Abstract
CD4+ T cells deficient in signaling lymphocyte activation molecule (SLAM)-associated protein (SAP) exhibit a selective impairment in adhesion to antigen presenting B cells but not dendritic cells (DC), resulting in defective germinal center formation. However, the nature of this selective adhesion defect remained unclear. We found that whereas T:DC interactions were primarily integrin-dependent, T:B cell interactions had both an early integrin-dependent phase and a sustained phase that also required SAP. We further found that the SLAM family member, CD84, was required for prolonged T:B cell contact, optimal T follicular helper function, and germinal center formation in vivo. Moreover, both CD84 and another SLAM member, Ly108, mediated T cell adhesion and participated in stable T:B cell interactions in vitro. Our results reveal insight into the dynamic regulation of T:B cell interactions and identify SLAM family members as critical components of sustained T:B cell adhesion required for productive humoral immunity.
Introduction
Long-term antibody-mediated immunity depends, in part, on the generation of germinal centers (GCs), which require T:B cell cooperation for their effective formation and maintenance. Such T-dependent antibody responses are initiated when CD4+ T cells are activated by antigen presenting dendritic cells (DCs). Once activated, these T cells deliver contact-dependent helper signals to antigen-activated B cells, promoting their proliferation and differentiation. Activated B cells that have received such early T cell help re-enter the B cell follicle, proliferate, and establish a GC (Allen et al., 2007). Activated antigen-specific CD4+ T cells that have acquired CXCR5 expression also relocate from the T cell zone into the B cell follicle and reside within the developing GC to help induce and maintain GC formation: these cells have been referred to as T follicular helper (Tfh) cells (Fazilleau et al., 2009).
Insight into the control of effective delivery of T cell help to B cells for GC formation has come from analysis of patients with X-linked lymphoproliferation (XLP) disease and its mouse model. Inactivating mutations in Sh2d1a, which encodes the adaptor protein signal lymphocyte activation molecule (SLAM)-associated protein (SAP), underlie most cases of XLP. XLP is characterized by fulminant infectious mononucleosis, lymphoma, and dysgammaglobulinemia (Calpe et al., 2008; Ma et al., 2007). Analyses of SAP-deficient (Sh2d1a−/−) mice revealed a T cell-intrinsic defect in humoral responses following infection or immunization with T-dependent antigens, characterized by poor GC formation, low antibody titers, and a scarcity of memory B cells as well as long-lived plasma cells. Similar findings have been confirmed in SAP-deficient XLP patients (Ma et al., 2007).
Using intravital imaging, we recently demonstrated that Sh2d1a−/− CD4+ T cells interact with and are activated effectively by antigen-bearing DCs, resulting in proliferation, upregulation of activation markers, and migration into B cell follicles, similar to WT CD4 T cells. However, Sh2d1a−/− CD4+ T cells fail to maintain stable conjugates with antigen-specific B cells in vivo (Qi et al., 2008). Thus, despite their expression of key markers characteristic of Tfh cells (CXCR5, CD40L, ICOS) and required for B cell help, Sh2d1a−/− CD4+ T cells are unable to deliver contact-dependent signals to antigen-specific B cells. Moreover, Sh2d1a−/− CD4+ T cells do not show effective antigen-dependent recruitment into or retention within GCs and thus are unable to act as functional Tfh cells to help sustain the GC reaction (Qi et al., 2008). These data implicated SAP in adhesive processes that selectively affect durable cognate T:B interactions required for GC formation, but not T:DC contacts involved in T cell activation. Nonetheless, how SAP contributes to effective adhesion between T and B cells has not been elucidated.
Itegrins are well characterized as critical mediators of cellular adhesion (Burbach et al., 2007) and the decreased binding of Sh2d1a−/− T cells to antigen-activated B cells could result from SAP-mediated effects on integrin function. Alternatively, SAP may participate in distinct mechanisms of cell adhesion involving other surface adhesive receptors. SAP is almost entirely composed of a single SH2 domain that binds to a conserved tyrosine-based motif in the cytoplasmic tails of SLAM family receptors, including SLAM, Ly9 (CD229), 2B4 (CD244), CRACC (CD319), Ly108 (NTB-A in human), and CD84 (Calpe et al., 2008). With the exception of 2B4, these receptors engage in homophilic interactions and several self-associate with high affinity (Cao et al., 2006; Yan et al., 2007). Interestingly, there is a structural similarity between these molecules and CD2, which is known to contribute to T cell adhesion (Calpe et al., 2008). Following SLAM or 2B4 engagement, SAP binds these receptors, and recruits the tyrosine kinase Fyn, leading to receptor phosphorylation and initiation of signal transduction (Ma et al., 2007). However, GC formation can be rescued by a mutant of SAP with greatly impaired Fyn binding (Cannons et al., 2006; McCausland et al., 2007), suggesting that SAP participates in additional signaling pathways, perhaps downstream of other SLAM family receptors.
To better understand how SAP influences T cell interactions with diverse antigen-presenting cells (APC), we examined in detail the contributions of integrins and SLAM family members to stable cell association at multiple times after cell-cell encounter. We found that T cells initially adhered to both activated, antigen-presenting B cells and DCs in an integrin-dependent manner. However, unlike long-lived T:DC interactions, prolonged T:B cell associations had both an integrin-dependent and a SAP-dependent component. We further found that GC B cells and Tfh cells expressed high amounts of the SLAM family members CD84 and Ly108 and that activated CD4 cells could adhere to CD84 and Ly108 in a SAP-dependent manner. Importantly, Cd84−/− mice exhibited partial defects in antibody-mediated responses to T-dependent antigens that correlated with impaired Tfh cell function and decreased duration of cognate T:B contacts as imaged in vivo. Strikingly, simultaneous interference with homotypic intercellular binding of both CD84 and Ly108 markedly reduced stable T:B conjugation in vitro. Our findings provide compelling genetic evidence that CD84 is a SAP-dependent adhesive receptor that specifically promotes cognate T:B cell interactions, and thereby is required for optimal GC formation. Moreover, our data suggest that multiple SLAM-related receptors collectively contribute to the stabilization of integrin-initiated cognate T:B cell conjugates, providing new insights into the requirements for cell collaboration during humoral immune responses.
Results
Integrin-mediated adhesion in SAP-deficient CD4+ T cells
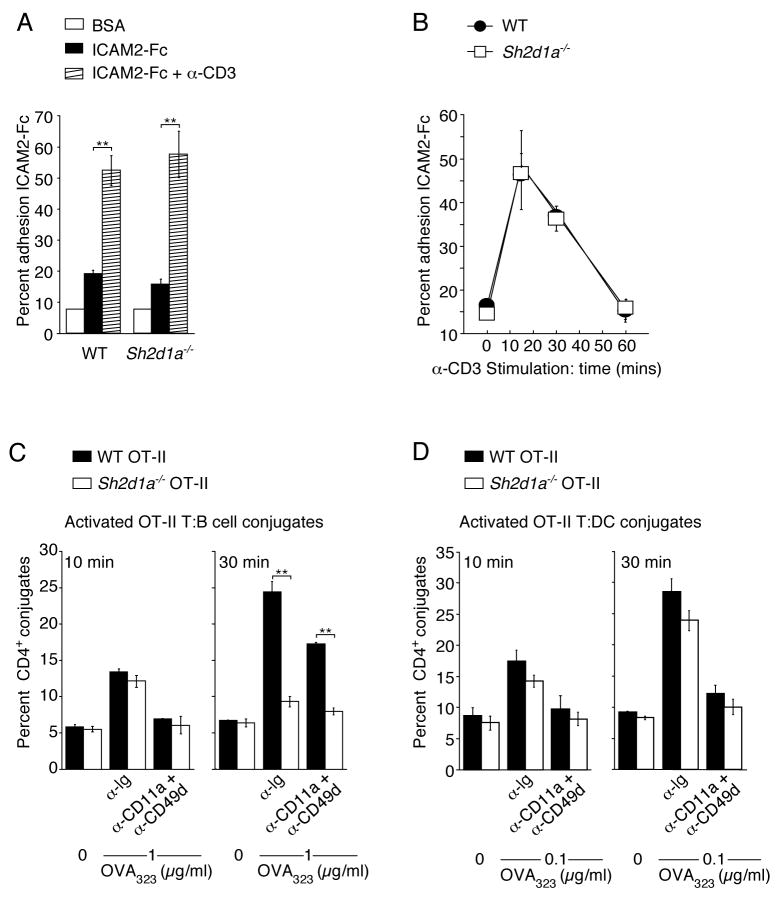
SAP deficiency in CD4 T cells severely impairs their ability to form stable conjugates with cognate B cells while leaving their antigen-specific interactions with DCs grossly intact (Qi et al., 2008). However, the basis for this T cell-intrinsic adhesive defect has remained undefined. Integrins are adhesive receptors that are critical for lymphocyte trafficking into and through tissues as well as for T cell adhesion to APCs. The predominant integrin dimers on T cells are αLβ2 (LFA-1) and α4β1 (VLA-4), which adhere to ICAMs and VCAMs, respectively. Activation through the T cell receptor (TCR) induces rapid increases in integrin affinity and avidity (inside-out signaling) that promote ligand binding, altered cell morphology, and increased adhesion (Burbach et al., 2007). To examine whether SAP is involved in inside-out signaling and integrin-mediated T cell adhesion, we first employed a static adhesion assay that utilizes a ligand-coated surface. When previously activated WT and Sh2d1a−/− OT-II T cells were plated onto ICAM-2 or VCAM-coated surfaces, adhesion was similarly enhanced following α-CD3 stimulation, with a peak at 10–15 min and a steady decline thereafter (Figure 1A and B and data not shown). Similar results were obtained using naïve CD4+ T cells (data not shown). These results suggest that SAP is not required as a general regulator of integrin-mediated adhesion and TCR-induced inside-out signaling in CD4+ T cells.
