In man the haptoglobin (Hp) gene is polymorphic with two common alleles denoted 1 and 2.1 We have recently demonstrated in multiple independent longitudinal studies that there is an up to 500% increase in the incidence of cardiovascular disease in individuals with the Hp 2-2 genotype and diabetes mellitus (DM).2–6 The normal function of Hp is to bind extracorpuscular hemoglobin (Hb) and promote its clearance after it is released into either the blood secondary to red cell intravascular hemolysis (6 g per day in a normal man) or into the extravascular compartment after hemorrhage (i.e., after hemorrhage into the atherosclerotic plaque).1 We and others have previously demonstrated that the Hp 1 allele protein product is superior to the Hp 2 allele protein product in blocking Hb induced oxidation of multiple substrates including LDL and HDL.7–9 The major pathway for clearance of the Hp-Hb complex from the blood as well as from the atherosclerotic plaque is via the monocyte/macrophage CD163 receptor.10 We have demonstrated that the Hp 1-Hb complex is cleared more rapidly by the CD163 receptor than the Hp 2-Hb complex.11 Furthermore, the amount of the CD163 receptor expressed on monocyte/macrophages is decreased in Hp 2-2 and DM individuals.12 In this study we set out to test the hypothesis that the Hp genotype regulates the turnover and oxidative activity of Hb in the atherosclerotic plaque.
All protocols were approved by the Animal Care and Use Committee of the Technion. The characterization and generation of Hp 1 and Hp 2 mice in a C57Bl/6 Apo E−/− genetic background has been previously described in detail.13 Briefly, the murine Hp 1 allele is the wild type murine Hp allele. The Hp 2 allele exists only in man and murine Hp 2 allele was genetically engineered in vitro and its insertion targeted at the Hp locus by homologous recombination. Previous characterization of these mice has demonstrated that there are no differences between Hp 1 and Hp 2 mice in Hp concentration, total cholesterol and HDL cholesterol.
Plaque iron in frozen 5 mm sections of brachiocephalic arteries from Hp 1 or Hp 2 Apo E−/− mice was detected and quantified using Perl’s stain as previously described. Immunofluorescent identification of hemoglobin in the atherosclerotic plaque was performed in frozen 5 mm sections of brachiocephalic arteries from Hp 1 or Hp 2 Apo E−/− mice. Hemoglobin was identified with a rabbit antiserum to mouse hemoglobin (MP Biochemicals) using a Cy3 conjugated secondary antibody (Jackson Immunoresearch) for antigen detection. As a control for specificity of the fluorescent signal from Hb, sections were also incubated in the presence of an excess of Hb (4.7 ug/ul) during the immunostaining. Quantitation of signal intensity was performed using Image Pro-Plus. For studies attempting to show the colocalization of Hb with plaque iron, immunofluorescence was performed prior to staining for iron.
In situ assessment of plaque oxidation state was assessed by incubating 5 mm frozen sections of brachiocephalic arteries from Hp 1 or Hp 2 Apo E mice in the presence of dihydrorhodamine (DHR). The time dependent oxidation of DHR was evident by the time dependent increase in fluorescence of the plaque. Plaques from different mice were compared at the 10 minute timepoint after the DHR was applied to the slide as this was found to be the maximal time in which the change in DHR fluorescence remained linear with time. Quantitation of fluorescent intensity was done using Image Pro-plus software. For studies involving use of an iron chelator to block iron induced oxidation of the DHR, desferroxamine was incubated with the tissue slice at a concentration of 50 mM for 60 minutes prior to applying the DHR. For studies in which DHR fluorescence was colocalized with plaque iron, the iron stain was performed first and then the DHR was applied to the slide.
