Abstract
Loss of neurons or neuronal functions over time has been hypothesized to contribute to the dysregulation of autonomic functions observed in aging. In this study, we evaluated the total number of the hypothalamic hypocretin (orexin) immunopositive neurons in 100, 400, 800 and 1,000 day old male and female C57Bl/6 mice that are commonly used in aging studies in vertebrates. Males had 15-20% more hypocretin immunopositive neurons (HIN) than females at all ages examined. Neuronal number for both sexes was stable in the first 400 days of life, but started declining between 400 and 800 days with rates of approximately 1 neuron per day. The rate of loss doubled in males between 800 and 1000 days of age. The total average number of HIN for males was 2251 ± 139 at 100 days, 2235 ± 112 at 400 days, 1914 ± 81 at 800 days, and 1596 ± 301 at 1,000 days. The total average number of HIN for females was 1805 ± 76 at 100 days, 1887 ± 118 at 400 days, and 1521± 181 at 800 days. Evaluation of the time-dependent decline in the number of hypocretin immunopositive neurons may help to explain the physiological changes in sleep or energy homeostasis regulation during aging.
Loss of hypothalamic neurons or their function over time has been proposed to contribute to the autonomic decline observed with aging [11, 24]. Although a thorough evaluation of neuronal loss is typically difficult, it is possible for discrete populations like hypocretin (orexin) neurons. These cells are found exclusively in the lateral hypothalamus and their relatively small number enables the whole population to be quantified as opposed to taking representative sections [10, 30]. Hypocretins (hypocretin 1 and hypocretin 2 or orexin-A and orexin-B), are neuropeptides derived from a common precursor (preprohypocretin) that bind to two G protein-coupled receptor subtypes (hypocretin receptor-1 and 2) [10, 30]. A substantial number of studies have identified the hypocretin system as an important regulator of sleep/wakefulness, energy homeostasis and reward seeking (for recent comprehensive reviews see [2, 9, 15]).
A mutation in one of the hypocretin receptors is responsible for narcolepsy in dogs and genetic ablation of the hypocretin gene or selective hypocretin neuronal loss induces a narcolepsy phenotype in mice [6, 13, 22]. Almost 90% of human narcoleptic patients show decreased levels of hypocretin-1 in their cerebrospinal fluid most likely due to a loss of hypocretin neurons [23, 26]. Levels of hypothalamic hypocretin-1 are higher during waking and REM sleep compared to slow wave sleep, providing additional evidence for the role of hypocretin in sleep/wakefulness [20].
The hypocretin system may also be responsible for the correlation between sleep and metabolic disturbances [18]. Hypocretin null mice develop late onset obesity and narcolepsy in humans is often associated with being overweight and an increased susceptibility to type 2 diabetes [13, 17, 21, 27, 31]. The excitability of hypocretin neurons was demonstrated to be influenced by glucose, ghrelin and leptin, which suggests that these cells can act as primary sensors of nutrients and participate in energy homeostasis [4, 5, 29, 34, 35]. Recently it has been demonstrated that hypocretin null mice develop age dependent insulin resistance both centrally and peripherally [33].
Previous studies in rats showed age-dependent reductions in the level of preprohypocretin, hypocretin receptors mRNA, hypocretin and the number of hypocretin axonal fibers [28]. Decreased numbers of hypocretin fibers were also found in the locus coeruleus of cats and rhesus macaques and in the magnocellular preoptic nucleus and the vertical and horizontal limb of the diagonal band of Broca in guinea pigs [12, 22, 36, 37]. In the rhesus macaques age was associated with a decrease of hypocretin immunopositive axon density but the reduction of thetotal number of hypocretin immunopositive neurons (HIN) in the lateral hypothalamus was not statistically significant [12]. In mice, increased age has been reported to be associated with a reduction of hypocretin receptor 1 and 2 mRNA levels in different brain regions [32]. To further assess the effects of aging on the hypocretin system, we investigated the total number of HIN in the lateral hypothalamus of young (∼100 days), adult (∼400 days), and old (∼800 to 1000 days) male and female C57BL/6 mice.