Figure 1. Integrin-mediated adhesion is critical for initial but not prolonged T:B cell conjugation.
(A–B) Adhesion of pre-activated WT and Sh2d1a−/− CD4+ T cells to recombinant ICAM2-Fc following α-CD3 stimulation (A) 10 min. and (B) 15–60 min. Results presented as the mean percent adhesion (A) n=3 ± SEM. (B) n=6 ± SEM. (C–D) Conjugation efficiency after pre-incubation with blocking integrin antibodies then incubation for 10–30 min with OVA323 pulsed B cells (C), mean ± SEM frequency of CD4+CD19+ conjugates in total CD4+ events (n=3) or (D) DCs, mean ± SEM frequency of CD4+CD11c+ conjugates in total CD4+ events (n=3). **p<0.005.
To explore the possibility that SAP could play a more subtle role in integrin-mediated T cell adhesion to B cells, we used live B cells in a FACS-based adhesion assay. Two time points were analyzed following the onset of T:B cell interactions, one corresponding to the peak (10 min) and the second to the declining phase (30 min) of integrin-mediated adhesion in the static plate-binding assay (Figure 1B). The latter time also corresponds to a duration longer than SAP-deficient T cells adhere to antigen-presenting B cells in vivo, whereas many WT T cells continue their interactions well beyond this time (Okada et al., 2005; Qi et al., 2008). Both WT and SAP-deficient OT-II T cells showed antigen-specific, dose-dependent conjugate formation of comparable magnitude with B cells after 10 min of incubation (Figure 1C and Figure S1A). However, only WT T cells maintained such associations at 30 min, as previously reported (Qi et al., 2008).
SAP could still regulate a distinct late-phase of T cell adhesion to B cells that is mediated by integrins. Alternatively, SAP might operate in a partially integrin-independent manner during this late phase of T:B adhesion. To differentiate between these possibilities, CD4+ T cells were pre-incubated with LFA-1 (CD11a)- and VLA-4 (CD49d)-blocking antibodies. At the 10 min time point (Figure 1C), this treatment largely abrogated T:B cell conjugation. However, it only partially blocked conjugate formation between WT T and B cells at 30 min (Figure 1C). The incomplete blocking did not result from insufficient concentrations of the antibody, because the same treatment reduced T:DC conjugation to near background at both 10- and 30-min time points (Figure 1D). Thus, sustained T:B adhesion does not depend solely on LFA-1 and VLA-4, the dominant integrins expressed by activated T cells, but rather also requires an additional SAP-dependent pathway. These conclusions were supported by results using antigen-activated MD4 B cells, a condition in which Sh2d1a−/− OT-II T cells showed low but measurable conjugate pairing at 30 min (Figure S1B and S1C). Notably, this residual adhesion of Sh2d1a−/− CD4+ T cells to MD4 B cells was completely blocked by integrin antibodies, whereas adhesion of WT CD4+ T cells was only partially blocked, arguing that a distinct SAP-dependent adhesion pathway operates in WT cells during prolonged T:B cell contact. Thus, unlike T:DC adhesion, which is primarily integrin-dependent, T:B cell adhesion has two stages: an early integrin-dependent phase and a later phase that also requires SAP-dependent pathways.
Requirements for SAP-mediated control of T:B adhesion
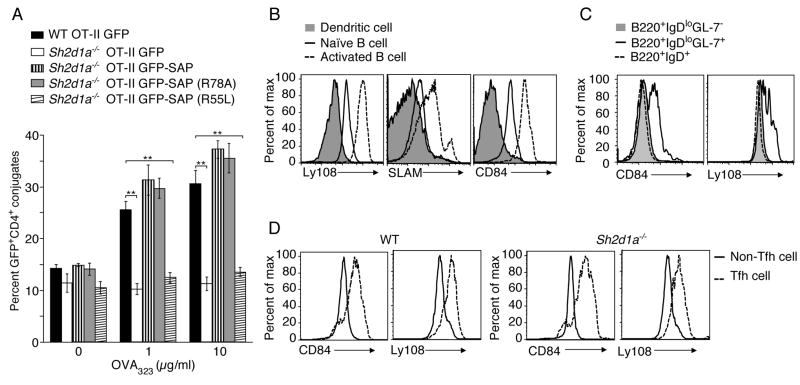
SAP binds to tyrosine-based motifs in the cytoplasmic tail of SLAM family proteins via its SH2 domain. To test whether such binding is required for SAP-mediated control of T:B conjugation, we examined the SAP R55L mutant that prevents SAP from binding SLAM (Calpe et al., 2008; Chen et al., 2004; Li et al., 2003; Ma et al., 2007). We also evaluated the SAP R78A mutant, which shows impaired Fyn binding but can still associate with SLAM family members and rescue GC formation in Sh2d1a−/− mice (Cannons et al., 2006; McCausland et al., 2007). GFP-tagged WT and mutant SAP molecules were introduced into Sh2d1a−/− CD4+ T cell blasts and assayed for conjugation efficiency between OT-II GFP+ T cells and activated, peptide-pulsed B cells. In accord with previous results, the SAP R78A mutant rescued adhesion between Sh2d1a−/− T cells and B cells as efficiently as WT SAP (Qi et al., 2008). However, when expressed at similar amounts, the SAP R55L mutant failed to support T:B conjugate formation at any peptide concentration tested (Figure 2A and Figure S2). These data suggest that interactions with SLAM family molecules via SAP’s SH2 phosphotyrosine binding pocket are required for SAP-mediated control of T:B cell conjugation. Moreover, because the SAP R78A mutant does not permit SLAM or 2B4 phosphorylation and signal transduction but efficiently rescues T:B cell adhesion, it is likely that other SLAM family member(s) are involved this process.
Figure 2. Importance of SAP in T:B adhesion is consistent with SLAM family member expression on activated B cells.
(A) OT-II T:B cell conjugation assays were conducted using CD4+ T cells transiently transfected with DNA constructs expressing either GFP, GFP-SAP, GFP-SAP(R78A), or GFP-SAP(R55L), n=6, mean ± SEM frequency of CD4+CD19+ conjugates in total GFP+CD4+ events, **p<0.005 (See Figure S2 for western blot and representative FACS plot). (B) CD11c+ splenic DCs, splenic B cells and LPS-activated B cells were assessed for SLAM, Ly108, and CD84 expression (n=3). (C–D) Day 9 post-NP-OVA in alum immunization, (C) B cells or (D) Tfh (CD4+CD44+CXCR5hiPD-1hi) and non-Tfh (CD4+CD44+CXCR5loPD-1lo) cells were evaluated for Ly108 and CD84 expression (n=2, 2–5 mice/genotype).
Expression of pattern of SLAM family members on activated B and T cell
DCs, but not B cells, actively contribute to the maintenance of T:APC synapses by mechanisms involving cytoskeletal and membrane dynamics (Al-Alwan et al., 2001; Benvenuti et al., 2004). However, whether T cell adhesion to DCs and B cells involves distinct adhesive receptors has not been examined. It is therefore of interest that we observed higher expression of several SLAM family members, including Ly108, SLAM, and CD84 on resting B cells, as compared to splenic CD11c+ DCs. Activation of B cells further increased the expression of these molecules (Figure 2B) whereas minimal changes in expression occurred on LPS-treated CD11c+ splenic DCs (data not shown). Following immunization with a T-dependent protein antigen, B220+IgDloGL-7+ GC B cells displayed increased expression of CD84 and Ly108 relative to either B220+IgDloGL-7− or B220+IgDhi follicular B cells (Figure 2C). Although CD4+ T cells constitutively express CD84 and Ly108, following immunization activated CD4+CD44+ cells expressing Tfh markers (CXCR5hiPD-1+) from both WT and Sh2d1a−/− mice displayed elevated CD84 and Ly108 expression (Figure 2D), (despite reduced Tfh cells in Sh2d1a−/− mice, see below). These findings suggested that SLAM, CD84, and Ly108 are all potential adhesive ligand/receptors involved in SAP-mediated T:B cell interactions and generation of humoral responses. However, to date, analyses of SLAM-deficient (Slamf1−/−) and Ly108-deficient (Slamf6−/−) mice have not revealed humoral immune defects (Howie et al., 2005; McCausland et al., 2007; Wang et al., 2004). We therefore chose to examine whether CD84 is involved in T:B cell interactions and the regulation of antibody-mediated responses.