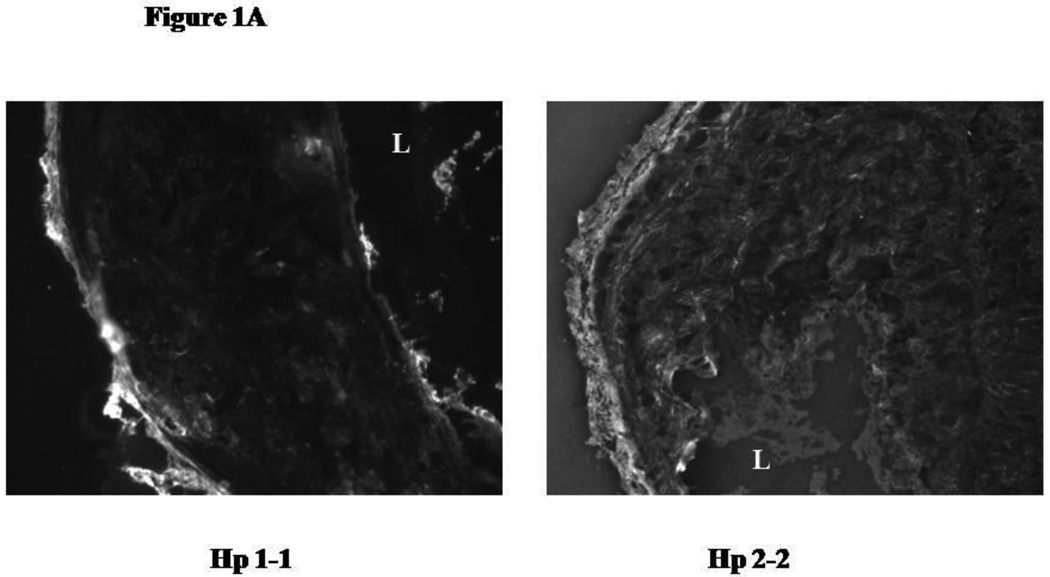
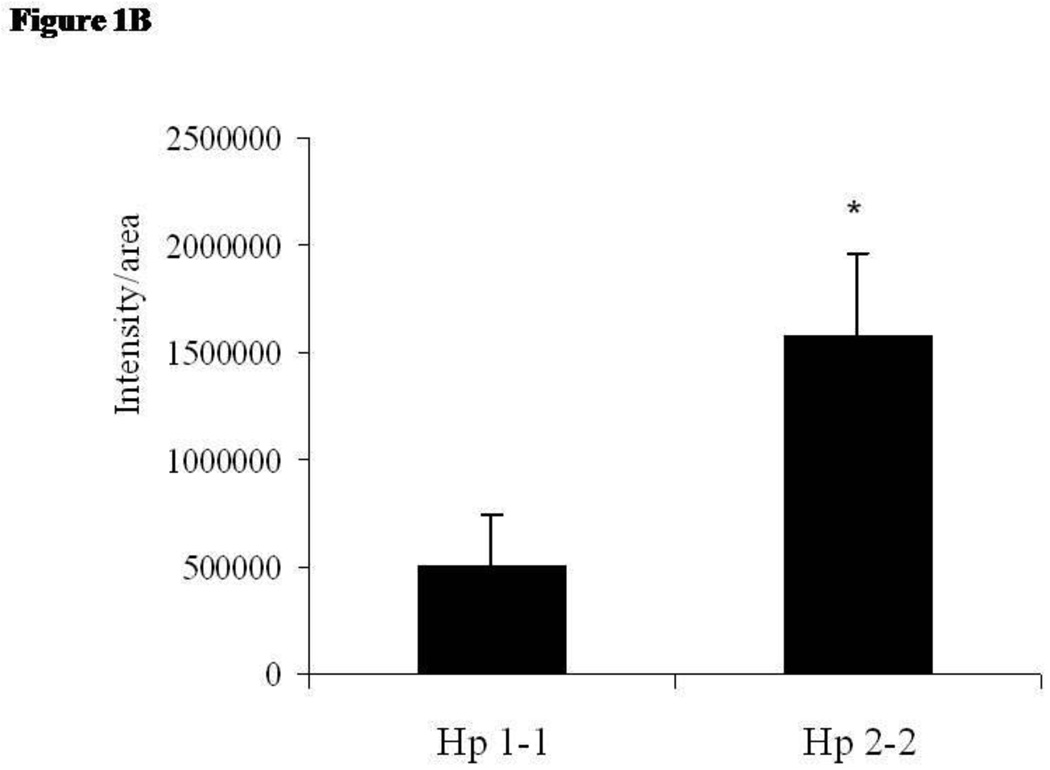
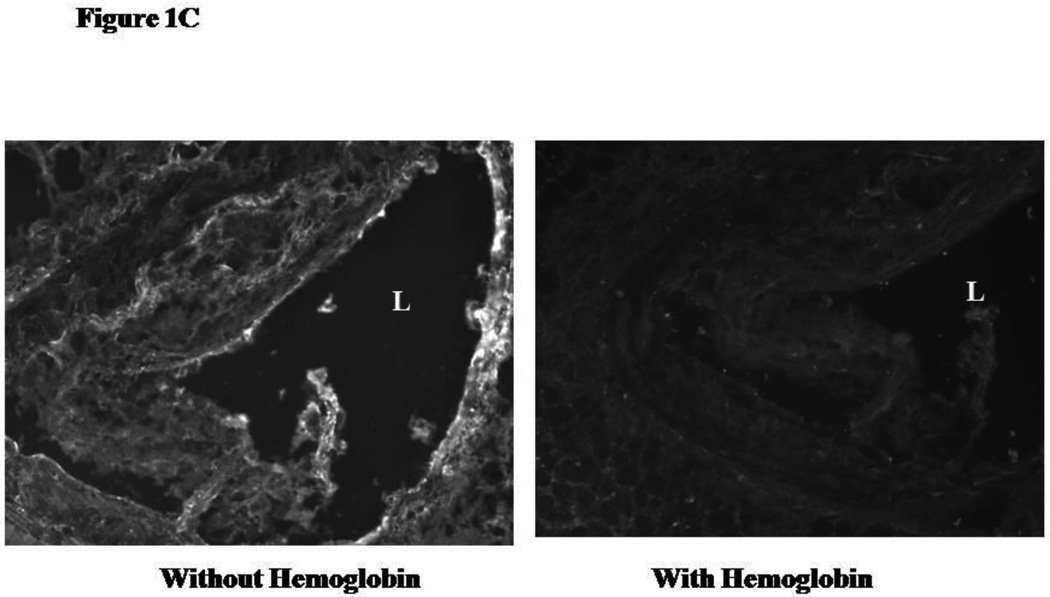
We have previously demonstrated that there is an increased amount of iron identified by Perl's stain in Hp 2-2 plaques.13 We sought to determine if this could be attributed to an increased amount of Hb in these plaques identified by immunofluorescence. The intensity of Hb staining in the plaques of Apo E−/− Hp 2-2 mice was markedly greater than that found in the plaques of ApoE−/− Hp 2-2 mice (Figure 1A and 1B). Specificity of the signal for Hb obtained in this immunofluorescent analysis was demonstrated by showing that an excess of Hb in the binding buffer could block the fluorescent signal obtained using rabbit antimouse Hb and antirabbit Cy3 antiserum (Figure 1C).
Figure 1.
