Abstract
In this study, we attempted to clarify whether distractibility in ADHD might arise from increased sensory-driven interference or from inefficient top-down control. We employed an attentional filtering paradigm in which discrimination difficulty and distractor salience (amount of image “graying”) were parametrically manipulated. Increased discrimination difficulty should add to the load of top-down processes, whereas increased distractor salience should produce stronger sensory interference. We found an unexpected interaction of discrimination difficulty and distractor salience. For difficult discriminations, ADHD children filtered distractors as efficiently as healthy children and adults; as expected, all three groups were slower to respond with high vs. low salience distractors. In contrast, for easy discriminations, robust between-group differences emerged: ADHD children were much slower and made more errors than either healthy children or adults. For easy discriminations, healthy children and adults filtered out high salience distractors as easily as low salience distractors, but ADHD children were slower to respond on trials with low salience distractors than they did on trials with high salience distractors. These initial results from a small sample of ADHD children have implications for models of attentional control, and ways in which it can malfunction. The fact that ADHD children exhibited efficient attentional filtering when task demands were high, but showed deficient and atypical distractor filtering under low task demands suggests that attention deficits in ADHD may stem from a failure to efficiently engage top-down control rather than an inability to implement filtering in sensory processing regions.
Keywords: selective attention, distractor filtering, children, ADHD
Attention Deficit Hyperactivity Disorder (ADHD) is the most common childhood mental disorder, affecting between 5–10% of children worldwide (Faraone, Sergeant, Gillberg, & Biederman, 2003). Hyperactivity, impulsivity, and inattention are all major behavioral symptoms of ADHD. However, while many studies have documented that ADHD children are impaired in executive functions, including response inhibition, working memory, and conflict resolution (Bush et al., 1999; Casey et al., 1997; Doyle, 2006; Pliszka et al., 2006; Rubia, Smith, Brammer, Toone, & Taylor, 2005; Schulz et al., 2004; Vaidya et al., 2005), the nature and extent of attention deficits in ADHD remain controversial. Although ADHD children are typically slower and more variable to respond to cued targets (Nigg, Swanson, & Hinshaw, 1997; Novak, Solanto, & Abikoff, 1995; van der Meere & Sergeant, 1988), ADHD children have not previously been reported to be impaired at filtering out irrelevant distractors. Healthy and ADHD children exhibit similar patterns of slowed responses to relevant targets when distractors are present (Booth et al., 2005; Huang-Pollock & Nigg, 2003; Huang-Pollock, Nigg, & Carr, 2005; Huang-Pollock, Nigg, & Halperin, 2006; Mason, Humphreys, & Kent, 2003, 2005; Nigg, Swanson, & Hinshaw, 1997; Novak, Solanto, & Abikoff, 1995; Oberlin, Alford, & Marrocco, 2005; van der Meere & Sergeant, 1988). This has led several researchers to question whether selective attention is a core deficit in ADHD or whether attentional problems are secondary to deficits of alertness (Huang-Pollock et al., 2005) or other executive processes, including inhibition (Barkley, 1997). The current study aimed to better characterize the nature of attention deficits in ADHD, viewed from the context of the biased competition model of attention (Desimone & Duncan, 1995; Kastner & Ungerleider, 2000, 2001).
According to this model, limited neural and cognitive resources necessitate privileged processing of some sensory inputs and associated responses at the expense of others. Limited capacity of cortical sensory regions leads to bottom-up, perceptual interference from competing stimuli (Desimone & Duncan, 1995; Kastner, De Weerd, Desimone, & Ungerleider, 1998; Moran & Desimone, 1985; Reynolds, Chelazzi, & Desimone, 1999) such that distractors reduce the magnitude and efficiency of neural and behavioral responses. However, stimulus-driven sensory competition can be overcome by top-down, intentional feedback from a network of prefrontal and parietal regions (Kastner, Pinsk, De Weerd, Desimone & Ungerleider, 1999; Kastner & Ungerleider, 2000, 2001). But while prefrontal and parietal cortex can mediate sensory competition in visual regions, these top-down sources have their own capacity limits. For example, performance on tasks that draw heavily on executive functions--such as tasks with high working memory load--can deteriorate due to insufficient prefrontal capacity to support efficient attentional filtering. (Lavie & DeFockert, 2003; Lavie et al., 2005). In the current study, we hoped to gain insight into the functional locus of attention deficits in ADHD. Specifically, is distractibility caused by increased competition in sensory cortex, decreased capacity of cognitive control regions, or deficient feedback from control areas to sensory regions? Furthermore, if ADHD can be shown to selectively impair bottom-up or top-down processes, then our findings would provide evidence for modularity and independence of sensory competition and top-down attentional control.
To isolate sensory-level and top-down components of distractor filtering, distractor salience and task difficulty were both parametrically manipulated in an orthogonal fashion. To probe sensory interactions, we manipulated perceptual load by varying distractor salience. Increasing distractor salience has been shown to diminish perceptual responses to target stimuli in ventral stream visual areas (Desimone & Duncan, 1995; Moran & Desimone, 1985; Reynolds et al., 1999). To test the integrity or efficiency of top-down control regions, we manipulated discrimination difficulty in a face discrimination task. We believe that task difficulty was a measure of cognitive load because 1) the face discrimination task involved a comparison of a presented face to an iconic image; 2) the judgment was based on slight differences between morphed face images rather than low-level visual features such as oriented edges or shape; and 3) perceptual decision-making has been shown to be mediated by regions of frontal cortex (Heekeren, et al., 2004).
Healthy children (age 8–13), ADHD children (age 8–13), and healthy adults practiced a face discrimination task and their perceptual threshold was measured in a staircase procedure. This allowed us to tailor task difficulty to each individual’s perceptual threshold. What behavioral patterns were expected for ADHD children? First, if distractibility in ADHD children results from deficient filtering mechanisms in sensory areas, we would expect to see distractor-dependent behavioral deficits. These would manifest as greater interference from high salience distractors than from low salience distractors in ADHD, compared to controls. An inability to filter out the suppressive effects of distractors in sensory areas should produce steeper RT × distractor salience slopes in ADHD than healthy subjects, similar to the effects of lesions of extrastriate visual processing areas V4 and TEO (Buffalo, Bertini, Ungerleider, & Desimone, 2005; De Weerd, Peralta, Desimone, & Ungerleider, 1999; Gallant, Shoup, & Mazer, 2000). We would not expect sensory-driven filtering deficits to be influenced by discrimination difficulty. Alternatively, if distractibility in ADHD results from decreased prefrontal and parietal capacity for top-down modulation, we would expect a different pattern of results. Specifically, more challenging tasks should create more competition for limited resources, and, in turn, greater decrements in distractor filtering in ADHD relative to healthy children. Similarly, if distractibility is due to diminished strength of top-down control, then high salience distractors, which cause the largest sensory interference, would require the strongest top-down control. Thus performance in ADHD relative to healthy children would be most affected by high salience distractors, especially for resource-intensive difficult discriminations. Finally, if distractibility is not due to diminished capacity or strength of top-down signals, but instead reflects a heightened threshold for recruiting top-down control, then ADHD children should be more distractible when deployment of selective attention is under endogenous control and not task-driven. In this instance, we expected that ADHD children would be most distractible when performing easy compared to hard discriminations.
