Abstract
Total parenteral nutrition (TPN) results in a number of derangements to the intestinal epithelium, including a loss of epithelial barrier function (EBF). As TPN supplemented with glutamine has been thought to prevent this loss, this article further defined the impact of glutamine on EBF, and investigated potential mechanisms that contributed to the preservation of EBF. C57BL/6J male mice were randomized to enteral nutrition (control), TPN, or TPN supplemented with glutamine (TPN+GLN). Changes in intraepithelial lymphocyte (IEL)-derived cytokine expression were measured, and EBF was assessed with electrophysiologic methods and assessment of junctional protein expression. TPN resulted in a significant decline in EBF, and this loss of EBF was significantly prevented in the TPN+GLN group. Coincident with these changes was a loss of intraepithelial lymphocyte (IEL, mucosal lymphocyte)-derived IL-10 and increase in interferon-γ (IFN-γ) expression, and a decline in IEL numbers in the TPN group. A prevention in the increase in IFN-γ and decline in IL-10 expression was seen in the TPN+GLN group. To determine the mechanism responsible for these glutamine-associated cytokine changes, we tested whether blockade of the IL-7 signaling pathway between epithelial cells (EC) and IEL would prevent these changes; however, blockade failed to influence IEL-derived cytokine changes. Glutamine-supplemented TPN leads to a specific IEL-derived cytokine profile, which may account for the preservation of EBF; and such action may be due to a direct action of glutamine on the IEL.
Introduction
Total parenteral nutrition (TPN) is required for patients with intestinal dysfunction. While the use of TPN may be lifesaving, TPN has been shown to impair small intestinal epithelial barrier function (EBF), decrease mucosal immune function, and increase bacterial translocation (Zhang and others 2003; Sun and others 2006). Many of these problems may have tremendous clinical relevance as administration of TPN may lead to an increase in wound and upper respiratory infections as well as septicemia (Moore and others 1989; Kudsk and others 1996). It is possible that a loss of EBF may lead to the transmigration of luminal toxins and microorganisms that may contribute to the increase in infections.
The mucosal epithelial immune system is predominately comprised of intraepithelial lymphocytes (IEL), which are located between mucosal epithelial cells (EC) (Ernst and others 1985). IEL act as the initial lymphoid defense layer against intraluminal foreign antigens (Fukui and others 1997), and may be of critical importance for proper functioning of the mucosal immune system (Watanabe and others 1995). It has been previously shown that in normal conditions IEL express several cytokines and growth factors, which help to support intestinal EC proliferation and maintain intestinal barrier function (Fujihashi and others 1993). Conversely, the EC may also have an important role in the support of the mucosal immune response by helping to regulate IEL phenotype and function (Hayday and others 2001). Such interactions may well work through a group of ligands between IEL and EC, particularly those located along the EC basolateral aspect of the cell. One such example is the surface expression of CD103 on IEL and E-cadherin on EC. Expression of CD103 is a requisite for IEL homing and localization to the mucosal epithelium (Benmerah and others 1994; Schon and others 1999). Additionally, EC-derived IL-7 directly interacts and binds to IL-7 receptors (IL-7R) on IEL (Yang and others 2008a, 2008b), and this interaction sustains IEL cytokine expression and proliferation.
Our laboratory has shown that administration of TPN leads to a decline in EC proliferation and loss of EBF (Yang and others 2002a; Yang and Teitelbaum 2003). TPN is also associated with a decrease in IEL numbers, and significant changes in the phenotypic subpopulations of the IEL, including marked changes in cytokine expression (Kiristioglu and Teitelbaum 1998). TPN leads to a significant increase in IEL-derived interferon-gamma (IFN-γ) and a decline in IL-10 expression (Yang and Teitelbaum 2003; Sun and others 2008); and the changes in the abundance of these cytokines contribute to a loss of EBF (Yang and others 2002a; Yang and Teitelbaum 2003; Sun and others 2008). Our group and others have also shown that TPN significantly decreased EC-derived IL-7 (Fukatsu and others 2005; Yang and others 2007), and this reduction in IL-7 may play a significant role in the alterations in the IEL (Yang and others 2008a).
It is possible that these IEL changes may be due to a nutritional deficiency. A significant line of evidence in the recent literature shows that the amino acid glutamine can ameliorate both TPN-associated mucosal changes and the systemic immune dysfunction seen with TPN (Berard and others 2000; Nagafuchi and others 2000; Zarzaur and others 2002). Glutamine is not normally a component of TPN, but glutamine supplementation may have several benefits. In fact, glutamine has been shown to be an essential metabolic component for the proliferative response of intestinal enterocytes, and is one of the preferred fuel sources for the small intestine. This is best demonstrated by the fact that glutamine may account for over one-third of the total CO2 produced in the small intestine (Souba and others 1990; Ziegler and others 2000). Glutamine can reduce the development of EC apoptosis (Evans and others 2005) and help preserve gut barrier function in models of TPN and sepsis (Van Der Hulst and others 1993; Helton 1994; Buchman and others 1995). Additionally, glutamine can prevent the loss of gut-associated lymphocyte tissue (GALT) during TPN administration (Alverdy 1990; Zarzaur and others 2002). Functionally, intestinal IL-4 and IL-10 were shown to increase with glutamine-supplemented TPN (DeWitt and others 1999; Fukatsu and others 2001). Although glutamine may signal EC via a number of amino acid transporters (Avissar and others 2008), it is unknown which intestinal immune cell population is responsible for the expression of these cytokines. As well, it is unknown whether these glutamine-associated cytokine changes may be associated with EBF loss. On the basis of these findings, we hypothesized that TPN supplemented with glutamine would improve EBF by a prevention of TPN-associated changes to IEL phenotype and function. We further hypothesized that the mechanism by which glutamine acted was via a signaling through the IL-7/IL-7R pathway between EC and IEL.
Materials and Methods
Parenteral nutrition model
Animals. Male, specific pathogen-free, adult C57Bl/6J mice (Jackson Laboratories, Bar Harbor, ME) were maintained under temperature-, humidity-, and light-controlled conditions. Mice were initially fed ad libitum with standard mouse chow and water and allowed to acclimate. During the administration of intravenous solutions, mice were housed in metabolic cages to prevent coprophagia. The studies conformed to the guidelines for the care and use of laboratory animals established by the University Committee on Use and Care of Animals at the University of Michigan, and protocols were approved by that committee (UCUCA No. 7703).
Operative procedures and study groups. Administration of TPN was delivered as previously described (Kiristioglu and Teitelbaum 1998). Mice were infused with a crystalloid solution at an initial rate of 4 mL/day. After 24 h, mice were randomized into three groups (N = 6 per group). The control group received the same intravenous saline solution at 7 mL/24 h, in addition to standard laboratory mouse chow and water ad libitum. The TPN group received a standard TPN solution intravenously at 7 mL/24 h with no oral intake. Composition of the TPN solution has been described in detail previously (Kiristioglu and Teitelbaum 1998), and it contained a balanced mixture of amino acids, lipids, and dextrose in addition to electrolytes, trace elements, and vitamins. The glutamine-administered mouse group (TPN+GLN group) was given 2% alanyl glutamine solution, which was isonitrogenous and isocaloric to the standard TPN solution. Percent of glutamine delivery was based on previous publications in mice that demonstrated beneficial effects on barrier function (Li and others 1994). In previous experiments, we have measured energy intake from enteral feeding, and ensured matched energy delivery between TPN and control groups; additionally, energy delivery was based on estimates of caloric intake by control and from previous reports (Li and others 1995), so that energy delivery was essentially the same in all groups. TPN solutions with or without glutamine were formulated and prepared by a PN supplier (HomeMed of the University of Michigan Health System). All animals were euthanized on study day 7 using CO2 asphyxia.
Intestinal morphology. A 5-mm section of jejunum was fixed in 10% formaldehyde, dehydrated, and embedded in paraffin. Sections were cut (5 μm) and stained with hematoxylin and eosin. Villus length and crypt depth for each specimen were measured. Measurements were based on a mean of at least 10 crypt–villus complexes with well-oriented sections.
Mucosal cell isolation
Small bowel IEL and ECs were isolated as previously described (Kiristioglu and Teitelbaum 1998; Yang and others 2008a, 2008b). In brief, the small bowel was placed in tissue culture medium and mesenteric fat and Peyer’s patches were removed. The intestine was then opened longitudinally and agitated to remove mucus and fecal material. The intestine was then cut into 5-mm pieces and incubated in an IEL extraction buffer with continuous brisk stirring at 37°C for 20 min. The supernatant was then filtered rapidly through a glass wool column. Magnetic beads conjugated with antibody to CD45 (lymphocyte-specific) were used to isolate lymphoid from non-lymphoid cells (BioMag SelectaPure Anti-Mouse CD 45R antibody particles, Polyscience Inc, Warrington, PA; allowing for the retrieval of all activated T cells, representing the vast majority of the IEL population). Cells bound to beads were considered purified IEL; unbound EC remained in the supernatant. Flow cytometry confirmed purity of sorted IEL, which was greater than 95%, based on a control sample stained with anti-CD45 antibody, or anti-G8.8 antibody (specific for EC) (Farr and others 1991). In some experiments, CD4+ and CD8+ IEL were sorted on an Epics Elite flow sorter (Coulter Pharmaceutical, Miami, FL) to study individual IEL populations.
