Abstract
Cholangiocarcinoma is a devastating cancer of biliary origin with limited treatment options. Symptoms are usually evident after blockage of the bile duct by the tumor, and at this late stage, they are relatively resistant to chemotherapy and radiation therapy. Therefore, it is imperative that alternative treatment options are explored. We have previously shown that serotonin metabolism is dysregulated in cholangiocarcinoma leading to an increased secretion of serotonin, which has growth-promoting effects. Because serotonin and dopamine share the degradation machinery, we evaluated the secretion of dopamine from cholangiocarcinoma and its effects on cell proliferation. Using 4 cholangiocarcinoma cell lines and human biopsy samples, we demonstrated that there was an increase in mRNA and protein expression of the dopamine synthesis enzymes tyrosine hydroxylase and dopa decarboxylase in cholangiocarcinoma. There was increased dopamine secretion from cholangiocarcinoma cell lines compared to H69 and HIBEC cholangiocytes and increased dopamine immunoreactivity in human biopsy samples. Furthermore, administration of dopamine to all cholangiocarcinoma cell lines studied increased proliferation by up to 30% which could be blocked by the pretreatment of the D2 and D4 dopamine receptor antagonists, whereas blocking dopamine production by α-methyldopa administration suppressed growth by up to 25%. Administration of α-methyldopa to nude mice also suppressed cholangiocarcinoma tumor growth. The data presented here represent the first evidence that dopamine metabolism is dysregulated in cholangiocarcinoma and that modulation of dopamine synthesis may represent an alternative target for the development of therapeutic strategies.
Keywords: Tyrosine hydroxylase, biliary cancer, Dopa decarboxylase, Monoamine oxidase A, biogenic amines
INTRODUCTION
Cholangiocarcinoma arises from the neoplastic transformation of the epithelial cells or cholangiocytes that line the bile ducts. It accounts for about 3% of all gastrointestinal cancers and represent the second most common primary liver tumor after hepatocellular carcinoma 1. Biliary tumors are extremely aggressive and display a poor prognosis 1-4. The lack of therapeutic tools for such a devastating disease is due, at least in part, to the lack of knowledge regarding the mechanisms regulating cholangiocarcinoma growth 2-4. However, increasing evidence has shown that neuropeptides and neuroendocrine hormones are amongst those factors that are able to affect cholangiocarcinoma biology, either promoting or inhibiting its growth 1, 4-7.
We recently demonstrated that cholangiocarcinoma over-produces and secretes serotonin, which has growth promoting effects in an autocrine manner 6. Specifically, compared to a non-malignant human cholangiocyte cell line, cholangiocarcinoma cells express higher levels of tryptophan hydroxylase 1, the rate-limiting enzyme in serotonin biosynthesis, whereas there is a marked decrease in expression of monoamine oxidase A (MAO A), the enzyme responsible for the degradation of various biogenic amines, including serotonin 6. This pattern of expression was also confirmed in human biopsy samples. Furthermore, treatment of cholangiocarcinoma cells with serotonin increased proliferation, whereas inhibiting serotonin synthesis decreased proliferation in vitro and reduced tumor volume in vivo. Because MAO A is also responsible for the degradation of dopamine 8, we wished to determine the relative levels of dopamine in cholangiocarcinoma compared to non-malignant tissue and to evaluate the consequences of these changes on cell growth.
Dopamine is synthesized mainly by nervous tissue and adrenal glands, first by the enzymatic conversion of tyrosine to DOPA ( 3 , 4-dihydroxyphenylalanine) by tyrosine hydroxylase (TH) and then by the decarboxylation of DOPA by Dopa decarboxylase (DDC) 8. The dopamine receptors (DR) are a class of metabotropic G protein-coupled receptors and, to date, there are 5 types; D1-D5 9. Activation of these receptors has differing effects on signal transduction pathways. For example, the D1DR interacts with the Gs complex to activate adenylyl cyclase, whereas the D2DR interacts with Gi to inhibit cAMP production9. As with serotonin, dopamine is rapidly cleared from the extracellular space by a dopamine specific re-uptake transporter where it is then degraded, predominantly by MAO A8.
Several opposing effects of dopamine on tumor growth have been reported. Lung carcinoma patients have 5-fold more circulating dopamine levels, which, it is speculated, may inhibit the proliferation, and cytotoxicity of specific T-cells via a D1DR-mediated mechanism, thereby assisting in the malignant process 10, 11. Conversely, dopamine levels are depleted in malignant colon tissue 12 and gastric cancer tissue 13. In addition, modulation of dopamine receptors is being proposed as a possible treatment of pituitary tumors due to the suppressive effects of dopamine on prolactin secretion 14. It may also have a role in the treatment of neuroblastoma cells, where D1DR agonists have a toxic effect on cell proliferation which appears to be neuronal specific 15. To date, nothing is known about the involvement of dopamine in the neoplastic transformation and growth of cholangiocarcinoma.
In the present study, we show a dysregulation of the cellular machinery responsible for the metabolism of dopamine in cholangiocarcinoma cell lines and human samples, which results in an increased production and secretion of dopamine from cholangiocarcinoma. Furthermore, we show that the increased secretion of dopamine has growth-promoting effects on cholangiocarcinoma cells and that inhibiting dopamine synthesis significantly blocks cholangiocarcinoma cell proliferation in vitro and in vivo.
METHODS
In vitro
Cell Lines
We used four human cholangiocarcinoma cell lines (Mz-ChA-1, HuCC-T1, CCLP1, and SG231) with different origins. Mz-ChA-1 cells, from human gallbladder 16 were a gift from Dr. G. Fitz (University of Texas Southwestern Medical Center, Dallas, TX). CCLP-1 17, HuCC-T1 18 and SG231 19 all from intrahepatic bile ducts were a kind gift from Dr AJ Demetris (University of Pittsburg, PA) and were cultured as described 17-19. The human immortalized, nonmalignant cholangiocyte cell line, H69 (from Dr. G.J Gores, Mayo Clinic, Rochester, MN), was cultured as described 20. In addition, the primary human intrahepatic cholangiocyte cell line (HIBEC) was purchased from Sciencell (Carlsbad, CA) and cultured according to the manufacturer’s instructions.
Real time PCR
RNA was extracted from all cell lines using the RNeasy Mini Kit (Qiagen Inc, Valencia, CA) according to the instructions provided by the vendor and reverse transcribed using the Reaction Ready™ First Strand cDNA synthesis kit (SA Bioscience, Frederick, MD). These reactions were used as templates for the PCR assays using a SYBR Green PCR master mix (SA Bioscience, Frederick, MD) in the real-time thermal cycler (ABI Prism 7900HT sequence detection system) using commercially available primers designed against human TH, DDC and the specific dopamine receptor subtypes (SA Bioscience, Frederick MD). A ΔΔCT analysis was performed using the normal cholangiocytes as the control sample. Data are expressed as relative mRNA levels ± SEM (n=3).
Immunoblotting
Following trypsinization, all cell lines (1×106 cells) were resuspended in lysis buffer 21 and sonicated. Immunoblots to detect TH, DDC and β-actin were performed as previously described22 using specific antibodies against each protein (Santa Cruz Biotechnology, Santa Cruz, CA). Data are expressed as fold change (mean ± SEM) of the relative expression after normalization with β-actin.