The current study focuses primarily on differences between healthy and ADHD children. However, neurocognitive deficits in ADHD have been attributed to neurodevelopmental immaturity. Thus, for two reasons, inclusion of data from healthy adults also clarifies the nature of any detected performance differences between ADHD and healthy children. First, because selective attention has been studied more extensively with adults than children, most theories of attention are based on data from adults. Inclusion of healthy adults in the current study provides a benchmark against which healthy and ADHD children can be compared, facilitating integration of current theories focused narrowly on attention and on ADHD. Second, data in healthy adults clarifies potential developmental influences on task performance, which in turn shapes views of ADHD as arising from neurodevelopmental immaturity (Shaw, et al., 2007).
2. Methods
2.1 Participants
The study was approved by the NIMH IRB. We tested three groups of subjects: 15 ADHD children (age 8–13, mean=10.3 ± 1.5 years, 11 male), 14 healthy (i.e. typically developing) children (age 9–13, mean=11.6 ± 1.2 years, 9 male), and 15 healthy adults (age 22–42, mean=28.5 ± 5.7 years, 8 male). An additional healthy child was tested, but was discarded from further analysis because his response times were 3.8 standard deviations from the group mean. Consent was obtained from adult subjects and parents/guardians of minor subjects, and assent was obtained from minor subjects. Mental health clinicians, with established inter-rater reliability (kappa > 0.70), conducted a semi-structured psychiatric interview (Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime) with each child and his/her parent or guardian, and psychiatrists reviewed the child’s medical history. Additional behavioral assessments of children included the Wechsler Abbreviated Scale of Intelligence and the Conners’ Parent Rating Scale-Revised-Long. Exclusion criteria common to all groups were non-corrected visual disturbances, IQ<75, any developmental or neurological disorder, and use of steroid-based asthma or allergy medication.
Healthy children had no past or present psychiatric disorder. ADHD children met the DSM-IV criteria for ADHD. The ADHD group included 7 Combined type and 8 Inattentive type patients. Exclusion criteria for ADHD patients were: any DSM-IV Axis I disorder other than oppositional defiant disorder (ODD), separation or generalized anxiety disorder, and use of medication other than short-acting stimulants. The ADHD cohort was made up of 9 children with no comorbid illnesses, 3 children with comorbid ODD, and 3 children with comorbid separation anxiety. The ADHD and healthy children did not differ in IQ (ADHD: range=75–139, mean IQ=108 ± 18; healthy children: range=83–135, mean IQ=109 ± 17). The ADHD group included 11 children who were receiving stimulant medication and 4 children who were not currently receiving medication. For those children receiving stimulant medication, medication was withheld for 48 hours before behavioral testing. In accordance with the recommendations of the NIMH IRB, a 48-hour period of medication withdrawal was chosen to minimize interference with academic performance and behaviour, since children took their last dose of medication on Friday morning and were tested on Sunday afternoon.
2.2 Stimuli and Procedure
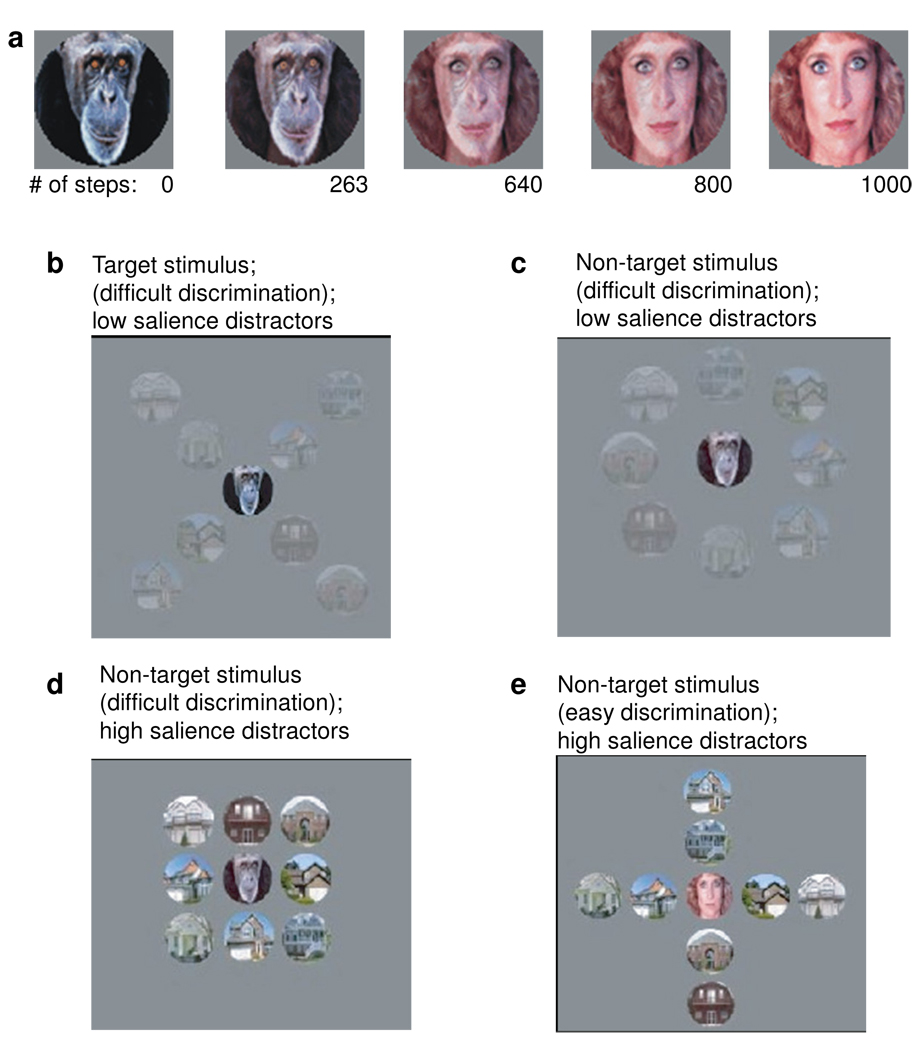
Subjects participated in a single testing session which was divided into two parts: training on a perceptual discrimination task and subsequent performance of the task with stimuli customized for each subject’s performance, as described below. Figure 1 depicts examples of stimuli and typical displays. A stimulus set was created by morphing (in 1000 steps) the image of an ape’s face with the image of a woman’s face. (Figure 1a) During training, the face stimuli (2 deg) were presented in blocks by themselves or surrounded by 8 distractors (2 deg each) which were images of houses. The attended face image appeared at a constant retinotopic location, but the distractor houses appeared randomly in one of 8 spatial configurations. All displays were presented in the upper left visual field, centered approximately 5 degrees peripherally, while subjects maintained fixation on a central red dot. The upper left visual field was chosen to maximize distractor interference, based upon other related behavioral studies (unpublished) in our laboratory. On each trial, the fixation dot appeared first and was joined, after 500 ms, by the stimulus display. Subjects indicated whether the item in the center of each stimulus configuration was the target (original ape) or a non-target (one of the morphs). Stimulus displays remained visible for a maximum of 2000 ms or were terminated by subject response. Perceptual thresholds (75% correct) were determined using a staircase procedure (8 reversals per staircase), in two staircases with no distractors present and two staircases with high salience distractors. The average of the latter two thresholds (from trials which included distractors) was used during subsequent testing.
Fig. 1.
Examples of stimuli and stimulus configurations. 1a) Examples of target images created from morphing an image of an ape with an image of a woman, in 1000 steps. Perceptual thresholds could be calculated using the number of morphing steps to quantify the images. 1b–d) Examples of stimulus groupings, illustrating different levels of distractor salience and spatial configurations.