Generation and phenotype of Cd84−/− mice
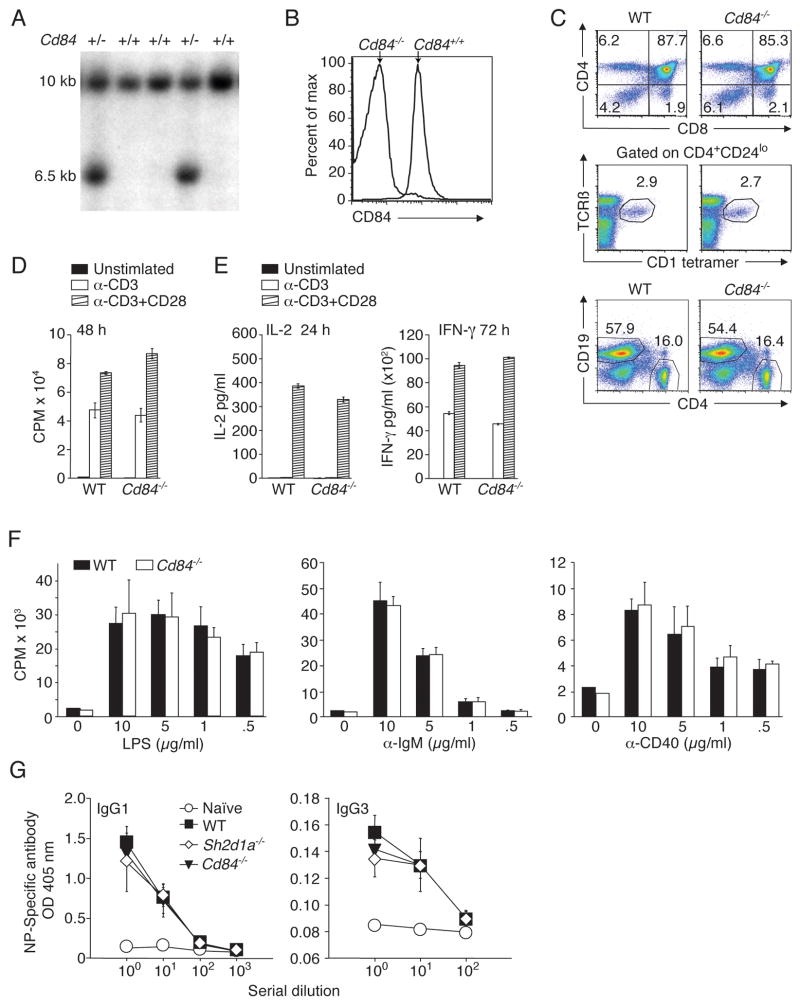
A region on mouse chromosome 1 that contains the Slam gene cluster has been identified by genetic mapping as a lupus susceptibility locus associated with the development of anti-nuclear antibodies: 129 mice carry the lupus-prone haplotype (Chan et al., 2006). To avoid complications from the genetic contribution of the Slam locus from the 129 background, we generated mice with a targeted disruption that introduced multiple stop codons into the second exon of the Cd84 gene by homologous recombination in C57BL/6J ES cells (Figure S3). Gene targeting was confirmed by Southern analyses and the lack of CD84 expression was verified by flow cytometry (Figure 3A and B). Cd84−/− mice were born at expected Mendelian frequencies and were viable, fertile, and grossly morphologically indistinguishable from WT littermates.
Figure 3. Targeted disruption of mouse Cd84 gene does not affect T and B cell development or in vitro stimulation.
(A) Screening for homologous recombination by Southern blot of NcoI digested DNA. WT allele:10kb. Disrupted allele:6.5kb. Targeting vector and locus are shown in Figure S3A. (B) Surface CD84 expression on WT and Cd84−/− splenic B cells. (C) Top panel: thymocytes stained with α-CD4 and α-CD8. Middle panel: thymocytes stained with CD1d-αGalCer tetramers, α-CD4, α-CD24, and α–TCRβ to evaluate NKT cells. Bottom panel: splenocytes stained with α-CD19 and α-CD4 (n=3, 3 mice/genotype). (D) WT and Cd84−/− T cells were stimulated with α-CD3+/− α-CD28 and evaluated for proliferation and (E) IL-2 and IFN-γ production (n=3). (F) WT and Cd84−/− B cells were stimulated for 48 h with LPS, α-IgM, or α-CD40 and evaluated for proliferation (n=4). (G) WT, Sh2d1a−/−, and Cd84−/− mice were immunized with the type II T-independent antigen, NP-Ficoll, and assessed for NP-specific antibody production, day 21 (n=2, 5 mice/genotype).
Cd84−/− mice showed normal thymic cellularity, normal development of conventional CD4+ and CD8+ T cells, and typical numbers of NKT cells, a cell population absent in SAP-deficient mice and XLP patients (Ma et al., 2007) (Figure 3C). Splenic and lymph node cellularity and CD4+ and CD8+ T cell phenotypes were also similar to WT mice (Figure S3B and data not shown). TCR-mediated proliferation and cytokine secretion of purified splenic Cd84−/− T cells were comparable to WT T cells (Figure 3D and E). In addition, T cell activation by peptide-pulsed DCs also appeared grossly normal, as both WT OT-II and Cd84−/− OT-II CD4+ T cells proliferated equivalently as evaluated by CFSE dilution, and secreted similar amounts of IL-2 (Figure S3C and D). Thus, other than a slight reduction in IFN-γ production by Cd84−/− T cells in this latter assay, CD84 deficiency did not overtly alter T cell development and activation.
Although CD84 is constitutively expressed on B cells (Figure 2B), we observed no alterations in bone marrow cellularity, composition, or B cell development in Cd84−/− mice (Figure 3C bottom panel and Figure S3E–G). In response to either LPS, α-IgM, or α-CD40 stimulation, Cd84−/− B cells proliferated comparably to WT B cells (Figure 3F). Furthermore, Cd84−/− mice produced normal levels of serum NP-specific antibodies following immunization with NP-Ficoll, a type II T-independent antigen (Figure 3G). Therefore, Cd84−/− B cells do not exhibit intrinsic defects in responding to antigen or mitogen stimulation in vitro or in vivo. Together, these results suggested that the development and function of B and T lymphocytes from Cd84−/− mice were grossly normal.
CD84 is required for optimal germinal center responses
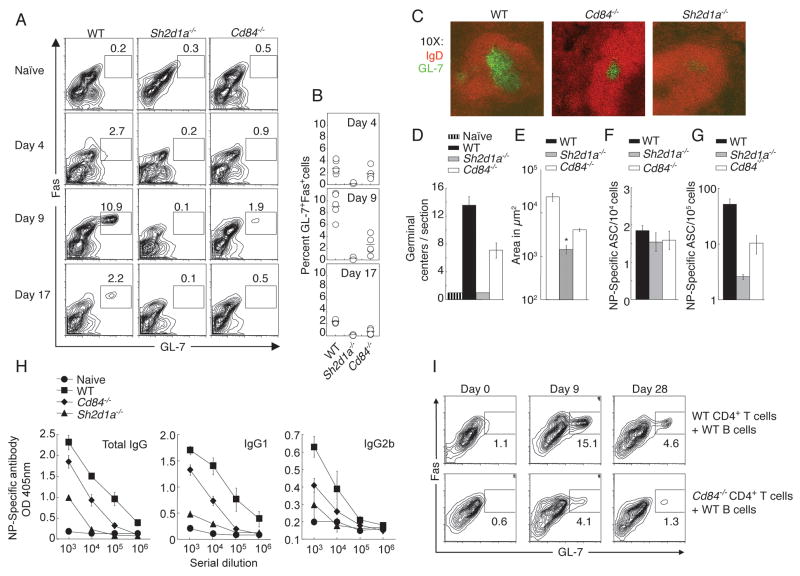
To determine whether CD84 is important for T-dependent humoral responses, mice were immunized i.p. with a T-dependent antigen, NP-OVA. Following immunization, Cd84−/− mice had reduced numbers of B220+IgDloFashiGL-7+ GC B cells at multiple time points examined, as evaluated by flow cytometry (Figure 4A and B). Histological analyses of spleens at day 9 post-immunization revealed fewer GC structures that were also reduced in size in Cd84−/− mice compared to WT mice (Figure 4C, D and E). Similar results were obtained using either alum or Ribi as adjuvants (Figure 4 and data not shown). We did not observe differences in the extent of B cell apoptosis (data not shown), suggesting that the decreases in GC B cells numbers were not secondary to increased cell death.
Figure 4. Impaired humoral response in the absence of CD84.
