Abstract
Neurotensin receptor type 1 (NTS1) is known to mediate a variety of biological functions of neurotensin (NT) in the central nervous system. In this study, we found that NTS1 null mice displayed decreased sensitivity to the ataxic effect of ethanol on the rotarod and increased ethanol consumption when given free choice between ethanol and tap water containing bottles. Interestingly, the administration of NT69L, a brain-permeable NT analog, increased ethanol sensitivity in wild-type littermates but had no such effect in NTS1 null mice, suggesting that NTS1 contributes to NT-mediated ethanol intoxication. Furthermore, the daily treatment of NT69L, for 4 consecutive days, significantly reduced alcohol preference and consumption in wild-type littermates but had no such effects in NTS1 null mice in a two-bottle drinking experiment. Our study provides evidence for one possible pharmacological role of NT69L in which it increases sensitivity to the ataxic effect, and decreases voluntary consumption, of ethanol. Our study also demonstrates NTS1-mediated behavioral effects of NT69L. Therefore, our findings will be useful for understanding some aspects of alcoholism as well as to develop novel pharmacological therapeutic options for humans.
Keywords: neurotensin, NT69L, alcohol, intoxication, sensitivity, consumption
1. Introduction
Neurotensin (NT) is a 13 amino acid peptide that acts as a neurotransmitter or a neuromodulator in the brain and is expressed in both the central and peripheral nervous systems (Caceda et al., 2006; Carraway and Leeman, 1973; Tyler-McMahon et al., 2000a). Three NT receptors, NT receptor types 1, 2 and 3 (NTS1, NTS2 and NTS3), have been cloned (Vincent et al., 1999). NTS1 is abundantly expressed in the rat (Tanaka et al., 1990) and human brain (Vita et al., 1993). Based on sequence homology with NTS1, NTS2 has been cloned in rats (Chalon et al., 1996), mice (Mazella et al., 1996) and humans (Vita et al., 1998). NTS2 is also highly expressed in the brain (Chalon et al., 1996). Unlike NTS1, NTS2 has a high affinity to levocabastine, a histamine H1 receptor antagonist (Chalon et al., 1996). Both NTS1 and NTS2 are G-protein coupled receptors with a sequence homology of 64% in rats (Chalon et al., 1996; Vincent et al., 1999). Structurally unrelated, NTS3 has been cloned in mouse (Mazella et al., 1988) and human (Zsurger et al., 1994). NTS3 is a single transmembrane protein (Mazella et al., 1998). Both full length NT (1–13) and short form NT (8–13), which contains amino acid fragments 8 to 13, have the highest affinity for NTS1, followed by NTS2 and NTS3. These peptides have over a 1000-fold lower affinity for NTS3 as compared to that for NTS1.
NT is known to enhance behavioral sensitivity to ethanol including ethanol-induced loss of righting response and hypothermia (Luttinger et al., 1981). Interestingly, alcohol-preferring rats show significantly lower concentrations of NT in the frontal cortex when compared to alcohol non-preferring rats (Ehlers et al., 1999). However, only centrally injected NT shows biological effectiveness, not when systematically injected; therefore, endogenous NT is not an ideal pharmacological agent to investigate activation of NT-mediated signaling via systemic injection (Boules et al., 2006).
Interestingly, NT69L, a synthetic NT analog that contains non-biogenic amino acids, N-Me-Arg and L-neo-Trp, is brain permeable and resistant to degradation when injected systematically (Boules et al., 2006). Although NT is known to affect some behavioral effects of ethanol, the functional roles of NTS1 in ethanol intoxication remain unknown. Here we demonstrate behavioral effects mediated by NTS1 in response to ethanol and NT69L, which are implicated in ethanol preference and consumption. Our findings provide possible behavioral mechanisms underlying increased alcohol consumption in NTS1 null mice.
2. Materials and methods
2.1. Animals
NT receptor type 1 (NTS1) null mice were generated and kindly provided by Dr. Rudy Shreiber’s group at Roche (Palo Alto, CA) (Mechanic et al., 2009). The colony was maintained in a C57BL/6J background for over 10 generations (> 99. 9% C57BL/6J background) before we generated F2 generation null mice. Since the phenotypes we examined, such as ethanol-induced ataxia and consumption, can vary across genetic backgrounds (Crawley, 1999; Rustay et al., 2003), we generated F2 null mice that have a ~50% C57BL/6J and ~50% 129X1/SvJ genetic background in order to minimize the risk of false positives and false negatives in behavioral phenotypes, as recommended by the Banbury Conference on Genetic Background in Mice (Banbury Conference on Genetic Background in Mice, 1997). To generate F2 NTS1 null mice, heterozygous NTS1 mice in a C57BL/6J background were crossed with 129X1/SvJ wild-type mice, and then F1 heterozygous mice were crossed. We obtained approximately 25% wild-type, 25% NTS1 null, and 50% heterozygous mice. There was no significant difference in spontaneous mortality (< 2%) between genotypes. Mice were genotyped by PCR utilizing purified tail DNA (DNeasy Kit, Qiagen) with two NTS1-specific primers (5′CAG GAG TGC AGA GAA CCA ACC ACA G 3′, 5′GTT CAC GTC CAG GTT GCT GTT 3′) and a neo primer (5′CCT TCT TGA CGA GTT CTT CTG AG 3′) which produced 488 bp for wild-type and 351 bp for NTS1 null alleles as described (Mechanic et al., 2009). Only male mice were used in experiments when they reached 6~8 weeks old. All mice used, with the exception of the group in the two-bottle choice experiment, were group-housed (4–5 mice per group) in standard Plexiglas cages with rodent chow and water available ad libitum. Independent sets of mice were used to examine the effect of 1) ethanol alone, 2) ethanol in combination with NT69L, and 3) NT69L alone on open-field locomotor activity, rotarod performance, two-bottle choice drinking, and blood alcohol clearance. Cages were maintained on a 12-h light/dark cycle with lights on at 6:00 a.m. Animal care and handling procedures were approved by Mayo Clinic Institutional Animal Care and Use Committee in accordance with NIH guidelines.