After perceptual thresholds were determined, participants were presented with 3–4 blocks of trials (total=240–300) where distractor salience (low, medium, or high) and face discrimination difficulty (easy or difficult) were randomly varied across trials, but counterbalanced within each block. (Figure 1b–e). Distractor salience was manipulated by “graying” the stimuli, by moving the red (r), green (g) , and blue (b) values equally closer to the values of the gray background (130, 130, 130).1
Task difficulty was also varied: Easy discriminations were defined as those in which the woman’s face was presented. Difficult discriminations were defined as those trials in which the original ape or the morph corresponding to the subject’s perceptual threshold appeared. By customizing the stimuli to each subject’s perceptual threshold, we aimed to equate task difficulty between all participants and all subject groups.
During experimental blocks of trials, we measured response time and error rates. Our primary aim was to investigate how task difficulty interacted with distractor salience in the three subject groups. For this analysis, response times and error rates from the mixed display experiment were measured and submitted to analysis with repeated measure ANOVA, with subject group as a between subject factor and discrimination difficulty and distractor salience as within subjects factors. Specific contrasts between factors were also tested with t-tests. Additionally, because we expected response time to be linearly dependent on distractor salience, we quantified the “cost” of distractor filtering for each subject by calculating a difference score of response time in the presence of high salience distractors vs. response time in the presence of low salience distractors. We used these within-subject difference scores to examine the interaction of subject group and task difficulty with distractor interference.
Small but significant differences in age occurred between the groups of ADHD and healthy children (t=2.54, p<.01). Accordingly, we included age as a covariate. There was no main effect of age on response time, distractor cost, or error rate, nor were there any significant interactions of age with distractor salience or task difficulty, for response time, cost, or error rate. Therefore, we report the results of ANOVA, excluding age as a covariate, in the results discussed below. Lastly, ADHD subjects in many prior studies exhibited greater intra-individual variance than healthy subject. As a result, we also calculated the coefficient of variation (standard deviation of RT/mean RT) for each subject, in order to explore whether intra-individual variability was correlated with group, distractor salience, or task difficulty.
3. Results
During perceptual training, we found that healthy adults had significantly lower perceptual thresholds than healthy children (t=2.87, p=.004) and ADHD children (t=1.92, p=.033), but ADHD and healthy children did not differ. Thus, when searching for targets, adults discriminated more subtle differences among morphed images than healthy and ADHD children, who required bigger differences between the stimuli in order to discriminate between targets and non-targets.
Turning next to the experimental blocks with randomly varying distractor salience, we found a significant main effect of task difficulty (response times, F(1,41)=129.5, p<.001; error rates, F(1,41)=231.9, p<.001). Participants took longer to respond (802 ms ± 20) and made more errors (28% ± 1) for difficult relative to easy discriminations (600 ms ± 15; 3% ± 0) . We also found a significant main effect of subject group (response times, F(2,41)=4.47, p=.017; error rates, F(2,41)=8.0, p=.001). ADHD children responded significantly slower (809 ms ± 20) than healthy children (631 ms ± 26, t=5.43, p<.00001) or adults (661 ms ± 21, t=5.08, p<.00001). There was no significant difference in response time between healthy children and adults. Both ADHD and healthy children made more errors (ADHD: 17% ± 2, t=2.76, p=.003; healthy children: 19% ± 2, t=3.48, p=.0003) than healthy adults (11% + 1) but did not differ from each other. There was no main effect of distractor salience.
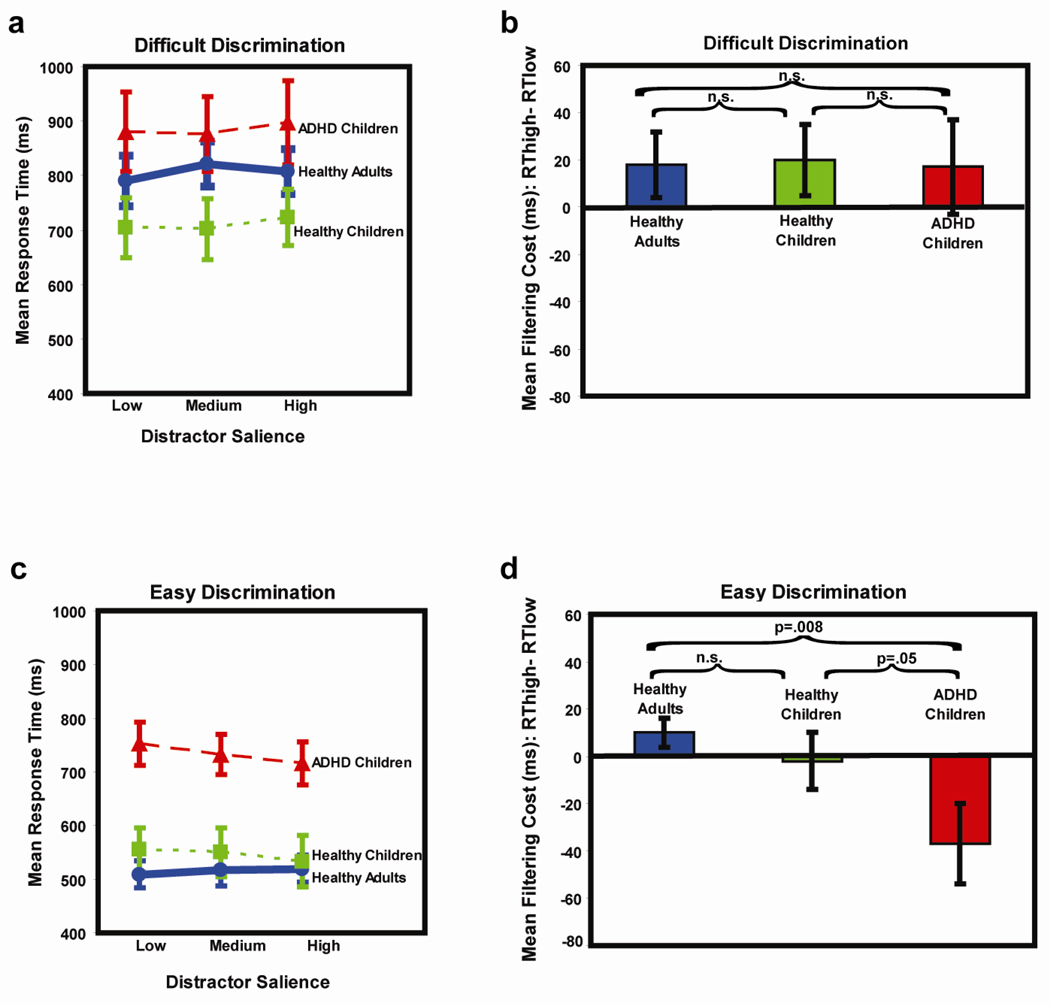
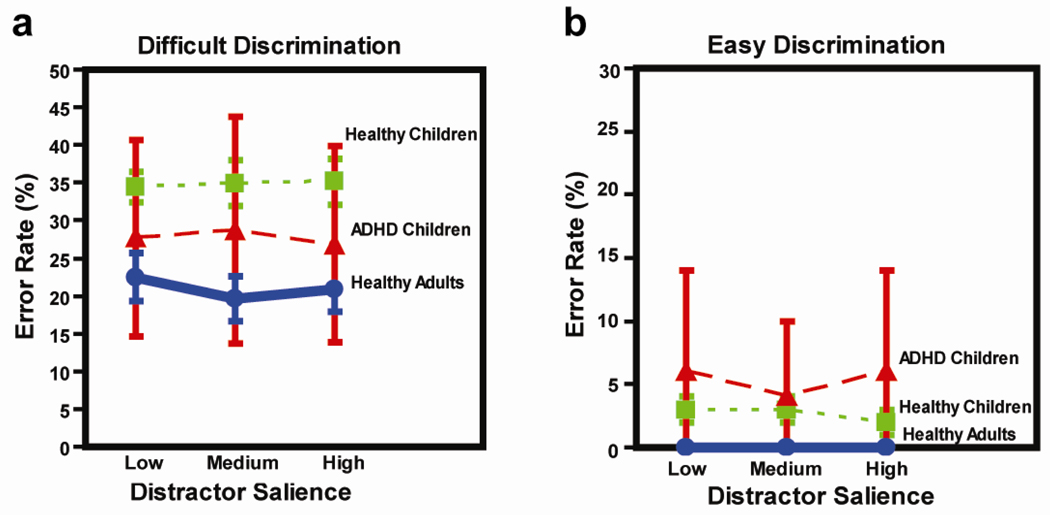
There was a significant interaction of discrimination difficulty with subject group (response times, F(2,41)=6.88, p=.003; error rates, F(2,41)=4.5, p=.017). This interaction reflected the fact that the largest differences between ADHD children and other subject groups emerged during easy, compared to difficult, discriminations. For difficult discriminations (Fig 2a), ADHD children responded slower (884 ms ± 31) than healthy adults (806 ms ± 24, t=2.01, p=.024) and children (709 ms ± 41, t=3.40, p<.001), whereas healthy children responded significantly faster than healthy adults (t=−2.01, p=.024). For difficult discriminations (Fig 3a), ADHD children made more errors (28% ± 2) than healthy adults (21% ± 2, t=2.60, p<.001) but fewer errors than healthy children (35% ± 1, t=−2.86, p<.005). The difference in error rate between healthy children and healthy adults was also significant (t=6.49, p<.00001). For easy discriminations (Fig 2c), ADHD children were much slower to respond (734 ms ± 22) than healthy adults (515 ms ± 15; t=8.16, p<.000001) and healthy children (553 ± 25; t=5.37, p<.000001). For easy discriminations, there were no significant differences in response time between healthy children and adults. For easy discriminations (Fig 3b), ADHD children made significantly more errors (5% ± 1) than healthy adults (0%, t=4.58, p<.0001) and children (3% ± 0; t=2.18, p=.016). The difference between healthy children and adults was also significant (t=5.41, p<.0001). As noted above, these between-group differences for easy discriminations were larger than those found for difficult discriminations, as reflected in the group-by-difficulty interactions.
