Abstract
Purpose
To determine the presence of four clinically relevant bacterial endosymbionts in Acanthamoeba isolates obtained from patients with Acanthamoeba keratitis (AK) and the possible contribution of endosymbionts to the pathogenesis of AK.
Design
Experimental study
Participants
Acanthamoeba isolates (N=37) recovered from cornea and contact lens paraphernalia of 23 patients with culture proven AK and 1 environmental isolate.
Methods
Acanthamoeba isolates were evaluated for the presence of microbial endosymbionts belonging to the bacterial genera Legionella, Pseudomonas, Mycobacteria and Chlamydia using molecular techniques (Polymerase chain reaction and sequence analysis, fluorescent in situ hybridization) and transmission electron microscopy. Corneal toxicity and virulence of Acanthamoeba isolates with and without endosymbionts were compared using a cytopathic effect (CPE) assay of human corneal epithelial cells in vitro. Initial visual acuity (VA), location and characteristics of the infiltrate, time to detection of the infection and symptoms duration at presentation were evaluated in all patients.
Main Outcome Measures
Prevalence and potential pathobiology of bacterial endosymbionts detected in Acanthamoeba isolates recovered from AK.
Results
Twenty-two of the 38 (59.4%) cultures examined contained at least one bacterial endosymbiont. One isolate contained two endosymbionts, Legionella and Chlamydia, confirmed by fluorescence in situ hybridization. Corneal toxicity (CPE) was significantly higher for Acanthamoebae hosting endosymbionts compared to isolates without endosymbionts (p<0.05). Corneal pathogenic endosymbionts such as Pseudomonas and Mycobacterium enhanced Acanthamoeba CPE significantly more than Legionella (p<0.05). In the presence of bacterial endosymbionts, there was a trend toward worse initial VA (p>0.05), central location (p<0.05), absence of radial perineuritis (p<0.05), delayed time to detection (p>0.05) and longer symptoms duration at presentation (p>0.05).
Conclusion
The majority of Acanthamoeba isolates responsible for AK harbors one or more bacterial endosymbionts. The presence of endosymbionts enhances the corneal pathogenicity of Acanthamoeba isolates and might impact detection time and clinical features of AK.
Introduction
Acanthamoeba keratitis (AK) is a painful, sight-threatening and difficult to treat corneal infection caused by pathogenic Acanthamoeba.1, 2 Although it is considered a rare corneal affection, the incidence of AK has exponentially increased over the past two decades as a consequence of the increasing use of contact lenses.3 AK primarily affects otherwise healthy contact lens wearers and patients with a history of trauma.1, 2 Clinical features are often misleading and clinical course is usually protracted despite aggressive treatment with anti-amoebic drugs.2
The genus Acanthamoeba encompasses at least 15 species of free living amoebae that have been isolated from a wide range of environments ranging from natural habitats like soil, salt water and fresh water, to domestic sources like tap water, air conditioning units and sewage systems. 1, 4-7
Acanthamoeba undergoes two stages during its life cycle: a vegetative trophozoite and a dormant resistant cyst stage.1, 8 During the trophozoite stage, Acanthamoeba actively feed on bacteria, fungi, yeasts, algae or small organic particles.8 However, a wide range of bacteria have developed strategies to resist phagocytosis, survive intracellularly and exploit Acanthamoeba for multiplication, and are therefore defined as endosymbionts.9-11 These bacterial endosymbionts are usually able to survive encystment of the amoeba, and the intracellular lifestyle protects the bacteria from adverse environmental conditions.11 This adaptation makes the amoeba a potential vehicle of virulence for pathogenic bacteria.9, 12
The association between bacterial endosymbionts and their amoeba hosts can be either transient (in the case of facultative intracellular bacteria) or stable (in the case of obligate intracellular bacteria).9 Stable associations of bacteria with amoebae leading to long term symbiotic interactions have been described for members of four evolutionary lineages within the domain Bacteria: the Alphaproteobacteria, the Betaproteobacteria, the Bacteroidetes and the Chlamydiae.13-17 None of these bacterial endosymbionts have the ability to survive and cannot be cultured outside their amoebic host cells. Such interactions may be of clinical relevance, since Acanthamoeba might be able to protect bacterial endosymbionts and release them under certain conditions. In fact, co-infections with other microrganisms have been reported in patients with culture proved AK.18 These include HSV, Adenovirus and Pseudomonas species.19-21 Because of the relationship of bacterial communities and free-living amoebae in the environment, the potential for dual human infections is increased.
The purpose of this study was to determine the prevalence of bacterial endosymbionts in Acanthamoeba isolates recovered from keratitis and to assess their potential in the pathogenesis of the disease.
Materials and Methods
Isolates
Thirty-eight Acanthamoeba isolates were recovered and examined for the presence of endosymbionts. Thirty-seven of the 38 (97%) were cultured from corneal scrapings, corneal biopsies, corneal buttons, contact lenses, or lens cases from 23 patients presenting with AK at our institution between January 2006 and February 2008. One environmental sample was cultured from tap water taken at the laboratory. All cultures were grown on agar-agar plates seeded with heat-killed Escherichia coli or Peptone Yeats Glucose (PYG) broth. Subsequently, amoebae were grown axenically for two weeks in 1× Page's saline solution (NaCl 120 mg; MgSO4 4 mg; Na2HPO4 142 mg; KH2PO4 136 mg; CaCl2 4 mg; 100 ml H2O).
DNA Isolation and Genotyping
Acanthamoebae were rinsed in phosphate-buffered saline (pH 7.4), and amoeba and bacterial DNA extracted using the UNSET method.22 Amplification and sequencing of the 16S-23S internally transcribed spacer (ITS) with primers Sp1 (5′-ACCTCCTTTCTAAGGAGCACC-3′) and Mb23S.44n (5′-TCTCGATGCCAAGGCATCCACC) was used to detect Mycobacterium endosymbionts.23, 24 Legionella and Pseudomonas endosymbionts were detected by amplification and sequencing with rRNA primers targeting the variable 23S-5S intergenic spacer (IGS): 23S (5′-TGAAGCCCGTTGAAGACTAC-3′) and 5S (GGAAGCCTCACACTATCAT-3′).25 The 23S primer was not an exact match to the Pseudomonas genus with two mismatches and an insertion all at the 5′ half of the primer. Detection of endosymbionts belonging to the Chlamydiales family utilized primer set Momp1 (5′-ATGAAAAAACTCTTGAAATCGG-3′) and Momp2 (5′-GCTCCTAAAGTTGCACA-3′) that target the major outer membrane protein (MOMP) gene.
Sequencing, Nucleotide Alignment and Phylogenetic Reconstruction
Sequences derived from the strains used in this study were analyzed along with sequences from strains available in GenBank. Several of the isolates are epidemiologically linked by being isolated from the same individual at different times or from contact lens paraphernalia of the same individual. The nucleotide sequences reported in this study were deposited in the GenBank database under accession numbers FJ444796 to FJ444819.