A: Immunofluorescent identification of Hb in the atherosclerotic plaque of Hp 1-1 and Hp 2-2 ApoE−/− mice. Hb was identified with a rabbit antiserum to mouse Hb and a Cy3 conjugated antirabbit secondary antibody. L, artery lumen.
B: Quantitative image analysis showing that Hb is increased in Hp 2-2 atherosclerotic plaques. Fluorescent immunostaining of Hb in plaques was quantified by Image Pro-Plus software. Hb staining in plaques from Hp 2-2 ApoE−/− mice was significantly greater than in Hp 1-1 ApoE−/− mice when scored as the average intensity/total plaque area (n=6 for each group, P<0.02).
C: Specificity of immunostaining for Hb in the plaque. In order to demonstrate that the fluorescent signal obtained for Hb by immunostaining was specific for Hb, sections were incubated in the presence of an excess of Hb (4.7µg/µl) during the immunostaining. L, artery lumen.
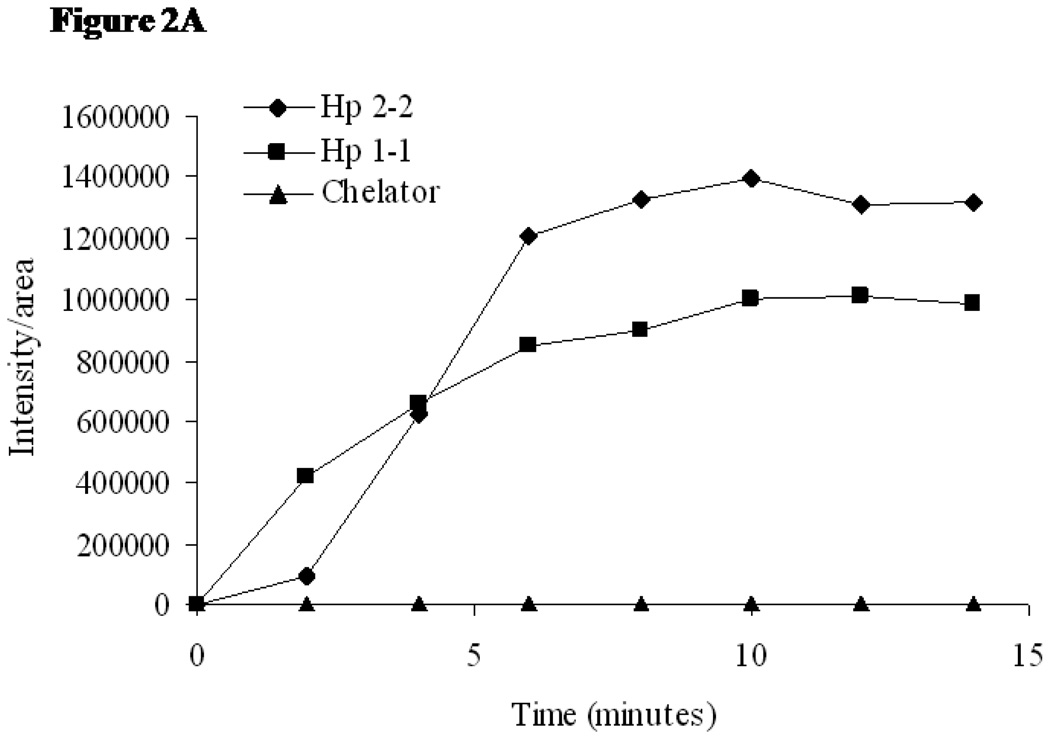
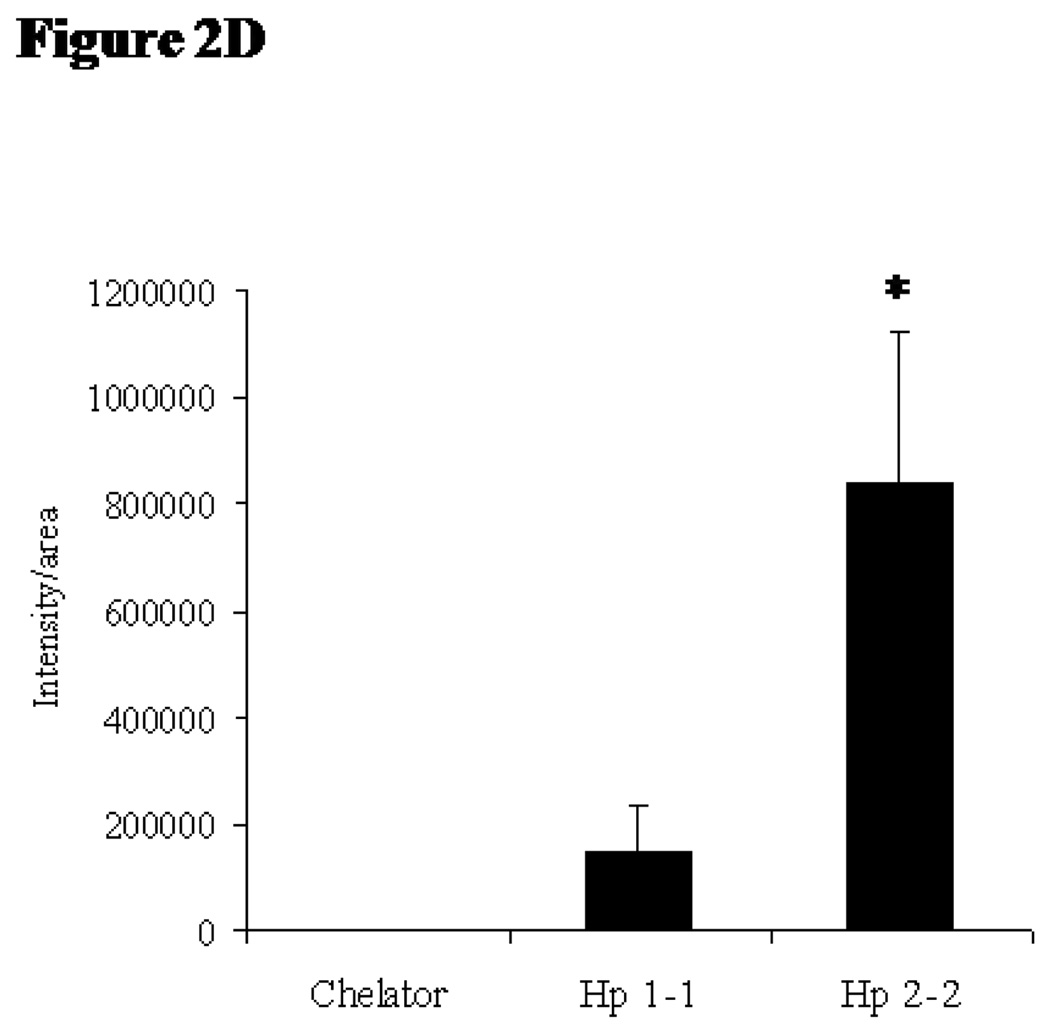
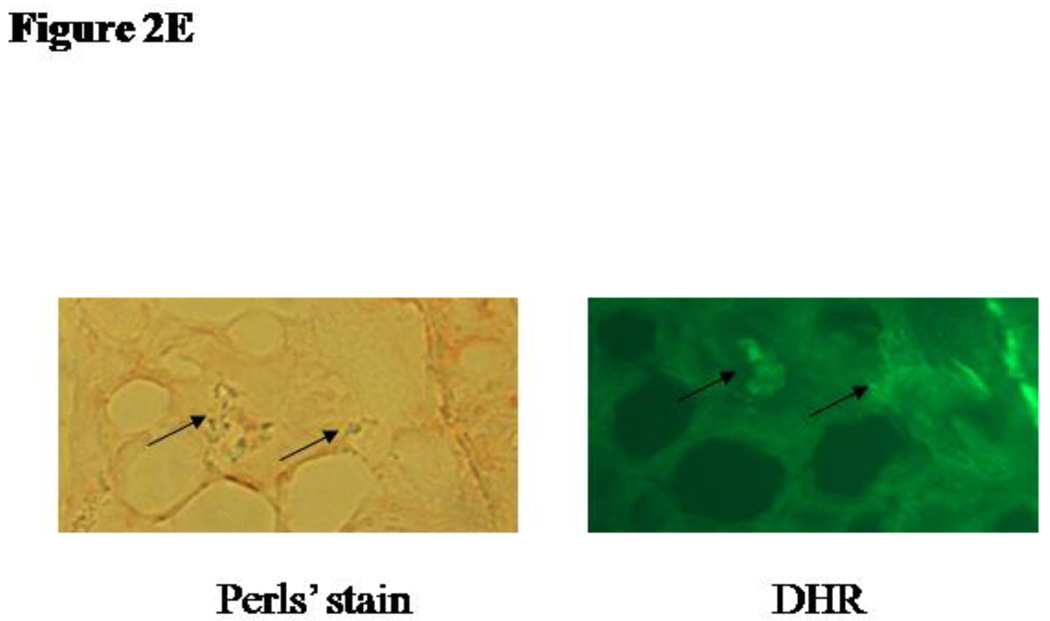
We then sought to determine if the increased oxidative stress in Hp 2-2 atherosclerotic plaques is due to iron. DHR becomes fluorescent when it becomes oxidized.14 We assessed oxidative stress in atherosclerotic plaques by monitoring the time dependent oxidation of DHR incubated with the plaques (Figure 2A). The time dependent increase in fluorescence was linear up to 10 minutes after the addition of the DHR to tissue thin sections containing the plaque and therefore all quantitative comparisions between groups were done at this 10 minute time point. We found markedly greater fluorescent activation in Hp 2-2 as compared to Hp 1-1 plaques and this oxidation was inhibited by iron chelation with desferroxamine (Figure 2B–D). Further supporting a direct role for iron induced oxidation of DHR we demonstrated colocalization of iron by Perl’s stain with DHR fluorescence (Figure 2E).
Figure 2.
A: Time course of DHR fluorescence signal intensity in atherosclerotic plaques of Hp 2-2 mice (■) and Hp 1-1 mice (●) incubated with DHR Thin sections of atherosclerotic plaques from Hp 1-1 or Hp 2-2 ApoE−/− mice were incubated with DHR and the time dependent increase in fluorescent signal intensity/plaque area determined using Image Pro-Plus software (n=6 per group). The oxidation of DHR was inhibited by incubation of the thin tissue section with 50mM of the iron chelator desferroxamine for 1 hour prior to applying the DHR (▲).
