Abstract
Background
Mean maximum carotid intima-media thickness (CIMT) is associated with both coronary artery disease and cerebral thromboembolism. Thoracic aortic calcification (TAC) detected by computed tomography (CT) is also highly associated with vascular disease and cardiovascular risk. No previous study has examined the relationship between CIMT and TAC in a large patient cohort. We performed a cross-sectional study to determine whether, at baseline, there is a relationship between CIMT and CT-determined TAC score.
Methods
In the Multi-Ethnic Study of Atherosclerosis, the study cohort included a population based sample of four ethnic groups (Chinese, White, Hispanic and African-American) of 6814 women and men ages 45-84 years. After exclusion of 198 persons due to incomplete information, we compared results of 6616 participants with both CIMT and TAC. TAC was measured from the lower edge of the pulmonary artery bifurcation to the cardiac apex. CIMT at the common carotid artery site was represented as the mean maximal CIMT of the right and left near and far walls, respectively. Multivariable relative risk regression analysis was used to evaluate relationships between TAC and CIMT.
Results
The prevalence of TAC was 28% (n=1846) and the mean maximum (±SD) CIMT was 0.87±0.19 mm. A higher prevalence of TAC was noted across increasing CIMT quartiles (1st: 12%, 2nd: 21%, 3rd: 30%, 4th: 49%, P<0.0001). One standard deviation increase in CIMT was associated with a 16% higher likelihood for presence of TAC after adjusting for demographics and cardiovascular disease (CVD) risk factors (95% CI: 1.12-1.26). In addition, individuals with CIMT in the highest quartile, as compared to those with CIMT in the first quartile, had a 76% higher likelihood for presence of TAC (prevalence ratio [PR]: 1.76, 95% CI: 1.37-2.26). In race-ethnic stratified analyses, similar associations were seen in all groups. Among those with TAC>0, a higher CIMT was significantly associated with continuous TAC scores (log transformed) in the overall population as well as among all ethnic-racial groups.
Conclusions
Our study demonstrates that TAC is associated with increasing severity of carotid atherosclerotic burden as measured by CIMT. The combined utility of these two noninvasive measures of subclinical atherosclerosis for CVD risk assessment needs to be determined in future studies.
Keywords: Atherosclerosis, carotid IMT, aortic calcification, ethnic, cardiac CT
Introduction
Numerous studies have reported on the correlations between atherosclerosis in the coronary and extra-coronary vascular beds.1,2 However, no previous study has examined the relationship between extra-coronary calcification and carotid intima-media thickness (CIMT) as measured by B-mode ultrasonography which has been widely used to evaluate cardiovascular risk, and many studies have shown a close relationship between carotid atherosclerosis and coronary artery calcification (CAC)3,4,5,6,7,8. Calcific aortic disease in the thoracic aorta is common in the elderly, and population based prospective studies have shown that patients with thoracic aortic calcification (TAC) have significantly higher cardiovascular disease (CVD) events and stroke9,10,11,12. Allison et al in a moderate-sized cohort using whole-body electron beam tomography (EBT) showed that the incidence and progression of calcification in various vascular beds differed13. Aortic involvement rapidly increased by age 60,6,14 while carotid involvement showed the slowest increases among the various arterial beds. Moreover, studies evaluating coronary artery calcium (CAC) and CIMT are only moderately correlated, with a stronger relationship of CVD events using CAC15,16,17. Kallikazaros et al, using a small cohort of patients without a history of atherosclerotic CVD, demonstrated that these individuals had a high prevalence of both aortic and carotid plaques18. In the current study, we compared carotid atheroma and aortic calcification using CIMT and TAC, respectively, in a multi-ethnic population.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) was initiated in July 2000 to investigate the prevalence, correlates and progression of subclinical cardiovascular disease in individuals without known CVD19. This prospective cohort study includes 6,814 women and men ages 45-84 years old recruited from six U.S. communities (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; northern Manhattan, NY; and St. Paul, MN). There are 38% White (N=2624), 28% African American (N=1895), 22% Hispanic (N=1492), and 12% Chinese (N=803) individuals. All participants underwent both non-enhanced cardiac CT for evaluation of CAC and carotid ultrasound. All participants provided written informed consent. An ancillary study, supported by the National Institutes of Health, was performed to measure aortic and valvular calcification on the CAC scans obtained for the MESA study. The study was approved by the Institutional Review Board of all participating institutions. Of 6,814 participants, 198 were excluded due to incomplete CIMT (N=197) or TAC (N=1) data.