Fig. 2.
In 2a and 2c, response time is plotted as a function of distractor salience for difficult and easy discriminations, for each of the three participant groups. In 2b and 2d, filtering cost (the intra-individual difference in response time for trials with high salience distractors and response time for trials with low salience distractors) is plotted for difficult and easy discriminations, for each subject group.
Fig. 3.
Error rate as a function of distractor salience for easy and difficult discriminations.
There was also a significant interaction of task difficulty with distractor salience (response times, F(2,82)=3.71, p=.029; error rates, n.s.) This interaction reflects the fact that reponse times increased as a function of distractor salience for difficult disciminations (low salience distractors: 793 ms ± 35; medium salience distractors: 802 ms ± 33; high salience distractors: 811 ms ± 34), but there was no linear increase in response time for easy disciminations (low salience distractors: 607 ms ± 26; medium salience distractors: 601 ms ± 26; high salience distractors: 597 ms ± 25). The difference in response time between difficult discriminations with low and high salience distractors was significant (t=−1.95, p=.029); no other contrasts reached significance.
Inspection of Fig 2a and 2c, suggests that the subject groups showed different patterns of interaction of distractor salience and task difficulty. Nonetheless, the triple interaction of difficulty × salience × subject group did not reach significance. However, the main effect of task difficulty indicated that there were very large differences in response times between easy and difficult discriminations. This large main effect of task difficulty and inter-subject variability may have obscured smaller differences in response times between trials with low and high salience distractors. Consequently, in further analysis, we focused on the within-subject “filtering costs”.
For difficult and easy discriminations, we calculated the “filtering cost” imposed by distractors of increasing salience, as described in the methods. Discrimination difficulty and subject group did not have significant main effects on filtering cost, but there was a significant interaction of the two factors (F(2,39)=3.82, p<.05). For difficult discriminations, all three groups showed the same pattern of results with increasing response times as a function of distractor salience (Fig 2a) and positive filtering costs (Fig 2b). There was no significant difference between groups in the magnitude of filtering costs for difficult discriminations (healthy adults: 18 ± 14 ms; healthy children: 20 ± 15 ms; ADHD children: 17 ± 20 ms, all p’s>.40). However, groups manifested distinct performance patterns for easy discriminations (Fig 2d). Healthy adults and children did not pay a significant filtering cost during easy discriminations: Their filtering costs (adults: 10 ± 5 ms; children: −2 ± 12 ms) did not differ from each other (t=−0.88 , p=.20 ) and were not significantly greater than zero (adults: t=1.7, p>.10; children: t=−.1, p>.10). Unexpectedly, ADHD children had a negative filtering cost (−37 ± 17 ms) which was significantly less than zero (t=−2.15, p<.05), indicating that they were slower to respond on trials with low salience distractors than on trials with high salience distractors. For easy discriminations, the filtering costs of ADHD children were significantly different than those of healthy children (t=−1.69 , p=.05 ) and adults (t=−2.58 p<.01 ), as shown in Fig. 2d.
We also looked for differences in response variability between ADHD children and healthy peers, especially because intra-individual variability has been noted as a very reliable behavioral marker of ADHD (Castellanos et al., 2005; Castellanos & Tannock, 2002). Table 1 lists the coefficient of variation (CV) for all 3 subject groups as a function of discrimination difficulty and distractor salience. There was a main effect of group (F=6.49, p<.005), with healthy adults exhibiting lower intra-individual variability (mean CV, collapsed across experimental factors: .255) than healthy children (mean CV:.370, t=3.09, p<.005) and ADHD children (mean CV: .398, t=5.15, p<.0001). Although there was a trend toward ADHD children showing more variability than healthy children, there was no statistical difference in average CV for the two groups (t=0.65, p=.26). Intra-individual variability was not correlated with task difficulty or distractor salience (i.e. no main effects), nor did these experimental factors significantly interact with the main effect of group, although there was a trend toward an interaction of group and task difficulty (F=1.86, p=.17), with increased variability in ADHD children relative to healthy children when performing easy discriminations.
Table 1.
