Abstract
While preserving keratinocyte proliferative ability, arsenite suppresses cellular differentiation markers by preventing utilization of AP1 transcriptional response elements. In present experiments, arsenite had a dramatic effect in electrophoretic mobility supershift analysis of proteins binding to an involucrin promoter AP1 response element. Without arsenite treatment, binding of JunB and Fra1 was readily detected in nuclear extracts from preconfluent cultures and was not detected a week after confluence, while c-Fos was detected only after confluence. By contrast, band shift of nuclear extracts from arsenite treated cultures showed only JunB and Fra1 binding in postconfluent as well as preconfluent cultures. Immunoblotting of cell extracts showed that arsenite treatment prevented the loss of Fra1 and the increase in c-Fos proteins that occurred after confluence in untreated cultures. Chromatin immunoprecipitation assays demonstrated substantial reduction of c-Fos and acetylated histone H3 at the proximal and distal AP1 response elements in the involucrin promoter and of coactivator p300 at the proximal element. Alteration of AP1 transcription factors was also examined in response to treatment with four metal containing compounds (chromate, vanadate, hemin, divalent cadmium) that also suppress involucrin transcription. These agents all influenced transcription at AP1 elements in a transcriptional reporter assay, but exhibited less effect than arsenite on binding activity assessed by mobility shift and chromatin immunoprecipitation and displayed variable effects on AP1 protein levels. These findings help trace a mechanism by which transcriptional effects of arsenite become manifest and help rationalize the unique action of arsenite, compared to the other agents, to preserve proliferative ability.
Keywords: ChIP assays, Differentiation, c-Fos, Fra-1, Histone H3, Mobility shift
Introduction
The extensive worldwide exposure to arsenic in drinking water (Nordstrom, 2002) and the prevalence of serious consequent health effects, emphasize an urgent need to link target tissue doses of active arsenic metabolites to key mechanistic events producing adverse responses (Hughes et al., 2007). The mechanism by which chronic inorganic arsenic exposure results in human carcinogenesis has long been elusive (Kitchin, 2001), complicated by the realization that arsenite methylation may not give effective detoxification (Wildfang et al., 2001) and could even yield more toxic metabolites (Stýblo et al., 2002). Although arsenite appears not to exhibit a direct genotoxic mode of action (Klein et al., 2007), it is a transplacental carcinogen in mice for several organs that are targets in humans (Waalkes et al., 2007). Recent work has helped focus attention on arsenite inhibition of DNA damage repair proteins with zinc finger DNA binding domains such as poly (ADP-ribose) polymerase, likely an important contributing factor in its action as a co-carcinogen (Ding et al., 2009). Moreover, arsenite exposure results in generation of reactive oxygen, which has a myriad of downstream effects (Kumagai and Sumi, 2007). In addition to producing skin cancer, inorganic arsenic now is recognized to increase the risk from exposure to sunlight in humans (Chen et al., 2006) and in mice (Rossman et al., 2004), an interaction that may arise through enhanced generation of reactive oxygen from NADPH oxidase and mitochondrial sources (Cooper et al., 2009).
Although transplacental arsenite exposure alone does not produce skin cancer in mice, it enhances tumorigenicity in Tg.AC mice treated with the tumor promoter tetradecanoyl phorbol acetate after weaning (Waalkes et al., 2008). This effect, attributed in part to an increase in the stem cell population as a target for carcinogenesis, parallels the observed preservation of stem cell character in human epidermal cultures treated with arsenite (Patterson et al., 2005). Preservation of proliferative potential in such cultures reflects suppression of downstream effects of insulin/IGF1 receptor signaling (Patterson and Rice, 2007), including reduced activation of the keratinocyte tumor suppressor Notch 1 (Reznikova et al., 2009). A key indicator of this action originally observed was the suppression of differentiation markers in keratinocyte culture, first noted for involucrin (Kachinskas et al., 1994) and then more generally (Kachinskas et al., 1997). Consistent with observations of functional AP1 response elements in the promoters of numerous genes expressed during keratinocyte terminal differentiation, two AP1 elements were noted to mediate arsenite and arsenate suppression of involucrin expression (Jessen et al., 2001).
The possibility that arsenic targets a key element in programming, such as a common transcription factor, is attractive. Numerous reports have appeared of arsenite effects on transcription driven by AP1 response elements and the proteins themselves, which are known to be redox sensitive. For example, chronic arsenite treatment led to a persistent increase in AP1 response element binding in nuclear extracts from mouse bladder epithelia (Simeonova et al., 2001). Other skin carcinogens such as ultraviolet light (Cooper and Bowden, 2007) and polycyclic aromatic hydrocarbons (Ding et al., 2006) also affect AP1 signaling. Numerous laboratories have demonstrated elevated activity of AP1 in cell cultures resulting from acute arsenite exposure, a response that is particularly striking at elevated concentrations, e.g., ≥ 10 μM in keratinocytes (Zhang et al., 2009). The activity observed after long term treatment, by contrast, has not been elevated and even has been slightly reduced (Kachinskas et al., 1994; Hu et al., 2002), consistent with a reduced contribution of AP1 response elements to differentiation marker regulation.
Finding the mechanism by which differentiation is suppressed could help elucidate how alteration of an upstream signaling pathway has multiple downstream effects. For this purpose, cultured human keratinocytes offer a good model for examining effects on target cells of arsenite and related agents, since the cells mimic well in culture salient aspects of growth and differentiation in vivo (Green, 1979). Before confluence, cells rapidly proliferate and have a protein complement similar to that of basal cells in living epidermis. After confluence, the cells differentiate and accumulate proteins present in suprabasal, terminally differentiated epidermis.
Exposure to chromate, vanadate, cadmium and hemin resemble arsenite in suppressing keratinocyte differentiation in culture (Rea et al., 2003), but they do not preserve the cellular proliferative potential (Patterson et al., 2005). Present experiments revealing effects on transcription factor binding to AP1 response elements provide insight into their distinctive actions.
Materials and Methods
Cell culture
SCC9 human squamous carcinoma cells were cultured in the presence of a lethally irradiated 3T3 feeder layer using a 2:1 mixture of Dulbecco’s modified Eagle’s and Ham’s F-12 media supplemented with 5% fetal bovine serum, 0.4 μg/ml hydrocortisone, 5 μg/ml insulin, 5 μg/ml transferrin, 20 pM triiodothyronine and 0.18 mM adenine (Allen-Hoffmann and Rheinwald, 1984). Treatments with sodium arsenate, sodium arsenite, cadmium chloride, potassium chromate, sodium vanadate or hemin at concentrations previously shown to be effective (Rea et al., 2003; Patterson et al., 2005) were initiated at confluence unless otherwise indicated and continued until 7–17 days after confluence. The medium was changed at 3-day intervals until harvesting. The malignant SCC13, premalignant SCC12F2 (Rheinwald and Beckett, 1981) and spontaneously immortalized keratinocyte (SIK) lines (Rice et al., 1993) were cultured similarly, except that the latter was treated with 10 ng/ml EGF until confluence.