Baseline medical history, anthropometric measurements, and laboratory data for the present study were taken from the first examination of the MESA cohort (July 2000 to August 2002). These self-administered questionnaires were available in English, Spanish, and Chinese. Information about age, gender, ethnicity, and medical history were obtained by questionnaires. Resting blood pressure was measured three times in the seated position, and the average of the 2nd and 3rd readings was recorded. Hypertension was defined as a systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or use of baseline blood pressure lowering medication. Use of antihypertensive and other medications were based on clinic staff entry of prescribed medications. Body mass index was calculated from the equation weight (kg)/ height (m2). Total and high density lipoprotein (HDL-C) were measured from blood samples obtained after a 12-hour fast. Low density lipoprotein (LDL-C) was estimated by the Friedewald equation with triglycerides measured in fasting state18. Current smoking was defined as having smoked a cigarette in the last 30 days.
Diabetes mellitus was defined as a fasting glucose ≥126 mg/dL or use of hypoglycemic medications. C reactive protein (CRP) was measured using the BNII nephelometer (N High Sensitivity CRP; Dade Behring Inc., Deerfield, IL) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT). Analytical intra-assay CVs ranged from 2.3 – 4.4% and inter-assay CVs ranged from 2.1 – 5.7%.
Thoracic aortic calcification assessment
All participants underwent two consecutive CT scans at baseline. Three sites used an Imatron C-150XL CT scanner (GE-Imatron, San Francisco, CA), and three sites used a multidetector CT scanner (four slice). The method has been reported previously20. Image slices were obtained with the participant supine, with no couch angulation. A minimum of 35 contiguous images with a 2.5- or 3-mm slice thickness was obtained, starting above the left main coronary artery to the bottom of both ventricles. Each scan was obtained in a single breath hold. Section thickness of 3 mm, field of view of 35 cm, and matrix of 512 × 512 were used to reconstruct raw image data. The nominal section thickness was 3.0 mm for electron beam CT and 2.5 mm for four-detector row CT. Spatial resolution can be described by the smallest volume element, or voxel, for the protocol for each system: 1.15 mm3 for four-detector row CT (0.68 × 0.68 × 2.50 mm) and 1.38 mm3 for electron beam CT (0.68 × 0.68 × 3.00 mm). Thoracic aortic wall calcification (TAC) in the segment of the ascending and/or descending thoracic aorta adjacent to the heart (imaged on every study of coronary calcium) was scored by using the same lesion definition. The sum of all TAC was reported for each study. This region of the thoracic aorta was included on the images of every study of coronary calcium and was quantified by using the same lesion definition (threshold and minimum lesion size) as used for coronary calcification.21 The absence of TAC was assigned a score of 0.
Carotid intima-media thickness assessment
In concordance with the consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force, we used CIMT of the common carotid artery22. Trained technicians in each field center performed B-mode ultrasonography of the right and left near and far walls of the common carotid artery. They used the Logiq 700 ultrasound device (General Electric Medical Systems, Waukesha, WI) to record images. An ultrasound reading center (Department of Radiology, Tufts-New England Medical Center, Boston, Massachusetts) measured maximal CIMT of the common carotid site as the mean of the maximum CIMT of the near and far walls of the right and left sides.