Intra-Individual Variability: Mean (SEM) Coefficient of Variation.
| group | difficult discrimination, low salience distractors: |
difficult discrimination, medium salience distractors |
difficult discrimination, high salience distractors |
easy discrimination, low salience distractors |
easy discrimination, medium salience distractors |
easy discrimination, high salience distractors: |
|---|---|---|---|---|---|---|
| healthy adults |
.259 (.015) | .269 (.012) | .251 (.012) | .243 (.031) | .238 (.022) | .247 (.019) |
| healthy children |
.362 (.034) | .378 (.026) | .388 (.042) | .332 (.036) | .359 (.041) | .383 (.045) |
| ADHD children |
.378 (.029) | .376 (.028) | .390 (.033) | .404 (.026) | .419 (.030) | .396 (.031) |
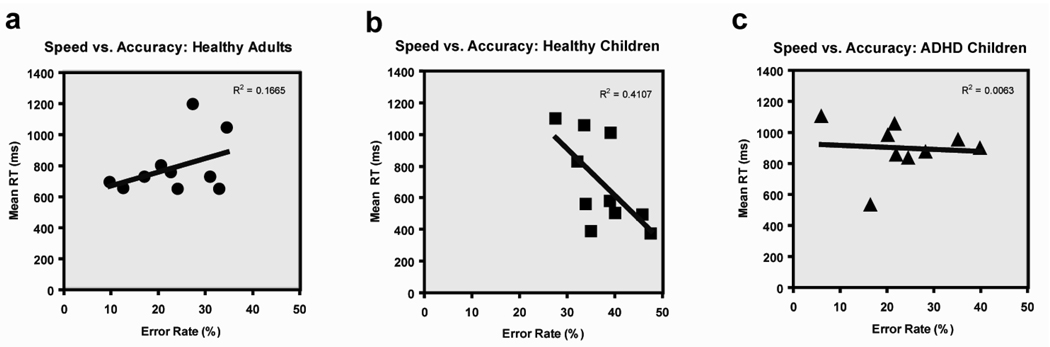
Because we found significant differences in error rates between groups—namely that ADHD children made more errors than healthy children and adults for easy discriminations (Fig 3b) and that healthy children made more errors than adults and ADHD children for difficult discriminations (Fig 3a)—we explored whether response time differences were due to speed-accuracy trade-offs. Error rates were inversely correlated with response time (Fig 4b) for healthy children (r2=0.41), but not for healthy adults (r2=.17; Fig 4a), suggesting that healthy children but not adults employed a strategy with a speed-accuracy tradeoff. Unlike healthy children, ADHD children did not exhibit a speed-accuracy trade-off (r2=.01; Fig 4c). Hence, the increased error rate of ADHD children for easy discriminations reflects greater distractor interference on target processing rather than a change in motor response strategies (i.e. more impulsive button pressing).
Fig. 4.
Analysis of speed-accuracy trade-off for healthy adults, healthy children, and ADHD children.
4. Discussion
4.1 Implications of Main Findings for Functional Locus of Filtering Deficits in ADHD
We conducted an experiment in which distractor salience and perceptual decision difficulty were manipulated in order to characterize and compare the attentional filtering abilities of children with ADHD, healthy children, and healthy adults. We were interested in testing whether distractibility in ADHD results from increased bottom-up sensory competition or from deficient top-down attentional control. Our experimental manipulations of distractor salience and discrimination difficulty revealed that ADHD children exhibited the most differences from healthy children and adults when they were performing easy discriminations. When performing easy tasks, ADHD children were much slower and made more errors than healthy adults and children. Moreover, there was a striking interaction of distractor salience and task difficulty: When performing difficult discriminations, ADHD children were able to filter out distractors as efficiently as healthy adults and children, as evidenced by the fact that all three groups exhibited similar “filtering costs” as a consequence of increasing distractor salience. For easy discriminations, by contrast, there was a markedly different pattern: healthy children and adults filtered out high salience distractors as easily as low salience distractors, but ADHD children were slower to respond on trials with low salience distractors. These results were unexpected because they imply that distractors which were so dim they almost fade into the background caused more behavioral interference for ADHD children than distractors which were bright and obvious.
How does this pattern of results relate to our hypotheses regarding whether distractibility arises from bottom-up or top-down processes? This pattern of findings suggests that distractibility does not reflect greater bottom-up sensory competition. If increased sensory competition underlies distractibility, then one would expect that filtering impairments would increase linearly as a function of distractor salience. Instead, we found that ADHD children were most distractible with low salience distractors. Also, if filtering difficulties were due to bottom-up mechanisms, one would not expect an interaction of salience with task difficulty. But we found that ADHD children exhibited different “filtering costs” from healthy children and adults when engaged in easy discriminations, but not difficult discriminations.
Alternatively, how consistent are our findings with the hypothesis that distractibility in ADHD is caused by deficits in top-down control? Here, there are two possibilities to consider: 1) that ADHD children have decreased capacity or efficiency of top-down control; or 2) that ADHD children have a higher threshold for activating top-down control of attention. Our findings suggest that ADHD children can filter as efficiently as healthy adults and children when engaged in difficult discriminations. This would argue against a general deficiency in the effectiveness of top-down control. It also argues against capacity limitations because one would expect that capacity limitations would cause a linearly increasing deficit as cognitive load increases, whereas we found no increased filtering deficit with the high load, difficult discrimination. Counter-intuitively, we found that ADHD children had difficulty filtering out distractors on trials with easy discriminations and low salience distractors. This finding is consistent with a heightened threshold for “triggering” top-down control mechanisms. Thus, when distractors are very salient or the behavioral task is difficult, then perceptual and task demands drive the activation of top-down control processes. However, when distractors are of low salience and the task is easy, then ADHD children do not recruit top-down control as readily as their healthy peers or adults. These data suggest that during less-demanding easy discriminations, ADHD children spread their attention over multiple objects and apparently process distractors rather than filter them out. If their attention were divided between target and distractors, then the low salience distractors would be more difficult to perceive, and thus would cause longer response times than high salience distractors, which is exactly what was found.
4.2 Consistency of Study Findings with Attentional Load Theory
While historical debate in the attention literature pitted “early” vs. “late” target selection (Driver, 2001), current theories acknowledge that task demands influence the anatomical locus of target selection and distractor filtering (Lavie, 2005; Lavie & DeFockert, 2003). Filtering capacity is dependent on load, but the nature of the load determines at what stage attention must be deployed to overcome resource impasses. When perceptual load is high (for example, when distractors are numerous or featurally similar to the target), then target selection occurs in sensory processing regions and distractors are not processed in depth, as evidenced by less response interference and smaller visually evoked brain activations to distractor stimuli (Lavie & DeFockert, 2003; Rees, Frith, & Lavie, 1997). On the other hand, when cognitive load is high (for example, when subjects must memorize random strings of many letters or numbers), then bottlenecks in resources occur later in processing, presumably because working memory and selective attention both engage fronto-parietal control regions. Under these conditions, distractors cause more behavioral interference and evoke robust brain activations in visual areas (Lavie, 2005; Yi, Woodman, Widders, Marois, & Chun, 2004), consistent with late distractor filtering, subsequent to early sensory processing.
How do the behavioral patterns of ADHD children relate to the load-dependent model of attentional filtering? If distractibility is the result of bottom-up bottlenecks such as an inability to process multiple sensory inputs, then ADHD children would be more sensitive to manipulations of perceptual load than healthy children. Previous studies testing this hypothesis have failed to find perceptual load deficits in ADHD (Huang-Pollock et al., 2005; Mason et al., 2003). Our findings also argue against impaired bottom-up filtering mechanisms in ADHD: Children with ADHD were not impaired at distractor filtering for difficult discrimination trials and response time was inversely correlated with distractor salience on easy discrimination trials. On the other hand, if distractibility is the result of late top-down processing limitations, then manipulations of cognitive load would reveal bottlenecks due to shared frontal lobe circuits for distractor filtering and executive function. We did, in fact, find that cognitive load (i.e. discrimination difficulty) interacted with distractibility. However, the interaction did not implicate smaller capacity/weaker modulation as the underlying mechanism for attentional deficits in ADHD children. Instead, ADHD children had the most difficulty ignoring distractors with the combination of low perceptual load (low salience distractors) and low cognitive load (easy discriminations). Of interest, these results are consistent with a study that examined individual differences in distractor interference in adults who scored high on self-report measures of inattentiveness, but were not clinically diagnosed with ADHD. Forster & Lavie (2007) found that, when perceptual load was low, more distractible individuals showed greater distractor interference than their more attentive peers, but performance did not differ between groups when perceptual load was high.