Dopamine secretion
All cell lines were trypsinized and the resulting cell pellet was resuspended in Hank’s-buffered saline buffer (1 × 107 cells/mL). Cells were then incubated for 6 hr at 37°C and the amount of dopamine released into the media was assayed using a commercially available dopamine ELISA kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
MTS cell proliferation assays
Cell lines were seeded into 96 well plates (10,000 cells/well) in a final volume of 200 μl of growth medium and allowed to adhere to the plate overnight. Cells were serum-starved for 24 hr prior to stimulation with dopamine (10-9 M to 10-5 M) or L-(-)-α-methyldopa (a specific DDC inhibitor; 10-6 M to 10-8 M; Ki = 39.3 μM; 23) for 48 hours. In parallel experiments, cells were pretreated with commercially available specific dopamine receptor antagonists all at 10 nM (D1DR antagonist, LE 300 24; D2DR antagonist, L-741,626 25; D3DR antagonist, NGB 2904 26; D4DR antagonist L-745,870 trihydrochloride 27, all purchased from Tocris Bioscience., (Ellisville, MI), for 1 hr prior to the addition of dopamine (10-7 M). Cell proliferation was assessed using a colorimetric cell proliferation assay (CellTiter 96 AQueous; Promega Corp., Madison, WI) and absorbance was measured at 490 nm by a microplate spectrophotometer (Versamax; Molecular Devices, Sunnyvale, CA). In all cases, data were expressed as the fold change of treated cells compared to vehicle-treated controls.
Bromodeoxyuridine (BrdU) incorporation assays
BrdU assays were performed as described previously 22 using Mz-ChA-1 cells stimulated with dopamine (100 nM) and α-Methyldopa (10-6 M) for 48 hr. The number of BrdU-positive nuclei was counted and expressed as a percentage of total cells in 5 random fields for each treatment group. Data is average ± SEM of 5 fields in 3 independent experiments.
Animal Model
Nude mice treatment
In vivo experiments were performed as described previously 28. Male balb/c 8-week-old nude (nu/nu) mice were kept in a temperature-controlled environment (20-22°C) with a 12-hour light-dark cycle with free access to drinking water and to standard mouse chow. Mz-ChA-1 cells (5 × 106) were suspended in 0.25 mL of extracellular matrix gel and injected subcutaneously in the left back flank of these animals. After the establishment of the tumors, mice received α-Methyl dopa (100 mg/kg/day ip 29, 30) injected 3 times per week. Tumor parameters were measured twice a week by an electronic calliper and volume determined as: tumor volume (mm3) = 0.5 × [length (mm) × width (mm) × height (mm)]. After approximately 2 months, mice were anesthetized with sodium pentobarbital (50 mg/kg ip) and sacrificed according to institutional guidelines. Serum was collected and AST and ALT levels were measured using a Dimension® RxL Max Integrated Chemistry system (Dade Behring Inc., Deerfield IL) by the Scott & White Hospital’s, Chemistry Department.
Tumor tissues were excised from the flank of these mice, fixed in formalin, and embedded in paraffin. Tumor protein selectively expressed by cholangiocytes was evaluated after CK-19 immunohistochemical staining. The local levels of dopamine were assessed by immunohistochemical staining using a dopamine-specific antibody. Proliferating cell nuclear antigen (PCNA) immunoreactivity in tumor sections was also evaluated and in parallel, PCNA mRNA expression was evaluated in tumor tissue by real time PCR.
In each case, sections were counterstained with hematoxylin prior to analysis. Light microscopy and immunohistochemistry observation were performed on the BX-40 light microscope (Olympus, Tokyo, Japan) with a videocam (Model No U-PMTVC; Olympus, Tokyo, Japan) and processed with Image Capturing Software (DP2-BSW Olympus, Tokyo, Japan). Three pathologists independently performed the analysis in a blind manner.
Human studies
Cholangiocarcinoma tissue analysis
Immunoreactivity for dopamine, TH, and DDC was assessed in commercially available Accumax tissue arrays (Isu Abxis Co, LTD, Seoul, Korea) and in a limited number (n=6) of cholangiocarcinoma tissues compared to the surrounding non-cancerous liver tissue containing normal cholangiocytes by immunohistochemistry as described 6 using specific antibodies. The tissue arrays contain 48 well-characterized cholangiocarcinoma biopsy samples from a variety of tumor differentiation grades as well as 4 control liver biopsy samples. Semi-quantitative analysis was performed by three independent observers, in a blind fashion, using the following parameters. Staining intensity was assessed on a scale from 1-4 (1=no staining, 4= intense staining) and the abundance of positively stained cells was given a score from 1 to 5 (1= no cells stained, 5 = 100% stained). The staining index was then calculated by the staining intensity multiplied by the staining abundance that gave a range from 1 to 20.
Dopamine secretion
Serum samples were obtained from cholangiocarcinoma patients and age-matched controls and were stored at -80°C until further analysis. Hepatic bile also was collected aseptically from T-tube drainage during post-operative day 1-3 from patients with intrahepatic stones or gallstones in association with common bile duct stones (n=25) and from cholangiocarcinoma patients (n=22). Bile samples were immediately frozen at -80°C until analysis for dopamine content via EIA kits as outlined above.
Statistical Analysis
All data are expressed as mean ± SEM. Differences between groups were analyzed by the Student unpaired t-test when two groups were analyzed and ANOVA when more than two groups were analyzed, followed by an appropriate post hoc test. A p value of less than 0.05 was used to indicate statistical significance. For the human studies, power analysis was performed using the G*Power3 software31.