Transfections
Log phase SCC9 cultures were stably transfected using calcium phosphate with a modified pREP9 episomal vector (Invitrogen, Carlsbad, CA) and selection with G418 (Jessen et al., 2001). In the constructs, the proximal involucrin promoter (−2466 to +41), either intact or with the two functional AP1 sites inactivated by double mutations (T to C transitions at −2119 and −2115 and T to A transversions at positions −124 and −120), was used to drive a firefly luciferase reporter (Kachinskas et al., 1997). Cultures were treated in triplicate starting when the cells were nearly at confluence and continued for 17 days, at which time they were harvested for measurement of luciferase activity (Luciferase Assay System, Promega, Madison, WI). After subtracting the activity of a promoterless luciferase plasmid assayed in parallel, luciferase values were normalized to protein content and illustrated as means ± standard deviations.
Preparation of nuclear extracts
Nuclear extracts were prepared by minor modification of a standard method (Dignam et al., 1983). Briefly, cultures were scraped from the dishes into isotonic phosphate buffered saline, collected by centrifugation at 1850 × g for 5 min, then incubated in hypotonic buffer [10 mM HEPES (pH 7.9 at 4°C), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol (DTT)], and lysed by homogenization. Nuclei were pelleted by centrifugation at 3300 × g for 15 min and extracted by addition of one volume of high salt buffer [20 mM HEPES (pH 7.9 at 4°C), 25% (v/v) glycerol, 1.5 mM MgCl2, 0.6 M KCl, 0.2 mM EDTA, 0.5 mM DTT] equal to the volume of nuclei. Both the hypotonic and the high salt buffer contained the protease inhibitor phenylmethyl sulfonyl fluoride (0.2 mM) and the phosphatase inhibitors sodium pyrophosphate (30 mM) and sodium vanadate (50 μM). Extracts were cleared by centrifugation at 16000 × g for 15 min at 4°C, then stored at −80°C.
Electrophoretic mobility shift assay
Binding reactions (20 μl) contained 5–10 μg nuclear extract protein and 500 ng of double stranded poly[d(I-C)] in a solution of 12.5 mM HEPES (pH 7.9 at 4°C); 0.2 mM EDTA; 0.01% NP-40; 0.5 mM DTT; 100 mM NaCl and 10% glycerol. Assays of gel filtration column fractions also contained 1 mM CaCl2. After 10 min incubation at room temperature, 10 fmol of 32P-labeled probe encompassing the involucrin proximal promoter AP1 site (101 to 130 bp upstream of the transcription start site: 5′GCTGTGGTGAGTCAGGAAGGGGTTAGAGGAA3′) was added and incubation was continued for 20 min. DNA protein complexes were separated on 4% polyacrylamide gels in buffer containing 25 mM Tris HCl, 190 mM glycine and 1 mM EDTA. Supershift analysis of Jun (c-Jun, JunB, JunD) and Fos (c-Fos, FosB, Fra1, Fra2) family member binding was done by adding 2 μg of antibody (sc-45x, sc-46x, sc-74x, sc-52x, sc-48x, sc-605x, sc-604x, respectively, from Santa Cruz Biotechnology, Santa Cruz, CA) to binding reactions, followed by incubation on ice for 3 hr before probe addition. An indication of specific binding, a 100 fold excess of unlabeled oligonucleotide prevented detection of radioactive protein-oligonucleotide complexes.
Immunoblotting
Cells were scraped into ice-cold isotonic phosphate buffered saline and lysed in buffer containing 62.5 mM Tris-HCl, pH 6.8; 2% SDS; 10% glycerol; 50mM DTT with further disruption of samples by sonication. Lysates were centrifuged at 10,000 × g for 15 min. Protein samples were submitted to SDS polyacrylamide gel electrophoresis, transferred to polyvinyldifluoride membranes, blocked with nonfat dry milk in TBST (25 mM Tris-HCl, pH 7.5; 150 mM NaCl; 0.05% Tween-20), incubated with the indicated primary antibodies and then detected using the ECL Plus chemiluminescence detection reagent (GE Healthcare, Piscataway, NJ). Primary antibodies used to detect Fra-1, c-fos (rabbit polyclonal antibodies), JunB (mouse monoclonal), actin (goat polyclonal) were from Santa Cruz Biotechnology. Involucrin rabbit polyclonal antibody is described in (Rice and Green, 1979).
mRNA quantitation
Total RNA was extracted from each culture dish using TRIZOL reagent (Invitrogen) and then purified by phenol/chloroform extraction followed by ethanol precipitation. RNA concentration was calculated by A260. Then 2 μg of total RNA were treated with DNase Free reagent (Ambion, Austin, TX) for 60 min and reverse-transcribed with Superscript II (Invitrogen, Carlsbad, CA) at 37°C for 120 min using random primers. The volume of the resultant cDNA mixture was adjusted to 200 μl by adding distilled water and stored at −20°C until further use. Using 5 μl of the reverse transcription reaction as a template, quantitative real time PCR was performed using ABI 7700 or 7500 real time PCR instruments and Taqman reagents (Applied Biosystems, Foster City, CA). 18S RNA, hypoxanthine phosphoribosyl transferase (HPRT) and glucuronidase β (GusB) were used as internal controls to normalize expression of genes of interest by the ΔΔCt method (Wong and Medrano, 2005).
Chromatin immunoprecipitation (ChIP)
ChIP assays were done using a ChIP-IT Express kit from Active Motif (Carlsbad, CA), with modifications. Briefly, cells were fixed in 1% formaldehyde for 15 min at room temperature and rinsed with phosphate buffered saline. Fixation was stopped by addition of glycine, and cells were scraped in phosphate buffered saline with protease inhibitors. After centrifugation and washing of the cell pellet with phosphate buffered saline, cells were lysed with 10 mM Tris-HCl, pH 7.5, 10 mM NaCl, 3 mM MgCl2, 0.05% NP-40, 1 mM phenylmethyl sulfonyl fluoride and protease inhibitors (Active Motif). Nuclei were obtained by centrifugation at 13,000 × g for 1 min, and the pellet was resuspended in shearing buffer (Active Motif). Chromatin was sheared by sonication (12 pulses for 30 seconds at 50% power using a sonicator from Sonics and Materials, Danbury, CT), and centrifuged at 13,000 × g for 10 min at 4C, then stored at −80C. After purification of DNA from an aliquot of each chromatin sample, analysis by agarose gel electrophoresis showed that the bulk of the DNA was sheared to fragments of 100 to 500 bp in length. Chromatin was immunoprecipitated using antibodies to the transcription factors JunB, c-Fos and Fra1, to the transcriptional coactivators CBP and p300 (Santa Cruz, sc-73x, sc-52x, sc-605x, sc-369x and sc-584x respectively), to acetyl-histone H3 (06-599 from Millipore, Billerica, MA) and with non-specific rabbit IgG (Sigma Chemical, St. Louis, MO) overnight at 4°C. Antibody-protein complexes were purified with protein G-coated magnetic beads (Active Motif) according to the supplier protocol. After reversal of cross-links and proteinase K digestion, DNA was analyzed by real time PCR using custom Taqman assays (Applied Biosystems) to detect the human involucrin promoter proximal and distal AP1 sites. Some samples were also analyzed for presence of glyceraldehyde-phosphate dehydrogenase, keratin 1, keratin 10 and keratin 14 promoters using Sybr Green detection. Sequences of real time primers and probes are listed in Supplemental Methods. Aliquots of DNA from input chromatin before immunoprecipitation were analyzed in parallel to adjust for differences in chromatin yield between samples. Enrichment values, which are a measure of specific association of proteins with DNA, were obtained by dividing the amounts of PCR product obtained after precipitation with specific antibodies by amounts obtained with non-specific IgG and ranged from 2 to 200 fold.