Statistical Methods
CIMT was categorized into quartiles for analyses. TAC was dichotomized as present (Agatston score > 0) or absent (Agatston score =0). Distributions of demographics and cardiovascular risk factors were compared across these groups. A simple approach to separate the qualitative difference between absence and presence of TAC from the quantitative effect of TAC was used. TAC was modeled as a dichotomous variable (TAC=0 versus TAC>0) and as a continuous variable (Ln(TAC)) among those with detectable TAC. Because the prevalence of TAC in our cohort is greater than 10%, odds ratios (ORs) overestimate the prevalence ratio (PR). Therefore, PR estimates are presented from the regression model y=exp(XTβ). The exponentiated parameters β are interpreted as relative risks. We assumed Gaussian error and used robust standard error estimates. Among individuals with detectable TAC, the relation of increasing CIMT and continuous TAC (log transformed), robust linear regression was employed. Covariates entered into the regression model included demographics (age, gender, race and site), cardiovascular risk factors (BMI, HDL, LDL, lipid lowering medication, smoking, hypertension, diabetes mellitus, family history of heart attack and cholesterol lowering medications) and a marker of inflammation (CRP) in a hierarchal fashion. Two-way interactions between CIMT and race with TAC as the outcome were examined. Statistical analyses were performed with STATA 10.0 for Windows (Stata Co, College Station, TX).
Results
Overall there were 6617 participants with completed CIMT measurements. TAC could not be assessed in one participant due to the presence of an atherosclerotic aneurysm in the descending thoracic aorta, yielding 6616 remaining participants (3129 men and 3478 women, mean age 61+/-10). The prevalence of TAC was 28% (n=1846). The mean maximum CIMT in the study population was 0.87±0.19 mm.
Table 1 demonstrates the baseline characteristics of the cohort according to the ethnic groups. Overall Hispanics were slightly younger among the 4 ethnic groups whereas no gender differences were noted. As far as risk factors are concerned, Chinese were least likely to be current smoker, hypertensive, had a lower LDL and BMI; whereas African Americans tend to be have a higher BMI and hypertension. Diabetes was more prevalent among both African Americans and Hispanics.
Table 1. Baseline Characteristics of Study Population Across Ethnic/Racial Groups.
| Variable | Whites | Chinese | African American | Hispanics | P value |
|---|---|---|---|---|---|
| Age (years) | 63±10 | 62±10 | 62±10 | 61±10 | 0.0012 |
| Gender (males) | 48% | 49% | 45% | 48% | 0.081 |
| Current smoker | 11% | 6% | 18% | 14% | <0.0001 |
| Hypertension | 38% | 37% | 59% | 41% | <0.0001 |
| Diabetes Mellitus | 7% | 15% | 19% | 19% | <0.0001 |
| Family history of heart attack | 51% | 20% | 42% | 41% | <0.0001 |
| LDL-C (mg/dl) | 117±30 | 115±28 | 117±33 | 120±33 | <0.0001 |
| HDL-C (mg/dl) | 52±16 | 50±13 | 52±15 | 47±13 | <0.0001 |
| BMI kg/m2 | 28±5 | 24±3 | 30±6 | 29±5 | <0.0001 |
| Lipid lowering meds | 17% | 15% | 16% | 14% | 0.028 |
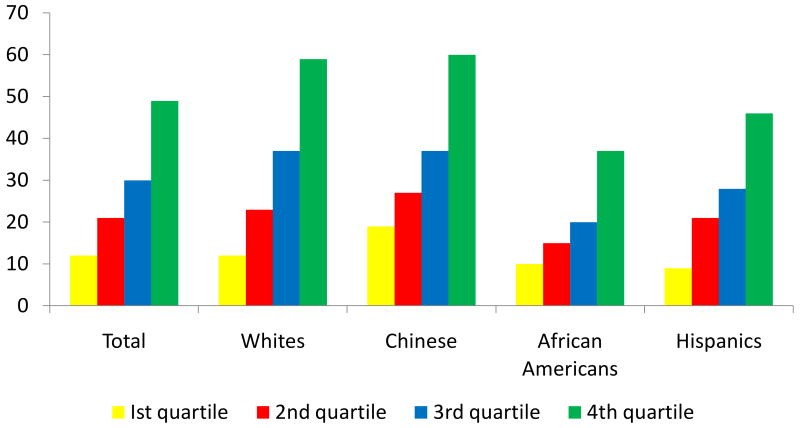
Figure 1 shows the relative prevalence of TAC in each race by CIMT quartiles. Only 12% of individuals in the lowest quartile of CIMT had TAC compared to 49% among those with CIMT in the highest quartile (p<0.0001). Similar trends were observed in all race-ethnic groups (figure 1) with increasing CIMT. Over 70% of all MESA participants were free of TAC.