RESULTS
In vitro studies
Expression of metabolic enzymes for dopamine is dysregulated in cholangiocarcinoma
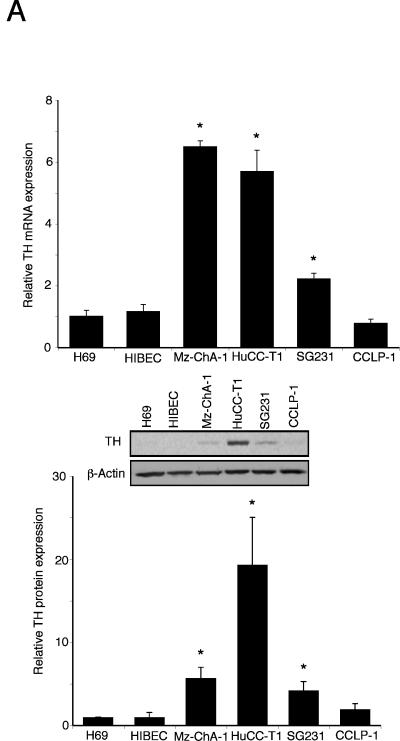
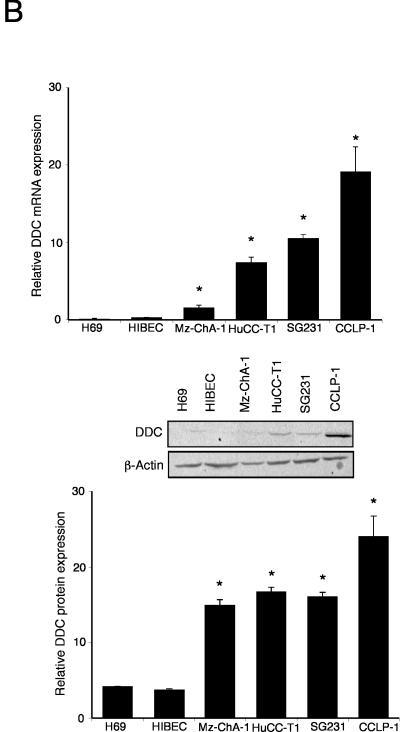
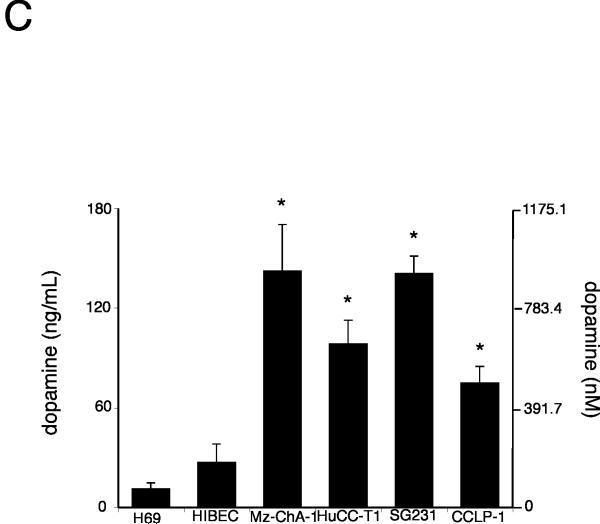
The de novo synthesis of dopamine is via the sequential reactions catalyzed by TH and DDC. The expression of TH mRNA was significantly upregulated (from 2 to 7 fold) in 3 out of 4 cholangiocarcinoma cell lines when compared to the non-malignant H69 and HIBEC cell lines (Figure 1A). This trend was confirmed by TH protein expression as demonstrated by immunoblotting (Figure 1A). Similarly, the mRNA expression of DDC was significantly increased (from 4 to 20 fold) in all cholangiocarcinoma cell lines studied when compared to non-malignant H69 and HIBEC cell lines (Figure 1B), which was paralleled in DDC protein expression as demonstrated by immunoblotting (Figure 1B). Considering the increase in expression of dopamine synthesis enzymes and decrease in the degradation enzyme MAO A shown previously 6, it would be reasonable to expect an overall increase in dopamine production and secretion from cholangiocarcinoma cells. Indeed, the secretion of dopamine was increased in all cholangiocarcinoma cell lines (Figure 1C).
Figure 1.
Enzymes responsible for dopamine synthesis are upregulated in cholangiocarcinoma cells in vitro. TH (A) and DDC (B) expression was assessed by real time PCR and immunoblotting in four cholangiocarcinoma cell lines as well as the non-malignant cholangiocyte cell lines H69 and HIBEC. In each case, data are expressed as average ± SEM (n=3). Asterisk denotes significance (p<0.05) compared with expression in H69 cells. Dopamine levels were also assessed in the supernatant of cell suspensions of cholangiocarcinoma cell lines and the non-malignant cholangiocyte cell lines H69 and HIBEC by EIA after 6 hr (C). Data are expressed as average dopamine concentration (ng/mL left axis and nMol, right axis) ± SEM (n=3). Asterisk denotes significance (p<0.05) compared with dopamine levels secreted from H69 cells.
Increased local dopamine secretion has implications on cholangiocarcinoma cell growth in vitro
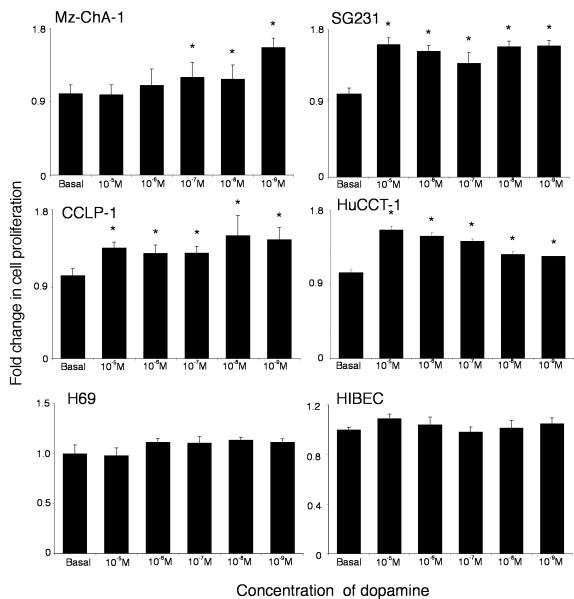
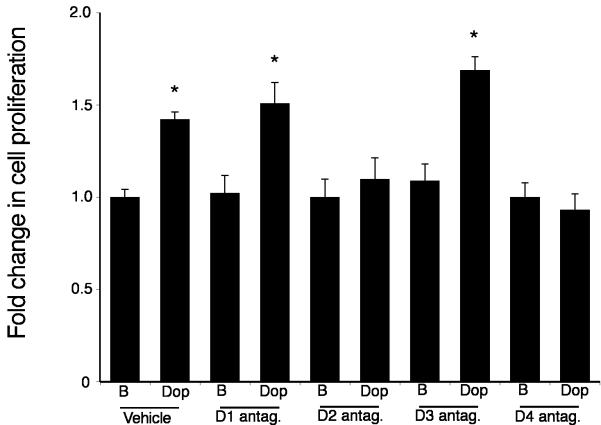
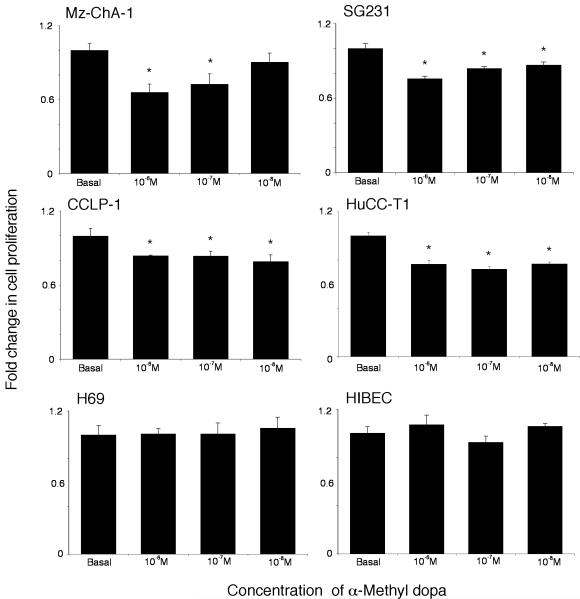
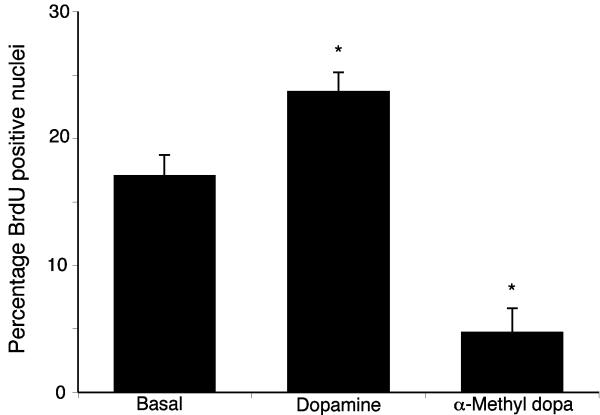
The potential implications of the increased local dopamine secretion were explored in vitro using the cholangiocarcinoma cell lines. Treatment of human cholangiocyte cell lines, H69 and HIBEC, with various concentrations of dopamine had no significant impact on cell proliferation (Figure 2), whereas treatment of all of the cholangiocarcinoma cell lines with dopamine (10-5 to 10-9 M) caused a significant increase in cell proliferation after 48 hr as demonstrated by MTS cell proliferation assays (Figure 2). Every dopamine receptor subtype is expressed in both cholangiocytes and cholangiocarcinoma cells, the relative levels of which are summarized in Table 1. The proliferative effects of dopamine could be blocked by pretreatment with D2 and D4 dopamine receptor inhibitors (Figure 3). In contrast, blocking dopamine synthesis using α-Methyl dopa, a specific DDC inhibitor, markedly inhibited cholangiocarcinoma cell proliferation, at concentrations from 10-6 M to 10-8 M (Figure 4). Once again, no effect was observed in H69 or HIBEC cells. These data were confirmed using a BrdU incorporation assay as an indication of cell cycle progression. Further, dopamine treatment increased BrdU incorporation, while α-Methyl dopa decreased BrdU incorporation (Figure 5). Taken together, these data indicate that the increased dopamine secretion from cholangiocarcinoma cells may have a growth promoting effect on tumor progression.