Statistics
One way ANOVA statistical testing (with Bonferroni corrections) was performed using Stata/SE9.2 software for Windows except for Table 1. In the latter, significance of differences between specifically and nonspecifically immunoprecipitated samples, after log transformation of the measured values, were submitted to least square difference and Dunnett tests using the SAS program.
Results
AP1 element-mediated transcriptional responses to treatments with arsenic and metal compounds
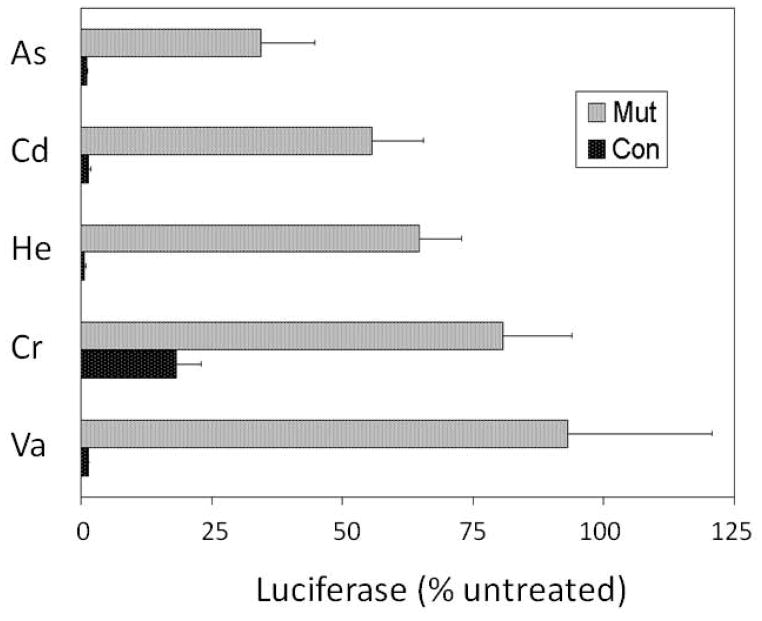
Constructs containing the proximal human involucrin promoter, wild type or mutated at both AP1 sites, were tested for their transcriptional activity in stably transfected SCC9 cells. Luciferase reporter activities driven by the wild type and mutated promoters were compared with and without treatment with arsenate, chromate, vanadate, cadmium or hemin (Figure 1). As shown previously for both arsenite and arsenate (Jessen et al., 2001), arsenate dramatically suppressed the relative transcriptional activity of the wild type promoter, in the present case to ≈ 2% of that in its absence. By contrast, the activity of the mutant promoter was suppressed to ≈ 30% of that in untreated cultures. Similar results were obtained with the other agents where, except for chromate, the degree of suppression was 10–20 fold greater for the wild type than for the mutant promoter. For the latter, the difference in suppression was ≈ 4 fold.
Fig. 1.
Effects of treatments on involucrin promoter activity. Confluent cultures stably transfected with the proximal involucrin promoter (−2466 to +41), either wild type (black bars) or with the two functional AP1 sites inactivated by double mutations (gray bars), were treated for 17 days with 6 μM arsenate (As), 10 μM hemin (He), 10 μM cadmium (Cd), 3 μM chromate (Cr) or 10 μM vanadate (Va), as indicated. Luciferase activities as percentages of untreated parallel cultures (mean ± standard deviation) are illustrated for 3 determinations. The response of cultures expressing the control and AP1 mutant promoters were significantly different for each treatment (p < 0.05).
Altered regulation of AP1-binding protein levels
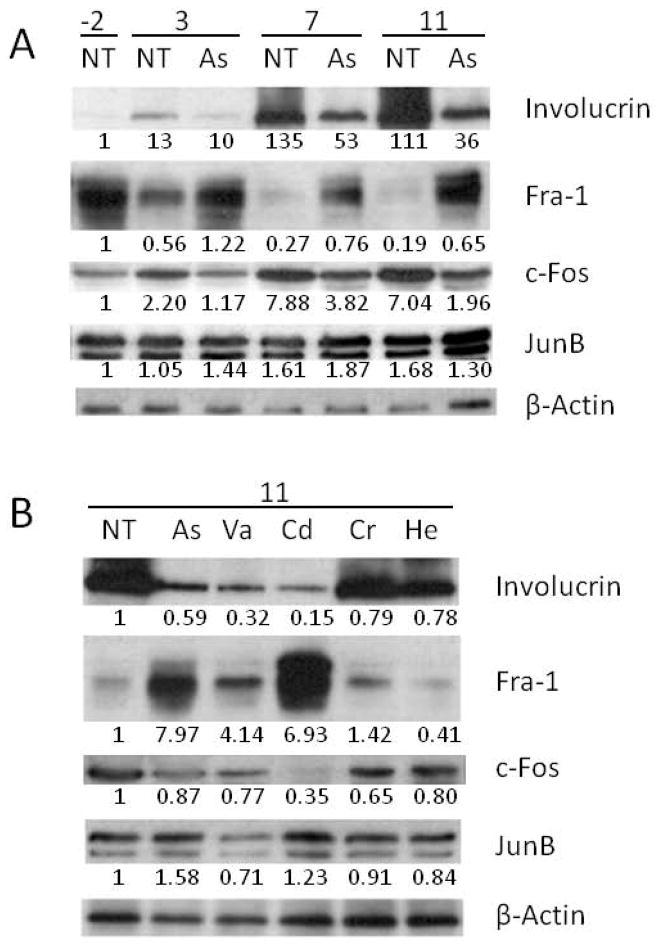
Alterations in involucrin promoter transcription could be the result of changes in levels of AP1 transcription factors. A survey of AP1 binding proteins and the differentiation marker, involucrin, during differentiation and after arsenite treatment was performed by immunoblotting. As shown in Figure 2A, Fra1 protein levels dropped to very low levels over a period of 11 days after confluence in untreated cultures, but were less affected in arsenite-treated cultures. During this time period, c-Fos levels increased substantially in untreated cells, an effect that was reduced noticeably by arsenite treatment. By contrast, levels of JunB were affected relatively little either by time or treatment. Changes in involucrin protein levels were seen to be reduced by arsenite in parallel with the mRNA changes reported previously (Jessen et al., 2001).