Alignments and phylogenetic reconstructions were performed using the phylogenetic computer program MEGA4 (Molecular Evolutionary Genetic Analysis software, ver. 4; http://www.megasoftware.net. Accessed February 2, 2008).26 Gene trees were generated using maximum-parsimony, neighbor-joining, Unweighted Pair Group Method with Arithmetic mean (UPGMA) or minimum evolution methods in MEGA4. The evolutionary distances were computed using the Kimura 2-parameter distance algorithm and are in the units of the number of base substitutions per site. All positions containing alignment gaps and missing data were eliminated in pairwise sequence comparisons.27 Confidence levels for the branching pattern were estimated by a bootstrap resampling of the data. Bootstrap values for the trees were obtained from a consensus tree based on 1,000 replicates.28
Fluorescent in situ hybridization (FISH)
For FISH, a protocol previously described was used.10 Briefly, Acanthamoebae (>95% trophozoites) harvested from axenic cultures were washed and resuspended in 100 μl of 1× Page's saline. 20 μl aliquots of amoebic suspension were incubated on poly-L-lysine slides for 20 minutes at 45°C to allow attachment of amoebae and fixed with 20 μl of 4% paraformaldeyde for 20 minutes at room temperature. The slides were subsequently washed for three times in 1× PBS and dehydrated in ethanol (96%, 70%, 50%) for 3 minutes. Slides were then incubated for and hour at 45°C with an aliquot (20 μl) of hybridizing buffer (20% formamide, 0.9 M NaCl, 0.01% SDS, e 20 mM Tris/HCl, pH 7.6) containing 100 nanograms of the specific probe (Pseudomonas: 5′-GGTTAGCTCAACGCCTCACAACGCTTACACACCCA-3′; Legionella 5′-CGCTATGGTCGCCAGGAAAACTGGTTT-3′; Mycobacterium 5′-TCACGACCAAGCTTTCCAG-3′; Chlamydia 5′-CGATTTCAAGAGTTTTTTCAT-3′). Slides were then gently washed with washing buffer (20 mM Tris/HCl, pH 7.6, 180 mM NaCl e 0.01% SDS), re-incubated for 15 minutes at 45°C covered with 500 μl of washing buffer. Slides were then washed with distilled water, dried at room temperature and mounted (Vectashield mounting medium, Vector Laboratories, Burlingame, CA). Images were acquired using a confocal microscope (Leica TC S SP5, Leica Microsystems Inc.).
Transmission electron microscopy (TEM)
Acanthamoeba cysts and trophozoites harvested from axenic cultures were fixed in 2% glutaraldehyde in 0.1M PO4/100mM sucrose overnight at 4°C. Then, they were rinsed with 0.15M PO4 for three times. The samples were post fixed in 2% phosphate buffered osmium tetroxide for 1 hour, followed by 3 rinses with 0.15M PO4. Samples were then dehydrated in an ascending series of ethanol up to 100%, infiltrated overnight with a 1:1 mixture of propylene oxide:epon-araldite resin and then embedded in the resin. Images were acquired with a Phillips CM-10 transmission electron microscope.
In vitro corneal pathogenicity assay
The cytopathic effect (CPE) of Acanthamoeba isolates was tested on human corneal epithelial cells (HCEC) in vitro as described elsewhere.29 Briefly, 500 μl of 2 × 104 CFU/mL Acanthamoebae (>95% trophozoites) suspended in KSFM were added to confluent HCECs in a 24-well plate and incubated at 37° for 8 h. After incubation, all plates were washed three times with PBS, stained with Giemsa (Fisher Scientific Company, Kalamazoo, MI) and solubilized with 400 μl of 5% SDS. Optical density (OD) was read at 590 nm in a microplate reader. Percent of CPE was calculated according to the following formula: %CPE= 100-[(OD experimental well − OD amoebae alone/OD control HCEC alone) × 100].
Clinical data
Clinical data were available for 22/23 patients. Initial visual acuity (VA), location and characteristics of the infiltrate, time to detection (defined as the time between first presentation at our institution and culture positivity) and symptoms duration at presentation (defined as the time between the symptoms onset and the first presentation at our institution) were examined and compared between patients infected by Acanthamoebae with endosymbionts and without endosymbionts.
Statistical analysis
Statistical analyses were performed using SPSS software version 15.0 (SPSS Inc, Chicago, Illinois, USA). Tests of significance were two-tailed with p ≤0.05 for all tests.
Results
Endosymbionts
Twenty-two of the 38 (59.4%) Acanthamoeba cultures from 12/23 patients (52.2%) examined yielded endosymbionts (Table 1, available at http://aaojournal.org).
Table 1. Acanthamoeba Isolates with detected endosymbionts.
| Culture Designationa | Culture Source | Endosymbiont designation | Endosymbiont GenBank Accession Number |
|---|---|---|---|
| BP:P2:CB | Corneal Button | Legionella | FJ444813 |
| Chlamydia | FJ444796 | ||
| BP:P2:CS | Corneal Scrape | Mycobacterium | FJ444817 |
| BP:P3:RCL | Right Contact Lens | Pseudomonas | FJ444800 |
| BP:P3:RLC | Right Lens Case | Pseudomonas | FJ444805 |
| BP:P3:LLC | Left Lens Case | Pseudomonas | FJ444804 |
| BP:P6:LCS | Left Corneal Scrape | Pseudomonas | FJ444806 |
| BP:P7:LCL | Left Contact Lens | Mycobacterium | FJ444814 |
| BP:P7:RCL | Right Contact Lens | Mycobacterium | FJ444815 |
| BP:P9:LCS | Left Corneal Scrape | Pseudomonas | FJ444807 |
| BP:P9:RCL | Right Contact Lens | Pseudomonas | FJ444808 |
| BP:P9:LCL | Left Contact Lens | Pseudomonas | FJ444809 |
| BP:P11:RCS | Right Corneal Scrape | Legionella | FJ444810 |
| BP:P14:LCS | Left Corneal Scrape | Pseudomonas | FJ444802 |
| BP:P14:LC | Lens Case | Pseudomonas | FJ444803 |
| BP:P16:RCS | Right Corneal Scrape | Pseudomonas | FJ444797 |
| BP:P16:LC | Lens Case | Pseudomonas | FJ444798 |
| BP:P16:LC[2] | Lens Case | Pseudomonas | FJ444799 |
| BP:P17:LCS | Left Corneal Scrape | Pseudomonas | FJ444801 |
| BP:P19:RCS | Right Corneal Scrape | Mycobacterium | FJ444818 |
| BP:P20:LCS | Left Corneal Scrape | Mycobacterium | FJ444816 |
| BP:P23:LCS | Left Corneal Scrape | Legionella | FJ444811 |
| BP:C:TW | Tap Water | Mycobacterium | FJ444819 |
| Legionella | FJ444812 |
Culture designation: BP, Bascom Palmer: P, Patient: Acronyms - RCS, Right Corneal Scrape; CB, Corneal Button; LCS, Left Corneal Scrape; LLC, Left Lens Case; RLC, Right Lens Case; LC, Lens Case; LCL, Left Contact Lens; RCL, Right Contact Lens; TW, Tap Water: C, control.