Figure 2.
Dopamine increases cholangiocarcinoma cell proliferation in vitro. Cholangiocarcinoma cells and the non-malignant cholangiocyte cell lines, H69 and HIBEC, were treated with various concentrations of dopamine (10-9 M to 10-5 M) for 48 hr. Cell proliferation was assessed using an MTS cell proliferation assay. Data are expressed as fold change in proliferation (average ± SEM, n=7) and the asterisk denotes p<0.05 compared to basal treatment within each cell line.
Table 1.
Fold change in dopamine receptor subunit expression in cholangiocarcinoma compared to H69 cholangiocyte cells
| Receptor | Mz-ChA-1 | HuCCT-1 | SG231 | CCLP-1 |
|---|---|---|---|---|
| D1DR | 5.43 ± 1.25* | 0.66 ± 0.11* | 0.85 ± 0.08 | 0.48 ± 0.05* |
| D2DR | 3.92 ± 0.72* | 2.14 ± 0.21* | 0.16 ± 0.03* | 3.13 ± 0.21* |
| D3DR | 2.87 ± 0.76* | 1.01 ± 0.03 | 0.79 ± 0.03 | 0.49 ± 0.03* |
| D4DR | 0.38 ± 0.06* | 0.49 ± 0.02* | 0.27 ± 0.02* | 0.76 ± 0.04* |
| D5DR | 2.45 ± 0.71* | 0.27 ± 0.03* | 0.49 ± 0.05* | 0.18 ± 0.02* |
● p<0.05 compared to non -malignant cholangiocytes (H69)
● significant increases is shaded in purple, significant decrease are shaded in gray, no shading indicates no significance.
Figure 3.
Specific dopamine receptor antagonists inhibit the growth-promoting effects of dopamine. Mz-ChA-1 cells were pretreated with specific antagonists of the dopamine receptors indicated (all at 10 nM), prior to the addition of dopamine (100 nM) for 48 hr. Cell proliferation was assessed using an MTS cell proliferation assay. Data are expressed as fold change in proliferation (average ± SEM, n=7) and the asterisk denotes p<0.05 compared to antagonist alone.
Figure 4.
Inhibition of dopamine synthesis decreases cholangiocarcinoma cell proliferation in vitro. Cholangiocarcinoma cells and the non-malignant cholangiocyte cell lines, H69 and HIBEC, were treated with various concentrations of α-methyl dopa (10-6 M to 10-8 M) for 48 hr. Cell proliferation was assessed using an MTS cell proliferation assay. Data are expressed as fold change in proliferation (average ± SEM, n=7) and the asterisk denotes p<0.05 compared to basal treatment within each cell line.
Figure 5.
BrdU labeling of cholangiocarcinoma cells indicates changes in cell cycle progression after treatment with dopamine and α-methyl dopa. Mz-ChA-1 cells were treated with dopamine (10-7M) and CPA (10-6M) for 48 hr and BrdU uptake was determined. The number of BrdU-positive cells was expressed as a percentage of total cells. Data was expressed as the average ± SEM from 5 random fields from 3 independent experiments. Asterisk denotes significance (p<0.05) when compared to basal treatment.
Animal model of cholangiocarcinoma
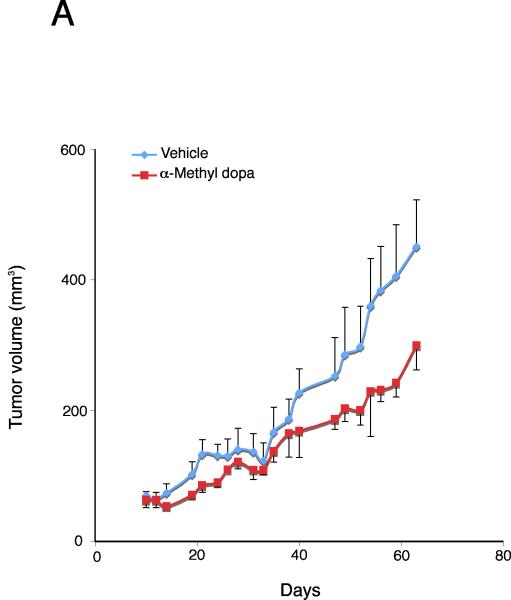
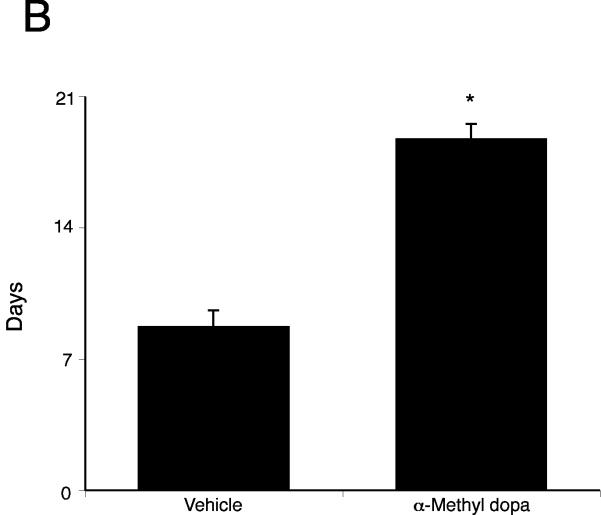
Inhibition of dopamine synthesis inhibits cholangiocarcinoma tumor growth in vivo
By treating an in vivo xenograft model of cholangiocarcinoma tumors with the DDC inhibitor, we significantly suppressed tumor growth (Figure 6A). In addition, the latency of tumor growth (i.e., time taken for tumor volume to increase to 150% of the original size) was increased after α-Methyl dopa treatment compared to vehicle treatment (Figure 6B). Analysis of liver enzymes in the serum revealed that there was no significant difference in AST (Vehicle, 93.3 ± 13.5 vs α-Methyl dopa, 102.0 ± 12.6) and ALT levels (Vehicle, 27.0 ± 12.6 vs α-Methyl dopa, 31.3 ± 4.6) between α-Methyl dopa-treated and vehicle-treated animals, both of which fell within normal range, suggesting that the α-Methyl dopa treatment was well tolerated and did not cause liver damage.
