Abstract
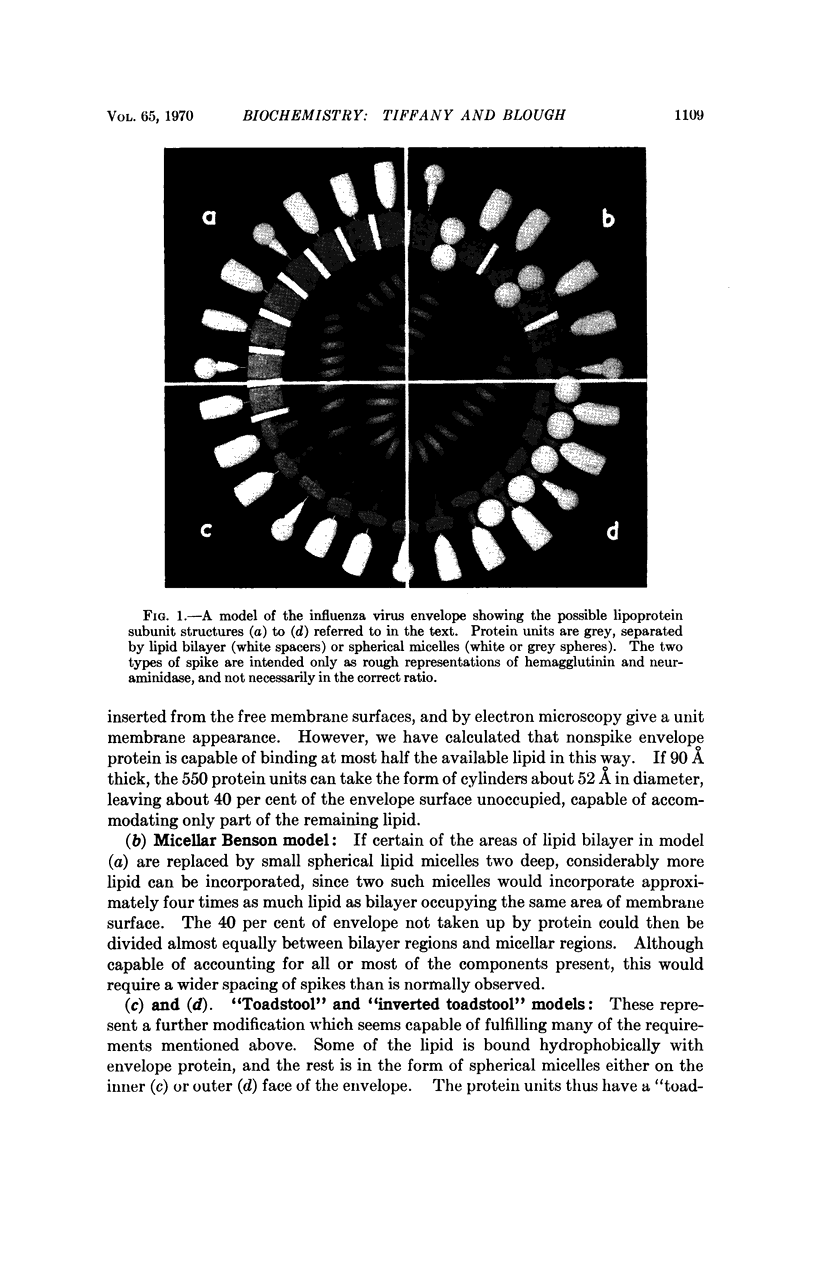
Possible models of the structure of the influenza virus envelope are considered in terms of the known chemical composition. Models incorporating lipid in the form of a bimolecular leaflet are shown to be unlikely on geometrical grounds. A model having „inverted toadstool” protein units separated by spherical lipid micelles is favored, and is capable of explaining the appearance of the virus in the electron microscope and differences between normal and incomplete (von Magnus) forms of the virus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., GOTTSCHALK A. The component sugars of the influenza-virus particle. Biochem J. 1956 Apr;62(4):686–689. doi: 10.1042/bj0620686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARCHETTI I., BOCCIARELLI D. S. On the structure of filamentous forms of influenza virus. Arch Gesamte Virusforsch. 1962;11:599–606. doi: 10.1007/BF01241310. [DOI] [PubMed] [Google Scholar]
- Almeida J. D., Waterson A. P. Some observations on the envelope of an influenza virus. J Gen Microbiol. 1967 Jan;46(1):107–110. doi: 10.1099/00221287-46-1-107. [DOI] [PubMed] [Google Scholar]
- Apostolov K., Flewett T. H. Further observations on the structure of influenza viruses A and C. J Gen Virol. 1969 Apr;4(3):365–370. doi: 10.1099/0022-1317-4-3-365. [DOI] [PubMed] [Google Scholar]
- Benson A. A. On the orientation of lipids in chloroplast and cell membranes. J Am Oil Chem Soc. 1966 May;43(5):265–270. doi: 10.1007/BF02609671. [DOI] [PubMed] [Google Scholar]
- Blough H. A., Lawson D. E. The lipids of paramyxoviruses: a comparative study of Sendai and Newcastle disease viruses. Virology. 1968 Oct;36(2):286–292. doi: 10.1016/0042-6822(68)90146-3. [DOI] [PubMed] [Google Scholar]
- Blough H. A., Merlie J. P., Tiffany J. M. The fatty acid composition of incomplete influenza virus. Biochem Biophys Res Commun. 1969 Mar 31;34(6):831–834. doi: 10.1016/0006-291x(69)90255-1. [DOI] [PubMed] [Google Scholar]
- Blough H. A., Weinstein D. B., Lawson D. E., Kodicek E. The effect of vitamin A on myxoviruses. II. Alterations in the lipids of influenza virus. Virology. 1967 Nov;33(3):459–466. doi: 10.1016/0042-6822(67)90121-3. [DOI] [PubMed] [Google Scholar]
- Bächi T., Gerhard W., Lindenmann J., Mühlethaler K. Morphogenesis of influenza A virus in Ehrlich ascites tumor cells as revealed by thin-sectioning and freeze-etching. J Virol. 1969 Nov;4(5):769–776. doi: 10.1128/jvi.4.5.769-776.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S., CHOPPIN P. W. Attachment and penetration of influenza virus. Virology. 1962 Nov;18:489–493. doi: 10.1016/0042-6822(62)90041-7. [DOI] [PubMed] [Google Scholar]
- DE BERNARD L. Associations moléculaires entre les lipides. II. Lecithine et cholestérol. Bull Soc Chim Biol (Paris) 1958;40(1):161–170. [PubMed] [Google Scholar]
- Drzeniek R., Frank H., Rott R. Electron microscopy of purified influenza virus neuraminidase. Virology. 1968 Dec;36(4):703–707. doi: 10.1016/0042-6822(68)90209-2. [DOI] [PubMed] [Google Scholar]