Legionella
Legionella endosymbionts were found in 3 patients and in one environmental sample. Legionella endosymbionts had highest sequence similarity to Legionella cherrii ORW (Z30537) between 94-95%, and greater than 99% similarity to each other (Table 1 and 2, available at http://aaojournal.org). Phylogenetic analysis supported this grouping with a bootstrap value of 98% (Fig. 1A, available at http://aaojournal.org).
Table 2. Differences in the sequences between the endosymbiont isolates.
| [1] | [2] | [3] | [4] | [5] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [1] | BP:P11:RCS | * | 2 | 3 | 2 | 17 | ||||||
| [2] | BP:P2:CB | 99.3 | * | 3 | 2 | 17 | ||||||
| [3] | BP:P23:LCS | 99 | 99 | * | 1 | 16 | ||||||
| [4] | BP:C:TW | 99.3 | 99.3 | 99.7 | * | 15 | ||||||
| [5] | L.cherrii ORW | 94.3 | 94.3 | 94.6 | 94.9 | * | ||||||
| [6] | [7] | [8] | [9] | [10] | [11] | [12] | [13] | [14] | [15] | [16] | ||
| [6] | BP:P3:RCL | * | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| [7] | BP:P3:RLC | 100 | * | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| [8] | BP:P3:LLC | 100 | 100 | * | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| [9] | BP:P6:LCS | 99 | 99 | 99 | * | 3 | 3 | 3 | 3 | 3 | 3 | 2 |
| [10] | BP:P14:LCS | 100 | 100 | 100 | 99 | * | 0 | 0 | 0 | 0 | 0 | 1 |
| [11] | BP:P14:LC | 100 | 100 | 100 | 99 | 100 | * | 0 | 0 | 0 | 0 | 1 |
| [12] | BP:P16:RCS[2] | 100 | 100 | 100 | 99 | 100 | 100 | * | 0 | 0 | 0 | 1 |
| [13] | BP:P16:LC | 100 | 100 | 100 | 99 | 100 | 100 | 100 | * | 0 | 0 | 1 |
| [14] | BP:P16:LC[2] | 100 | 100 | 100 | 99 | 100 | 100 | 100 | 100 | * | 0 | 1 |
| [15] | BP:P17:LCS | 100 | 100 | 100 | 99 | 100 | 100 | 100 | 100 | 100 | * | 1 |
| [16] | P. aeruginosa UCBBPP-PA 14 | 99.7 | 99.7 | 99.7 | 99.3 | 99.7 | 99.7 | 99.7 | 99.7 | 99.7 | 99.7 | * |
| [17] | [18] | [19] | [20] | |||||||||
| [17] | BP:P9:RCL | * | 0 | 0 | 10 | |||||||
| [18] | BP:P9:LCL | 100 | * | 0 | 10 | |||||||
| [19] | BP:P9:LCS | 100 | 100 | * | 10 | |||||||
| [20] | P. putida W619 | 96.4 | 96.4 | 96.4 | * | |||||||
| [21] | [22] | [23] | [24] | [25] | [26] | [27] | [28] | [29] | [30] | |||
| [21] | BP:P2:CS | * | 0 | 4 | ||||||||
| [22] | BP:P20:LCS | 100 | * | 4 | ||||||||
| [23] | M. sp. 1371 | 98 | 98 | * | ||||||||
| [24] | BP:P7:LCL | * | 6 | 7 | ||||||||
| [25] | BP:P7:RCL | 98 | * | 2 | ||||||||
| [26] | M. fortuitum K7594-03 | 97 | 98.8 | * | ||||||||
| [27] | BP:P19:RCS | * | 0 | 1 | 1 | |||||||
| [28] | M. gordonae Tropicalis-2 | 100 | * | 1 | 1 | |||||||
| [29] | BP:C:TW | 99.2 | 99.2 | * | 0 | |||||||
| [30] | M. gordonae (L42258) | 99.2 | 99.2 | 100 | * | |||||||
| [31] | [32] | |||||||||||
| [31] | BP:P2:CB | * | 13 | |||||||||
| [32] | C. trachomatis DK-K40 | 98.9 | * | |||||||||
Culture designation: BP, Bascom Palmer: P, Patient: Acronyms - RCS, Right Corneal Scrape; CB, Corneal Button; LCS, Left Corneal Scrape; LLC, Left Lens Case; RLC, Right Lens Case; LC, Lens Case; LCL, Left Contact Lens; RCL, Right Contact Lens; TW, Tap Water: C, control. Numbers above the diagonal are nucleotide differences. Numbers below the diagonal are percent similarities. Numbers not shown comparing the different Mycobacterium species were greater than 15% and 70 base pairs different.
Figure 1.
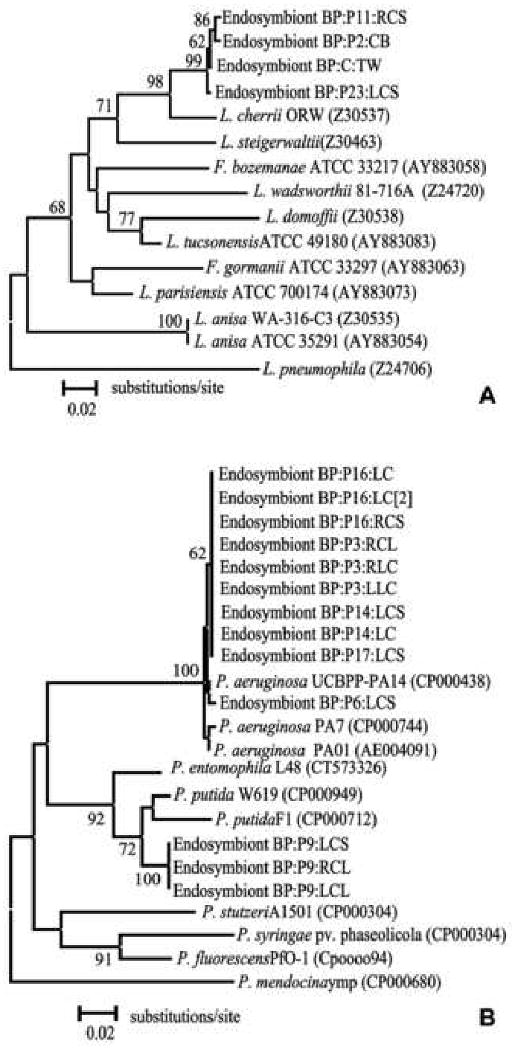
A Distance Neighbor-joining tree based on 23S-5S IGS analysis showing relationship of Acanthamoeba endosymbionts to representative members of the Legionella genus. The tree was rooted by using the L. pneumophila (Z24706) strain. B Distance Neighbor-joining tree based on 23S-5S IGS analysis showing relationship of Acanthamoeba endosymbionts to representative members of the Pseudomonas genus. The tree was rooted by using the P. mendocina ymp strain. The scale bar represents percent difference per average site. Bootstrap values based on 1000 replicates are given at nodes to which they apply, values below 50 are not shown.