Figure 6.
Inhibition of dopamine synthesis decreases tumor growth in an in vivo xenograft model of cholangiocarcinoma. Mz-ChA-1 cells were injected into the flank of athymic mice. After tumors were established, mice were treated with 100 mg/kg/day (ip) α-methyl dopa, three days per week for 70 days and tumor volume assessed (A). Tumor latency was assessed as the time taken for the tumor to grow to 150% of the original size (B). Data are expressed as average latency (days ± SEM) and the asterisk denotes significance (p<0.05) from vehicle-treated tumors.
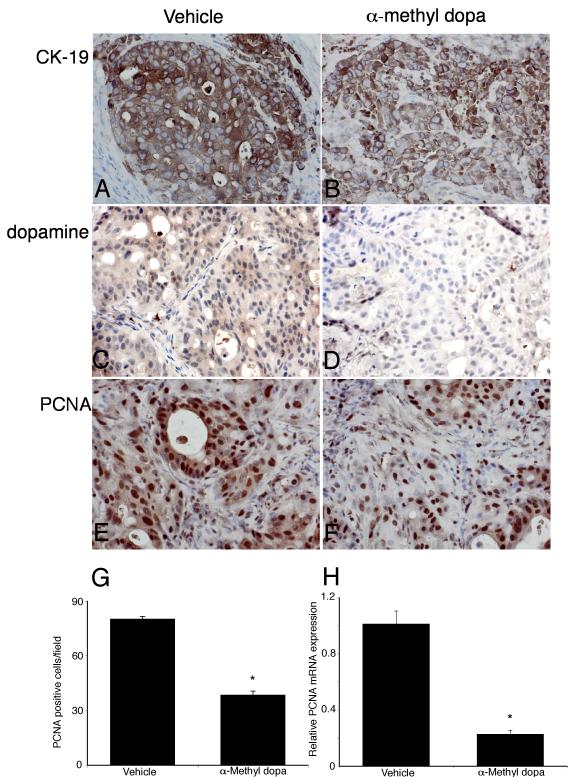
Histological analysis of the excised tumors revealed that all cells within tumors from α-Methyl dopa-treated and vehicle-treated animals were CK-19 positive, indicating cholangiocyte phenotype (Figure 7). As expected, α-Methyl dopa treatment significantly decreased the dopamine immunoreactivity (Figure 7). Using PCNA immunoreactivity as a marker of proliferative capacity, α-Methyl dopa treatment decreased the number of PCNA positive nuclei compared to vehicle treatment (Figure 7). This decrease in PCNA expression was confirmed by real time PCR analysis of RNA extracted from the excised tumors (Figure 7).
Figure 7.
Immunohistological analysis of tumors. Immunohistochemistry on tumors from vehicle- (A, C, E) and α-methyl dopa-treated mice was performed using specific antibodies against CK-19 (A, B), dopamine (C, D) and PCNA (E, F). Representative photomicrographs of the immunoreactivity are shown (magnification X40). Semi-quantitative analysis of PCNA immunoreactivity was performed and data was expressed as average (± SEM) PCNA positive nuclei per field (G) and the asterisk denotes significance (p<0.05) compared to vehicle-treated tumors. PCNA expression in the tumors was also assessed by real time PCR (H). Data are expressed as average ± SEM (n=3). Asterisk denotes significance (p<0.05) compared to vehicle-treated tumors.
Human studies
Dopamine secretion is increased in cholangiocarcinoma
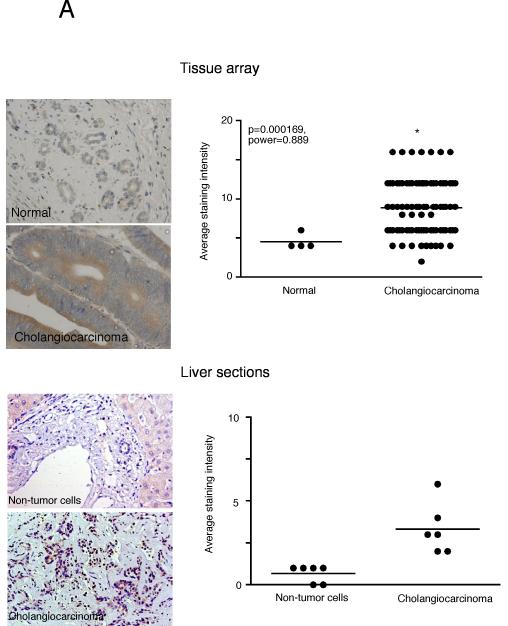
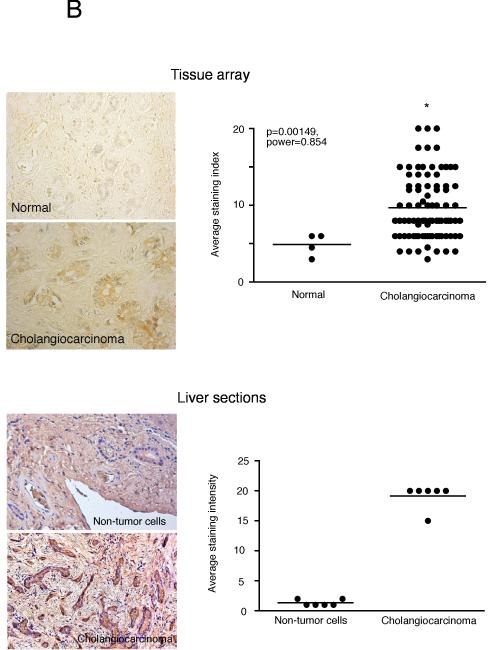
Immunohistochemical analysis of human liver biopsy samples (tissue arrays) indicated an increased TH immunoreactivity in cholangiocarcinoma samples compared to controls as assessed by three independent observers (Figure 8A; data not shown). We acknowledge that these commercially available tissue arrays are limited in the number and appropriateness of the control samples; therefore we also performed a parallel pilot study in a limited number of cholangiocarcinoma sections that also contained surrounding non-cancerous liver cells as the control. Once again, TH immunoreactivity was increased in cholangiocarcinoma tissue compared to that in normal cholangiocytes in the surrounding non-cancerous liver (Figure 8A). Similarly, immunohistochemical analysis of human liver biopsy samples from both the tissue arrays and the cholangiocarcinoma sections indicated that there is also increased DDC immunoreactivity in cholangiocarcinoma samples compared to control as assessed by three independent observers (Figure 8B; data not shown).
Figure 8.
Enzymes responsible for dopamine synthesis are upregulated in cholangiocarcinoma tumor biopsy samples. TH (A) and DDC (B) immunoreactivity was assessed in biopsy samples from 48 cholangiocarcinoma patients and 4 healthy controls by immunohistochemistry (upper panel) and in paraffin-embedded sections containing cholangiocarcinoma and the surrounding non-malignant liver tissue (n=6; lower panel). Representative photomicrographs of the TH (A) and DDC (B) immunoreactivity are shown (magnification X40). Staining intensity was assessed as described in the methods. P values and the power analysis values are shown in parentheses.