WT, Sh2d1a−/−, and Cd84−/− mice were immunized with T-dependent antigen NP-OVA in alum or Ribi. (A) GC B cell development evaluated by gating on B220+IgDlo cells: GC B cells are FashiGL-7+. (B) Percentage of B cells with GC markers at day 4, 9 and 17 post-immunization. (C) Splenic GC were detected via staining with IgD and GL-7 day 9. (D) GC number/section, day 9. (E) GC size, day 9. * Size of the rare (2) GC identified in Sh2d1a−/− sections. (F) NP-specific IgG ASCs in the spleen, day 9. (G) Long-lived NP-specific plasma cell responses were measured in bone marrow, day 30. (H) NP-specific [NP-(30)], day 30 (See Figure S4A for high affinity NP-specific [NP-(3)] responses). Results are shown from mice immunized with NP-OVA in alum (A, B and H, n=2, 3–5 mice/genotype/time point) or NP-OVA in Ribi (C, D, E, F and G n=3, 6–8 mice/genotype/time point). Similar results were obtained with either adjuvant. (I) Cd84−/− or WT sorted naïve CD4+CD62L+CD44lo T cells and WT B cells were co-transferred into Rag2−/− hosts. Day 25 following reconstitution, Rag2−/− mice were immunized with SRBCs and evaluated for GC development (See Figure S4B for antibody titres), n=2, 4 mice/genotype/time point.
In contrast to short-lived antibody secreting cells (ASC), long-lived plasma cells are typically derived from GC B cells and are severely reduced in Sh2d1a−/− mice (Crotty et al., 2003). These plasma cells migrate to the bone marrow and continue to secrete antibody for extended periods of time (Allen et al., 2007). Although both Sh2d1a−/− and Cd84−/− mice developed a considerable NP-specific ASC response at day 9 (Figure 4F), they had fewer NP-specific ASCs in the bone marrow 30 days post-immunization (Figure 4G). This reduction in long-lived plasma cell generation correlated with lower total amounts of NP-specific antibodies in serum at day 30 (Figure 4H). Interestingly, Cd84−/− mice did develop high affinity NP-specific antibodies, albeit at lower titers than WT mice (Figure S4A). Together, these data demonstrate that CD84 is required for maximal GC formation and T-dependent antibody production. However, in absolute magnitude, the defects displayed by Cd84−/− mice were intermediate relative to the robust WT responses and the extremely poor responses of Sh2d1a−/− mice.
Cd84−/− mice have T cell intrinsic defects
A large body of data indicates that the humoral defects in Sh2d1a−/− mice are T-cell intrinsic (Calpe et al., 2008; Schwartzberg et al., 2009). To determine if there was a T-cell intrinsic component to the impaired GC response in Cd84−/− mice, we reconstituted Rag2−/− mice with either WT or Cd84−/− CD4+ T cells along with WT B cells, waited 25 days to minimize effects of homeostasic expansion, and immunized with sheep red blood cells (SRBCs). Under these conditions, WT T cells provided help to generate robust GC responses. However, Cd84−/− T cells were less efficient at supporting GC and long-term antibody responses (Figure 4I and Figure S4B). Similarly results were obtained with adoptive transfer of WT, Sh2d1a−/−, or Cd84−/− OT-II GFP+ CD4+ T cells into SAP-deficient hosts that were subsequently immunized with NP-OVA. Although Cd84−/− OT-II T cells rescued some GC formation, the extent of this rescue was reduced relative to WT cell transfers (Figure S4C). Although these results do not rule out a B cell component to this phenotype, and indeed we would expect there to be one since CD84 is its own ligand, our results demonstrate that Cd84−/− T cells have an intrinsic defect in supporting help for GC formation.
Evaluation of Tfh cell differentiation and function
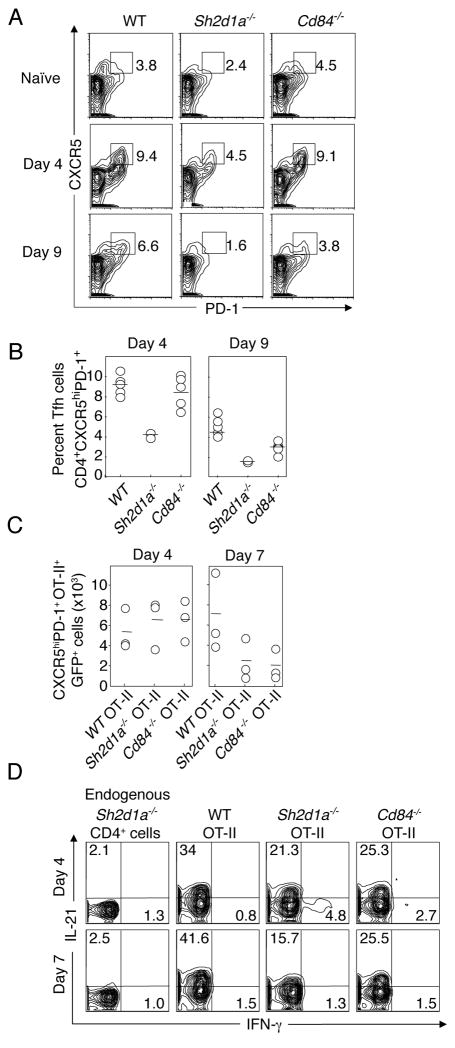
To determine if the sub-optimal GC response observed in Cd84−/− mice was a result of impaired Tfh cell differentiation or function, we evaluated the expression of the Tfh cell markers, CXCR5 and PD-1, on activated T cells in WT, Sh2d1a−/−, or Cd84−/− mice following immunization. Both Sh2d1a−/− and Cd84−/− CD4+ T cells were able to upregulate Tfh cell markers early in the response (day 4). While there was a reduction in the percentage of cells expressing Tfh cell markers in Sh2d1a−/− mice, this reduction was much less severe than the subsequent defect in GC formation (Figure 4A). However, at time points when GC formation was maximal (days 7–9), there was a clear decrease in CD4+ T cells expressing Tfh cell markers in both Sh2d1a−/− and Cd84−/− mice (Figure 5A and B). Similar results were observed when WT, Sh2d1a−/−, or Cd84−/− OT-II GFP+ T cells were transferred into Sh2d1a−/− hosts and Tfh cell markers evaluated following NP-OVA immunization (Figure 5C). In these experiments, we observed no differences between the genotypes early in the response, yet clear differences in Tfh cells at the time of maximal GC formation. These results are reminiscent of our previous findings where Sh2d1a−/− CD4+ T cells initially expressed markers consistent with Tfh differentiation yet were unable to deliver contact-dependent B cell help and were neither efficiently recruited into nor retained within the GC (Qi et al., 2008). Interestingly, relative to WT cells there was a reduction in the percentage of both Sh2d1a−/− and Cd84−/− OT-II GFP+ T cells producing IL-21 (Figure 5D), a cytokine that is highly expressed by Tfh cells and serves as a potent B cell stimulator (Fazilleau et al., 2009). Similar results were observed with T cells from immunized WT, Sh2d1a−/− and Cd84−/− mice (Figure S5). Although IL-21 is produced by multiple effector CD4+ T cell lineages, IL-21 is highly expressed by Tfh cells (Figure S5 (Fazilleau et al., 2009; Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009)) suggesting there may be defects in Tfh cell differentiation and/or function in the absence of either SAP or CD84. Thus, despite the initial expression of activation markers consistent with Tfh cell development, both Sh2d1a−/− and Cd84−/− mice have reduced numbers of Tfh cells at the time of maximal GC development, suggesting a defect in final Tfh differentiation or maintenance.
Figure 5. Evaluation of Tfh cells.