Pseudomonas
Pseudomonas species were documented in 6 patients (13 Acanthamoeba isolates) (Table 1, available at http://aaojournal.org). Ten of the endosymbionts showed highest sequence similarity to P. aeruginosa UCBPP-PA 14 (CP000438) at 99% or more. All but one endosymbiont sequences from this group were identical (Table 2, available at http://aaojournal.org). The phylogenetic grouping of these endosymbionts with members of the P. aeruginosa clade was supported by a bootstrap value of 100% (Fig.1B, available at http://aaojournal.org). The remaining three endosymbionts were identical to each other and had the closest sequence similarity to P. putida W619 (CP000949) at 96.4% sequence identity (Table 2, available at http://aaojournal.org). The phylogenetic grouping of these endosymbionts with members of the Pseudomonas putida clade was supported by a bootstrap value of 92% (Fig. 1B, available at http://aaojournal.org).
Mycobacterium
Four patients (5 Acanthamoeba isolates) and one environmental isolate with endosymbionts had sequences similar to bacteria in the Mycobacterium genus (Table 1, available at http://aaojournal.org). Two of the endosymbionts (BP:P2:CS and BP:P20:LCS) were identical to each other and showed highest sequence similarity to M. sp. 1371 (AY646435) at 98% sequence identity (Table 2, available at http://aaojournal.org). Phylogenetic analysis grouped these endosymbionts in the M. mucogenicum clade with 96% supported value (Fig. 2A, available at http://aaojournal.org). Endosymbionts of isolates BP:P19:RCS and BP:C:TW were 99.2% similar to each other and identical to M. gordonae Tropicalis-2 (EU497913) and M. gordonae (L42258), respectively (Table 2, available at http://aaojournal.org). The remaining Acanthamoeba isolates BP:P7:LCL and BP:P7:RCL contained endosymbionts with sequences at 98% identity to each other and closest sequence similarity to M. fortuitum K7594-03 (AM709726) at 97-98% sequence identity (Table 2; Fig. 2A, available at http://aaojournal.org).
Figure 2.
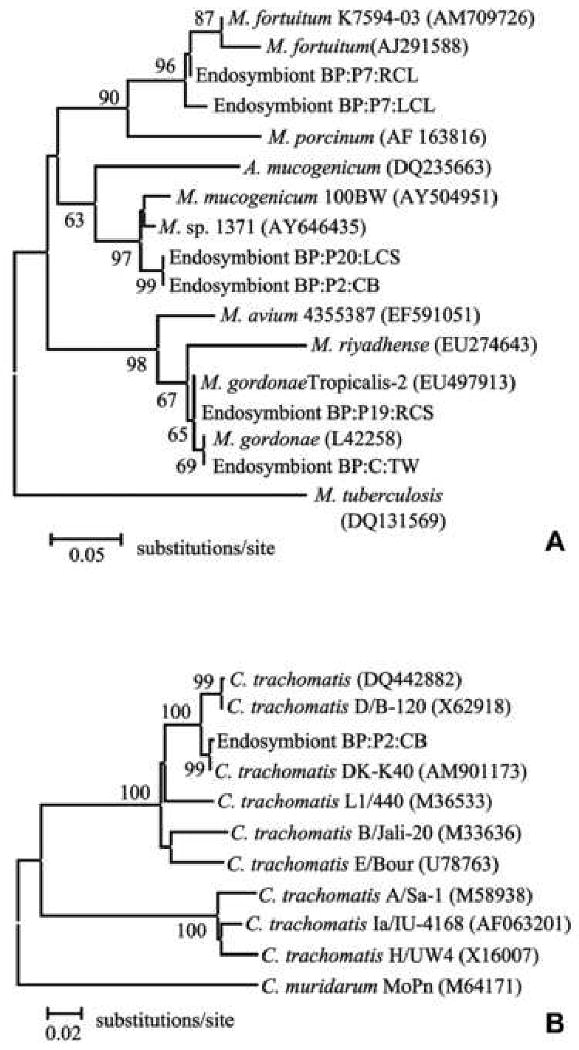
A Distance Neighbor-joining tree based on 16S-23 ITS analysis showing relationship of Acanthamoeba endosymbionts to representative members of the Mycobacterium genus. The tree was rooted by using the M. tuberculosis (DQ131569) strain. The scale bar represents percent difference per average site. Bootstrap values based on 1000 replicates are given at nodes to which they apply, values below 50 are not shown. B Distance Neighbor-joining tree based on analysis of partial sequence of the MOMP gene, showing relationship of Acanthamoeba endosymbionts to representative members of the Chlamydia genus. The tree was rooted by using the C. muridarum MoPn strain. The scale bar represents percent difference per average site. Bootstrap values based on 1000 replicates are given at nodes to which they apply, values below 50 are not shown.
Chlamydia
A single isolate from one patient (BP:P2:CB) was identified as a member of the Chlamydophila family The endosymbiont was most similar to C. trachomatis DK-K40 (AM901173) serovar D at 99% sequence identity (Table 2, available at http://aaojournal.org). Phylogenetically it grouped among other members of the C. trachomatis serovar D clade (Fig. 2B, available at http://aaojournal.org). This isolate was unique in that it also contained Legionella-like bacterial endosymbionts.
Isolates of BP:C:TW
Two Acanthamoeba sequences were detected in tap water cultured from the laboratory; and two endosymbiont sequences were amplified from the genomic DNA of this culture, Legionella and Mycobacterium (Table 1, 2, available at http://aaojournal.org). The origin of the two endosymbionts could not be determined by PCR and images from FISH and TEM were not available.
Fluorescence in situ hybridization (FISH)
Positive hybridization reactions were obtained with the specific fluorescent DNA probes for all four species of bacterial endosymbionts (Fig 3). Bacterial endosymbionts were dispersed throughout the cytoplasm of the Acanthamoeba host, and were present in all amoeba cells in the population. Co-localization of phylogenetically different endosymbionts strains was also detected by FISH in the amoeba isolate that had Legionella and Chlamydia colonization (Fig 3D).
Figure 3.
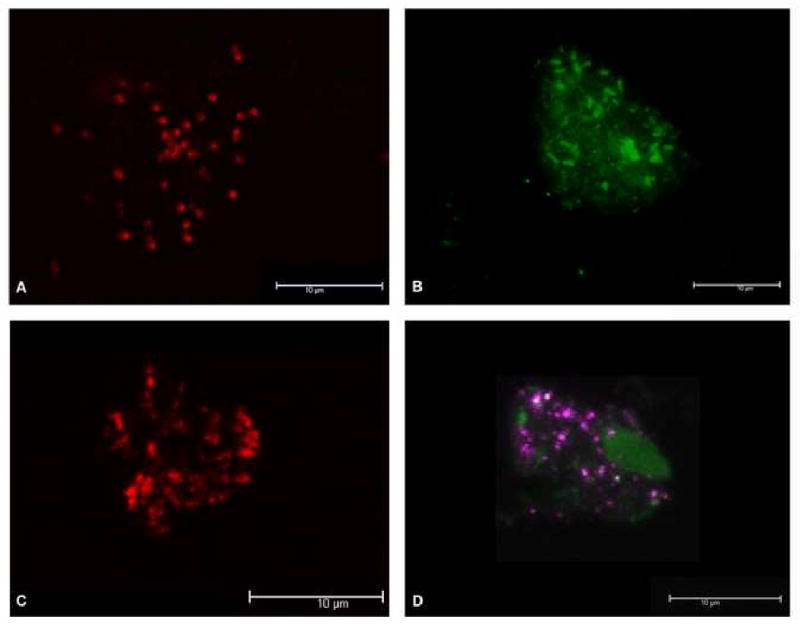
Specific fluorescent DNA probes targeting Pseudomonas species (A, Texas Red, in red, 63×), Legionella species (B, FAM labeling, in green, 63×), Mycobacterium species (C, Cy3, in red, 63×). D Co-existence of Legionella and Chlamydia species in a single Acanthamoeba isolate DNA probes marked with, respectively (FAM/Legionella and Cy5/Chlamydia labeling, in green and purple respectively, 63×).