Intracellular cytokine staining and flow cytometry
Intracellular staining of IL-10 and IFN-γ was performed to define which IEL subpopulation(s) were their predominant source, with previously described techniques (Yang and others 2004). Cell surface staining used anti-mouse CD4, CD8-α, and CD8-β (BD PharMingen, San Diego, CA), conjugated with fluorescein isothiocyanate (FITC) or Texas red. Intracellular staining was performed with anti-mouse IL-10 and IFN-γ antibodies conjugated with phycoerythrin (PE) (Pharmingen). Acquisition and analysis were performed on FACSCalibur (Becton-Dickinson, Mountain View, CA) using CellQuest software (Becton-Dickinson). The IEL population was identified by forward and side scatter characteristics. Quantification of IEL subpopulations was based on its percentage of the gated IEL population compared to the total IEL population. Expression of IL-10 or IFN-γ-positive IEL was based on the percent expression over the negative-gated population of PE-conjugated nonspecific, isotype control antibody. Other surface markers studied with flow cytometry included CD103 and IL-7 receptor (IL-7R) expression.
Real-time PCR
Methods of RNA isolation, purification, and PCR are identical to those previously described (Yang and others 2003a). Oligomers were designed using an optimization program (Lasergene 6, DNAStar, Inc., Madison, WI). Sequences of specific primers are described as follows (GeneBank Accession number; forward; reverse primers): IL-10 (NM_010548): 5′-AAT AAG AGC AAG GCA GTG GA-3′; 5′-GGG ATG ACA GTA GGG GAA CC-3′. IFN-γ (K00083): 5′-AAT AAG AGC AAG GCA GTG GA-3′; 5′-GGG ATG ACA GTA GGG GAA CC-3′. β-Actin (M12481): 5′-AAT CGT GCG TGA CAT CAA A-3′; 5′-AAG GAA GGC TGG AAA AGA GC-3′. Real-time PCR was performed using a Smart Cycler thermocycler (Cepheid, Sunnyvale, CA). Specificity of the real-time PCR products was documented with gel electrophoresis and resulted in a single product with the desired length. Additionally, cDNA was extracted by using a centrifugal filter device (Millipore), and sequencing of the products showed that they matched targeted GeneBank mRNA sequences. Expression of results were normalized to β-actin expression using the ΔΔCtT method, by which the change (delta, Δ) in Ct values between each study group were compared to the Ct value of β-actin (ΔCt); and the ΔΔCt is the difference between the ΔCt’s from the TPN groups and the control group, taken as 2−(ΔΔCt) (Livak and Schmittgen 2001).
Epithelial barrier function studies
Ussing chambers (Physiologic Instruments, Inc, San Diego, CA) were used to assess intestinal barrier function, and methods are identical to previous publications (Yang and others 2003a). For all reported experiments only jejunal small bowel was studied, as we have previously found this segment to demonstrate the greatest changes in EBF with TPN (Yang and others 2003b).
Electrical measurement. Transmembrane resistance (TER) was measured after a 30-min equilibration period and determined using Ohm’s law.
Intestinal permeability experiments. Permeability of the small intestine was also assessed with 3H-mannitol (183 Da, diameter 6.7Å) as a tracer molecule. After a 30-min equilibration period, 3H-mannitol (3 μCi/mL; Sigma Chemical, St. Louis, MO) was added to the mucosal compartment of Ussing chambers. One-milliliter samples were taken every 15 min from the serosal compartment for analysis of 3H-mannitol and replaced with 1 mL fresh Krebs buffer. Incubations were carried out for 90 min after equilibration. 3H-mannitol was measured in a scintillation counter. The permeability of the isotope was then calculated as the apparent permeability coefficient (Papp) by standard equation (Madara and Trier 1982; Grass and Sweetana 1988).
Junctional protein expression
Antibodies. The following antibodies were used for Western blotting: goat anti-JAM1 (Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-occludin Ab (Santa Cruz; extracellular/cytoplastic domain), and mouse anti-β-actin (Sigma Aldrich, St. Louis, MO). Additionally, for immunofluorescent studies (see below) goat anti-ZO-1 (Santa Cruz) was used. Secondary antibodies were rabbit anti-goat HRP (Sigma) and goat anti-mouse-HRP (Santa Cruz).
Western blot. Western immunoblots of occludin and adherence junctional molecule 1 (JAM1) was performed. Each was selected because of their ability to sustain EBF, and the previously identified association of both JAM and occludin with IEL (Liu and others 2000; Inagaki-Ohara and others 2005). Jejunal protein extracts underwent immunoblotting using a Micro BCA™ protein assay kit (Pierce, Rockford, IL), as previously described (Yang and others 2002b). Blots were then stripped and re-probed with monoclonal mouse anti-β-actin antibody to equalize protein loading. Quantification of results was performed using Kodak 1D image software. Results of immunoblots are expressed as the relative expression of proteins to β-actin expression.
Immunofluorescent microscopy. To more precisely examine the expression of tight junctions in our TPN model, immunofluorescent staining for two proteins, ZO-1 and occludin, was performed. These two proteins were selected as ZO-1 and occludin have been shown to have a direct association with each other when localized to the apical membrane, and both have been shown to alter their intracellular distribution when exposed to proinflammatory cytokines (Furuse and others 1994; Wang and others 2006). A 0.5-cm section of fresh jejunum was cut and embedded in an optimum cutting temperature compound (PELCO International), and frozen. Cryosections (5 µm) were fixed, blocked in 10% goat serum (1 h), and incubated with primary antibodies (mouse monoclonal anti-ZO-1 1:500 or mouse monoclonal anti-occludin 1:250) at 4°C overnight. Slides were then incubated with corresponding secondary antibodies for 1 h, and mounted with anti-fade permanent mounting gel (Invitrogen, Carlsbad, CA). Fluorescence was analyzed with an Olympus fluorescent microscope (BX-51).
Data analysis
All data are expressed as mean ± standard deviation (SD). Statistical analysis was carried out using the t-tests for comparison of two means and a one-way ANOVA for comparison of multiple groups (with a Bonferroni post hoc analysis to assess statistical differences between groups). Prism software was used (GraphPad Software, Inc., San Diego, CA); and statistical significance was defined as P < 0.05.
Results
General description of mice
Body weights were recorded before initiation of the study and after mice were euthanized. After surgery, all animals had a mild weight loss, which rapidly reversed in the control group. Body weight changes were −0.7 ± 0.2 g for the control group, −2.3 ± 0.4 g for the TPN group, and −1.4 ± 0.2 g for the TPN+GLN group, respectively (P > 0.05).
Morphometric studies
Villus length and crypt depth in the TPN group were significantly lower compared to controls (P < 0.05). This was partially prevented in the TPN+GLN group, and values in this latter group were significantly higher than in the TPN group (Table 1).
Table 1. .
Morphometric Studies
| Groups | Villus length (μm) | Crypt depth (μm) |
|---|---|---|
| Control | 397.4 ± 49.5 | 121.4 ± 27.2 |
| TPN | 259.5 ± 58.3* | 56.6 ± 8.9* |
| TPN + GLN | 328.9 ± 24.8** | 92.3 ± 13.8** |
Changes in the jejunal villus length and crypt depth after 7 days of TPN, or TPN supplemented with glutamine, compared with control mice. The TPN group received a standard TPN intravenous solution with no oral intake. Controls received physiologic saline in addition to standard laboratory mouse chow and water ad libitum.
*P < 0.05 compared with the control group. **P < 0.05 compared with TPN group. Data are expressed as mean ± SD.
Abbreviations: TPN, total parenteral nutrition; TPN+GLN, TPN with 2% alanyl glutamine.
Changes in IEL numbers and IEL-derived cytokine expression
IEL numbers. The number of IEL significantly declined (35%) in the TPN group versus controls (Table 2). Glutamine supplementation prevented this entire decline in cell numbers, and numbers of IEL in the TPN+GLN group were not significantly different from controls.
Table 2. .
IEL Cell Numbers and Expression of IEL-Derived IL-10 and IFN-γ Using Intracellular Staining and Flow Cytometry
|
Cell population |
Control |
TPN |
TPN + GLN |
|---|---|---|---|
|
Total IEL yield |
7.9 ± 1.3 × 106 |
5.1 ± 1.4 × 106* |
8.4 ± 1.7× 106† |
| Percent of gated IEL | Percent of gated IEL | Percent of gated IEL | |
| IL-10+, CD4+ | 20.1 ± 5.3 | 20.1 ± 8.9 | 62.0 ± 2.3*† |
| IL-10+, CD8-αα+ | 23.9 ± 10.9 | 13.6 ± 6.2* | 82.6 ± 8.9*† |
| IL-10+, CD8-αβ+ | 26.5 ± 7.5 | 11.6 ± 0.6* | 83.2 ± 8.4*† |
| IFN-γ+, CD8-αα+ | 0.7 ± 0.2 | 1.0 ± 0.4 | 0.6 ± 0.3 |
| IFN-γ+, CD8-αβ+ | 12.8 ± 1.8 | 13.7 ± 7.1 | 5.9 ± 3.5 |
IEL yield represents the number of isolated IEL per entire length of mouse small intestine. IEL subpopulation values are the percent of IL-10-positive cells from each IEL subpopulation. Thus, for control mice, 20.1% of the CD4+ IEL are IL-10+. *P < 0.05 vs. control group. †P < 0.05 vs. TPN group.
