Abstract
Introduction
Pericardial fat is emerging as an important parameter for cardiovascular risk stratification. We extended previously developed quantitation of thoracic fat volume (TFV) from non-contrast coronary calcium (CC) CT scans to also quantify pericardial fat volume (PFV) and investigated the associations of PFV and TFV with CC and the Metabolic Syndrome (METS).
Methods
TFV is quantified automatically from user-defined range of CT slices covering the heart. Pericardial fat contours are generated by spline interpolation between 5-7 control points, placed manually on the pericardium within this cardiac range. Contiguous fat voxels within the pericardium are identified as pericardial fat. PFV and TFV were measured from non-contrast CT for 201 patients. In 105 patients, abdominal visceral fat area (VFA) was measured from an additional single-slice CT. In 26 patients, images were quantified by 2 readers to establish inter-observer variability. TFV and PFV were examined in relation to Body Mass Index (BMI), waist circumference and VFA, standard coronary risk factors (RF), CC (Agatston score >0) and METS.
Results
PFV and TFV showed excellent correlation with VFA (R=0.79, R=0.89, p <0.0001), and moderate correlation with BMI (R=0.49, R=0.48, p<0.0001). In 26 scans, the inter-observer variability was greater for PFV (8.0 ± 5.3 %) than for TFV (4.4 ± 3.9 %, p = 0.001). PFV and TFV, but not RF, were associated with CC [PFV: p=0.04, Odds Ratio 3.1; TFV: p<0.001, OR 7.9]. PFV and TFV were also associated with METS [PFV: p<0.001, OR 6.1; TFV p<0.001, OR 5.7], unlike CC [OR=1.0 p =NS] or RF. PFV correlated with low-HDL and high-glucose; TFV correlated with low-HDL, low-adiponectin, and high glucose and triglyceride levels.
Conclusions
PFV and TFV can be obtained easily and reproducibly from routine CC scoring scans, and may be important for risk stratification and monitoring.
Keywords: Pericardial fat, Thoracic fat, non-contrast CT, coronary calcium, metabolic syndrome
Introduction
Regional visceral fat distribution may contribute to an unfavorable metabolic and cardiovascular risk profile 1, 2. Pericardial fat, a local visceral fat depot surrounding coronary arteries, may contribute to the development of coronary atherosclerosis through local production of inflammatory cytokines 3-5 and is emerging as an important parameter for further cardiovascular risk stratification 1, 2.
Non-contrast cardiac computed tomography (CT) has been increasingly used during the past 15 years, with the principal goal of identifying patients at risk of having obstructive coronary artery disease based on the presence and severity of coronary calcium, a marker of subclinical coronary atherosclerosis. Pericardial and thoracic fat are routinely imaged by non-contrast CT. It has been shown that thoracic fat volume (TFV) correlates with abdominal visceral fat, a known cardiovascular risk factor 6, 7. Pericardial fat quantified from non-contrast CT was shown to be associated with the presence of coronary calcium 3, 5 and coronary artery disease 8. Ding et al have shown that pericardial fat quantified manually from non-contrast CT from a 45 mm-slab about the left main coronary artery was independently associated with coronary calcium 5. Recently, Mahabadi et al have reported that pericardial fat volume (PFV) quantified from non-contrast CT is independently associated with cardiovascular events 9. To date, epidemiological studies of pericardial and thoracic fat used manual quantification of fat volumes, a time-consuming process subject to inter-observer variability. Currently, no software tool for quantification of pericardial fat is available. We have previously developed a software method (QFAT) for semi-automated quantification of TFV from non-contrast cardiac CT, and demonstrated that TFV quantified by this method shows excellent correlation with abdominal visceral fat 7. In this study, we extended our previously developed method thoracic fat quantification to compute PFV separately using fast tracing of pericardial contours and investigated the associations of CT-measured PFV and TFV with coronary calcium and the metabolic syndrome.
Methods
Patients and Imaging protocol
Our study was a retrospective analysis of 201 sequential non-contrast CT scans, from patients scanned under the EISNER (Early Identification of Subclinical Atherosclerosis using Non-invasivE Imaging Research) study performed at the Cedars-Sinai Medical Center. In the EISNER study, clinical, biochemical, and non-contrast CT data from individuals with coronary risk factors but without known coronary artery disease (CAD) were collected. Table 1 lists the patient characteristics. Non-contrast CT scans were acquired using either an Electron Beam CT (EBCT) scanner (e-Speed, manufactured by GE Healthcare, Milwaukee, WI) or a Multislice CT (MSCT) scanner (Somatom Volumezoom, manufactured by Siemens Medical Solutions, Forcheim, Germany). The study was conducted according to the guidelines of the Cedars-Sinai Medical Center Institutional Review Board, and all patients gave written informed consent for retrospective use of their data.
Table 1.