Transmission electron microscopy (TEM)
The ultrastructure and intracellular niche of endosymbionts were further investigated by transmission electron microscopy (Fig 4). Bacteria were observed inside both trophozoites and cysts. None of the endosymbionts was enclosed in phagosomal or phagolysosomal vacuoles, as observed by other laboratories.30-33 Pseudomonas endosymbionts (0.7-0.9 × 0.4 μm in size; Fig. 4 A,B) had a typical rod-shaped appearance, and were surrounded by an electron-translucent area probably corresponding to their capsule. They formed small clusters randomly distributed within the host cytoplasm. Legionella (0.8-1 × 0.3 μm; Fig. C,D) and Mycobacterium (1.3-1.6 × 0.2-0.3 μm; Fig. 4 E,F) endosymbionts were also rod-shaped, and were randomly distributed within cysts and throphozoites cytoplasm. No EM image was available for the one isolate that showed presence of a Chlamydia endosymbiont.
Figure 4.
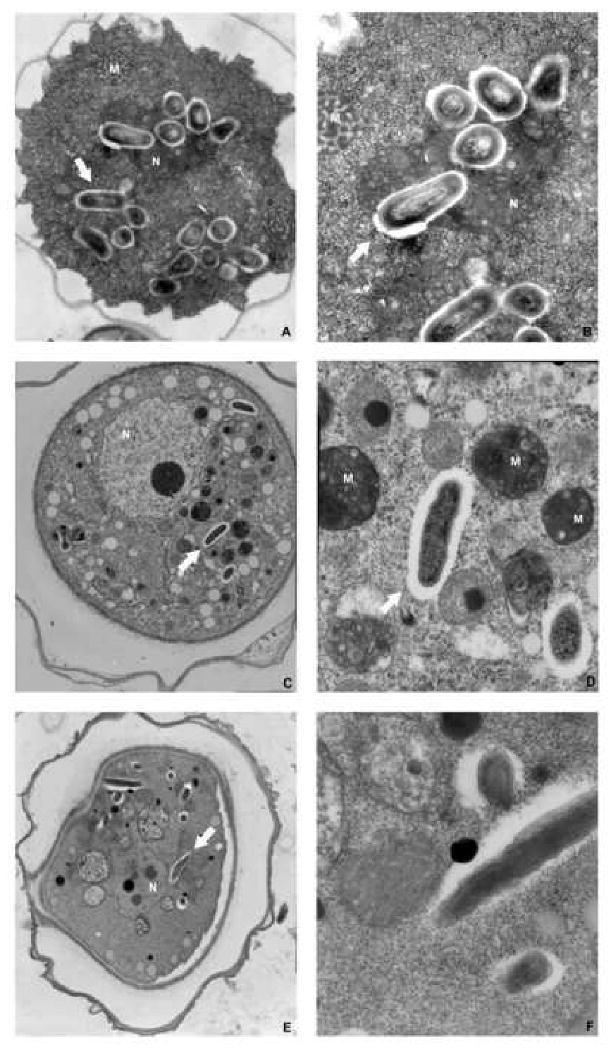
Transmission electron micrographs of Acanthamoeba isolates with endosymbionts. Acanthamoeba cysts containing Pseudomonas (A), Legionella (C) and Mycobacteria (E). Ultrastructural feature of Pseudomonas (B), Legionella (D) and Mycobacteria (F). Notice absence of phagolysosomal membrane surrounding the endosymbiont bacteria. N: nucleus; M: mytocondria.
Cytopathic effect
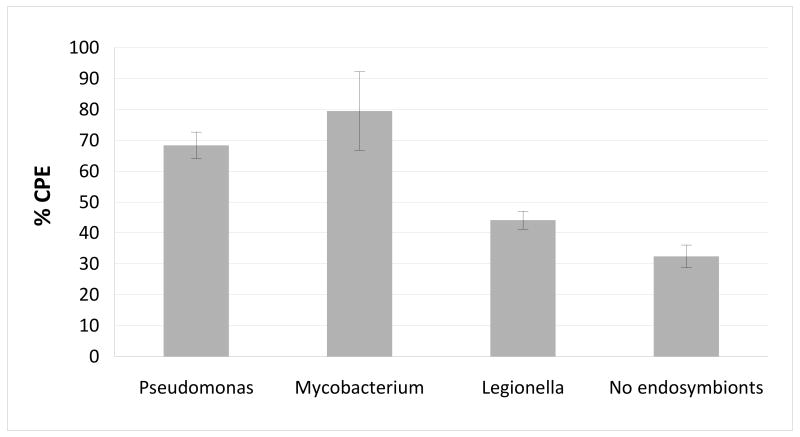
CPE on HCEC was significantly higher for Acanthamoeba hosting endosymbionts compared to isolates without endosymbionts (p<0.05, T-test, Fig. 5). Also, Pseudomonas and Mycobacterium enhanced Acanthamoeba CPE significantly more than Legionella (p<0.05, T-test).
Figure 5.
Cytopathic effect (CPE) on human corneal epithelial cells for Acanthamoebae hosting different endosymbionts. Amoebae harboring bacterial endosymbionts were more pathogenic than isolates without endosymbionts (p<0.05). Bacteria capable of corneal infections, such as Pseudomonas and Mycobacteria, were able to confer to Acanthamoebae additional pathogenicity when compared to Legionella (p<0.05). Data are expressed as mean ±SD results of triplicate experiments.
Clinical data
A trend towards a worse initial VA (p>0.05) and a preferred central location of the infiltrate (p<0.05) was observed in patients affected by Acanthamoeba with bacterial endosymbionts (Table 3). Interestingly, radial perineuritis was only reported in patients with Acanthamoeba isolates without endosymbionts (60% vs 0%, p<0.01). In the presence of endosymbionts, a delayed time to culture positivity and presentation to our clinic were also noted (p>0.05, Table 3).
Table 3. Comparison of patients outcomes with and without endosymbionts.
| Endosymbionts Present (12) | Endosymbionts Not Present (10) | P value (95 CI) | |
|---|---|---|---|
| Initial VA | 1.11± 0.5 (20/260) | 0.56 ± 0.2 (20/72) | 0.9a |
| Location of the infiltrate | Central (91.7%, 11/12) | Central (50%, 5/10) | 0.04b |
| Radial perineuritis | 0% (0/12) | 60% (6/10) | 0.002b |
| Time to detection (days) Less than 7 days |
7.6 ± 2.5 33% (4/12) |
5.4 ± 1.6 70% (7/10) |
0.8a 0.08b |
| Symptoms duration at presentation | 50.2 ± 30.4 | 21 ± 5.8 | 0.9a |
Data are expressed as mean ± standard error of the mean (SEM). VA: visual acuity; CI: confidence interval.