Mice were housed four per cage with a 12:12 hr light/dark cycle with lights on at 6:00 am, were fed ad libitum and had unlimited access to water. Males were 100 (100-157) (n=5), 400 (400-484) (n=3), 800 (775-897) (n=7), and 1000 (1000-1157) (n=3) days old; females were 100 (100-121) (n=8), 400 (400-559) (n=5), and 800 (750-889) (n=5) days old] All procedures were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. Tissue collection occurred between the hours of 10:00 am and 2:00 pm. Immunohistochemistry was performed using antibody specific for hypocretin-1(SC-8070, Santa Cruz Biotechnology) as previously described [8].
Given the relatively small number of hypocretin neurons and their discrete localization we opted for counting the cell number in all consecutive sections containing HIN rather than in sections selected by systematic sampling. We were thus able to determine the absolute number of HIN without assuming that each section was typical or representative of the entire population. To the best of our knowledge this is the first study counting directly the total number of HIN in mouse. To determine the total number of HIN, mice were deeply anesthetized using 4-5% isoflurane and subsequently perfused with a 0.1M phosphate buffer (PB) wash followed by 4% paraformaldehyde. Brains were removed and fixed in 4% paraformaldehyde for two days, before being transferred to 30% sucrose for another two days. Frozen brain tissue was cut into 35 μm coronal sections using a cryostat. In order to evaluate the total cell number all consecutive sections encompassing the lateral hypothalamus (Bregma +2, -3 mm) were collected. Groups of 3 consecutive sections were placed in 12 wells CoorsTek ceramic plates for free floating immunohistochemistry. On the same day, slices were incubated with 0.3% hydrogen peroxide for 30 minutes, rinsed twice in 0.1M PBS and 1% BSA, pre-incubated with 0.1M PBS, 0.5% BSA, and Triton-100X for 30 minutes, rinsed twice in 0.1M PBS and 0.5% BSA, and incubated overnight with a primary antibody specific for hypocretin-1 (SC-8070, Lot# A2604, Santa Cruz Biotechnology). The following day, slices were rinsed twice in 0.1M PBS and 0.5% BSA, incubated in perioxidase secondary antibody for 60 minutes, rinsed twice in 0.1M PBS and 0.5% BSA, incubated with Vectastain ABC (Vector Labs, Burlingame, CA) for 60 minutes, rinsed twice in 0.1M PB, and reacted with diamino benzidine (DAB) for 15 minutes for visualization. All consecutive section slices were mounted on microscope slides. The raw number of hypocretin immunopositive neurons was determined by two independent investigators counting the number of DAB stained cell bodies using a regular optical microscope and a manual cell counter. HIN were counted in coronal sections beginning and ending with at least 3 consecutive sections that were void of immunopositive neurons. Representative sections of hypocretin immunopositive neurons are shown in Figure 1(a-c). The actual number of hypocretin neurons was calculated using the Abercrombie correction with the formula Actual Number = Raw Number × Section Thickness / (Section Thickness + Cell Diameter) where Section Thickness was 35 μm and Cell Diameter was 20 μm [1, 7, 10]. Statistical analysis was done using single factor ANOVA and values are expressed as the mean ± SEM.
Figure 1.