Abbreviations: GLN, glutamine; IEL, intraepithelial lymphocyte; IFN, interferon; IL, interleukin; TPN, total parenteral nutrition.
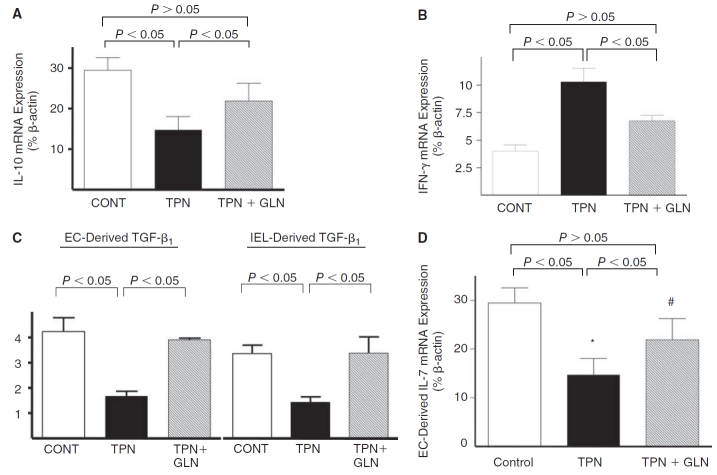
mRNA expression. IEL-derived IL-10 mRNA abundance was significantly decreased in the TPN group compared with controls (P < 0.05), and this expression returned to nearly control levels in the TPN+GLN group (Fig. 1A). IEL-derived IFN-γ mRNA expression significantly increased with TPN compared to controls, and this increased expression was partially prevented with the addition of glutamine to the TPN solution (Fig. 1B). TGF-β1, another cytokine that can modulate EBF (Planchon and others 1994; Di Leo and others 2002), was also examined. As TGF-β1 is expressed in both intestinal EC and IEL, both populations were examined (Fig. 1C). A decline in TGF-β1 abundance was found in the TPN group versus the control group in both cell populations. Supplementation with glutamine resulted in a return to levels not significantly different from the control group. IL-7 expression in intestinal EC was found to significantly decline with TPN, as previously reported (Yang and others 2003c; Yang and others 2007). Loss of IL-7 was prevented in the TPN+GLN group (Fig. 1D).
FIG. 1. .
Expression of mucosal-derived cytokines using real-time PCR. Results are expressed as 2−(ΔΔCT) in relation to β-actin gene expression. (A) IEL-derived IL-10 mRNA; (B) IEL-derived IFN-γ mRNA; (C) epithelial cell (EC)-derived and IEL-derived TGF-β mRNA; (D) EC-derived IL-7 mRNA. Note that IEL-derived IL-10 mRNA expression significantly decreased with TPN; and IFN-γ expression significantly increased with TPN. These expressions returned to nearly control levels with glutamine (#P > 0.05). Similar trends were seen with TGF-β and IL-7 expression. Statistical analysis: compared with controls: *P < 0.05 control vs. TPN; #P > 0.05 TPN vs. TPN+GLN. N = minimum of five per group. Abbreviations: EC, epithelial cells; IEL, intraepithelial lymphocytes; CONT, control; IFN, interferon; IL, interleukin; TGF, transforming growth factor; TPN, total parenteral nutrition; and TPN+GLN, TPN with glutamine supplementation.
Cytokine expression in IEL subpopulations. The IEL is a unique and heterogeneous population of lymphocytes, each with its own phenotype and cytokine profile. IL-10 and IFN-γ in IEL subpopulations are shown in Table 2. Interestingly, a substantial portion of both CD4+ and CD8+ IEL expressed IL-10 (Fig. 2). However, a significant decline in IL-10 was only noted in CD8+ IEL in the TPN group. This decline occurred in the CD8-αα as well as the CD8-αβ subpopulations (Table 2 and Fig. 2B and 2C). This was interesting, as we have previously noted that the number of CD8+ IEL decreased only slightly with TPN administration, yet the number of CD4 IEL significantly decline (Kiristioglu and Teitelbaum 1998). The percentage of IEL-expressing IL-10 rose significantly in both CD4+ and CD8+ IEL in the TPN+GLN group. Additionally, with glutamine the percent of IL-10+ IEL was far greater than that in either control or TPN group (Fig. 2).
FIG. 2. .
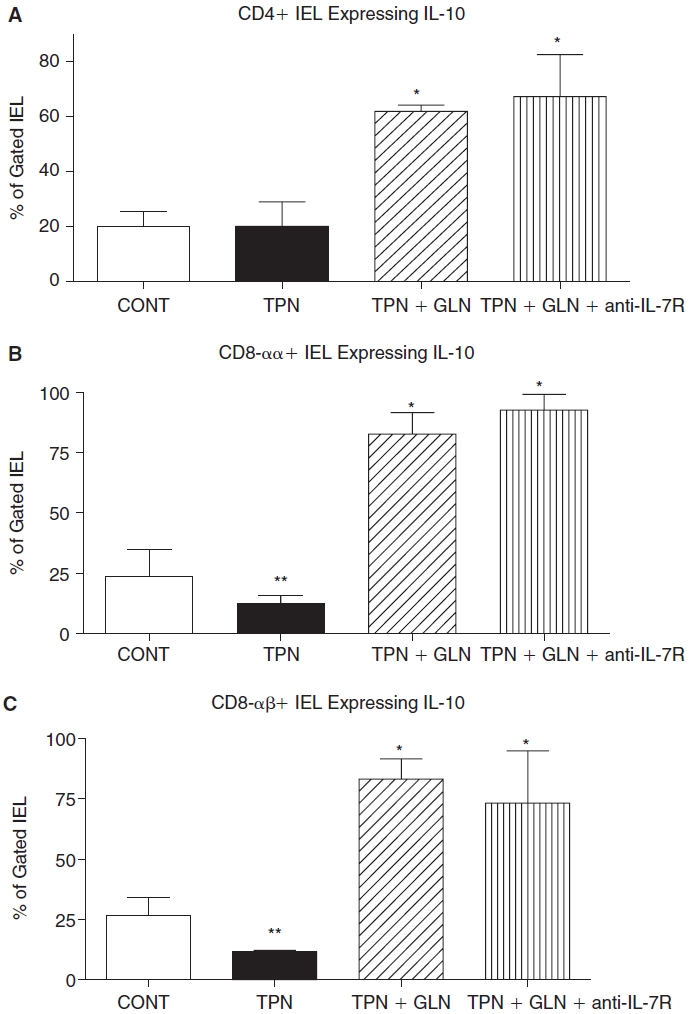
Flow cytometric results of intracellular staining for IL-10 for gated IEL subpopulations for each study group. Cell populations are expressed as the percentage of gated cells with (A) CD4; (B) CD8-α; and (C) CD8-β surface markers. TPN administration significantly decreased the percentage of CD8-αα+ and CD8-αβ+ IEL subpopulations expressing IL-10 when compared with controls. Glutamine administration significantly increased the percentage of IEL-expressing IL-10 in each subpopulation, and this increase resulted in a much greater percent expressing IL-10 compared to enteral controls. Note that TPN+GLN mice treated with IL-7R blocking antibody (TPN+GLN+anti-IL-7R) failed to change the marked increase in IEL-expressing IL-10 associated with the supplementation with glutamine. **P < 0.05 control vs. TPN; *P < 0.01 TPN+GLN (both groups) vs. control and TPN groups. N = minimum of five per group. Abbreviations: CONT, control; IEL, intraepithelial lymphocyte; IL, interleukin; TPN, total parenteral nutrition; TPN+GLN, TPN with glutamine supplementation.
Similar to mRNA data, IFN-γ expression increased in the TPN group compared to controls (Fig. 3), and this was found to be significantly elevated for the CD8-αβ subpopulation, whereas little IFN-γ was detected in CD4+ IEL. Glutamine supplementation prevented this increase in IFN-γ expression, such that levels in the TPN+GLN group were not significantly different from control values. The overall percent of nonstimulated IEL, which were detected by intracellular staining for TGF-β1, was below 2% in all subpopulations and in all study groups, and thus results are not reported.
FIG. 3. .