2.2. Open-Field Activity
Spontaneous locomotor activity was measured in brightly lit (500 lux) Plexiglas chambers (41 cm × 41 cm) as described (Chen et al., 2007). The chambers were located in sound-attenuating cubicles and equipped with two sets of 16 pulse-modulated infrared photobeams to record X–Y ambulatory movements at a 100 ms resolution (Med Associates, Lafayette, IN). To examine the pharmacological effects of ethanol alone, ethanol in combination with NT69L, and NT69L alone on locomotor activity, mice were given 1) a 1.0 or 1.5 g/kg ethanol injection (20% ethanol in 0.9% NaCl; i.p.), 2) 1.0 or 1.5 g/kg ethanol i.p. injection 15 min after a o.0 (saline; 0.9% NaCl), 0.5, 1.0, or 2.0 mg/kg dose of NT69L (0.12 mg/ml in 0.9% NaCl; i.p.), and 3) 1.0 mg/kg NT69L was injected (i.p.) one-day after the saline-treated experiment, respectively. NT69L was synthesized and provided by Elliott Richelson’s lab in Mayo Clinic Jacksonville (Tyler-McMahon et al., 2000b). Mice were observed in open-field chambers for 1 h. Horizontal distance traveled (cm) was recorded for 1 h. Distance traveled in 20 min intervals was calculated from the locomotor activity data.
2.3. Ataxia
A standard mouse rotarod treadmill (UGO Basile, Verese, Italy) operating at a fixed speed of 20 rpm was used to evaluate ethanol-induced ataxia by observing latency to fall from the treadmill (Choi et al., 2002). Mice were acclimatized to the rotarod treadmill by placing them on the apparatus 2–3 times prior to the actual experiment. Only mice that were able to pass this initial screening (remained on the treadmill for 180 s; time point 0 min) were used for the rotarod experiments. To examine the pharmacological effects of ethanol alone, ethanol in combination with NT69L, and NT69L alone on ataxia, mice were given 1) a 1.0 or 1.5 g/kg ethanol injection (20% ethanol in 0.9% NaCl; i.p.), 2) 1.0 or 1.5 g/kg ethanol i.p. injection 15 min after a o (saline), 0.5, 1.0, or 2.0 mg/kg dose of NT69L (0.12 mg/ml in 0.9% NaCl; i.p.), and 3) 1.0 mg/kg NT69L was injected (i.p.) one-day after the saline-treated experiment, respectively. Latency to fall was evaluated for each mouse in 15 min intervals for 1 h, totaling 4 trials, following the ethanol or NT69L injection (15, 30, 45, and 60 min).
2.4. Blood Ethanol Clearance
Mice were injected with 3.6 g/kg ethanol (20% ethanol in 0.9% NaCl; i.p.) or 1.0 g/kg (i.p.) ethanol 30 min after 1.0 mg/kg NT69L (0.12 mg/ml in 0.9% NaCl; i.p.) and immediately returned to their home cages (Choi et al., 2002). Approximately 50 μl of blood was collected from each mouse 1, 2, and 3 h after the ethanol injection via tail bleeding. Plasma ethanol levels were determined using Analox AM1 (Analox Instrument USA, Lunenburg, MA).
2.5. Alcohol Self-Administration
Oral alcohol self-administration and preference were examined using a two-bottle choice experiment (Choi et al., 2004). Mice were given one week to acclimatize to individual housing conditions and handling. Mice were then given 24 h access to two bottles, one containing plain tap water and the other containing an ethanol solution. The concentration of ethanol was raised every fourth day, increasing from 3 to 6 to 10 % (v/v) ethanol in tap water. The positions of the bottles were changed every 2 days to control position preference. Also, we examined potential differences in taste preference, which could influence alcohol consumption. Four weeks after the ethanol self-administration procedure, the same mice were tested for saccharin (sweet) and quinine (bitter) fluid intake and preference in an order-balanced experimental design that can detect taste neophobias (Crabbe et al., 1996). Saccharin sodium salt and quinine hemisulphate salt (Sigma, St. Louis, Missouri) were dissolved in tap water. These sweet and bitter solutions were used because of their strong tastes, lack of caloric value and absence of confounding pharmacological effects. The concentration of saccharin (0.03 and 0.06%) and quinine (30 and 60 μM) were raised every fourth day and the positions of the bottles were changed every 2 days to control position preference. Throughout the experiments, fluid intake and body weight were measured every 2 days using an analytical balance (Denver Instrument, Arvada, CO) with a precision of 0.01 g. During the alcohol self-administration experiment, average ethanol consumption per day was obtained for each ethanol concentration. To obtain an accurate measure of ethanol consumption that corrected for individual physical differences in mouse weight, grams of ethanol consumed per kilogram of body weight per day were calculated for each mouse. A measure of relative ethanol preference (%) was calculated at each ethanol concentration by dividing the total ethanol solution consumption by the total fluid (ethanol plus water) consumption. Similarly, relative taste preference (%) was calculated at each concentration by dividing the total saccharin or quinine solution consumption by total fluid consumption.
To examine the effect of NT69L on ethanol consumption and preference, a new group of mice was given 1.0 mg/kg NT69L (i.p.) every 12 h for four additional days after the 10% ethanol period continuously in a two-bottle drinking experiment. Fluid intake and body weight were measured every 2 days and the positions of the bottles were changed every 2 days to control position preference. Relative ethanol consumption (%) and preference (%) were calculated by dividing the average of ethanol consumption or preference of pre-, NT69- and post-injection periods by the average of the pre-injection period (four days each).
2.6. Statistical Analysis
All data were expressed as mean ± s.e.m. For the rotarod, basal open-field locomotor activity, blood alcohol concentration, and the two-bottle choice experiments, statistical analyses were carried out by two-way repeated measures ANOVA followed by Tukey post-hoc test. To examine the effect of NT69L during the rotarod and two-bottle choice experiment, one-way repeated measures ANOVA followed by Tukey post-hoc test was used. All other data were analyzed using unpaired two-tailed t-test. Criterion for statistical significance was p < 0.05.