4.3 Relevance of Current Findings to Influential Models of ADHD
Our results thus indicate that attentional filtering in ADHD children is subject to failures of top-down deployment of selective attention. The inefficient engagement of attention in ADHD under low cognitive load, as found in this study, is consistent with other studies that have pointed to deficits in vigilance or alertness in ADHD. (Huang-Pollock & Nigg, 2003; Huang-Pollock et al., 2007; Sergeant, 2005). The slow and/or variable responding of ADHD subjects has been shown in numerous studies and this has been interpreted as evidence for deficient alertness (Booth et al., 2005; Huang-Pollock & Nigg, 2003; Huang-Pollock et al., 2005, 2006; Mason et al., 2003, 2005; Nigg et al., 1997; Novak et al., 1995; Oberlin et al., 2005; van der Meere & Sergeant, 1988). Meta-analysis of behavioral studies of ADHD children point to robust differences in sustained attention, but, in contrast to the current study, only weak and inconsistent deficits in selective attention (Huang-Pollock, et al., 2005; Huang-Pollock & Nigg, 2003). Our findings, however, implicate a way in which sustained and selective attention interact since low cognitive load leads to loss of focus on relevant items and increased attention to irrelevant stimuli. When task demands do not force ADHD children to deploy selective attention, they have higher thresholds for the volitional activation of top-down modulation.
Thus, our results are consistent with “multiple pathway” or integrative models of ADHD which posit that ADHD reflects multiple, interacting behavioral and neural differences (Castellanos & Tannock, 2002; Sagvolden et al, 2005; Sergeant, 2005; Sergeant, Oosterlaan, & van der Meere, 1999; Sonuga-Barke, 2005;). Sergeant’s cognitive-energetic model of ADHD is especially relevant to our findings. Within the context of Sergeant’s model, easy discrimination trials may fail to engage ADHD children and thus result in lower “energetic pools” for effort and activation. The energetic pools interact with executive processes such as cognitive control and selective attention. Thus, low “energy” would fail to trigger executive control. Similarly, difficult discriminations may spur ADHD children to exert more effort and may cause more tonic activation, which, in turn, would aid in recruiting executive top-down control processes.
While multiple pathway models of ADHD may provide a theoretical framework for understanding how task difficulty and selective attention interact in ADHD children, we also found a surprising interaction of bottom-up distractor salience. Why do low salience distractors cause the most interference in easy discriminations? A recent brain imaging study of healthy adults suggests a way in which bottom-up information (such as distractor salience) may interact with energetic pools and central executive functions. Tsushima et al., (2006) found that task-irrelevant, subthreshold coherent-motion stimuli led to stronger disturbance of task performance than did suprathreshold motion stimuli. Furthermore, the subthreshold relative to suprathreshold stimuli were associated with greater activation of visual cortex and diminished activation of the lateral prefrontal cortex—a cortical region important for top-down control. These findings imply an important relationship between distractor salience and activation of inhibitory processes. Applying these results to our study, the easy discrimination with low salience distractors combines two non-optimal experimental factors to form the “perfect storm” scenario for ADHD children: low salience distractors and easy (or seemingly “effortless”) discrimination may together contribute to reduced activation of control processes.
4.4 Intra-individual Response Variability
We also measured intra-individual response variability, but failed to find a difference between ADHD and healthy children. Elevated response variability in ADHD has been reported widely and has been hypothesized to result from temporal processing deficits, a putative endophenotype of ADHD (Castellanos et al., 2005; Castellanos & Tannock, 2002). Intra-individual response variability has been frequently measured by the coefficient of variation of trial response times, but more nuanced analysis has been possible by separating short and long response time trials (Williams et al., 2007) or by calculating the power spectrum (Castellanos et al., 2005; Di Martino et al., 2008; Gilden & Hancock, 2007; Johnson et al, 2006). Not only have ADHD subjects been found to have increased variability relative to healthy peers, but intra-individual response variability has been found to be correlated with specific dopaminergic-system gene variants (Bellgrove, Hawi, Kirley, Gill, & Robertson, 2005) and brain activation patterns (Rubia, Smith, Brammer, Toone, & Taylor., 2007; Suskauer, et al., 2008) . Given the preponderance of evidence for increased intra-individual response variability in ADHD, it is surprising that we did not find a significant difference in variability between ADHD and healthy children, although both groups had significantly elevated response variability relative to healthy adults. One possibility is that the lack of significance reflected low power due to multiple experimental factors (e.g. discrimination difficulty, distractor salience). This possibility is bolstered by the fact that there was a trend supporting an interaction of discrimination difficulty and group (i.e. ADHD children had greater variability than healthy children on easy discrimination trials). A second reason, however, why group differences in intra-individual response variability was not found in this study may concern a fundamental difference in the task used in this study vs. previous investigations: We utilized a selective attention task that involved a complex perceptual discrimination, whereas most other studies have used tasks targeted at response inhibition or interference such as the Stop task, Go/No-Go, or Ericksen flanker (Bellgrove et al, 2005; Castellanos et al., 2005, de Zeeuw et al., 2008; Di Martino et al, 2008; Johnson et al., 2006; Rubia et al., 2007; Suskauer et al., 2008; Williams, Strauss, Hultsch, Hunter, & Tannock, 2007). Increased response variability in ADHD may be specific to motor control and output, rather than reflecting variability in the temporal dynamics of other cognitive processes, such as perceptual decision-making or selective attention.
4.5 Relevance of Current Findings for Models of Typical Development
In addition to investigating the attentional deficits of ADHD children, the current study also provided us with an opportunity to explore attentional filtering in typical development and to compare bottom-up and top-down processes within each subject. During perceptual training, we found that healthy children had lower perceptual thresholds for face discrimination than did healthy adults. This finding is consistent with many behavioral studies that have documented that face recognition continues to improve during childhood (Carey & Diamond, 1977; Carey, Diamond, & Woods, 1980; Mondloch, Dobson, Parsons, & Maurer, 2004 ) and with more recent brain imaging studies showing that the development of face expertise in the transition from childhood to adolescence is accompanied by increases in the size and decreases in inter-subject spatial variability of face-selective regions (Golarai et al., 2007; Scherf, Behrmann, Humphreys, & Luna, 2007 ). There was no difference in perceptual thresholds of ADHD vs. healthy children, indicating that the ADHD children did not have perceptual deficits, nor did they have global developmental delays. In the attention task, we found that healthy children made more errors than healthy adults and were faster than adults to respond on difficult discrimination trials, but that these reduced response times reflected a speed-accuracy trade-off.
There were no significant differences in “filtering cost” between healthy children and adults, suggesting that in typical development, children attain mature levels of attentional filtering by middle childhood to early adolescence (age 8–12). These results complement previous developmental studies of selective attention which mostly focussed on children younger than 8 years old. For example, several studies found mature exogenous orienting of attention by age 6 (Enns & Brodeuer, 1989; Rueda et al, 2004). In contrast to early development of exogenous orienting, endogenous orienting of attention, measured by cueing paradigms, continues to mature into middle childhood (Brodeur & Enns, 1997, ages 6–10). Likewise, attentional filtering appears to continue to improve in school age children. Children are more susceptible than adults to perceptual interference from distractors (Enns & Akhtar, 1989, ages 4–7, and 20; Hommel, Li, & Li, 2004, age 6–89), and also show some evidence for greater response interference on tasks which rely heavily on consistent response mapping (Enns, 1993, ages 7, 10, and 22). Endogenous orienting, distractor filtering and conflict resolution appear to be three distinct processes, with separate, but interacting, developmental trajectories (Akhtar & Enns, 1989; Enns & Cameron, 1987).