(A–B) Flow cytometric analysis of cells expressing Tfh markers from WT, Sh2d1a−/−, and Cd84−/− mice at day 4 and 9 post-immunization with NP-OVA in alum. (A) Representative FACS plots (gated on CD4+ cells). (B) Percent Tfh cells, n=2, 2–5 mice/genotype/time point. (C–D) WT, Sh2d1a−/−, or Cd84−/− OT-II GFP+ T cells were transferred into Sh2d1a−/− hosts subsequently immunized with NP-OVA in alum (n=2, 3 mice/genotype/time point). (C) Number of WT, Sh2d1a−/−, or Cd84−/− OT-II GFP+ Tfh (CD4+CXCR5hiPD-1hi) cells day 4 and 7 post-immunization. (D) IL-21 production from WT, Sh2d1a−/−, or Cd84−/− OT-II GFP+ cells. (See Figure S5 for Tfh IL-21 production)
Cd84−/− CD4+ T cells are defective in adhesion to cognate B cells
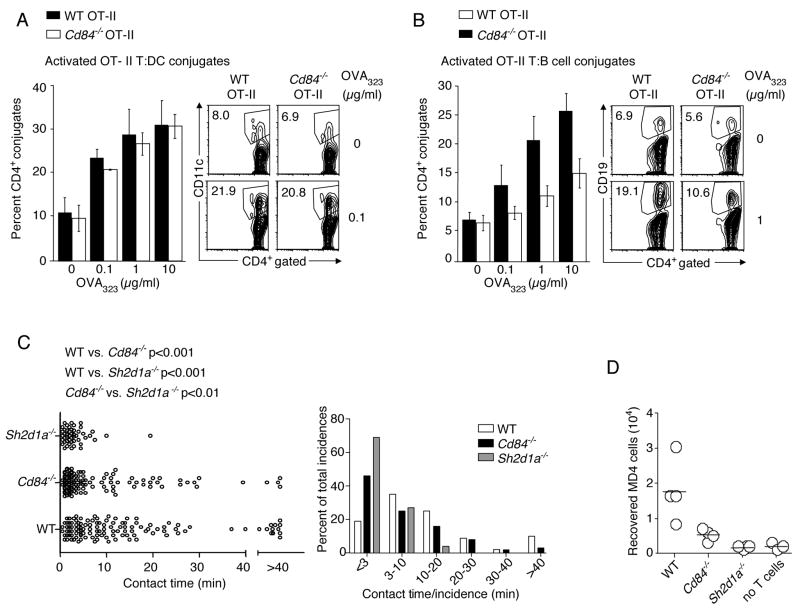
To examine whether CD84, like SAP, contributes to GC formation by affecting the stability of T:B cell interactions, we evaluated conjugation between activated OT-II CD4+ T cells and OVA323 pulsed DCs or LPS-activated B cells in vitro. Dose-dependent increases in T:DC conjugate frequency were observed with both WT and Cd84−/− OT-II T cells (Figure 6A). Similar results were obtained using naïve OT-II T cells (data not shown). However, when B cells were used as the APCs, Cd84−/− OT-II T cells were significantly less efficient (*p<0.05; 1 μg/ml, **p<0.005; 10 μg/ml) in forming conjugates as compared to their WT counterparts (Figure 6B). Thus, CD84 expression on T cells contributes to optimal antigen-specific T:B adhesion in vitro. Nonetheless, Cd84−/− T cells were better at forming conjugates with B cells than Sh2d1a−/− T cells, especially at higher peptide concentrations (compare Figures 6B and 7E).
Figure 6. Cd84−/− OT-II T cells are defective in adhesion to cognate B cells.
(A-B) Conjugation efficiency of pre-activated WT and Cd84−/− OT-II T cells with OVA323 pulsed splenic (A) CD11c+ DCs, mean ± SEM frequency of CD4+CD11c+ conjugates in total CD4+ events (n=4, representative FACS plots, right panel), (B) LPS-activated B cells, mean ± SEM frequency of CD4+CD19+ conjugates in total CD4+ events (n=4, representative FACS plots, right panel) (*p<0.05, **p<0.005). (C) In vivo contact durations between T cells of indicated genotypes and MD4 B cells as measured by intravital microscopy between 60–72 h after immunization with HEL-OVA in alum. Individual contact durations (left) and their distribution (right) are shown. A total of 125, 120, and 49 contacts pooled from 3 experiments for WT, Cd84−/−, and Sh2d1a−/− T cells, respectively. Mean contact times: WT 14.5+/−1.2 min; Cd84−/− 8.5+/−2.1 min; Sh2d1a−/− 3.5+/−1.6 min. The p values were calculated by nonparametric one-way ANOVA. (D) Recovery of MD4 B cells from draining lymph nodes 96 h post-HEL-OVA immunization with or without exogenous OT-II T cells of indicated genotypes. Each symbol represents 1 of 4 mice/group. Data from 1 of 3 experiments with similar results are shown.
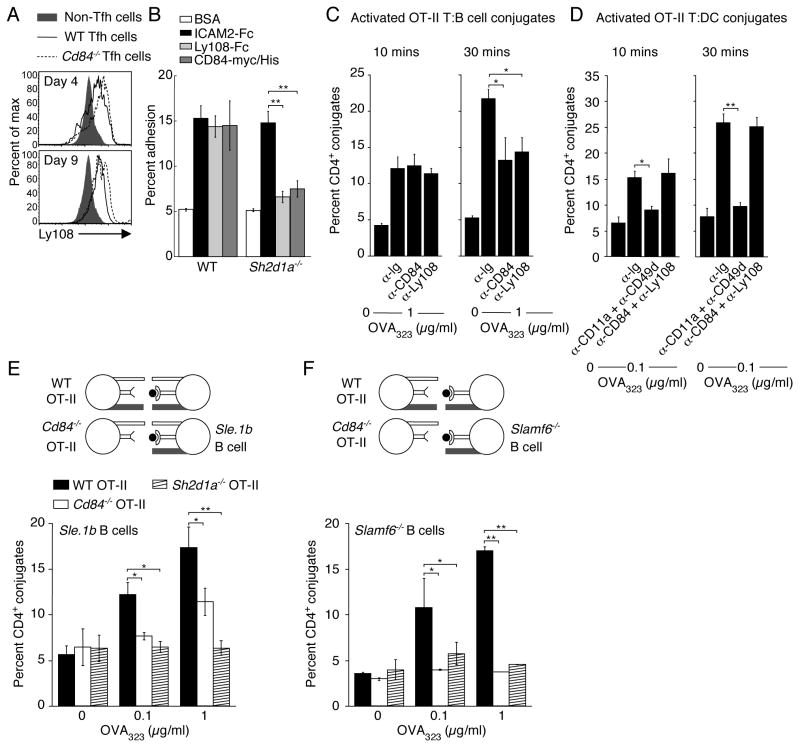
Figure 7. CD84 and Ly108 are adhesive receptors and contribute to long-lived stable T:B cell conjugates.
(A) Ly108 expresssion on Tfh (CD4+CXCR5hiPD-1hi) cells from WT and Cd84−/− mice post-NP-OVA in alum immunization. (B) Evaluation of pre-activated WT and Sh2d1a−/− T cell adhesion to recombinant ICAM2-Fc, Ly108-Fc, and CD84-myc.HIS assessed for 30 min (n=4). (C-D) Conjugation efficiency of blasted WT OT-II T cells, pre-incubated with α-CD84 or α-Ly108, with OVA323-pulsed LPS-activated B cells (C), mean ± SEM frequency of CD4+CD19+ conjugates in total CD4+ events (n=4) or DCs (D), mean ± SEM frequency of CD4+CD11c+ conjugates in total CD4+ events (n=3). (E–F) Conjugation frequencies of pre-activated WT, Sh2d1a−/−, and Cd84−/− OT-II T cells with OVA323-pulsed LPS-activated B cells for 30 min from (E) Sle.1b congenic or (F) Slamf6−/− mice, mean ± SEM frequency of CD4+CD19+ conjugates in total CD4+ events, n=3 (See Figure S6 for representative FACS plot). (*p<0.05, **p<0.005).
To determine whether these observations apply in vivo, we examined the dynamics of cognate T:B interactions using 2-photon intravital imaging. WT, Cd84−/−, or Sh2d1a−/− OT-II GFP+ T cells were transferred along with WT MD4 CFP+ B cells into recipient mice that were subsequently immunized with HEL-OVA conjugate antigen. Under these conditions, Sh2d1a−/− OT-II T cells typically made contacts with MD4 B cells that mostly lasted less than 10 min and they were incapable of forming long-lasting conjugates with these B cells (>30 min duration), in contrast to their WT counterparts (Figure 6C and Movie S1) (Qi et al., 2008). Although the bulk of the contacts involving SAP-deficient T cells lasted less than 3 min, these cell contacts still lasted longer on average than non-cognate interactions (Qi et al., 2008). Cd84−/− OT-II T cells, however, exhibited an intermediate phenotype. Relative to WT, Cd84−/− OT-II T cells had a reduced ability to form the longest-lasting conjugates seen with WT T cells and MD4 B cells, although they were more efficient than SAP-deficient T cells in maintaining contacts with B cells lasting longer than 10 min. These findings are consistent with the notion that CD84 on T cells contributes to the efficiency of cognate adhesion with B cells in a physiologic lymphoid environment. This intermediate defect in adhesion to B cells correlated with a reduced capacity of Cd84−/− OT-II T cells to promote MD4 B cell clonal expansion in this co-transfer system (Figure 6D). Thus, our results suggest that CD84, like SAP, contributes to a normal GC response by promoting cognate T:B cell interactions and the efficient delivery of contact-dependent T cell help to B cells required for B cell expansion and GC formation.