Fig. 2.
Alteration of transcription factor protein levels by treatments. Immunoblots of nuclear extract were performed after treating cultures with (A, B) 2 μM arsenite (As), or (B) 10 μM vanadate (Va), 10 μM cadmium (Cd), 3 μM chromate (Cr) or 10 μM hemin (He) as indicated. Cultures were harvested for analysis 2 days before confluence or 3, 7 or 11 days after confluence as indicated (−2, 3, 7 11) (A) or 7 days after confluence (B). β-Actin was used as a loading control. The numbers below the lanes show amounts of protein (detected by scanning the blots) which are normalized to β-actin and expressed relative to the untreated value (NT) at day −2 (A) or 11 (B).
Effects of treatments with other metal-containing compounds that suppressed involucrin promoter activity were compared at 11 days after confluence. The most striking differences were seen in Fra1 levels. Compared to untreated cultures, those treated with arsenite or cadmium or to a lesser extent vanadate exhibited much higher levels; whereas levels in cultures treated with chromate were changed little and with hemin were lower (Figure 2B). Cadmium produced a substantial decrease in c-Fos, an effect also seen (but not as markedly) by the other agents. JunB appeared affected relatively little by all the treatments. The reduction of involucrin protein was most obvious with treatment by arsenite, vanadate or cadmium.
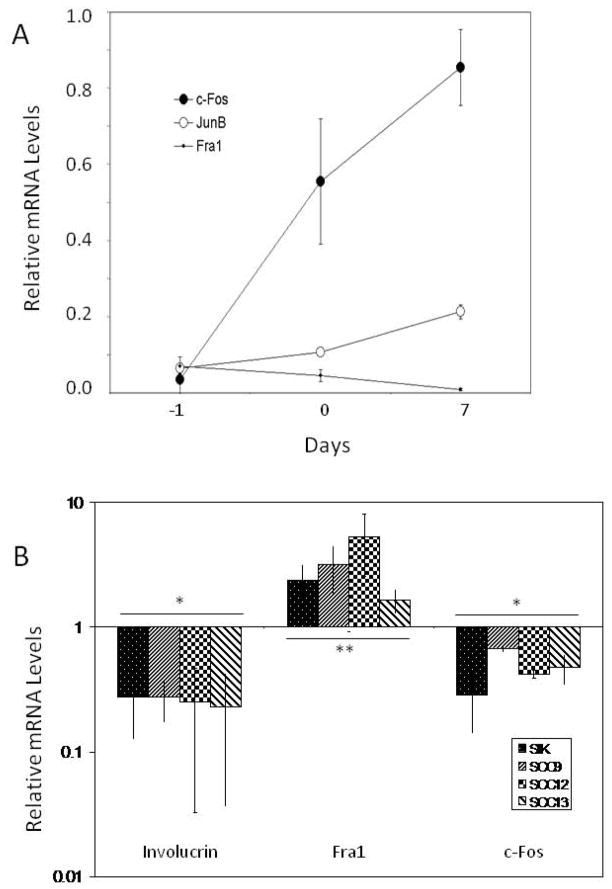
To determine whether the observed alterations in AP1 proteins by arsenite reflected changes in their transcription, mRNA levels were measured by real time PCR. As shown in Figure 3A, JunB mRNA levels increased 3–4 fold from preconfluence to a week after confluence. The increase in c-Fos mRNA levels was more dramatic, increasing ≥20 fold, while Fra1 mRNA declined by a similar factor. mRNA and protein changes were roughly parallel with large changes over time in c-Fos and Fra1 and less change in JunB. Arsenite treatment also resulted in parallel trends in protein and mRNA levels. As shown in Figure 3B, Fra1 mRNA levels were increased substantially by arsenite treatment. By contrast, mRNA levels of c-Fos were consistently suppressed by arsenite treatment, as were mRNA levels of involucrin. Levels of JunB mRNA were essentially unaffected by arsenite treatment (not shown). These effects were observed not only in SCC9, but in two other squamous carcinoma lines, SCC13 and SCC12F2, and in a minimally deviated spontaneously immortalized keratinocyte line (SIK) as well.
Fig. 3.
Alteration of transcription factor mRNA levels with time and by treatments. (A) Real time PCR measurements of the indicated mRNAs in untreated SCC9 cultures were performed a day before confluence (−1), at confluence (0) and 7 days after confluence (7). (B) Arsenite treatment began at confluence. Samples were harvested 3 days later for measurement of Fra1 and c-Fos mRNAs or 7 days after confluence for measurement of involucrin mRNA. mRNA levels in parallel untreated control cultures were measured and assigned a value of 1 for comparison. Shown are the means ± standard deviations of 4–8 experiments, which differed significantly from untreated control cultures (*, p < 0.01; **, p = 0.05).
Effects of differentiation and treatment with arsenite or metal compounds on binding of AP1 transcription factors
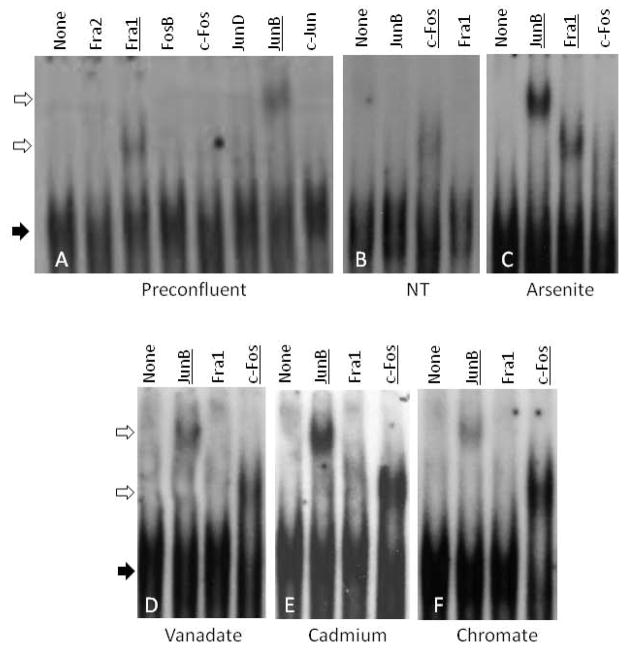
Electrophoretic mobility supershift experiments were performed to assess the major AP1 binding proteins in untreated and treated cells. Nuclear extracts were prepared from preconfluent (growing) cultures and from differentiated cultures one week after confluence, when they have accumulated substantial amounts of involucrin and other differentiation markers. Untreated cells exhibited a reproducible change in the pattern of proteins binding to the involucrin proximal promoter AP1 response element. In growing cells, Fra-1 and JunB were the major AP1 proteins detected (Figure 4A). A week after confluence, however, these proteins were not evident, but c-Fos was clearly detected (Figure 4B). Treatment of the cultures with arsenite for one week starting at confluence prevented this change so that JunB and Fra-1 were clearly seen, but c-Fos was detected weakly if at all (Figure 4C). By contrast, parallel treatments with chromate, vanadate or cadmium gave an intermediate result; JunB and c-Fos were evident but Fra-1 was not detected (Figure 4D, E and F).