Although there were statistically significant differences between healthy and ADHD children, especially for mean response time and for “filtering cost” during easy discriminations, there was also some overlap between the two groups, rather than a crisp boundary. Inspection of the individual data from healthy children reveals that some of them also exhibited negative (greater response time for low salience vs. high salience distractors) cost for easy discriminations, leading to a group average of near-zero cost. None of the adult subjects showed this pattern of response. Most of the ADHD subjects had large negative costs. Thus, one intriguing possibility is that inefficient recruitment of endogenous attention is a general developmental trend, with ADHD children exhibiting more severe problems or perhaps even a lag in development. This is an interesting avenue for future investigations, with a large subject sample.
4.6 Study Limitations and Conclusions
Sample size was a limitation of the present study. Clinical populations are heterogeneous in severity and type of behavioral symptoms. For ADHD, this is particularly evident, because there are multiple subtypes. With a larger sample size, it would have been possible to compare across subtypes of ADHD. Additionally, even in typical development, there is variation in developmental trajectories. With a larger sample, it would have also been possible to look at smaller periods of development within the range represented by the current study (age 8–12). Lastly, although the ADHD subjects were tested when they were medication-free, they were not medication-naïve: It is possible that long-term use of stimulants could be a factor in cognitive and behavioral differences between ADHD and healthy children.
In spite of the limitations of the present study, these initial behavioral findings suggest that attention problems in ADHD may implicate a failure to endogenously deploy attentional filtering under low task demands, rather than a failure in distractor-driven or top-down control mechanisms. In future research, we hope to replicate these findings with a larger sample, uncover the neural correlates of distractibility in ADHD children, and also explore in more detail what turns the “ignition key” for selective attention.
Acknowledgments
We thank J. Snow, W. Wheeler, A. Zametkin, K. Towbin, J. Cameron, L. Justement, S. Smith, and M. Ritter for skilled clinical screening; C. Sims for patient recruitment and scheduling; A. Chronis, N. Beers, and J. Joseph for patient referral; and B. Kaplan for scientific encouragement. This work was supported by the NIMH Intramural Research Program and NSF Independent Research and Development funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Specifically, look-up table values were altered according to the following algorithm: “new [r,g,b]=original [r,g.b] + (([130, 130, 130]-original [r,g,b])* percent_gray_in_decimal_format.)” For a stimulus that was not grayed, percent gray would equal “0” and the new {r,g,b] would be equivalent to the old. For a stimulus that was completely grayed, percent gray would equal “1”, which would render the new [r,g,b] to be [130,130,130], the same color as the background. For the experiments reported in this paper, high salience distractors were not grayed at all, medium salience distractors underwent 40% graying, and low salience distractors underwent 80% graying.
References
- Akhtar N, Enns JT. Relations between covert orienting and filtering in the development of visual attention. Journal of Experimental Child Psychology. 1989;48:315–334. doi: 10.1016/0022-0965(89)90008-8. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral Inhibition, Sustained Attention, and Executive Functions: Constructing a Unifying Theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Hawi Z, Kirley A, Gill M, Robertson IH. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: Sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43:1847–1857. doi: 10.1016/j.neuropsychologia.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Luzzatti C. Unilateral neglect of representational space. Cortex. 1978;14:129–133. doi: 10.1016/s0010-9452(78)80016-1. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD) The Journal of Child Psychology and Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- Brodeur DA, Enns JT. Covert visual orienting across the lifespan. Canadian Journal of Experimental Psychology. 1997;51:20–35. doi: 10.1037/1196-1961.51.1.20. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Bertini G, Ungerleider LG, Desimone R. Impaired filtering of distractor stimuli by TE neurons following V4 and TEO lesions in macaques. Cerebral Cortex. 2005;5:141–151. doi: 10.1093/cercor/bhh117. [DOI] [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biological Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Butter CM. Varieties of attention and disturbances of attention: A neuropsychological analysis. In: Jeannerod M, editor. Neurophysiological and Neuropsychological Aspects of Spatial Neglect. North-Holland: Amsterdam; 1987. pp. 1–24. [Google Scholar]
- Carey S, Diamond R. From piecemeal to configurational representation of faces. Science. 1977;195:312–314. doi: 10.1126/science.831281. [DOI] [PubMed] [Google Scholar]
- Carey S, Diamond R, Woods B. The development of face recognition—a maturation component? Developmental Psychology. 1980;16:257–269. [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Vauss YC, Vaituzis AC, Dickstein DP, Sarfatti SE, Rapoport JL. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biological Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit hyperactivity disorder: The search for endophenotypes. Nature Reviews Neuroscience. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- De Weerd P, Peralta MR, 3rd, Desimone R, Ungerleider LG. Loss of attentional selection after extrastriate cortical lesions in macaques. Nature Neuroscience. 1999;2:753–758. doi: 10.1038/11234. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- De Zeeuw P, AArnoudse-Moens C, Biljhout J, Koenig C, Uiterweer AP, Papanikolau A, Hoogenraad C, Imandt L, De Been D, Sergeant JA, Oosterlaan J. Inhibitory performance, response speed, intraindividual variability, and response accuracy in ADHD. Journal of American Academy of Child Adolescent Psychiatry. 2008;47:808–816. doi: 10.1097/CHI.0b013e318172eee9. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Ghaffari M, Curchack J, Reiss P, Hyde C, Vannucci M, Petkova E, Klein DF, Castellanos FX. Decomposing intra-subject variability in children with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;64:607–614. doi: 10.1016/j.biopsych.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle AE. Executive functions in attention-deficit/hyperactivity disorder. Journal of Clinical Psychiatry. 2006;67:21–26. [PubMed] [Google Scholar]
- Driver J. A selective review of selective attention research from the past century. British Journal of Psychology. 2001;92:53–78. [PubMed] [Google Scholar]
- Enns JT. What can be learned about attention from studying its development? Canadian Psychology. 1993;34:271–281. [Google Scholar]
- Enns JT, Akhtar N. A developmental study of filtering in visual attention. Child Development. 1989;60:1188–1199. [PubMed] [Google Scholar]
- Enns JT, Brodeur DA. A developmental study of covert orienting to peripheral visual cues. Journal of Experimental Child Psychology. 1989;48:171–189. doi: 10.1016/0022-0965(89)90001-5. [DOI] [PubMed] [Google Scholar]
- Enns JT, Cameron S. Selective attention in young children: The relations between visual search, filtering, and priming. Journal of Experimental Child Psychology. 1987;44:38–63. doi: 10.1016/0022-0965(87)90021-x. [DOI] [PubMed] [Google Scholar]
- Farah M. Disorders of object recognition and what they tell us about normal vision. Bradford Books: MIT Press; 1990. [Google Scholar]
- Faraone SW, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2:104–113. [PMC free article] [PubMed] [Google Scholar]
- Forster S, Lavie N. High perceptual load makes everybody equal: eliminating individual differences in distractibility with load. Psychological Science. 2007;18:377–381. doi: 10.1111/j.1467-9280.2007.01908.x. [DOI] [PubMed] [Google Scholar]
- Friedman-Hill SR, Robertson LC, Desimone R, Ungerleider LG. Posterior parietal cortex and the filtering of distractors. Proceedings of the National Academy of Sciences, USA. 2003;100:4263–4268. doi: 10.1073/pnas.0730772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant JL, Shoup RE, Mazer JA. A human extrastriate cortical area that is functionally homologous to Macaque area V4. Neuron. 2000;27:227–235. doi: 10.1016/s0896-6273(00)00032-5. [DOI] [PubMed] [Google Scholar]