Figure 1. Prevalence of TAC According to Common CIMT Quartiles.
* Mean Maximum CIMT
Table 2 demonstrates the baseline characteristics of the cohort according to the CIMT quartiles. Participants with higher CIMT quartiles were more likely to be men, older, whites and have a higher prevalence of traditional CVD risk factors (table 1). The distribution of CIMT quartiles among each ethnic group was as follows (Whites: 27%, 24%, 25%, 24%; Chinese: 33%, 27%, 24%, 16%; African Americans: 18%, 23%, 27%, 32%; Hispanic: 27%, 28%, 23%, 22%; p value for differences>0.0001). When the analyses was limited to those without any cholesterol lowering medication no differences was seen in LDL levels (1st quartile: 108±32, 2nd quartile: 107±31, 3rd quartile: 105±29, 4th quartile: 107±29 mg/dl, p=0.65) as well as HDL levels (1st quartile: 51±14, 2nd quartile: 51±15, 3rd quartile: 50±13, 4th quartile: 50±13 mg/dl, p=0.56) across increasing CIMT quartiles.
Table 2. Baseline Characteristics of Study Population According to Increasing CIMT Levels.
| Variable | 1st Quartile (≤0.735) | 2nd Quartile (0.736-0.845) | 3rd Quartile (0.846-0.975) | 4th Quartile (>0.975) | P value |
|---|---|---|---|---|---|
| Age (years) | 56±9 | 61±10 | 64±9 | 68±9 | <0.0001 |
| Gender (males) | 40% | 44% | 49% | 55% | <0.0001 |
| Race | |||||
| Whites | 41% | 37% | 39% | 38% | |
| Chinese | 16% | 13% | 11% | 8% | <0.0001 |
| African Americans | 20% | 25% | 30% | 35% | |
| Hispanics | 23% | 25% | 20% | 19% | |
| Current smoker | 14% | 15% | 12% | 12% | 0.02 |
| HTN | 26% | 39% | 50% | 63% | <0.0001 |
| DM | 10% | 11% | 13% | 22% | <0.0001 |
| Family history of heart attack | 39% | 43% | 44% | 45% | <0.0001 |
| LDL-C (mg/dl) | 116±30 | 117±32 | 116±31 | 121±32 | <0.0001 |
| HDL-C (mg/dl) | 53±15 | 51±15 | 51±14 | 49±145 | <0.0001 |
| BMI kg/m2 | 27±5 | 28±5 | 29±5 | 29±5 | <0.0001 |
| Lipid lowering meds | 11% | 14% | 16% | 22% | <0.0001 |
Table 3 shows the multivariable adjusted relationship between the presence of TAC and increasing CIMT levels. After adjustment for demographics (age, gender, race and site of examination), one standard deviation increase in CIMT was associated with a 19% (PR 1.19, 95% CI: 1.16-1.22) higher likelihood for presence of TAC. The relationship remained robust (PR: 1.16, 95% CI: 1.12-1.26) with further adjustment of CVD risk factors (model 2). In addition, individuals with CIMT in the highest quartile as compared to those with CIMT in the first quartile had a 76% higher likelihood for presence of TAC (PR: 1.76, 95% CI: 1.37-2.26). In race-ethnic stratified analyses, similar associations were seen in all groups. No statistical interaction between CIMT and ethnicity was observed on either regression model for presence of TAC (P=NS). As shown in table 4, among those with TAC>0, a higher CIMT was significantly associated with continuous TAC scores (log transformed) in the overall population as well as when assessed among all ethnic-racial groups. Similarly, no interaction between higher CIMT and ethnicity was noted for increasing TAC scores among those with detectable thoracic aorta calcification (p=NS).