Patient Characteristics
| Age (yrs) | 61 ± 10 |
| Male gender | 110/201 (55%) |
| BMI (Kg/m2) | 27.1 ± 4 |
| Framingham Risk score | 12 ± 8 (intermediate to high) |
| Family history of CAD | 66/201 (33%) |
| HDL (mg/dl) | 53 ±17 |
| LDL (mg/dl) | 123±42 |
| Triglycerides (mg/dl) | 139 ± 137 |
| Smoking | 23/201 (11%) |
| Arterial hypertension | 118/201 (59%) |
| Diabetes | 36/201 (18%) |
| Agatston calcium score | 419 ± 700 |
Imaging and coronary calcium scoring
Each complete scan contained 50-60 contiguous, non-overlapping, 512×512 matrix slices over a 35-cm field of view. The pixel size was 0.68×0.68 mm. For each patient, the scan was obtained in a single breath-hold and extended from the aortic arch to the level of the diaphragm. Depending on the heart rate, ECG triggering was set to 45%-60% of the RR interval. For EBCT, images were obtained with 100-ms exposure time and 3-mm-thick slices. For MSCT, 120 kVp was used 10, and the slice thickness was 2.5 mm. MSCT scans were acquired with prospective ECG-gating. Each scanner was calibrated daily using both air and water phantoms. All CT images were reviewed by an experienced cardiac imaging physician. Each scan was analyzed using semi-automatic commercially available calcium scoring software (ScImage, Inc., Los Altos, CA). The total Agatston coronary calcium score (CCS) 11 for each scan was measured as the sum of calcified plaque scores of all the coronary arteries. In 105 patients, an additional single transverse CT-slice was acquired following cardiac CT acquisition, at the L4 to L5 level of the abdomen for abdominal fat assessment, as described previously 7, 12, 13. DICOM images were then transferred to a research workstation for fat quantification.
Pericardial, Thoracic and Abdominal Visceral Fat Quantification
We defined pericardial and thoracic fat as follows: Pericardial fat refers to all adipose tissue enclosed by the pericardium, including the epicardial fat surrounding the coronary arteries 3, 9. As in most recent studies, it is defined as the fat depot surrounding the coronary arteries. Total thoracic fat refers to the adipose tissue surrounding the heart enclosed by the rib-cage and above the diaphragm, and includes pericardial fat 9.
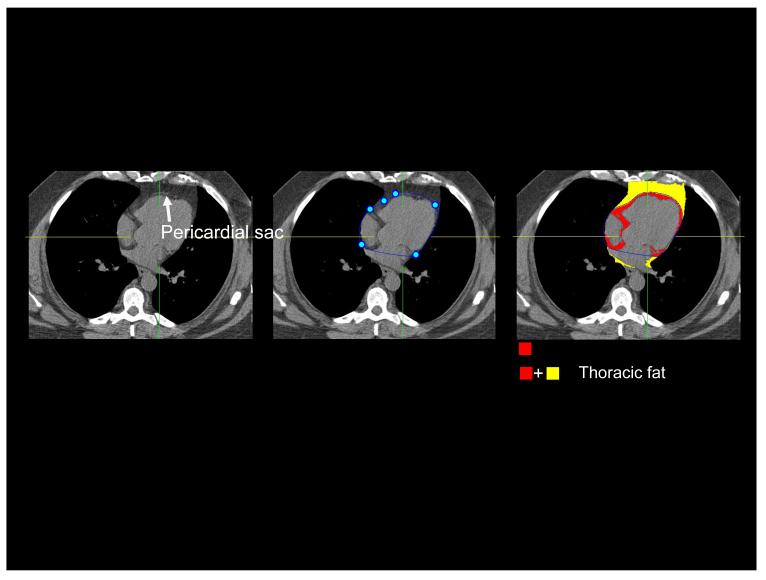
Pericardial and thoracic fat quantitation was performed by QFAT software developed at the Cedars-Sinai Medical Center. The software is written in C++ includes algorithms for automatic segmentation of the thoracic cavity and heart, and quantification of pericardiac or thoracic fat, which we have already described 7. In our algorithm, thoracic fat is limited in the posterior by the descending aorta or the top of the spine, whichever is higher. To allow quantification of the pericardial and thoracic fat at the same time, image data was processed as follows. First, the upper slice limit, marked by bifurcation of the pulmonary trunk, and lower slice limit, identified as the last slice containing any portion of the heart, were manually chosen from visual review of the CT images. Next, an experienced reader scrolled through the slices between upper and lower heart limit and if the pericardium was visualized, placed 5-7 control points on the pericardium in transverse view. From the control points, piecewise cubic Catmull-Rom spline functions 14 were automatically generated to obtain a smooth closed pericardial contour (Figure 1(a)). If in a particular slice the pericardium was not visualized (for example, in the superior slices close to the upper limit), this interaction was not necessary, and fat voxels adjacent to the heart were identified automatically following automated cardiac segmentation7. Following selection of cardiac limits and placing of control points, pericardial and thoracic fat quantification was automated, initiated with a single button-click. Contiguous 3D voxels between the Hounsfield Units (HU) limits of (−190, −30) were defined as fat voxels by default; this CT attenuation range for adipose tissue has been validated by previous investigators 6, 12, 13, 15 and could be modified by the user if needed. PFV and TFV were reported in cm3.