3. Results
3.1. NTS1 null mice showed reduced ethanol-induced ataxia and locomotor activity
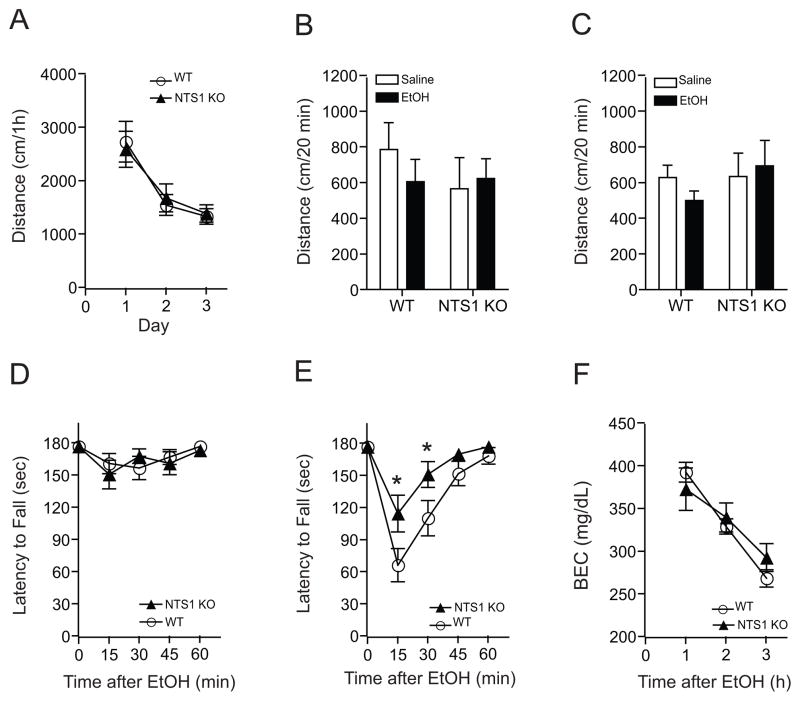
We examined ethanol-induced spontaneous locomotor activity of mice lacking the NTS1 gene. There were no differences in basal spontaneous locomotion (Fig. 1A) or initial ethanol-induced locomotion when NTS1 null mice were given 1.0 or 1.5 g/kg ethanol (Fig. 1B and C) compared to their wild-type littermates. Next, we investigated ethanol-induced ataxia using a constant velocity rotarod (Choi et al., 2008). While there was no difference between genotypes at a 1.0 g/kg ethanol dose (Fig. 1D), NTS1 null mice showed a significantly reduced sensitivity to ethanol-induced ataxia in response to an acute 1.5 g/kg ethanol i.p. injection compared to their wild-type littermates (Fig. 1E). Two-way repeated measures ANOVA showed significant effects of genotype (F(1,148) = 5.147, p = 0.029) and time (F(4,148) = 29.094, p < 0.001), but no significant effect of the interaction between genotype and time (F(4,148) = 2.406, p = 0.052).
Figure 1.
Effect of ethanol-induced locomotor activity and ataxia in NTS1 null mice. (A) Basal locomotor activity was similar between genotypes (n = 22 for wild-type and n = 16 for NTS1 null mice). (B) 1.0 g/kg (i.p.) ethanol (n = 11 for wild-type and n = 7 for NTS1 null mice) and (C) 1.5 g/kg (i.p.) ethanol did not induce any changes in initial locomotor activation (n = 11 for wild-type and n = 9 for NTS1 null mice). In the rotarod test, (D) 1.0 g/kg ethanol had no effect (n = 19 for wild-type and n = 15 for NTS1 null mice), but (E) at a 1.5 g/kg ethanol dose, NTS1 null mice (n = 18) showed a significant reduction in ethanol-induced ataxia in a rotarod test compared to their wild-type littermates (n = 21). *p < 0.05 compared to the wild-type littermates at same time after ethanol injection (Tukey test). (F) Blood ethanol clearances after acute administration of 3.6 g/kg ethanol were similar between genotypes (n = 11 for wild-type and n = 9 for NTS1 null mice). p > 0.05 by Tukey test. All data are expressed as mean ± s.e.m.
Since individual differences in ethanol clearance could contribute to altered acute ethanol responses, we measured blood ethanol concentrations 1, 2 and 3 h after an injection of 3.6 g/kg ethanol (i.p.). We found that blood ethanol concentrations were similar between NTS1 null mice and their wild-type littermates (Fig. 1F), indicating that differences in ethanol-induced ataxia were not due to alterations in blood ethanol concentrations. Two-way repeated measures ANOVA showed no effect of genotype (F(1,36) = 0.064, p = 0.803), but showed significant effects of time (F(2,36) = 95.128, p < 0.001) and the interaction between genotype and time (F(2,36) = 4.701, p = 0.015).
3.2. NTS1 null mice consume more alcohol voluntarily
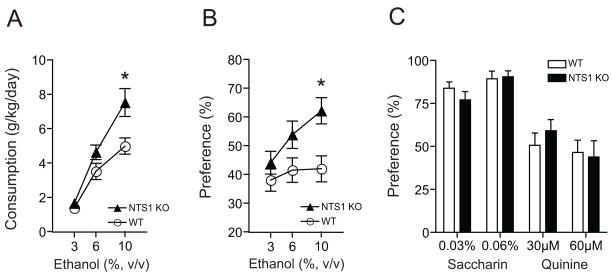
Next, we examined whether absence of NTS1 could alter ethanol consumption and preference using a two-bottle choice drinking experiment (Choi et al., 2004; Choi et al., 2002). NTS1 null mice consumed significantly more ethanol during the 10% ethanol period compared to their wild-type littermates (Fig. 2A). Two-way repeated measures ANOVA mice showed significant effects of genotype (F(1,55) = 6.888, p = 0.013) and ethanol concentrations (F(2,55) = 53.067, p < 0.001), but did not show any significant effect of the interaction between genotype and ethanol concentrations (F(2,55) = 3.097, p = 0.053). Furthermore, NTS1 null mice showed greater ethanol preference at 10% ethanol compared to their wild-type littermates (Fig. 2B). Two-way repeated measures ANOVA showed significant effects of genotype (F(1,47) = 4.533, p = 0.041) and ethanol concentrations (F(2,47) = 3.818, p = 0.029), but did not show a significant effect of the interaction between genotype and ethanol concentrations (F(2,47) = 2.891, p = 0.065). Additionally, we confirmed that the increased ethanol drinking behavior was not due to alterations in water intake, or body weight (data not shown).