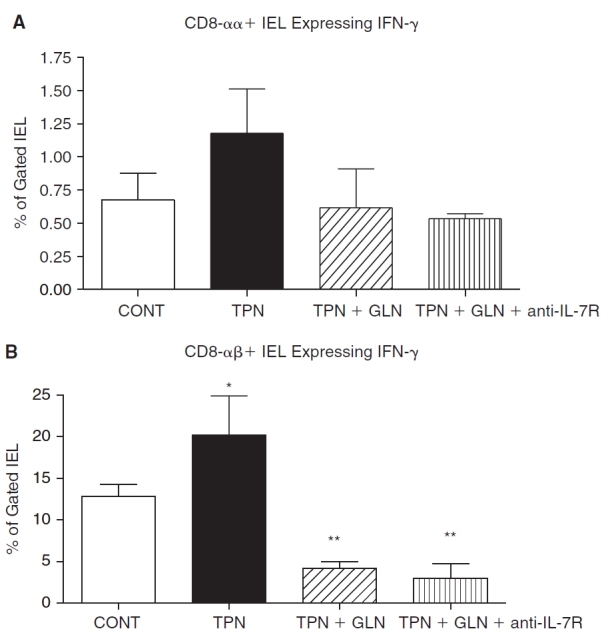
Flow cytometric results of intracellular staining for IFN-γ for gated IEL populations for each study group. Cell populations are expressed as the percentage of gated cells with (A) CD8-αα and (B) CD8-αβ subpopulations of CD8+ IEL. As virtually all IFN-γ were detected in CD8+ IEL, CD4 data is not shown. TPN administration led to a significant increase in the expression of IFN-γ, and the supplementation of glutamine led to a marked prevention of this increase. Note that TPN+GLN mice treated with IL-7R-blocking antibody (TPN+GLN+anti-IL-7R) failed to change the suppression in IFN-γ expression with glutamine. *P < 0.05 control vs. TPN; **P < 0.01 TPN+GLN (both groups) vs. control and TPN groups. N = minimum of five per group. Abbreviations: CONT, control; IEL, intraepithelial lymphocyte; IFN, interferon; IL, interleukin; TPN, total parenteral nutrition; TPN+GLN, TPN with glutamine supplementation.
Epithelial barrier function
As alterations in IL-10, IFN-γ, and TGF-β1 are known to modulate EBF, and TPN administration can markedly decrease EBF (Yang and others 2002a; Sun and others 2008), we next examined whether the administration of glutamine could prevent the TPN-associated loss of EBF.
Transepithelial resistance. Transepithelial resistance (TER) values remained relatively stable over the 90-min incubation period (Fig. 4). Baseline TER (Ω·cm2) after an equilibration period was 82.9 ± 6.1 Ω·cm2 and 48.6 ± 4.8 Ω·cm2 in control and TPN groups, respectively. At all time points, TER in the TPN group was significantly (P < 0.05) lower than controls. This was partially attenuated in the TPN+GLN group; however, TER remained lower than controls at all time points (66.6 ± 6.9 Ω·cm2, P < 0.05 compared to control values, and compared to the TPN group, Fig. 4).
FIG. 4. .
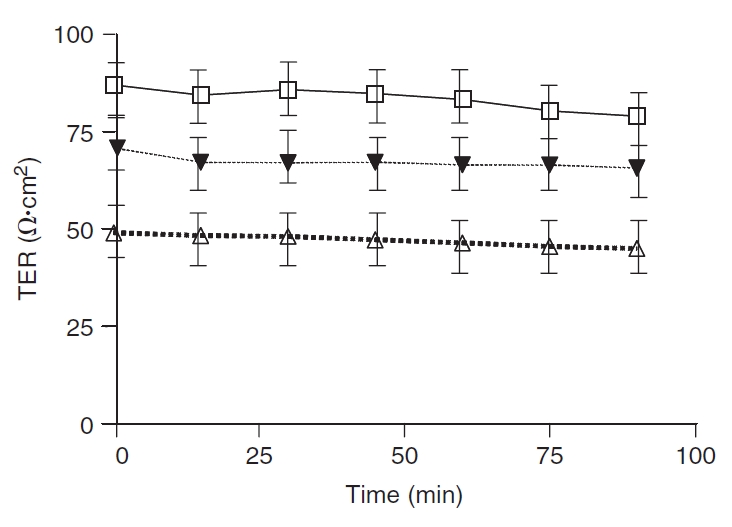
Basal intestinal transepithelial resistance (TER; Ω·cm2) in jejunum. Control (□), total parenteral nutrition (TPN; Δ), and TPN–glutamine (▾) study groups. Results are represented as mean ± SD. Time 0 represents the time at which all tissues were mounted in Ussing chambers following a 30-min equilibration period to achieve steady-state conditions. At all time points, TER in the TPN group was significantly (P < 0.05) lower than in the control group. The loss of TER for TPN mice was significantly ameliorated with glutamine administration; however, TER values did not return to the level of control mice. N = 6 per group. Not shown (because of the overlapping of lines) was that the blockade with anti-IL-7R antibody administration in TPN mice failed to impact TER.
Epithelial permeability of 3H-Mannitol. Intestinal EBF was further assessed by determining permeation of 3H-mannitol. After an equilibration period, there was a constant permeation of 3H-mannitol in each group during the 90-min incubation period. At all time points, 3H-mannitol permeability in the TPN group was significantly higher than controls (Fig. 5). The permeability in the TPN+GLN group was significantly lower than in TPN mice. The cumulated permeation of 3H-mannitol was 0.21% ± 0.09% in the controls. TPN significantly increased permeability values (0.56% ± 0.16%) compared with controls (P < 0.05; Fig. 5). Glutamine-supplemented TPN markedly prevented 3H-mannitol permeability (0.32% ± 0.14%, vs. TPN alone P < 0.05). The permeability coefficient (Papp, expressed as 10−6 x cm x sec−1) for 3H-mannitol increased in the TPN group (18.7 ± 4.1) compared to controls (7.4 ± 2.8), and Papp returned to control levels in the TPN+GLN group (9.8 ± 3.1, P > 0.05).
FIG. 5. .
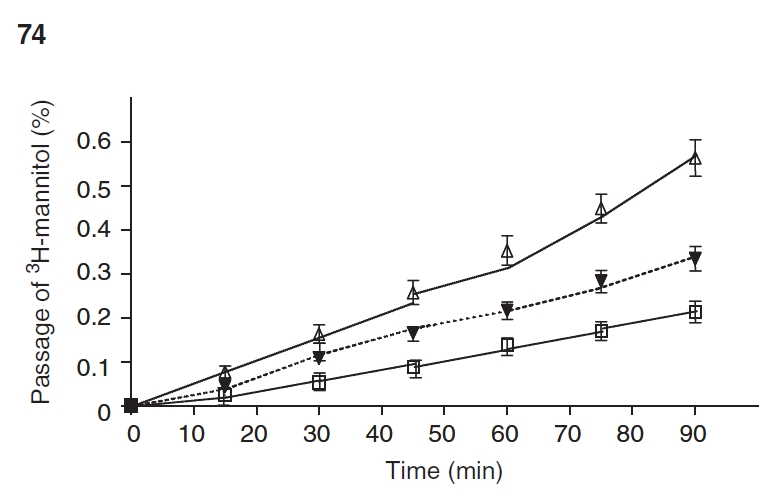
Intestinal permeability as measured by 3H-mannitol in Ussing chambers. Cumulative transmucosal permeation of 3H-mannitol (mean% ± SD%) in jejunum of mice during a 90-min incubation in Ussing chambers. Control (□), total parenteral nutrition (TPN; ▵), and TPN–glutamine (▾). Time 0 represents the start of the incubation after an initial 30-min equilibration phase. There was a steady linear permeation increment during the incubation in all groups. The cumulative permeation of 3H-mannitol was noted to be significantly increased in mice receiving TPN compared to controls. With glutamine administration, 3H-mannitol permeability was markedly reduced (P < 0.05 vs. TPN without glutamine) at all time points starting at the 50-min time period to the end of the experiment N = 6 per group. See text for the permeability coefficient data (Papp).
Changes in tight junctional protein expression
A major mechanism by which cytokines mediate alterations in EBF is via changes in epithelial tight junctional protein abundance and localization. We next examined the expression of these junctional proteins, and how glutamine supplementation affected this. Western immunoblotting was used to measure the tight junctional molecules, occludin, and adherence junctional molecule 1 (JAM1). The expression of both proteins were significantly (P < 0.05) decreased in TPN mice compared with controls: occludin: 0.42 ± 0.04 vs. 0.21 ± 0.03; and JAM1: 0.32 ± 0.05 vs. 0.14 ± 0.04; control vs. TPN, respectively (values expressed as a % of β-actin, Fig. 6). The decline in junctional proteins was partially prevented in the TPN+GLN group. Abundance of occludin and JAM1 rose to values not significantly (P > 0.05) different from controls: occludin: 0.35% ± 0.03% and JAM1: 0.24% ± 0.04% (Fig. 6).
FIG. 6. .