Figure 1.
(a) Figure illustrating pericardial and thoracic fat quantification for a 65-year old asymptomatic male patient from the EISNER study. Left: white arrow points to the pericardial sac as a thin band enveloping the heart. Middle: pericardial sac (closed curve in blue) is traced by an expert observer by placing 5-7 control points (shown as blue circles) on the pericardium. Right: Fat quantification results. Red overlay represents pericardial fat enclosed by the pericardium. Yellow overlay represents fat outside the pericardium. Color overlay (Red + Yellow) represents total thoracic fat.
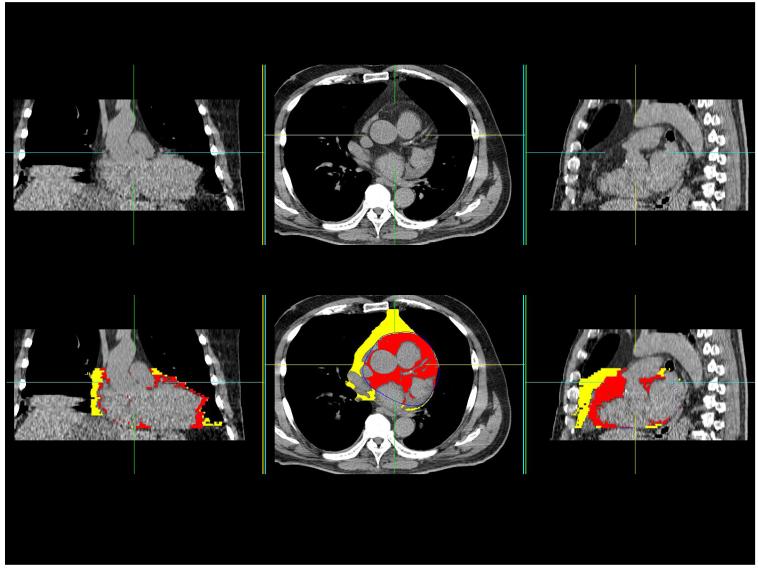
(b) CT study of a 71-year old male patient with a history of hypertension and no prior cardiovascular disease. Coronal, transverse and sagittal slices from the non-contrast CT scan are shown in top panel. Results of pericardial fat quantitation are shown in bottom panel. Red overlay represents pericardial fat enclosed by the pericardium. Yellow overlay represents thoracic fat outside the pericardium. PFV was 224 cm3 and TFV was 470 cm3.
Abdominal visceral fat processing was performed manually by an expert reader on the single-slice abdominal CT series, as described previously 7. The manual step consisted of drawing a closed region-of-interest (ROI) to separate subcutaneous and visceral fat. Connected voxels within the CT attenuation range of −190 to −30 HU were identified as fat 12, 16. Fat voxels inside the drawn ROI were classified as visceral fat and those outside were classified as subcutaneous fat. For each patient with single-slice CT, visceral fat area (VFA) and subcutaneous fat area (SFA) in cm2 were quantified.
Assessment of Risk factors and the Metabolic Syndrome
Prior to CT imaging, a fasting lipid profile (total cholesterol, HDL cholesterol, and triglycerides, with calculated LDL cholesterol) and glucose were obtained on each study participant using a Cholestech (Hayward, California) desktop chemical analyzer. Weight, height (for calculation of body mass index (BMI) – weight in kg/ height squared in meters), and two readings of blood pressure (with mean systolic and diastolic readings used for analysis) were also measured. A brief medical history was collected to assess prior history of cardiac disease, diabetes, typical cardiovascular event risk factors, and medication usage. Diabetes was defined as a self-reported history of being told by a physician that diabetes was present or having a fasting glucose of 126 mg/dl or greater. Smoking was defined as self-reported history of current smoking. Waist circumference measurements (measured by tape to the nearest 0.1 cm) were available for 127 patients.
Presence of metabolic syndrome (METS) was defined as recommended by the recent joint American Heart Association-National Heart Lung Blood Institute statement 17, based on the Third Adult Treatment Panel (ATP III) criteria of the National Cholesterol Education Program (NCEP) 18, with the modification of replacing waist circumference cutpoints with body-mass index (BMI), as previously described by our center 19 Therefore, patients were required have at least three of the following criteria to have METS: i) BMI of 30 kg/m2 or greater (in lieu of using ATP waist circumference cutpoints, which were not available for all patients in our study sample); ii) serum triglycerides of at least 150 mg/dl or drug treatment for elevated triglycerides ; iii) HDL cholesterol levels of <40 mg/dl in men and <50 mg/dl in women or drug treatment for reduced HDL cholesterol ; iv) impaired fasting glucose of at least 100 mg/dl or drug treatment for elevated glucose; or v) blood pressure of at least 130/85 mm Hg or drug treatment for hypertension 17. A published analysis of the Third National Health and Nutrition Examination Survey showed a high concordance of obesity at or above our BMI cutpoint and a high waist circumference as defined by NCEP III criteria 20, lending support to the validity of this modification. Blood serum was available for 100 of the 201 patients. For these patients, C-reactive protein and adiponectin were measured from blood serum by Biosite (San Diego, CA).