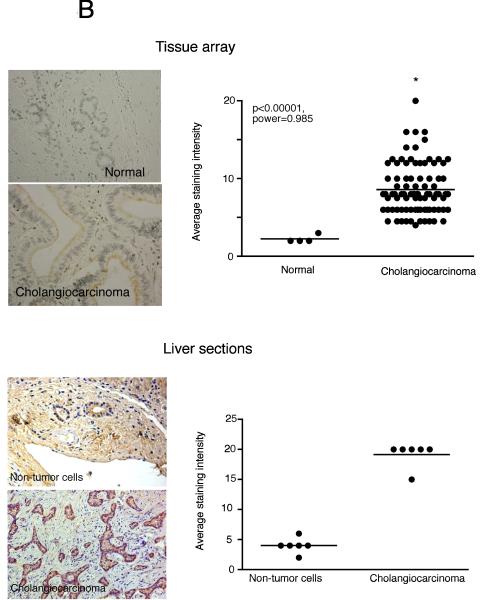
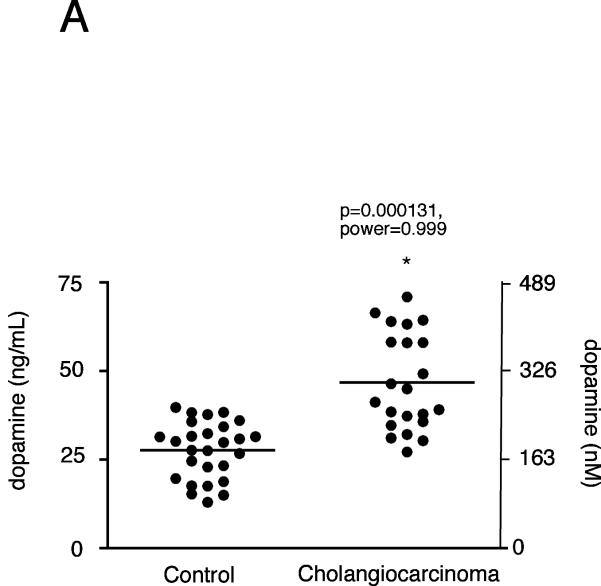
Analysis of serum samples from cholangiocarcinoma patients versus age-matched controls revealed no significant difference in dopamine levels (data not shown), which is not surprising given that the normal tissue surrounding the tumor presumably has normal degradation machinery (i.e., MAO A expression) and would possibly take up the excess dopamine and metabolize it. We then performed a pilot study with a limited number of bile samples taken from cholangiocarcinoma patients. As controls, we used bile samples collected from patients affected by intrahepatic cholelithiasis (e.g. nonmalignant disease). Analysis of these treatment groups revealed an increase in dopamine levels in cholangiocarcinoma patients compared to “controls” (p<0.05, Figure 9A). In addition, dopamine immunoreactivity was also increased in the cholangiocarcinoma tissue compared to the control sections from both the commercially available tissue arrays as well as the cholangiocarcinoma sections as assessed by 3 independent observers (Figure 9B and data not shown).
Figure 9.
Dopamine secretion in cholangiocarcinoma. Dopamine levels in bile samples from cholangiocarcinoma patients and patients with intrahepatic cholelithiasis were determined by EIA (A). Data are expressed in a scatter plot of dopamine concentration (ng/mL left axis and nMol right axis). Dopamine immunoreactivity was assessed in biopsy samples from 48 cholangiocarcinoma patients and 4 healthy controls by immunohistochemistry (upper panel) and in paraffin-embedded sections containing cholangiocarcinoma and the surrounding non-malignant liver tissue (n=6; lower panel). Representative photomicrographs of the dopamine immunoreactivity are shown (B; magnification X40). Staining intensity was assessed as described in the methods. P values and the power analysis values are shown in parentheses.
DISCUSSION
The major findings presented here relate to the dysregulation of dopamine metabolism in cholangiocarcinoma. We demonstrated that the expression of the dopamine synthesis enzymes TH and DDC are markedly increased in cholangiocarcinoma, which together with the suppression of the degradation machinery shown previously 6, results in an increased dopamine production in cholangiocarcinoma cell lines and tumor tissue, and increased dopamine in the bile of cholangiocarcinoma patients. Treatment of cholangiocarcinoma cell lines with dopamine increased cell proliferation in vitro, and inhibition of dopamine synthesis decreased cell proliferation in vitro and in an in vivo xenograft model of cholangiocarcinoma. These data suggest that the dysregulation of dopamine metabolism may be a key feature associated with the progression of cholangiocarcinoma, and that the modulation of this metabolic pathway may be targeted for the development of effective adjunct therapies to treat this deadly disease.
In support of the observations that dopamine metabolism is dysregulated in cholangiocarcinoma, increased dopamine production and secretion can be found in a number of other tumors. There is an increased circulating level of dopamine in lung tumors, which seems to play a protective role by inhibiting cytotoxic t-cell proliferation thereby preventing their ability to mount an adequate attack on the tumor cells 10, 11. Furthermore, dopamine secretion is increased in some cases of the rare malignancy, pheochromocytoma 32-34 and in carcinoid tumors 35, although the consequences of this secretion on tumor growth or progression is unclear. However, agents such as dexamethasone that increase pheochromocytoma dopamine content also increase cell proliferation36, suggesting that there may be a causal link between increased dopamine content and cell proliferation in these tumor cells. Conversely, dopamine levels are depleted in malignant colon tissue 12 and gastric cancer tissues 13, whereas dopamine treatement slows tumor growth, presumably by decreasing the expression of vascular epithelial growth factor and subsequent angiogenesis 13, 37. We have previously shown that cholangiocarcinoma, while not strictly classified as a carcinoid tumor, displays many features of a neuroendocrine phenotype, such as Chromogranin A, neuron specific enolase expression, and enhanced serotonin secretion 6. Because some carcinoid tumors also produce higher levels of dopamine 35, our observations of increased dopamine production and secretion are conceivable. Serotonin and dopamine have been shown to interact in both an antagonistic38, 39 and synergistic40 manner. We have clearly demonstrated both serotonin and dopamine are overproduced in cholangiocarcinoma and subsequently exert growth-promoting effects, however, whether both dopamine and serotonin interact in a synergistic manner in this tumor type is largely unknown and will be a topic of future research in our laboratory.
The expression of TH and DDC mRNA in blood or bone marrow has been shown to be higher in patients with neuroblastoma, which can be correlated with poor prognosis of this disease 41. Furthermore, increased TH expression can also be demonstrated in pheochromocytomas and midgut carcinoid tumors 42. The data presented here support the notion that the expression of dopamine synthesizing enzymes may be involved in the process of tumor progression, and perhaps in controlling cell proliferation.
In our study, we demonstrated an antiproliferative effect of α-Methyl dopa on cholangiocarcinoma cell growth. Chronic treatment of mice bearing cholangiocarcinoma tumors decreased the amount of dopamine produced by the tumor cells and slowed tumor growth over the period of time studied. The notion of targeting dopamine synthesis as an anticancer treatment is not without precedent. Treatment of small cell lung carcinoma cells and pulmonary carcinoid cells with another DDC inhibitor caused a selective cytotoxicity 43.
