Abstract
Objective
This study was designed to evaluate the associated risk of RLS with pregnancy in relation to family history and age of symptom onset of RLS.
Methods and subjects
Data from a prior RLS family history study in which 1019 subjects (527 males, 492 females) were interviewed provided a diagnosis and characterization of RLS and determination of pregnancy status on which the current study analysis was undertaken.
Results
In the family members of RLS probands, the prevalence of RLS was significantly higher for parous women than for nulliparous women (49.5% vs. 33.7%, OR = 1.92, 95% CI = 1.16-3.19) or for men (49.5% vs. 30.0%, OR 2.29, 1.69-3.10), but no different for nulliparous women compared to men (33.7% vs. 30.0%, OR 2.29, 1.69-3.10). When only those whose RLS started at or after age 30 was considered, similar differences occurred. These differences were not observed among family members of control probands.
Conclusions
These data indicate pregnancy has a major impact on the risk of developing RLS for those with a family history of RLS. This pregnancy effect appears to account totally for the gender differences often reported in overall RLS prevalence data.
Keywords: Pregnancy, RLS, Restless Legs Syndrome, Familial RLS, Prevalence
Introduction
In most epidemiological studies, restless legs syndrome (RLS) has been reported to be about 1.5 to 2 times as common for women as for men (1-3). This, however, appears to only apply to adults over 30. Moderately severe RLS is only slightly more common for females in adult population under 30 (1) and there is no gender difference in prevalence for pediatric RLS (4). It had been suggested that these gender differences relate to either iron or hormonal differences that have been considered to possibly cause or exacerbate RLS (5), but both hormonal and iron differences are fully present in adolescence when there is no gender difference for RLS (4). Thus, neither of these factors explains the interaction of gender difference with age. Berger et al. (6) reported data from a large epidemiological study showing nulliparous women had the same incidence of RLS as men. The gender difference may relate only to pregnancy and this would, of course, explain the increase in the gender effect with age. If this is the case, it would identify pregnancy as a major environmental factor for RLS risk and thus deserve further study. Furthermore, an environmental risk factor would seem more likely to lead to expression of RLS when there is an increased genetic risk such as is assumed for familial RLS. In the Berger et al. study (6), there was no separation of the patients by family history.
Data from a large RLS family history study, which included information on RLS diagnosis, RLS family history and pregnancy, were available for analysis. Our goals in this study were to replicate the results of Berger et al. (6) in regard to the impact of pregnancy on prevalence of RLS for familial RLS. We hypothesized that for family members of an RLS patient, pregnancy would increase the risk of RLS and nulliparous women would have the same risk as males.
Methods
Data from a family history study of RLS, conducted at Johns Hopkins and described in more detail elsewhere (7, 8), were the primary source material for this study. The population included in this study was only family members of identified probands. RLS case probands were recruited from sequential patients presenting to the Neurology and Sleep clinics of the Hopkins Bayview Medical Center. Control probands were non-patients screened to be free of RLS and to have low PLMS rates, i.e., PLMS/h < 15 if they were less than 80 years old and <25 if over 80. This was determined as the maximum of 3 nights of recording using the PAM-RL, a device that has been validated for use with RLS patients (9). All first degree family members of all probands and second degree family members of RLS case probands who were available were interviewed by a single expert diagnostician (WAH) using the validated Hopkins diagnostic telephone interview (HDTI) (8). This interviewer was blinded both to the diagnosis of the family member's proband and to the relationship between the interviewee and the proband. All probands signed a written consent approved by the Johns Hopkins Institutional Review Board and provided contact information for their families. Family members consented over the phone at the time of the interview.
The HDTI includes a series of specific diagnostic questions derived from the diagnostic features of RLS (10). In addition, it includes a number of questions about sleep and pregnancy (when appropriate), as well as situations in which RLS symptoms are likely to occur. The HTDI supports making the diagnoses of definite, probable, possible RLS or not RLS. Both definite and probable RLS require that the subject explicitly meets all 4 diagnostic criteria for RLS (7, 8). Definite RLS further requires that the subject satisfy all criteria for a “typical case”; this includes symptoms while lying down as well as sitting. The minimum frequency of symptoms for diagnosis of RLS was set at 20 lifetime events, or events at least once a month for three consecutive months in order to detect acute secondary RLS as with pregnancy. In the current analysis, we have classified as RLS both those diagnosed with probable and those with definite RLS. Those with possible RLS were excluded as “unknowns.” All subjects with definite secondary RLS, including women who had RLS only during pregnancy, were also excluded from the analysis.