Statistical Methods
Statistical analyses were performed using Analyse-it (www.analyse-it.com, Analyse-it Software Ltd, Leeds, UK) and STATA (Version 10, www.stata.com, StataCorp LP, Texas, USA) software packages. All continuous variables were expressed as mean ± standard deviation (s.d.). Groupwise comparisons were performed with one-way analysis of variance (ANOVA), with Bonferroni correction between pairs. PFV and TFV were not normally distributed and were log-transformed to base 2; the Agatston score was normally distributed. Multivariable logistic regression was performed to examine association of PFV and TFV with CCS (Agatston score >0), METS and presence of combined METS and Diabetes Mellitus. Separate multivariable statistical logistic regression tests were needed to examine association of PFV and TFV with the outcome variables; since TFV and PFV are dependent measures (TFV includes PFV). These tests have been termed “TFV Analysis” and “PFV Analysis” in the tables. For the regressions using METS as outcome, HDL, triglycerides were omitted since they were part of the METS definition. Presence of diabetes could not be used as outcome since only 36/201 (18%) of patients had diabetes in our study. Presence of arterial hypertension was adjusted by self-reported usage of medication for lowering hypertension. A p-value of <0.05 was considered statistically significant.
Results
Processing time ranged from 7-11 minutes for pericardial contour tracing and was less than 20 seconds for automated quantitation of TFV on a standard 2.5 GHz personal computer running Windows XP. Figure 1 (b) shows an example of fat quantification from our study. Mean PFV and TFV values for all 201 studies were 87.3 ± 43.7 cm3 and 189.6 ± 109.1 cm3, respectively. There was excellent correlation between PFV and TFV (R=0.87, p<0.0001), with a best-fit linear relationship of y=2.14x. In the 26 scans analyzed by both observers, the inter-observer variability was significantly greater for PFV (8.0 ± 5.3 %) than for TFV (4.4 ± 3.9 %, p = 0.001). For TFV, each observer was required to only choose the limits of the heart. For PFV, each observer needed to trace pericardial contours after choosing the heart limits, which resulted in higher observer variability and longer processing time.
Figure 2(a) shows the correlation of PFV and TFV with VFA quantified from single-slice abdominal CT. PFV and TFV showed strong correlation with VFA, with TFV showing significantly stronger correlation (R=0.89 vs. R=0.79, p < 0.01). Correlation with BMI was moderate for both PFV and TFV (R=0.49, R=0.48, p<0.0001). PFV and TFV correlated similarly with CCS. TFV had greater correlation to the coronary calcium score compared to PFV (R = 0.29 for TFV, R = 0.21 for PFV, p < 0.0001). However, this difference in correlation coefficient was not statistically significant (p = 0.39). Similar correlation has been shown by Ding et al (R = 0.32, p < 0.0001) for 159 subjects from the MESA study 5.
Figure 2.
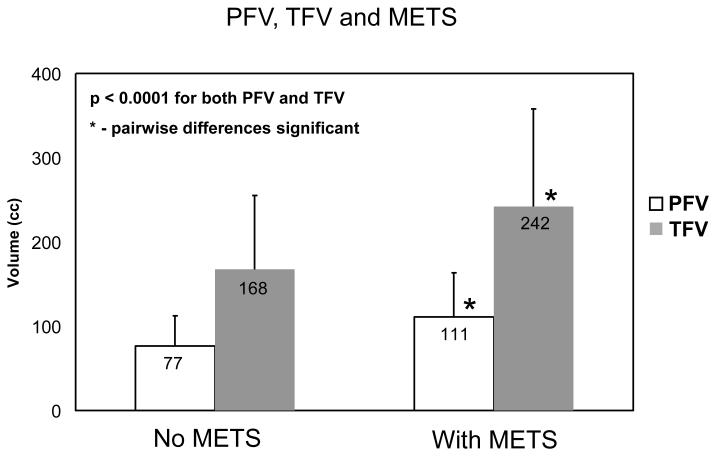
(a) Correlation of CT-measured (a) pericardial fat volume (PFV) and (b) thoracic fat volume (TFV) with abdominal visceral fat area (VFA) (N=105). (b) PFV and TFV for patients with and without coronary calcium (p=0.006). Pairwise differences were significant for both. (c) PFV and TFV for patients with and without METS (p<0.0001). Pairwise differences were significant for both.