CD84 and Ly108 are adhesive receptors that contribute to sustained T:B adhesion
The intermediate phenotype observed with Cd84−/− CD4+ T cells relative to WT and Sh2d1a−/− CD4+ T cells both in vitro and in vivo suggested that other SLAM family receptors participate in SAP-dependent regulation of T:B cell interactions. Ly108 is an intriguing candidate, because like CD84, it shows homophilic interactions, self-associates with high affinity (Cao et al., 2006; Yan et al., 2007), and is upregulated on GC B cells and Tfh cells (Figure 2C and E). Intriguingly, Ly108 was expressed slightly higher on Cd84−/− compared to WT Tfh cells suggesting partial functional compensation between these SLAM family members (Figure 7A). To examine whether CD84 and Ly108 can directly mediate adhesion, we compared binding of pre-activated WT and Sh2d1a−/− CD4+ T cells to recombinant CD84, Ly108, and ICAM-2. WT CD4+ T cells bound to plastic surfaces coated with any of the 3 ligands. However, despite similar Ly108 and CD84 expression on WT and Sh2d1a−/− CD4+ T cells, adhesion to CD84 or Ly108 but not ICAM-2, was clearly decreased in Sh2d1a−/− cells (Figure 7B). Stimulation of WT and Sh2d1a−/− CD4+ T cells with α-CD3 had no effect on binding to either CD84 or Ly108 at multiple time points examined, suggesting that their adhesion does not change upon TCR engagement (data not shown).
To evaluate the possibility that both CD84 and Ly108 contribute to stabilization of T:B cell interactions, CD4+ T cell blasts were pre-incubated with either α-CD84 or α-Ly108 prior to conjugate formation. Addition of α-CD84 or α-Ly108 significantly (p<0.05) reduced T:B cell conjugate efficiency at 30 min but not at 10 min (Figure 7C), yet did not appreciably affect T:DC conjugation at any time point tested (Figure 7D). Together, our results provide evidence that both CD84 and Ly108 can function as SAP-dependent adhesion receptors that specifically promote sustained T:B cell conjugation.
CD84 and Ly108 cooperate in promoting T:B cell conjugation
The ability of CD4+ T cells to bind either CD84 or Ly108 in a SAP-dependent manner raises the interesting possibility that coordinated action by CD84 and Ly108 contributes to the profound defect in prolonged T:B cell adhesion exhibited by Sh2d1a−/− T cells. Ideally, this could be tested in mice deficient in both CD84 and Ly108. However, these two genes are located next to each other on mouse chromosome 1, making it difficult to generate the desired double deficiency. To overcome this obstacle, we took advantage of the homophilic properties of both CD84 and Ly108 molecules (Cao et al., 2006; Yan et al., 2007). We paired Cd84−/− CD4+ T cells and Ly108-deficient (Slamf6−/−) B cells in the in vitro conjugate assay, thus creating a functional “T:B double mutant” where neither homophilic receptor could be engaged in trans between the two lymphocyte types (see diagrams in Figure 7E and F). Because Slamf6−/− mice were generated on a 129 background, their SLAM locus expresses the 129 alleles, even though they are partially backcrossed to C57Bl/6 mice (confirmed by staining for Ly9 polymorphic epitopes, data not shown). We therefore used B cells isolated from Sle.1b congenic mice (C57Bl/6 mice expressing the 129 SLAM locus) as controls in this assay (Wandstrat et al., 2004). Sh2d1a−/− OT-II T cells failed to form stable conjugates with control Sle.1b congenic B cells while Cd84−/− OT-II T cells again demonstrated reduced conjugate formation (Figure 7E). WT OT-II T cells formed conjugates with Sle.1b congenic B cells; however, the percentage formed was slightly reduced relative to B6 activated B cells (compare Figure 7E to 1C and D), despite similar expression of SLAM, CD84, and Ly108 (data not shown). Strikingly, although WT OT-II T cells formed conjugates with LPS-activated Slamf6−/− B cells in a similar dose-dependent manner to that seen using Sle.1b congenic B cells, Cd84−/− OT-II T cells formed very few conjugates with Slamf6−/− B cells at any peptide concentration examined, analogous to the defect observed with SAP-deficient OT-II T cells (Figure 7F and Figure S6). Thus, multiple SLAM family members collectively contribute to T:B conjugation in vitro and may functionally compensate for each other during T:B lymphocyte interactions.
Discussion
Proper functioning of the adaptive immune system requires effective intercellular co-operation involving direct physical interactions among lymphocytes and myeloid lineage cells. Our previous studies revealed that the small adapter protein SAP plays a critical role in GC formation and that a major effect of SAP deficiency is to limit the duration of effective T:B contact required for delivery of helper signals, without a comparable effect on initiation of T cell immunity involving DCs (Qi et al., 2008). Here we explored the mechanistic basis for these observations and provide evidence that while integrins, in particular LFA-1 and VLA-4, are major adhesive receptors involved in stable, antigen-dependent T:DC interactions and early T:B associations, sustained T:B cell contacts are also dependent on SAP and the associated SLAM family member CD84. Thus, without the proper functioning of SAP and SLAM family receptors, integrin-dependent interactions with B cells are insufficient to sustain effective delivery of T cell help required for GC formation.
SLAM family members are a series of receptors on hematopoietic cells that bind to the adaptor SAP to initiate downstream signaling cascades. The homophilic nature of many of these receptors, combined with high affinity binding (Cao et al., 2006; Yan et al., 2007) and homology to CD2 (Calpe et al., 2008), suggests potential roles as cell adhesion receptors, although direct demonstration of such functionality has been limited (Howie et al., 2002; Nanda et al., 2005). Our results provide clear evidence that T cells adhere to CD84 in a SAP-dependent manner. Moreover, the observation that Cd84−/− T cells display a reduced ability to support GC formation, suggests that CD84 is critical for the sustained T:B cell interactions required for this process. These data furthermore indicate that the initiation and the maintenance phases of stable T:B cell interaction have distinct requirements for different receptor families, with the initial phase primarily controlled by integrins and the later phase also requiring SAP and SLAM family members. The requirement for SAP and SLAM family members in this sustained phase is therefore a major difference between T cell interactions with antigen-presenting DCs versus B cells. Our results further raise the possibility that these differences result in part from the selective expression patterns of the SLAM family receptors on these distinct cell populations.
Our study provides evidence that deficiency of a specific SLAM family receptor, CD84, partially phenocopies the humoral defects seen in the absence of SAP by affecting T:B cell adhesion and GC formation. Of note, both T:B cell interactions and GC formation are markedly improved when Sh2d1a−/− cells are reconstituted with SAP R78A mutant (Cannons et al., 2006; McCausland et al., 2007; Qi et al., 2008). Interestingly, CD84 can be tyrosine phosphorylated in SAP-deficient T cells from XLP patients (Tangye et al., 2003) raising the possibility that CD84 does not require SAP R78-mediated Fyn recruitment for receptor tyrosine phosphorylation, while still requiring SAP for downstream signal transduction.
The partial nature of the humoral defects in Cd84−/− mice suggested potential collaboration between SLAM family members. Indeed, we found that Ly108 is also a SAP-dependent adhesive receptor that contributes to T:B cell conjugation in vitro. Interestingly, Ly108 phosphorylation, albeit greatly reduced, is detected in thymocytes expressing the SAP R78A mutant, indicating that Ly108 can undergo some tyrosine phosphorylation independent of direct SAP R78-mediated Fyn contact (Zhong and Veillette, 2008). This study also demonstrated Vav1 phosphorylation in response to Ly108 engagement (Zhong and Veillette, 2008). Vav proteins are central mediators of cytoskeletal reorganization that act as exchange factors for the Rho family GTPases, Cdc42, Rac and Rho (Burbach et al., 2007). Previous data from NK cells demonstrated cooperative synergistic binding to insect target cells expressing ICAM-1 and CD48 (the ligand for 2B4) (Barber and Long, 2003; Bryceson et al., 2005) leading to increased Vav phosphorylation (Riteau et al., 2003). Thus, NK:target and T:B cell contacts provide interesting parallels where potential signaling cross-talk between integrins and SLAM-related receptors may help coordinate cytoskeletal rearrangement and adhesion. Although we have not seen evidence for “inside-out signaling” to integrins from these SLAM family receptors in our assays, it is possible that such cross-talk also adds to amplification of adhesion processes or is required for sustained adhesion in vivo.
Our in vitro data support direct roles for both CD84 and Ly108 in promoting and/or stabilizing prolonged T:B cell contacts. These results are reminiscent of those recently observed for NKT cell development where chimeric mice that were functional double knockouts revealed that both SLAM and Ly108 participate in this process and are partially redundant, despite minimal to no defects in NKT cell development in gene-targeted mice affecting the individual receptors (Griewank et al., 2007). While it is plausible that other SLAM family members, including SLAM and Ly9, contribute to stable T:B cell interactions, adhesion between Cd84−/− CD4+ T cells and Slamf6−/− B cells in vitro is as defective as that seen in the absence of SAP, suggesting that these are major SLAM family members involved in T:B cell interactions required for delivery of T cell help and productive GC formation.