T-test.
Fisher exact
Discussion
The presence of a variety of endosymbiont bacteria in Acanthamoeba hosts has been known for a long time.34 In this study, we examined 37 Acanthamoeba isolates obtained from patients with AK and one tap water isolate for the presence of four medically important bacterial agents: Legionella, Mycobacterium, Pseudomonas and Chlamydiae.
On a total of 23 AK patients, 12 were found infected by Acanthamoebae hosting bacterial endosymbionts (52.2%). Of the 38 isolates examined, 22 (59.4%) were found to possess at least one bacteria as an endosymbiont, a twofold greater number than the 26% endosymbiont presence observed by Fritsche et al.30 The higher endosymbiont presence may be due to the sensitive detection method of PCR. Additionally, the 59% observance may be low due to the restricted search for endosymbionts of interest to our laboratory.
The predominant endosymbiont of the four genera examined belonged to the genus Pseudomonas with 13 of the 22 endosymbionts (6/12 patients) reported here. Pseudomonas species are commonly responsible for acute-onset and highly destructive keratitis.35 Previously, it was assumed that Pseudomonas aeruginosa and Acanthamoeba were mutually exclusive ocular pathogens, since Pseudomonas expressed amoebicidal activity in co-cultivation.36 More recently, in vitro studies have showed that Pseudomonas can actually cooperate in the pathogenesis of contact lens related AK: in fact, it increases the resistance of Acanthamoeba to contact lens disinfecting solutions and creates a biofilm on contact lenses surface that enhances amoebae retention.37 Pseudomonas-Acanthamoeba co-infections have also been described in keratitis patients, along with the presence of Pseudomonas aeruginosa intracellularly in environmental Acanthamoebae.20, 38
The second most common endosymbionts belonged to the Mycobacteria family. Corneal infection by atypical Mycobacteria is a relevant clinical challenge, mostly occurring after laser in situ keratomileusis (LASIK) procedures.39 Mycobacteria have been previously reported as endosymbionts in an environmental Acanthamoebae, but this study is the first to report on Mycobacteria in clinical samples.33 Mycobacteria can penetrate the amoeba cell, multiply inside the cytoplasm and resist amoeba encystation.40 It has also been shown that Acanthamoeba can protect Mycobacteria from chlorine and antibiotics and enhance Mycobacteria virulence.41, 42 Five clinically related Acanthamoeba isolates and the tap water sample harbored Mycobacteria species. The Mycobacterium endosymbionts observed segregated into to 3 different Mycobacteria species cluster: M. gordonae, M. fortuitum and M. mucogenicum. Patient BP:P7 was unusual in that the Acanthamoeba isolated from the right and left contact lens cases were closely related, but not identical (unpublished data). Interestingly, both housed Mycobacteria that were very closely related.
Legionella type endosymbionts were also identified in 3 out of 12 patients. The symbiosis between Acanthamoeba and Legionella has been extensively characterized.43 In fact, intracellular replication within amoeba is considered a prerequisite to infection in humans in Legionella pneumophila, a facultative intracellular pathogen causing a severe pneumonia called Legionnaire disease.44
A Chlamydia type endosymbiont was observed in only one Acanthamoeba host (one patient), BP:P2:CB, from this dataset. This was surprising since endosymbionts belonging to the Chlamydiales family are quite common in free-living amoeba, and may reflect a lower affinity of Chlamydia endosymbionts for clinically relevant amoebae.12 Moreover, the identification of an intra-amoebal form of Chlamydia trachomatis opens the door to a possible role of Acanthamoeba in the developmental cycle and spreading of this important ocular pathogen, responsible in humans for the occurrence of inclusion conjunctivitis and sight-threatening trachoma.45
Interestingly, the BP:P2:CB isolate was also the only isolate observed to contain two of the bacteria types investigated, Chlamydia and Legionella. Occurrence of multiple endosymbionts in a clinical isolate has never been reported. Recovery of more than one endosymbiont has only recently been documented in an environmental Acanthamoeba strain.46 Co-colonization of different bacteria may result from the ability of each bacterial type to exploit a different niche within the amoeba. In fact, Proteobacteria, like Legionella, survive within the cytoplasm of amoeba, while Chlamydiales symbionts are sequestered within vacuoles.46
Patient BP:P2 was interesting in that two Acanthamoeba isolates isolated from the cornea at different times hosted different endosymbionts. This could be explained by the fact that the two isolates grew from a corneal scraping done before starting specific amoebicidal topical therapy (chlorexidine and polyhexamethylbiguanide) and from the corneal button subsequently obtained during therapeutic keratoplasty. The anti-amoebic therapy and/or the change in microenvironment could have determined loss or selection of specific endosymbionts. A reversal of this observation is also seen, in that Acanthamoeba from different patients possess endosymbionts with identical sequences. For example, Acanthamoeba sp. BP:P3:RCL and Acanthamoeba sp. BP:P16:RCS possess Pseudomonas sp. type endosymbionts with identical sequences. Although bacteria-Acanthamoeba symbiosis has been reported and analyzed in multiple studies, the real contribution of endosymbionts to the pathogenesis of amoebal infections has not been clarified yet.
Endosymbionts may influence Acanthamoeba pathogenicity, virulence or susceptibility to antiamoebic drugs. An enhancement of Acanthamoeba cytopathogenicity following the acquisition of bacterial endosymbionts has been described in vitro.47 The presence of the endosymbiont may modify Acanthamoeba phenotype making the protozoa more pathogenic or resistant to therapy. Changes in gene expression and protein profiles have been observed resulting from the amoeba/bacteria interaction in Hartmannella and Naegleria.48, 49 In our study, we described an increased pathogenicity of Acanthamoebae harboring bacterial endosymbionts on corneal epithelial cells. Moreover, amoeba isolates hosting corneal pathogenic bacteria (Pseudomonas and Mycobacteria) as endosymbionts were significantly more pathogenic than Legionella.
Our preliminary clinical observations also suggested that endosymbionts may influence Acanthamoeba virulence and AK clinical features. In AK patients, the worse VA at presentation and the central location of the infiltrate observed in the presence of endosymbionts could be considered as a consequence of the increased corneal pathogenicity of Acanthamoeba. The absence of radial perineuritis and the delay in culture positivity of amoebae with endosymbionts might be due to modification of Acanthamoeba life cycle or AK pathogenesis induced by the endosymbionts. Moreover, the fact that endosymbionts were most frequently found in cases where the interval between symptoms onset and first medical examination was longer could indicate that a longer persistence of Acanthamoeba in the eye increases the chances of bacterial uptake and endosymbiosis. Unfortunately, only a small number of patients were included in this study due to the low prevalence of this infection, and most of them were only referred to our tertiary center for diagnosis and subsequently lost to follow-up. Further studies with a larger cohort of patients and longer follow-up are needed to better understand the contribution of endosymbionts to the natural history of AK.