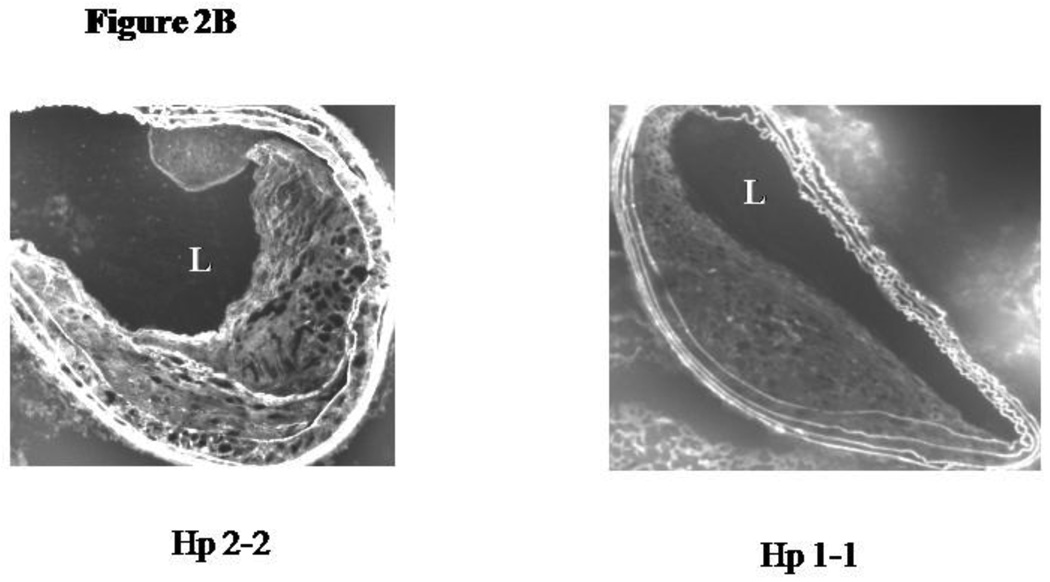
2B: DHR immunofluorescence of representative plaques from Hp 1-1 ApoE−/− and Hp 2-2 ApoE−/− mice incubated with DHR for 10 minutes. L, artery lumen.
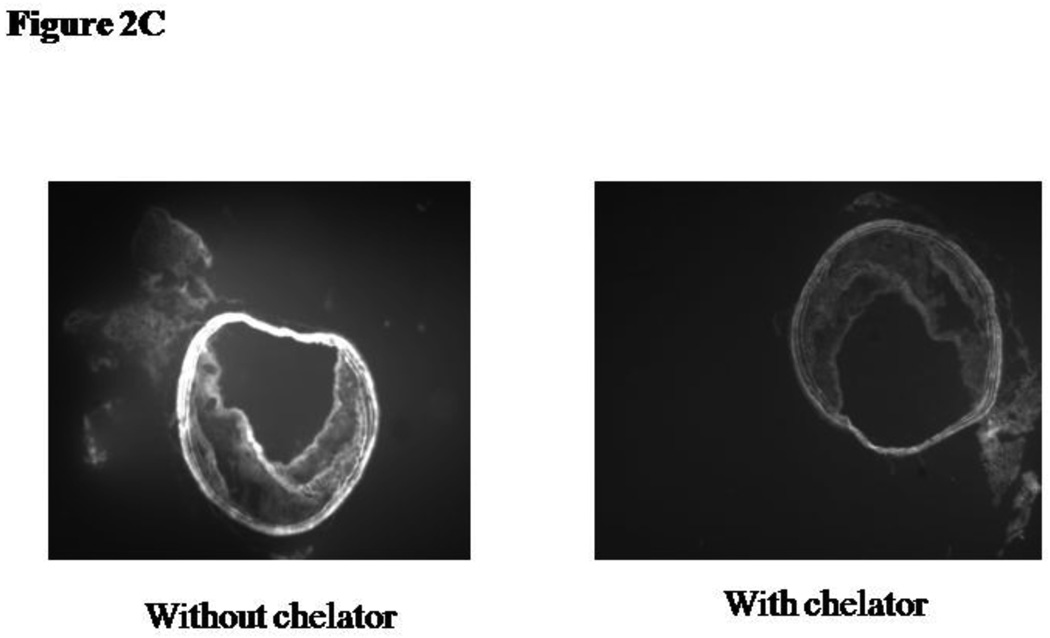
2C: Plaque induced oxidative activation of DHR fluorescence is blocked by the iron chelator desferroxamine. 50mM desferroxamine was added 1 hour prior to applying the DHR to the tissue slice.
2D: DHR fluorescent signal intensity is increased by Hp 2-2 Apo E−/− atherosclerotic plaques. Quantitation of fluorescent intensity 10 minutes after DHR was added to the thin sections using Image Pro-Plus software and scored as the average intensity/plaque area. DHR fluorescence at this 10 minute time point in plaques from Hp 2-2 ApoE−/− mice was significantly greater than in Hp 1-1 ApoE−/− mice (n=6 per group, P<0.05). Oxidation induced fluorescent activation of DHR was blocked by preincubation of the tissue slices with the iron chelator desferroxamine.
2E: Colocalization of DHR fluorescence and iron staining in the atherosclerotic plaque. Plaque iron was detected using Perl's stain and subsequently DHR was applied to the tissue section on the slide. Arrows indicate colocalization of Perl’s iron stain and DHR fluorescence in the same field.