These latter findings are consistent with our data showing that both CD84 and Ly108 are highly expressed on both GC B cells and CD4 Tfh cells, as well as previous gene-expression studies showing that Tfh cells express high levels of SAP and CD84 (Chtanova et al., 2004; Vinuesa et al., 2005). Tfh cells are recognized as a specialized subclass of effector CD4+ T cells that reside within GCs and are critical for their formation and maintenance. In addition to expressing molecules important for GC development, including CD40L and ICOS, this effector population is characterized by IL-21 production and Bcl-6 expression (Fazilleau et al., 2009; Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009). Although Sh2d1a−/− mice have T-cell intrinsic defects in GC formation, the effects of SAP-deficiency on Tfh cell differentiation are less clear. While SAP-deficiency decreased percentages of Tfh cells in a model of chronic autoimmunity (Linterman et al., 2009), we found, using intact animals and cell transfers that at early time points post-immunization, Sh2d1a−/− and Cd84−/− activated CD4+ T cells can upregulate the Tfh cell markers, CXCR5 and PD-1. However, at the time of maximal GC formation, Tfh cells are reduced in the absence of either SAP or CD84. Moreover, we observed a reduction in IL-21 production from Sh2d1a−/− and Cd84−/− CD4+ T cells. Because Tfh cells are associated with high IL-21 expression, these findings support the idea that full differentiation of functional Tfh cells may require sustained T:B cell contact (Haynes et al., 2007; Nurieva et al., 2008; Qi et al., 2008). Our observations therefore raise the possibility that Tfh cell differentiation is a multi-step process and it is the later phase that is most affected by SAP deficiency.
Recent data indicate that SLAM family members contribute to the development of autoimmunity and increased anti-nuclear antibody production (Chan et al., 2006). Thus, it is intriguing that we observed differences in conjugate efficiencies between B6 WT CD4+ T cells and B cells isolated from B6 and Sle.1b congenic mice despite comparable surface expression of SLAM family members. These results highlight the importance of the genetic background of mice deficient in SLAM family members for evaluating their function. It is also of note that SAP deficiency ameliorates disease in several models of autoimmunity (Chan et al., 2006; Linterman et al., 2009). While the use of SAP-based therapeutics would be fraught with potential complications from EBV or malignant B cells, the milder defects observed in Cd84−/− mice raise the possibility of CD84 as an attractive alternative therapeutic target for autoimmune disorders with a humoral component.
Finally, SLAM family members have diverse roles in immune cell development and function (Calpe et al., 2008; Ma et al., 2007). Our results provide evidence that a SLAM family member, CD84, is involved in GC formation and the generation of humoral immunity, and moreover, suggest that multiple family members cooperate in this process. Our results further indicate that SLAM family members participate in these processes by specifically affecting adhesion between lymphocytes as opposed to binding between T cells and other cell types involved in MHC-dependent antigen presentation. It is thus highly intriguing that many of the phenotypes of Sh2d1a−/− mice and SAP-deficient XLP patients are related to cellular interactions that occur specifically between lymphocytes, including impaired T and NK cell killing of virally-infected or transformed B lymphocytes, impaired T cell help for B cells, and impaired generation of “innate” T lymphocytes selected by recognition of ligand on other thymocytes, such as is the case for NKT cells (Qi et al., 2008; Schwartzberg et al., 2009). By providing support for a direct role of SLAM family members in lymphocyte:lymphocyte interactions, our results provide a conceptual framework for understanding these diverse phenotypes.
Experimental Procedures
Mice
Cd84 DNA was isolated from a C57BL/6J BAC clone and inserted into pPNT double loxP so that the second exon of the Cd84 gene encoding the IgV ecotodomain of CD84 was replaced with 2 stop codons followed by PGK-Neo. The targeting vector was linearized with NotI and electroporated into HGTC-8 C57BL/6J ES cells (Cheng et al., 2004), which were selected in G418 and FIAU. DNA isolated from ES cell clones were screened by Southern blot using NcoI digestion to generate a 10kb band from the endogenous Cd84 allele, and a 6.5kb band from the targeted allele. Single integration was confirmed by screening for Neo. Cd84+/− ES cell clones were injected into Balb/c blastocytes and chimeric mice bred to C57BL/6J mice to generate pure C57BL/6J F1 mice that were either bred to homozygosity or bred to C57BL/6J for one further generation before additional breeding. Sh2d1a−/− mice (Czar et al., 2001) were backcrossed to B6 for 10 generations. C57BL/6, Rag2−/−, Ub-GFP-expressing, CFP-expressing transgenic, HEL-specific Ig-transgenic MD4 and OVA323–339-specific TCR-transgenic OT-II mice were purchased from Jackson. Slamf6−/− mice on a mixed 129 × B6 F1 were purchased from the MMRRC and were backcrossed to C57Bl/6 2 generations prior to breeding to homozygosity. Sle.1b congenic mice were described (Wandstrat et al., 2004). All mice were maintained under specific-pathogen free conditions, and used in accordance of NIH institutional guidelines for animal welfare.
Adhesion assay
96-well Nunc MaxiSorp plates were coated with mouse rICAM-2/Fc (0.3μg/well), rVCAM/Fc (0.3μg/well), rLy108-1/Fc (0.6μg/well) (R&D) or rCD84-myc/HIS tagged protein (0.6μg/well). Assays were performed using preactivated CD4+ T cells as described (Finkelstein et al., 2005).
Generation and purification of recombinant CD84
The portion of the gene encoding the extracellular domain of CD84 was amplified by RT-PCR from C57Bl/6 splenic RNA and subcloned into pcDNA3.1/myc.His (Invitrogen). Constructs were transiently transfected into CHO cells using Lipofectamine 2000 (Invitrogen) and expression confirmed by intracellular staining. CD84-myc.His tagged protein was purified using the Probond Ni NTA purification system for His-tagged recombinant proteins (Invitrogen) and concentrated via centricon YM-30 (Amicon) at 5000G. Recombinant protein was dialysed against PBS and purification evaluated by SDS-PAGE stained with Coomassie and immunoblotting using α-myc (Cell Signaling Technology).
Proliferation, differentiation and cytokine production
T cells purified by mouse T cell enrichment column (R&D) were stimulated with immobilized α-CD3 (1μg/ml) +/− α-CD28 (5μg/ml). Proliferation evaluated by [3H]-thymidine pulse 24–72 h post-stimulation. Supernatants were isolated at indicated times for cytokine analysis via ELISA (R&D). CD4+ T cells were purified by negative selection using CD4 isolation kits (Miltenyi Biotec). To isolate DCs, spleens were digested with Liberase CI (Roche) and purified using CD11c microbeads (Miltenyi Biotec). Lineage differentiation was evaluated by restimulating cells with PMA (10ng/ml) and ionomycin (1μg/ml) for 5 h and staining for intracellular cytokine production using α-IFN-γ antibody (BD Biosciences) while IL-21 was detected using IL-21RFc (R&D) (Suto et al., 2008). Polyclonal or MD4 B cells were isolated by naïve CD43 B-cell isolation kit (Miltenyi Biotec). B cells were stimulated with varying concentrations of LPS, soluble α-CD40 (BD Bioscience) or α-IgM (Jackson ImmunoResearch) and evaluated for proliferation via [3H]-thymidine incorporation.
Immunization, ELISAs and ELISPOT
For T-dependent responses mice were injected i.p. with 100μg of NP-OVA (Biosearch Technologies Inc.) in Ribi (Corixa) or alum (Pierce) or immunized with SRBCs (Colorado Serum Company). For T-independent responses, mice were immunized with 100μg NP-Ficoll (Biosearch Technologies Inc.) in 0.2 ml HBSS. ELISAs were performed using 96-well flat-bottom Immuno Plates (Nunc) (Cannons et al., 2006). Goat α–mouse IgG+M+A (Caltag Laboratories) or [NP-(18)-BSA] was used to capture total or NP-specific antibody-secreting cell ELISPOTs (Cannons et al., 2006).
Flow cytometry and microscopy
Antibodies were from the following sources: anti-Fas, GL-7, IgMa, CXCR5, CD44, CD24, TCRβ (BD Bioscience), IgD (SouthernBiotech), CD84, SLAM (Biolegend), Ly108, B220, CD19, CD4, CD11c, CD11a, CD49d, PD-1 (eBioscience). Flow cytometry data analysis was performed using Flojo software (Treestar). GCs were identified as described (Cannons et al., 2006). The number GCs (GL-7+IgD− areas in the follicular mantle) were determined by systematic examination of the entire spleen section through the microscope ocular. GC images were captured at 20 X and Zeiss AIM (v 4.2) software was employed to determine GC area in μm2.
In vitro cell conjugation assay
OT-II T cells (5×105/well) were incubated for 10–30 min (37°C) in 96-well U-bottom plates with DCs (106/well) or LPS-activated B cells (2×106/well) pulsed with antigen OVA323 (AnaSpec) or antigen-(HEL-OVA or HEL-BSA)-activated MD4 B cells (2×106/well). Conjugate frequencies were enumerated by flow cytometry after the cell mixture was stained at 4°C for CD4, CD11c and CD19. For blocking experiments, T cells were pre-incubated for 30 min at 4°C prior to conjugation with 20μg/ml of α-CD11a, α-CD49d, α-CD84, or α-Ly108, which were included throughout the conjugation. Transfection of CD4+ T cells was performed via Amaxa nucleofection, as previously described (Qi et al., 2008).