In these analyses, we examined all family members who provided information on the number of pregnancies and all male family members. We stratified the sample by proband type (RLS patient vs. control), age at interview, and family member diagnosis (definite and probable vs. not RLS). The age groups were formed based on the strata defined by Berger et al. (6). Statistics were performed by odds ratios, presented with 95% confidence limits, to assess the relative risks of RLS associated with pregnancy and by t-tests to evaluate differences between nulliparous and parous women.
We specifically hypothesized that 1) pregnancy would increase the risk of RLS in family members of RLS probands, and 2) nulliparous women who were family members of RLS probands would have the same risk for RLS as men. Finally, we explored the effects of number of pregnancies, age and limiting the population to exclude RLS onset before age 30. We also examined the same data for a small set of family members from control probands.
Results
There were 527 men interviewed and 492 women interviewed who had pregnancy information available. The mean (± sd) age was 50.2 (± 15.9). General population characteristics for the RLS and control probands are shown in Table 1.
Table 1.
Population Characteristics
| RLS Proband | ||||
|---|---|---|---|---|
| Overall | Not RLS | RLS | ||
| Sample Size | Total | 830 | 516 | 314 |
| Men | 430 | 301 | 129 | |
| Women | 400 | 215 | 185 | |
| Average Age | Total | 49.7±16.5 | 48.3±16.8 | 50.2±15.9 |
| Men | 49.0±16.3 | 49.5±17.0 | 47.5±14.5 | |
| Women | 51.3±16.7 | 50.1±16.5 | 52.6 sd±16.8 | |
| Percent Female | 48.2% | 41.7% | 58.9% | |
| Control Proband | ||||
| Overall | Not RLS | RLS | ||
| Sample Size | Total | 189 | 167 | 22 |
| Men | 97 | 87 | 10 | |
| Women | 92 | 80 | 12 | |
| Average Age | Total | 52.2±17.1 | 49.7±16.8 | 53.2±19.4 |
| Men | 50.9±16.5 | 51.4±16.0 | 51.2±22.2 | |
| Women | 48.0±16.6 | 45.9±17.7 | 47.1±10.7 | |
| Percent Female | 48.7% | 47.9% | 54.5% | |
We found the overall rate of RLS to be 32.9%, but this high rate occurred only for family members of RLS probands (37.8%). The rate for family members of control probands (11.6%) was close to that for the general population data for RLS at any frequency (1).
Testing our first hypothesis, we found that for family members of RLS probands the prevalence of RLS was higher for parous than nulliparous women (49.5% vs. 33.7%, OR = 1.92, 95% CI = 1.16-3.19). Table 2 shows the prevalence of RLS in relation to age (stratified into under 40, between 40 and 59, and 60 and over groups), gender and pregnancy status for these subjects. The prevalence was higher for parous than nulliparous women for each of the separate age groups, but the differences in each age group were not statistically significant.
Table 2.
RLS Prevalence of Family Members of RLS Probands By Age
| All Ages | <40 | 40-59 | 60+ | |
|---|---|---|---|---|
| Men | 30% (129/430) | 30.7% (42/137) | 31% (58/187) | 27.4% (29/106) |
| All Women | 46.2% (185/400) | 40.4% (44/109) | 44.9%(79/176) | 53.9% (62/115) |
| Nulliparous | 33.7% (28/83) | 34.8% (16/46) | 33.3% (9/27) | 30% (3/10) |
| Parous | 49.5% (157/317) | 44.4% (28/63) | 46.9% (70/149) | 56.2% (59/105) |
| OR | 1.92 (1.16-3.19) | 1.5 (0.68-3.29) | 1.77 (0.75-4.19) | 2.99 (0.73-12.21) |
Testing our second hypothesis, we found that the prevalence of RLS for family members of RLS probands were essentially the same for nulliparous women and men (33.7 % vs. 30.0%, OR 1.19, 0.72-1.96) but significantly greater for parous women than men (49.5% vs. 30.0%, OR 2.29, 1.69-3.10). Men and nulliparous women within each of the age groups had consistently lower rates of RLS than parous women (see Figure 1). We found no statistically significant differences in the RLS prevalence related to number of pregnancies beyond the first (see Figure 2).