Table 3. Prevalence Ratios (95% CI) For Presence of TAC with Increasing Burden of Mean Maximum Common Intimal Medial Thickness.
| Mean Maximum Common Carotid Artery | Per One SD (0.19) Increase | 1st Quartile (≤0.735) | 2nd Quartile (0.736-0.845) | 3rd Quartile (0.846-0.975) | 4th Quartile (>0.975) |
|---|---|---|---|---|---|
| MODEL 1 | |||||
| Total | 1.19 (1.16-1.22) | 1 (ref) | 1.08 (0.85-1.37) | 1.37 (1.10-1.70) | 2.22 (1.80-2.75) |
| Caucasians | 1.21 (1.17-1.26) | 1 (ref) | 0.97 (0.68-1.39) | 1.30 (0.94-1.79) | 2.23 (1.60-3.08) |
| Chinese | 1.29 (1.18-1.41) | 1 (ref) | 1.03 (0.61-1.77) | 1.42 (1.70-5.12) | 2.98 (1.70-5.16) |
| African Americans | 1.15 (1.10-1.20) | 1 (ref) | 1.07 (0.61-1.91) | 1.35 (0.79-2.30) | 2.03 (1.21-3.43) |
| Hispanics | 1.19 (1.12-1.26) | 1 (ref) | 1.23 (0.77-2.00) | 1.33 (0.82-2.15) | 1.94 (1.20-3.15) |
| MODEL 2 | |||||
| Total | 1.16 (1.13-1.19) | 1 (ref) | 1.02 (0.79-1.32) | 1.19 (0.93-1.52) | 1.76 (1.37-2.26) |
| Caucasians | 1.17 (1.12-1.22) | 1 (ref) | 1.07 (0.69-1.66) | 1.23 (0.83-1.83) | 1.83 (1.21-2.77) |
| Chinese | 1.28 (1.17-1.40) | 1 (ref) | 0.77 (0.43-1.38) | 0.99 (0.54-1.83) | 2.20 (1.15-4.20) |
| African Americans | 1.12 (1.06-1.17) | 1 (ref) | 0.89 (0.45-1.76) | 1.19 (0.64-2.22) | 1.71 (1.10-1.15) |
| Hispanics | 1.16 (1.09-1.25) | 1 (ref) | 1.25 (0.69-2.26) | 1.20 (0.67-2.12) | 1.59 (0.89-2.86) |
Model 1: Adjusted for age, gender, race and site
Model 2: Adjusted for age, gender, race, site, body mass index, LDL, HDL, hypertension, systolic blood pressure, diabetes mellitus, cigarette smoking, family history of heart attack, cholesterol lowering medications, C Reactive Protein
Note: Race not adjusted in ethnic specific analyses
Table 4. Beta coefficients (95% CI) For Increasing TAC scores (TAC>0) with Increasing Burden of Mean Maximum Common Intimal Medial Thickness.
| Mean Maximum Common Carotid Artery Per One SD (0.19) Increase | MODEL 1 | MODEL 2 |
|---|---|---|
| MODEL 1 | ||
| Total | 0.09 (0.07-0.11) | 0.08 (0.06-0.10) |
| Caucasians | 0.08 (0.05-0.10) | 0.06 (0.03-0.09) |
| Chinese | 0.08 (0.06-0.96) | 0.08 (0.02-0.15) |
| African Americans | 0.11 (0.07-0.15) | 0.08 (0.03-0.13) |
| Hispanics | 0.11 (0.06-0.16) | 0.09 (0.04-0.15) |
Model 1: Adjusted for age, gender, race and site
Model 2: Adjusted for age, gender, race, site, body mass index, LDL, HDL, hypertension, systolic blood pressure, diabetes mellitus, cigarette smoking, family history of heart attack, cholesterol lowering medications, C Reactive Protein
Note: Race not adjusted in ethnic specific analyses
Discussion
Our study demonstrates that a higher TAC is associated with presence and severity of CIMT in all racial groups and this relationship persisted even after taking into account traditional CVD risk factors. Given the increased ethnic diversity of the current US population, our findings add to the current body of evidence about CIMT and its association with TAC.