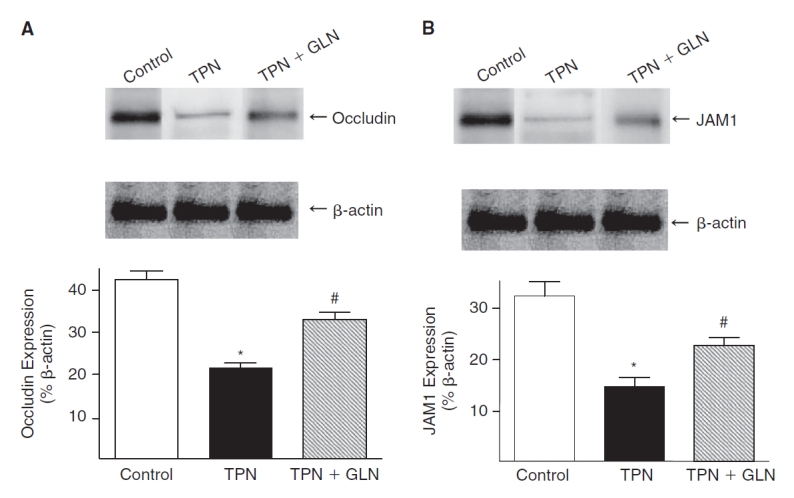
Expression of occludin (A) and JAM1 (B) proteins was examined by Western immunoblots. Results are expressed as the ratio of the tight junctional protein expression to the β-actin protein expression. The expression of occludin and JAM1 were significantly decreased with total parenteral nutrition (TPN) administration compared with controls. With glutamine (GLN) administration, the expression of these proteins increased, and levels of expression were not significantly different from control levels (*P < 0.05; #P > 0.05).
To further examine the expression of tight junctions, two proteins, ZO-1 and occludin were examined (Fig. 7). Immunofluorescent staining demonstrated a marked loss of both junctional proteins in intestinal EC with TPN. An additional observation was an internalization of these junctional proteins with TPN administration. Interestingly, glutamine supplementation prevented much of the loss of ZO-1 expression along the apical surface of intestinal EC. However, glutamine failed to completely prevent the internalization of occludin observed in the TPN group (Fig. 7).
FIG. 7. .
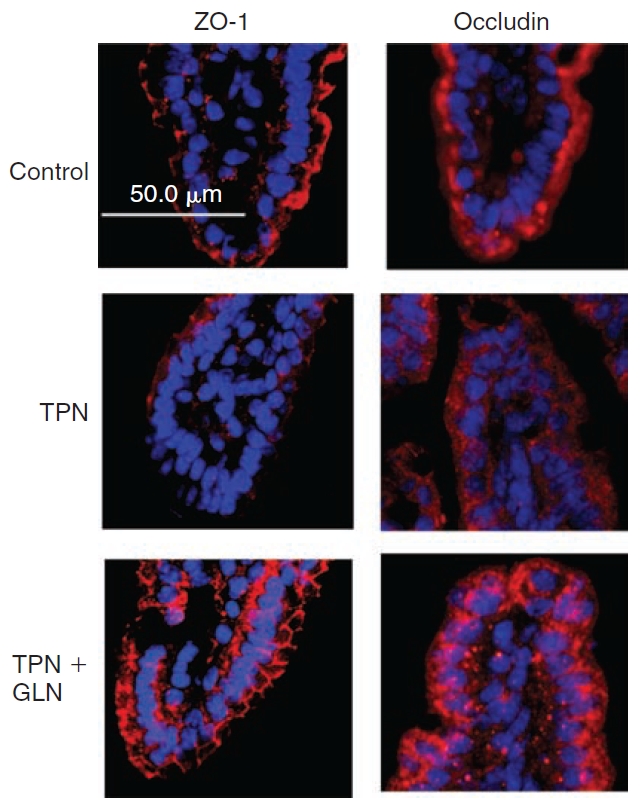
Occludin and ZO-1 immunofluorescent staining (both stained red; tissues were counterstained with nuclear staining (DAPI, blue)). Note the diffuse loss of immunofluorescent staining for both junctional proteins, particularly at the distal half of the villi with total parenteral nutrition (TPN) administration. As well both proteins show an internalization of signal from the apical membrane into the cytoplasm. Administration of TPN with glutamine supplementation led to an increase in junctional expression. Although ZO-1 demonstrated a return to the apical surface of epithelial cells, occludin continued to be distributed within the cytoplasm.
Mechanisms leading to altered IEL function with glutamine supplementation
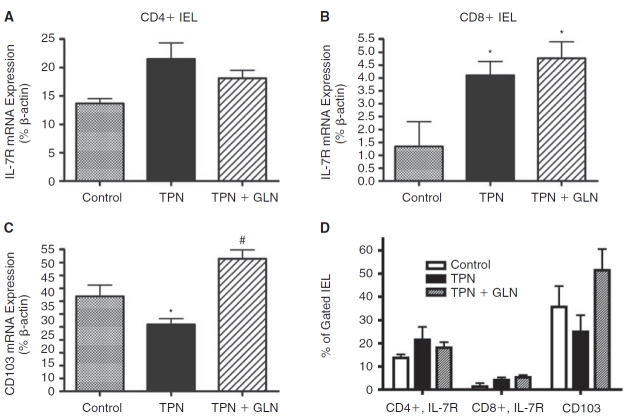
IEL ligand expression. In order to understand the mechanisms that could account for the changes in IEL numbers and alteration in cytokine expression, we next investigated alterations in surface expression of IL-7 receptor (IL-7R on IEL) and CD103 (on EC); both of these being key ligands for IEL function and the ability of IEL to home to intestinal EC, respectively (Benmerah and others 1994; Fujihashi and others 1996; Yang and others 2005; Yang and others 2007). mRNA abundance showed a significant increase in IL-7R expression with TPN, and this was noted on both CD4+ and CD8+ IEL populations. For the CD4+ IEL group, the changes were not significant; however, levels significantly rose in the CD8+ IEL population. TPN supplemented with glutamine actually resulted in a further increase in IL-7R abundance in the CD8+ IEL subpopulation (Fig. 8A and 8B). mRNA levels of CD103 expression significantly decreased with TPN administration, and returned to levels not significantly different from control values in the TPN+GLN group (Fig. 8C). Four of the most common glutamine receptors were studied on IEL, and a significant decline was noted in the abundance of SLC1M4 and SLC1M7 (Table 3). Glutamine-supplemented TPN did prevent this decline in receptor abundance, suggesting that other mechanisms may be driving down the abundance of these receptors aside from a deficiency in this glutamine itself.
FIG. 8. .
Interleukin-7 receptor (IL-7R) and CD103 expression are shown as measured by mRNA and flow cytometric analysis. (A) IL-7R mRNA expression on sorted CD4+ IEL. Note an increase in IL-7R in the total parenteral nutrition (TPN) group, and a slightly lower amount of increase in the TPN+glutamine (TPN+GLN) group. Differences were not significant. (B) IL-7R mRNA expression on sorted CD8+ IEL. Note a significant increase in IL-7R in the TPN group, and a sustained increased expression of IL-7R in the TPN+GLN group. (C) Epithelial cell CD103 mRNA expression. Note a significantly decreased expression in the TPN group, and a prevention of this decline in the TPN+GLN group. (D) Flow cytometric analysis of gated IEL and EC populations. Surface expression of IL-7R is given for both the CD4+ and CD8+ IEL populations. Although IL-7R increased with TPN, and CD103 declined, the changes were not significantly different. Values are expressed as the percent of gated cells positive for each target population. *P < 0.05 vs. control; #P < 0.05 vs. TPN group.
Table 3. .
Glutamine Receptor Expression on IEL
| Glutamine receptor | Control | TPN | TPN + GLN |
|---|---|---|---|
| SLC1M4 | 0.006 ± 0.004 | 0.001 ± 0.001* | 0.002 ± 0.001* |
| SLC1M7 | 0.026 ± 0.003 | 0.007 ± 0.002* | 0.007 ± 0.003* |
| SLC6M14 | 0.004 ± 0.000 | 0.0004 ± 0.0002 | 0.0006 ± 0.0004 |
| SLC7M5 | 0.005 ± 0.002 | 0.008 ± 0.003 | 0.006 ± 0.003 |
*P < 0.05, compared to control.
Abbreviations: GLN, glutamine; IEL, intraepithelial lymphocytes; TPN, total parenteral nutrition.
Alterations in surface expression of IL-7R and CD103 were next examined using flow cytometry. Similar to mRNA results, TPN led to a decline in CD103 (25.8 ± 7.2 vs. 35.6 ± 9.2, TPN vs. control, respectively; Fig. 8D); however, the change was not statistically significant. The addition of glutamine to the TPN solution led to an increase in CD103 surface expression (51.4 ± 12.2); and although not significantly different from the other study groups, values exceeded those in the control group. Percent of gated IEL-expressing IL-7R rose with TPN administration (Fig. 8D), and this was true for both the CD4+ and CD8+ IEL subpopulations. Although not reaching significant difference from the control group, this may have been a compensatory increase to compensate for the significant decline in EC-derived IL-7 expression. Administration of glutamine did not significantly change IL-7R expression from levels in the control group.
Relation of IL-7 receptor signaling and glutamine-associated cytokine expression. As stated earlier, IL-7 declined with TPN and rose with glutamine, and IL-7/IL-7R has a critical role in sustaining IEL proliferation and function. Because of this, we next assessed whether the mechanism by which glutamine supplementation in TPN resulted in the observed change in IEL-derived cytokine expression was via the IL-7/IL-7R signaling pathway. This pathway was blocked by the systemic administration of an IL-7R-blocking antibody given continually during the entire TPN course, and this has been previously shown by our group and others to result in a complete blockade of this pathway (Kunisawa and others 2002; Yang and others 2007). Cytokine expressions of IL-10 and IFN-γ are shown in Figures 2 and 3. Despite IL-7R blockade, glutamine supplementation continued to result in a marked increase in IL-10 expression and prevented the TPN-associated rise in IFN-γ expression. Additionally, no change in TER was detected with IL-7R blockade (data not shown). This suggested that glutamine may well act directly on the IEL, as opposed to signaling through the adjacent EC population.