Fig. 4.
Supershift analysis of proteins binding to involucrin promoter proximal AP1 response element. Nuclear extract was prepared from untreated cultures the day before confluence (preconfluent) or 7 days after confluence either with no treatment (NT), or treated with 2 μM arsenite, 10 μM vanadate, 10 μM cadmium or 3 μM chromate as indicated. The band patterns were examined after incubation in the absence (None) or presence of antibodies to the indicated transcription factors. Solid arrows point to protein complexes with labeled AP1 oligonucleotide, and open arrows show the supershifted bands. Antibody addition resulted in supershifted bands for the underlined AP1 proteins.
Chromatin immunoprecipitation assays were used to assess effects of treatments on the relative amounts of the major AP1 binding proteins detected by electrophoretic mobility supershift which associate with the involucrin promoter proximal and distal AP1 sites in living cells. In untreated, differentiating cells (1 week after confluence), JunB, c-Fos and Fra1 were all detected in chromatin surrounding both AP1 sites. As observed in supershift assays, cells treated for one week with arsenite had much less c-Fos associated with both AP1 sites than in parallel cultures without arsenite (Table 1), while treatment with vanadate, chromate or cadmium did not result in altered amounts of c-Fos. In contrast, amounts of JunB and Fra1 associated with AP1 sites were not significantly altered by any of the treatments.
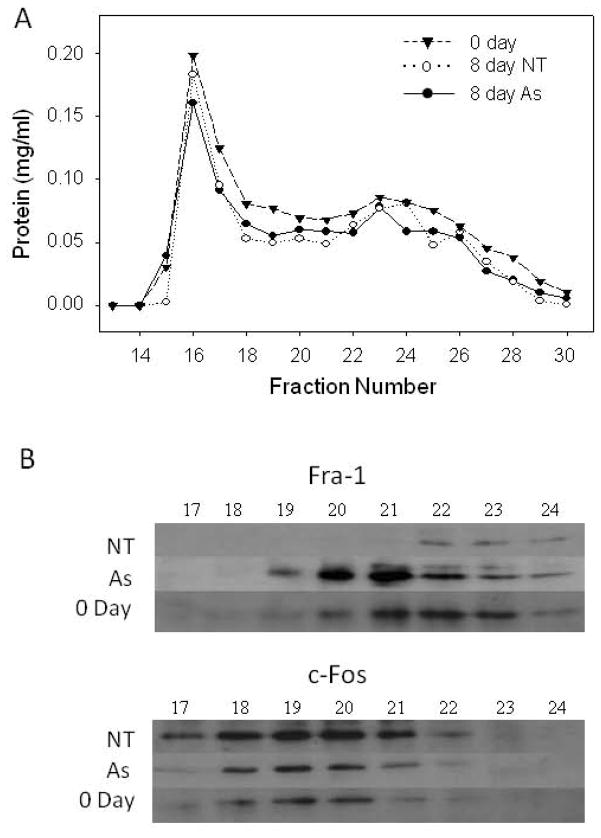
Fra1 has been reported to act in a multiprotein complex with the co-activator, p300, to stimulate involucrin transcription (Crish and Eckert, 2008). The possibility that arsenite treatment prevented the participation of Fra1 or c-Fos in large complexes was examined. Separation of nuclear extract proteins by gel filtration revealed that AP1 binding activity eluted near the void volume of Sephacryl S-300, corresponding to a molecular weight in the vicinity of ≈ 1 million for globular protein. Immunoblotting across the column fractions confirmed the changes seen in Figure 2B in protein level as a result of arsenite treatment of the cultures, but little if any change was seen in the elution positions of c-Fos or Fra1 (Figure 5).
Fig. 5.
Nuclear extracts were prepared from cultures treated with 2 μM arsenite (As) or not (NT) for 8 days and compared to extracts from newly confluent untreated cultures (0 Day). Extract (1 ml) containing 2 mg of protein was applied to a 45 ml column of Sephacryl S-300 (Sigma-Aldrich) that had been equilibrated with 20 mM Hepes buffer (pH 7.2) containing 0.3 M KCl and 1 mM DTT. Fractions of 1 ml were collected (A) and analyzed by immunoblotting (B). AP1-binding activity monitored by mobility shift eluted in a broad band from fractions 17–23. In parallel runs, peak concentrations of bovine catalase (240 kDa) and equine myoglobin (17 kDa) eluted at fractions 23 and 30, respectively, and blue dextran eluted in the void volume, fractions 16–17.
Reduction of Co-activator p300 and acetylated histone H3 at involucrin promoter AP1 sites
ChIP assays were employed to determine the association of co-activators and acetylated histone in the vicinity of the involucrin promoter AP1 sites in living cells. Previous results that p300 localizes at or near the distal AP1 site were confirmed, although the amount was not significantly altered by arsenite treatment (Table 1). In contrast, half as much p300 was associated with the region containing the proximal AP1 site after 1 week of arsenite treatment, while vanadate, chromate and cadmium treatments did not significantly alter p300 association with either AP1 site. Immunoprecipitations were also performed with antibody to the related co-activator, CBP, but amounts associated with AP1 regions were not significantly different in untreated and arsenite treated cells.
Since p300 has intrinsic acetyltransferase activity, we compared amounts of acetyl histone H3 localized near each involucrin promoter AP1 site in chromatin prepared from treated and untreated cells. Although p300 was decreased by arsenite only at the proximal site, acetyl histone H3 levels were decreased to about half the amount present in untreated cells in the vicinity of both AP1 sites. Arsenite effects on acetyl histone H3 were specific for the involucrin promoter since amounts associated with the glyceraldehyde-phosphate dehydrogenase and keratin promoters were little affected by treatment (Supplemental Table 1) and total cellular acetyl histone H3 levels were essentially unchanged (arsenite/untreated = 1.18 ± 0.34 after 1 week of treatment). Vanadate treatment decreased acetyl histone H3, but only at the proximal AP1 site, while cadmium and chromate had little effect.
Discussion
Differentiation of cultured keratinocytes is suppressed by chronic treatment with inorganic arsenic and several metal-containing compounds (Kachinskas et al., 1997). In the case of arsenite, with three fold the potency of arsenate but indistinguishable in its effects on keratinocytes (Patterson et al., 2003; Rea et al., 2003), maximal suppression of the differentiation marker, involucrin, was shown to require intact AP1 sites (Jessen et al., 2001). This observation prompted present experiments demonstrating a similar AP1 mediation of suppression by vanadate, chromate, cadmium and hemin. AP1 is a particularly important transcription factor promoting keratinocyte differentiation in general (Eckert et al., 1997; Rossi et al., 1998) and involucrin transcription in particular (Welter et al., 1995; Lopez-Bayghen et al., 1996; Kachinskas et al., 1997). The involucrin gene promoter contains two AP1 sites, separated by 2 kb of intervening DNA, mediating much (but not all) of the gene regulation. The proximal site mediates phorbol ester responsiveness (Efimova et al., 1998; Takahashi et al., 1998) and the distal site confers responsiveness to calcium, vitamin D and oxysterols (Hanley et al., 2000; Ng et al., 2000; Bikle et al., 2002; Deucher et al., 2002).
