the human, broadly neutralizing anti-human immunodeficiency virus type 1 antibody b12. J Virol. 2001;75(14):6692–6699. doi: 10.1128/JVI.75.14.6692-6699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menendez A, Chow KC, Pan OC, Scott JK. Human immunodeficiency virus type 1-neutralizing monoclonal antibody 2F5 is multispecific for sequences flanking the DKW core epitope. J Mol Biol. 2004;338(2):311–327. doi: 10.1016/j.jmb.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 48.Menendez A, Calarese DA, Stanfield RL, Chow KC, Scanlan CN, Kunert R, et al. A peptide inhibitor of HIV-1 neutralizing antibody 2G12 is not a structural mimic of the natural carbohydrate epitope on gp120. FASEB J. 2008;22(5):1380–1392. doi: 10.1096/fj.07-8983com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sette A, Moutaftsi M, Moyron-Quiroz J, McCausland MM, Davies DH, Johnston RJ, et al. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity. 2008;28(6):847–858. doi: 10.1016/j.immuni.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Berardinis P, D'Apice L, Prisco A, Ombra MN, Barba P, Del Pozzo G, et al. Recognition of HIV-derived B and T cell epitopes displayed on filamentous phages. Vaccine. 1999;17(11–12):1434–1441. doi: 10.1016/s0264-410x(98)00377-6. [DOI] [PubMed] [Google Scholar]
- 51.De Berardinis P, Sartorius R, Fanutti C, Perham RN, Del Pozzo G, Guardiola J. Phage display of peptide epitopes from HIV-1 elicits strong cytolytic responses. Nat Biotechnol. 2000;18(8):873–876. doi: 10.1038/78490. [DOI] [PubMed] [Google Scholar]
- 52.Wan Y, Wu Y, Bian J, Wang XZ, Zhou W, Jia ZC, et al. Induction of hepatitis B virus-specific cytotoxic T lymphocytes response in vivo by filamentous phage display vaccine. Vaccine. 2001;19(20–22):2918–2923. doi: 10.1016/s0264-410x(00)00561-2. [DOI] [PubMed] [Google Scholar]
- 53.Rubinchik E, Chow AW. Recombinant expression and neutralizing activity of an MHC class II binding epitope of toxic shock syndrome toxin-1. Vaccine. 2000;18(21):2312–2320. doi: 10.1016/s0264-410x(99)00554-x. [DOI] [PubMed] [Google Scholar]
- 54.van Houten NE. Strategies for the design of epitope targeting vaccines [dissertation] Burnaby: Simon Fraser University; 2007. [Google Scholar]
- 55.Simitsek PD, Campbell DG, Lanzavecchia A, Fairweather N, Watts C. Modulation of antigen processing by bound antibodies can boost or suppress class II major histocompatibility complex presentation of different T cell determinants. J Exp Med. 1995;181(6):1957–1963. doi: 10.1084/jem.181.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manca F, Fenoglio D, Cambiaggi C, Celada F. Paratope interference with processing can explain selective help for different epitopes in beta-galactosidase. In: Smith-Gill SJ, Sercarz EE, National Cancer Institute (U.S.), editors. The Immune response to structurally defined proteins : the lysozyme model : proceedings of a workshop sponsored by the National Cancer Institute of NIH, held at the Mary Woodard Lasker Center for Health Research and Education; June 13–15, 1988; Bethesda, MD. Schenectady, NY: Adenine Press; 1989. [Google Scholar]
- 57.Bergmann-Leitner ES, Leitner WW, Tsokos GC. Complement 3d: from molecular adjuvant to target of immune escape mechanisms. Clin Immunol. 2006;121(2):177–185. doi: 10.1016/j.clim.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271(5247):348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 59.Barton GM. Viral recognition by Toll-like receptors. Semin Immunol. 2007;19(1):33–40. doi: 10.1016/j.smim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Macagno A, Napolitani G, Lanzavecchia A, Sallusto F. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends Immunol. 2007;28(5):227–233. doi: 10.1016/j.it.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 61.Batista FD, Neuberger MS. B cells extract and present immobilized antigen: implications for affinity discrimination. EMBO J. 2000;19(4):513–520. doi: 10.1093/emboj/19.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colino J, Chattopadhyay G, Sen G, Chen Q, Lees A, Canaday DH, et al. Parameters underlying distinct T cell-dependent polysaccharide-specific IgG responses to an intact gram-positive bacterium versus a soluble conjugate vaccine. J Immunol. 2009;183(3):1551–1559. doi: 10.4049/jimmunol.0900238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.