Discussion
In this study, the effects of glutamine administration on IEL cytokine expression and EBF were investigated in a mouse TPN model. The study also showed that glutamine significantly attenuated the TPN-associated loss of EBF. Glutamine-supplemented TPN prevented the increase in IEL-derived expression of IFN-γ and prevented the decline in IEL-derived IL-10 expression. Further, glutamine resulted in a significant attenuation in TPN-associated intestinal mucosal atrophy. It is important to note that this study has only quantified this attenuation of mucosal atrophy by somewhat crude morphometric studies. Future additional work on enterocyte turnover will be necessary to more accurately quantify these results. Unlike previous investigations on the subject of glutamine-supplemented TPN, the current work demonstrated that the changes observed in intestinal cytokine expression (Fukatsu and others 2001) are derived from specific IEL subpopulations. Additionally, this study also demonstrated some of the detailed molecular changes in the tight junction, which sustained EBF with the addition of glutamine. Finally, the fact that blocking the IL-7/IL-7R signaling pathway, the main signaling pathway for maintaining IEL numbers and functionality, failed to influence glutamine’s action on cytokine expression, suggests that glutamine had a direct effect on the IEL.
The intraepithelial lymphocytes (IEL) are closely associated with the intestinal epithelium, and are a rich source of several cytokines (Hayday and others 2001). The expression of these cytokines is a major aspect of IEL regulatory function. The IEL may well function to maintain EC function (Croitoru and Ernst 1993), as well as to down-regulate immunologic sensitization to foreign antigen. Our laboratory and others have previously shown that the IEL undergoes phenotypic and functional changes with TPN administration (Kiristioglu and others 1999; Fukatsu and others 2001; Kiristioglu and others 2002). TPN administration resulted in a significant decrease in IEL-derived IL-10 (Sun and others 2008) and increased expression of IEL-derived IFN-γ (Kiristioglu and Teitelbaum 1998). A similar finding of a decline in intestinal IL-10 with TPN administration has also been observed by Fukatsu and others (2001); however, the precise population of the intestine producing this cytokine was not fully defined. In our present study we concentrated our investigation to the IEL, as we were interested in the close cross communication between this lymphoid population and the adjacent EC. Our work showed that this decline in IL-10 and increase in IFN-γ occurred predominately in the two heterodimeric CD8+ IEL populations (CD8-αα and CD8-αβ). Our group previously observed a significant decline in CD4+ IEL with TPN (35); however, despite the decline in numbers of CD4+ IEL, the percentage of CD4+ IEL that expressed IL-10 did not change. The number of CD8+ IEL did not significantly change with TPN; however, it has been shown that CD8+ IEL plays a key role in generation of IL-10 and the prevention of intestinal inflammatory conditions (22). It may well be that although the number of CD8+ IEL cells declined only slightly with TPN, their functional role is markedly altered, contributing to the loss of IL-10.
The decline in IL-10 and increase in IFN-γ with TPN has been shown to be closely associated with a loss of EBF (Yang and others 2002a; Sun and others 2008). Additionally, TGF-β1 is known to sustain EBF, and prevent proinflammatory cytokine-mediated loss of barrier function (Planchon and others 1999). Thus it was quite interesting how each of these cytokines shifted toward a profile that would promote a loss of EBF. As well, the finding that TPN with glutamine resulted in dramatic changes in these IEL-derived cytokines suggests that this may well be the mechanism that drives EBF preservation with glutamine administration. Maintenance of EBF is essential to prevent bacterial endotoxins from entering the host from the intestinal lumen. Glutamine has been previously reported to play an important role in the modulation of EBF (Li and others 1994; Ding and Li, 2003), and this can prevent not only TPN-induced loss of EBF but also disruption of the intestinal epithelium due to exposure to either lipopolysaccharide (Ding and Li 2003) or acetaldehyde (Seth and others 2004). Much of the literature regarding the action of glutamine-supplemented TPN on EBF relate to transit of tracer particles across the intestinal wall, and do not examine the detailed alterations in EBF and tight junctional expression. As well, other reports have reported on bacterial translocation, which may also be similarly attenuated with glutamine supplementation (Gianotti and others 1995; Ding and others 2003). However, the mechanisms that promote or prevent bacterial translocation are distinct from changes in tight junction function, and bacterial translocation may actually work via a transcellular route (O’Brien and others 2002). Our present work suggests that the action of glutamine may well involve a complex mechanism of altering mucosal cytokine expression, with the subsequent observed loss and internalization of junctional proteins. Clearly, glutamine may function in other ways to achieve preservation of barrier function including preservation of oxidative stress or driving heat shock protein expression (Ziegler and others 2000; Singleton and Wischmeyer 2008).
The mechanisms that accounted for how glutamine administration attenuated the decline in tight junction protein expression and prevented much of the TPN-induced loss of EBF are not known. Although other investigators have shown a preservation of EBF with TPN supplemented with glutamine, our Ussing chamber findings have better quantified this loss of barrier function. Additionally, the finding of the sustained expression of JAM1 and occludin, as well as prevention of the internalization of ZO-1, provides for potential mechanisms by which glutamine sustains EBF. Because of the close cross talk between ECs and adjacent IEL, we investigated whether glutamine’s action on modulating IEL cytokine expression relied on this intercommunication.
The decline in CD103 expression in the TPN group could well account for the loss in total number of IEL (Table 2), and the prevention of the loss of CD103 expression in the TPN+GLU group may help to explain the sustained number of IEL. The decline in EC-derived IL-7 expression with TPN, and the prevention of this loss of IL-7 expression with glutamine, may also explain some of the IEL functional changes seen with TPN. Additionally, the rise in IL-7R expression on CD8+ IEL may be in response to the loss of IL-7 expression. Because of these findings, we tested whether the observed changes in IEL-derived cytokine expression with glutamine supplementation were due to signaling via this IL-7/IL-7R pathway. Our finding that blockade of IL-7R did not influence TER- or IEL-derived cytokine expression suggests that glutamine was acting directly on the IEL to induce these cytokine changes. However, the actual mechanism may be far more complex. A recent in vitro study where isolated IEL were incubated with glutamine led to the expression of markedly proinflammatory cytokines (TH1 type), and not our observed increase in IL-10 or decline in IFN-γ (Horio and others 2008). This suggests that other signaling pathways may have a role in modulating IEL function. Additionally, the fact that the exogenous administration of glutamine was not able to completely return barrier function to the normal level suggests that other factors contribute to EBF loss with TPN, and IEL-derived cytokine changes may only be one of several contributory mechanisms.
In conclusion, this study demonstrated that exogenous glutamine administration in mice receiving TPN partially prevented the TPN-associated changes to IEL phenotype and function, including IEL-derived cytokine expression. Coincident with these IEL changes, glutamine led to a significant improvement in EBF, an up-regulation in the expression of tight junction proteins, and a prevention of the internalization of ZO-1. It may well be that these actions were mediated through glutamine’s direct action on the IEL to prevent the TPN-induced cytokine changes to this lymphoid population. Future work will need to be done to better address the link between these two actions noted with glutamine supplementation of TPN.
Contributor Information
Keisuke Nose, Section of Pediatric Surgery, Department of Surgery, the University of Michigan Medical School and the C.S. Mott Children’s Hospital, Ann Arbor, Michigan..
Hua Yang, Section of Pediatric Surgery, Department of Surgery, the University of Michigan Medical School and the C.S. Mott Children’s Hospital, Ann Arbor, Michigan.; Xinqiao Hospital, Third Military Medical University, Chongqing, People’s Republic of China.
Xiaoyi Sun, Section of Pediatric Surgery, Department of Surgery, the University of Michigan Medical School and the C.S. Mott Children’s Hospital, Ann Arbor, Michigan..
Satoko Nose, Section of Pediatric Surgery, Department of Surgery, the University of Michigan Medical School and the C.S. Mott Children’s Hospital, Ann Arbor, Michigan..
Hiroyuki Koga, Section of Pediatric Surgery, Department of Surgery, the University of Michigan Medical School and the C.S. Mott Children’s Hospital, Ann Arbor, Michigan..
Yongjia Feng, Section of Pediatric Surgery, Department of Surgery, the University of Michigan Medical School and the C.S. Mott Children’s Hospital, Ann Arbor, Michigan..
Eiichi Miyasaka, Section of Pediatric Surgery, Department of Surgery, the University of Michigan Medical School and the C.S. Mott Children’s Hospital, Ann Arbor, Michigan..
Daniel H. Teitelbaum, Section of Pediatric Surgery, Department of Surgery, the University of Michigan Medical School and the C.S. Mott Children’s Hospital, Ann Arbor, Michigan.