Calcification in the blood vessels is part of a complicated atherosclerotic lesion and appears later than atheroma. Aortic calcification has been demonstrated to be an actively regulated process,23,24 representing aortic atherosclerosis. As for a relationship between carotid and aortic atherosclerosis, Kallikazaros et al25 demonstrated a close relationship between atheroma, of the carotid artery and the ascending aorta by using B-mode and transesophageal ultrasound. Kardys et al,26 in the population-based Rotterdam study, demonstrated the gender differences between CAC, CIMT, carotid plaque and aortic calcification in the abdominal aorta using radiography. Carotid plaque and aortic calcification had the closest relationships. There were minimal gender differences for relationships of CIMT and aortic calcification.21 Allison et al6 demonstrated relationships among coronary and extra-coronary arterial calcifications by CT. They showed similar prevalence of carotid calcification and TAC in men, while in women, the prevalence of carotid calcification was significantly lower than TAC.
Measures of subclinical atherosclerosis are increasingly being proposed as a method to improve risk stratification for CV events. Recently prospective studies such as MESA have demonstrated that CIMT has predictive power for future CV events, independent of traditional CVD risk factors, although less than CAC, while CIMT was a better predictor of stroke3. TAC is easily detectable by non-enhanced CT, whether assessed during CAC scanning or non-cardiac applications27. We previously demonstrated a strong relationship between CAC and TAC28. The racial-ethnic distribution, in particular, was similar between these two measures14,18. Eisen et al,7 in a prospective study, demonstrated that TAC had predictive power for future CV events in patients with stable angina, independent of CAC. In the MESA cohort, Budoff et al29 demonstrated that TAC was an independent predictor of future CV events. It would be interesting to observe whether the relationship of future cardiac events associated with TAC as well CIMT can be explained independently of each other, as well whether there is a role for identification of atherosclerosis in specific vascular beds to establish the potential role for multi-modality atherosclerotic imaging.
Our study findings need to be interpreted in light of the following limitations. As noted earlier, the MESA sample may not be completely generalizable to the entire population, as individuals participating tended to be healthier overall than the general population [13]. Also, overall only 12% of our cohort was Chinese, so these estimates are more variable than those for the other race-ethnicities. In addition, the cross sectional nature of the study limits the inference whether the probability of TAC development is higher with increased CIMT burden, and will need to be evaluated in follow-up studies. An important limitation of our study is our measurement of aortic calcium only in the ascending and descending aorta. Although higher prevalence of aortic calcium have been reported in the abdominal aorta, where most aortic calcium is known to occur. In addition as also noted by others.30 TAC is only one component of overall aortic calcium burden and does not include calcification in the aortic arch, abdominal aorta, or prebifurcation aorta; findings regarding overall aortic calcium may differ from those in this study. However an advantage of TAC is that it can be measured during a standard CAC scan, without requiring additional scanning, and could reflect the presence and extent of overall aortic calcium. The real utility of TAC will be proven if it is able to demonstrate added prognostic value above and beyond CAC; further follow-up of the current study will be able to shed light on this very important issue.
In summary, a strong association of CIMT and TAC was noted in our study of multiethnic cohort of individuals free of known cardiovascular disease. This relationship was seen across all ethnic groups. Participants with higher CIMT had a significant relative risk for the presence of TAC. However, nearly half of patients with the highest CIMT level were free of TAC. Both atherosclerotic measures are fairly independent of each other and therefore may both provide complementary value for cardiovascular risk assessment.