Adoptive transfer, intravital imaging, and assay of B cell expansion in vivo
A total of 1×105 OT-II T cells of a given genotype were transferred into Sh2d1a−/− recipients that were then immunized subcutaneously with 50μg of NP-OVA in alum. For intravital imaging, 5×104 T cells of one given genotype and 2×105 B cells/mouse were co-transferred into naïve B6 or CD45.1 congenic B6 recipients, which were then immunized with 20–30μg chemical conjugates of OVA and HEL or BSA and HEL mixed in alum and 0.2μg LPS. Intravital imaging of the draining lymph node was conducted 60–72 h later, and MD4 B cell expansion was measured by flow cytometry 96 h post-immunization. Hardware and software methods used for imaging data acquisition and analysis have been described (Qi et al., 2008).
Statistical analysis
For intravital imaging analysis the non-parametric Mann-Whitney rank sum test was used when data points exhibited highly skewed and non-Gaussian distributions. For Gaussian-like distribution, t tests were used to compare endpoint means of different groups. Statistical differences between analyzed groups were calculated with the paired Student’s t test. Values of p <0.05 are considered significant. Calculation and graphing were done in Prism (GraphPad) and Excel (Microsoft).
Supplementary Material
Acknowledgments
We thank R. Handon and A. Venegas for invaluable technical assistance; M. Kirby and S. Anderson for cell sorting; J. Fekecs for graphics assistance and K. Nichols and S-H, Hwang for mouse reagents. This work was funded by the intramural research programs of the NHGRI and NIAID, NIH, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Alwan MM, Rowden G, Lee TDG, West KA. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol. 2001;166:1452–1456. doi: 10.4049/jimmunol.166.3.1452. [DOI] [PubMed] [Google Scholar]
- Allen CDC, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DF, Long EO. Coexpression of CD58 or CD48 with intracellular adhesion molecule 1 on target cells enhances adhesion of resting NK cells. J Immunol. 2003;170:294–299. doi: 10.4049/jimmunol.170.1.294. [DOI] [PubMed] [Google Scholar]
- Benvenuti F, Hugues S, Walmsley M, Ruf S, Fetler L, Popoff M, Tybulewicz VLJ, Amigorena S. Requirements of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305:1150–1153. doi: 10.1126/science.1099159. [DOI] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1101–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T-cell receptor signaling to integrins. Immunol Rev. 2007;281:65–81. doi: 10.1111/j.1600-065X.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- Calpe S, Wang N, Romero X, Berger SB, Lanyi A, Engel P, Terhorst C. The SLAM and SAP gene families control innate and adaptive immune responses. Adv Immunol. 2008;97:177–250. doi: 10.1016/S0065-2776(08)00004-7. [DOI] [PubMed] [Google Scholar]
- Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, Anderson S, Cheever AW, Sher A, Schwartzberg PL. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao E, Raagopal UA, Fedorov A, Fedorov E, Yan Q, Lary JW, Cole JL, Nathenson SG, Almo SC. NTB-A receptor crystal structure: insights into homophilic interactions in the signaling lymphocytic activation molecule receptor family. Immunity. 2006;25:559–570. doi: 10.1016/j.immuni.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Chan AY, Westcott JM, Mooney JM, Wakeland EK, Schatzle JD. The role of the SAP and SLAM family in autoimmunity. Curr Opin Immunol. 2006;18:656–664. doi: 10.1016/j.coi.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Chen R, Relouzat F, Roncagalli R, Aoukaty A, Tan R, Latour S, Veillette A. Molecular dissection of 2B4 signaling: implications for signal transduction by SLAM-related receptors. Mol Cell Biol. 2004;24:5144–5156. doi: 10.1128/MCB.24.12.5144-5156.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Dutra A, Takesono A, Garrett-Beal L, Schwartzberg PL. Improved generation of C57BL/6J mouse embryonic stem cells in a defined serum-free media. Genesis. 2004;39:100–104. doi: 10.1002/gene.20031. [DOI] [PubMed] [Google Scholar]
- Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinct transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, Chen A, Sher A, Duckett CS, Ahmed R, Schwartzberg PL. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci USA. 2001;98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazilleau N, Mark L, MacHeyzer-Williams LJ, MacHeyzer-Williams MG. Follilcular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein LD, Shimizu Y, Schwartzberg PL. Tec kinases regulate TCR-mediated recruitment of signaling molecules and integrin-dependent cell adhesion. J Immunol. 2005;175:5923–5930. doi: 10.4049/jimmunol.175.9.5923. [DOI] [PubMed] [Google Scholar]
- Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes NM, Allen CDC, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- Howie D, Laroux FS, Morra M, Satoskar AR, Rosas LE, Faubion WA, Julien A, Rietdijk S, Coyle AJ, Fraser C, Terhorst C. The SLAM family receptor Ly108 controls T cell and neutrophil functions. J Immunol. 2005;174:5931–5935. doi: 10.4049/jimmunol.174.10.5931. [DOI] [PubMed] [Google Scholar]
- Howie D, Simarro M, Sayos J, Guirado M, Sancho J, Terhorst C. Molecular dissection of the signaling and costimulatory functions of CD150 (SLAM): CD150/SAP and CD150-mediated costimulation. Blood. 2002;99:957–965. doi: 10.1182/blood.v99.3.957. [DOI] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Iosef C, Jia CYH, Gkourasas T, Han VKM, Li SSC. Disease-causing SAP mutants are defective in ligand binding and protein folding. Biochem. 2003;42:14885–14892. doi: 10.1021/bi034798l. [DOI] [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Nichols KE, Tangye SG. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Ann Rev Immunol. 2007;25:337–379. doi: 10.1146/annurev.immunol.25.022106.141651. [DOI] [PubMed] [Google Scholar]
- McCausland MM, Yusuf I, Tran H, Ono N, Yanagi Y, Crotty S. SAP regulation of follicular helper CD4 T cell development and humoral immunity is independent of SLAM and Fyn kinase. J Immunol. 2007;178:817–828. doi: 10.4049/jimmunol.178.2.817. [DOI] [PubMed] [Google Scholar]
- Nanda N, Andre P, Bao M, Clauser K, Deguzman F, Howie D, Conley PB, Terhorst C, Phillips DR. Platelet aggregation induces platelet aggregate stability via SLAM family receptor signaling. Blood. 2005;106:3028–3034. doi: 10.1182/blood-2005-01-0333. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang Y-h, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2 or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O’Garra A, Cahalan MD, Cyster JG. Antigen-engaged B cells undergo chemotaxis toward the T zone and form mobile conjugates with helper T cells. PLOS Biol. 2005;3:1047–1061. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlies germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riteau B, Barber DF, Long EO. Vav1 phosphorylation is induced by β2 integrin engagement on natural killer cells upstream of actin cytoskeleton and lipid raft reorganization. J Exp Med. 2003;198:469–474. doi: 10.1084/jem.20021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg PL, Mueller KL, Qi H, Cannons JL. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat Rev Immunol. 2009;9:39–46. doi: 10.1038/nri2456. [DOI] [PubMed] [Google Scholar]
- Suto A, Kashiwakuma D, Kagami S-i, Hirose K, Watanabe N, Yokotte K, Saito Y, Nakayama T, Grusby MJ, Iwamoto I, Nakajima H. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205:1369–1376. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye SG, Nichols KE, Hare NJ, Weerdt BCMvd. Functional requirements for interactions between CD84 and src homology 2 domain-containing proteins and their contribution to human T cell activation. J Immunol. 2003;171:2485–2495. doi: 10.4049/jimmunol.171.5.2485. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- Wandstrat AE, Nguyen C, Limaye N, Chan AY, Subramanian S, Tian XH, Yim YS, Pertsemlidis A, Jr, HRG, Morel L, Wakeland EK. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004;21:769–780. doi: 10.1016/j.immuni.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Wang N, Satoskar A, Faubion W, Howie D, Okamoto S, Feske S, Gullo C, Clarke K, Sosa MR, Sharpe AH, Terhorst C. The cell surface receptor SLAM controls T cell and macrophage functions. J Exp Med. 2004;199:1255–1264. doi: 10.1084/jem.20031835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Malashkevich VN, Fedorov A, Fedorov E, Cao E, Lary JW, Cole JL, Nathenson SG, Almo SC. Structure of CD84 provides insight into SLAM family function. Proc Natl Acad Sci USA. 2007;104:10583–10588. doi: 10.1073/pnas.0703893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Zhong MC, Veillette A. Control of T lymphocyte signaling by Ly108, a signaling lymphocytic activation molecule receptor implicated in autoimmunity. J Biol Chem. 2008;283:19255–19264. doi: 10.1074/jbc.M800209200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.