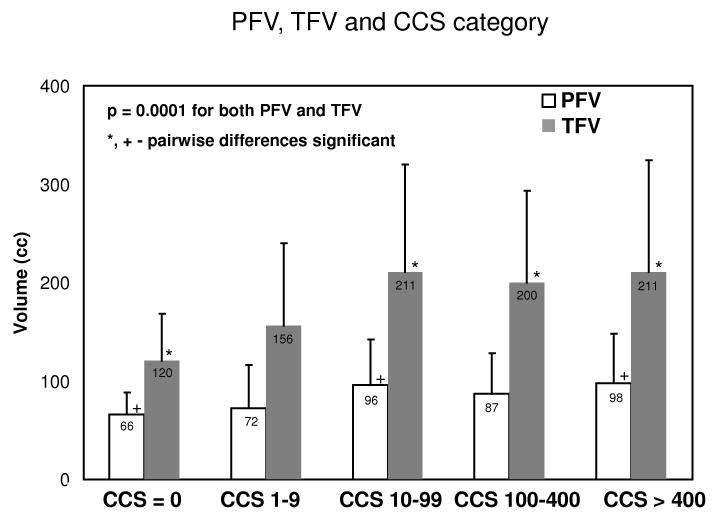
Figure 2(b) shows PFV and TFV for patients grouped according to presence of coronary calcium. PFV and TFV were significantly different for patients with and without coronary calcium (p = 0.006). Figure 3 (appendix) shows PFV and TFV for patients with no coronary calcium and with coronary calcium in the four standard CCS categories: 1-9, 10-99, 100-399, 400 and greater; PFV and TFV were significantly different across the groups (p=0.0001).
Figure 3 (appendix).
PFV and TFV for coronary calcium score categories (p = 0.0001 from ANOVA analysis). Significant pairwise differences are shown by + for PFV and by * for TFV.
Table 2(a) shows the association of PFV and TFV with presence of coronary calcium in comparison with standard pre-scan cardiovascular risk factors and obesity parameters. From Table 2, a doubling of PFV and TFV showed higher association with coronary calcium than standard risk factors (p = 0.01, odds ratio 2.4 for log2(PFV); p < 0.001, odds ratio 3.8 for log2(TFV) ).
Table 2 (a).
Association of PFV and TFV with coronary calcium in comparison with pre-scan cardiovascular risk factors (* indicates significant). +
| Odds Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|
| PFV Analysis | |||
| Family history of CAD | 0.9 | 0.4-2.2 | 0.8 |
| Smoking | 2.0 | 0.4-9.8 | 0.4 |
| Arterial hypertension | 0.6 | 0.2-1.3 | 0.2 |
| Diabetes Mellitus | 2.2 | 0.4-10.6 | 0.3 |
| LDL | 1.0 | 0.9-1.0 | 0.3 |
| HDL | 1.0 | 0.9-1.0 | 0.5 |
| Triglycerides | 1.0 | 0.9-1.0 | 0.5 |
| log2( PFV ) | 2.4 | 1.2-4.8 | 0.01* |
| TFV Analysis | |||
| Family history of CAD | 0.9 | 0.4-2.3 | 0.9 |
| Smoking | 2.0 | 0.4-10.6 | 0.4 |
| Arterial hypertension | 0.5 | 0.2-1.2 | 0.1 |
| Diabetes Mellitus | 2.0 | 0.4 -10.0 | 0.4 |
| LDL | 1.0 | 0.9-1.0 | 0.3 |
| HDL | 1.0 | 0.9-1.0 | 0.8 |
| Triglycerides | 1.0 | 0.9-1.0 | 0.5 |
| log2( TFV ) | 3.8 | 2.0-7.2 | <0.001* |
- Corrected for age and gender.
Separate multivariable logistic regression analyses were performed for TFV, PFV since they are dependent measures.
In our patient population, 60/201 patients had the metabolic syndrome and 75/201 patients had metabolic syndrome or diabetes. Figure 2(c) shows PFV and TFV for patients grouped according to presence of the metabolic syndrome. PFV and TFV were significantly different for patients with and without METS (p=< 0.0001). Table 2(b) shows association of PFV and TFV with METS in comparison with standard pre-scan risk factors and CCS. Table 2(c) shows association of PFV and TFV with combined METS and diabetes compared with standard risk factors and CCS. PFV and TFV showed a significantly higher association with both METS and combined METS and diabetes than standard risk factors or CCS. In all patients, TFV and PFV showed significantly higher association with coronary calcium than BMI (p=0.008, odds ratio 2.5 for log2 (PFV); p<0.001, odds ratio 4.3 for log2 (TFV)). In 127 patients, waist circumference was measured with tape measure prior to the scan and we considered the association of PFV, TFV with coronary calcium compared with BMI, measured waist circumference and waist-height ratio. In these patients, TFV showed significant association with coronary calcium compared to these three obesity measures (p = 0.003, odds ratio 3.3 for log2 (TFV)) but PFV was not significant (p = 0.13, odds ratio 1.8 for log2 (PFV)).
Table 2 (b).