Acknowledgments
This work was supported by NIH grant 5R01 AI044076-10 (to D.H.T.). The work was also supported with the help of the National Cancer Institute through the University of Michigan’s Cancer Center Support Grant (5 P03 CA46592).
References
- Alverdy JC. Effects of glutamine-supplemented diets on immunology of the gut. JPEN J Parenter Enteral Nutr. 1990;14((4 Suppl)):109S–113S. doi: 10.1177/014860719001400415. [DOI] [PubMed] [Google Scholar]
- Avissar NE, Sax HC, Toia L. In human entrocytes, GLN transport and ASCT2 surface expression induced by short-term EGF are MAPK, PI3K, and Rho-dependent. Dig Dis Sci. 2008;53((8)):2113–2125. doi: 10.1007/s10620-007-0120-y. [DOI] [PubMed] [Google Scholar]
- Benmerah A, Badrichani A, Ngohou K, Mégarbané B, Bègue B, Cerf-Bensussan N. Homotypic aggregation of CD103 (alpha E beta 7)+ lymphocytes by an anti-CD103 antibody, HML-4. Eur J Immunol. 1994;24((9)):2243–2249. doi: 10.1002/eji.1830240946. [DOI] [PubMed] [Google Scholar]
- Bérard MP, Zazzo JF, Condat P, Vasson MP, Cynober L. Total parenteral nutrition enriched with arginine and glutamate generates glutamine and limits protein catabolism in surgical patients hospitalized in intensive care units. Crit Care Med. 2000;28((11)):3637–3644. doi: 10.1097/00003246-200011000-00016. [DOI] [PubMed] [Google Scholar]
- Buchman AL, Mestecky J, Moukarzel A, Ament ME. Intestinal immune function is unaffected by parenteral nutrition in man. J Am Coll Nutr. 1995;14((6)):656–661. doi: 10.1080/07315724.1995.10718556. [DOI] [PubMed] [Google Scholar]
- Croitoru K, Ernst P. New York: Raven Press, Ltd; 1993. Intraepithelial lymphocyte lineage and function. The interactions between the intestinal epithelium and the intraepithelial lymphocyte. In: Kiyono H, McGhee J, eds. Mucosal immunology: intraepithelial lymphocytes; pp. 79–88. [Google Scholar]
- DeWitt RC, Wu Y, Renegar KB, Kudsk KA. Glutamine-enriched total parenteral nutrition preserves respiratory immunity and improves survival to a Pseudomonas Pneumonia. J Surg Res. 1999;84((1)):13–18. doi: 10.1006/jsre.1999.5592. [DOI] [PubMed] [Google Scholar]
- Di Leo V, Yang PC, Berin MC, Perdue MH. Factors regulating the effect of IL-4 on intestinal epithelial barrier function. Int Arch Allergy Immunol. 2002;129((3)):219–227. doi: 10.1159/000066778. [DOI] [PubMed] [Google Scholar]
- Ding L, Li J. Effects of glutamine on intestinal permeability and bacterial translocation in TPN-rats with endotoxemia. World J Gastroenterol. 2003;9:1327–1332. doi: 10.3748/wjg.v9.i6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding LA, Li JS. Effects of glutamine on intestinal permeability and bacterial translocation in TPN-rats with endotoxemia. World J Gastroenterol. 2003;9((6)):1327–1332. doi: 10.3748/wjg.v9.i6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst PB, Petit A, Befus AD, Clark DA, Rosenthal KL, Ishizaka T, Bienenstock J. Murine intestinal intraepithelial lymphocytes II. Comparison of freshly isolated and cultured intraepithelial lymphocytes. Eur J Immunol. 1985;15((3)):216–221. doi: 10.1002/eji.1830150303. [DOI] [PubMed] [Google Scholar]
- Evans ME, Jones DP, Ziegler TR. Glutamine inhibits cytokine-induced apoptosis in human colonic epithelial cells via the pyrimidine pathway. Am J Physiol Gastrointest Liver Physiol. 2005;289((3)):G388–G396. doi: 10.1152/ajpgi.00072.2005. [DOI] [PubMed] [Google Scholar]
- Farr A, Nelson A, Truex J, Hosier S. Epithelial heterogeneity in the murine thymus: a cell surface glycoprotein expressed by subcapsular and medullary epithelium. J Histochem Cytochem. 1991;39((5)):645–653. doi: 10.1177/39.5.2016514. [DOI] [PubMed] [Google Scholar]
- Fujihashi K, Kawabata S, Hiroi T, Yamamoto M, McGhee JR, Nishikawa S, Kiyono H. Interleukin 2 (IL-2) and interleukin 7 (IL-7) reciprocally induce IL-7 and IL-2 receptors on gamma delta T-cell receptor-positive intraepithelial lymphocytes. Proc Natl Acad Sci USA. 1996;93((8)):3613–3618. doi: 10.1073/pnas.93.8.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihashi K, Yamamoto M, McGhee JR, Beagley KW, Kiyono H. Function of alpha beta TCR+ intestinal intraepithelial lymphocytes: Th1- and Th2-type cytokine production by CD4+CD8- and CD4+CD8+ T cells for helper activity. Int Immunol. 1993;5((11)):1473–1481. doi: 10.1093/intimm/5.11.1473. [DOI] [PubMed] [Google Scholar]
- Fukatsu K, Kudsk KA, Zarzaur BL, Wu Y, Hanna MK, DeWitt RC. TPN decreases IL-4 and IL-10 mRNA expression in lipopolysaccharide stimulated intestinal lamina propria cells but glutamine supplementation preserves the expression. Shock. 2001;15((4)):318–322. doi: 10.1097/00024382-200115040-00012. [DOI] [PubMed] [Google Scholar]
- Fukatsu K, Moriya T, Maeshima Y, Omata J, Yaguchi Y, Ikezawa F, Mochizuki H, Hiraide H. Exogenous interleukin 7 affects gut-associated lymphoid tissue in mice receiving total parenteral nutrition. Shock. 2005;24((6)):541–546. doi: 10.1097/01.shk.0000183237.32256.78. [DOI] [PubMed] [Google Scholar]
- Fukui T, Katamura K, Abe N, Kiyomasu T, Iio J, Ueno H, Mayumi M, Furusho K. IL-7 induces proliferation, variable cytokine-producing ability and IL-2 responsiveness in naive CD4+ T-cells from human cord blood. Immunol Lett. 1997;59((1)):21–28. doi: 10.1016/s0165-2478(97)00093-x. [DOI] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127((6 Pt 1)):1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianotti L, Alexander JW, Gennari R, Pyles T, Babcock GF. Oral glutamine decreases bacterial translocation and improves survival in experimental gut-origin sepsis. JPEN J Parenter Enteral Nutr. 1995;19((1)):69–74. doi: 10.1177/014860719501900169. [DOI] [PubMed] [Google Scholar]
- Grass GM, Sweetana SA. In vitro measurement of gastrointestinal tissue permeability using a new diffusion cell. Pharm Res. 1988;5((6)):372–376. doi: 10.1023/a:1015911712079. [DOI] [PubMed] [Google Scholar]
- Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2((11)):997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- Helton WS. The pathophysiologic significance of alterations in intestinal permeability induced by total parenteral nutrition and glutamine. JPEN J Parenter Enteral Nutr. 1994;18((4)):289–290. doi: 10.1177/014860719401800401. [DOI] [PubMed] [Google Scholar]
- Horio Y, Osawa S, Takagaki K, Hishida A, Furuta T, Ikuma M. Glutamine supplementation increases Th1-cytokine responses in murine intestinal intraepithelial lymphocytes. Cytokine. 2008;44((1)):92–95. doi: 10.1016/j.cyto.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Inagaki-Ohara K, Sawaguchi A, Suganuma T, Matsuzaki G, Nawa Y. Intraepithelial lymphocytes express junctional molecules in murine small intestine. Biochem Biophys Res Commun. 2005;331((4)):977–983. doi: 10.1016/j.bbrc.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Kiristioglu I, Antony P, Fan Y, Forbush B, Mosley RL, Yang H, Teitelbaum DH. Total parenteral nutrition-associated changes in mouse intestinal intraepithelial lymphocytes. Dig Dis Sci. 2002;47((5)):1147–1157. doi: 10.1023/a:1015066813675. [DOI] [PubMed] [Google Scholar]
- Kiristioglu I, Forbush B, Teitelbaum D, Heidelberg K. Loss of enteral nutrition results in alterations in the intestinal intraepithelial lymphocytes. Gastroenterology. 1999;114:G4135. [Google Scholar]
- Kiristioglu I, Teitelbaum DH. Alteration of the intestinal intraepithelial lymphocytes during total parenteral nutrition. J Surg Res. 1998;79((2)):91–96. doi: 10.1006/jsre.1998.5408. [DOI] [PubMed] [Google Scholar]
- Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg. 1996;223((6)):629–635. doi: 10.1097/00000658-199606000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisawa J, Takahashi I, Okudaira A, Hiroi T, Katayama K, Ariyama T, Tsutsumi Y, Nakagawa S, Kiyono H, Mayumi T. Lack of antigen-specific immune responses in anti-IL-7 receptor alpha chain antibody-treated Peyer’s patch-null mice following intestinal immunization with microencapsulated antigen. Eur J Immunol. 2002;32((8)):2347–2355. doi: 10.1002/1521-4141(200208)32:8<2347::AID-IMMU2347>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Li J, Gocinski B, Henken B, Kudsk K. Effects of parenteral nutrition on gut-associated lymphoid tissue. J Traum. 1995;39:44–52. doi: 10.1097/00005373-199507000-00006. [DOI] [PubMed] [Google Scholar]