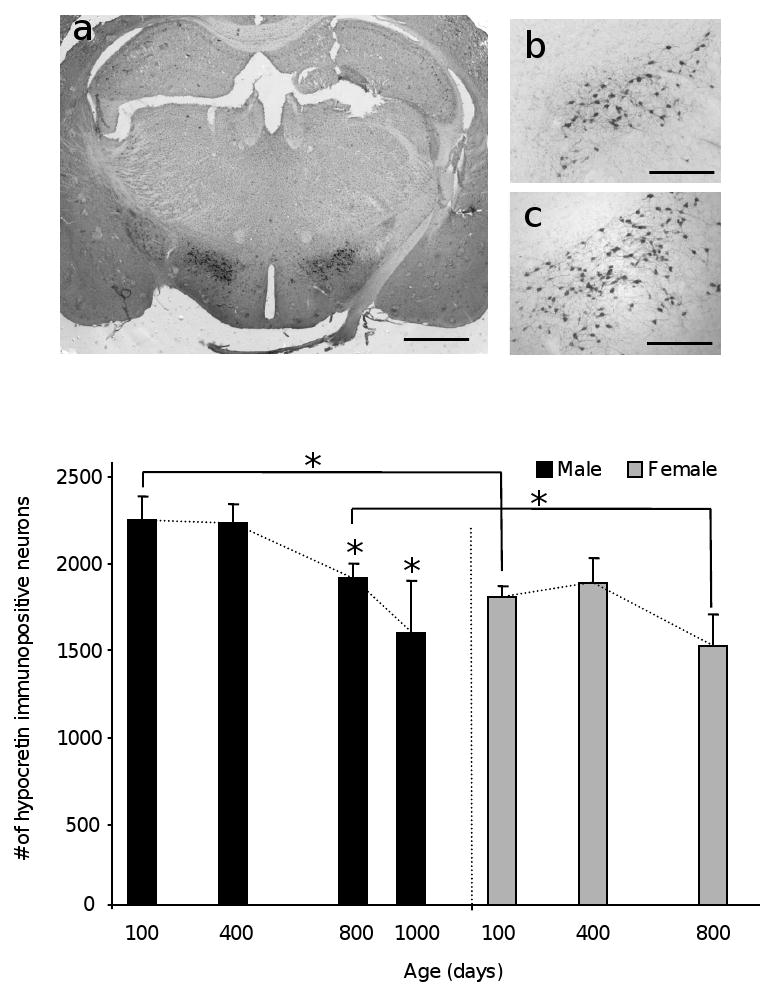
Representative hypocretin immunohistochemistry on 35 μm thick brain coronal sections of male (a and b) and Female (c left) C57Bl/6 mice. Bar is 1 mm in a and 0.25 mm in b and c. The histograms show the total number of HIN in male and female mice at different ages as indicated. (d) Gender-specific age-dependent decline; The slope of neuronal numbers over time is shown as dotted line (*p<0.05).
The total number of hypocretin immunopositive neurons was summarized in Table I. In males, the number of neurons remained unchanged between 100 days and 400 days. It declined by 15% (p< 0.05) between 400 and 800 days and by 29% (p=0.37) between 800 and 1,000 days (this decline was marginally statistically significant most likely due to the low number of 1,000 day old animals available (Figure 1d)). The rate at which immunopositive neurons were lost was 24 neurons/month (or 0.8 neurons per day) between 400 and 800 days, and doubled to 47 neurons/month (or 1.58 neurons/day) between 800 and 1000 days (Table II). Despite a trend towards a decline in the number of HIN in females and an overall 16% loss observed between 100 and 800 days, the difference was not statistically significant (Figure 1d). The rate of decline between 400 and 800 days for females was 27 neurons/month (or 0.91 neurons/day). No 1,000 day old females were available as absolute lifespan for female C57Bl/6 mice is shorter than males [3].
Table I.
Summary of actual individual as well as mean neuronal number for male and female mice at different ages as indicated. SEM, Standard Error of the Mean.
| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| n | 100 day | 400 day | 800 day | 1000 day | 100 day | 400 day | 800 day |
| 1 | 2733 | 2380 | 1604 | 1810 | 1856 | 1742 | 1586 |
| 2 | 2357 | 2310 | 2172 | 1977 | 2043 | 1488 | 853 |
| 3 | 1980 | 2015 | 1692 | 1001 | 1857 | 2377 | 1955 |
| 4 | 1998 | 1892 | 1929 | 1807 | 1572 | ||
| 5 | 2186 | 1862 | 1525 | 2018 | 1640 | ||
| 6 | 2064 | 1608 | |||||
| 7 | 2107 | 1877 | |||||
| 8 | 1749 | ||||||
| Mean | 2251 | 2235 | 1914 | 1596 | 1805 | 1887 | 1521 |
| SEM | 139 | 112 | 80 | 301 | 76 | 118 | 181 |
Table II.