Figure 2.
Ethanol consumption, preference and taste preference of NTS1 null mice. (A) Ethanol consumption and (B) preference of NTS1 null mice (n = 18) in a two-bottle choice experiment. *p < 0.05 compared to their wild-type littermates (n = 14) at the same ethanol concentration (Tukey test). (C) No differences (p > 0.05 by Tukey test) in genotype-associated taste (saccharin for sweet and quinine for bitter tastes) preference in NTS1 null mice (n = 9) compared to their wild-type littermates (n = 11). All data are expressed as mean ± s.e.m.
Since differential taste reactivity may affect alcohol consumption and preference, we performed a taste preference experiment using saccharin (sweet) and quinine (bitter) solutions (Crabbe et al., 1996). In this experiment, NTS1 null mice did not show any significant differences in saccharin or quinine preference over water compared to their wild-type littermates (Fig. 2C). For saccharin solutions, two-way repeated measures ANOVA did not show any significant effects of genotype (F(1,18) = 0.352, p = 0.561) or the interaction between genotype and saccharin concentrations (F(1,18) = 1.497, p = 0.237), but showed a significant effect of saccharin concentrations (F(1,18) = 8.505, p = 0.009). For quinine solutions, two-way repeated measures ANOVA did not show any significant effects of genotype (F(1,18) = 0.087, p = 0.772) or the interaction between genotype and saccharin concentrations (F(1,18) = 2.393, p = 0.139), but showed a significant effect of saccharin concentrations (F(1,18) = 7.476, p = 0.014). Our results indicated NTS1 null mice preferred and voluntarily consumed more ethanol than their wild-type littermates, without any genotype-associated taste preference differences.
3.3. NTS1 mediates NT-mediated ataxia
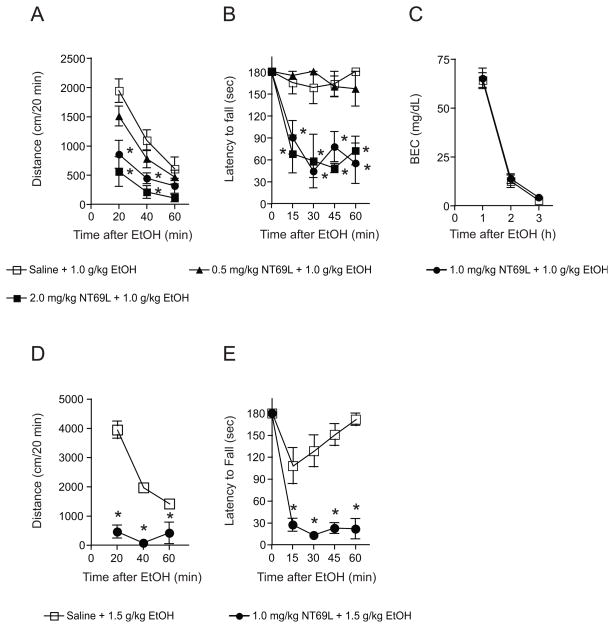
We examined the dose-dependent effect of NT69L on ethanol-induced spontaneous locomotion and ataxia using 0, 0.5, 1.0, or 2.0 mg/kg NT69L doses. For the open-field locomotor test (Fig. 3A), when mice were given 1.0 and 2.0 mg/kg NT69L, two-way repeated measures ANOVA showed a significant effect of treatment (F(3,40) = 8.121, p < 0.001), time (F(2,40) = 63.773, p < 0.001), and interaction between treatment and time (F(6,40) = 3.789, p = 0.004) compared to the saline treated group. For the ataxia test (Fig. 3B), the two-way repeated measures ANOVA showed a significant effect of treatment (F(3, 60) = 28.481, p < 0.001), time (F(4, 60) = 13.436, p < 0.001) and an interaction between these factors (F(12, 60) = 3.388, p < 0.001) at NT69L doses of 1.0 and 2.0 mg/kg when compared to the saline treated group. For blood ethanol clearance in response to 1.0 g/kg ethanol and 1.0 mg/kg NT69L (Fig. 3C), two-way repeated measures ANOVA did not show any effects on either the treatment (F(1,20) = 0.247, p = 0.630) or the interaction between treatment and time (F(2,20) = 0.00402, p = 0.996), but did show a significant effect of time (F(2,20) = 218.034, p < 0.001). Interestingly, at a 1.5 g/kg ethanol dose, mice pretreated 1.0 mg/kg NT69L dose displayed a severe reduction of locomotor activity (Fig. 3D) and motor coordination (Fig. 3E) compared to the saline-pretreated group. For locomotor activity (Fig. 3D), two-way repeated measures ANOVA showed a significant effect of treatment (F(3,28) = 93.354, p < 0.001), time (F(2,28) = 25.820, p < 0.001), and interaction between treatment and time (F(2,28) = 19.920, p < 0.001). For the motor coordination experiment (Fig. 3E), the two-way repeated measures ANOVA showed a significant effect of the treatment (F(1, 56) = 46.275, p < 0.001), time (F(4, 56) = 45.852, p < 0.001) and interaction between these factors (F(4, 56) = 18.573, p < 0.001).