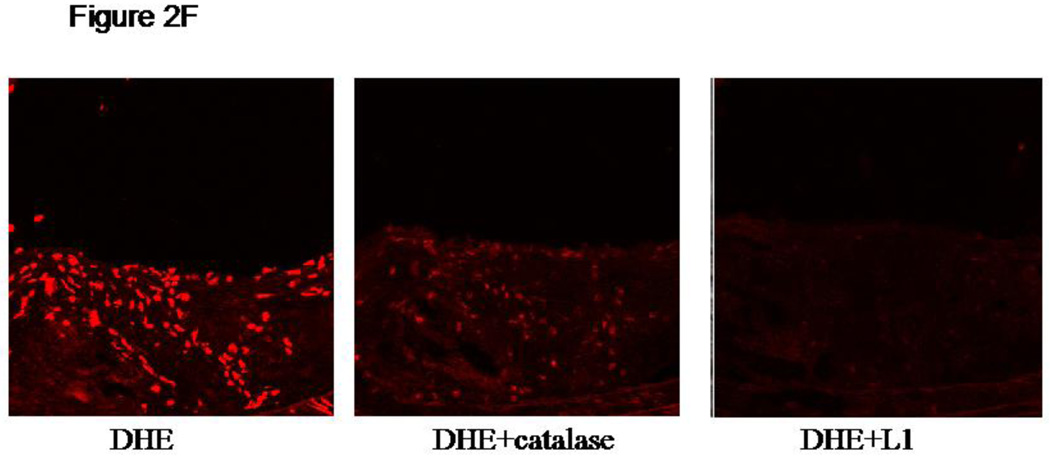
2F: Oxidative stress detected by DEA in Hp 2-2 plaques is blocked by iron chelation or by catalase. 5 micron frozen sections of brachiocephalic arteries from Hp 2-2 ApoE−/− mice were preincubated in phosphate buffered saline in the absence or presence of PEG-catalase (Sigma) 2500 U/ml or 50 mM L1 for one hour at 37C and then exposed to dihydroethidium (DEA, 5 uM) (Molecular Probes) in the dark for 1 hour at 37C. Slides were rinsed and examined in a Zeiss LSM 510 META Laser scanning confocal microscope (excitation wavelength 530 nm, emission wavelength 610 nm).
In order to confirm that the increased DHR fluorescence described above seen in Hp 2-2 plaques was indeed due to increased oxidative stress and that this increased oxidative stress was due to hemoglobin derived iron we performed additional analysis using an alternative probe (dihydrothidium (DEA)) to detect oxidative stress in the presence or absence of the iron chelator deferiprone (Figure 2F). Similar to our studies with DHR we found that iron chelation prevented oxidation induced DEA fluorescence. Furthermore, consistent with the proposed mechanism by which hemoglobin mediates oxidation via Fenton chemistry15, DEA oxidation was blocked by catalase (Figure 2F).
In this study we have demonstrated that there is an increase in the amount of redox active hemoglobin derived iron in the atherosclerotic plaque in Hp 2-2 mice. We have recently demonstrated the relevance of these studies to the human atherosclerotic plaque by showing that there is increased iron deposition in Hp 2-2 DM human atherosclerotic plaques.16 These results are therefore consistent with our previous in vitro mechanistic studies showing that both the protection against hemoglobin induced oxidation and the clearance mechanism for hemoglobin in the atherosclerotic plaque is Hp genotype dependent.11,12,14
The major source of Hb in the atherosclerotic plaque is from intraplaque hemorrhage.17 Intraplaque hemorrhage has been suggested to play an important role in determining plaque stability.17 These results are consistent with our hypothesis that the Hp genotype determines the response to intraplaque hemorrhage by determining if Hb released from red blood cells into the plaque will result in oxidative stress or inflammation.
In conclusion, these studies provide direct evidence that the increased oxidative stress which we have previously demonstrated in Hp 2-2 plaques13 is due to Hb derived iron. Agents which prevent iron induced oxidation may have considerable value in decreasing oxidation and inflammation in Hp 2-2 plaques thereby reducing the risk of plaque rupture and atherothrombosis in Hp 2-2 DM individuals.