In conclusion, the data presented here indicate a dysregulated dopamine metabolic pathway in cholangiocarcinoma compared with nonmalignant cholangiocytes. Specifically, there is an increase in the expression of TH and DDC, the enzymes responsible for dopamine synthesis. This leads to an increased accumulation and secretion of dopamine from cholangiocarcinoma and an increase in dopamine in the bile from cholangiocarcinoma patients. Specific inhibition of dopamine production leads to a suppression of tumor growth in a xenograft model of cholangiocarcinoma, which suggests that agents that modulate the metabolism of dopamine may be useful therapeutic tools for the treatment of this devastating cancer.
Acknowledgements
We sincerely thank Dr. Gianfranco Alpini for his assistance and expert advice in the development of this manuscript. Also, we thank Ms. Julie Venter and Ms. Shelley Kopriva for their assistance with the animal studies. This work was supported by an NIH K01 grant award (DK078532) to Dr. DeMorrow.
Abbreviations
- CK-19
Cytokeratin19
- DDC
Dopa decarboxylase
- DR
Dopamine receptor
- HIBEC
human intrahepatic biliary epithelial cells
- MAO A
monoamine oxidase A
- PCNA
proliferating cell nuclear antigen
- TH
tyrosine hydroxylase
REFERENCES
- 1.Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology. 2003;37:961–9. doi: 10.1053/jhep.2003.50200. [DOI] [PubMed] [Google Scholar]
- 2.Blechacz B, Gores GJ. Tumor-specific marker genes for intrahepatic cholangiocarcinoma: utility and mechanistic insight. J Hepatol. 2008;49:160–2. doi: 10.1016/j.jhep.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blechacz BR, Gores GJ. Cholangiocarcinoma. Clin Liver Dis. 2008;12:131–50. ix. doi: 10.1016/j.cld.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- 5.Fava G, Alpini G, Rychlicki C, Saccomanno S, DeMorrow S, Trozzi L, Candelaresi C, Venter J, Di Sario A, Marzioni M, Bearzi I, Glaser S, et al. Leptin enhances cholangiocarcinoma cell growth. Cancer Res. 2008;68:6752–61. doi: 10.1158/0008-5472.CAN-07-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alpini G, Invernizzi P, Gaudio E, Venter J, Kopriva S, Bernuzzi F, Onori P, Franchitto A, Coufal M, Frampton G, Alvaro D, Lee SP, et al. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res. 2008;68:9184–93. doi: 10.1158/0008-5472.CAN-08-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeMorrow S, Francis H, Gaudio E, Venter J, Franchitto A, Kopriva S, Onori P, Mancinelli R, Frampton G, Coufal M, Mitchell B, Vaculin B, et al. The endocannabinoid anandamide inhibits cholangiocarcinoma growth via activation of the noncanonical Wnt signaling pathway. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1150–8. doi: 10.1152/ajpgi.90455.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shih JC, Chen K, Ridd MJ. Role of MAO A and B in neurotransmitter metabolism and behavior. Pol J Pharmacol. 1999;51:25–9. [PubMed] [Google Scholar]
- 9.Callier S, Snapyan M, Le Crom S, Prou D, Vincent JD, Vernier P. Evolution and cell biology of dopamine receptors in vertebrates. Biol Cell. 2003;95:489–502. doi: 10.1016/s0248-4900(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 10.Saha B, Mondal AC, Basu S, Dasgupta PS. Circulating dopamine level, in lung carcinoma patients, inhibits proliferation and cytotoxicity of CD4+ and CD8+ T cells by D1 dopamine receptors: an in vitro analysis. Int Immunopharmacol. 2001;1:1363–74. doi: 10.1016/s1567-5769(01)00068-6. [DOI] [PubMed] [Google Scholar]
- 11.Saha B, Mondal AC, Majumder J, Basu S, Dasgupta PS. Physiological concentrations of dopamine inhibit the proliferation and cytotoxicity of human CD4+ and CD8+ T cells in vitro: a receptor-mediated mechanism. Neuroimmunomodulation. 2001;9:23–33. doi: 10.1159/000049004. [DOI] [PubMed] [Google Scholar]
- 12.Basu S, Dasgupta PS. Decreased dopamine receptor expression and its second-messenger cAMP in malignant human colon tissue. Dig Dis Sci. 1999;44:916–21. doi: 10.1023/a:1026644110737. [DOI] [PubMed] [Google Scholar]
- 13.Chakroborty D, Sarkar C, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin Cancer Res. 2004;10:4349–56. doi: 10.1158/1078-0432.CCR-04-0059. [DOI] [PubMed] [Google Scholar]
- 14.Ivan G, Szigeti-Csucs N, Olah M, Nagy GM, Goth MI. Treatment of pituitary tumors: dopamine agonists. Endocrine. 2005;28:101–10. doi: 10.1385/endo:28:1:101. [DOI] [PubMed] [Google Scholar]
- 15.Chan AS, Ng LW, Poon LS, Chan WW, Wong YH. Dopaminergic and adrenergic toxicities on SK-N-MC human neuroblastoma cells are mediated through G protein signaling and oxidative stress. Apoptosis. 2007;12:167–79. doi: 10.1007/s10495-006-0524-8. [DOI] [PubMed] [Google Scholar]
- 16.Knuth A, Gabbert H, Dippold W, Klein O, Sachsse W, Bitter-Suermann D, Prellwitz W, Meyer zum Buschenfelde KH. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J Hepatol. 1985;1:579–96. doi: 10.1016/s0168-8278(85)80002-7. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu Y, Demetris AJ, Gollin SM, Storto PD, Bedford HM, Altarac S, Iwatsuki S, Herberman RB, Whiteside TL. Two new human cholangiocarcinoma cell lines and their cytogenetics and responses to growth factors, hormones, cytokines or immunologic effector cells. Int J Cancer. 1992;52:252–60. doi: 10.1002/ijc.2910520217. [DOI] [PubMed] [Google Scholar]
- 18.Miyagiwa M, Ichida T, Tokiwa T, Sato J, Sasaki H. A new human cholangiocellular carcinoma cell line (HuCC-T1) producing carbohydrate antigen 19/9 in serum-free medium. In Vitro Cell Dev Biol. 1989;25:503–10. doi: 10.1007/BF02623562. [DOI] [PubMed] [Google Scholar]
- 19.Storto PD, Saidman SL, Demetris AJ, Letessier E, Whiteside TL, Gollin SM. Chromosomal breakpoints in cholangiocarcinoma cell lines. Genes Chromosomes Cancer. 1990;2:300–10. doi: 10.1002/gcc.2870020408. [DOI] [PubMed] [Google Scholar]
- 20.Grubman SA, Perrone RD, Lee DW, Murray SL, Rogers LC, Wolkoff LI, Mulberg AE, Cherington V, Jefferson DM. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol. 1994;266:G1060–70. doi: 10.1152/ajpgi.1994.266.6.G1060. [DOI] [PubMed] [Google Scholar]