Conversely, Acanthamoeba can affect endosymbionts pathogenicity.11 In fact, intracellular growth of Mycobacteria and Legionella in Acanthamoeba enhances invasiveness and virulence of the bacteria.41, 50, 51 Acanthamoeba can also protect endosybiont bacteria from antibiotics, disinfectant and hostile environmental conditions.40, 42, 52 Detection of bacterial and fungal contaminants in contact lens cases of patients with AK is common. In a contact lens-related keratitis scenario, Acanthamoeba may be able to uptake bacteria from the contact lens surface or an improperly cleaned case, protect them from the disinfecting solutions and antibiotics through encystation, and deliver them to the cornea. The release of bacterial endosymbionts upon the demise of the amoeba host is an equally important point of interest. Bacterial endosymbionts may be not be capable of inducing an infectious keratitis themselves, but the presence of pro-inflammatory bacterial components in a compromised cornea may boost corneal inflammation and further exacerbated the outcome of keratitis.
Obviously, there is a definite need to better understand the interactions between amoebae and their endosymbionts. More data on the effect of amoebic endosymbionts influence on virulence factors, clinical outcome, drug susceptibility and role in human/host interactions need to be acquired. Additionally, to what degree is the amoeba/endosymbiont relationship important in dissemination of medically important bacteria worldwide? These are only a few of the questions that need to be addressed to better understand and develop measures for disease control.
Acknowledgments
Financial support: Research to Prevent Blindness (P30 grant EY014801)
Footnotes
Part of this work has been presented at:
Association for Research in Vision and Ophthalmology (ARVO) meeting, May 2008
Ocular Microbiology and Immunology Group (OMIG) meeting, November 2008;
Harry Hirsch Leiter Award winning presentation
American Academy of Ophthalmology (AAO) meeting, November 2008
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khan NA. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev. 2006;30:564–95. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- 2.Chong EM, Dana MR. Acanthamoeba keratitis. Int Ophthalmol Clin. 2007 Spring;47(2):33–46. doi: 10.1097/IIO.0b013e318036bcf4. [DOI] [PubMed] [Google Scholar]
- 3.Foulks GN. Acanthamoeba keratitis and contact lens wear: static or increasing problem? Eye Contact Lens. 2007;33:412–4. doi: 10.1097/ICL.0b013e318157e8be. discussion 424-5. [DOI] [PubMed] [Google Scholar]
- 4.Nwachuku N, Gerba CP. Health effects of Acanthamoeba spp. and its potential for waterborne transmission. Rev Environ Contam Toxicol. 2004;180:93–131. doi: 10.1007/0-387-21729-0_2. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Zaragoza S. Ecology of free-living amoebae. Crit Rev Microbiol. 1994;20:225–41. doi: 10.3109/10408419409114556. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder JM, Booton GC, Hay J, et al. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. J Clin Microbiol. 2001;39:1903–11. doi: 10.1128/JCM.39.5.1903-1911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong HJ, Lee SJ, Kim JH, et al. Acanthamoeba: keratopathogenicity of isolates from domestic tap water in Korea. Exp Parasitol. 2007;117:357–67. doi: 10.1016/j.exppara.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 9.Horn M, Wagner M. Bacterial endosymbionts of free-living amoebae. J Eukaryot Microbiol. 2004;51:509–14. doi: 10.1111/j.1550-7408.2004.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz-Esser S, Toenshoff ER, Haider S, et al. Diversity of bacterial endosymbionts of environmental Acanthamoeba isolates. Appl Environ Microbiol. 2008;74:5822–31. doi: 10.1128/AEM.01093-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greub G, Raoult D. Microorganisms resistant to free-living amoebae. Clin Microbiol Rev. 2004;17:413–33. doi: 10.1128/CMR.17.2.413-433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molmeret M, Horn M, Wagner M, et al. Amoebae as training grounds for intracellular bacterial pathogens. Appl Environ Microbiol. 2005;71:20–8. doi: 10.1128/AEM.71.1.20-28.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horn M, Fritsche TR, Gautom RK, et al. Novel bacterial endosymbionts of Acanthamoeba spp. related to the Paramecium caudatum symbiont Caedibacter caryophilus. Environ Microbiol. 1999;1:357–67. doi: 10.1046/j.1462-2920.1999.00045.x. [DOI] [PubMed] [Google Scholar]
- 14.Horn M, Fritsche TR, Linner T, et al. Obligate bacterial endosymbionts of Acanthamoeba spp. related to the beta-Proteobacteria: proposal of ‘Candidatus Procabacter acanthamoebae’ gen. nov., sp. nov. Int J Syst Evol Microbiol. 2002;52:599–605. doi: 10.1099/00207713-52-2-599. [DOI] [PubMed] [Google Scholar]
- 15.Horn M, Harzenetter MD, Linner T, et al. Members of the Cytophaga-Flavobacterium-Bacteroides phylum as intracellular bacteria of acanthamoebae: proposal of ‘Candidatus Amoebophilus asiaticus’. Environ Microbiol. 2001;3:440–9. doi: 10.1046/j.1462-2920.2001.00210.x. [DOI] [PubMed] [Google Scholar]
- 16.Horn M, Wagner M, Muller KD, et al. Neochlamydia hartmannellae gen. nov., sp. nov. (Parachlamydiaceae), an endoparasite of the amoeba Hartmannella vermiformis. Microbiology. 2000;146:1231–9. doi: 10.1099/00221287-146-5-1231. [DOI] [PubMed] [Google Scholar]
- 17.Birtles RJ, Rowbotham TJ, Michel R, et al. ‘Candidatus Odyssella thessalonicensis’ gen. nov., sp. nov., an obligate intracellular parasite of Acanthamoeba species. Int J Syst Evol Microbiol. 2000;50:63–72. doi: 10.1099/00207713-50-1-63. [DOI] [PubMed] [Google Scholar]
- 18.Tu EY, Joslin CE, Nijm LM, et al. Polymicrobial keratitis: Acanthamoeba and infectious crystalline keratopathy. Am J Ophthalmol. 2009;148:13–9. doi: 10.1016/j.ajo.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gajdatsy AD, Kosmin A, Barrett GD. Coexistent adenoviral keratoconjunctivitis and Acanthamoeba keratitis. Clin Experiment Ophthalmol. 2000;28:434–6. doi: 10.1046/j.1442-9071.2000.00352.x. [DOI] [PubMed] [Google Scholar]
- 20.Ziak P, Ondriska F, Mrva M. Acanthamoeba keratitis after use of soft contact lenses--case report [in Slovak] Cesk Slov Oftalmol. 2003;59:352–8. [PubMed] [Google Scholar]
- 21.Rumelt S, Cohen I, Rehany U. Spontaneous corneal graft ulcerative perforation due to mixed Acanthamoeba and herpes simplex keratitis: a clinicopathologic study. Cornea. 2000;19:240–2. doi: 10.1097/00003226-200003000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Hugo ER, Stewart VJ, Gast RJ, Byers TJ. Purification of amoeba mtDNA using the UNSET procedure. In: Lee JJ, Soldo AJ, editors. Protocols in Protozoology. Lawrence, KS: Society of Protozoologists; 1992. pp. D7.1–7.2. [Google Scholar]