Rates of daily decline of hypocretin immunopositive across different ages expressed as slope of the neuronal loss curve and as average number of neurons lost per day.
| Males | Females | |
|---|---|---|
| 100 - 400 days | -0.05 | 0.27 |
| 400 - 800 days | -0.8 (0.8/day) | -0.91 (0.9/day) |
| 800 -1000 days | -1.58 (1.6/day) |
Finally, the number of HIN showed a gender-specific difference of approximately 20%. Females had consistently fewer HIN than males: 19.8 %, 15.6% and 20.5% at 100*, 400 and 800* days, respectively (*p<0.05) (Figure 1e).
Thus, reduction of the total number of HIN effectively occurs with a late onset in a progressive and cumulative fashion. These features are consistent with the theory that biological aging is the result of progressive accumulation of molecular damage eventually leading to loss of biological function [16], which is possibly mediated by free radicals [14]. In this scenario, molecular damage to hypocretin neurons was reduced or simply did not reach biological significance during the first 400 days (or approximately one year) of life, but began showing its effects thereafter. Although a more complete evaluation of the rate of decline would require the measurement of neuronal numbers at shorter time intervals, the data collected here for males between age 800 and 1,000 days highlights the progressive and cumulative nature of the mechanisms of decline.
Whether the reduction in neuronal number was due to neuronal cell death or loss of hypocretin expression was not evaluated in this study. Both phenomena are believed to contribute to age-related declines in cognitive and physiological function [25]. The gender specific differences in hypocretin immunopositive cell number were particularly interesting. Male mice not only had a higher number of immunopositive neurons at all ages examined, but also the 800 day old males that had already lost 15% of their total HIN population had a higher number of HIN than the 100 day old females. A previous study investigating gender specific differences in the rat did not measure cell number, but found that the levels of hypocretin-1 peptide and prepro-orexin mRNA were higher in females [19].
Aging is associated with decline of autonomic functions, which affect several homeostatic systems including sleep, energy balance, stress and reproduction. The reduction of HIN observed here, alone or in conjunction with age-dependent reduction of other neuronal groups regulating similar functions and not investigated in this study, may contribute to this decline. Due to its relatively modest lifespan and the availability of numerous transgenic models, the mouse is the most commonly used model to investigate aging in vertebrates. The profile of HIN loss over time in both female and male C57Bl/6 mice will serve as a comparative parameter for studies investigating aging in other hypothalamic neuronal populations and their possible contribution to autonomic dysfunctions.
Acknowledgments
Support was provided by the Ellison Medical Foundation, NIA AG028040 and the National Science Foundation Graduate Fellowship. The authors have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 2.Adamantidis A, de Lecea L. The hypocretins as sensors for metabolism and arousal. J Physiol. 2009;587:33–40. doi: 10.1113/jphysiol.2008.164400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali SS, Xiong C, Lucero J, Behrens MM, Dugan LL, Quick KL. Gender differences in free radical homeostasis during aging: shorter-lived female C57BL6 mice have increased oxidative stress. Aging Cell. 2006;5:565–574. doi: 10.1111/j.1474-9726.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 4.Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O'Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 6.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 7.Chen CT, Dun SL, Kwok EH, Dun NJ, Chang JK. Orexin A-like immunoreactivity in the rat brain. Neurosci Lett. 1999;260:161–164. doi: 10.1016/s0304-3940(98)00977-x. [DOI] [PubMed] [Google Scholar]