Chart 1.
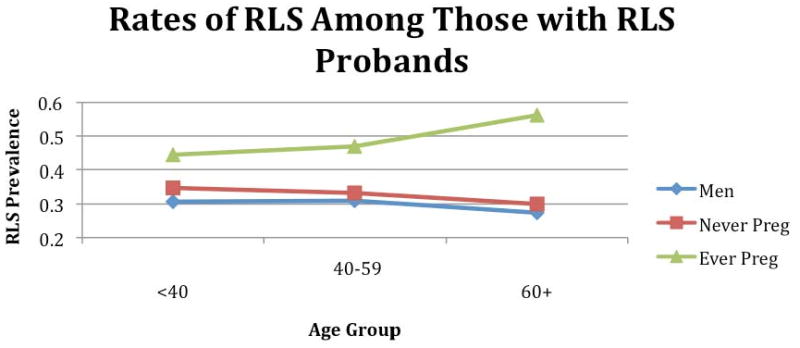
RLS prevalence for those with RLS probands based on sex, age, parous status
Chart 2.
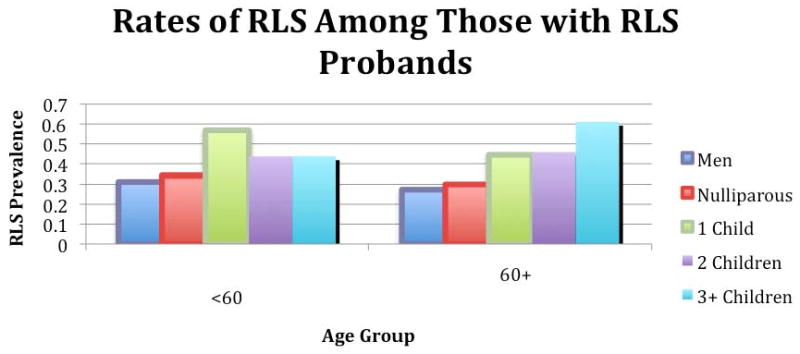
RLS prevalence for those with RLS probands based on sex, age (over/under 60), and number of pregnancies
The age at which RLS symptoms first occurred was significantly later for parous than for nulliparous family members of RLS probands (mean ± sd: 34.3±16.4 and 26.6±11.7, t=2.62, p=0.013). The family members of RLS probands whose RLS symptoms started at age 30 or older showed a significantly increased prevalence of RLS for parous compared to nulliparous women (28.7% vs. 16.4% respectively, OR 2.05, 1.06 – 3.98). The prevalence of RLS for male family members of RLS probands ages 30 or older was 24.0%, which fell between the values for parous (24% vs 28.7%, OR 1.28, 0.91- 1.79) and nulliparous women (24% vs. 16.4%, OR 0.62, 0.32-1.21) and was not significantly different for either comparison.
Limiting the sample to women who reported no RLS symptoms during pregnancy reduced our sample size to 221 parous women and 83 nulliparous women. The difference in rates between parous and nulliparous women were no longer significant (38.0% vs. 33.7%, OR 1.204, 0.71-2.05). However, in this sample, men still had significantly lower rates than parous women (30.0% vs. 38.0%, OR 0.70, 0.50-0.98) and did not differ significantly from nulliparous women (30.0% vs. 33.7%, OR 0.84, 0.51-1.39).
RLS prevalence for family members of control probands was not significantly greater for females than males [13.0% (12/92) vs. 10.3% (10/97), OR 1.31, 0.53 – 3.19]. Of all the parous women within this group 12.9% (11/85) had RLS, compared to 14.3% (1/7) among nulliparous women (OR of 0.89 and a 95% CI of 0.1-8.13). There was also no significant difference in prevalence between parous women and males. The control proband sample size was too small to be stratified into age groups.