- Eckert E. A. Characterization of a low molecular weight antigenic protein from the envelope of influenza virus. J Bacteriol. 1966 Nov;92(5):1430–1434. doi: 10.1128/jb.92.5.1430-1434.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRISCH-NIGGEMEYER W. Absolute amount of ribonucleic acid in viruses. Nature. 1956 Aug 11;178(4528):307–308. doi: 10.1038/178307b0. [DOI] [PubMed] [Google Scholar]
- FRISCH-NIGGEMEYER W., HOYLE L. The nucleic acid and carbohydrate content of influenza virus A and of virus fractions produced by ether disintegration. J Hyg (Lond) 1956 Jun;54(2):201–212. doi: 10.1017/s0022172400044466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROMMHAGEN L. H., KNIGHT C. A., FREEMAN N. K. The ribonucleic acid, lipid, and polysaccharide constituents of influenza virus preparations. Virology. 1959 Jun;8(2):176–197. doi: 10.1016/0042-6822(59)90003-0. [DOI] [PubMed] [Google Scholar]
- Green D. E., Perdue J. F. Membranes as expressions of repeating units. Proc Natl Acad Sci U S A. 1966 May;55(5):1295–1302. doi: 10.1073/pnas.55.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross L. Active membranes for active transport. J Theor Biol. 1967 Jun;15(3):298–306. doi: 10.1016/0022-5193(67)90139-7. [DOI] [PubMed] [Google Scholar]
- HORNE R. W., WATERSON A. P., WILDY P., FARNHAM A. E. The structure and composition of the myxoviruses. I. Electron microscope studies of the structure of myxovirus particles by negative staining techniques. Virology. 1960 May;11:79–98. doi: 10.1016/0042-6822(60)90056-8. [DOI] [PubMed] [Google Scholar]
- HOYLE L., DAVIES S. P. Amino acid composition of the protein components of influenza virus A. Virology. 1961 Jan;13:53–57. doi: 10.1016/0042-6822(61)90031-9. [DOI] [PubMed] [Google Scholar]
- HOYLE L., HORNE R. W., WATERSON A. P. The structure and composition of the myxoviruses. II. Components released from the influenza virus particle by ether. Virology. 1961 Apr;13:448–459. doi: 10.1016/0042-6822(61)90276-8. [DOI] [PubMed] [Google Scholar]
- HOYLE L. Structure of the influenza virus; the relation between biological activity and chemical structure of virus fractions. J Hyg (Lond) 1952 Jun;50(2):229–245. doi: 10.1017/s0022172400019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Taylor J. The stability and properties of bimolecular lipid leaflets in aqueous solutions. J Theor Biol. 1963 May;4(3):281–296. doi: 10.1016/0022-5193(63)90007-9. [DOI] [PubMed] [Google Scholar]
- Ji T. H., Benson A. A. Association of lipids and proteins in chloroplast lamellar membrane. Biochim Biophys Acta. 1968 Jun 11;150(4):686–693. doi: 10.1016/0005-2736(68)90058-8. [DOI] [PubMed] [Google Scholar]
- Kendal A. P., Apostolov K., Belyavin G. The effect of protease treatment on the morphology of influenza A, B and C viruses. J Gen Virol. 1969 Jul;5(1):141–143. doi: 10.1099/0022-1317-5-1-141. [DOI] [PubMed] [Google Scholar]
- Kendal A. P., Biddle F., Belyavin G. Influenza virus neuraminidase and the viral surface. Biochim Biophys Acta. 1968 Oct 15;165(3):419–431. doi: 10.1016/0304-4165(68)90221-3. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Chemical composition of the parainfluenza virus SV5. Virology. 1969 Jan;37(1):155–157. doi: 10.1016/0042-6822(69)90321-3. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Lipids of plasma membranes of monkey and hamster kidney cells and of parainfluenza virions grown in these cells. Virology. 1969 Jun;38(2):255–268. doi: 10.1016/0042-6822(69)90367-5. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Structure of biological membranes. Science. 1966 Sep 23;153(3743):1491–1498. doi: 10.1126/science.153.3743.1491. [DOI] [PubMed] [Google Scholar]