Figure 3.
Effect of 1.0 g/kg ethanol on NT69L pretreated mice in open-field locomotor activity and rotarod ataxia experiments. (A) NT69L-treated mice traveled less in the open field activity compared to saline-treated mice in a dose-dependent fashion after ethanol administration (i.p.) to observe the effect of NT69L in ethanol-mediated spontaneous locomotor activity. The Tukey test showed reduced spontaneous locomotor activity from 20 min to 40 min (at 1.0 and 2.0 mg/kg NT69L + 1.0 g/kg ethanol) after NT69L administration in wild-type mice. (B) Dose-dependency of increased alcohol-induced ataxic responses of NT69L-treated wild-type mice. The Tukey test showed that NT69L treatment was effective from 15 min to 1 h after the ethanol treatment at doses of 1.0 mg/kg and 2.0 mg/kg NT69L. Conditions were 1.0 g/kg ethanol and various concentrations of NT69L (saline, 0.5, 1.0, and 2.0 mg/kg NT69L) for both experiments. n = 5–6 for each treatment. *p < 0.05 compared to the saline+ethanol-injected mice at the same time after injection. (C) No significant difference (p > 0.05 by Tukey test) between blood ethanol clearances after acute administration of NT69L. Conditions were 1.0 g/kg ethanol and 1.0 mg/kg NT69L for blood ethanol clearance experiment (n = 6). At 1.5 g/kg ethanol-injected mice showed sedative-like phenotype when pretreated with 1.0 mg/kg NT69L (n = 8) both in (D) locomotor activity and (E) ataxia compared to saline-treated mice (n = 8). *p < 0.05 by Tukey test. All data are expressed as mean ± s.e.m.
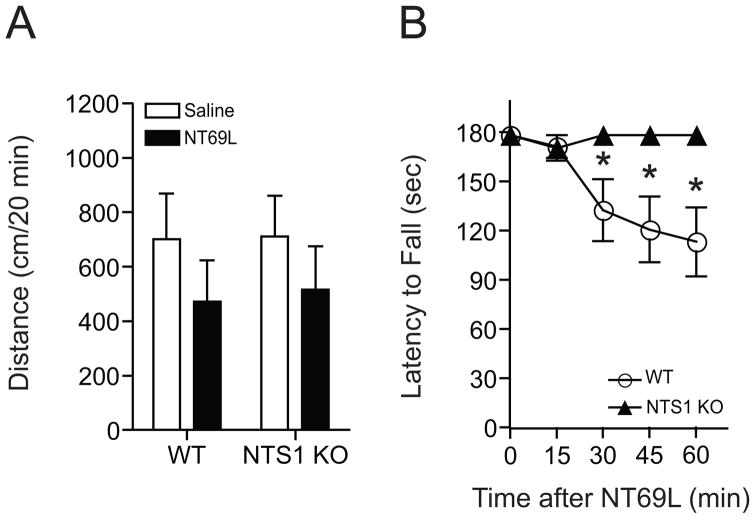
We measured spontaneous locomotor activity after mice were given either 1.0 mg/kg NT69L or equivalent volume of saline (i.p. injection) and placed in open-field chambers for 1 h. Similar to 1.5 g/kg ethanol administration, 1.0 mg/kg acute NT69L (i.p.) did not show any significant change of locomotor activation in NTS1 null mice as compared to their wild-type littermates (p > 0.05) (Fig. 4A). These results indicate that NTS1 receptor is not responsible for NT-mediated spontaneous locomotor activity in the open-field chamber.
Figure 4.
Effect of NT69L-induced locomotor activity and ataxia in NTS1 null mice. (A) 1.0 mg/kg NT69L (i.p. in saline) did not induce any significant alteration of initial locomotor activation compared to their saline-treated groups both in NTS1 null mice (n = 6) and wild-type littermates (n = 9; p > 0.05 by t-test). (B) However, NTS1 null mice stayed on the rotarod significantly longer than the wild-type littermates from 30 min after NT69L injection (n = 12 for each genotype). *p < 0.05 compared to their saline-treated groups both in NTS1 null mice and wild-type littermates (Tukey test). All data are expressed as mean ± s.e.m.
Next, we examined whether NT69L-induced ataxia is absent in NTS1 null mice. Indeed, NTS1 null mice showed no NT69L-induced ataxia after an i.p. injection of 1.0 mg/kg NT69L, while wild-type mice showed an ataxic effect at this dose of NT69L (Fig. 4B). One-way repeated measures ANOVA showed a significant ataxic effect of NT69L in the wild-type littermates (F(4,44) = 6.998, p < 0.001), but not in NTS1 null mice (F(4,44) = 1.000, p = 0.418). These findings suggest that NT-mediated ataxia is mainly attributed to NTS1.