Discussion
We confirmed both of our primary hypotheses. In this sample of family members of RLS probands, the parous women had significantly greater prevalence of RLS then either nulliparous women or men. In contrast, nulliparous women had about the same RLS prevalence as men. These results in general confirm the findings by Berger et al. (6), except they indicate that the pregnancy risk factor may be limited to women with a family history of RLS. It was particularly striking that for the family members of control probands the prevalence of RLS was not significantly greater for females than males. This would be consistent with the concept that the environmental factor has more impact when there is greater genetic susceptibility, supporting the expression of the disease. With a weakened genetic contribution, the pregnancy effect could have less effect and the RLS female predominance should decrease. Thus, in this study the female:male prevalence ratio was 1.54 for family members of RLS probands compared to 1.26 for family members of control probands. It should be noted, however, that our control probands were selected not only to have no RLS symptoms but also to have minimal PLM. Selecting a population with few PLMS would likely also select a population with less of the risk alleles for RLS (11). The risk alleles could still be present even in these control probands and also from other progenitors in control families not screened for PLM or RLS. Nonetheless, the probability of significant genetic factors contributing to RLS will be somewhat reduced for family members of control probands. Thus, even those with RLS are less likely to have the same degree of genetic contribution to their RLS if they are family members of a control proband rather than an RLS proband. This may account in part for the reduced pregnancy effect in this population. It seems likely that there remains some pregnancy effect for RLS from families with reduced PLMS but the effect size would require a much larger sample size than in this study.
We also failed to find any difference in RLS prevalence related to the number of pregnancies more than one. This, however, may also be a problem of sample size, but clearly any effect with increased number of pregnancies is much smaller than that from having just one pregnancy.
The obvious major question is whether or not the increased incidence of RLS in parous women occurred because the RLS started in pregnancy and then continued thereafter. Since we do not have the women's ages when they became pregnant we cannot directly answer this question, but it seems unlikely for several reasons. First, the age of onset of RLS was significantly older for parous than nulliparous women. Second, the pregnancy effect was clearly present even when considering only RLS that started after age 30. The average age for first pregnancy is about 25 and most pregnancies occur before age 30 (12). Third, and most significantly, when the data set were limited only to those women who denied RLS symptoms during their pregnancies some significant findings remained despite the smaller sample size: a significant difference in prevalence between parous women and men but not between nulliparous women and men. The RLS prevalence was higher for parous than nulliparous women, but this difference was no longer statistically significant.
Thus, overall the findings suggest that child bearing induces some long lasting effects leading to development of RLS that may occur several years after pregnancy. Two obvious factors to consider would be the large iron or hormonal changes during pregnancy. Either or both of these could produce some lasting biological changes relevant to the development of RLS.
The loss of significance between RLS rates of nulliparous and parous women when excluding those who displayed RLS symptoms during pregnancy can be attributed to a decrease in sample size. Despite this decrease, the results comparing men with both nulliparous and parous women are consistent with the primary results.
One recent study noted that childhood restless sleep was related to increased risk of RLS in adulthood (13) and, like pregnancy, it has been associated with iron deficiency (14). Taken together, these findings of an increased risk of RLS with childhood restless sleep and pregnancy occurring years after these events could be seen as indicating that any major disruption of the normal iron balance increases the risk of RLS later in life for those with genetic susceptibility to RLS.
Since pregnancy is such a large factor contributing to the expression of RLS, parous and nulliparous RLS women should probably be evaluated as potentially separate RLS phenotypes. Future studies should look at their RLS characteristics compared to each other and to men.
Conclusion
These data support the findings by Berger et al. (6), i.e., pregnancy is a major non-genetic factor increasing the prevalence of RLS, and that this effect clearly occurs for familial RLS, but, contrary to the findings by Berger et al. (6), does not appear to get much more pronounced, if at all, with more pregnancies. The effect also occurs later in life years after the pregnancy. This suggests that one pregnancy alone is enough to create a long-lasting alteration in some biological factors in the body that later lead to RLS.
Table 3.