- Li J, Langkamp-Henken B, Suzuki K, Stahlgren LH. Glutamine prevents parenteral nutrition-induced increases in intestinal permeability [see comments] JPEN J Parenter Enteral Nutr. 1994;18:303–307. doi: 10.1177/014860719401800404. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, Parkos CA. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113((Pt 13)):2363–2374. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25((4)):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Madara JL, Trier JS. Structure and permeability of goblet cell tight junctions in rat small intestine. J Membr Biol. 1982;66((2)):145–157. doi: 10.1007/BF01868490. [DOI] [PubMed] [Google Scholar]
- Moore FA, Moore EE, Jones TN, McCroskey BL, Peterson VM. TEN versus TPN following major abdominal trauma—reduced septic morbidity. J Trauma. 1989;29:916–922. doi: 10.1097/00005373-198907000-00003. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S, Totsuka M, Hachimura S, Goto M, Takahashi T, Yajima T, Kuwata T, Kaminogawa S. Dietary nucleotides increase the proportion of a TCR gammadelta+ subset of intraepithelial lymphocytes (IEL) and IL-7 production by intestinal epithelial cells (IEC); implications for modification of cellular and molecular cross-talk between IEL and IEC by dietary nucleotides. Biosci Biotechnol Biochem. 2000;64((7)):1459–1465. doi: 10.1271/bbb.64.1459. [DOI] [PubMed] [Google Scholar]
- O’Brien DP, Nelson LA, Kemp CJ, Williams JL, Wang Q, Erwin CR, Hasselgren PO, Warner BW. Intestinal permeability and bacterial translocation are uncoupled after small bowel resection. J Pediatr Surg. 2002;37((3)):390–394. doi: 10.1053/jpsu.2002.30807. [DOI] [PubMed] [Google Scholar]
- Planchon S, Fiocchi C, Takafuji V, Roche JK. Transforming growth factor-beta1 preserves epithelial barrier function: identification of receptors, biochemical intermediates, and cytokine antagonists. J Cell Physiol. 1999;181((1)):55–66. doi: 10.1002/(SICI)1097-4652(199910)181:1<55::AID-JCP6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Planchon SM, Martins CA, Guerrant RL, Roche JK. Regulation of intestinal epithelial barrier function by TGF-beta 1. Evidence for its role in abrogating the effect of a T cell cytokine. J Immunol. 1994;153((12)):5730–5739. [PubMed] [Google Scholar]
- Schön MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, Olson S, Lefrancois L, Brenner MB, Grusby MJ, Parker CM. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162((11)):6641–6649. [PubMed] [Google Scholar]
- Seth A, Basuroy S, Sheth P, Rao RK. L-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol. 2004;287((3)):G510–G517. doi: 10.1152/ajpgi.00058.2004. [DOI] [PubMed] [Google Scholar]
- Singleton KD, Wischmeyer PE. Glutamine induces heat shock protein expression via O-glycosylation and phosphorylation of HSF-1 and Sp1. JPEN J Parenter Enteral Nutr. 2008;32((4)):371–376. doi: 10.1177/0148607108320661. [DOI] [PubMed] [Google Scholar]
- Souba WW, Herskowitz K, Salloum RM, Chen MK, Austgen TR. Gut glutamine metabolism. JPEN J Parenter Enteral Nutr. 1990;14((4 Suppl)):45S–50S. doi: 10.1177/014860719001400403. [DOI] [PubMed] [Google Scholar]
- Sun X, Spencer AU, Yang H, Haxhija EQ, Teitelbaum DH. Impact of caloric intake on parenteral nutrition-associated intestinal morphology and mucosal barrier function. JPEN J Parenter Enteral Nutr. 2006;30((6)):474–479. doi: 10.1177/0148607106030006474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Yang H, Nose K, Nose S, Haxhija EQ, Koga H, Feng Y, Teitelbaum DH. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2008;294((1)):G139–G147. doi: 10.1152/ajpgi.00386.2007. [DOI] [PubMed] [Google Scholar]
- van der Hulst RR, van Kreel BK, von Meyenfeldt MF, Brummer RJ, Arends JW, Deutz NE, Soeters PB. Glutamine and the preservation of gut integrity. Lancet. 1993;341((8857)):1363–1365. doi: 10.1016/0140-6736(93)90939-e. [DOI] [PubMed] [Google Scholar]
- Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, Abraham C, Turner JR. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology. 2006;131((4)):1153–1163. doi: 10.1053/j.gastro.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Ueno Y, Yajima T, Iwao Y, Tsuchiya M, Ishikawa H, Aiso S, Hibi T, Ishii H. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J Clin Invest. 1995;95((6)):2945–2953. doi: 10.1172/JCI118002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte gamma delta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol. 2004;172((7)):4151–4158. doi: 10.4049/jimmunol.172.7.4151. [DOI] [PubMed] [Google Scholar]
- Yang H, Fan Y, Teitelbaum DH. Intraepithelial lymphocyte-derived interferon-gamma evokes enterocyte apoptosis with parenteral nutrition in mice. Am J Physiol Gastrointest Liver Physiol. 2003a;284:G629–G637. doi: 10.1152/ajpgi.00290.2002. [DOI] [PubMed] [Google Scholar]
- Yang H, Finaly R, Teitelbaum DH. Alteration in epithelial permeability and ion transport in a mouse model of total parenteral nutrition. Critical Care Medicine. 2003b;31:1118–1125. doi: 10.1097/01.CCM.0000053523.73064.8A. [DOI] [PubMed] [Google Scholar]
- Yang H, Gumucio D, Teitelbaum D. Intestinal specific over-expression of interleukin-7 attenuates the alternation of intestinal intraepithelial lymphocytes after TPN administration. Ann Surg. 2008a;248:849–856. doi: 10.1097/SLA.0b013e31818a1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Kiristioglu I, Fan Y, Forbush B, Bishop DK, Antony PA, Zhou H, Teitelbaum DH. Interferon-gamma expression by intraepithelial lymphocytes results in a loss of epithelial barrier function in a mouse model of total parenteral nutrition. Ann Surg. 2002a;236:226–234. doi: 10.1097/00000658-200208000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Kiritioglu I, Forbush B, Bishiop D, Antony P, Teitelbaum D. Total parenteral nutrition results in altered expression of cytokines in intestinal intraepithelial lymphocytes and a loss of epithelial barrier function in the mouse. Ann Surg. 2002b;236:226–234. doi: 10.1097/00000658-200208000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Madison B, Gumucio D, Teitelbaum D. Specific overexpression of IL-7 in the intestinal mucosa: the role in intestinal intraepithelial lymphocyte development. Am J Physiol Gastrointest Liver Physiol. 2008b;294:G1421–G1430. doi: 10.1152/ajpgi.00060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Spencer AU, Teitelbaum DH. Interleukin-7 administration alters intestinal intraepithelial lymphocyte phenotype and function in vivo. Cytokine. 2005;31((6)):419–428. doi: 10.1016/j.cyto.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Yang H, Sun X, Haxhija EQ, Teitelbaum DH. Intestinal epithelial cell-derived interleukin-7: A mechanism for the alteration of intraepithelial lymphocytes in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2007;292((1)):G84–G91. doi: 10.1152/ajpgi.00192.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Tazuke Y, Ko K, Teitelbaum D. TPN administration alters intestinal epithelial cell derived IL-7: Potential mechanism for alteration in intraepithelial lymphocyte changes. JPEN. 2003c;27:S6. [Google Scholar]
- Yang H, Fan Y, Teitelbaum DH. Intraepithelial lymphocyte-derived interferon-gamma evokes enterocyte apoptosis with parenteral nutrition in mice. Am J Physiol Gastrointest Liver Physiol. 2003;284((4)):G629–G637. doi: 10.1152/ajpgi.00290.2002. [DOI] [PubMed] [Google Scholar]
- Zarzaur BL, Ikeda S, Johnson CD, Le T, Sacks G, Kudsk KA. Mucosal immunity preservation with bombesin or glutamine is not dependent on mucosal addressin cell adhesion molecule-1 expression. JPEN J Parenter Enteral Nutr. 2002;26((5)):265–70. doi: 10.1177/0148607102026005265. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Wang W, Ren WY, He BM, Zhou K, Zhu WN. Effect of cisapride on intestinal bacterial and endotoxin translocation in cirrhosis. World J Gastroenterol. 2003;9((3)):534–538. doi: 10.3748/wjg.v9.i3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TR, Bazargan N, Leader LM, Martindale RG. Glutamine and the gastrointestinal tract. Curr Opin Clin Nutr Metab Care. 2000;3((5)):355–362. doi: 10.1097/00075197-200009000-00005. [DOI] [PubMed] [Google Scholar]