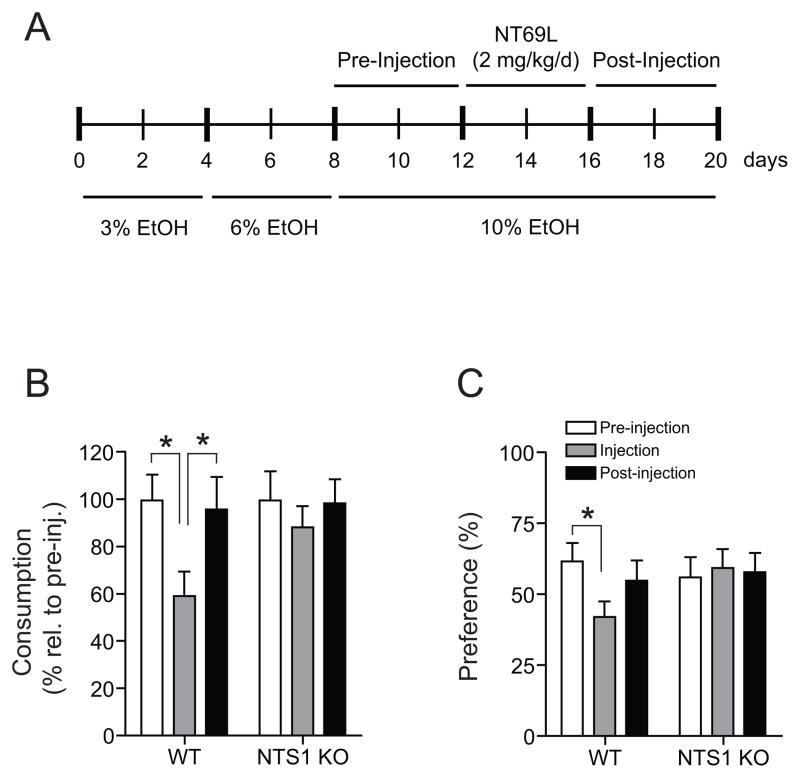
3.4. NTS1 is responsible for NT69L-induced decrease in voluntary alcohol consumption
To examine whether NT analog NT69L could regulate ethanol consumption and preference, we evaluated oral ethanol self-administration using a modified two-bottle choice experiment (Fig. 5A). As shown in Fig. 5B, wild-type littermates consumed significantly less ethanol during the NT69L injection period as compared to the pre- and post-treatment periods. Interestingly, NT69L had no effect on alcohol consumption in NTS1 null mice (Fig. 5B). One-way repeated measures ANOVA for wild-type littermates showed a significant effect of NT69L treatment (F(2,16) = 26.79, p < 0.0001), but did not for NTS1 null mice (F(2,16) = 0.97, p = 0.39). For ethanol preference during the modified two-bottle choice drinking experiment, one-way repeated measures ANOVA showed a significant effect of NT69L in wild-type littermates (F(2,14) = 4.346, p = 0.034), but did not in NTS1 null mice (F(2,20) = 0.353, p = 0.707) (Fig. 5C). These findings strongly suggest that NTS1 mediates the pharmacological effect of NT69L in reducing ethanol consumption and preference.
Figure 5.
Effect of NT69L on ethanol consumption and preference in NTS1 null mice. (A) Experiment design scheme for the modified two-bottle choice experiment to examine pharmacological effect of NT69L. (B) Ethanol consumption and (C) preference for ethanol are significantly reduced in the wild-type littermates (n = 9) but not changed in NTS1 null mice (n = 11). *p < 0.05 compared to the pre- or post-injection period as indicated by Tukey test. All data are expressed as mean ± s.e.m.
4. Discussion
Our present study provides a novel role of NTS1 in response to ethanol and a brain-permeable NT analog, NT69L. Notably, mice lacking NTS1 are resistant to ethanol-induced and NT69L-induced ataxia on the rotarod. Furthermore, NTS1 null mice are insensitive to one of the pharmacological effects of NT69L in reducing ethanol drinking, indicating that NTS1 contributes to ethanol intoxication and consumption. Our findings suggest that NTS1 contributes to the ataxic effects of ethanol and ethanol consumption.
Since NT69L is a specific agonist for NTS1, the activation of NT receptors via systemic injection could mimic microinjection of NT or NT analogs. We found that NT69L mimics ethanol-mediated ataxia and also reduced alcohol consumption in wild-type littermates. In contrast, NTS1 null mice were insensitive to NT69L-induced ataxia and the reduction of alcohol consumption and preference, indicating that activation of NTS1-mediated signaling promotes ethanol-induced ataxia and reduces ethanol consumption. Our results are consistent with the finding that alcohol preferring rats show a significantly lower concentration of endogenous NT in the frontal cortex, which might lead to reduced NTS1-mediated signaling when compared to non-alcohol preferring rats (Ehlers et al., 1999). Taken together, activation of NTS1 signaling appears to be inversely correlated with ethanol consumption and preference.
Notably, we found a significant difference between 1.0 and 1.5 g/kg ethanol doses on locomotor activity and ataxia when mice were pretreated with 1.0 mg/kg NT69L. This difference might be due to the biphasic pharmacological effect of ethanol on glutamate release at the striatum, which regulates motor function. Since low ethanol doses (< 1.0 g/kg) promote extracellular glutamate levels, whereas high ethanol doses (> 2.0 g/kg) inhibit glutamate levels in the striatum (Moghaddam and Bolinao, 1994), a significant reduction in glutamate levels could be correlated with a notable difference between 1.0 and 1.5 g/kg ethanol doses in our study.
Interestingly, a behavioral study showed that NTS1 null mice do not display NT-induced inhibition of feeding (Remaury et al., 2002). This finding suggests that reduced ethanol consumption might be due to reduced appetite to ethanol without altering taste preference or water consumption since ethanol intake is related to dietary homeostasis (Forsander, 1998). NT69L can activate NTS1-mediated behavioral functions in various brain regions since NTS1 is expressed in most brain regions including the substantia nigra (SN), ventral tegmental area (VTA), lateral septum, anterior cingulate, and insular cortex (Boudin et al., 1996; Fassio et al., 2000). Moreover, NTS1 exerts its function through the PLCβ-PKC pathway (Perron et al., 2007). Therefore, further studies on brain region-specific function or downstream signaling of NTS1 would clarify the molecular mechanisms of how NTS1-regulated signaling contributes to ethanol-induced ataxia and preference.