Although suppression of involucrin mRNA level by arsenite, chromate, vanadate, cadmium and hemin involves the same promoter AP1 sites, the mechanisms for disruption appear to differ between arsenite and the others. Examination of AP1 DNA binding activity by supershift assay using extracts from treated cells demonstrated that only arsenite preserved a pattern similar to that obtained with extract from undifferentiated (preconfluent) cultures, consistent with being unique in preserving the cellular proliferative capability (Patterson et al., 2005). Immunoblotting of AP1 proteins present in cell extracts showed little relation to binding activities, indicating that binding assays are affected by factors other than the simple presence or absence of the protein. Likely possibilities are exclusion from the nucleus (not present in the nuclear extracts used for in vitro binding assays) or post-translational modifications, such as phosphorylation, which may differ with various treatments. Participation of c-Fos or Fra1 in large protein complexes was evident by gel filtration but was not detectably altered by arsenite treatment.
Although mRNA and protein levels for JunB, c-Fos and Fra1 were parallel in untreated and arsenite-treated cells, DNA binding activities corresponded to protein and mRNA levels only for Fra1 and c-Fos. This observation prompted ChIP experiments to determine which factors are associated with the involucrin proximal and distal AP1 regions in living cells. JunB was found to be associated with both AP1 sites, and the amount was similar in the presence and absence of arsenite, paralleling the protein content of the cultures. Similarly, c-Fos association with AP1 sites in chromatin from treated and untreated cells paralleled relative intracellular amounts of protein, where less was associated with the involucrin AP1 sites in cells treated with arsenite than in untreated cells. Although immunoblotting showed much higher levels of Fra1 in post-confluent cells treated with arsenite than in untreated cells, there was little difference in the amount associated with either the proximal or distal AP1 sites, a result obtained with two different antibodies to Fra1. This differs from results of in vitro DNA binding supershift assays, likely due to additional proteins present in intact chromatin that impact binding of transcription factors. Overall, ChIP experiments demonstrated that arsenite affects recruitment of c-Fos, but not of JunB or Fra1.
In contrast to arsenite treatment, vanadate, chromate or cadmium treatment did not alter association of Fra1, c-Fos or JunB with either the proximal or distal involucrin promoter AP1 sites as assessed by ChIP. Since none of those treatments affected in vitro binding of Fra1 or c-Fos, DNA binding in living cells and in cell extracts were parallel. In most cases, Fra1 and c-Fos binding did not correlate with relative amounts measured by Western blotting, possibly due to alteration of post-translational modifications which affect binding activity. Vanadate, chromate and cadmium had little effect on amounts of JunB or on binding activity either in living cells or in extracts. We conclude from these studies that although AP1 sites mediate suppression of involucrin expression by several metal compounds and by arsenite, this occurs by a mechanism other than alteration of binding of the major AP1 transcription factors associated with those sites in living cells, with the exception of decreased binding of c-Fos after arsenite treatment.
Since co-activators have been demonstrated to influence keratinocyte differentiation (Missero et al., 1995; Kawabata et al., 2002; Tran and Crowe, 2004), and p300 has been shown to be associated with the involucrin promoter distal AP1 site (Crish and Eckert, 2008), we investigated the influence of arsenite and several metal compounds on association of the co-activators, CBP and p300, with the involucrin promoter proximal and distal AP1 sites. Both co-activators showed specific association with these sites. While CBP association was unaltered in arsenite-treated cells, p300 was decreased. In contrast, none of the metals affected p300 association with either AP1 site.
Because p300 has intrinsic histone acetyl-transferase activity, we next investigated effects of arsenite treatment on acetyl histone H3 localization. Treatment resulted in about half the acetyl histone H3 in the vicinity of each AP1 site. This reduction was not a general effect of arsenite to reduce cellular histone H3, since immunoblotting showed similar amounts in treated and untreated cells. Furthermore, arsenite treatment did not significantly change amounts of acetyl histone H3 associated with the glyceraldehyde phosphate dehydrogenase and three keratin promoters. Notably, however, arsenite and monomethylarsonous acid have been shown to produce epigenetic remodeling of cultured human bladder epithelial cells during malignant transformation, where histone H3 acetylation was significantly increased in some genes and decreased in others, a possible contributing factor in tumorigenesis (Jensen et al., 2008). Of the metal compounds, only vanadate showed a tendency toward altering acetyl histone H3 association and only in the region encompassing the proximal AP1 site. Since vanadate did not alter p300 association, vandate and arsenite likely produce their effects on histone H3 acetylation by different mechanisms.
Coactivator recruitment is regulated by epigenetic modification of chromatin, a factor that can affect cell proliferative potential by, for example, Ezh2-mediated prevention of premature AP1-dependent differentiation in keratinocyte stem cells (Ezhkova et al., 2009). Thus, arsenite could influence the stem cell target population for carcinogenesis through epigenetic means. Coactivator recruitment is also regulated by growth factor dependent phosphorylation (Zanger et al., 2001; Chen et al., 2007; Tsai et al., 2008). Phosphorylation and acetylation of AP1 transcription factors have also been demonstrated to affect coactivator binding (Narayanan et al., 2004; Wang et al., 2006). Since arsenite has been shown to impact EGF and insulin/IGF receptor signaling (Patterson and Rice, 2007), exploration of downstream effects on AP1 transcription factor and co-activator phosphorylation merit future investigation.
Supplementary Material
Supplementary Methods. Real time PCR Primer and Probe Sequences.
Supplementary Table 1. ChIP analyses of acetyl histone H3 at GAPDH, K1 K10 and K14 promoters.
Acknowledgments
We thank Dr. Neil H. Willits, Department of Statistics, University of California, Davis, for performing the statistical testing in Table 1. This research was supported by US Public Health Service grants P42 ES04699 and T32 ES07059 from the National Institute of Environmental Health Sciences.