Acknowledgements
This work was supported by grants from the US Israel Binational Science Foundation (to APL and PRM), the Israel Science Foundation (to APL), the Juvenile Diabetes Research Foundation (to APL), the Kennedy Leigh Charitable Trust (to APL) and the National Institutes of Health (NIH R01DK085226) (to APL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bowman BH, Kurosky A. Haptoglobin: the evolutionary product of duplication, unequal crossing over, and point mutation. Adv Hum Genet. 1982;12:189–261. doi: 10.1007/978-1-4615-8315-8_3. [DOI] [PubMed] [Google Scholar]
- 2.Levy AP, Hochberg I, Jablonski K, et al. Haptoglobin phenotype and the risk of cardiovascular disease in individuals with diabetes: The Strong Heart Study. J Am Coll Card. 2002;40:1984–1990. doi: 10.1016/s0735-1097(02)02534-2. [DOI] [PubMed] [Google Scholar]
- 3.Roguin A, Koch W, Kastrati A, Aronson D, Schomig A, Levy AP. Haptoglobin genotype is predictive of major adverse cardiac events in the one year period after PTCA in individuals with diabetes. Diabetes Care. 2003;26:2628–2631. doi: 10.2337/diacare.26.9.2628. [DOI] [PubMed] [Google Scholar]
- 4.Suleiman M, Aronson D, Asleh R, et al. Haptoglobin polymorphism predicts 30-day mortality and heart failure in patients with diabetes and acute myocardial infarction. Diabetes. 2005;19:2802–2806. doi: 10.2337/diabetes.54.9.2802. [DOI] [PubMed] [Google Scholar]
- 5.Milman U, Blum S, Shapira C, et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both Type 2 Diabetes Mellitus and the Haptoglobin 2-2 genotype: a prospective, double-blinded clinical trial. Art Thromb Vasc Biol. 2008;28:341–347. doi: 10.1161/ATVBAHA.107.153965. [DOI] [PubMed] [Google Scholar]
- 6.Costacou T, Ferrell RE, Orchard TJ. Haptoglobin genotype: a determinant of cardiovascular complication risk in Type I Diabetes. Diabetes. 2008;57:1702–1706. doi: 10.2337/db08-0095. [DOI] [PubMed] [Google Scholar]
- 7.Bamm VV, Tsemakhovich VA, Shaklai M, Shaklai N. Haptoglobin phenotypes differ in their ability to inhibit heme transfer from hemoglobin to LDL. Biochemistry. 2004;43:3899–3906. doi: 10.1021/bi0362626. [DOI] [PubMed] [Google Scholar]
- 8.Frank M, Lache O, Enav B, et al. Structure/function analysis of the anti-oxidant properties of haptoglobin. Blood. 2001;98:3693–3698. doi: 10.1182/blood.v98.13.3693. [DOI] [PubMed] [Google Scholar]
- 9.Asleh R, Miller-Lotan R, Aviram M, et al. Haptoglobin Genotype is a Regulator of Reverse Cholesterol Transport in Diabetes In Vitro and In Vivo. Circ Res. 2006;99:1419–1425. doi: 10.1161/01.RES.0000251741.65179.56. [DOI] [PubMed] [Google Scholar]
- 10.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the hemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 11.Asleh R, Marsh S, Shiltruck M, et al. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res. 2003;92:1193–1200. doi: 10.1161/01.RES.0000076889.23082.F1. [DOI] [PubMed] [Google Scholar]
- 12.Levy AP, Purosothaman KR, Levy NS, et al. Downregulation of the hemoglobin scavenger receptor in individuals with diabetes and the Hp 2-2 genotype: implications for the response to intraplaque hemorrhage and plaque vulnerability. Circ Res. 2007;101:106–110. doi: 10.1161/CIRCRESAHA.107.149435. [DOI] [PubMed] [Google Scholar]
- 13.Levy AP, Levy JE, Kalet-Litman S, et al. Haptoglobin genotype is a determinant of iron, lipid peroxidation and macrophage accumulation in the atherosclerotic plaque. Art Thromb Vasc Biol. 2007;27:134–140. doi: 10.1161/01.ATV.0000251020.24399.a2. [DOI] [PubMed] [Google Scholar]
- 14.Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP. Haptoglobin genotype and diabetes dependent differences in iron mediated oxidative stress in vitro and in vivo. Circ Res. 2005;96:435–441. doi: 10.1161/01.RES.0000156653.05853.b9. [DOI] [PubMed] [Google Scholar]
- 15.Van Dyke BR, Saltman P. Hemoglobin: a mechanism for the generation of hydroxyl radicals. Free Rad Biol Med. 1996;20:985–989. doi: 10.1016/0891-5849(95)02186-8. [DOI] [PubMed] [Google Scholar]
- 16.Moreno PM, Purosothaman KR, Levy NS, et al. Haptoglobin genotype is a major determinant of iron in the human atherosclerotic plaque. J Am Coll Card. 2008;52:1049–1051. doi: 10.1016/j.jacc.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Levy AP, Moreno PR. Intraplaque hemorrhage. Curr Mol Med. 2006;6:479–488. doi: 10.2174/156652406778018626. [DOI] [PubMed] [Google Scholar]