Association of PFV and TFV with METS in comparison with risk factors and CCS (* indicates significant). +
| Odds Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|
| PFV Analysis | |||
| Age | 1.0 | 0.9-1.0 | 0.7 |
| Gender | 0.7 | 0.3-1.7 | 0.5 |
| Smoking | 2.6 | 0.9-7.1 | 0.06 |
| Arterial hypertension | 2.2 | 1.0-4.6 | 0.04* |
| LDL | 1.0 | 0.9-1.0 | 0.2 |
| CCS | 1.0 | 0.9-1.0 | 0.3 |
| log2( PFV ) | 3.5 | 2.0-6.2 | <0.001* |
| TFV Analysis | |||
| Age | 1.0 | 0.9-1.0 | 0.6 |
| Gender | 0.5 | 0.2-1.2 | 0.1 |
| Smoking | 2.7 | 0.9-7.3 | 0.05 |
| Arterial hypertension | 2.0 | 1.0-4.3 | 0.06 |
| LDL | 1.0 | 0.9-1.0 | 0.3 |
| CCS | 1.0 | 0.9-1.0 | 0.4 |
| log2( TFV ) | 3.3 | 1.9-5.8 | <0.001* |
Separate multivariable logistic regression analyses were performed for TFV, PFV since they are dependent measures.
Table 2(c).
Association of PFV and TFV with combined METS and diabetes in comparison with risk factors and CCS (* indicates significant). +
| Odds Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|
| PFV Analysis | |||
| Age | 1.0 | 0.9-1.0 | 0.6 |
| Gender | 0.8 | 0.4-1.7 | 0.5 |
| Smoking | 1.7 | 0.6-4.6 | 0.3 |
| Arterial hypertension | 1.9 | 0.9-3.7 | 0.06 |
| LDL | 1.0 | 0.9-1.0 | 0.8 |
| CCS | 1.0 | 0.9-1.0 | 0.3 |
| log2( PFV ) | 3.4 | 1.2-5.9 | <0.001* |
| TFV Analysis | |||
| Age | 1.0 | 0.9-1.0 | 0.5 |
| Gender | 0.5 | 0.2-1.2 | 0.2 |
| Smoking | 1.7 | 0.6-4.6 | 0.3 |
| Arterial hypertension | 1.8 | 0.9-3.5 | 0.08 |
| LDL | 1.0 | 0.9-1.0 | 0.8 |
| CCS | 1.0 | 0.9-1.0 | 0.4 |
| log2( TFV ) | 2.9 | 1.7-4.9 | <0.001* |
Separate multivariable logistic regression analyses were performed for TFV, PFV since they are dependent measures.
Table 3 shows the Spearman rank correlation of PFV and TFV with serum biomarkers. Correlation with serum biomarkers, when significant (p<0.05), was moderate to weak, similar to a recent study by Greif et al 21. PFV showed inverse correlation with HDL (R = −0.27, p = 0.0001) and correlation with glucose (R = 0.31, p< 0.0001). TFV showed inverse correlation with HDL (R = −0.33, p = 0.0001) and adiponectin (R = −0.21, p=0.03), correlation with triglycerides (R = 0.17, p = 0.01), and glucose (R = 0.31, p < 0.0001).
Table 3.
Spearman Rank Correlation of PFV and TFV with serum markers (p-value in brackets).
| PFV Rank correlation | TFV Rank correlation | |
|---|---|---|
| HDL | −0.27 (0.0001)* | −0.33 (0.0001)* |
| LDL | −0.01(0.9) | −0.02 (0.7) |
| Triglycerides | 0.13 (0.07) | 0.17 (0.01)* |
| Glucose | 0.31 (<0.0001)* | 0.31 (<0.0001*) |
| Adiponectin | −0.15 (0.13) | −0.21 (0.03)* |
| CRP | −0.04 (0.7) | −0.01 (0.9) |
Discussion
Our study indicates that computer-aided quantification of PFV and TFV can be performed from non-contrast coronary calcium scans with TFV being performed almost automatically with high reproducibility and PFV requiring moderate user interaction. We have previously demonstrated the accuracy of the semi-automated quantitation, TFV, showing excellent agreement with QFAT and expert manual processing 7. “Pericardiac fat” in the previous paper actually equates to TFV in the current manuscript; we revised this term to TFV to correctly differentiate it from PFV and to be more consistent with subsequent publications on this topic 3, 9. PFV is essentially manual measurement by drawing closed pericardial contours, followed by fat quantitation using standard preset fat thresholds. PFV measurement was not available in QFAT at the time of our previous publication7; this is its first description. To our knowledge, this is the first report of a tool which allows fast quantification of both PFV and TFV from the non-contrast CT scan at the same time; such a tool could potentially advance existing techniques for cardiovascular risk assessment, both in clinical research and in clinical practice. We showed that PFV and TFV correlate similarly with CCS, and both PFV and TFV are strongly associated with the presence of coronary calcium, unlike standard risk factors. Additionally, PFV and TFV are strongly associated with METS and combined METS and diabetes, unlike standard risk factors and CCS. Compared to PFV, TFV has the advantage of automatic quantification as soon as simple limits of the heart are chosen, which results in lower observer variability.