- LAVER W. G. STRUCTURAL STUDIES ON THE PROTEIN SUBUNITS FROM THREE STRAINS OF INFLUENZA VIRUS. J Mol Biol. 1964 Jul;9:109–124. doi: 10.1016/s0022-2836(64)80094-2. [DOI] [PubMed] [Google Scholar]
- LUZZATI V., HUSSON F. The structure of the liquid-crystalline phasis of lipid-water systems. J Cell Biol. 1962 Feb;12:207–219. doi: 10.1083/jcb.12.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Valentine R. C. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969 May;38(1):105–119. doi: 10.1016/0042-6822(69)90132-9. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., MOORE D. H. Structure and development of viruses observed in the electron microscope. III. Influenza virus. J Exp Med. 1956 Aug 1;104(2):171–182. doi: 10.1084/jem.104.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rose H. M. Structure and development of viruses as observed in the electron microscope. 8. Entry of influenza virus. J Virol. 1968 Sep;2(9):925–936. doi: 10.1128/jvi.2.9.925-936.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M. W., Hirst G. K. Polyacrylamide gel electrophoresis of influenza virus RNA. Virology. 1968 Feb;34(2):385–388. doi: 10.1016/0042-6822(68)90257-2. [DOI] [PubMed] [Google Scholar]
- ROBERTSON J. D. The ultrastructure of cell membranes and their derivatives. Biochem Soc Symp. 1959;16:3–43. [PubMed] [Google Scholar]
- Reimer C. B., Baker R. S., Newlin T. E., Havens M. L. Influenza virus purification with the zonal ultracentrifuge. Science. 1966 Jun 3;152(3727):1379–1381. doi: 10.1126/science.152.3727.1379. [DOI] [PubMed] [Google Scholar]
- Robertson J. D. Granulo-fibrillar and globular substructure in unit membranes. Ann N Y Acad Sci. 1966 Jul 14;137(2):421–440. doi: 10.1111/j.1749-6632.1966.tb50174.x. [DOI] [PubMed] [Google Scholar]
- Tiffany J. M., Blough H. A. Myxovirus envelope proteins: a directing influence on the fatty acids of membrane lipids. Science. 1969 Feb 7;163(3867):573–574. doi: 10.1126/science.163.3867.573. [DOI] [PubMed] [Google Scholar]
- VALENTINE R. C., ISAACS A. The structure of influenza virus filaments and spheres. J Gen Microbiol. 1957 Feb;16(1):195–204. doi: 10.1099/00221287-16-1-195. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Darlington R. W. Disruption of myxoviruses with Tween 20 and isolation of biologically active hemagglutinin and neuraminidase subunits. J Virol. 1969 Aug;4(2):182–187. doi: 10.1128/jvi.4.2.182-187.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Kilbourne E. D. Reactions of antibodies with surface antigens of influenza virus. J Gen Virol. 1968 Dec;3(3):315–326. doi: 10.1099/0022-1317-3-3-315. [DOI] [PubMed] [Google Scholar]
- von MAGNUS P. Propagation of the PR8 strain of influenza A virus in chick embryos. II. The formation of incomplete virus following inoculation of large doses of seed virus. Acta Pathol Microbiol Scand. 1951;28(3):278–293. doi: 10.1111/j.1699-0463.1951.tb03693.x. [DOI] [PubMed] [Google Scholar]