- 8.Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, de Lecea L, Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–828. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- 9.de Lecea L. A decade of hypocretins: past, present and future of the neurobiology of arousal. Acta Physiol (Oxf) 2009 doi: 10.1111/j.1748-1716.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- 10.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516(Pt 2):611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downs JL, Dunn MR, Borok E, Shanabrough M, Horvath TL, Kohama SG, Urbanski HF. Orexin neuronal changes in the locus coeruleus of the aging rhesus macaque. Neurobiol Aging. 2007;28:1286–1295. doi: 10.1016/j.neurobiolaging.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 14.Harman D. Free radical theory of aging: an update: increasing the functional life span. Ann N Y Acad Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- 15.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Hayflick L. Biological aging is no longer an unsolved problem. Ann N Y Acad Sci. 2007;1100:1–13. doi: 10.1196/annals.1395.001. [DOI] [PubMed] [Google Scholar]
- 17.Honda Y, Doi Y, Ninomiya R, Ninomiya C. Increased frequency of non-insulin-dependent diabetes mellitus among narcoleptic patients. Sleep. 1986;9:254–259. doi: 10.1093/sleep/9.1.254. [DOI] [PubMed] [Google Scholar]
- 18.Horvath TL, Gao XB. Input organization and plasticity of hypocretin neurons: possible clues to obesity's association with insomnia. Cell Metab. 2005;1:279–286. doi: 10.1016/j.cmet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Johren O, Neidert SJ, Kummer M, Dominiak P. Sexually dimorphic expression of prepro-orexin mRNA in the rat hypothalamus. Peptides. 2002;23:1177–1180. doi: 10.1016/s0196-9781(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 20.Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lammers GJ, Pijl H, Iestra J, Langius JA, Buunk G, Meinders AE. Spontaneous food choice in narcolepsy. Sleep. 1996;19:75–76. doi: 10.1093/sleep/19.1.75. [DOI] [PubMed] [Google Scholar]
- 22.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 23.Melberg A, Ripley B, Lin L, Hetta J, Mignot E, Nishino S. Hypocretin deficiency in familial symptomatic narcolepsy. Ann Neurol. 2001;49:136–137. [PubMed] [Google Scholar]
- 24.Miles LE, Dement WC. Sleep and aging. Sleep. 1980;3:1–220. [PubMed] [Google Scholar]
- 25.Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 26.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 27.Nishino S, Ripley B, Overeem S, Nevsimalova S, Lammers GJ, Vankova J, Okun M, Rogers W, Brooks S, Mignot E. Low cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsy. Ann Neurol. 2001;50:381–388. doi: 10.1002/ana.1130. [DOI] [PubMed] [Google Scholar]
- 28.Porkka-Heiskanen T, Alanko L, Kalinchuk A, Heiskanen S, Stenberg D. The effect of age on prepro-orexin gene expression and contents of orexin A and B in the rat brain. Neurobiol Aging. 2004;25:231–238. doi: 10.1016/s0197-4580(03)00043-5. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92 doi: 10.1016/s0092-8674(02)09256-5. 1 page following 696. [DOI] [PubMed] [Google Scholar]
- 31.Schuld A, Hebebrand J, Geller F, Pollmacher T. Increased body-mass index in patients with narcolepsy. Lancet. 2000;355:1274–1275. doi: 10.1016/S0140-6736(05)74704-8. [DOI] [PubMed] [Google Scholar]
- 32.Terao A, Apte-Deshpande A, Morairty S, Freund YR, Kilduff TS. Age-related decline in hypocretin (orexin) receptor 2 messenger RNA levels in the mouse brain. Neurosci Lett. 2002;332:190–194. doi: 10.1016/s0304-3940(02)00953-9. [DOI] [PubMed] [Google Scholar]
- 33.Tsuneki H, Murata S, Anzawa Y, Soeda Y, Tokai E, Wada T, Kimura I, Yanagisawa M, Sakurai T, Sasaoka T. Age-related insulin resistance in hypothalamus and peripheral tissues of orexin knockout mice. Diabetologia. 2008;51:657–667. doi: 10.1007/s00125-008-0929-8. [DOI] [PubMed] [Google Scholar]
- 34.Williams RH, Alexopoulos H, Jensen LT, Fugger L, Burdakov D. Adaptive sugar sensors in hypothalamic feeding circuits. Proc Natl Acad Sci U S A. 2008;105:11975–11980. doi: 10.1073/pnas.0802687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang JH, Sampogna S, Morales FR, Chase MH. Age-related changes in hypocretin (orexin) immunoreactivity in the cat brainstem. Brain Res. 2002;930:206–211. doi: 10.1016/s0006-8993(02)02240-0. [DOI] [PubMed] [Google Scholar]
- 37.Zhang JH, Sampogna S, Morales FR, Chase MH. Age-related changes of hypocretin in basal forebrain of guinea pig. Peptides. 2005;26:2590–2596. doi: 10.1016/j.peptides.2005.05.003. [DOI] [PubMed] [Google Scholar]