Footnotes
Conflict of Interest
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen-Hoffmann BL, Rheinwald JG. Polycyclic aromatic hydrocarbon mutagenesis of human epidermal keratinocytes in culture. Proc Natl Acad Sci USA. 1984;81:7802–7806. doi: 10.1073/pnas.81.24.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Ng D, Oda Y, Hanley K, Feingold K, Xie Z. The vitamin D response element of the involucrin gene mediates its regulation by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2002;119:1109–1113. doi: 10.1046/j.1523-1747.2002.19508.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Graziano JH, Parvez F, Hussain I, Momotaj H, van Geen A, Howe GR, Ahsan H. Modification of risk of arsenic-induced skin lesions by sunlight exposure, smoking, and occupational exposures in Bangladesh. Epidemiol. 2006;17:459–467. doi: 10.1097/01.ede.0000220554.50837.7f. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Wang YN, Chang WC. ERK2-mediated C-terminal serine phosphorylation of p300 is vital to the regulation of epidermal growth factor-induced keratin 16 gene expression. J Biol Chem. 2007;282:27215–27228. doi: 10.1074/jbc.M700264200. [DOI] [PubMed] [Google Scholar]
- Cooper KL, Liu KJ, Hudson LG. Enhanced ROS production and redox signaling with combined arsenite and UVA exposure: Contribution of NADPH oxidase. Free Radical Biol Med. 2009;47:381–388. doi: 10.1016/j.freeradbiomed.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SJ, Bowden GT. Ultraviolet B regulation of transcription factor families: Roles of nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Current Cancer Drug Targets. 2007;7:325–334. doi: 10.2174/156800907780809714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crish JF, Eckert RL. Synergistic activation of human involucribn gene expression by Fra1 and p300 - evidence for the presence of a multiprotein complex. J Invest Dermatol. 2008;128:530–541. doi: 10.1038/sj.jid.5701049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deucher A, Efimova T, Eckert RL. Calcium-dependent involucrin expression is inversely regulated by protein kinase C (PKC)α and PKCδ. J Biol Chem. 2002;277:17032–17040. doi: 10.1074/jbc.M109076200. [DOI] [PubMed] [Google Scholar]
- Dignam JD, Lebowitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucl Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Li J, Chen J, Chen H, Ouyang W, Zhang R, Xue C, Zhang D, Amin S, Desai D, Huang C. Effects of polycyclic aromatic hydrocarbons (PAHs) on vascular endothelial growth factor induction through phosphatidylinositol 3-kinase/AP-1-dependent, HIF-1alpha-independent pathway. J Biol Chem. 2006;281:9093–9100. doi: 10.1074/jbc.M510537200. [DOI] [PubMed] [Google Scholar]
- Ding W, Liu W, Cooper KL, Qin XJ, de Souza Bergo PL, Hudson LG, Liu KJ. Inhibition of poly(ADP-ribose) polymerase-1 by arsenite interferes with repair of oxidative DNA damage. J Biol Chem. 2009;284:6809–6817. doi: 10.1074/jbc.M805566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Banks EB, Welter JF. The epidermis: genes on - genes off. J Invest Dermatol. 1997;109:501–509. doi: 10.1111/1523-1747.ep12336477. [DOI] [PubMed] [Google Scholar]
- Efimova T, LaCelle P, Welter JF, Eckert RL. Regulation of human involucrin promoter activity by a protein kinase C, Ras, MEKK1, MEK3, p38/RK, AP1 signal transduction pathway. J Biol Chem. 1998;273:24387–24395. doi: 10.1074/jbc.273.38.24387. [DOI] [PubMed] [Google Scholar]
- Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su IH, Hannon G, Tarakhovsky A, Fuchs E. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell. 2009;136:1122–1135. doi: 10.1016/j.cell.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. The keratinocyte as differentiated cell type. Harvey Lect. 1979;74:101–138. [PubMed] [Google Scholar]
- Hanley K, Ng DC, He SS, Lau P, Min K, Elias PM, Bikle DD, Mangelsdorf DJ, Williams ML, Feingold KR. Oxysterols induce differentiation in human keratinocytes and increase Ap-1-dependent involucrin transcription. J Invest Dermatol. 2000;114:545–553. doi: 10.1046/j.1523-1747.2000.00895.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Jin X, Snow ET. Effect of arsenic on transcription factor AP-1 and NF-kappaB DNA binding activity and related gene expression. Toxicol Lett. 2002;133:33–45. doi: 10.1016/s0378-4274(02)00083-8. [DOI] [PubMed] [Google Scholar]
- Hughes MF, Kenyon EM, Kitchin KT. Research approaches to address uncertainties in the risk assessment of arsenic in drinking water. Toxicol Appl Pharmacol. 2007;222:399–404. doi: 10.1016/j.taap.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Jensen TJ, Novak P, Eblin KE, Gandolfi AJ, Futscher BW. Epigenetic remodeling during arsenical-induced malignant transformation. Carcinogenesis. 2008;29:1500–1508. doi: 10.1093/carcin/bgn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen BA, Qin Q, Phillips MA, Phillips DL, Rice RH. Keratinocyte differentiation marker suppression by arsenic: Mediation by AP1 response elements and antagonism by tetradecanoylphorbol acetate. Toxicol Appl Pharmacol. 2001;174:302–311. doi: 10.1006/taap.2001.9227. [DOI] [PubMed] [Google Scholar]
- Kachinskas DJ, Phillips MA, Qin Q, Stokes JD, Rice RH. Arsenate perturbation of human keratinocyte differentiation. Cell Growth Differ. 1994;5:1235–1241. [PubMed] [Google Scholar]
- Kachinskas DJ, Qin Q, Phillips MA, Rice RH. Arsenate suppression of human keratinocyte programming. Mutation Res. 1997;386:253–261. doi: 10.1016/s1383-5742(97)00015-x. [DOI] [PubMed] [Google Scholar]
- Kawabata H, Kawahara K, Kanekura T, Araya N, Daitoku H, Hatta M, Miura N, Fukamizu A, Kanzaki T, Maruyama I, Nakajima T. Possible role of transcriptional coactivator P/CAF and nuclear acetylation in calcium-induced keratinocyte differentiation. J Biol Chem. 2002;277:8099–8105. doi: 10.1074/jbc.M108250200. [DOI] [PubMed] [Google Scholar]
- Kitchin KT. Recent advances in arsenic carcinogenesis: Modes of action, animal model systems and methylated arsenic metabolites. Toxicol Sci. 2001;172:249–261. doi: 10.1006/taap.2001.9157. [DOI] [PubMed] [Google Scholar]
- Klein CB, Leszczynska J, Hickey C, Rossman TG. Further evidence against a direct genotoxic mode of action for arsenic-induced cancer. Toxicol Appl Pharmacol. 2007;222:289–297. doi: 10.1016/j.taap.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai Y, Sumi D. Arsenic: signal transduction, transcription factor, and biotransformation involved in cellular response and toxicity. Ann Rev Pharmacol Toxicol. 2007;47:243–262. doi: 10.1146/annurev.pharmtox.47.120505.105144. [DOI] [PubMed] [Google Scholar]