- Gilden DL, Hancock H. Response variability in attention-deficit disorders. Psychological Science. 2007;18:796–802. doi: 10.1111/j.1467-9280.2007.01982.x. [DOI] [PubMed] [Google Scholar]
- Golarai G, Chahremani DG, Whitfield-Gabrieli S, Reiss A, Eberhardt JL, Gabrieli JDE, Grill-Spector K. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nature Neuroscience. 2007;10:512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B, Li KZH, Li S-C. Visual search across the lifespan. Developmental Psychology. 2004;40:545–558. doi: 10.1037/0012-1649.40.4.545. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Nigg JT. Searching for the attention deficit in attention deficit hyperactivity disorder: the case of visuospatial orienting. Clinical Psychology Review. 2003;23:801–830. doi: 10.1016/s0272-7358(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Nigg JT, Carr TH. Deficient attention is hard to find: applying the percpetual load model of selective attention to attention deficit hyperactivity disorder subtypes. The Journal of Child Psychology and Psychiatry. 2005;46:1211–1218. doi: 10.1111/j.1469-7610.2005.00410.x. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Nigg JT, Halperin JM. Single dissociation findings of ADHD deficits in vigilance but not anterior or posterior attention systems. Neuropsychology. 2006;20:420–429. doi: 10.1037/0894-4105.20.4.420. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Kelly SP, Bellgrove MA, Barry E, Cox M, Gill M, Robertson IH. Response variability in attention deficit hyperactivity disorder: Evidence for neuropsychological heterogeneity. Neuropsychologia. 2007;45:630–638. doi: 10.1016/j.neuropsychologia.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Kastner S, De Weerd P, Desimone R, Ungerleider LG. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. The neural basis of biased competition in human visual cortex. Neuropsychologia. 2001;39:1263–1276. doi: 10.1016/s0028-3932(01)00116-6. [DOI] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: Selective attention under load. Trends in Cognitive Sciences. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lavie N, DeFockert JW. Contrasting effects of sensory limits and capacity limits in visual selective attention. Perception & Psychophysics. 2003;62:202–212. doi: 10.3758/bf03194795. [DOI] [PubMed] [Google Scholar]
- Mason DJ, Humphreys GW, Kent LS. Exploring selective attention in ADHD: visual search through space and time. The Journal of Child Psychology and Psychiatry. 2003;44:1158–1176. doi: 10.1111/1469-7610.00204. [DOI] [PubMed] [Google Scholar]
- Mason DJ, Humphreys GW, Kent L. Insights into the control of attentional set in ADHD using the attentional blink paradigm. The Journal of Child Psychology and Psychiatry. 2005;46:1345–1353. doi: 10.1111/j.1469-7610.2005.01428.x. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;4:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Dobson KS, Parsons J, Maurer D. Why 8-year-olds cannot tell the difference between Steve Martin and Paul Newman: factors contributing to the slow development of sensitivity to the spacing of facial features. Journal of Experimental Child Psychology. 2004;89:159–181. doi: 10.1016/j.jecp.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Nachev P, Husain M. Disorders of visual attention and the posterior parietal cortex. Cortex. 2006;42:766–773. doi: 10.1016/s0010-9452(08)70415-5. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Swanson JM, Hinshaw SP. Covert visual spatial attention in boys with attention deficit hyperactivity disorder: lateral effects, methylphenidate response, and results for parents. Neuropsychologia. 1997;35:165–176. doi: 10.1016/s0028-3932(96)00070-x. [DOI] [PubMed] [Google Scholar]
- Novak GP, Solanto M, Abikoff H. Spatial orienting and focused attention in attention deficit hyperactivity disorder. Neuropsychology. 1995;32:546–559. doi: 10.1111/j.1469-8986.1995.tb01231.x. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Alford JL, Marrocco RT. Normal attention orienting but abnormal stimulus alerting and conflict effect in combined subtype of ADHD. Behavioural Brain Research. 2005;165:1–11. doi: 10.1016/j.bbr.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Glahn DC, Semrud-Clikeman M, Franklin C, Perez R, 3rd, Xiong J, Liotti M. Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naïve or in long-term treatment. The American Journal of Psychiatry. 2006;163:1052–1060. doi: 10.1176/ajp.2006.163.6.1052. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal R. Effects of parietal injury on convert orienting of visual attention. Journal of Neuroscience. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Frith CD, Lavie N. Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science. 1997;278:1616–1619. doi: 10.1126/science.278.5343.1616. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. Journal of Neuroscience. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naïve adolescents with ADHD. The American Journal of Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Temporal lobe dysfunction in medication-naïve boys with attention-deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biological Psychiatry. 2007;62:999–1006. doi: 10.1016/j.biopsych.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, Posner MI. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Aase H, Johansen EP, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences. 2005;28:397–468. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Sergeant J. Modeling attention-deficit/hyperactivity disorder: A critical appraisal of the cognitive-energetic model. Biological Psychiatry. 2005;57:1248–1255. doi: 10.1016/j.biopsych.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Oosterlaan J, van der Meere JJ. Information processing and energetic factors in attention-deficit/hyperactivity disorder. In: Quay HC, Hogan A, editors. Handbook of Disruptive Behavior Disorders. New York: Plenum Press; 1999. pp. 75–104. [Google Scholar]
- Scherf KS, Behrmann M, Humphreys K, Luna B. Visual category-selectivity for faces, places, and objects emerges along different developmental trajectories. Developmental Science. 2007;10:F15–F30. doi: 10.1111/j.1467-7687.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Fan J, Tang CY, Newcorn JH, Buchsbaum MS, Cheung AM, Halperin JM. Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: an event-related fMRI study. The American Journal of Psychiatry. 2004;161:1650–1657. doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Science, USA. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJS. Causal models of attention-deficit/hyperactivity disorder: From common simple deficits to multiple developmental pathways. Biological Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Suskauer SJ, Simmonds DJ, Caffo BS, Denckla MB, Pekar JJ, Mostofsky SH. fMRI of intrasubject variability in ADHD: Anomalous premotor activity with prefrontal compensation. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:1141–1150. doi: 10.1097/CHI.0b013e3181825b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsushima Y, Sasaki Y, Watanabe T. Greater disruption due to failure of inhibitory control of an ambiguous distractor. Science. 2006;314:1786–1788. doi: 10.1126/science.1133197. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JD. Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. The American Journal of Psychiatry. 2005;162:1605–1613. doi: 10.1176/appi.ajp.162.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meere J, Sergeant J. Focused attention in pervasively hyperactive children. Journal of Abnormal Child Psychology. 1988;16:627–639. doi: 10.1007/BF00913474. [DOI] [PubMed] [Google Scholar]
- Williams BR, Strauss EH, Hultsch DF, Hunter MA, Tannock R. Reaction time performance in adolescents with attention deficit/hyperactivity disorder: Evidence of inconsistency in the fast and slow portions of the RT distribution. Journal of Clinical and Experimental Neuropsychology. 2007;29:277–289. doi: 10.1080/13803390600678020. [DOI] [PubMed] [Google Scholar]
- Yi DJ, Woodman GF, Widders D, Marois R, Chun MM. Neural fate of ignored stimuli: Dissociable effects of perceptual and working memory load. Nature Neuroscience. 2004;7:992–996. doi: 10.1038/nn1294. [DOI] [PubMed] [Google Scholar]