TFV correlated with low adiponectin and low HDL levels and with high glucose and triglyceride levels. It has been shown that human epicardial adipose tissue expresses adiponectin and that adiponectin expression is significantly higher in epicardial fat from subjects with normal coronary arteries than in patients with severe coronary artery disease 1. Interestingly, there was no correlation with CRP. However, CRP is a non-specific marker of inflammation and plasma inflammatory biomarkers may not adequately reflect local tissue inflammation in all patients 22. High-sensitivity CRP values were not available in our patient population.
Although semi-automated quantitation of thoracic fat have been described 7, 23, to our knowledge, ours is the first report of fast, simultaneous quantitation PFV and TFV from non-contrast CT, and subsequent direct comparison of these measures. Our reproducibility was lower than that recently reported by Grief et al who reported inter-observer variability of 15% for PFV and 8% for TFV from manual quantification of coronary CT Angiography scans 21. Our study adds to several recent studies underscoring the clinical importance of pericardial fat. Ding et al have also shown that pericardial fat, quantified manually from a 45 mm thick slab around the origin of the left main artery, was independently associated with calcified coronary plaque in 159 individuals from the MESA study, they did not, however, quantify TFV from the same slices 5. Grief et al, manually quantified TFV from contrast-enhanced coronary CT angiography (CCTA) scans from 286 consecutive patients and found that patients with atherosclerotic coronary plaque on CCTA had significantly larger TFV than patients without plaque, and elevated TFV strongly predicted the presence of coronary atherosclerosis as imaged by CCTA and were correlated with hypoadiponectimia 21. Rosito et al found that pericardial and intra-thoracic fat volume, quantified manually in 1155 participants of the Framingham Heart Study, were associated with vascular calcification, suggesting that these fat depots may exert local toxic effects on the vasculature 3. Interestingly, the same group has recently reported that pericardial fat, but not thoracic fat, was independently associated with cardiovascular events 9. Since our automated algorithm quantifies TFV within a bounding box about the heart, and excludes posterior fat 7, it centers more on the heart than previously-described methods 3, 9. While the patients in this study were a consecutive cohort of 201 patients without cardiovascular event information, we have recently completed an outcome analysis of 232 matched patients from 2751 asymptomatic patients without known CAD (enrolled in the EISNER study), with prospective 4-year post-scan follow-up for major adverse cardiovascular events (MACE); the MACE events were cardiac death, myocardial infarction, stroke, and late revascularization24. We compared 58 cases who experienced MACE to 174 event-free controls (1:3 MACE-to-control ratio), matched by gender and propensity score to account for age, traditional risk factors and coronary calcium score. PFV and TFV were measured in these 232 patients as described in this manuscript. Our results showed that MACE patients had significantly higher mean PFV, higher mean TFV and higher frequencies of PFV > 125 cm3 and TFV > 250 cm3. In multivariable regression analysis, doubling of PFV and TFV were both associated with MACE (odds ratio 1.74, p = 0.038 for log2(PFV); odds ratio 1.78, p = 0.047 for log2(TFV)).
Limitations
Our study had several limitations. We used CCS as the reference marker for CAD and did not have invasive angiographic data in these patients to further describe coronary artery disease severity. Our algorithm for quantifying PFV and TFV was not completely automatic and still required user interaction. Our patient sample size was small (201). Single-slice abdominal CT is an additional CT scan, and was available only for a subset of the 201 patients; however, conclusive results could be reached in this subgroup. Additionally, blood serum was only available in a subset of the 201 patients. Our patient population was at an intermediate clinical risk for CAD, with intermediate-to-high Framingham Risk Scores and high CCS. In our study population without prior cardiovascular disease, it was not possible to compare PFV or TFV to other modalities, such as echocardiography, Magnetic Resonance Imaging (MRI), or to contrast-enhanced CT coronary angiography. Prognostic studies with event follow-up are needed to prove the incremental predictive impact of PFV and TFV over CCS and standard risk factors.
Conclusions
Volumetric measures of pericardial and thoracic fat can be obtained reproducibly from routine coronary calcium scoring scans. Thoracic (pericardiac) fat volumes can be obtained automatically and are more reproducible than pericardial fat volumes. Both parameters correlate similarly with CCS, and are strongly associated with presence of CC, METS and combined METS and diabetes, and will be important for risk stratification and monitoring.