Acknowledgments
This research was supported by R01 HL071739 and contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yamamoto H, Shavelle DM, Takasu J, Lu B, Mao SS, Fisher H, Budoff MJ. Valvular and Thoracic Aortic Calcium as a Marker of the Extent and Severity of Angiographic Coronary Artery Disease. Am Heart J. 2003;146:153–9. doi: 10.1016/S0002-8703(03)00105-4. [DOI] [PubMed] [Google Scholar]
- 2.Budoff MJ, Nasir K, Mao S, Tseng PH, Chau A, Liu ST, Flores F, Blumenthal RS. Ethnic differences of the presence and severity of coronary atherosclerosis. Atherosclerosis. 2006;185:394–399. doi: 10.1016/j.atherosclerosis.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Folsom AR, Kronmal RA, Detrano RC, O'Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manolio TA, Arnold AM, Post W, et al. Ethnic differences in the relationship of carotid atherosclerosis to coronary calcification: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.02.030. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman AB, Naydeck BL, Sutton-Tyrrell K, et al. Relationship between coronary artery calcification and other measures of subclinical cardiovascular disease in older adults. Arterioscler Thromb Vasc Biol. 2002;22:1674–1679. 6. doi: 10.1161/01.atv.0000033540.89672.24. [DOI] [PubMed] [Google Scholar]
- 6.Oei HHS, Vliegenthart R, Hak AE, et al. The association between coronary calcification assessed by electron beam compute tomography and measures of extracoronary atherosclerosis. The Rotterdam Coronary Calcification Study. J Am Coll Cardiol. 2002;39:1745–1751. 7. doi: 10.1016/s0735-1097(02)01853-3. [DOI] [PubMed] [Google Scholar]
- 7.Wagenknecht LE, Langefeld CD, Carr JJ, et al. Race-specific relationships between coronary and carotid artery calcification and carotid intimal medial thickness. Stroke. 2004;35:e97–e99. doi: 10.1161/01.STR.0000127081.99767.1d. [DOI] [PubMed] [Google Scholar]
- 8.Barrett-Connor E, Laughlin GA, Connor C. Coronary artery calcium versus intima-media thickness as a measure of cardiovascular disease among asymptomatic adults (from the Rancho Bernardo Study) Am J Cardiol. 2007;99:227–231. doi: 10.1016/j.amjcard.2006.07.085. [DOI] [PubMed] [Google Scholar]
- 9.Witteman JC, Kannel WB, Wolf PA, Grobbee DE, Hofman A, D'Agostino RB, Cobb JC. Aortic calcified plaques and cardiovascular disease (the Framingham Study) Am J Cardiol. 1990;66:1060–1064. doi: 10.1016/0002-9149(90)90505-u. [DOI] [PubMed] [Google Scholar]
- 10.Witteman JC, Kok FJ, van Saase JL, Valkenburg HA. Aortic calcification as a predictor of cardiovascular mortality. Lancet. 1986;2:1120–1122. doi: 10.1016/s0140-6736(86)90530-1. [DOI] [PubMed] [Google Scholar]
- 11.Hollander M, Hak AE, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Witteman JC, Breteler MM. Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study. Stroke. 2003 Oct;34:2367–2372. doi: 10.1161/01.STR.0000091393.32060.0E. [DOI] [PubMed] [Google Scholar]
- 12.Eisen A, Tenenbaum A, Koren-Morag N, Tanne D, Shemesh J, Imazio M, Fisman EZ, Motro M, Schwammenthal E, Adler Y. Calcification of the thoracic aorta as detected by spiral computed tomography among stable angina pectoris patients: association with cardiovascular events and death. Circulation. 2008;118:1328–34. doi: 10.1161/CIRCULATIONAHA.107.712141. [DOI] [PubMed] [Google Scholar]
- 13.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–336. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 14.Tejada C, Gore I, Strong JP, McGill HC., Jr Comparative severity of atherosclerosis in Costa Rica, Guatemala, and New Orleans. Circulation. 1958;18:92–97. doi: 10.1161/01.cir.18.1.92. [DOI] [PubMed] [Google Scholar]