- 23.Roth A, Reischl U, Streubel A, et al. Novel diagnostic algorithm for identification of mycobacteria using genus-specific amplification of the 16S-23S rRNA gene spacer and restriction endonucleases. J Clin Microbiol. 2000;38:1094–104. doi: 10.1128/jcm.38.3.1094-1104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth A, Fischer M, Hamid ME, et al. Differentiation of phylogenetically related slowly growing mycobacteria based on 16S-23S rRNA gene internal transcribed spacer sequences. J Clin Microbiol. 1998;36:139–47. doi: 10.1128/jcm.36.1.139-147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grattard F, Ginevra C, Riffard S, et al. Analysis of the genetic diversity of Legionella by sequencing the 23S-5S ribosomal intergenic spacer region: from phylogeny to direct identification of isolates at the species level from clinical specimens. Microbes Infect. 2006;8:73–83. doi: 10.1016/j.micinf.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–9. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 27.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–20. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 28.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–91. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 29.Alizadeh H, Neelam S, Niederkorn JY. Effect of immunization with the mannose-induced Acanthamoeba protein and Acanthamoeba plasminogen activator in mitigating Acanthamoeba keratitis. Invest Ophthalmol Vis Sci. 2007;48:5597–604. doi: 10.1167/iovs.07-0407. [DOI] [PubMed] [Google Scholar]
- 30.Fritsche TR, Gautom RK, Seyedirashti S, et al. Occurrence of bacterial endosymbionts in Acanthamoeba spp. isolated from corneal and environmental specimens and contact lenses. J Clin Microbiol. 1993;31:1122–6. doi: 10.1128/jcm.31.5.1122-1126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xuan YH, Yu HS, Jeong HJ, et al. Molecular characterization of bacterial endosymbionts of Acanthamoeba isolates from infected corneas of Korean patients. Korean J Parasitol. 2007;45:1–9. doi: 10.3347/kjp.2007.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yagita K, Matias RR, Yasuda T, et al. Acanthamoeba sp. from the Philippines: electron microscopy studies on naturally occurring bacterial symbionts. Parasitol Res. 1995;81:98–102. doi: 10.1007/BF00931612. [DOI] [PubMed] [Google Scholar]
- 33.Yu HS, Jeong HJ, Hong YC, et al. Natural occurrence of Mycobacterium as an endosymbiont of Acanthamoeba isolated from a contact lens storage case. Korean J Parasitol. 2007;45:11–8. doi: 10.3347/kjp.2007.45.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall J, Voelz H. Bacterial endosymbionts of Acanthamoeba sp. J Parasitol. 1985;71:89–95. [PubMed] [Google Scholar]
- 35.Yilmaz S, Saklamaz A, Maden A. Pseudomonas keratitis [letter] Ophthalmology. 2006;113:883–4. doi: 10.1016/j.ophtha.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Qureshi MN, Perez AA, II, Madayag RM, Bottone EJ. Inhibition of Acanthamoeba species by Pseudomonas aeruginosa: rationale for their selective exclusion in corneal ulcers and contact lens care systems. J Clin Microbiol. 1993;31:1908–10. doi: 10.1128/jcm.31.7.1908-1910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cengiz AM, Harmis N, Stapleton F. Co-incubation of Acanthamoeba castellanii with strains of Pseudomonas aeruginosa alters the survival of amoeba. Clin Experiment Ophthalmol. 2000;28:191–3. doi: 10.1046/j.1442-9071.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- 38.Michel R, Burghardt H, Bergmann H. Acanthamoeba, naturally intracellularly infected with Pseudomonas aeruginosa, after their isolation from a microbiologically contaminated drinking water system in a hospital [in German] Zentralbl Hyg Umweltmed. 1995;196:532–44. [PubMed] [Google Scholar]
- 39.John T, Velotta E. Nontuberculous (atypical) mycobacterial keratitis after LASIK: current status and clinical implications. Cornea. 2005;24:245–55. doi: 10.1097/01.ico.0000151565.63107.64. [DOI] [PubMed] [Google Scholar]
- 40.Steinert M, Birkness K, White E, et al. Mycobacterium avium bacilli grow saprozoically in coculture with Acanthamoeba polyphaga and survive within cyst walls. Appl Environ Microbiol. 1998;64:2256–61. doi: 10.1128/aem.64.6.2256-2261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cirillo JD, Falkow S, Tompkins LS, Bermudez LE. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect Immun. 1997;65:3759–67. doi: 10.1128/iai.65.9.3759-3767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miltner EC, Bermudez LE. Mycobacterium avium grown in Acanthamoeba castellanii is protected from the effects of antimicrobials. Antimicrob Agents Chemother. 2000;44:1990–4. doi: 10.1128/aac.44.7.1990-1994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barker J, Brown MR. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology. 1994;140:1253–9. doi: 10.1099/00221287-140-6-1253. [DOI] [PubMed] [Google Scholar]
- 44.Abu Kwaik Y, Gao LY, Stone BJ, et al. Invasion of protozoa by Legionella pneumophila and its role in bacterial ecology and pathogenesis. Appl Environ Microbiol. 1998;64:3127–33. doi: 10.1128/aem.64.9.3127-3133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.An BB, Adamis AP. Chlamydial ocular diseases. Int Ophthalmol Clin. 1998 Winter;38(1):221–30. doi: 10.1097/00004397-199803810-00017. [DOI] [PubMed] [Google Scholar]
- 46.Heinz E, Kolarov I, Kastner C, et al. An Acanthamoeba sp. containing two phylogenetically different bacterial endosymbionts. Environ Microbiol. 2007;9:1604–9. doi: 10.1111/j.1462-2920.2007.01268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fritsche TR, Sobek D, Gautom RK. Enhancement of in vitro cytopathogenicity by Acanthamoeba spp. following acquisition of bacterial endosymbionts. FEMS Microbiol Lett. 1998;166:231–6. doi: 10.1111/j.1574-6968.1998.tb13895.x. [DOI] [PubMed] [Google Scholar]
- 48.Abu Kwaik Y, Fields BS, Engleberg NC. Protein expression by the protozoan Hartmannella vermiformis upon contact with its bacterial parasite Legionella pneumophila. Infect Immun. 1994;62:1860–6. doi: 10.1128/iai.62.5.1860-1866.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michel R, Muller KD, Hauroder B, Zoller L. A coccoid bacterial parasite of Naegleria sp. (Schizopyrenida: Vahlkampfiidae) inhibits cyst formation of its host but not transformation to the flagellate stage. Acta Protozool. 2000;39:199–207. [Google Scholar]
- 50.Cirillo JD, Cirillo SL, Yan L, et al. Intracellular growth in Acanthamoeba castellanii affects monocyte entry mechanisms and enhances virulence of Legionella pneumophila. Infect Immun. 1999;67:4427–34. doi: 10.1128/iai.67.9.4427-4434.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cirillo JD, Falkow S, Tompkins LS. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect Immun. 1994;62:3254–61. doi: 10.1128/iai.62.8.3254-3261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kilvington S, Price J. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J Appl Bacteriol. 1990;68:519–25. doi: 10.1111/j.1365-2672.1990.tb02904.x. [DOI] [PubMed] [Google Scholar]