Acknowledgements
The authors would like to thank Romalisa Miranda-Peats, Abhishek Shah, Olga Guzovsky for their assistance with patient data analysis. This study was supported by NIH grant number R21EB006829-01A2 (PI: Damini Dey) from the National Institute Of Biomedical Imaging And Bioengineering (NIBIB), and also in part by a grant from the Glazer Foundation (IRB 6318). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIBIB or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest: none
Financial disclosure: N/A
References
- 1.Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, Gallo P, di Gioia CR. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005 Mar 21;29(6):251–255. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Kortelainen ML. Myocardial infarction and coronary pathology in severely obese people examined at autopsy. Int J Obes Relat Metab Disord. 2002 Jan;26(1):73–79. doi: 10.1038/sj.ijo.0801852. [DOI] [PubMed] [Google Scholar]
- 3.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Pericardial Fat, Visceral Abdominal Fat, Cardiovascular Disease Risk Factors, and Vascular Calcification in a Community-Based Sample: The Framingham Heart Study. Circulation. 2008 February 5;117(5):605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. 2008. [DOI] [PubMed] [Google Scholar]
- 4.Gorter PM, van Lindert ASR, de Vos AM, Meijs MFL, van der Graaf Y, Doevendans PA, Prokop M, Visseren FLJ. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197(2):896–903. doi: 10.1016/j.atherosclerosis.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Ding J, Kritchevsky SB, Harris TB, Burke GL, Detrano RC, Szklo M, Carr J Jeffrey. The Association of Pericardial Fat With Calcified Coronary Plaque. Obesity. 2008;16(8):1914–1919. doi: 10.1038/oby.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheeler GL, Shi R, Beck SR, Langefeld CD, Lenchik L, Wagenknecht LE, Freedman BI, Rich SS, Bowden DW, Chen MY, Carr JJ. Pericardial and visceral adipose tissues measured volumetrically with computed tomography are highly associated in type 2 diabetic families. Invest Radiol. 2005 Feb;40(2):97–101. doi: 10.1097/00004424-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Dey D, Suzuki Y, Suzuki S, Ohba M, Slomka PJ, Polk D, Shaw LJ, Berman DS. Automated Quantitation of Pericardiac Fat From Noncontrast CT. Invest Radiol. 2008 Feb;43(2):145–153. doi: 10.1097/RLI.0b013e31815a054a. [DOI] [PubMed] [Google Scholar]
- 8.Taguchi R, Takasu J, Itani Y, Yamamoto R, Yokoyama K, Watanabe S, Masuda Y. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis. 2001 Jul;157(1):203–209. doi: 10.1016/s0021-9150(00)00709-7. [DOI] [PubMed] [Google Scholar]
- 9.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O’Donnell CJ, Fox CS, Hoffmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. European Heart Journal. 2009 January 9; doi: 10.1093/eurheartj/ehn573. 2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniell ALFJ, Ben-Yosef N, Wong ND, Miranda-Peats L, Ventresca MTHS, Sciammarella MG, Berman DS. Concordance of Coronary Calcium Estimation between Multi-Detector and Electron Beam CT. Circulation. 2002;106(II):479. [Google Scholar]
- 11.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990 Mar 15;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 12.Yoshizumi T, Nakamura T, Yamane M, Islam AH, Menju M, Yamasaki K, Arai T, Kotani K, Funahashi T, Yamashita S, Matsuzawa Y. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999 Apr;211(1):283–286. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 13.Kvist H, Chowdhury B, Grangard U, Tylen U, Sjostrom L. Total and visceral adiposetissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988 Dec;48(6):1351–1361. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 14.Catmull E, Rom R. A class of local interpolating splines. Proc. Of International Conference on Computer Aided Geometric Design; 1974.pp. 317–326. [Google Scholar]
- 15.Sjostrom L, Kvist H, Cederblad A, Tylen U. Determination of total adipose tissue and body fat in women by computed tomography, 40K, and tritium. Am J Physiol. 1986 Jun;250(6 Pt 1):E736–745. doi: 10.1152/ajpendo.1986.250.6.E736. [DOI] [PubMed] [Google Scholar]
- 16.Wajchenberg B. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr., Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005 Oct 25;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 18.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001 May 16;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 19.Wong ND, Sciammarella MG, Polk D, Gallagher A, Miranda-Peats L, Whitcomb B, Hachamovitch R, Friedman JD, Hayes S, Berman DS. The metabolic syndrome, diabetes, and subclinicalatherosclerosis assessed by coronary calcium. Journal of the American College of Cardiology. 2003;41(9):1547–1553. doi: 10.1016/s0735-1097(03)00193-1. [DOI] [PubMed] [Google Scholar]
- 20.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med. 2002 Oct 14;162(18):2074–2079. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- 21.Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Brödl U, Tittus J, Parhofer K, Becker C, Reiser M, Knez A, Leber AW. Pericardial Adipose Tissue Determined by Dual Source CT Is a Risk Factor for Coronary Atherosclerosis. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.108.180653. in press. [DOI] [PubMed] [Google Scholar]
- 22.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003 Nov 18;108(20):2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 23.Bandekar A, Naghavi M, Kakadiaris IA. Automated Pericardial Fat Quantification in CT Data. Proc. of the 28th Annual Intl. Conf. of the IEEE Engineering in Medicine and Biology Society, (EMBS’06); New York. Aug 30 - Sep 3. 2006; [DOI] [PubMed] [Google Scholar]
- 24.Cheng V, Dey D, Tamarappoo B, Nakazato R, Gransar H, Ramesh A, Slomka PJ, Berman DS. Pericardial and thoracic fat burden in asymptomatic patients who experience adverse cardiovascular events after noncontrast CT for calcium scoring. Submitted to the American Heart Association Annual Meeting; 2009. [Google Scholar]