- 21.Kanno N, Glaser S, Chowdhury U, Phinizy JL, Baiocchi L, Francis H, LeSage G, Alpini G. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2+-dependent protein kinase C-alpha. J Hepatol. 2001;34:284–91. doi: 10.1016/s0168-8278(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 22.DeMorrow S, Glaser S, Francis H, Venter J, Vaculin B, Vaculin S, Alpini G. Opposing actions of endocannabinoids on cholangiocarcinoma growth: Recruitment of fas and fas ligand to lipid rafts. J Biol Chem. 2007;282:13098–113. doi: 10.1074/jbc.M608238200. [DOI] [PubMed] [Google Scholar]
- 23.Bertoldi M, Dominici P, Moore PS, Maras B, Voltattorni CB. Reaction of dopa decarboxylase with alpha-methyldopa leads to an oxidative deamination producing 3,4-dihydroxyphenylacetone, an active site directed affinity label. Biochemistry. 1998;37:6552–61. doi: 10.1021/bi9718898. [DOI] [PubMed] [Google Scholar]
- 24.Kassack MU, Hofgen B, Decker M, Eckstein N, Lehmann J. Pharmacological characterization of the benz[d]indolo[2,3-g]azecine LE300, a novel type of a nanomolar dopamine receptor antagonist. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:543–50. doi: 10.1007/s00210-002-0641-z. [DOI] [PubMed] [Google Scholar]
- 25.Bowery BJ, Razzaque Z, Emms F, Patel S, Freedman S, Bristow L, Kulagowski J, Seabrook GR. Antagonism of the effects of (+)-PD 128907 on midbrain dopamine neurones in rat brain slices by a selective D2 receptor antagonist L-741,626. Br J Pharmacol. 1996;119:1491–7. doi: 10.1111/j.1476-5381.1996.tb16063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan J, Chen X, Brodbeck R, Primus R, Braun J, Wasley JW, Thurkauf A. NGB 2904 and NGB 2849: two highly selective dopamine D3 receptor antagonists. Bioorg Med Chem Lett. 1998;8:2715–8. doi: 10.1016/s0960-894x(98)00469-7. [DOI] [PubMed] [Google Scholar]
- 27.Bristow LJ, Kramer MS, Kulagowski J, Patel S, Ragan CI, Seabrook GR. Schizophrenia and L-745,870, a novel dopamine D4 receptor antagonist. Trends Pharmacol Sci. 1997;18:186–8. doi: 10.1016/s0165-6147(97)01066-3. [DOI] [PubMed] [Google Scholar]
- 28.Fava G, Marucci L, Glaser S, Francis H, De Morrow S, Benedetti A, Alvaro D, Venter J, Meininger C, Patel T, Taffetani S, Marzioni M, et al. gamma-Aminobutyric acid inhibits cholangiocarcinoma growth by cyclic AMP-dependent regulation of the protein kinase A/extracellular signal-regulated kinase 1/2 pathway. Cancer Res. 2005;65:11437–46. doi: 10.1158/0008-5472.CAN-05-1470. [DOI] [PubMed] [Google Scholar]
- 29.Kapoor V, Chalmers J. Correlation between fall in blood pressure and in vivo amine release after alpha-methylDOPA. Eur J Pharmacol. 1989;164:531–8. doi: 10.1016/0014-2999(89)90261-6. [DOI] [PubMed] [Google Scholar]
- 30.Sivagnanam G, Adithan C, Thakur LC, Raveendran R, Bapna JS. Alphamethyldopa analgesia: its possible mechanism of action. Arch Int Pharmacodyn Ther. 1985;277:168–76. [PubMed] [Google Scholar]
- 31.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 32.Awada SH, Grisham A, Woods SE. Large dopamine-secreting pheochromocytoma: case report. South Med J. 2003;96:914–7. doi: 10.1097/01.SMJ.0000077069.95831.62. [DOI] [PubMed] [Google Scholar]
- 33.Tam V, Ng KF, Fung LM, Wong YY, Chan MH, Lam CW, Tam S. The importance of the interpretation of urine catecholamines is essential for the diagnosis and management of patient with dopamine-secreting paraganglioma. Ann Clin Biochem. 2005;42:73–7. doi: 10.1258/0004563053026916. [DOI] [PubMed] [Google Scholar]
- 34.Yasunari K, Kohno M, Minami M, Kano H, Ohhira M, Nakamura K, Yoshikawa J. A dopamine-secreting pheochromocytoma. J Cardiovasc Pharmacol. 2000;36(Suppl 2):S75–7. doi: 10.1097/00005344-200000006-00016. [DOI] [PubMed] [Google Scholar]
- 35.Kema IP, de Vries EG, Slooff MJ, Biesma B, Muskiet FA. Serotonin, catecholamines, histamine, and their metabolites in urine, platelets, and tumor tissue of patients with carcinoid tumors. Clin Chem. 1994;40:86–95. [PubMed] [Google Scholar]
- 36.Yang TT, Tsao CW, Li JS, Wu HT, Hsu CT, Cheng JT. Changes of dopamine content and cell proliferation by dexamethsone via pituitary adenylate cyclase-activating polypeptide in PC12 cell. Neurosci Lett. 2007;426:45–8. doi: 10.1016/j.neulet.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar C, Chakroborty D, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Dopamine in vivo inhibits VEGF-induced phosphorylation of VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H1554–60. doi: 10.1152/ajpheart.00272.2004. [DOI] [PubMed] [Google Scholar]
- 38.Olvera-Cortes ME, Anguiano-Rodriguez P, Lopez-Vazquez MA, Alfaro JM. Serotonin/dopamine interaction in learning. Prog Brain Res. 2008;172:567–602. doi: 10.1016/S0079-6123(08)00927-8. [DOI] [PubMed] [Google Scholar]
- 39.Di Giovanni G, Di Matteo V, Pierucci M, Esposito E. Serotonin-dopamine interaction: electrophysiological evidence. Prog Brain Res. 2008;172:45–71. doi: 10.1016/S0079-6123(08)00903-5. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Burgos I, Feria-Velasco A. Serotonin/dopamine interaction in memory formation. Prog Brain Res. 2008;172:603–23. doi: 10.1016/S0079-6123(08)00928-X. [DOI] [PubMed] [Google Scholar]
- 41.Trager C, Vernby A, Kullman A, Ora I, Kogner P, Kagedal B. mRNAs of tyrosine hydroxylase and dopa decarboxylase but not of GD2 synthase are specific for neuroblastoma minimal disease and predicts outcome for children with high-risk disease when measured at diagnosis. Int J Cancer. 2008;123:2849–55. doi: 10.1002/ijc.23846. [DOI] [PubMed] [Google Scholar]
- 42.Meijer WG, Copray SC, Hollema H, Kema IP, Zwart N, Mantingh-Otter I, Links TP, Willemse PH, de Vries EG. Catecholamine-synthesizing enzymes in carcinoid tumors and pheochromocytomas. Clin Chem. 2003;49:586–93. doi: 10.1373/49.4.586. [DOI] [PubMed] [Google Scholar]
- 43.Gilbert JA, Frederick LM, Ames MM. The aromatic-L-amino acid decarboxylase inhibitor carbidopa is selectively cytotoxic to human pulmonary carcinoid and small cell lung carcinoma cells. Clin Cancer Res. 2000;6:4365–72. [PubMed] [Google Scholar]