Risk of Developing RLS Compared to Men, RLS Probands Only
| RLS Probands | OR (95% CI) | |
|---|---|---|
| Men | 1.00 (reference) | |
| All Ages | Preg Women | 2.29 (1.69-3.10) |
| Non-Preg Women | 1.19 (0.72-1.96) | |
| Men | 1.00 (reference) | |
| <40 | Preg Women | 1.81 (0.98-3.35) |
| Non-Preg Women | 1.21 (0.59-2.45) | |
| Men | 1.00 (reference) | |
| 40-59 | Preg Women | 1.97 (1.26-3.08) |
| Non-Preg Women | 1.11 (0.47-2.62) | |
| Men | 1.00 (reference) | |
| 60+ | Preg Women | 3.41 (1.92-6.05) |
| Non-Preg Women | 1.14 (0.28-4.70) | |
| Men | 1.00 (reference) | |
| <60 | Preg Women | 1.93 (1.35-2.76) |
| Non-Preg Women | 0.77 (0.46-1.28) | |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen RP, Walters AS, Montplaisir J, Hening W, Myers A, Bell TJ, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005 Jun 13;165(11):1286–92. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 2.Bjorvatn B, Leissner L, Ulfberg J, Gyring J, Karlsborg M, Regeur L, et al. Prevalence, severity and risk factors of restless legs syndrome in the general adult population in two Scandinavian countries. Sleep Med. 2005 Jul;6(4):307–12. doi: 10.1016/j.sleep.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Tison F, Crochard A, Leger D, Bouee S, Lainey E, El Hasnaoui A. Epidemiology of restless legs syndrome in French adults: a nationwide survey: the INSTANT Study. Neurology. 2005 Jul 26;65(2):239–46. doi: 10.1212/01.wnl.0000168910.48309.4a. [DOI] [PubMed] [Google Scholar]
- 4.Picchietti D, Allen RP, Walters AS, Davidson JE, Myers A, Ferini-Strambi L. Restless legs syndrome: prevalence and impact in children and adolescents--the Peds REST study. Pediatrics. 2007 Aug;120(2):253–66. doi: 10.1542/peds.2006-2767. [DOI] [PubMed] [Google Scholar]
- 5.Manconi M, Govoni V, De Vito A, Tiberio Economou N, Cesnik E, Mollica G, et al. Pregnancy as a risk factor for restless legs syndrome. Sleep Medicine. 2004;5(3):305–8. doi: 10.1016/j.sleep.2004.01.013. 2004/5. [DOI] [PubMed] [Google Scholar]
- 6.Berger K, Luedemann J, Trenkwalder C, John U, Kessler C. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med. 2004 Jan 26;164(2):196–202. doi: 10.1001/archinte.164.2.196. [DOI] [PubMed] [Google Scholar]
- 7.Mathias RA, Hening W, Washburn M, Allen RP, Lesage S, Wilson AF, et al. Segregation analysis of restless legs syndrome: possible evidence for a major gene in a family study using blinded diagnoses. Hum Hered. 2006;62(3):157–64. doi: 10.1159/000096443. [DOI] [PubMed] [Google Scholar]
- 8.Hening WA, Allen RP, Washburn M, Lesage S, Earley CJ. Validation of the Hopkins telephone diagnostic interview for restless legs syndrome. Sleep Med. 2008 Jul 16;9(3):283–9. doi: 10.1016/j.sleep.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Sforza E, Johannes M, Claudio B. The PAM-RL ambulatory device for detection of periodic leg movements: a validation study. Sleep Med. 2005 Sep;6(5):407–13. doi: 10.1016/j.sleep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003 Mar;4(2):101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 11.Stefansson H, Rye DB, Hicks A, Petursson H, Ingason A, Thorgeirsson TE, et al. A Genetic Risk Factor for Periodic Limb Movements in Sleep. N Engl J Med. 2007 Jul 18; doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- 12.Martin J, Hamilton B, Sutton P, Ventura S, Menacker G, Kimeyer S, et al. Births: Final Data for 2005. National Vital Statistics Reports. 2007 December 5;56(6):1–104. 2007. [PubMed] [Google Scholar]
- 13.Gamaldo CE, Benbrook AR, Allen RP, Scott JA, Henning WA, Earley CJ. Childhood and adult factors associated with restless legs syndrome (RLS) diagnosis. Sleep Med. 2007 Nov;8(78):716–22. doi: 10.1016/j.sleep.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Simakajornboon N, Gozal D, Vlasic V, Mack C, Sharon D, McGinley BM. Periodic limb movements in sleep and iron status in children. Sleep. 2003 Sep 15;26(6):735–8. doi: 10.1093/sleep/26.6.735. [DOI] [PubMed] [Google Scholar]