- Lopez-Bayghen E, Vega A, Cadena A, Granados SE, Jave LF, Gariglio P, Alvarez-Salas LM. Transcriptional analysis of the 5′-noncoding region of the human involucrin gene. J Biol Chem. 1996;271:512–520. doi: 10.1074/jbc.271.1.512. [DOI] [PubMed] [Google Scholar]
- Missero C, Calautti E, Eckner R, Chin J, Tsai LH, Livingston DM, Dotto GP. Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc Natl Acad Sci USA. 1995;92:5451–5455. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan K, Srinivas R, Peterson MC, Ramachandran A, Hao J, Thimmapaya B, Scherer PE, George A. Transcriptional regulation of dentin matrix protein 1 by JunB and p300 during osteoblast differentiation. J Biol Chem. 2004;279:44294–44302. doi: 10.1074/jbc.M403511200. [DOI] [PubMed] [Google Scholar]
- Ng DC, Shafaee S, Lee D, Bikle DD. Requirement of an AP-1 site in the calcium response region of the involucrin promoter. J Biol Chem. 2000;275:24080–24088. doi: 10.1074/jbc.M002508200. [DOI] [PubMed] [Google Scholar]
- Nordstrom DK. Worldwide occurrences of arsenic in ground water. Science. 2002;296:2143–2144. doi: 10.1126/science.1072375. [DOI] [PubMed] [Google Scholar]
- Patterson TJ, Ngo M, Aronov PA, Reznikova TV, Green PG, Rice RH. Biological activity of inorganic arsenic and antimony reflects oxidation state in cultured keratinocytes. Chem Res Toxicol. 2003;16:1624–1631. doi: 10.1021/tx034146y. [DOI] [PubMed] [Google Scholar]
- Patterson TJ, Reznikova TV, Phillips MA, Rice RH. Arsenite maintains germinative state in cultured human epidermal cells. Toxicol Appl Pharmacol. 2005;207:69–77. doi: 10.1016/j.taap.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Patterson TJ, Rice RH. Arsenite and insulin exhibit opposing effects on epidermal growth factor receptor and keratinocyte proliferative potential. Toxicol Appl Pharmacol. 2007;221:119–128. doi: 10.1016/j.taap.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea MA, Gregg JP, Qin Q, Phillips MA, Rice RH. Global alteration of gene expression in human keratinocytes by inorganic arsenic. Carcinogenesis. 2003;24:747–756. doi: 10.1093/carcin/bgg010. [DOI] [PubMed] [Google Scholar]
- Reznikova TV, Phillips MA, Rice RH. Arsenite suppresses Notch1 signaling in human keratinocytes. J Invest Dermatol. 2009;129:155–161. doi: 10.1038/jid.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous carcinomas. Cancer Res. 1981;41:1657–1663. [PubMed] [Google Scholar]
- Rice RH, Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: Activation of the cross-linking by calcium ions. Cell. 1979;18:681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Rice RH, Steinmann KE, deGraffenried LA, Qin Q, Taylor N, Schlegel R. Elevation of cell cycle control proteins during spontaneous immortalization of human keratinocytes. Molec Biol Cell. 1993;4:185–194. doi: 10.1091/mbc.4.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AJSI, Ceci R, Steinert PM, Markova NG. Effect of AP1 transcription factors on the regulation of transcription in normal human epidermal keratinocytes. J Invest Dermatol. 1998:110. doi: 10.1046/j.1523-1747.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- Rossman TG, Uddin AN, Burns FJ. Evidence that arsenite acts as a cocarcinogen in skin cancer. Toxicol Appl Pharmacol. 2004;198:394–404. doi: 10.1016/j.taap.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Simeonova PP, Wang S, Kashon ML, Kommineni C, Crecelius E, Luster MI. Quantitative relationship between arsenic exposure and AP-1 activity in mouse urinary bladder epithelium. Toxicol Sci. 2001;60:279–284. doi: 10.1093/toxsci/60.2.279. [DOI] [PubMed] [Google Scholar]
- Stýblo M, Drobná Z, Jaspers I, Lin S, Thomas DJ. The role of biomethylation in toxicity and carcinogenicity of arsenic: a research update. Environ Hlth Perspect. 2002;110(suppl 5):767–771. doi: 10.1289/ehp.110-1241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Asano K, Manabe A, Kinouchi M, Ishida-Yamamoto A, Iizuka H. The α and η forms of protein kinase C stimulate transcription of human involucrin gene. J Invest Dermatol. 1998;110:218–222. doi: 10.1046/j.1523-1747.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- Tran NQ, Crowe DL. Regulation of human involucrin gene promoter by co-activator proteins. Biochem J. 2004;381:267–273. doi: 10.1042/BJ20031653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LN, Ku TK, Salib NK, Crowe DL. Extracellular signals regulate rapid coactivator recruitment at AP-1 sites by altered phosphorylation of both CREB binding protein and c-jun. Molec Cell Biol. 2008;28:4240–4250. doi: 10.1128/MCB.01489-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Diwan BA. Transplacental arsenic carcinogenesis in mice. Toxicol Appl Pharmacol. 2007;222:271–280. doi: 10.1016/j.taap.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Germolec DR, Trempus CS, Cannon RE, Tokar EJ, Tennant RW, Ward JM, Diwan BA. Arsenic exposure in utero exacerbates skin cancer response in adulthood with contemporaneous distortion of tumor stem cell dynamics. Cancer Res. 2008;68:8278–8285. doi: 10.1158/0008-5472.CAN-08-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YN, Chen YJ, Chang WC. Activation of extracellular signal-regulated kinase signaling by epidermal growth factor mediates c-Jun activation and p300 recruitment in keratin 16 gene expression. Molec Pharmacol. 2006;69:85–98. doi: 10.1124/mol.105.016220. [DOI] [PubMed] [Google Scholar]
- Welter JF, Crish JF, Agarwal C, Eckert RL. Fos-related antigen (Fra-1), junB and junD activate human involucrin promoter transcription by binding to proximal and distal AP1 sites to mediate phorbol ester effects on promoter activity. J Biol Chem. 1995;270:12614–12622. doi: 10.1074/jbc.270.21.12614. [DOI] [PubMed] [Google Scholar]
- Wildfang E, Radabaugh TR, Aposhian HV. Enzymatic methylation of arsenic compounds. IX. Liver arsenite methyltransferase and arsenate reductase activities in primates. Toxicol. 2001;168:213–221. doi: 10.1016/s0300-483x(01)00481-4. [DOI] [PubMed] [Google Scholar]
- Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. BioTechniques. 2005;39:75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- Zanger K, Radovick S, Wondisford FE. CREB binding protein recruitment to the transcription complex requires growth factor-dependent phosphorylation of its GF box. Molec Cell. 2001;7:551–558. doi: 10.1016/s1097-2765(01)00202-7. [DOI] [PubMed] [Google Scholar]
- Zhang D, Li J, Gao J, Huang C. c-Jun/AP-1 pathway-mediated cyclin D1 expression participates in low dose arsenite-induced transformation in mouse epidermal JB6 Cl41 cells. Toxicol Appl Pharmacol. 2009;235:18–24. doi: 10.1016/j.taap.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods. Real time PCR Primer and Probe Sequences.
Supplementary Table 1. ChIP analyses of acetyl histone H3 at GAPDH, K1 K10 and K14 promoters.