- 15.Newman AB, Naydeck BL, Sutton-Tyrrell K, Edmundowicz D, O'Leary D, Kronmal R, Burke GL, Kuller LH. Relationship between coronary artery calcification and other measures of subclinical cardiovascular disease in older adults. Arterioscler Thromb Vasc Biol. 2002;22:1674–1679. doi: 10.1161/01.atv.0000033540.89672.24. [DOI] [PubMed] [Google Scholar]
- 16.Oei HH, Vliegenthart R, Hak AE, Iglesias del Sol A, Hofman A, Oudkerk M, Witteman JC. The association between coronary calcification assessed by electron beam computed tomography and measures of extracoronary atherosclerosis: the Rotterdam Coronary Calcification Study. J Am Coll Cardiol. 2002;39:1745–1751. doi: 10.1016/s0735-1097(02)01853-3. [DOI] [PubMed] [Google Scholar]
- 17.Wagenknecht LE, Langefeld CD, Carr JJ, Riley W, Freedman BI, Moossavi S, Bowden DW. Race-specific relationships between coronary and carotid artery calcification and carotid intimal medial thickness. Stroke. 2004;35(5):e97–e99. doi: 10.1161/01.STR.0000127081.99767.1d. [DOI] [PubMed] [Google Scholar]
- 18.Kallikazaros IE, Tsioufis CP, Stefanadis CI, Pitsavos CE, Toutouzas PK. Closed relation between carotid and ascending aortic atherosclerosis in cardiac patients. Circulation. 2000;102(19 Suppl 3):III263–8. doi: 10.1161/01.cir.102.suppl_3.iii-263. [DOI] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 20.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 22.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS, American Society of Echocardiography Carotid Intima-Media Thickness Task Force Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Tintut Y, Demer LL. Recent advances in multifactorial regulation of vascular calcification. Curr Opin Lipidol. 2001;12:555–60. doi: 10.1097/00041433-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–74. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 25.Kallikazaros IE, Tsioufis CP, Stefanadis CI, Pitsavos CE, Toutouzas PK. Closed relation between carotid and ascending aortic atherosclerosis in cardiac patients. Circulation. 2000;102(19 Suppl 3):III263–268. doi: 10.1161/01.cir.102.suppl_3.iii-263. [DOI] [PubMed] [Google Scholar]
- 26.Kardys I, Vliegenthart R, Oudkerk M, Hofman A, Witteman JC. The female advantage in cardiovascular disease: do vascular beds contribute equally? Am J Epidemiol. 2007;166:403–412. doi: 10.1093/aje/kwm115. [DOI] [PubMed] [Google Scholar]
- 27.Takasu J, Katz R, Nasir K, Carr JJ, Wong N, Detrano R, Budoff MJ. Relationships of thoracic aortic wall calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2008;155:765–771. doi: 10.1016/j.ahj.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takasu J, Budoff MJ, O'Brien KD, Shavelle DM, Probstfield JL, Jeffrey Carr J, Katz R. Relationship between coronary artery and descending thoracic aortic calcification as detected by computed tomography: The Multi-Ethnic Study of Atherosclerosis. 2009;204:440–6. doi: 10.1016/j.atherosclerosis.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budoff MJ, Nasir K, Katz R, Takasu J, Carr JJ, Wong ND, Allison M, Lima JA, Detrano R, Blumenthal RS, Kronmal R. Thoracic Aortic Calcification and Coronary Heart Disease Events: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2008;118:S689. doi: 10.1016/j.atherosclerosis.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong ND, Sciammarella M, Arad Y, et al. Relation of Thoracic Aortic and Aortic Valve Calcium to Coronary Artery Calcium and Risk Assessment. Am J Cardiol. 2003;92:951–955. doi: 10.1016/s0002-9149(03)00976-7. [DOI] [PubMed] [Google Scholar]