As recommended by a consortium on mice genetic background for neuro-behavioral studies (Banbury Conference on Genetic Background in Mice, 1997), we used F2 generation hybrid mice with C57BL/6J × 129X1/SvJ genetic background to minimize the risk of false positives or negatives in behavioral phenotypes that could be influenced by a C57BL/6J background. Nevertheless, there are still potential caveats of using this mixed genetic background since co-segregated genes near the knockout locus could contribute to the phenotypes attributed to the null allele. Furthermore, inbred C57BL/6J mice are less sensitive to the ataxic and sedating effects of ethanol and display higher ethanol consumption than inbred 129X1/SvJ mice (Crabbe, 2001; Tordoff et al., 2002). Therefore, to ensure that our findings are not due to background alleles from either genetic background, but instead are due to the knockout mutation, further testing with inbred 129X1/SvJ or C57BL/6J mice lacking NTS1 would clarify the effects of genetic backgrounds (Banbury Conference on Genetic Background in Mice, 1997). However, our experiments indicate that complications from genetic backgrounds or possible co-segregated genes nearby NTS1 seem not to influence the phenotypes we observed because NTS1 null mice prefer ethanol compared to wild-type littermates, and conversely NT69L reduced alcohol consumption significantly in wild-type littermates but showed no significant effect in NTS1 null mice.
Our locomotor activity and ataxia study suggests that activation of NT receptors may be associated with the motor functions of alcohol. Because young adults with a low level of response to acute alcohol intake are known to be associated with a higher risk of developing an alcohol use disorder later in life (Schuckit, 1998), reduced ethanol sensitivity due to the lack of NT signaling could promote, or be a permissive factor, for increased ethanol intake.
Importantly, the unique pharmacological profile of NT69L, including brain permeability, extended half-life, and low-toxicity without any addictive properties (Boules et al., 2006; Fantegrossi et al., 2005) allowed us to examine its neuropharmacological effects via systemic injection. Given extracranially to rats, NT69L reduced the hyperactivity caused by cocaine (Boules et al., 2005). In addition, NT69L also reverses the disruption of prepulse inhibition caused by several different agents (Caceda et al., 2006; Feifel et al., 2003). Thus, activating NTS1 may be effective in regulating addictive behaviors.
In summary, our findings suggest that NTS1 contributes to the ataxic effect of ethanol and NT69L, a synthetic neurotensin analog. Injections of NT69L (2 mg/kg/d, i.p.), for four consecutive days, significantly reduced ethanol preference and consumption when mice steadily consumed 10% ethanol during a two-bottle choice experiment. Such findings indicate that activating NTS1 and its associated signaling pathways could potentially be a novel therapeutic option for treating human alcoholism.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health (NIH) to D.-S.C. (AA015164, AA018779, and AA017830-Project 1) and by Samuel Johnson Foundation for genomics of drug addiction program at Mayo Clinic to D.-S.C. We thank S. Choi for mouse husbandry, S. Johng for technical assistance and M. Boules for providing NT69L compounds. We thank D. Frederixon for preparing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banbury Conference on Genetic Background in Mice. Mutant Mice and Neuroscience: Recommendations Concerning Genetic Background. Neuron. 1997;19:755–759. doi: 10.1016/s0896-6273(00)80958-7. [DOI] [PubMed] [Google Scholar]
- Boudin H, Pelaprat D, Rostene W, Beaudet A. Cellular distribution of neurotensin receptors in rat brain: immunohistochemical study using an antipeptide antibody against the cloned high affinity receptor. J Comp Neurol. 1996;373:76–89. doi: 10.1002/(SICI)1096-9861(19960909)373:1<76::AID-CNE7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Boules M, Fredrickson P, Richelson E. Bioactive analogs of neurotensin: focus on CNS effects. Peptides. 2006;27:2523–2533. doi: 10.1016/j.peptides.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Boules M, Fredrickson P, Richelson E. Neurotensin agonists as an alternative to antipsychotics. Expert Opin Investig Drugs. 2005;14:359–369. doi: 10.1517/13543784.14.4.359. [DOI] [PubMed] [Google Scholar]
- Caceda R, Kinkead B, Nemeroff CB. Neurotensin: role in psychiatric and neurological diseases. Peptides. 2006;27:2385–2404. doi: 10.1016/j.peptides.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248:6854–6861. [PubMed] [Google Scholar]
- Chalon P, Vita N, Kaghad M, Guillemot M, Bonnin J, Delpech B, Le Fur G, Ferrara P, Caput D. Molecular cloning of a levocabastine-sensitive neurotensin binding site. FEBS Lett. 1996;386:91–94. doi: 10.1016/0014-5793(96)00397-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Rinaldo L, Lim SJ, Young H, Messing RO, Choi DS. The type 1 equilibrative nucleoside transporter regulates anxiety-like behavior in mice. Genes Brain Behav. 2007;6:776–783. doi: 10.1111/j.1601-183X.2007.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, Diamond I, Bonci A, Messing RO. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci. 2004;7:855–861. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- Choi DS, Wang D, Dadgar J, Chang WS, Messing RO. Conditional rescue of protein kinase C epsilon regulates ethanol preference and hypnotic sensitivity in adult mice. J Neurosci. 2002;22:9905–9911. doi: 10.1523/JNEUROSCI.22-22-09905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Wei W, Deitchman JK, Kharazia VN, Lesscher HM, McMahon T, Wang D, Qi ZH, Sieghart W, Zhang C, Shokat KM, Mody I, Messing RO. Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J Neurosci. 2008;28:11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC. Use of genetic analyses to refine phenotypes related to alcohol tolerance and dependence. Alcohol Clin Exp Res. 2001;25:288–292. [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN, Schafer GL. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet. 1996;14:98–101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Li TK, Lumeng L, Kinkead B, Owens MJ, Nemeroff CB. Neurontensin studies in alcohol naive, preferring and non-preferring rats. Neuroscience. 1999;93:227–236. doi: 10.1016/s0306-4522(99)00113-x. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Ko MC, Woods JH, Richelson E. Antinociceptive, hypothermic, hypotensive, and reinforcing effects of a novel neurotensin receptor agonist, NT69L, in rhesus monkeys. Pharmacol Biochem Behav. 2005;80:341–349. doi: 10.1016/j.pbb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Fassio A, Evans G, Grisshammer R, Bolam JP, Mimmack M, Emson PC. Distribution of the neurotensin receptor NTS1 in the rat CNS studied using an amino-terminal directed antibody. Neuropharmacology. 2000;39:1430–1442. doi: 10.1016/s0028-3908(00)00060-5. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. A systemically administered neurotensin agonist blocks disruption of prepulse inhibition produced by a serotonin-2A agonist. Neuropsychopharmacol. 2003;28:651–653. doi: 10.1038/sj.npp.1300083. [DOI] [PubMed] [Google Scholar]
- Forsander OA. Dietary influences on alcohol intake: a review. J Stud Alcohol. 1998;59:26–31. doi: 10.15288/jsa.1998.59.26. [DOI] [PubMed] [Google Scholar]
- Luttinger D, Nemeroff CB, Mason GA, Frye GD, Breese GR, Prange AJ., Jr Enhancement of ethanol-induced sedation and hypothermia by centrally administered neurotensin, beta-endorphin and bombesin. Neuropharmacol. 1981;20:305–309. doi: 10.1016/0028-3908(81)90139-8. [DOI] [PubMed] [Google Scholar]
- Mazella J, Botto JM, Guillemare E, Coppola T, Sarret P, Vincent JP. Structure, functional expression, and cerebral localization of the levocabastine-sensitive neurotensin/neuromedin N receptor from mouse brain. J Neurosci. 1996;16:5613–5620. doi: 10.1523/JNEUROSCI.16-18-05613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazella J, Chabry J, Kitabgi P, Vincent JP. Solubilization and characterization of active neurotensin receptors from mouse brain. J Biol Chem. 1988;263:144–149. [PubMed] [Google Scholar]
- Mazella J, Zsurger N, Navarro V, Chabry J, Kaghad M, Caput D, Ferrara P, Vita N, Gully D, Maffrand JP, Vincent JP. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J Biol Chem. 1998;273:26273–26276. doi: 10.1074/jbc.273.41.26273. [DOI] [PubMed] [Google Scholar]
- Mechanic JA, Sutton JE, Berson AE, Wu X, Kwan J, Schreiber R, Pang Z, Button DC. Involvement of the neurotensin receptor 1 in the behavioral effects of two neurotensin agonists, NT-2 and NT69L: lack of hypothermic, antinociceptive and antipsychotic actions in receptor knockout mice. Eur Neuropsychopharmacol. 2009;19:466–475. doi: 10.1016/j.euroneuro.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML. Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neurosci Lett. 1994;178:99–102. doi: 10.1016/0304-3940(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Perron A, Sharif N, Sarret P, Stroh T, Beaudet A. NTS2 modulates the intracellular distribution and trafficking of NTS1 via heterodimerization. Biochem Biophys Res Commun. 2007;353:582–590. doi: 10.1016/j.bbrc.2006.12.062. [DOI] [PubMed] [Google Scholar]
- Remaury A, Vita N, Gendreau S, Jung M, Arnone M, Poncelet M, Culouscou JM, Le Fur G, Soubrie P, Caput D, Shire D, Kopf M, Ferrara P. Targeted inactivation of the neurotensin type 1 receptor reveals its role in body temperature control and feeding behavior but not in analgesia. Brain Res. 2002;953:63–72. doi: 10.1016/s0006-8993(02)03271-7. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Wahlsten D, Crabbe JC. Assessment of genetic susceptibility to ethanol intoxication in mice. Proc Natl Acad Sci U S A. 2003;100:2917–2922. doi: 10.1073/pnas.0437273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Biological, psychological and environmental predictors of the alcoholism risk: a longitudinal study. J Stud Alcohol. 1998;59:485–494. doi: 10.15288/jsa.1998.59.485. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Masu M, Nakanishi S. Structure and functional expression of the cloned rat neurotensin receptor. Neuron. 1990;4:847–854. doi: 10.1016/0896-6273(90)90137-5. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Pilchak DM, Williams JA, McDaniel AH, Bachmanov AA. The maintenance diets of C57BL/6J and 129X1/SvJ mice influence their taste solution preferences: implications for large-scale phenotyping projects. J Nutr. 2002;132:2288–2297. doi: 10.1093/jn/132.8.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler-McMahon BM, Boules M, Richelson E. Neurotensin: peptide for the next millennium. Regul Pept. 2000a;93:125–136. doi: 10.1016/s0167-0115(00)00183-x. [DOI] [PubMed] [Google Scholar]
- Tyler-McMahon BM, Stewart JA, Farinas F, McCormick DJ, Richelson E. Highly potent neurotensin analog that causes hypothermia and antinociception. Eur J Pharmacol. 2000b;390:107–111. doi: 10.1016/s0014-2999(99)00877-8. [DOI] [PubMed] [Google Scholar]
- Vincent JP, Mazella J, Kitabgi P. Neurotensin and neurotensin receptors. Trends Pharmacol Sci. 1999;20:302–309. doi: 10.1016/s0165-6147(99)01357-7. [DOI] [PubMed] [Google Scholar]
- Vita N, Laurent P, Lefort S, Chalon P, Dumont X, Kaghad M, Gully D, Le Fur G, Ferrara P, Caput D. Cloning and expression of a complementary DNA encoding a high affinity human neurotensin receptor. FEBS Lett. 1993;317:139–142. doi: 10.1016/0014-5793(93)81509-x. [DOI] [PubMed] [Google Scholar]
- Vita N, Oury-Donat F, Chalon P, Guillemot M, Kaghad M, Bachy A, Thurneyssen O, Garcia S, Poinot-Chazel C, Casellas P, Keane P, Le Fur G, Maffrand JP, Soubrie P, Caput D, Ferrara P. Neurotensin is an antagonist of the human neurotensin NT2 receptor expressed in Chinese hamster ovary cells. Eur J Pharmacol. 1998;360:265–272. doi: 10.1016/s0014-2999(98)00678-5. [DOI] [PubMed] [Google Scholar]
- Zsurger N, Mazella J, Vincent JP. Solubilization and purification of a high affinity neurotensin receptor from newborn human brain. Brain Res. 1994;639:245–252. doi: 10.1016/0006-8993(94)91737-x. [DOI] [PubMed] [Google Scholar]